SUMMARY
This draft of the Official Round Table held during the 101st SIO National Congress is an updated review on sialoendoscopy, a technique used for diagnosis and treatment of obstructive pathologies of salivary glands in a minimally invasive fashion. This review treats many aspects of salivary gland endoscopy, starting from anatomy to deal with the more advanced surgical techniques and analyses the main decisional algorithms proposed in the literature. In addition, particular attention was directed to the current limitations of this technique and to the potential developments that sialoendoscopy could have in the near future.
KEY WORDS: Sialoendoscopy, Salivary glands, Endoscopic surgery, Sialoadenitis, Sialolithiasis, Salivary duct
RIASSUNTO
Questo testo è un estratto della Tavola Rotonda Istituzionale tenutasi durante il 101° Congresso Nazionale SIO. Si tratta di una revisione aggiornata sulla scialoendoscopia, metodica che mira all'esplorazione e al trattamento mini-invasivo delle patologie ostruttive delle ghiandole salivari maggiori. Il lavoro proposto analizza i molteplici aspetti dell'endoscopia dei dotti salivari, a partire dall'anatomia endoscopica per approfondire le tecniche chirurgiche più avanzate, passando per l'analisi dei principali algoritmi decisionali proposti in letteratura. Particolare attenzione è inoltre stata rivolta ai limiti attuali della metodica e ai potenziali sviluppi che l'endoscopia delle ghiandole salivari maggiori potrà vivere nel prossimo futuro.
Introduction
Sialoendoscopy is a recently developed technique for diagnosis and treatment of obstructive pathologies of the major salivary gland. Described for the first time in the early 1990s by Katz 1, this minimally invasive technique has become widespread in the last 15 years, especially thanks to two European schools (Geneva and Erlangen) and to the school of Ashkelon, Israel. The clinical research by the pioneers of this technique, associated with technological advances, have deeply modified the management of salivary ductal pathologies, changing the treatment of obstructive sialoadenitis.
This review treats many aspects of salivary gland endoscopy, starting from anatomy, to focus on the more advanced surgical techniques and analyses the main decisional algorithms proposed in literature. The objective of this article is to describe the main features of salivary gland endoscopy and provide an overview of its current and future fields of application.
Anatomy of the salivary ducts
The parotid excretory duct (Stensen's duct, SD), first described by Niels Stensen, forms from the convergence of second and third-order tributary ducts that arise from the deep and superficial lobe, joining near the anterior border of the gland and leaving the gland in its anterosuperior third. SD runs forward over the lateral surface of the masseter muscle and turns around its anterior border, approximately a finger's breadth below the zygomatic arch, and then passes through the buccal fat pad, the buccopharyngeal fascia and the buccinator muscle. It runs forward obliquely for a short distance between the buccinator and oral mucosa before opening on it with a small papilla at the level of the maxillary second molar.
The surface markings of the duct are obtained by joining a line from the anterior point of the tragus with the midpoint of a line drawn between the lowest point of the alar cartilage and the angle of the mouth. When dividing this line into three equal parts, the middle section corresponds to the position of the parotid duct. The duct lies approximately 1 cm below the transverse facial vessels.
The length of SD varies from 4 to 7 cm with an average diameter of 1.4 mm at the hilum, 1.2 mm passing through the buccinator muscle and 0.5 mm at the papilla orifice 2. Some authors have reported the existence of small muscle fibres originating from the buccinator muscle which fit on the outer layer of the distal portion of the SD, thus playing a role in the regulation of salivary secretion and acting as a passive sphincter system 3-5.
The submandibular duct (Wharton's duct, WD) was first described by Thomas Wharton. It forms from the joining of numerous branches arising from the deep surface of the gland, running backwards along the inferior border of the mylohyoid muscle. Once it reaches the posterior border of the muscle, it turns upward forming the WD genu. Then, the WD runs forward laterally to the hyoglossus and genioglossus and medially to the attachment of the mylohyoid muscle to the mandible along the medial side of the sublingual gland. It runs superiorly to the hypoglossal nerve and at the anterior border of the hyoglossus. It is crossed laterally by the lingual nerve.
WD then opens into the oral cavity through a narrow orifice, with a diameter of 0.1-0.5 mm, on the top of the sublingual caruncle behind the lower incisor. WD is approximately 4-6 cm long, with an average diameter of 1.5 mm 2 6 7. Sometimes the major sublingual duct is joined to WD 8.
WD genu is defined as the angle between the main duct and the main intraglandular duct, but it represents the change in angulation when the duct turns around the posterior free margin of the mylohyoid muscle 13. The genu angle varies significantly from 24° to 178°, but this variability does not appear to be associated with sialolithiasis or sialadenitis 6.
In both SD and WD, the epithelium lining is smooth and pale pink, showing the blood vessels in transparency. Furthermore, the sphincter function of the ducts is reflected by the presence of circular ridges on the mucosa lining, especially in the papillary region. Along the main duct, numerous accessory ducts may be opened and may have numerous patterns of bifurcation of the first order ducts at the hilar level, which at the intraparenchymal level become second and third order ducts.
Diagnostic and operative sialoendoscopy: equipment and basic technique
The first endoscopic approach to the salivary glands requires specific instruments (sialoendoscopes), which have notably evolved since the first model described by Katz 1. The miniaturised optical fibres can be introduced inside sheaths of varying shape and diameter or be included in so-called "all-in-one" endoscopes. These semi-flexible tools are made from nitinol and guarantee great resistance, manoeuvrability and fine optical resolution. There are two types of "all-in-one" endoscopes: diagnostic and operative. While the diagnostic sialoendoscope has only an irrigation channel, operative sialoendoscopes have also a working channel for the insertion of dedicate tools (baskets, forceps or balloons). The size of the working channel determines the overall diameter of the "all-in-one" sialoendoscope.
Sialoendoscopes are connected to optical devices (cold lighting source, video camera and monitor) and to irrigation systems. Irrigation is mandatory in order to expand the duct, thus avoiding its collapse under periductal pressure. It is usually performed by connecting a 20 ml syringe filled with saline to the irrigation channel of the endoscope.
Many miniaturised tools can be introduced into the working channels: wire baskets are used for removing stones and foreign bodies, mini-grasping forceps can be used to remove debris or smaller stones without a basket and high-pressure balloons are useful for duct stenosis dilation. Laser fibres and microdrills are helpful in stone fragmentation.
The sialoendoscopic procedure could be divided into two phases: salivary duct access (through the papilla or the duct wall) and endoluminar phase 9. The procedure can be carried out under local or under general anaesthesia. Local anaesthesia is usually performed with topical lidocaine before salivary duct dilation. Local infiltration with anaesthetic and vasoconstrictor can be performed in some cases, to show a difficult papilla or when papillotomy is required. General anaesthesia should be recommended for more complex and lengthy procedures or in non-compliant patients 9-15.
Several techniques were proposed to access the main salivary duct. The classic technique is achieved by progressively expanding the papilla with salivary probes having progressive diameters in order to expand the duct to reach the endoscope diameter 9. A conic dilator completes dilation with the probes.
Currently, several techniques have been proposed to simplify the introduction of the endoscope through the papilla. These include the introduction of a guidewire into the papilla and its dilation using sheaths in metal or in plastic materials. Then, without removing the guidewire, the dilator is directly replaced by the endoscope whose operating channel is taken up by the guidewire 10.
The retropapillary technique, first proposed by Nahlieli, is utilised when the papilla of submandibular gland cannot be localised 11. An incision is made using preventive infiltration at the level of the oral pelvis parallel to the axis of the duct, searching for it carefully on the medial face of the sublingual gland. Once the duct is detected, a 1 mm cut is performed to allow endoscope insertion.
On the other hand, when the papilla is localised but there is a stenosis of the duct, atraumatic endoscope insertion may be difficult. A mini-papillotomy, a lengthwise cut of the distal part of the papilla, allows solving this problem. The mini-papillotomy should not be longer than 3-4 mm in order to avoid postsurgical papillar stenosis and makes the procedure more technically challenging due to leakage of the irrigation. This technique is reserved for cases where an atraumatic approach is not possible, such as papillary hypertrophy, papillary stenosis or extremely small ductal orifices 12 13.
After endoscope insertion, the entire ductal system is explored from the main duct to the peripheral branches. Certain tracts of the gland are more difficult to access and to explore and, consequently, to pass through with the endoscope. These are commonly referred to as the "comma area" of WD, where the duct turns inferiorly at the posterior border of the mylohyoid muscle; in the case of SD, most difficulties are encountered in the area posterior to the duct's curvature (around the masseter muscle) and when the duct passes through the buccinator muscle 14. The presence of an assistant is useful to provide correct visualisation of the surgical field by continuous irrigation and to support the surgeon in managing the operative instruments.
The technique for sialolith removal depends on size, shape, mobility and location of the stone. Stones floating in the duct having regular contours and a major axis parallel to the main duct are usually extracted with wire baskets. The basket is placed behind the stone, opened entrapping it and then gently retracted. Final exploration of all the branches of the salivary duct is mandatory to detect the presence of other residual stones. Sialoliths impacted in the ductal lumen require lithotripsy 12. A combined approach is reserved to those stones that for size and position cannot be fragmented 12-14.
Management of stenosis often requires endoluminal dilation, which can easily be achieved with high-pressure balloons, microdrills, forceps, or simply with a larger endoscope 15. Defining the exact location and extension of a stenosis is challenging. Placement of an intra-ductal stent is sometimes necessary. After appropriate exploration of the gland ducts, endoluminal irrigation with steroid solution under endoscopic control may be useful to remove mucous plugs and alleviate ductal inflammation.
Learning curve in sialoendoscopy
There are few reports in the literature regarding the learning curve for sialoendoscopy. This procedure, like all endoscopic techniques, requires specific skills. According to Luers 16, a shorter learning curve can be assumed since otolaryngologists commonly have experience with endoscopic procedures in general and an experienced supervisor can support the process by direct feedback and practical help. However, sialoendoscopy differs from other endoscopic procedures in many ways (smaller endoscopes, newer instruments, endoscopy in a fluid-filled branched system). The actual endpoint of the individual learning curve with performance results, operating times and rate of complications similar to those reported in the literature, can be reached in approximately 50 cases 16 17.
As with any new form of technology, there are several barriers in beginning a successful sialoendoscopy program 18. Kroll, in a statistical survey regarding the prevalence of sialoendoscopy in ENT clinics, documented how, in 2009, it was performed in only a minority (24%) of ENT Departments in Germany. Its diffusion was hampered by technical problems, a lack of cost benefits, a lack of adequate instrumentation and a small number of patients 19.
The first difficulty encountered when beginning this new technique is the elevated initial costs of the sialoendoscopes and related equipment 18 19. Technically, the first problem is related to difficulties in canalising and dilating the duct to allow appropriate endoscopic use, bypassing and dilating strictures 18. Vairel et al. found it impossible to catheterise in 6 cases of the first 101 (5.9%) in their experience 17. When initial identification and dilation of the punctum seems challenging, it may be useful to perform it under magnification with loupes or, as reported by other authors, with a microscope 18.
Sialoendoscopic treatment of salivary stones may improve with increased surgical experience. Modest et al. reported their experience in two consecutive groups of patients presenting sialolithiasis. In the first group of 43 patients, endoscopic removal occurred in 20% of cases and gland resection was required in 34.3% of patients, while in the second group of 39 patients, endoscopic removal increased to 35.9% of patients and gland resection was reduced to 0% 20.
Another parameter in the advancement of the learning curve can be defined by the need to perform the sialoendoscopic procedure under general anaesthesia less and less frequently. Operating on the first cases under general anaesthesia may be helpful to avoid patient discomfort due to longer procedure times. According to Vairel et al., with an increase in experience, a higher number of interventional sialoendoscopies can be performed under local anaesthesia, limiting the use of general anaesthesia to more complex cases 17.
Indications of sialoendoscopy and review of decisionmaking algorithms
The goal of sialoendoscopy is to resolve the obstructive condition preserving at the same time a physiologically intact gland 21-23. Over the years, several treatment algorithms for sialolithiasis and obstructive pathologies have been proposed to provide a more accurate selection of cases.
These algorithms have generally shown consensus concerning the size of stones, site of obstruction and technologies available 21 24 25.
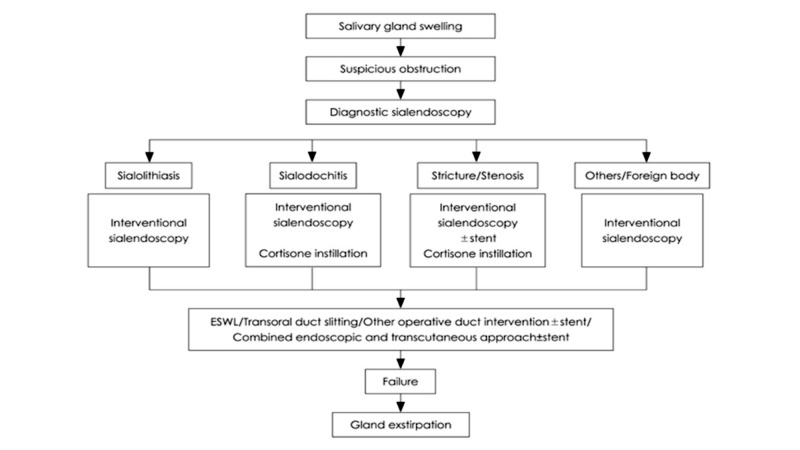
The current clinico-diagnostic algorithm for any glandular swelling includes ultrasound and the use of diagnostic and therapeutic sialoendoscopy (Fig. 1). The actual indications of sialoendoscopy are sialolithiasis, stenosis, foreign bodies, polyps, recurrent sialoadenitis and sialoadenosis.
Fig. 1.
Treatment plan and therapeutic strategy for obstructive salivary gland diseases according to Koch et al. 2009 24, mod.
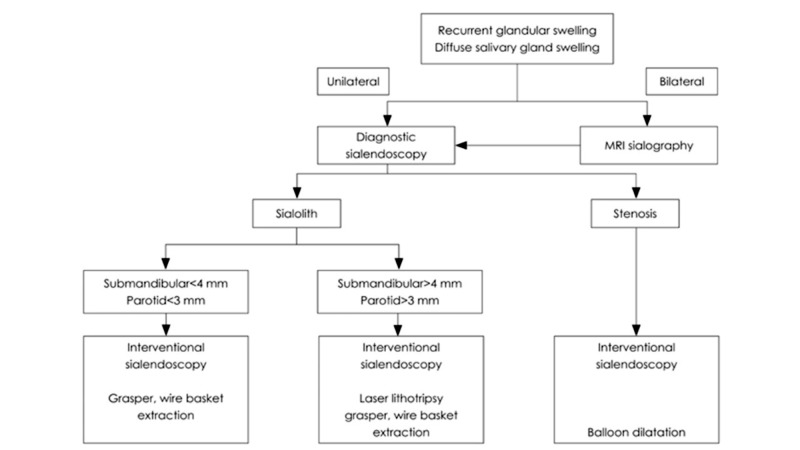
In detail, analysing the sialolithiasis: small, mobile stones of 3-4 mm or less can be easily removed via simple basket extraction, while larger, impacted stones with diameters > 7 mm are generally treated with combined endoscopic and transoral/transfacial approaches 26. For stones between 4 and 7 mm, the best treatment depends on available technology. If stones are too large for simple basket retrieval, they need to be fragmented before endoscopic extraction (Fig. 2).
Fig. 2.
Decision algorithm for the evaluation and management of sialolithiasis (from Marchal and Dulguerov, 2003 23, mod.).
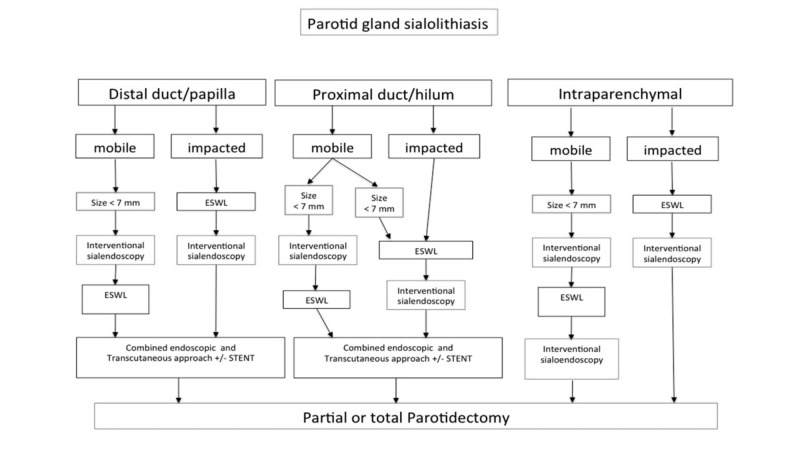
Concerning parotid sialolithiasis, some authors 24 26 27 described different approaches based on the size and location of the stones (Fig. 3):
Fig. 3.
Management of parotid salivary stones (from Koch et al. 2009 24, mod.).
Anterior third of SD (distal duct): interventional sialoendoscopy must be the first therapeutic option in case of stones < 7 mm, eventually combined with transoral removal.
Middle third, (middle, proximal duct): other options for stones > 3 mm include stone fragmentation by (extracorporeal shock wave lithotripsy, ESWL) or intracorporeal lithotripsy followed by interventional sialoendoscopy combined with transcutaneous or lifting approach 24 28-33.
In the posterior third of SD (intraparenchymal), sialoendoscopy combined with fragmentation techniques, combined surgery, or ESWL, are the only alternatives to parotidectomy.
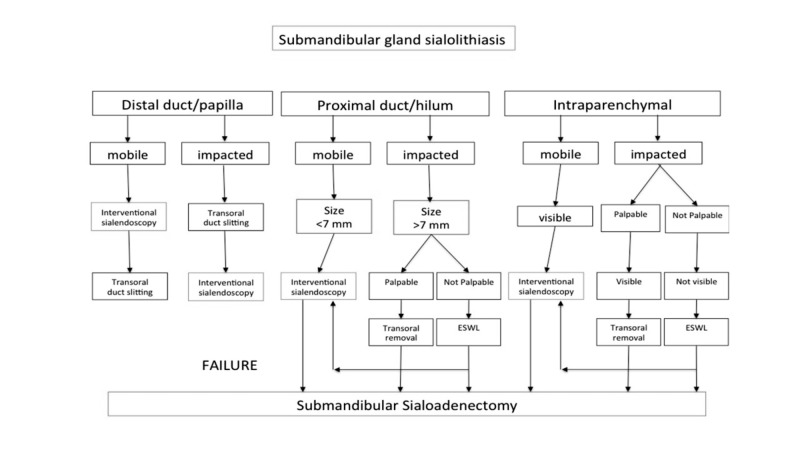
In submandibular gland sialoliths, the current algorithm is based on the location of the stones (Fig. 4):
Fig. 4.
Management of submandibular gland stones (from Koch et al. 2009 24, mod.).
Distal duct/papilla. If there are mobile ductal stones < 5 mm, sialoendoscopy with basket retrieval may be the first attempt, and papillotomy may be necessary; if the stones are impacted, transoral duct slitting is generally performed before interventional sialoendoscopy.
Proximal duct/hilum: in case of small, mobile stones < 5 mm attempting to remove the stone with a wire basket or grasping forceps is indicated; if stones are > 7 mm and palpable, a transoral duct incision or combined endoscopic-guided removal can be performed if fragmentation via ESWL or laser lithotripsy are not available.
Intraparenchymal: mobile stones < 7 mm can be removed via interventional sialoendoscopy if they are impacted; stones > 7 mm up to 10 mm can be fragmented with laser or ESWL allowing endoscopic removal.
In case of partial success or failure of sialoendoscopy, endoscopically-assisted transoral removal can be performed; however, sialoadenectomy still remains the definitive therapeutic solution even in case of failure as well as intraparenchymal stones not fragmented by ESWL 14.
The characteristics of the stenosis may be assessed by ultrasound or MRI, but in recent years sialoendoscopy has contributed to the introduction of the LSD (lithiasis, stenosis and dilatations) classification system (2007). LSD classifies the stenosis according to site, extension and number 21.
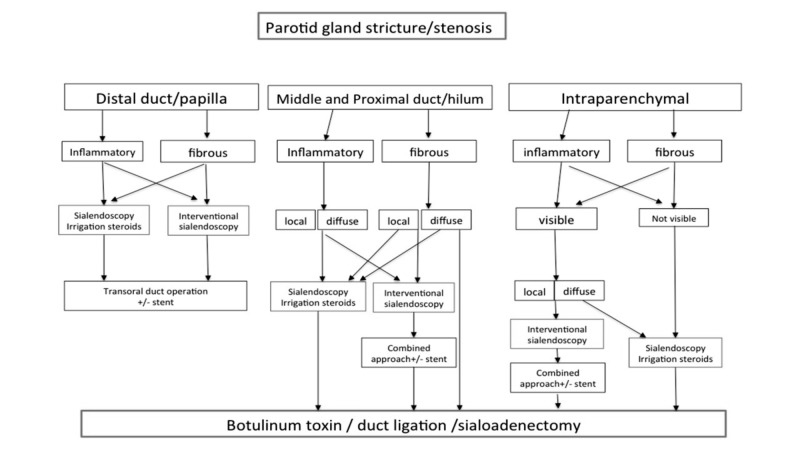
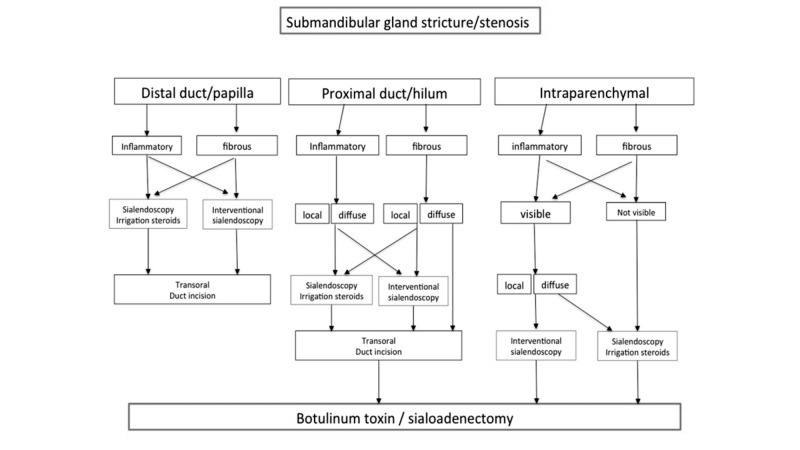
Sialoendoscopy has the advantage of direct assessment, allowing differentiation between an inflammatory reaction from a fibrous stenosis. The majority of the former may be successfully treated conservatively (irrigation and intraductal steroid instillation), whereas the latter can usually only be managed by endoscopically-controlled instrumental dilatation. Besides papillotomy and distal duct incision, resection of the affected segment and duct repair are generally successful. Stent implantation is important to prevent restenosis and many authors advocate it even if there is still no worldwide consensus on this issue regarding the time and positioning of stenting. In rare cases, ligation of the duct with subsequent parotid atrophy is an option and avoids parotidectomy, but with success rates of only around 50%. As an additional option, repeated intraglandular application of botulinum toxin may also be attempted as an alternative to gland removal. The diagnostic algorithm for stenosis or strictures of the submandibular glands and parotid glands is illustrated in detail in Figures 5 and 6.
Fig. 5.
Algorithm for management of parotid gland stricture/stenosis (from Koch et al., 2009 24, mod.).
Fig. 6.
Algorithm for management of submandibular stricture/stenosis (from Koch et al., 2009 24, mod.).
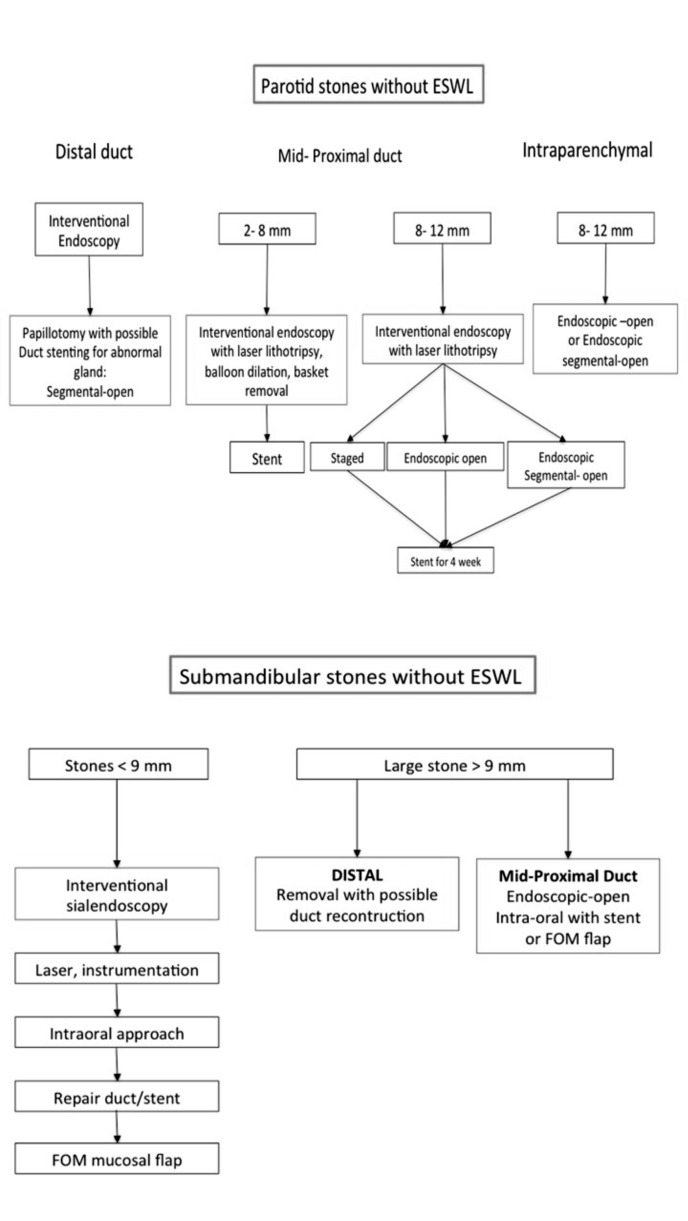
The correct integration with therapeutic options such as laser lithotripsy or ESWL obviously depends on the technologies available. For these reasons, there are differences in stone treatment algorithms used by physicians who have access to ESWL and those who do not 25, mainly in terms of stone dimensions (Fig. 7).
Fig. 7.
Algorithm for treatment of salivary glands obstruction without ESWL (from Fritsch 2009 25, mod.).
Lithiasic pathology
Intracorporeal lithotripsy
Since the mid-1980s, much thought has been given to apply lithotripsy to salivary glands similarly to that used for urinary tract stones. It is well-known, in fact, that salivary gland function can recover after stone removal alone. With the introduction of sialoendoscopy, intracorporeal lithotripsy has been proposed as a promising alternative to ESWL 35 36. The limitation of the endoscopic approach without laser lithotripsy is usually considered the size of the stone, which allows removal of stones no more than 4 mm in diameter with a wire basket or grasping forceps, whereas larger stones or stones impacted in the duct require fragmentation 36 37.
Endoscopic laser lithotripsy has the potential to treat many stones larger than 4 mm with minimal complications and allows preservation of a functional salivary gland. This technique was introduced in the early 1990s when laser, electrohydraulic probes and pneumatic lithotripsy were tested 30 31 38. Due to possible adverse effects, such as facile duct perforation and surrounding tissue damage, it was soon clear that both intracorporeal electrohydraulic and pneumatic lithotripsy were not feasible procedures for salivary stone treatment. Therefore, many authors focused on the use of laser lithotripsy and further case series were published on the clinical use of different lasers 9 28 32 39-44.Currently, there is still no consensus as to which laser is the most efficient in performing lithotripsy on salivary stones, although most studies have reported Ho:YAG laser as the first choice.
Ho:YAG laser creates pulsed energy with a wave length of 2080 nm which is near the peak for absorption of water; lithotripsy is achieved by a photomechanic effect with a collision of the particles of the stone and then a photothermic effect on the surface level. This associates the fragmentation effect with vaporisation of the stone's surface. It creates a shockwave when the laser is activated and the tip of the fibre is placed perpendicular to the stone's surface. Lithotripsy is then activated by a cavitation/fragmentation technique until the stone is completely fragmented, and then washed out or retrieved with a basket or grasping forceps without damaging the epithelium of the duct. Extreme care is necessary to avoid pieces that are sharp enough to damage the walls of the duct or remain encased during retrieval and to avoid activating the laser when the tip is in contact with the duct wall. However, there are no reports about side effects due to laser litotripsy in clinical practice 18 19 28 32 39-44.
Regardless of the type of laser used, intracorporeal lithotripsy overcomes the need for combined approaches or open interventions. The advantage of most lasers is that the fibres have small diameters, sometimes as small as 200 μm, allowing application of high-watt intensities for fragmentation of stones even in the proximal duct system or behind stenotic areas. Currently, the real limitations are represented by stones that are only partially visible due to the possible laser action to the ductal wall that may inadvertently cause perforation and by stones that are too large in size (> 15 mm) due to the excessive length of the procedure. Similarly, the hardness of stones can play an important role in fragmentation and, consequently, in the duration of the procedure 19.
Extra-corporeal lithotripsy for salivary stones: indications and limitations
The experience acquired with ESWL techniques have been widely described in the literature 34 35 45-55. The shockwaves produced by an extra-corporeal source (electromagnetic or piezoelectric) have the aim of fragmenting stones so that they can be flushed out by physiological saliva flowing out the duct. ESWL is usually performed under US control, which allows stone identification and targeted administration of the shockwave with real-time visualisation of the fragmentation process and avoiding any iatrogenic lesions of surrounding tissue.
The most frequently used ESWL energy source is electromagnetic as it is minimally invasive and can be used on an outpatient basis without anaesthesia. It was introduced in the 1980s for the treatment of renal calculi and gallstones. Electromagnetic and piezoelectric sources exploit the compressive and expansive waves generated by the difference in impedance at the stone-water interface and cause stone cavitation. After encouraging results with multiple animal and in vitro experiments, the first successful ESWL for human sialolithiasis was carried out by Iro et al. in 1989 using a device designed for renal lithotripsy 48. Since then, dedicated instruments have been designed and the use of ESWL has become increasingly widespread.
Ultrasonography is used to focus the shockwaves on the stone. Stones that can be identified ultrasonographically and have a diameter of at least 2.4 mm (diameter of the focus) are potentially amenable to treatment. Contraindications for ESWL are complete distal duct stenosis, pregnancy, and the presence of a cardiac pacemaker. Relative contraindications include acute sialadenitis or other acute inflammatory processes of the head and neck and treatment should be postponed in these cases. ESWL is considered safe, and only minor and self-limiting undesired effects have generally been reported, including pain of the treated area, glandular swelling, ductal bleeding and cutaneous petechiae.
As for the effectiveness of electromagnetic ESWL of salivary stones, it is quite difficult to compare published results directly due to differences in criteria used to define outcomes: definition of complete or partial success (< 2 mm and > 2 mm), or symptom status after lithotripsy. On the basis of the published findings, the success rate is higher in parotid stones than in submandibular stones: complete stone clearance has been reported in 39-69% of parotid stones, but only 26-42% of submandibular stones treated electromagnetically, and respectively, 33-81% and 29-40% of those treated piezoelectrically. Moreover, multivariate analysis of one of the previous studies showed that favourable outcomes were significantly associated with a younger age (< 46 years), parotid location (intraductal), stone diameter (< 7 mm) and a lower number of therapeutic sessions 35 54. However, despite the availability of specific indications for ESWL, US shows that a significant number of patients still have residual fragments in the affected gland, although most are asymptomatic and do not require additional procedures. It is well known that ultrasound is not accurate in stones with a diameter less than 1.5 mm. Consequently, the undetectable microliths (consequence of ESWL) can act as a nidus for recurrence; this is why, nowadays, ESWL is proposed in combination with interventional sialoendoscopy to verify and obtain cleansing of the salivary duct system 55.
In conclusion, the main indication of ESWL is for parotid calculi, but it can still be chosen for selected submandibular stones such as impalpable pure intraparenchymal ones as an alternative to sialadenectomy. The main limitations are the need for multiple sessions and the fact that residual stone fragments inside the duct system may require a multimodal approach (together with interventional sialoendoscopy and sialoendoscopy-assisted surgical removal) in some patients.
Combined approach to submandibular and parotid gland for calculi
Clinical experience with ESWL has shown that this technique is successful for most parotid stones. However, submandibular stones, in particular large stones (> 7 mm) and those in the hiloparenchymal region, are not responsive to this type of treatment 35. Sialoendoscopy is an adequate procedure for all mobile stones and for small (< 5 mm) stones of the submandibular and parotid duct system 9 56 57. About 10% of patients with parotid stones treated with ESWL and sialoendoscopy remain symptomatic and require further treatment 58. In recent years, an endoscopeassisted surgical approach has been proposed for the management of proximal duct and intraparenchymal stones of the submandibular gland and for large, palpable and fixed stones of the parotid gland 27 59-67.
An intraoral approach for submandibular stones, well known since 1968, has emerged as the primary treatment modality in the last 15 years. The results of experience acquired in dedicated centres show that successful stone retrieval may be achieved in the majority of patients if adequate preoperative assessment delineating the position and size of the stone is performed (manual palpation of the stone in the oral floor and ultrasound). In particular, ultrasonography is able to distinguish the position of the stone in the main duct and the hiloparenchymal region. The sialoendoscopic inspection of the duct system is extremely useful in guiding the surgeon during the search for deep intraparenchymal stones or to check the hilar cavity after the removal of the main stone for any residual sialoliths. The surgical procedure is preferably performed under general anaesthesia. A low rate of complications has been encountered, mainly represented by persistent or transitory lingual nerve damage, hilar stenosis and ranula 27 59-67. The few failures are limited to patients with non-palpable intraparenchymal calculi adherent to gland tissue 63. A relatively low number of symptomatic recurrence of calculi (16.3%) have been observed, in particular in patients who previously underwent ESWL; in these patients, secondary or tertiary minimally invasive procedures such as ESWL and interventional sialoendoscopy can be proposed to reduce the indication to traditional sialadenectomy 63.
A sialoendoscopy-assisted surgical approach for symptomatic parotid calculi has recently been described 60-62 65. Indications include large, palpable, fixed, duct and parenchymal stones, calculi not responding to minimally invasive approaches (ESWL or interventional sialoendoscopy) and strictures of SD that can impede stone removal by a minimally invasive approach 58. Two stone removal options are available: the modified rhytidectomy approach under general anaesthesia and facial nerve monitoring, and the cheek approach through a direct cutaneous incision over the palpable and superficial stone under local anaesthesia. In the former procedure, a parotid sialodrain is usually inserted along the duct after its incision to favour the release of the stone, and the parotid duct is sutured with 6/0 suture while the stent is sutured to the oral mucosa. A retrograde sialoendoscopic check with saline lavage is performed in both procedures to exclude any additional calculi. No facial nerve damage has been described after these surgical approaches, although a low number of sialoceles, stenosis and salivary fistula have been reported. Based on the experience of five major centres, a successful result is achieved in most of patients suggesting that an endoscope-assisted surgical approach to parotid calculi is a viable alternative to traditional parotidectomy.
Non-lithiasic pathologies
Autoimmune disorders of the major salivary glands
Autoimmune disorders of the major salivary glands can be divided in two categories. A first group includes the IgG4-correlated sclerosing disease (MS IgG4), such as Mikulicz Syndrome, Kuttner tumour and chronic sclerosing sialoadenitis, and another group including Sjögren's syndrome.
The aetiology of MS IgG4 is unknown, but it has an autoimmune pathogenesis with connective tissue invasion by T CD4+ lymphocytes, T CD8+ lymphocytes and IgG4- producing plasma cells. The salivary glands involved progressively reduce saliva production thus tending to cause stasis within the ducts due to stenosis and/or extraordinary dilatation induced by surrounding fibrosis. Sialolithiasis formation is frequent. Until recently, sialoendoscopies have not been described in these patients, and systemic steroids represent elective therapy 68 69.
In Sjögren's syndrome, 80% of the immune response is represented by CD4+ T lymphocytes; there is also a significant presence of interleukin and antibody production. The disease attacks the ducts of all exocrine glands including the salivary glands 70. Bilateral gland swelling is often present causing duct obstruction due to lymphocytic inflammatory infiltrates. Swelling is typically recurrent with complete remission intervals. Pain is moderate and increases during chewing. Sometimes gland swelling is absent, and various hypotheses have thus been proposed to explain the xerostomy (which is always present): these include gland damage and neuron degeneration consequent to vasculitis and neuron transmission inhibition by antimuscarinic antibodies 71. Autoantibodies may be present in the blood, although they are not specific for Sjögren's syndrome. At sialoendoscopy, the involved glands express, at the main and secondary duct levels, wall hyperaemia with a marked vascular reticule showing perivascular inflammation and congestion. Later, with progression of the substitute sclerosis of parenchymal tissue, the ducts seem pale and poorly vascularised. Mucous plugs are often present within the duct lumen and eventually obstruct the ducts partially or completely with saliva stasis and inflammation where the obstruction begins, causing temporary gland swelling and pain 72.
Juvenile recurrent parotitis
Juvenile recurrent parotitis (JRP) is the second most common disease of the parotid gland after mumps in children. It typically occurs between 3 and 6 years of age, more frequently in males and in most cases shows self-restraint at puberty. JRP is a non-specific sialoadenitis characterised by a non-obstructive, non-suppurative inflammatory process with acute episodes of unilateral (less frequently bilateral) parotid swelling and pain, lasting between few days and a couple of weeks, interspersed by varying disease- free periods. The aetiology of JRP remains unclear 73. The sialographic demonstration of duct ectasis combined with an accurate clinical and symptomatological evaluation still represents the diagnostic hallmark of JRP. Sialectasis is demonstrated by multiple radio-opaque dye among dilated interlobular ducts, typically detected in both parotids even when swelling is limited to one side, and the severity of which is not correlated with the clinical course of disease. Sialectasies tend to disappear after adolescence. Moreover, sialography may have a therapeutic effect due to the irrigation of ductal system (free-radicals flushing), and the action of antiseptic iodine into the ducts may be helpful in healing 74.
With the improvement of less-invasive imaging techniques, such as CT, US and MRI, diagnostic approaches different from sialography are available.
The first-line treatments of acute swelling episodes usually consist of the association of analgesics, anti-inflammatory drugs and antibiotics. Sialagogues and gland massages remain useful additional therapy. Corticosteroids are indicated in severe forms.
In the last few years, there are many reports on the striking role of sialoendoscopy in diagnosis and treatment of JRP. Recently, some authors have demonstrated that sialoendoscopic diagnosis is as significant as that made with conventional imaging. Furthermore, sialoendoscopic examination allows detecting characteristics of JRP that might be difficult to observe with US or other imaging techniques, such as the lack of natural vascularisation of the ductal wall 75-78. Finally, a recent work of Ardekian et al. evaluating a sample of 50 children affected by JRP showed a statistically significant correlation between sialoendoscopic findings and clinical outcome, also validating the sialoendoscopy as an effective treatment for this condition 79. Sialoendoscopy, in fact, may break the cycle of ductal inflammation by washing out intraductal debris and dilating the stenosis 75-79.
Recurrent parotitis and masticatory disorders
Masseter muscle hypertrophy (MH), also referred to as benign or idiopathic masseter hypertrophy, may play a role in the aetiology of recurrent obstructive parotitis. The aetiology of MH is still unknown, but several authors claim that emotional stress results in chronic overuse of the jaws due to clenching, bruxism, constant chewing, or temporomandibular joint disorder 80 81.
A relationship between parotitis and masseter hypertrophy has been recently described. In particular, Reddy et al. showed three cases of chronic parotitis secondary to an acute bend in SD caused by an enlargement of the masseteric space 82.
The diagnosis of this concomitant condition is made on clinical signs, imaging and sialoendoscopic findings 80-82. Patients typically show recurrent unilateral swelling of the face during meals, mastication with unilateral or bilateral tenderness, enlargement of the masseter muscles and dental wear facets consistent with bruxism. CT or cone beam 3D CT can be useful to detect bone abnormalities secondary to MH and to check for temporo-mandibular joints. Conventional MR and MR-sialography can be done to depict the relationship between the involved parotid gland, muscle hypertrophy and ductal dilation secondary to ab estrinseco compression of masseter muscles. Finally, electromyographic evaluation of masticatory muscles can be done to evaluate muscular activities 81. Diagnostic sialoendoscopy may be helpful to detect typical kinkings or acute angles of SD due to external muscle compression 9 77.
The treatment of this condition is usually multimodal and should consider the management of both conditions, namely MH and parotitis. Non-surgical therapy includes reassurance, muscle relaxants, injection of botulinum toxin type A, dental restorations and occlusal adjustments and nightly bite guard use 81. Surgical management includes an intraoral approach with reduction of deep masseter muscle and monocortical and bicortical ostectomy of the angle of the mandible.
Management of recurrent parotitis is based on interventional sialoendoscopy of the parotid glands with dilation and irrigation of the duct system with saline and steroid solution 9 82. Concomitant injections of botulinum toxin type A in the masseter muscle and parotid gland have been proposed to obtain functional silencing of the parotid gland and relaxation of masseter muscles 9.
Diagnosis of recurrent obstructive parotitis secondary to masseteric hypertrophy should be done every time diagnostic sialoendoscopy does not reveal intraluminal causes of obstruction but only duct kinkings, and clinical evaluation of the cheek reveals tenderness and enlargement of masseter muscles. In this case, an orthodontic diagnostic and therapeutic work-up should be planned to facilitate clinical recovery.
Radioiodine sialadenitis
Thyroid gland cancer management with radioactive iodine (131I) has led to the development of salivary gland injuries specifically related to the harmful effects of the radioisotope. According to the literature, up to 69% of post-radioiodine salivary dysfunction and more than 25% of radioiodine sialadenitis is present at 12 months after treatment 83-85.
Salivary gland tissues express the ability to concentrate iodine due to a sodium/iodide symporter placed in parenchymal and, prevalent mostly, in ductal cells. It has been assessed that the salivary iodine concentration ranges from 20 to 100 times the level detected in plasma and up to 24% of administered 131I is secreted into the saliva. Therefore, the salivary glands become a potential collateral target of radioactive iodine therapy, and obstructive sialadenitis is usually the first gland effects due to irradiation. Moreover, the vascular endothelium of salivary glands results in increased permeability because of 131I damage, leading to leakage of plasma proteins and electrolytes.
Since serous glands, and especially the excretory ductal system, are more frequently involved than mucous glands, radioiodine sialadenitis may be mainly defined as a ductal disease of the parotid gland.
As a physiopathological consequence of 131I exposure, the pivotal processes may be resumed as follows 86:
Ductal obstruction secondary to periductal inflammation and an inflammatory infiltrate;
Ascending gland infections related to the reduced ability to drain saliva;
Radioisotope diffusion into salivary gland parenchyma and biochemical salivary changes by through increased capillary permeability.
The overall described mechanisms determine salivary flow decrease, stagnation and mucus precipitation with mucous plugs formation. Furthermore, they trigger an inflammatory vicious circle that upgrades 131I retention. Recurrent inflammatory and/or infectious events may result in chronic gland sclerosis. Pain, swelling, distorted taste perception and subsequent xerostomia are common symptoms. Clinical presentation, essentially obstructive in nature with bilateral predominance, may occur early (within the first 48 hours after irradiation) or late at 3-6 months from the beginning of 131I treatment 85 86.
Historically, treatment of 131I sialadenitis included sialogogic agents followed by gland massages, heat, steroids, adequate daily fluid intake, mouthwashes, duct probing and antibiotics. According to Kim et al., the benefits perceived from conservative therapies have been estimated in no more than 71% of patients treated with 131I 85. Up to now, recalcitrant sialadenitis may only be submitted to adenectomy as the sole option available after failure of medical therapy.
In 2006, Nahlieli et al. published encouraging results concerning a novel employment of salivary gland videoendoscopy in 15 patients with radioiodine sialadenitis 87. Since then, three other international experiences have reported on the added advantages of sialoendoscopy as a minimally invasive procedure for both diagnostic and therapeutic purposes 83-85.
Results
Sialoendoscopy: analysis of outcomes
Many international experiences have reported on the effectiveness and safety of sialoendoscopy in both adult and paediatric patients 14 73 77 78. Herein, sialoendoscopic outcomes will be discussed with particular focus on the main causes of benign salivary duct obstructions.
Salivary stones
Sialolithiasis is undoubtedly the most frequent area of application, used in 60-70% of all sialoendoscopic treatments 24. In particular, video-endoscopic findings stress not only the interventional aspects, but also the diagnostic role of sialoendoscopy. Assessment of undiagnosed recurrent obstructive symptoms with sialoendoscopy reinforce that it is an additional tool that can fill the diagnostic gap between clinical suspicion and instrumental investigation 88. A monocentric retrospective study reviewed the results on 1154 patients submitted to sialoendoscopy after US 89. Provisional US diagnosis of salivary stones was excluded by video-endoscopy in 21% of parotid glands and in 7% of submandibular glands. Nahlieli et al. focused on the diagnostic gain produced by sialoendoscopy in 236 cases: stones were revealed only after sialoendoscopy in 63% of parotid glands and in 32% of submandibular glands 90. These findings have led some authors to reconsider the epidemiology of traditional salivary stones because of the relatively high percentage of US misdiagnosis involving parotid glands 36.
The working channel of salivary endoscopes allows both diagnostic and therapeutic operations, and there are many publications that have documented high success rates. A systematic review and meta-analysis searched all articles published since October 2010 77. Sialoendoscopy alone provided a success rate (symptom-free percentage) of 86% in 1213 patients (19 publications) which increased to 93% in 374 patients when sialoendoscopy was performed with a combined surgical approach (11 publications). Combined external surgical approaches comprised small or large transoral incisions and preauricular skin flaps. Salivary gland adenectomy was required in 0-11% of cases. Despite the meticulous work of the meta-analysis, the Authors considered that the main weakness of their study was due to the large heterogeneity of the articles included. More specifically, the results not only involved treatment of salivary stones, but also other causes of obstruction (e.g. ductal strictures or polyps) mixing both parotid and submandibular glands. Therefore, the pooled percentage of success may be considered susceptible to variation compared with "true outcomes".
A multicentre international observational study lasting 14 years on 4691 patients with sialolithiasis did not meet the selection criteria of the above meta-analysis. In fact, firstline treatments included not only sialoendoscopy, but also extracorporeal shockwave lithotripsy or stones removal under fluoroscopic/radiographic guidance 55. The overall success rate was 80.5% (complete calculi removal) and 16.7% (partial calculi removal) with an incidence of sialadenectomy of 2.9%. Appropriate patient selection allows high success rates and reduces adverse events: the size, site, shape and orientation of salivary stones strictly correlates with the probability of successful endoscopic stone removal 23 91 92.
Salivary duct anomalies
Strictures, polyps, kinks and foreign bodies are considered the second most frequent cause of benign salivary duct obstruction 93. Several miniaturised devices coupled with a sialoendoscope may be adopted to address these obstructive disorders such as balloons, grasping forceps and stents. As previously reported, no systematic review has been published on outcomes in salivary duct anomalies other than sialolithiasis. Ardekian et al. retrospectively analysed sialoendoscopic outcomes in 335 glands and found 87 cases of strictures 94. Sialoendoscopy was successful in 81.7% of the affected parotid glands with similar results to Nahlieli O. who documented a success rate of 80-81% for strictures and 100% for kinks 7 94.
Radioiodine sialadenitis
Three studies (33 patients) were included in the metaanalysis by Strychowsky's 77 84 87. The percentage of patients with complete resolution of symptoms ranged from 50-100%, with no major complications reported. One noteworthy finding is the high rate of inability (50% of cases) to cannulate gland ducts reported by Kim et al. 84 Salivary gland excision was only described by Prendes's experience in 9% of patients 83.
Juvenile recurrent parotitis
Updated to August 2013, a recent work overviewed the existing literature concerning sialoendoscopic outcomes in patients suffering from JRP 73. Despite the limits of the included studies (level of evidence 4, relatively small population, absence of long-term follow-up), the high success rate achieved (symptom free: 78%, partial regression: 22%) support the positive role of sialoendoscopy in prevention of recurrent attacks. International experiences have also confirmed the diagnostic benefits of sialoendoscopy, since direct endoscopic exploration allows for differential diagnosis among dissimilar causes of obstruction 79 95.
Autoimmune sialadenitis
Currently, a limited amount of information is available on salivary gland videoendoscopy in patients with autoimmune sialadenitis, and additional evidence is needed 72.
Contraindications and management of complications
Sialoendoscopy has few contraindications, and in almost all cases it is possible to perform the endoscopic procedure. From a review of the literature, an exclusive endoscopic procedure is contraindicated in acute sialoadenitis, complete distal duct stenosis, symptomatic intraparenchymal stone and limited mouth opening, even if the latter is a contraindication mainly in a combined endoscopically/ external or intraoral approach 14 22 23 27 37 59 93.
Complications in an exclusive endoscopic approach are rare, and most are minor: even temporary glandular swelling, routinely present in almost all procedures (88%) 14 22 23, is mainly considered a correlated effect rather than a true complication. The most frequent complications in an endoscopic approach are post-operative duct strictures, laceration of the duct (including device blocks or rupture) and infection 23.
Iatrogenic post-operative ductal stricture are not so common: they are less than 2% and in most cases is related to stone removal > 5 mm 41 42 56. Laceration of the duct is present in about 5% of cases 27 44, but long term salivary fistula is rarely related with laceration 14. Papilla infection is quite common, seen in around 23% of patients, while glandular infection is relatively infrequent (2.5%) 14 93. The breaking and blocking of endoscopic tools inside the duct is another possible complication, which is only rarely reported. It does not appear to be a major concern, as in all reported cases except one endoscopic removal of the instrument was possible. Complications are related to the duration of the procedure: longer procedures are associated with an increase rate in side effects 14 89 90 96.
As for a combined or external approach, the most frequent complications are pain of the floor of the mouth (8%) 27, temporary lingual nerve paresthesia (4%) 96, ranula formation (3%) 65 96 and definitive lingual nerve palsy (< 1%) 65. Considering the combined approach, the rate of complications such as fistula or duct stenosis/laceration 14 27 is not significantly higher than in the endoscopic group. The only different complication is temporary or definitive palsy of the lingual nerve 27 59 96, for which only medical treatment is required. Other related complications such as swelling of the floor of the mouth and ranulas are seen in less than 1% of cases, and no treatment is required 65.
Complications after sialoendoscopy usually resolve spontaneously: post-operative gland swelling shows complete recovery usually after few days (1-4) 23, except in rare cases of duct or hilar fistula in which compressive medication or botulinum treatment is needed. Post-operative duct stricture requires medical treatment, after which a second endoscopic approach may be necessary with duct dilatation and/or sialo-stent positioning 14 22 77.
In conclusion, sialoendoscopy (both endoscopic and combined approach) has a low rate of complications and side effects, which in most cases are easy to manage. At centres where the salivary endoscopy is performed, sialeadenectomies for obstructive pathologies are needed in less than 10% of all cases.
Conclusions
Is sialadenectomy still indicated in obstructive salivary pathologies?
Sialoadenectomy, while remaining the gold standard for salivary gland neoplasms, has greatly reduced its role in cases of obstructive diseases due to the introduction of interventional sialoendoscopy. Nevertheless, sialoadenectomy continues to have a role in all cases where, due to the size, location and number of stones or due to irreversible pathological conditions of the gland (massive fibrosis, multiple stenoses, chronic sialadenitis), an endoscopic technique may not lead to satisfactory results or does not prevent the appearance of relapses.
A limitation of sialoendoscopy alone, taking into account the size of stones, is the difficulty in removing stones with a diameter > 4 mm 14. This constraint has been overcome through the use of lithotripsy achieved by different types of intracorporeal lasers or by extracorporeal shock waves 97.
These techniques are time consuming and not readily available in all centres. Its best success rates up range from 75% for the parotid to 40% for the submandibular gland 35 46 and seem similar for both external and intraductal lithotripsy 98. On the other hand, holmium:YAG, and to a lesser degree thulium:YAG 15 laser fibres, may inadvertently cause damage to duct walls 23 while dye laser at 350 nm which are absorbed by the tissues are still expensive 6 2.
Marchal, in his cases series of interventional sialoendoscopy with laser fragmentation, reported 20% of failures due to large stones (6 mm or larger) and stenotic ducts, particularly in the parotid gland. In these cases, sialoadenectomy can be used to treat such failures 23.
The introduction by Nahlieli 65 of a combined technique for removal of stones, which consists in locating the stone endoscopically and extracting it with a minimally invasive technique, has further reduced the number of cases in which sialoadenectomy is necessary.
However, Marchal, in a selected case series of combined procedures, had to remove the submandibular gland shortly afterwards in 28% (8 of 29) of patients for postoperative closure of the mucosa of the floor of the mouth leading to continuous swelling of the submandibular gland after an initial period of remission. In the same series, failures of the combined technique for parotid obstructions were due to polycystic ductal disease and mega SD 62.
Giant salivary stones (≥ 15 mm) can be treated using a combined technique, although in some cases (from 14% to 43%) they require sialoadenectomy 99.
Zenk et al. conducted a case series of 1033 patients with sialolithiasis, the largest up to date, using transoral removal or endoscopy alone, respectively, in 92 and 5% of submandibular lithiasis with long-term success rates of ≥ 90%. Parotid stones were removed by salivary gland endoscopy (22%), combined endoscopy with an incisional technique (26%), or ESWL (52%), with long-term success rates of 98%, 89% and 79%, respectively. Submandibular or parotid glands had to be removed in 5% of cases 89. Similar gland excision rates (from 0 to 9%) are reported in recent literature 100.
Taking into account the location of the stones, another limitation of sialoendoscopy alone can be the difficulty in removing stones located in second and third ductal divisions, where combined approaches are not always efficient (in these cases the use of thulium laser lithotripsy may offer better results) or after an acute bend in the main duct 44.
Lastly, a contraindication for sialoendoscopy, and thus an indication for sialoadenectomy, is complete distal obliteration of the duct that is impenetrable by the endoscope which can occur in patients with a long-standing history of recurrent inflammations that leads to the impaction of the sialolith to the wall of the efferent duct 14 100.
In conclusion, sialoadenectomy should be considered in cases of failures of transoral removal of hilar stones > 7 mm or failures after an extra-corporeal shock-wave lithotripsy for an intraparenchymal stone in the submandibular gland 14.
Sialoendoscopy is a relatively new procedure, but in the last few years it has rapidly spread worldwide. Although it was first described as an alternative procedure for salivary stone removal, it is now considered as the treatment of choice for obstructive pathologies of the salivary ductal system. Its popularity is continuously growing because it represents a relatively simple procedure and since the last decade most otolaryngologists have become experienced in different areas of endoscopic surgery. Moreover, the basic equipment, although fragile, does not represent an excessively expensive tool in the era of minimally invasive surgery.
Sialeendoscopic procedures, in addition to combined minimally invasive external or transoral approaches, have now drastically reduced the indication for salivary gland removal.
Even in the field of research, sialoendoscopy seems to offer a new perspective in the medical treatment of some emerging neurologic and autoimmune diseases usually presenting quantitative and/or qualitative alterations of saliva such as sialorrhoea and xerostomia. The increasing number of studies on sialoendoscopy emerging from analysis of the literature in this review confirm the rising interest of otolaryngologists in this new field of research and treatment modality.
References
- 1.Katz P. Endoscopy of the salivary glands. Ann Radiol. 1991;34:110–113. [PubMed] [Google Scholar]
- 2.Horsburgh A, Massoud TF. The role of salivary duct morphology in the aetiology of sialadenitis: statistical analysis of sialographic features. Int J Oral Maxillofac Surg. 2013;42:124–128. doi: 10.1016/j.ijom.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Zenk J, Hosemann WG, Iro H. Diameters of the main excretory ducts of the adult human submandibular and parotid gland: a histologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:576–580. doi: 10.1016/s1079-2104(98)90294-3. [DOI] [PubMed] [Google Scholar]
- 4.Kang HC, Kwak HH, Hu KS, et al. An anatomicalstudy of the buccinator muscle fibres that extend to the terminal portion of the parotid duct, and their functional roles in salivary secretion. J Anat. 2006;208:601–607. doi: 10.1111/j.1469-7580.2006.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano K, Moriyama H, Shimada K, et al. Study of human adult parotid duct in the area of penetration through buccinator muscle and their functional relationship as a sphincter. Ital J Anat Embryol. 2013;118:6–18. [PubMed] [Google Scholar]
- 6.Drage NA, Wilson RF, McGurk M. The genu of the submandibular duct:is the angle significant in salivary gland disease? Dentomaxillofac Radiol. 2002;31:15–18. doi: 10.1038/sj/dmfr/4600653. [DOI] [PubMed] [Google Scholar]
- 7.Nahlieli O, Shacham R, Yoffe B, et al. Diagnosis and treatment of strictures and kinks in salivary gland ducts. J Oral Maxillofac Surg. 2001;59:484–490. doi: 10.1053/joms.2001.22667. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Xu H, Cai ZG, et al. Clinical and anatomic study on the ducts of the submandibular and sublingual glands. J Oral Maxillofac Surg. 2010;68:606–610. doi: 10.1016/j.joms.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 9.Marchal F, Becker M, Dulguerov, et al. Interventional sialoendoscopy. Laryngoscope. 2000;110:318–320. doi: 10.1097/00005537-200002010-00026. [DOI] [PubMed] [Google Scholar]
- 10.Chossegros C, Guyot L, Richard O, et al. A technical improvement in sialoendoscopy to enter the salivary ducts. Laryngoscope. 2006;116:842–844. doi: 10.1097/01.mlg.0000214665.74330.96. [DOI] [PubMed] [Google Scholar]
- 11.Nahlieli O, Shacham R, Bar T, et al. Endoscopic mechanical retrieval of sialoliths. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:396–402. doi: 10.1067/moe.2003.145. [DOI] [PubMed] [Google Scholar]
- 12.Lari N, Chossegros C, Thiery G, et al. Sialoendoscopy of the salivary glands. Rev Stomatol Chir Maxillofac. 2008;109:167–171. doi: 10.1016/j.stomax.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Nahlieli O, Baruchin AM. Sialoendoscopy: three years' experience as a diagnostic and treatment modality. J Oral Maxillofac Surg. 1997;55:912–918. doi: 10.1016/s0278-2391(97)90056-2. [DOI] [PubMed] [Google Scholar]
- 14.Capaccio P, Torretta S, Ottaviani F, et al. Modern management of obstructive salivary diseases. Acta Otorhinolaryngol Ital. 2007;27:161–172. [PMC free article] [PubMed] [Google Scholar]
- 15.Faure F, Boem A, Taffin C, et al. Diagnostic and interventional sialoendoscopy. Rev Stomatol Chir Maxillofac. 2005;106:250–252. doi: 10.1016/s0035-1768(05)85854-1. [DOI] [PubMed] [Google Scholar]
- 16.Luers JC, Damm M, Klussmann JP, et al. The learning curve of sialoendoscopy with modular sialoendoscopes: a single surgeon's experience. Arch Otolaryngol Head Neck Surg. 2010;136:762–765. doi: 10.1001/archoto.2010.109. [DOI] [PubMed] [Google Scholar]
- 17.Vairel B, Bonnecaze G, Shehri S, et al. Courbe d'apprentissage en sialoendoscopie: nos 101 premières procedures. Rev Laryngol Otol Rhinol. 2012;133:177–181. [PubMed] [Google Scholar]
- 18.Maresh A, Kutler DI, Kacker A. Sialoendoscopy in the diagnosis and management of obstructive sialadenitis. Laryngoscope. 2011;121:495–500. doi: 10.1002/lary.21378. [DOI] [PubMed] [Google Scholar]
- 19.Kroll T, Finkensieper M, Hauk H, et al. Sialoendoscopylearning curve and nation-wide survey in German ENT-departments. Laryngorhinootologie. 2012;91:561–565. doi: 10.1055/s-0032-1314880. [DOI] [PubMed] [Google Scholar]
- 20.Modest MC, Galinat L, Rabinowitz MR, et al. Learning progression in the use of sialoendoscopy for sialolithiasis: effect on gland preservation. Otolaryngol Head Neck Surg. 2014;151:240–245. doi: 10.1177/0194599814533658. [DOI] [PubMed] [Google Scholar]
- 21.Marchal F, Chossegros C, Faure F, et al. Salivary stones and stenosis. A comprehensive classification. Rev Stomatol Chir Maxillofac. 2008;109:223–226. doi: 10.1016/j.stomax.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 22.McGurk M, Escudier MP, Brown JE. Modern management of salivary calculi. Br J Surg. 2005;92:107–112. doi: 10.1002/bjs.4789. [DOI] [PubMed] [Google Scholar]
- 23.Marchal F, Dulguerov P. Sialolithiasis management: the state of the art. Arch Otolaryngol Head Neck Surg. 2003;129:951–956. doi: 10.1001/archotol.129.9.951. [DOI] [PubMed] [Google Scholar]
- 24.Koch M, Zenk J, Iro H. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin North Am. 2009;42:1173–1192. doi: 10.1016/j.otc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Fritsch MH. Algorithms for treatment of salivary gland obstructions without access to extracorporeal lithotripsy. Otolaryngol Clin North Am. 2009;42:1193–1197. doi: 10.1016/j.otc.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Foletti JM, Chossegros C, Salles F, et al. Transoral approach for Stensen's duct lithiasis. Laryngoscope. 2011;121:1893–1895. doi: 10.1002/lary.21792. [DOI] [PubMed] [Google Scholar]
- 27.Capaccio P, Bottero A, Pompilio M, et al. Conservative transoral removal of hilar submandibular salivary calculi. Laryngoscope. 2005;115:750–752. doi: 10.1097/01.mlg.0000161348.15505.11. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Baba S. Pulsed dye laser lithotripsy of submandibular gland salivary calculus. J laryngol Otol. 1996;110:218–221. doi: 10.1017/s0022215100135418. [DOI] [PubMed] [Google Scholar]
- 29.Konigsberger R, Freyh J, Goetz A, et al. Endoscopicallycontrolled electrohydraulic intracorporeal shock wave lithotripsy (EISL) of salivary stones. J Otolaryngol. 1993;22:12–13. [PubMed] [Google Scholar]
- 30.Iro H, Zenk J, Hosemann WG, et al. Electrohydraulic intracorporeal lithotripsy of salivary calculi. In vitro and in animal experimented studies. HNO. 1993;41:389–395. [PubMed] [Google Scholar]
- 31.Iro H, Benzel W, Gode U, et al. Pneumatic intracorporeal lithotripsy of salivary calculi. In vitro and in animal experimented studies. HNO. 1995;43:172–176. [PubMed] [Google Scholar]
- 32.Arzoz E, Santiago A, Esnal F, et al. Endoscopic intracorporeal lithotripsy for sialolithiasis. J Oral Maxillofac surg. 1996;54:847–850. doi: 10.1016/s0278-2391(96)90533-9. [DOI] [PubMed] [Google Scholar]
- 33.Ottaviani F, Capaccio P, Rivolta R, et al. Salivary gland stones: US evaluation in shock wave lithotripsy. Radiology. 1997;204:437–441. doi: 10.1148/radiology.204.2.9240532. [DOI] [PubMed] [Google Scholar]
- 34.Iro H, Zenk J, Waldfahrer F, et al. Extracorporeal shock wave lithotripsy of parotid stones. Results of a prospective clinical trial. Ann Otol Rhinol Laryngol. 1998;107:860–864. doi: 10.1177/000348949810701009. [DOI] [PubMed] [Google Scholar]
- 35.Capaccio P, Ottaviani F, Manzo R, et al. Extracorporeal lithotripsy for salivary calculi: a long-term clinical experience. Laryngoscope. 2004;114:1069–1073. doi: 10.1097/00005537-200406000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Nahlieli O, Baruchin AM. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J Oral Maxillofac Surg. 1999;57:1394–1402. doi: 10.1016/s0278-2391(99)90716-4. [DOI] [PubMed] [Google Scholar]
- 37.Marchal F, Dulguerov P, Becker M, et al. Specificity of parotid sialoendoscopy. Laryngoscope. 2001;111:264–271. doi: 10.1097/00005537-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Gundlach P, Scherer H, Hopf J, et al. Endoscopic-controlled laser lithotripsy of salivary calculi: in vitro studies and initial clinical use. HNO. 1990;38:247–250. [PubMed] [Google Scholar]
- 39.Iro H, Zenk J, Benzel W. Laser lithotripsy of salivary duct stones. Adv Otorhinolaryngol. 1995;49:148–152. doi: 10.1159/000424360. [DOI] [PubMed] [Google Scholar]
- 40.Kerr PD, Krahn H, Brodovsky D. Endoscopic laser lithotripsy of a proximal parotid duct calculus. J Otolaryngol. 2001;30:129–130. doi: 10.2310/7070.2001.19938. [DOI] [PubMed] [Google Scholar]
- 41.Martellucci S, Pagliuca G, Vincentiis M, et al. Ho:Yag laser for sialolithiasis of Wharton's duct. Otolaryngol Head Neck Surg. 2013;148:775–777. doi: 10.1177/0194599813479914. [DOI] [PubMed] [Google Scholar]
- 42.Sionis S, Caria RA, Trucas M, et al. Sialoendoscopy with and without holmium:YAG laser-assisted lithotripsy in the management of obstructive sialadenitis of major salivary glands. Br J Oral Maxillofac Surg. 2014;52:58–62. doi: 10.1016/j.bjoms.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Raif J, Vardi M, Nahlieli O, et al. An Er:YAG laser endoscopic fiber delivery system for lithotripsy of salivary stones. Lasers Surg Med. 2006;38:580–587. doi: 10.1002/lsm.20344. [DOI] [PubMed] [Google Scholar]
- 44.Durbec M, Dinkel E, Vigier S, et al. Thulium-YAG laser sialoendoscopy for parotid and submandibular sialolithiasis. Lasers Surg Med. 2012;44:783–786. doi: 10.1002/lsm.22094. [DOI] [PubMed] [Google Scholar]
- 45.Iro H, Schneider T, Nitsche N, et al. Extracorporeal piezoelectric lithotripsy of salivary calculi: initial clinical experiences. HNO. 1990;38:251–255. [PubMed] [Google Scholar]
- 46.Ottaviani F, Capaccio P, Campi M, et al. Extracorporeal electromagnetic shock-wave lithotripsy for salivary gland stones. Laryngoscope. 1996;106:761–764. doi: 10.1097/00005537-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Escudier MP, Brown JE, Drage NA, et al. Extracorporeal shockwave lithotripsy in the management of salivary calculi. Br J Surg. 2003;90:482–485. doi: 10.1002/bjs.4067. [DOI] [PubMed] [Google Scholar]
- 48.Iro H, Nitsche N, Schneider HT. Extracorporeal shockwave lithotripsy of salivary gland stones. Lancet. 1989;2:115–115. doi: 10.1016/s0140-6736(89)90365-6. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz S, Zengel P, Alvir I. Long-term evaluation of extracorporeal shock wave lithotripsy in the treatment of salivary stones. J Laryngol Otol. 2008;122:65–71. doi: 10.1017/S0022215107007396. [DOI] [PubMed] [Google Scholar]
- 50.Zenk J, Bozzato A, Winter M, et al. Extracorporeal shock wave lithotripsy of submandibular stones: evaluation after 10 years. Ann Otol Rhinol Laryngol. 2004;113:378–383. doi: 10.1177/000348940411300507. [DOI] [PubMed] [Google Scholar]
- 51.Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545–1549. doi: 10.1002/lary.21000. [DOI] [PubMed] [Google Scholar]
- 52.Zenk J, Koch M, Mantsopoulos K, et al. The significance of extracorporeal shock wave lithotripsy in sialolithiasis therapy. HNO. 2013;61:306–311. doi: 10.1007/s00106-013-2677-4. [DOI] [PubMed] [Google Scholar]
- 53.Nahlieli O, Shacham R, Zaguri A. Combined external lithotripsy and endoscopic techniques for advanced sialolithiasis cases. J Oral Maxillofac Surg. 2010;68:347–353. doi: 10.1016/j.joms.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Capaccio P, Torretta S, Pignataro L. Extracorporeal lithotripsy techniques for salivary stones. Otolaryngol Clin North Am. 2009;42:1139–1159. doi: 10.1016/j.otc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Iro H, Zenk J, Escudier MP, et al. Outcome of minimally invasive management of salivary calculi in 4,691 patients. Laryngoscope. 2009;119:263–268. doi: 10.1002/lary.20008. [DOI] [PubMed] [Google Scholar]
- 56.Chang J, Eisele DW. Limited distal sialodochotomy to facilitate sialoendoscopy of the submandibular duct. Laryngoscope. 2013;123:1163–1167. doi: 10.1002/lary.23801. [DOI] [PubMed] [Google Scholar]
- 57.Rahmati R, Gillespie MB, Eisele DW. Is sialoendoscopy an effective treatment for obstructive salivary gland disease? Laryngoscope. 2013;123:1828–1829. doi: 10.1002/lary.23958. [DOI] [PubMed] [Google Scholar]
- 58.Overton A, Combes J, McGurk M. Outcome after endoscopically assisted surgical retrieval of symptomatic parotid stones. Int J Oral Maxillofac Surg. 2012;41:248–251. doi: 10.1016/j.ijom.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Makdissi J, Escudier MP, Brown JE, et al. Glandular function after intra-oral removal of salivary calculi from the hilum of the submandibular gland. Br J Oral Maxillofac Surg. 2004;42:538–541. doi: 10.1016/j.bjoms.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Combes J, Karavidas K, McGurk M. Intraoral removal of proximal submandibular stones-an alternative to sialadenectomy? Int J Oral Maxillofac Surg. 2009;38:813–816. doi: 10.1016/j.ijom.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 61.Koch M, Bozzato A, Iro H, et al. Combined endoscopic and transcutaneous approach for parotid gland sialolithiasis: indications, technique, and results. Otolaryngol Head Neck Surg. 2010;142:98–103. doi: 10.1016/j.otohns.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Marchal F. A combined endoscopic and external approach for extraction of large stones with preservation of parotid and submandibular glands. Laryngoscope. 2007;117:373–377. doi: 10.1097/mlg.0b013e31802c06e9. [DOI] [PubMed] [Google Scholar]
- 63.Capaccio P, Clemente IA, McGurk M, et al. Transoral removal of hiloparenchymal submandibular calculi: a long-term clinical experience. Eur Arch Otorhinolaryngol. 2011;268:1081–1086. doi: 10.1007/s00405-011-1508-z. [DOI] [PubMed] [Google Scholar]
- 64.Pagliuca G, Martellucci S, Vincentiis M, et al. Wharton's duct repair after combined sialolithectomy: is ductoplasty necessary? Otolaryngol Head Neck Surg. 2013;148:775–777. doi: 10.1177/0194599813477839. [DOI] [PubMed] [Google Scholar]
- 65.Nahlieli O, London D, Zagury A, et al. Combined approach to impacted parotid stones. Oral Maxillofac Surg. 2002;60:1418–1423. doi: 10.1053/joms.2002.36097. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Escudier M, Brown J, et al. Long-term outcome after intraoral removal of large submandibular gland calculi. Laryngoscope. 2010;120:964–966. doi: 10.1002/lary.20839. [DOI] [PubMed] [Google Scholar]
- 67.Walvekar RR, Bomeli SR, Carrau RL, et al. Combined approach technique for the management of large salivary stones. Laryngoscope. 2009;119:1125–1129. doi: 10.1002/lary.20203. [DOI] [PubMed] [Google Scholar]
- 68.Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 69.Ohta N, Makihara S, Okano M, et al. Roles of IL-17, Th1, and Tc1 cells in patients with IgG4-related sclerosing sialadenitis. Laryngoscope. 2012;122:2169–2174. doi: 10.1002/lary.23429. [DOI] [PubMed] [Google Scholar]
- 70.MH, Guobis Z. Advances in the aetiophatgogenesis of Sjögren's Syndrome: a literature review. J Oral Maxillofac Res. 2012;3:e2–e2. doi: 10.5037/jomr.2012.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passafaro D, S. Reina S, Sterin-Borda L, et al. Cholinergic autoantibodies from primary Sjogren's syndrome modulate submandibular gland Na+/K+-ATPase activity via prostaglandin E2 and cyclic AMP. Eur J Oral Sciences. 2010;118:131–138. doi: 10.1111/j.1600-0722.2010.00716.x. [DOI] [PubMed] [Google Scholar]
- 72.Shacham R, Puterman MB, Ohana N, et al. Endoscopic treatment of salivary glands affected by autoimmune diseases. J Oral Maxillofac Surg. 2011;69:476–481. doi: 10.1016/j.joms.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Canzi P, Occhini A, Pagella F, et al. Sialoendoscopy in juvenile recurrent parotitis: a review of literature. Acta Otorhinolaryngol Ital. 2013;33:367–373. [PMC free article] [PubMed] [Google Scholar]
- 74.Galili D, Marmary Y. Juvenile recurrent parotitis: Clinicoradiologic follow-up study and the beneficial effect of sialography. Oral Surg Oral Med Oral Pathol. 1986;61:550–556. doi: 10.1016/0030-4220(86)90091-5. [DOI] [PubMed] [Google Scholar]
- 75.Capaccio P, Sigismund PE, Luca N, et al. Modern management of juvenile recurrent parotitis. J Laryngol Otol. 2012;126:1254–1260. doi: 10.1017/S0022215112002319. [DOI] [PubMed] [Google Scholar]
- 76.Konstantinidis I, Chatziavramidis A, Tsakiropoulou E, et al. Pediatric sialoendoscopy under local anesthesia: limitations and potentials. Int J Pediatr Otorhinolaryngol. 2011;75:245–249. doi: 10.1016/j.ijporl.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Strychowsky JE, Sommer DD, Gupta MK, et al. Sialoendoscopy for the management of obstructive salivary gland disease: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2012;138:541–547. doi: 10.1001/archoto.2012.856. [DOI] [PubMed] [Google Scholar]
- 78.McGurk M. Salivary gland disease. First International accord on modern management-Paris, July 4-5 2008. Acta Otorhinolaryngol Ital. 2008;28:269–272. [Google Scholar]
- 79.Ardekian L, Klein H, Abri R. Sialoendoscopy for the diagnosis and treatment of juvenile recurrent parotitis. Rev Stomatol Chir Maxillofac Chir Orale. 2014;115:17–21. doi: 10.1016/j.revsto.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Harbison JM, Liess BD, Templer JW, et al. Chronic parotitis: a challenge disease entity. Ear Nose Throat J. 2011;90:E13–E16. doi: 10.1177/014556131109000317. [DOI] [PubMed] [Google Scholar]
- 81.Özkan BT, Tabrizi R, Cigerim L. Management of bilateral masseter muscle hypertrophy. J Craniofac Surg. 2012;23:e14–e16. doi: 10.1097/SCS.0b013e31824207a2. [DOI] [PubMed] [Google Scholar]
- 82.Reddy R, White DR, Gillespie MB. Obstructive parotitis secondary to an acute masseteric bend. ORL J Otorhinolaryngol Relat Spec. 2012;74:12–15. doi: 10.1159/000334246. [DOI] [PubMed] [Google Scholar]
- 83.Prendes BL, Orloff LA, Eisele DW. Therapeutic sialoendoscopy for the management of radioiodine sialadenitis. Arch Otolaryngol Head Neck Surg. 2012;138:15–19. doi: 10.1001/archoto.2011.215. [DOI] [PubMed] [Google Scholar]
- 84.Bomeli SR, Schaitkin B, Carrau RL, et al. Interventional sialoendoscopy for treatment of radioiodine-induced sialadenitis. Laryngoscope. 2009;119:864–867. doi: 10.1002/lary.20140. [DOI] [PubMed] [Google Scholar]
- 85.Kim JW, Han GS, Lee SH, et al. Sialoendoscopic treatment for radioiodine induced sialadenitis. Laryngoscope. 2007;117:133–136. doi: 10.1097/01.mlg.0000247776.72484.62. [DOI] [PubMed] [Google Scholar]
- 86.Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13:265–271. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]
- 87.Nahlieli O, Nazarian Y. Sialadenitis following radioiodine therapy -a new diagnostic and treatment modality. Oral Dis. 2006;12:476–479. doi: 10.1111/j.1601-0825.2006.01223.x. [DOI] [PubMed] [Google Scholar]
- 88.Koch M, Zenk J, Bozzato A, et al. Sialoscopy in cases of unclear swelling of the major salivary glands. Otolaryngol Head Neck Surg. 2005;133:863–868. doi: 10.1016/j.otohns.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Zenk J, Koch M, Klintworth N, et al. Sialoendoscopy in the diagnosis and treatment of sialolithiasis: a study on more than 1000 patients. Otolaryngol Head Neck Surg. 2012;147:858–863. doi: 10.1177/0194599812452837. [DOI] [PubMed] [Google Scholar]
- 90.Nahlieli O, Baruchin AM. Long-term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope. 2000;110:988–993. doi: 10.1097/00005537-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 91.Luers JC, Grosheva M, Stenner M, et al. Sialoendoscopy: prognostic factors for endoscopic removal of salivary stones. Arch Otolaryngol Head Neck Surg. 2011;137:325–329. doi: 10.1001/archoto.2010.238. [DOI] [PubMed] [Google Scholar]
- 92.Walvekar RR, Carrau RL, Schaitkin B. Endoscopic sialolith removal: orientation and shape as predictors of success. Am J Otolaryngol. 2009;30:153–156. doi: 10.1016/j.amjoto.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Nahlieli O, Bar T, Shacham R, et al. Management of chronic recurrent parotitis: current therapy. J Oral Maxillofac Surg. 2004;62:1150–1155. doi: 10.1016/j.joms.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 94.Ardekian L, Shamir D, Trabelsi M, et al. Chronic obstructive parotitis due to strictures of Stensen's duct--our treatment experience with sialoendoscopy. J Oral Maxillofac Surg. 2010;68:83–87. doi: 10.1016/j.joms.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Martins-Carvalho C, Plouin-Gaudon I, Quenin S, et al. Pediatric sialoendoscopy: a 5-year experience at a single institution. Arch Otolaryngol Head Neck Surg. 2010;136:33–36. doi: 10.1001/archoto.2009.184. [DOI] [PubMed] [Google Scholar]
- 96.McGurk M, Makdissi J, Brown JE. Intra-oral removal of stones from the hilum of the submandibular gland: report of technique and morbidity. Int J Oral Maxillofac Surg. 2004;33:683–686. doi: 10.1016/j.ijom.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 97.Witt RL, Iro H, Koch M, et al. Minimally invasive options for salivary calculi. Laryngoscope. 2012;122:1306–1311. doi: 10.1002/lary.23272. [DOI] [PubMed] [Google Scholar]
- 98.Gutmann R, Ziegler G, Leunig A, et al. Endoscopic and extracorporeal shock wave lithotripsy of salivary calculi. Laryngorhinootologie. 1995;74:249–253. doi: 10.1055/s-2007-997732. [DOI] [PubMed] [Google Scholar]
- 99.Wallace E, Tauzin M, Hagan J, et al. Management of giant sialoliths: review of the literature and preliminary experience with interventional sialoendoscopy. Laryngoscope. 2010;120:1974–1978. doi: 10.1002/lary.21082. [DOI] [PubMed] [Google Scholar]
- 100.Felton M, Mamais C, Kumar BN, et al. Medico-legal aspects of introducing sialoendoscopy: a minimally invasive treatment for salivary gland obstruction. Clin Otolaryngol. 2012;37:213–220. doi: 10.1111/j.1749-4486.2012.02485.x. [DOI] [PubMed] [Google Scholar]