Abstract
The neuropeptide kisspeptin plays an important role in fertility and the onset of puberty, stimulating gonadotropin-releasing hormone (GnRH) neurons to activate the hypothalamic–pituitary–gonadal axis. Several studies have demonstrated a morphological interaction between kisspeptin- and GnRH-expressing neurons; however, few have addressed the interaction of kisspeptin with other neuronal subtypes. We recently showed that fibers immunoreactive for kisspeptin were densely distributed in the dorsal part of the arcuate nucleus. These fibers were found in close proximity to GnRH and tuberoinfundibular dopamine (TIDA) neurons. In the present study, using biotinylated kisspeptin, we established a visualization method for identifying kisspeptin binding sites on TIDA neurons. Biotinylated kisspeptin bound to the cell bodies of TIDA neurons and surrounding fibers, suggesting that TIDA neurons express sites of action for kisspeptin. Our assay also detected biotinylation signals from kisspeptin binding to GnRH fibers in the median eminence, but not to cell bodies of GnRH neurons in the medial preoptic area. Positive signals were completely eliminated by addition of excess non-labeled kisspeptin. This method enabled us to detect kisspeptin binding sites on specific neural structures and neuronal fibers.
Keywords: kisspeptin, tuberoinfundibular dopaminergic neuron, binding assay, dopamine, GnRH neuron
I. Introduction
The functional role of the neuropeptide kisspeptin has been thoroughly investigated over the last decade. Studies in kisspeptin receptor (Gpr54) knockout mice confirm that the kisspeptin–GPR54 system is essential in regulating the secretion of luteinizing hormone from the anterior pituitary via activation of gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus [5]. Kisspeptin also plays an important role in the initiation of puberty, sexual differentiation of the brain and behavior [5, 14, 15, 22, 29], and fertility and the seasonal control of reproduction via the hypothalamic–pituitary–gonadal (HPG) axis [28]. Furthermore, inhibition of kisspeptin neurons during lactation results in the inhibition of the entire HPG axis [34, 35]. Stress also suppresses kisspeptin–GPR54 signaling [16]. However, the morphological relationship between kisspeptin fibers and GnRH neurons remains unclear. In rats, only a small number of kisspeptin fibers have been observed around GnRH neuronal cell bodies in the medial preoptic region [12]. Furthermore, despite reports of direct contact between kisspeptin and GnRH fibers, there is no evidence of synaptic connectivity [32], suggesting that a proportion of transmission occurring between these two fibers is extrasynaptic. Evidence that GPR54 is located on the GnRH fiber is lacking. However, it is highly likely that kisspeptin acts on other target neurons via synaptic transmission [26, 27]. We therefore hypothesized that synaptic transmission of kisspeptin occurs in regions containing dense kisspeptin fibers.
It was recently shown that kisspeptin stimulates the secretion of prolactin from the anterior pituitary [24, 31]. Prolactin-producing lactotrophs are negatively regulated by dopamine secreted by tuberoinfundibular dopaminergic (TIDA) neurons located within the dorsal part of the arcuate nucleus (ArcD) [1, 2, 11]. We previously reported that TIDA neurons are another neuronal target of kisspeptin [26, 27]. In that report, we revealed that kisspeptin and neurokinin B fibers emanating from kisspeptin/neurokinin B/dynorphin neurons in the arcuate nucleus (Arc) are densely distributed around TIDA neurons. We also demonstrated that kisspeptin was coexpressed with synaptophysin on tyrosine hydroxylase (TH)-positive cell bodies, forming synapse-like structures between kisspeptin fibers and TIDA neurons.
In the present study, to acquire morphological evidence of kisspeptin receptors located on individual neurons, we developed a new binding assay using a biotinylated form of kisspeptin-10. Our assay revealed kisspeptin-binding sites on TIDA neurons, which we concluded are the input from kisspeptin fibers to these neurons. We also examined the differences between kisspeptin-binding sites on the GnRH cell body and neuronal fibers around terminals.
II. Materials and Methods
Animals
Female Wistar rats were purchased from Saitama Experimental Animal Supply Co., Ltd (Saitama, Japan). The animals were given food and water ad libitum and kept under controlled lighting conditions (14 hr light: 10 hr dark) prior to use. Rats demonstrating at least two consecutive 4- or 5-day estrus cycles were used in experiments, with the stage of estrus cycle established by daily vaginal smear. All experiments were carried out according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals, and with approval from the Committee for Animal Research in Nippon Medical School (approval number: 25-107, 26-060, 27-061).
Immunohistochemistry for kisspeptin and tyrosine : hydroxylase (TH)
Diestrus rats (9–10 weeks old, n=5) were anesthetized with sodium pentobarbital (70 mg/kg) and perfused transcardially with physiological saline followed by 150 ml of 0.1 M phosphate buffer (PB; pH 7.4) containing 4% paraformaldehyde. Whole brains were immediately removed and immersed in the same fixative at 4°C overnight, then cryoprotected by immersing in 0.1 M PB containing 30% sucrose for 3 days. To prepare for histology, brains were frozen rapidly with powdered dry ice and cut frontally into sections 30 μm thick using a cryostat (Leica 3050; Leica Microsystems GmbH, Watzlar, Germany). To eliminate cross-reactivity with RFamide related peptides, anti-kisspeptin antibody was absorbed with neuropeptide FF (NPFF). Immunofluorescent double-labeling was carried out using 0.45 μg/ml rabbit anti-kisspeptin antibody preabsorbed with 10 μg/mL NPFF [12] in combination with monoclonal anti-TH antibody (1:10,000; Sigma-Aldrich, St. Louis, MO, USA), a marker for dopaminergic neurons. Sections were incubated with antibodies diluted in 0.1 M PB saline (PBS) containing 0.3% Triton X-100 (PBST) at 4°C for 2 days, and washed several times in PBST. Alexa Fluor 488-conjugated anti-mouse IgG antibody (1:1,000; Life Technologies, Carlsbad, CA, USA) and Alexa Fluor 568-conjugated anti-rabbit IgG antibody (1:1,000; Life Technologies), both in PBST, were applied for 2 hr to sections as secondary antibodies for TH and kisspeptin, respectively. Sections were then mounted onto glass slides and examined under a confocal laser scanning microscope (LSM710, Carl Zeiss, Oberkochen, Germany). Scanning was performed separately for each secondary wavelength emission to minimize cross-interference. Optical sections were taken at 0.375 μm intervals. Images were deconvoluted using Huygens Essential (Scientific Volume Imaging B.V., Hilversum, Netherlands). Finally, the sections were stained with cresyl violet.
Binding assay for kisspeptin-10 in TIDA or GnRH neurons
Section preparation was performed as described above. Sections (10 μm) were placed on positively charged SuperFrost slides (Matsumani Glass Ind., Ltd., Osaka, Japan), and blocked with 1% bovine serum albumin (Sigma-Aldrich) in Elix water (Merck Millipore, Darmstadt, Germany) for 1 hr. After washing in Elix water, 30 or 50 μg/ml of biotinylated human kisspeptin-10 (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA), dissolved in 30% dimethyl sulfoxide (DMSO; Sigma-Aldrich), was added to the slides for 2 hr at room temperature. DMSO was used to dissolve biotinylated kisspeptin-10 because it is insoluble in water. As a negative control, 405 μg/ml of non-labeled human kisspeptin-10 (Peptide Institute, Osaka, Japan) and biotinylated kisspeptin-10 were simultaneously applied to the sections. The slides were washed in Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBST; pH 7.4) and immediately fixed with Bouin solution (71.5% saturated aqueous picric acid solution, 9.5% formalin, and 19% acetic acid) for 20 min at 4°C. Kisspeptin-10 binding was detected using the Vectastain Elite standard ABC kit (Vector Laboratories, Burlingame, CA, USA) and TSATM Plus Cyanine 3 System (PerkinElmer, Waltham, MA, USA). After several washes in TBST, slides were incubated with anti-TH antibody (Sigma-Aldrich; 1:10,000 in TBST) or anti-GnRH monoclonal antibody (LRH13, kindly provided by Dr. Park Min Kyun, University of Tokyo; 1:1,000 in TBST) [23] at 4°C overnight. After several washes in TBST, the secondary antibody, Alexa Fluor 488-conjugated anti-mouse IgG (Life Technologies; 1:1,000 in TBST), was applied to the slides for 2 hr. Sections were examined under a confocal laser scanning microscope.
III. Results
Morphological interaction between kisspeptin fibers and : TIDA neurons
Comparison of immunofluorescent images and cresyl violet staining indicated that the distribution of kisspeptin fibers was restricted to the Arc along the rostrocaudal axis. Fibers with intense kisspeptin immunoreactivity were primarily observed in the ArcD, an area containing TH-positive TIDA neurons. Notably, all TIDA neurons were surrounded by intense kisspeptin immunoreactivity (Fig. 1A). Proximity was further confirmed using serial optical sections at higher magnification, which revealed close apposition of kisspeptin fibers with TIDA neurons (Fig. 1B), with some fibers wound around these cells.
Fig. 1. .
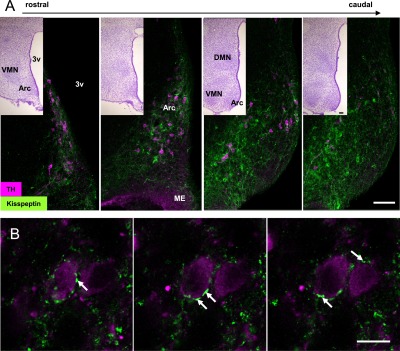
Double immunofluorescence for TH and kisspeptin in the arcuate nucleus (Arc) of female rats. (A) Images of sections arranged rostrocaudally (n=1), showing numerous kisspeptin-immunoreactive fibers (green) in close apposition to TIDA neurons (magenta). Inset, cresyl violet stain. Bars= 100 μm. (B) Serial optical sections of a TIDA neuron. Arrows: kisspeptin-immunoreactive fiber surrounding a TIDA neuron. 3v: third ventricle; Arc: arcuate nucleus, ME: median eminence.
Biotinylated kisspeptin binding assay in TIDA and GnRH neurons
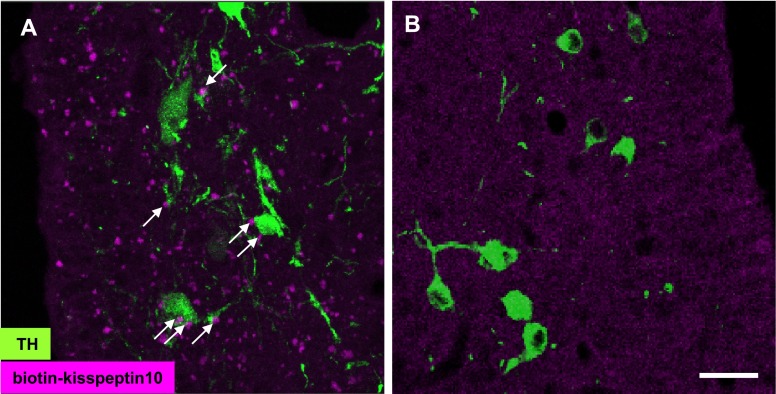
Kisspeptin-10 binding was detected as irregularly-shaped forms on cell bodies and fibers of TIDA neurons located within the ArcD (Fig. 2A). These signals were eliminated by excess application of non-labeled kisspeptin with labeled kisspeptin (Fig. 2B). When this binding assay was applied to GnRH neurons, biotinylated kisspeptin-10 was detected on GnRH fibers in the median eminence (ME) (Fig. 3A, B) and organum vasculosum of the lamina terminalis (Fig. 3D, E), but not on cell bodies in the medial preoptic nucleus (MPO) (Fig. 3F). Specificity of binding signals was confirmed in the ME using excess non-labeled kisspeptin similar to the assay for TIDA neurons (Fig. 3C).
Fig. 2. .
Biotinylated kisspeptin-10 binding signals on TIDA neurons. (A) Incubation with biotinylated kisspeptin-10 yields an accumulation of signal (magenta) on the cell bodies (double-arrows) and fibers (single-arrow) of TIDA neurons (green). (B) Incubation with biotinylated kisspeptin-10 and excess non-labeled kisspeptin-10 reveals no binding signal. Bar=20 μm.
Fig. 3. .
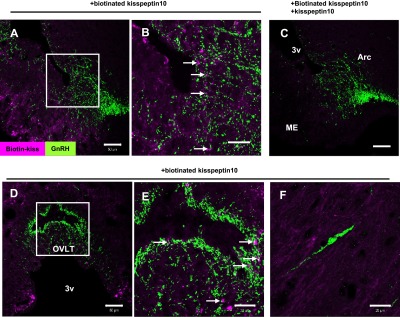
Biotinylated kisspeptin-10 binding signals on GnRH neurons. Signals in the median eminence (A–C), organum vasculosum of the lamina terminalis (D, E), and GnRH cell body in the medial preoptic area (F). Incubation with biotinylated kisspeptin-10 and excess non-labeled kisspeptin-10 yields no signal (C). The sections yield an accumulation of kisspeptin signals (magenta) on GnRH (green) fibers (A, B, D, E) but not on the cell body (F). Higher magnification views of (A) and (D) are shown in (B) and (E), respectively. Bars=50 μm (A, C, D); 20 μm (B, E, F).
IV. Discussion
In the present study, we examined whether TIDA neurons receive direct kisspeptin input. Our results demonstrate that kisspeptin fibers wind around TIDA neurons and that biotinylated kisspeptin is capable of binding to these cells. Kisspeptin binding signals manifested as small, irregular shapes in the binding assay, clearly distinguishable from the binding signals of the biotinylated forms of ghrelin or gastrin-releasing peptide [4, 25]. We considered that biotinylated kisspeptin-10 formed aggregates in 30% DMSO. Excess non-labeled kisspeptin-10 eliminated these binding signals, indicating that our results are specific for kisspeptin binding sites and suggesting that kisspeptin receptors are expressed on TIDA neurons. Part of the binding signal on the TIDA neurons may represent synaptic contacts between kisspeptin fibers and TIDA neurons. However, the possibility that extrasynaptic binding sites are also expressed on TIDA neurons cannot be excluded.
Similar to other RFamide peptides demonstrating cross-reactivity with various receptors, kisspeptin binds two receptor types, GPR54 and NPFFR2. NPFFR2 is one of two G-protein-coupled receptors for NPFF [3, 6]. As specific antibodies are not yet available, the distribution of GPR54 and NPFFR2 in the brain remains uncertain. mRNA for both Grp54 and NPFFR2 has been detected in the Arc [7, 10, 18], but its expression in TIDA neurons has yet to be examined. Thus, biotin signals detected in the present study suggest localization of at least one of two distinct kisspeptin binding receptors. Additional studies detailing the expression patterns of Grp54 and NPFFR2 mRNA in TIDA neurons will further elucidate whether there is direct kisspeptin input to TIDA neurons.
Intracerebroventricular injection of kisspeptin-10 reportedly increases plasma levels of prolactin while decreasing 3,4-dihydroxyphenylacetic acid, a degradation product of dopamine, in adult male and proestrus female rats [24, 31]. Prolactin-producing lactotrophs are known to be controlled by dopamine released from TIDA neurons and transported via the portal capillaries of the ME to the pituitary [1, 2, 11]. Kisspeptin may suppress axonal transport or release of dopamine by TIDA neurons. The specific binding sites detected on TIDA neurons in the present study suggest that kisspeptin is capable of acting directly upon these neurons to suppress the release of dopamine. GPR54 couples primarily with the Gαq/11-mediated signaling pathway to produce excitatory effects of GnRH neurons, in addition to activating several other second messenger systems [17, 20, 21, 30]. In contrast, NPFFR2 couples with Gi2, Gαi3, Gαo, and Gαs [8]. Excitatory neuromodulator was also reported to reduce dopamine release enhancing prolactin secretion [33]. The types of kisspeptin receptor and G protein that play roles in the suppression of dopamine release from TIDA neurons have yet to be elucidated.
Our present results also show that kisspeptin-10 binds to GnRH fibers in the ME, but not to cell bodies of GnRH neurons. While evidence suggests that GnRH neurons express Gpr54 mRNA [9, 13], localization of kisspeptin receptors within these neurons remains to be determined. We previously showed that kisspeptin fibers were distributed amongst GnRH fibers in the Arc, and that only a few surrounded the cell bodies of GnRH neurons in the MPO [12]. Uenoyama et al. [32] demonstrated the morphological and physiological interactions of kisspeptin with GnRH fibers in the ME. Together, these results suggest that kisspeptin acts mainly on GnRH fibers in the Arc or ME but not on cell bodies located in the MPO. Although GPR54 is widely expressed in the brain, few or no kisspeptin fibers are found in GPR54-expressing regions [10, 18, 19]. Therefore, synaptic contact between kisspeptin fibers and target neurons is not expected in these areas; kisspeptin may act via non-synaptic transmission. Conversely, ArcD contains a dense population of kisspeptin fibers. In this area, kisspeptin can function as neurotransmitter through synaptic connections.
Our method has enabled the visualization of kisspeptin binding sites, which were expected to be involved in both non-synaptic and synaptic transmission. As a further investigation, a new method to distinguish between binding sites of these two kinds of transmission should be developed.
V. Abbreviations
Arc, arcuate nucleus; ArcD, dorsal part of arcuate nucleus; GnRH, gonadotropin-releasing hormone; IR, immunoreactive; ME, median eminence; MPO, medial preoptic nucleus; NPFF, neuropeptide FF; TIDA, tuberoinfundibular dopamine; TH, tyrosine hydroxylase.
VI. Acknowledgments
We are grateful to Dr. Park Min Kyun from The University of Tokyo for kindly providing the monoclonal antibody against GnRH (LRH13).
This study was supported by Grants-in-Aid for Scientific Research (23659125 to N.I. and 22590230, 26460323 to H.O.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and Supported Program for the Strategic Research Foundation at Private Universities (#S0801035 to H.O.), Japan.
VII. References
- 1.Ben-Jonathan N. and Hnasko R. (2001) Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 22; 724–763. [DOI] [PubMed] [Google Scholar]
- 2.Björklund A., Moore R. Y., Nobin A. and Stenevi U. (1973) The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 51; 171–191. [DOI] [PubMed] [Google Scholar]
- 3.Bonini J. A., Jones K. A., Adham N., Forray C., Artymyshyn R., Durkin M. M., Smith K. E., Tamm J. A., Boteju L. W., Lakhlani P. P., Raddatz R., Yao W. J., Ogozalek K. L., Boyle N., Kouranova E. V., Quan Y., Vaysse P. J., Wetzel J. M., Branchek T. A., Gerald C. and Borowsky B. (2000) Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 275; 39324–39331. [DOI] [PubMed] [Google Scholar]
- 4.Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., Gaskin F. S., Nonaka F, N., Jaeger L. B., Banks W. A., Morley J. E. Pinto, S., Sherwin R. S., Xu L., Yamada K. A., Sleeman M. W., Tschöp M. H. and Horvath T. L. (2006) Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 9; 381–388. [DOI] [PubMed] [Google Scholar]
- 5.Dungan H. M., Gottsch M. L., Zeng H., Gragerov A., Bergmann J. E., Vassilatis D. K., Clifton D. K. and Steiner R. A. (2007) The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 27; 12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elhabazi K., Humbert J. P., Bertin I., Schmitt M., Bihel F., Bourguignon J. J., Bucher B., Becker J. A., Sorg T., Meziane H., Petit-Demoulière B., Ilien B. and Simonin F. (2013) Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology 75; 164–171. [DOI] [PubMed] [Google Scholar]
- 7.Gouardères C., Puget A. and Zajac J. M. (2007) Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: a comparative autoradiographic study. Synapse 51; 249–269. [DOI] [PubMed] [Google Scholar]
- 8.Gouardères C., Mazarguil H., Mollereau C., Chartrel N., Leprince J., Vaudry H. and Zajac J. M. (2007) Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPgammaS binding. Neuropharmacology 52; 376–386. [DOI] [PubMed] [Google Scholar]
- 9.Han S. K., Gottsch M. L., Lee K. J., Popa S. M., Smith J. T., Jakawich S. K., Clifton D. K., Steiner R. A. and Herbison A. E. (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25; 11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbison A. E., de Tassigny X. D., Doran J. and Colledge W. H. (2010) Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151; 312–321. [DOI] [PubMed] [Google Scholar]
- 11.Hökfelt T. (1967) The possible ultrastructural identification of tubero-infundibular dopamine containing nerve endings in the median eminence of the rat. Brain Res. 5; 121–123. [DOI] [PubMed] [Google Scholar]
- 12.Iijima N., Takumi K., Sawai N. and Ozawa H. (2011) An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. J. Mol. Neurosci. 43; 146–154. [DOI] [PubMed] [Google Scholar]
- 13.Irwig M. S., Fraley G. S., Smith J. T., Acohido B. V., Popa S. M., Cunningham M. J., Gottsch M. L., Clifton D. K. and Steiner R. A. (2004) Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80; 264–272. [DOI] [PubMed] [Google Scholar]
- 14.Kauffman A. S., Park J. H., McPhie-Lalmansingh A. A., Gottsch M. L., Bodo C., Hohmann J. G., Pavlova M. N., Rohde A. D., Clifton D. K., Steiner R. A. and Rissman E. F. (2007) The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J. Neurosci. 27; 8826–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman A. S. (2010) Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 324; 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsey-Jones J. S., Li X. F., Knox A. M., Wilkinson E. S., Zhu X. L., Chaudhary A. A., Milligan S. R., Lightman S. L. and O’Byrne K. T. (2009) Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J. Neuroendocrinol. 21; 20–29. [DOI] [PubMed] [Google Scholar]
- 17.Kotani M., Detheux M., Vandenbogaerde A., Communi D., Vanderwinden J. M., Le Poul E., Brézillon S., Tyldesley R., Suarez-Huerta N., Vandeput F., Blanpain C., Schiffmann S. N., Vassart G. and Parmentier M. (2001) The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 276; 34631–34636. [DOI] [PubMed] [Google Scholar]
- 18.Lee D. K., Nguyen T., O’Neill G. P., Cheng R., Liu Y., Howard. A. D., Coulombe N., Tan C. P., Tang-Nguyen A. T., George S. R. and O’Dowd B. F. (1999) Discovery of a receptor related to the galanin receptors. FEBS Lett. 446; 103–107. [DOI] [PubMed] [Google Scholar]
- 19.Lehman M. N., Hileman S. M. and Goodman R. L. (2013) Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv. Exp. Med. Biol. 784; 27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Lee K. and Herbison A. E. (2008) Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149; 4605–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muir A. I., Chamberlain L., Elshourbagy N. A., Michalovich D., Moore D. J., Calamari A., Szekeres P. G., Sarau H. M., Chambers J. K., Murdock P., Steplewski K., Shabon U., Miller J. E., Middleton S. E., Darker J. G., Larminie C. G., Wilson S., Bergsma D. J., Emson P., Faull R., Philpott K. L. and Harrison D. C. (2001) AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 276; 28969–28975. [DOI] [PubMed] [Google Scholar]
- 22.Oakley A. E., Clifton D. K. and Steiner R. A. (2009) Kisspeptin signaling in the brain. Endocr. Rev. 30; 713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, M. K. and Wakabayashi, K. (1986) Preparation of a monoclonal antibody to common amino acid sequence of LHRH and its application. Endocrinol. Jpn. 33: 257–272. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro A. B., Leite C. M., Kalil B., Franci C. R., Anselmo-Franci J. A. and Szawka R. E. (2015) Kisspeptin regulates tuberoinfundibular dopaminergic neurones and prolactin secretion in an oestradiol-dependent manner in male and female rats. J. Neuroendocrinol. 27; 88–99. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto H., Matsuda K., Zuloaga D. G., Hongu H., Wada E., Wada K., Jordan C. L., Breedlove S. M. and Kawata M. (2008) Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat. Neurosci. 11; 634–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawai N., Iijima N., Takumi K., Matsumoto K. and Ozawa H. (2012) Immunofluorescent histochemical and ultrastructural studies on the innervation of kisspeptin/neurokinin B neurons to tuberoinfundibular dopaminergic neurons in the arcuate nucleus of rats. Neurosci. Res. 74; 10–16. [DOI] [PubMed] [Google Scholar]
- 27.Sawai, N., Iijima, N., Ozawa, H. and Matsuzaki, T. (2014) Neurokinin B- and kisspeptin-positive fibers as well as tuberoinfundibular dopaminergic neurons directly innervate periventricular hypophyseal dopaminergic neurons in rats and mice. Neurosci Res. 84: 10–18. [DOI] [PubMed] [Google Scholar]
- 28.Simonneaux V., Ansel L., Revel F. G., Klosen P., Pévet P. and Mikkelsen J. D. (2009) Kisspeptin and the seasonal control of reproduction in hamsters. Peptides 30; 146–153. [DOI] [PubMed] [Google Scholar]
- 29.Smith J. T. and Clarke I. J. (2007) Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev. Endocr. Metab. Disord. 8; 1–9. [DOI] [PubMed] [Google Scholar]
- 30.Stafford L. J., Xia C., Ma W., Cai Y. and Liu M. (2002) Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 62; 5399–5404. [PubMed] [Google Scholar]
- 31.Szawka R. E., Ribeiro A. B., Leite C. M., Helena C. V., Franci C. R., Anderson G. M., Hoffman G. E. and Anselmo-Franci J. A. (2010) Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 151; 3247–3257. [DOI] [PubMed] [Google Scholar]
- 32.Uenoyama Y., Inoue N., Pheng V., Homma T., Takase K., Yamada S., Ajiki K., Ichikawa M., Okamura H., Maeda K. I. and Tsukamura H. (2011) Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J. Neuroendocrinol. 23; 863–870. [DOI] [PubMed] [Google Scholar]
- 33.Van den Pol A. N. (2010) Excitatory neuromodulator reduces dopamine release, enhancing prolactin secretion. Neuron 65; 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada S., Uenoyama Y., Kinoshita M., Iwata K., Takase K., Matsui H., Adachi S., Inoue K., Maeda K. I. and Tsukamura H. (2007) Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 148; 2226–2232. [DOI] [PubMed] [Google Scholar]
- 35.Yamada S., Uenoyama Y., Deura C., Minabe S., Naniwa Y., Iwata K., Kawata M., Maeda K. I. and Tsukamura H. (2012) Oestrogen-dependent suppression of pulsatile luteinizing hormone secretion and kiss1 mRNA expression in the arcuate nucleus during late lactation in rats. J. Neuroendocrinol. 24; 1234–1242. [DOI] [PubMed] [Google Scholar]