Key Points
We report a first-in-human dose-escalation study in relapsed/refractory B-cell malignancies with the potent BTK inhibitor ONO/GS-4059.
ONO/GS-4059 induced clinically durable responses in relapsed/refractory B-cell malignancies without significant toxicities.
Abstract
We report the results of a multicenter phase 1 dose-escalation study of the selective Bruton tyrosine kinase (BTK) inhibitor ONO/GS-4059 in 90 patients with relapsed/refractory B-cell malignancies. There were 9 dose-escalation cohorts ranging from 20 mg to 600 mg once daily with twice-daily regimens of 240 mg and 300 mg. Twenty-four of 25 evaluable chronic lymphocytic leukemia (CLL) patients (96%) responded to ONO/GS-4059, with a median treatment duration of 80 weeks; 21 CLL patients remain on treatment. Lymph node responses were rapid and associated with a concurrent lymphocytosis. Eleven of 12 evaluable patients with mantle cell lymphoma (92%) responded (median treatment duration, 40 weeks). Eleven of 31 non–germinal center B-cell diffuse large B-cell lymphoma patients (35%) responded but median treatment duration was 12 weeks due to development of progressive disease. ONO/GS-4059 was very well tolerated with 75% of adverse events (AEs) being Common Toxicity Criteria for Adverse Events version 4.0 grade 1 or grade 2. Grade 3/4 AEs were mainly hematologic and recovered spontaneously during therapy. One CLL patient experienced a grade 3 treatment-related bleeding event (spontaneous muscle hematoma) but no clinically significant diarrhea, cardiac dysrhythmias, or arthralgia were observed. No maximal tolerated dose (MTD) was reached in the CLL cohort. In the non-Hodgkin lymphoma cohort, 4 patients developed a dose-limiting toxicity, yielding an MTD of 480 mg once daily. ONO/GS-4059 has significant activity in relapsed/refractory B-cell malignancies without major drug-related toxicity. The selectivity of ONO/GS-4059 should confer advantages in combination therapies. This trial was registered at www.clinicaltrials.gov as #NCT01659255.
Introduction
Bruton tyrosine kinase (BTK) is a TEC family kinase1 that is broadly expressed in several hemopoietic lineages.2 BTK comprises 5 structural domains, including pleckstrin homology, TEC homology, Src homology 2 and Src homology 3, and kinase domains3; full BTK kinase activation is dependent on recruitment to the plasma membrane via the pleckstrin homology domain following phosphatidylinositol (3,4,5) triphosphate binding.4 In humans, BTK mutations result in arrested B-cell development leading to severe agammaglobulinemia.5 This, along with the observations of the central role of BTK in signaling from the B-cell receptor, has indicated that BTK is a functional therapeutic target and has led to the development of small-molecule BTK inhibitors for the treatment of B-cell malignancies and autoimmune conditions.6
An interesting feature of the adenosine triphosphate–binding pocket of BTK is the presence of a cysteine residue at 481; only 9 other kinases retain cysteine at comparable sites.7 It has been possible to design BTK inhibitors that target this exposed amino acid side chain specifically, in some instances irreversibly, through the formation of a covalent bond. The first-in-class irreversible BTK inhibitor, ibrutinib (Imbruvica; Pharmacyclics), has been approved for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and Waldenström macroglobulinemia (WM).8-10 The kinome of ibrutinib includes low nanomolar inhibition of several other key kinases such as epidermal growth factor receptor (EGFR), Janus kinase 3, and ErbB2.11 Such broad specificity may potentially have clinical utility; for example, inhibition by ibrutinib of interleukin-2–inducible T-cell kinase in T cells12 has resulted in a clinical trial in T-cell lymphoma (NCT02309580), whereas the inhibition of ErbB kinases may lead to application in solid malignancies.13 Most patients tolerate ibrutinib well. However, ibrutinib can also lead to significant toxicities, including bleeding, arthralgia, diarrhea, and atrial fibrillation (AF).9,14-17 Potent inhibition of EGFR by ibrutinib18 may explain the observed diarrhea, also seen in clinical practice with EGFR inhibitors.19
More selective BTK inhibitors have therefore been developed.6,20 ONO/GS-4059 is a selective and an irreversible inhibitor of BTK21; the structure and kinome of ONO/GS-4059 are shown in supplemental Figure 1 (see supplemental Data available on the Blood Web site). In vitro, BTK is potently inhibited by ONO/GS-4059 with a 50% inhibitory concentration of 2 nmol/L.21 ONO/GS-4059 induces classical apoptosis at nanomolar concentrations in the activated B-cell diffuse large B-cell lymphoma (DLBCL) cell line, TMD-822 and in a TMD-8 xenograft model, treatment with ONO/GS-4059 resulted in inhibition of tumor growth.23 These promising preclinical data prompted clinical evaluation of ONO/GS-4059. We report here clinical data from a multicenter phase 1 dose-escalation study of ONO/GS-4059 in 90 patients with relapsed and refractory B-cell malignancies.
Patients and methods
Ethical approval
Approval from the regulatory authorities was obtained prior to screening of the first patient, according to national and international regulations and guidelines. Each investigator had obtained written and dated approval from the Institutional Ethical Committee for the trial protocol, the written informed consent form, consent form updates, and all other written information provided to patients.
Patients
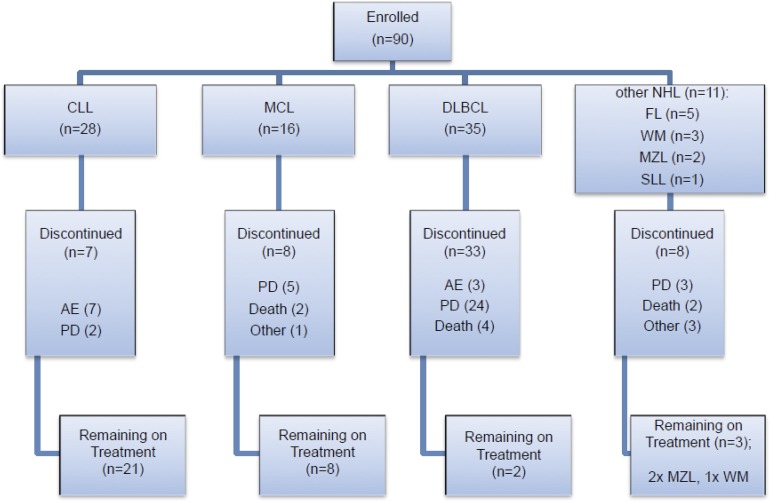
Eligible patients were aged 18 years or over with a diagnosis of relapsed or refractory non-Hodgkin lymphoma (DLBCL, MCL, follicular lymphoma [FL], small lymphocytic lymphoma [SLL], and WM confirmed by National Cancer Institute [NCI] Working Group Criteria24) or CLL. Inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, failure of ≥2 previous treatments, bidimensionally measurable disease >1.5 cm in its largest dimension, clinical indication for treatment and life expectancy >12 weeks. CLL patients were eligible if they met the following additional criteria: Binet stage C disease or Binet stage B disease requiring treatment for active disease as per NCI CLL Working Group Criteria. A summary of patients recruited according to the different diagnostic categories and their disposition in the trial is shown in Figure 1. Additional eligibility criteria included: 30 days from previous treatment, aspartate transaminase or alanine transaminase <2.5 times the upper limit of normal, total bilirubin <1.5 times the upper limit of normal, creatinine clearance ≥50 mL per minute, hemoglobin >10 g/dL, platelet count >50 × 109/L, and neutrophils >1.0 × 109/L. Exclusion criteria included: prior use of an investigational tyrosine kinase inhibitor (including BTK inhibitors) or investigational monoclonal antibody within 3 months of trial entry; prior history of pancreatitis; central nervous system lymphoma; documented history of Parkinson disease; cerebellar disorders or other movement-related disorders; history of malignancy that had been treated, but not with curative intent, unless in remission for ≥2 years without treatment; known active infection; positive results for HIV, hepatitis B or C; or significant uncontrolled concomitant diseases. A maximum dose of prednisolone 20 mg per day was permitted. There were no restrictions on anticoagulant use.
Figure 1.
Patient enrollment by disease subtype and disposition within the trial. Seven CLL patients were withdrawn due to AEs; 2 of the CLL patients withdrawn due to an AE also had concurrent PD. AEs were considered to be either related (R) or not related (NR) to study drug. In the CLL cohort, these AEs comprised: fever (NR), Escherichia coli septicemia (NR), worsening heart failure (NR), neutropenic sepsis (R), purpura, lymphocytic infiltration (R), spontaneous psoas hematoma (R), idiopathic thrombocytopenia (R). Three DLBCL patients were withdrawn due to AEs: myelodysplastic syndrome (NR), tubulopathy myeloma (renal tubular necrosis) (NR), and confusional state (NR). Two WM patients were withdrawn due to AEs: urticarial reaction (R), nonimmune drug reaction (R). Other reasons for discontinuation included investigator decision (2 DLBCL cases), patient decision (1 FL case), 1 case of MCL proceeding to allograft and DLTs in 2 WM patients. The majority of deaths especially in the DLBCL cohort were due to PD.
Study design and objective
The primary objective of this study was to determine the safety and tolerability of escalating oral doses of ONO/GS-4059 in patients with relapsed/refractory NHL and relapsed/refractory CLL. Each treatment cycle consisted of 28 days with continuous dosing. Study design was that of a 3 + 3 dose-escalation design with 2 parallel dose-escalation groups, 1 for NHL and 1 for CLL. Patients in both cohorts were treated with an initial dose of 20 mg once daily. Dosing for subsequent CLL cohorts was 40 mg, 80 mg, 160 mg, 320 mg, 400 mg, 500 mg, 600 mg once daily, and 300 mg twice daily. Subsequent dosing for NHL was 40 mg, 80 mg, 160 mg, 320 mg, 480 mg, 600 mg once daily, and 240 mg twice daily. Dose escalation continued until the maximal tolerated dose (MTD) was reached based on protocol-defined dose-limiting toxicities (DLTs) or the predetermined maximum dose of 600 mg was reached. DLTs were limited to drug-related toxicities occurring within the first 28 days of treatment. After the first patient in each new cohort reached day 15 without occurrence of a DLT, the remaining patients in the cohort were enrolled. All patients were followed for the occurrence of toxicities for 28 days from receiving their first dose of ONO/GS-4059 prior to the initiation of the next dose level/cohort. Where a DLT occurred, a further 3 patients were recruited to that dose level. DLTs were defined according to NCI–Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 as all CTC grade 4 ONO/GS-4059–related adverse events (AEs) and all CTC grade 3 ONO/GS-4059–related AEs with the exception of grade 3 lymphocytosis, which was considered an expected outcome of BTK inhibition.25
Study assessments
Frequency and intensity of AEs were graded throughout treatment, and toxicities were assessed by the NCI-CTCAE (version 4.0). Physical examination and blood samples for hematology, biochemistry, coagulation, and serum lipase/amylase were performed at every cycle. Leukocyte immunophenotyping and serum immunoglobulins (cycles 1, 3, and 5), urinalysis, and T-/B-cell counts were assessed on day 1 of each cycle. Disease response assessments included computed tomography (CT) imaging and physical examination. CT scans were performed during screening, at 3 monthly intervals for the first year and at 6 monthly intervals thereafter. All patients underwent CT scan at cycle 1 day 28 to check for possible drug-associated pancreatic changes. For CLL patients, analysis of prognostic markers was undertaken (including 17p deletions, 11q, 13q, trisomy 12, TP53 mutational status, IGHV mutational status) centrally. Determination of DLBCL subtype was performed locally using immunohistochemistry and the Hans algorithm.26 Best clinical response was determined based on Cheson et al for NHL patients,27 Owen et al for WM patients,28 and the International Workshop CLL (iwCLL) Working Guidelines 2008 for CLL patients.24 Progressive disease (PD) was defined from best response.
All patients were recommended to take ONO/GS-4059 in a fasted condition to minimize potential variability in absorption. Pharmacokinetic (PK) sampling was carried out for cycle 1 day 1 (predose then 30 minutes, 1, 2, 3, 4, 6, 8, and 12 hours postdose), cycle 1 days 2, 8, and 15: predose and 4 hours postdose. On cycle 1 day 28, PK sampling was taken predose, then 30 minutes 1, 2, 3, 4, 6, 8, and 12 hours postdose.
Statistical analysis
This was a dose-escalation trial designed to determine the recommended phase 2 dose. Safety analysis was based on all patients who were enrolled into the study and received at least 1 dose of ONO/GS-4059. Efficacy analysis is based on all patients who have undergone at least 1 response assessment. The best overall response was used for response analysis. Overall response rate (complete response [CR] plus partial response [PR], including PR with residual lymphocytosis) was calculated for the entire study population as well as for each treatment cohort and histology. Progression-free survival (PFS) was defined as the time from start of treatment until PD or relapse.
Results
From September 2012 to January 2015, 90 patients with relapsed or refractory B-cell malignancy were enrolled into the study and received 1 or more doses of ONO/GS-4059. Patients were recruited and data analyzed according to the 2 treatment groups: relapsed/refractory non-Hodgkin lymphoma (NHL) and CLL. Nine dose cohorts were evaluable for each treatment group ranging from 20 mg once daily to 600 mg once daily. A twice-daily regimen was also investigated in 2 cohorts (240 mg twice daily in DLBCL and 300 mg twice daily in CLL patients). Primary clinical data on all enrolled patients are shown in supplemental Tables 1-7. A summary of the distribution of patients according to the different diagnostic subgroups is shown in Figure 1.
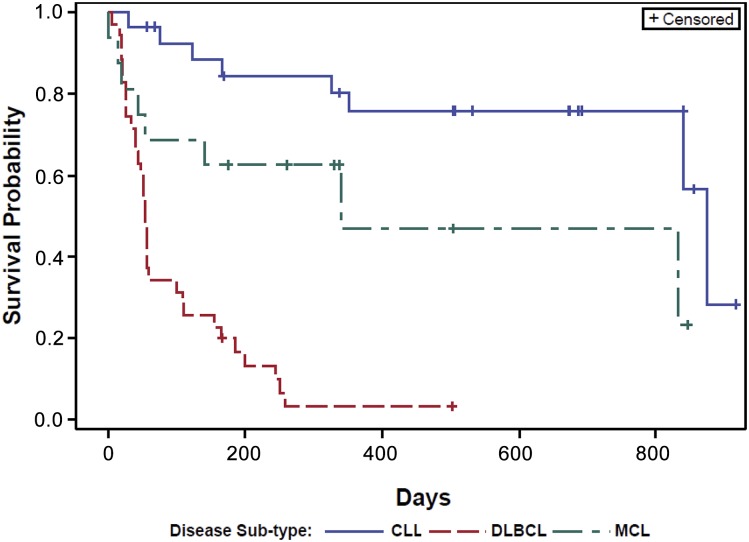
Overall, significant clinical responses were observed at all dose levels in both CLL and NHL treatment groups. Kaplan-Meier estimates of the median time to disease progression were calculated for each patient group; a plot of the Kaplan-Meier survival function is presented in Figure 2. The overall estimated mean PFS was as follows: CLL, 874 days; MCL, 341 days; and DLBCL, 54 days. The pattern and timing of responses included: rapid reduction in lymphadenopathy (and with concurrent lymphocytosis) observed in 23 of 28 patients with CLL (82%), 5 of 16 patients with MCL (31%), and 2 of 5 patients with FL. There was no obvious relationship between ONO/GS-4059 dose and duration and depth of response in either CLL or NHL cohorts (Figures 3-5; supplemental Table 8). At the data cutoff point (March 2, 2015), 34 patients continued treatment with ONO/GS-4059 (supplemental Table 8). These patients comprised 21 CLL patients and 13 NHL patients with the longest-treated patients on cycle 32 of therapy (Figure 3A). The median duration of follow-up for CLL, MCL, and DLBCL patients was 560, 309, and 60 days, respectively.
Figure 2.
PFS curves for CLL, MCL, and DLBCL patients. Results are shown for CLL, MCL, and DLBCL patients as separate curves. Estimated mean PFS for each cohort: CLL = 874 days, MCL = 341 days, and DLBCL = 54 days.
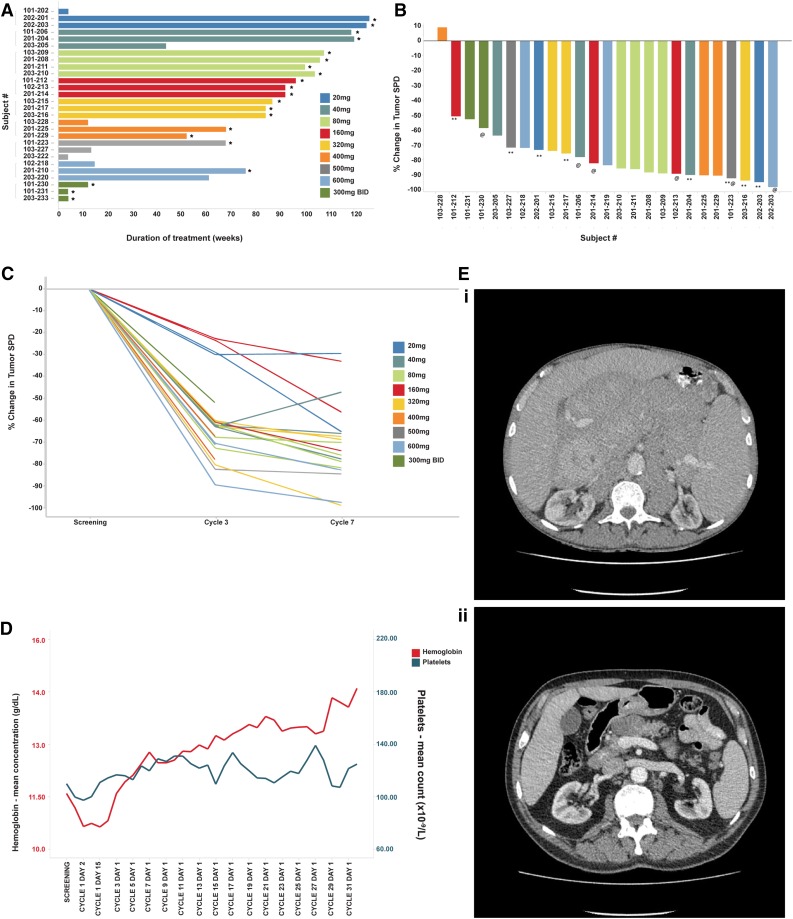
Figure 3.
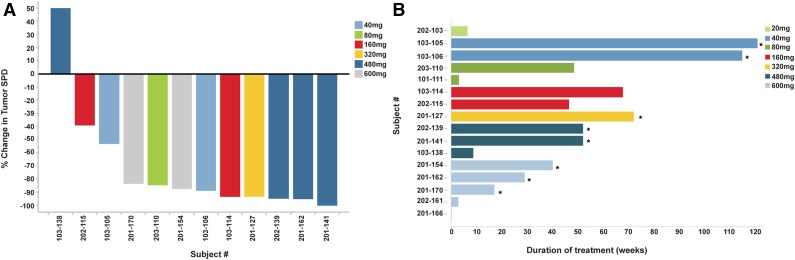
Efficacy of ONO/GS-4059 in patients with CLL. (A) Duration on treatment for all CLL patients (n = 28) according to dose cohort. *Ongoing patients. (B) Waterfall plot for all CLL patients by dose cohort (n = 25), showing response evaluated by CT imaging. Changes from baseline scan (sum of the largest diameter of each target lesion) are shown. Negative values indicate tumor response. **Patients with TP53/17p deletion; @patients with ATM/11q deletion. (C) Rate of response of lymph nodes in CLL patients up to cycle 7 (n = 23). Measured by the percentage change in tumor SPD. (D) Mean blood concentrations of hemoglobin (g/dL) and platelet count (×109/L) showing recovery of normal hemopoiesis in CLL patients up to cycle 31. (E) Case example: CT axial images from a CLL patient. (i) Pretreatment CT imaging showing large volume intra-abdominal lymphadenopathy. (ii) CT imaging during cycle 3 following treatment with ONO/GS-4059 600 mg once daily shows considerable reduction in lymphadenopathy. SPD, sum of perpendicular dimensions.
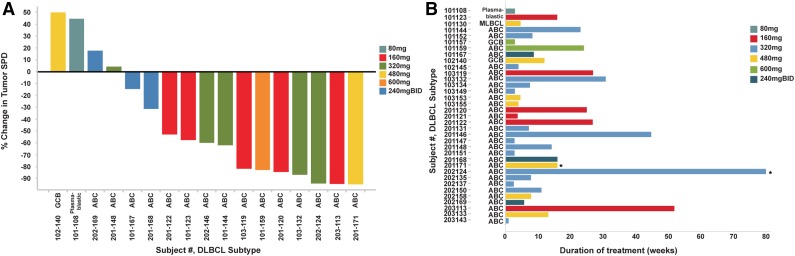
Figure 5.
Efficacy of ONO/GS-4059 in patients with DLBCL. (A) Waterfall plot for all DLBCL patients by dose cohort (n = 17), showing response evaluated by CT imaging. Changes from baseline scan (sum of the largest diameter of each target lesion) are shown. Negative values indicate tumor response. (B) Duration on treatment for all DLBCL patients (n = 35) according to dose cohort. *Ongoing patients.
Within the CLL cohort (comprising 28 patients), 2 patients discontinued study due to disease progression and 5 due to AEs; in 2 patients with AEs, disease progression occurred concurrently. In the NHL cohort (comprising 62 patients), 49 patients discontinued therapy with ONO/GS-4059: in 32 patients this was due to PD and in 5 due to AEs or DLTs. The other 12 NHL patients were discontinued for various reasons including decision by the investigator or patient themselves, 1 case proceeding to an allogeneic stem cell transplant and the rest were patient deaths due to PD.
Further exploration of the efficacy data for the 3 main patient groups, namely CLL, MCL, and DLBCL (28, 16, and 35 patients, respectively), is presented in the following three sections. Data from patients with FL, marginal zone lymphoma (MZL), SLL, and WM are not discussed further due to small patient numbers but primary data are presented in supplemental Tables 4-7, respectively.
CLL patients and responses
Twenty-eight CLL patients were enrolled with a median age of 67 years (range, 40-83 years); 23 patients were male (Figure 3; Table 1; supplemental Table 1). Fifteen patients were Binet stage B and 13 Binet stage C. The median number of previous treatments was 3.5 (range, 2-7) with 93% of patients having received prior rituximab and fludarabine-based immunochemotherapy. Eleven patients (39%) had chemotherapy-refractory disease. The median time from last treatment to commencing ONO/GS-4059 was 11 months (range, 1-99 months). Laboratory prognostic factors were evaluable for 25 CLL patients; notably, 9 patients exhibited loss of TP53 by fluorescent in situ hybridization, whereas an additional 4 patients were found to have TP53 mutation by Sanger DNA sequencing in the presence of normal fluorescent in situ hybridization signal. Twenty-one of 25 exhibited unmutated IGHV gene segments.
Table 1.
Characteristics of the 28 CLL patients enrolled on study
| Patient characteristics | Total |
|---|---|
| Age, median (range), y | 67 (40-83) |
| ≥65 y, n (%) | 16 (57.1) |
| Female, n (%) | 5 (17.9) |
| Binet stage at diagnosis, n = 28, n (%) | |
| Clinical stage B | 15 (53.6) |
| Clinical stage C | 13 (46.4) |
| ECOG performance status, n = 28, n (%) | |
| 0 | 10 (35.7) |
| 1 | 14 (50) |
| 2 | 4 (14.3) |
| With lymphadenopathy >5 cm, n (%) | 15 (53.5) |
| Interphase cytogenetic abnormalities, n = 25, n (%) | |
| with del (13)(q14.3) | 12 (48) |
| with del (11)(q22.3) | 7 (28) |
| with del (17)(p13.1) | 9 (36) |
| with trisomy 12 | 5 (20) |
| Unmutated IGHV gene segments, n (%) | 21 (84) |
| TP53 mutation (Sanger DNA sequencing), n (%) | 13 (52) |
| No cytogenetics available | 3 (10.7) |
| Prior no. of therapies, median (range) | 3.5 (2-7) |
| Relapsed, n (%) | 16 (57.1) |
| Refractory, n (%) | 11 (39.3) |
| Not known, n (%) | 1 (3.6) |
Please see supplemental Table 1 for additional clinical and laboratory details on this cohort.
Patients were treated with doses of ONO/GS-4059 escalating from 20 to 600 mg once daily (1 CLL cohort was also treated with 300 mg of ONO/GS-4059 twice daily). The median number of treatment cycles was 20 (range, 1-32). Twenty-five CLL patients were evaluable for tumor response and 21 of these remain on trial (Figure 3A). In respect of nonevaluable patients, 1 patient had not reached cycle 3 disease assessment at the time of data analysis, 1 patient developed early PD during cycle 1 (cohort 20 mg once daily) and 1 patient was withdrawn due to AE (idiopathic thrombocytopenia) prior to assessment. The patient with progression during cycle 1 was considered to have developed transformation to DLBCL, although this was not confirmed on biopsy. Twenty-four of 25 evaluable CLL patients (96%) had an objective response within the lymph nodes (Figure 3B). Rapid resolution of bulky lymphadenopathy was observed within the first 3 months of therapy (Figure 3C-D). However, further, slower improvement in lymphadenopathy continued for up to 18 months in most patients. Importantly, normal hemopoiesis recovered during ONO/GS-4059 therapy (Figure 3E). Only 2 of 25 CLL patients progressed during therapy with ONO/GS-4059. One patient progressed at cycle 12, the other at cycle 3 (patient 103-228); the latter event was associated with concomitant AEs as described in “Safety and toxicities associated with ONO/GS-4059.” Within the different prognostic subgroups it was noteworthy that of the 10 evaluable chemotherapy-refractory patients, all initially responded and 8 of 10 remained on therapy at the time of data cutoff; only 1 patient (203-220, this patient also had del17p/TP53 mutation) had PD on study having made an initial response (supplemental Table 1 sheet 2). Similarly, in patients with chromosome 17p deletions (9 patients) or TP53 mutation without chromosome 17p deletion (4 patients), all 12 evaluable patients responded; 9 patients remain on therapy (supplemental Table 1 sheet 3).
Four of the 25 evaluable CLL patients were withdrawn due to AEs, of which 1 patient had concomitant PD. AEs included neutropenic sepsis, purpura, spontaneous psoas hematoma in the presence of a normal platelet count, and idiopathic thrombocytopenia. One patient had worsening heart failure that was considered by the investigator to be unrelated to study drug. Apart from the 1 patient with early study withdrawal no other cases of Richter syndrome reported.
MCL patients and responses
Sixteen patients with MCL were enrolled. The median age was 64 years (range, 52-81 years) (Figure 4; supplemental Table 2). Twelve patients were male. Seven patients were refractory to their last course of immunochemotherapy and the median number of prior therapies was 3 (range, 2-7). Two patients had received previous autologous stem cell transplant, whereas a further 2 patients had received allogeneic stem cell transplants. Ten patients had lymph nodes of >5 cm in diameter. Eleven of 12 evaluable MCL patients (92%) responded to ONO/GS-4059 with 6 of responding MCL patients (54%) attaining a PR and 5 patients (46%) attaining a CR/complete response unconfirmed (CRu), including 1 patient who had previously received an allogeneic stem cell transplant. One patient who made a good response to ONO/GS-4059 subsequently underwent allogeneic stem cell transplantation. Eight patients remain on study. Four patients failed to respond and 3 had PD on ONO/GS-4059 following an initial clinical response.
Figure 4.
Efficacy of ONO/GS-4059 in patients with MCL. (A) Waterfall plot for all MCL patients by dose cohort (n = 12), showing response evaluated by CT imaging. Changes from baseline scan (sum of the largest diameter of each target lesion) are shown. Negative values indicate tumor response. (B) Duration on treatment for all MCL patients (n = 16) according to dose cohort. *Ongoing patients.
DLBCL patients and responses
Thirty-five patients with relapsed or refractory DLBCL were enrolled with a median age of 65 years (range, 28-85 years) (Figure 5; supplemental Table 3). Of the 35 patients, 24 were male. Thirty-one were classified as non–germinal center B-cell (non-GCB) DLBCL, 2 were classified as GCB DLBCL using the Hans immunohistochemical algorithm,26 with 1 case of primary mediastinal and 1 plasmablastic DLBCL; assessment of subtype was made locally without central review. Eighteen (51%) had nodal or extranodal masses >5 cm in diameter. Median number of prior treatments was 3 (range, 2-10) and 30 of 35 patients were refractory to their last line of chemotherapy. Ten patients (20%) had undergone autologous stem cell transplantation; none had undergone allogeneic transplantation. Eleven of 31 (35%) of the non-GCB subtype responded, with 2 confirmed CRs and 1 CRu, the remainder being PRs; all of the other patients had PD. Neither of the 2 GCB DLBCL patients nor the patients with primary mediastinal B cell lymphoma or plasmablastic DLBCL responded to therapy. Responses in this cohort were more frequently short (median duration, 54 days). The median number of cycles was 3.5 (range, 1-126 weeks). Fourteen patients underwent dose escalation, which was permitted after completion of 6 months of therapy at the investigator’s discretion; no additional responses were noted following escalation. For the 11 non-GCB DLBCL patients who responded, the median time on treatment was 12 weeks.
Peripheral blood lymphocytosis induced by ONO/GS-4059
Thirty patients (67%) exhibited a lymphocytosis (defined as ≥50% increase from baseline and above absolute lymphocyte count of 5 × 106/mL on initiation of therapy) with ONO/GS-4059, 23 patients with CLL, 4 with MCL, and 1 FL. Three of 4 MCL and the 1 FL patient with lymphocytosis had peripheral blood involvement at baseline. The mean fold increase above baseline was 4.5-fold for the CLL patients, 39-fold for the 4 MCL patients, and in the 1 FL patient, 24.4-fold. For CLL patients with lymphocytosis, peak lymphocytosis was not reached until cycle 3 and then resolved to baseline by cycle 6. Lymphocytosis peaked during the first month of therapy for the MCL patients and had normalized by cycle 6. Lymphocytosis was not associated with response depth or duration nor dose level.
Safety and toxicities associated with ONO/GS-4059
ONO/GS-4059 was generally very well tolerated (Table 2). A total of 171 AEs were reported on study and those occurring in >10% of patients are listed in Table 2. The most common (75% in the CLL cohort and 50% in the NHL cohort) were grade 1 or 2 in severity. However, 14.3% of patients treated with ONO/GS-4059 in the CLL cohort and 16.1% of those with NHL experienced a grade 3 or 4 toxicity that was drug related. Grade 3 or 4 toxicities were mainly hematologic and included neutropenia in 9 patients (10%), anemia in 12 patients (13.3%), and thrombocytopenia in 12 (13.3%). These toxicities occurred early during therapy and recovered spontaneously (Figure 3E). There was only 1 grade 3 episode of drug-related hemorrhage, in a CLL patient, which resulted in the formation of a psoas hematoma (with concomitant progressive infiltration with CLL) in the presence of a normal platelet count and subsequent trial withdrawal. Twenty-eight patients (31.1%) received anticoagulation therapy during the study period (18 prophylaxis for thromboembolic events, 5 in the context of AF, 4 for confirmed thrombosis, and 1 myocardial infarction), with no resulting increased risk of bleeding (supplemental Table 9). In the 5 patients with AF, in 4 this was a preexisting medical condition. The onset of AF in 1 patient while on ONO/GS-4059 occurred following an episode of pneumonia and resolved spontaneously with resolution of infection; it was therefore not considered related to study drug. Diarrhea and arthralgia were classified as a grade 2 toxicity in their most severe forms. Diarrhea occurred in 11% of CLL patients and 21% of NHL patients. Arthralgia occurred in 7% of CLL patients and 8% of NHL patients.
Table 2.
Adverse events
| AE | Grade 1-2 | Grade 1-2 | Grade 3-4 | Grade 3-4 | Total, N = 90 |
|---|---|---|---|---|---|
| CLL, N = 28, (%) | NHL, N = 62, (%) | CLL, N = 28, (%) | NHL, N = 62 (%) | No. of patients (%) | |
| Anemia | 4 (14) | 13 (21) | 3 (11) | 9 (15) | 29 (32) |
| Thrombocytopenia | 1 (4) | 3 (5) | 2 (7) | 10 (16) | 16 (18) |
| Diarrhea | 3 (11) | 13 (21) | 0 (0) | 0 (0) | 16 (18) |
| Petechiae | 5 (18) | 7 (11) | 1 (4) | 0 (0) | 13 (14) |
| Rash | 6 (21) | 9 (15) | 0 (0) | 1 (4) | 16 (18) |
| Pyrexia | 5 (18) | 5 (8) | 2 (7) | 0 (0) | 12 (13) |
| Nasopharyngitis | 4 (14) | 8 (13) | 0 (0) | 0 (0) | 12 (13) |
| Neutropenia | 0 (0) | 2 (3) | 4 (14) | 5 (8) | 11 (12) |
| Bruising | 6 (21) | 5 (8) | 0 (0) | 0 (0) | 11 (12) |
| Lower respiratory tract infection | 5 (18) | 1 (2) | 1 (4) | 3 (5) | 10 (11) |
| Cough | 4 (14) | 6 (10) | 0 (0) | 0 (0) | 10 (11) |
| Nausea | 2 (7) | 9 (15) | 0 (0) | 0 (0) | 11 (12) |
| Hematoma | 5 (18) | 3 (5) | 1 (4) | 0 (0) | 9 (10) |
Treatment-related AEs reported in ≥10% of patients according to treatment cohort on or before the data cutoff date of March 2, 2015.
An MTD of ONO/GS-4059 in the CLL cohort was not reached. In the NHL cohort, the MTD was reached at 480 mg once daily. Four NHL patients experienced a DLT including: 1 patient with WM enrolled at a dose of 320 mg (urticarial reaction after initial doses of ONO/GS-4059), 1 patient with DLBCL at 480 mg (recurrent and extensive maculopapular cutaneous rash), and 2 patients at 600 mg (including a WM patient experiencing a nonimmune drug reaction which recurred upon rechallenge with ONO/GS-4059 necessitating trial discontinuation and a MCL patient with cutaneous rash at a dose of 600 mg once daily who was able to continue study on a lower dose of 480 mg once daily).
Small animal studies had suggested that clinical doses of ONO/GS-4059 might induce pancreatic changes (T.Y., unpublished data). However, no pancreatic abnormalities were seen on CT imaging at cycle 1 day 28 and no changes in blood amylase and lipase levels were observed at any point during the study in any patient.
Pharmacokinetics of ONO/GS-4059
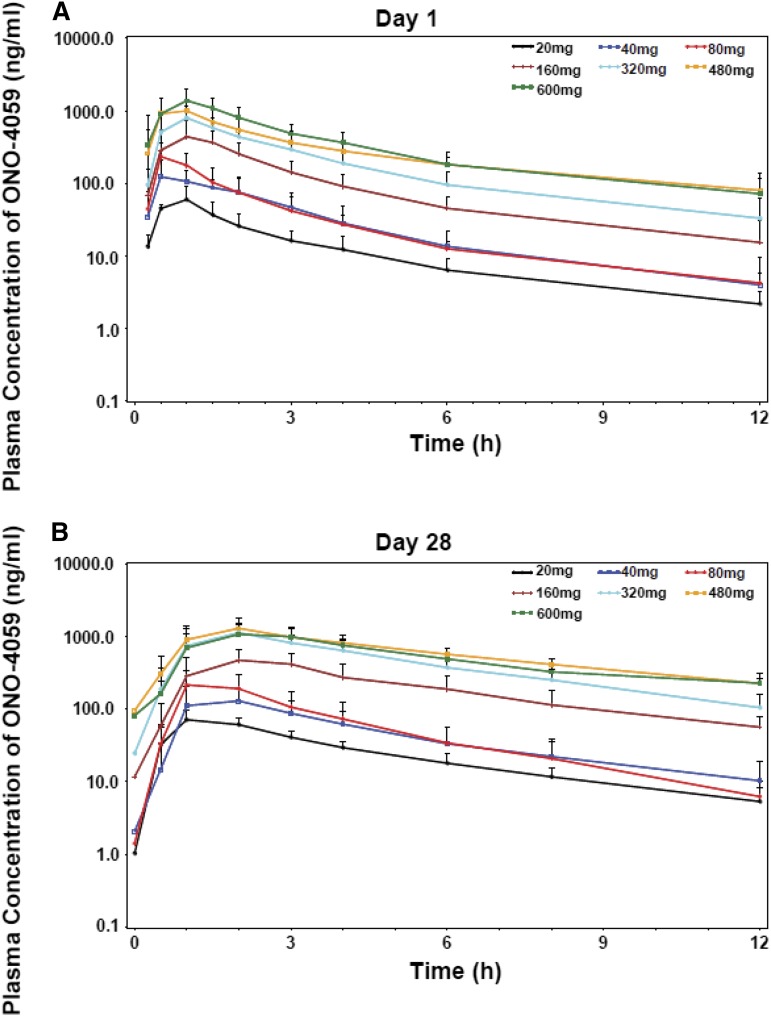
The pharmacokinetic parameters, maximum observed concentration (Cmax), time to Cmax (Tmax), area under the concentration-time curve from time 0 to the last sampling point (AUClast), terminal elimination half-life (T1/2), and oral clearance (CL/F) were calculated from plasma concentrations of ONO/GS-4059 (Figure 6; supplemental Table 10). Following multiple doses, ONO/GS-4059 was absorbed with maximal plasma concentrations reached between 2 and 3 hours postdose in all cohorts based on median Tmax values. Levels of ONO/GS-4059 in the plasma declined rapidly over the 24-hour interval sampling period with mean T1/2 values of 6.5 to 8 hours. (Figure 6; supplemental Table 10). ONO/GS-4059 systemic exposure as assessed by Cmax and AUClast increased in a dose proportional manner across all dose cohorts from 20 mg up to 600 mg once daily (Figure 6). A full pharmacokinetic profile was taken on both day 1 and day 28 of the first cycle of dosing though with the rapid clearance; little accumulation of drug was observed during this period (AUClast values between day 1 and day 28 were comparable). There were no obvious differences in plasma exposure of ONO/GS-4059 (as assessed Cmax or AUClast) in any of the patient groups in the trial (CLL and NHL) or between male and female patients. The pharmacokinetics of ONO/GS-4059 following a twice-daily regimen were as anticipated with the AUC time curve for the dosing interval (AUC0-12) on both day 1 and day 28 being equivalent to the same interval in the once-daily regimen.
Figure 6.
Pharmacokinetic studies of ONO/GS-4059. Plasma concentration-time profiles of ONO/GS-4059 on day 1 (A) and day 28 (B) after once-daily oral administration. Graphs show the mean plasma concentration of ONO/GS-4059 (ng/mL) for all patients in both CLL and NHL cohorts according to dose cohort. Error bars represent the standard deviation.
Discussion
The BTK inhibitor, ibrutinib, has shown remarkable efficacy in both CLL and MCL8,9 and in some cases of non-GCB DLBCL.29 In most patients, ibrutinib is well tolerated. However, ibrutinib exhibits a very broad kinome in vitro7,11 and this may cause toxicities arising from inhibition of structurally related kinases. Initial studies with ibrutinib have shown grade 1 and grade 2 toxicities to be common, with gastrointestinal toxicity occurring in up to 50% of patients, diarrhea being the most common. Grade 3 and 4 hematologic toxicity was also seen, with neutropenia, thrombocytopenia, and anemia reported.16,17,30 Bleeding events of CTCAE grade 3 or higher, including central nervous system hemorrhage, have been reported in 3.4% (17 of 506 CLL patients).17
Here, we report initial clinical data from a phase 1 dose-escalation study of a selective BTK inhibitor, ONO/GS-4059, in 90 patients with relapsed and refractory B-cell malignancies. A striking feature of this study was that ONO/GS-4059 across all disease subsets showed a low incidence of associated toxicities. Individual toxicity (all grades) was <30%. No grade 3 or 4 gastrointestinal toxicities were reported; grades 1 or 2 diarrhea were observed in only 18% of patients. Anemia and thrombocytopenia were the most common hematologic toxicities but, in most instances, were transient and recovered spontaneously. Only 1 grade 3 drug-related hemorrhagic episode was reported and, importantly, the use of anticoagulant therapy was not associated with an increased risk of bleeding. Grade 3 or 4 neutropenia occurred in 10% of patients, but was usually transient and associated with a low incidence of reported infections. Cutaneous toxicity with ONO/GS-4059 was observed and particularly at higher doses. The pathology was varied and included maculopapular rash, angioedema, and urticarial reactions. In the patient with MCL receiving the 600-mg dose of ONO/GS-4059, biopsy of the rash showed infiltration with polyclonal B cells that did not express cyclin D1 (H.S.W. and M.J.S.D., unpublished observations, November 11, 2014).
In terms of efficacy, at this early stage of development, the overall patterns of response with ONO/GS-4059 appear to be broadly similar to the published data with ibrutinib. Thus, CLL and MCL patients with chemotherapy-refractory disease, with chromosome 17p deletion and/or TP53 mutation, or following allogeneic stem cell transplantation responded rapidly, with concurrent lymphocytosis in the peripheral blood and rapid clinical resolution of lymphadenopathy within the first few months of starting therapy with ONO/GS-4059. These responses were frequently sustained; 29 of 44 patients with either CLL or MCL (66%) remain on study drug at the time of writing (median duration on therapy, 21 months). Most patients failed to attain a complete remission due to a persistent low level of malignant cells in the blood and/or bone marrow. In non–germinal center DLBCL, 11 of 17 evaluable patients responded; in contrast to CLL and MCL, responses were much less durable with most patients dying from PD.
Which BTK inhibitor is optimal remains unclear and will require head-to-head comparisons. However, ONO/GS-4059 may have significant advantages in terms of reduced toxicities arising from more selective kinase inhibition.
Acknowledgments
The authors thank all of the patients and their family members for participating in this clinical trial and the ONO/GS-4059POE001 Investigators and study site staff. They also thank Steve Hietschold and his colleagues at Orion for their help with data collection and entry.
Studies in Leicester were supported by the Leicester Experimental Cancer Medicine Centre (C325/A15575 Cancer Research UK/UK Department of Health).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.S.W., S.A.R., M.J.S.D., L.K., C.J., B.C., P.Q., N.S., C.V.H., G.C., F.M., C.F., and G.S. performed research and contributed data; H.H., K.D., J.B., V.J., N.C.-L., T.Y., J.S., T.O., S.A., and A.N. collected, assembled, and analyzed data; H.W.S., M.J.S.D., H.H., K.D., and N.C.-L. further analyzed and interpreted data and wrote the manuscript; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: M.J.S.D. receives research funding from ONO Pharmaceuticals. H.H., K.D., J.B., V.J., N.C.-L., T.Y., J.S., T.O., S.A., and A.N. are all employed by ONO Pharmaceuticals. J.B. is currently employed by Cresta Pharma and consults Acerta Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Martin J. S. Dyer, University of Leicester, Henry Wellcome Building, Room 3/57, Lancaster Rd, Leicester LE1 9HN, United Kingdom; e-mail: mjsd1@le.ac.uk.
References
- 1.Mohamed AJ, Yu L, Bäckesjö CM, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228(1):58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 2.de Weers M, Verschuren MC, Kraakman ME, et al. The Bruton’s tyrosine kinase gene is expressed throughout B cell differentiation, from early precursor B cell stages preceding immunoglobulin gene rearrangement up to mature B cell stages. Eur J Immunol. 1993;23(12):3109–3114. doi: 10.1002/eji.1830231210. [DOI] [PubMed] [Google Scholar]
- 3.Hyvönen M, Saraste M. Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. EMBO J. 1997;16(12):3396–3404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem. 2001;276(19):16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks RW, Bredius RG, Pike-Overzet K, Staal FJ. Biology and novel treatment options for XLA, the most common monogenetic immunodeficiency in man. Expert Opin Ther Targets. 2011;15(8):1003–1021. doi: 10.1517/14728222.2011.585971. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson CV, Dyer MJ. Breaking good: the inexorable rise of BTK inhibitors in the treatment of chronic lymphocytic leukaemia. Br J Haematol. 2014;166(1):12–22. doi: 10.1111/bjh.12895. [DOI] [PubMed] [Google Scholar]
- 7.Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 11.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabinski N, Ewald F. Ibrutinib (ImbruvicaTM) potently inhibits ErbB receptor phosphorylation and cell viability of ErbB2-positive breast cancer cells. Invest New Drugs. 2014;32(6):1096–1104. doi: 10.1007/s10637-014-0141-2. [DOI] [PubMed] [Google Scholar]
- 14.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 16.Smith MR. Ibrutinib in B lymphoid malignancies. Expert Opin Pharmacother. 2015;16(12):1879–1887. doi: 10.1517/14656566.2015.1067302. [DOI] [PubMed] [Google Scholar]
- 17.Tucker DL, Rule SA. A critical appraisal of ibrutinib in the treatment of mantle cell lymphoma and chronic lymphocytic leukemia. Ther Clin Risk Manag. 2015;11:979–990. doi: 10.2147/TCRM.S73559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Wang M, Wang L, et al. Selective antitumor activity of ibrutinib in EGFR-mutant non-small cell lung cancer cells. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harandi A, Zaidi AS, Stocker AM, Laber DA. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009 doi: 10.1155/2009/567486. 567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Paolo JA, Huang T, Balazs M, et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol. 2011;7(1):41–50. doi: 10.1038/nchembio.481. [DOI] [PubMed] [Google Scholar]
- 21.Yasuhiro T, Yoshizawa T, Daub H, Weber C, Narita M, Kawabata K. ONO-WG-307, a novel, potent and selective inhibitor of Bruton’s tyrosine kinase (Btk), results in sustained inhibition of the ERK, AKT and PKD signaling pathways. Cancer Res. 2012;72(suppl 8) Abstract 2021. [Google Scholar]
- 22.Kozaki R, Hutchinson C, Sandrine J, Dyer MJS. Kinome reprogramming in DLBCL by the BTK-specific inhibitor ONO-4059 highlights synergistic combinations for clinical application [abstract]. Haematologica. 2014;99(S1):137–138. Abstract P431. [Google Scholar]
- 23.Kozaki R, Yoshizawa T, Tohda S, et al. Development of a bruton’s tyrosine kinase (btk) inhibitor, ONO-WG-307: efficacy in ABC-DLBCL xenograft model potential treatment for B-cell malignancies [abstract]. Blood. 2011;118(21) Abstract 3731. [Google Scholar]
- 24.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 28.Owen RG, Kyle RA, Stone MJ, et al. VIth International Workshop on Waldenström macroglobulinaemia. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160(2):171–176. doi: 10.1111/bjh.12102. [DOI] [PubMed] [Google Scholar]
- 29.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhang LL, Champlin RE, Wang ML. Targeting Bruton’s tyrosine kinase with ibrutinib in B-cell malignancies. Clin Pharmacol Ther. 2015;97(5):455–468. doi: 10.1002/cpt.85. [DOI] [PubMed] [Google Scholar]