Abstract
Vascular dysfunction is thought to play a major role in the development of diabetic cardiovascular disease. The roles of endothelial dysfunction, oxidative stress, and dyslipidemia will be considered. Melatonin as well as L-carnitine were shown to possess strong antioxidant properties. Diabetes induced with high fat diet (for 8 weeks) and multipl low doses intraperitoneal injection of STZ (twice, 30 mg/kg/d i.p). The diabetic animals were randomly assigned to one of the experimental groups as follows: Control group (C), high fat diet (HFD), STZ-induced diabetic group (HFD+STZ) , HFD+STZ diabetic group received melatonin (10 mg/kg/d i.p), HFD+STZ diabetic group received L-carnitine (0.6 g/kg/d i.p), and HFD+STZ diabetic group received glibenclamide (5 mg/kg/d, oral). The serum fasting blood glucose, insulin, total cholesterol, HDL- cholesterol, LDL-cholesterol, triglyceride and malondialdehyde (MDA) levels were tested. Acetylcholine induced endothelium-dependent relaxation and sodium nitroprusside induced endothelium-independent relaxation were measured in aortas for estimating endothelial function. Also, glutathione peroxidase (GPx), superoxide dismutase (SOD) and nitric oxide (NO) levels activities were determined in rat liver. According to our results melatonin and L-carnitine treatment decreased fasting blood glucose, total cholesterol, and LDL levels. MDA levels significantly decreased with the melatonin treatment whereas SOD levels were not significantly changed between the groups. The results suggest that especially melatonin restores the vascular responses and endothelial dysfunction in diabetes.
Keywords: Type 2 diabetes, HFD, Oxidative stress, Melatonin, L-carnitine, Rat
Graphical abstract
Highlights
-
•
We use Type 2 diabetes model with low dose of streptozotocin on high-fat fed rats.
-
•
Melatonin decreased oxidative stress especially MDA levels.
-
•
L-carnitine partly improved the endothelial dependent relaxation in the diabetic aorta.
-
•
Melatonin and L-carnitine decreased fasting blood glucose, total cholesterol, LDL levels.
Diabetes Mellitus is a metabolic as well as vascular disease which causes important complications with gradually increasing frequency all over the world. Formation of the free radicals and association between oxidative stress and diabetes complications have recently gained importance in the field old diabetes.
It is well known that the interaction between free oxygen radicals and nitric oxide (NO) cause formation of peroxynitrite and, this cytotoxic oxidant leads to endothelial dysfunction by impairing the function of cellular proteins through the nitration of proteins. Endothelial dysfunction plays a role in especially NO bioavailability of vasodilators, impaired endothelium dependent vasodilation or increased endothelium derived contracting factors, resulting in emergence of diabetes complications [1].
L-Carnitine (3-Hydroxy 4-N-trimethylammonio – butyrate) is an amino acid-like agent involved in the transit of long chain fatty acids which are physically synthesized in the various tissues in living organisms and will be transferred from cytoplasm to the mitochondria matrix, through the inner mitochondrial membrane. In diabetic experimental animals, carnitine levels of pancreas have been found to decrease both in early and advanced stages of diabetes and excretion of carnitine with urine to increase [2]. There are studies suggesting that insulin sensitivity and use of glucose by peripheral tissues increase 3, 4. In the studies conducted with hypertensive and normotensive rats, L-carnitine has been demonstrated to induce endothelium dependent relaxation by increasing the production of nitric oxide [5].
Melatonin is one of the known most potent antioxidants and recent studies indicate that melatonin receptors found in the pancreas islet cells might be protective against harmful outcomes of hyperglycemia [6]. In the other studies, L-carnitine has been shown to effectively normalize impaired oxidative condition in diabetic rats induced with STZ [7].
1. Experimental procedures
1.1. Animal model and experimental groups
Thirty five male Wistar rats (250–350 g) had access to laboratory food and water ad libitum. They were housed in cages under standard laboratory conditions (light period between 07:00–19:00 h, 21_2 °C; relative humidity 55%). This study was approved by the Institutional Animal Care Ethics Committee of Ege University, Turkey (2010/137).
The diabetic rat model was developed using a high-fat diet plus multiple low doses of streptozotocin which was similar to that employed in previously studies [8]. The high-fat diet consisted of 22% fat, 48% carbohydrate, and 20% protein with total calorific value 44.3 kJ/kg ( Bilgen Lab. Istanbul, Turkey) and control rats were given regular chow consisting of 5% fat, 53% carbohydrate, and 23% protein with total calorific value 25 kJ/kg. Following 4 weeks of dietary intervention, the diabetic group was injected intraperitoneally (i.p.) with low doses of streptozotocin (Sigma, St. Louis, MO, USA, 30 mg/kg, dissolved in 0.1 M sodium citrate buffer, pH 4.4). One week later, blood samples were collected by tail cutting for fasting blood glucose measurements were measured by (Accu-Chek Active-glucometer). Rats with a fasting blood glucose of 7.8 mmol/L were injected with streptozotocin again (30 mg/kg). Control rats were given vehicle citrate buffer (pH 4.4) in a matched volume (0.25 ml/kg) via intraperitoneal injection. 4 weeks after the streptozotocin injection, the fasting blood glucose was measured again, and rats with a fasting blood glucose of ≥7.8 mmol/L were considered diabetic [9]. The diabetic rats were fed the high-fat diet for another 4 weeks, the control and diabetic rats were then randomly divided into 5 groups: [1] control group (CONN, rats treated with saline in a matched volume), [2] Non treated diabetic group (HFD+STZ ), [3] L-carnitine treated diabetic group (HFD+STZ +LC, 0.6 g/kg/d, oral), [4] melatonin treated diabetic group (HFD+STZ +MLT, 10 mg/kg/d, i.p) and [5] glibenclamide treated diabetic group (HFD+STZ+GB, 5 mg/kg/d, i.p.). Melatonin and L-carnitine were dissolved in distillated water and administered intraperitoneally glibenclamide was administrated via oral gavage daily for 2 weeks 10, 11, 12. All the rats were allowed to continue to feed on their respective diets until the end of the study.
1.2. Vasocontractile responses
At the end of treatment, the animals were anesthetized by using an intraperitoneal injection urethane (1000 mg/kg, 20%) after fasting 12 h. Thoracic aorta was removed, placed in cold Krebs-Henseleit solution, cleaned gently of adherent connective tissue and cut into rings (approximately 3 mm length). Blood samples from abdominal aorta and also liver tissue samples were collected, when rats were killed, for the measurement of glucose, insulin, NO, lipid metabolic parameters and some antioxidant enzyme levels.
General parameters of rats (fasting blood glucose and body weight) were measured and recorded at the beginning, before the melatonin and L–carnitine treatment and at the end of experiment.
The aortic rings were suspended under a resting tension 1 g in 20 ml organ chambers containing oxygenated (5% CO2, 95% O2) and warmed (37'C) Krebs solution (pH: 7.4) with the following composition (mM): NaCl 112, KCl 5, NaHCO3 25, NaH2PO4 1, MgCl2 0.5, CaCl2 2.5 and glucose 11.5. All preparations were fixed with two stainless steel wires, one was connected to a force displacement transducer (MAY FDT 05, COMMAT Ltd., Ankara, Turkey) for the measurement of isometric contractions and for record on computer using transducer data acquisition system (TDA 94, COMMAT).
After 1 h washing and equilibration period, contractile responses to phenylephrine were taken in rings to determine precontractile tone in vessels. Relaxations induced by acetylcholine (10−9–10−5 M ) and sodium nitroprusside (10−9–10−5 M) were obtained from aortic rings precontracted with EC80 concentration of phenylephrine. To determine endothelium-dependent relaxation induced by NO, the contractile responses to NG-nitro-L-arginine (L-NAME) (10−2 M) were recorded in rings precontracted with EC50 concentration of phenylephrine.
1.3. Measurement of total cholesterol, LDL, HDL, triglyceride, glucose, NO and insulin
The plasma was prepared with EDTA and separated by centrifugation (10 min, 3000 rpm). Plasma total cholesterol, triglyceride and high-density lipoprotein (HDL) were measured by a commercially available enzyme kit. Plasma LDL was determined using the Friedewald formula: LDL cholesterol=Total cholesterol- HDL-Triglyceride/5 [13]. The concentration of plasma glucose was measured using a glucose kit based on the glucose oxidase method. Insulin was measured by a rat insulin ELISA kit (Millipore, Merck). A nitric oxide fluorometric assay kit (Cayman Chemical, USA) that provides measurement of total nitrate/nitrite concentration was used to assay plasma NO levels.
1.4. MDA assay
The malondialdehyde (MDA) assay was conducted by the thiobarbituric acid method in liver homogenates and plasma. The MDA levels were determined using a molar extinction coefficient of 1.56×105 M−1 cm−1.
1.5. Measurement of tissue antioxidant levels
Liver tissues were washed in 0.9% NaCl solution and approximately 0.5 g tissue samples of liver were homogenized in 0.15 M KCl- 10 mM potassium phosphate buffer, pH:7,4 [tissue to buffer ratio, 1:10 w/v]. Homogenized samples were centrifuged (13000 g, 10 min.) and stored at −80'C until analysis.
Glutathione peroxidase and superoxide dismutase levels in liver tissues were assayed using commercially specific kits (Cayman chemical, USA) and liver catalase activity was determined as described by Aebi [14].
1.6. Statistical analyses
Statistical analyses were performed using SPSS 16.00 for windows software. Data were expressed as arithmetic mean±standard error (SE) in n number subjects. Difference between the values was considered statistically significant if p value<0.05. One way variance (Anova) analysis was used in evaluation of the isometric vascular responses and post hoc tukey test was used in comparison of the data having significance. Other non-normal distribution data of the experimental animals were subjected to nonparametric testing. Accordingly, first Kruskal–Wallis test was carried out and in case of difference between the groups Mann–Whitney U test was performed. Variance analysis was made in order to define differences and significances in the repeating measures (fasting blood glucose, body weight etc.).
2. Results
2.1. General parameters
Body weight and fasting blood glucose changes in normal and diabetic rats were shown in Table 1, Table 2. Body weight values were significantly increased after diabetes induced period by (HFD+STZ) and at the end of experiment in all groups except L-carnitine treated group (p<0.05). Final body weights significantly decreased in L-carnitine and melatonin treated groups compared with untreated diabetic group in 5th week. (p<0.05). Fasting blood glucose concentration of untreated diabetic rats were higher compared with those of control group (p<0.05). L-carnitine and melatonin treatment has ameliorative effect on blood glucose levels. In these groups, blood glucose levels significantly decreased after L-carnitine and melatonin treatment compared with 5th week levels and untreated diabetic group (p<0.05).
Table 1.
Body weight values of the experiment animals.
| Groups | Initial body weight (g) | Body weight after STZ/vehicle (i.p) (5th week) | Final body weight (g) |
|---|---|---|---|
| C (n=7) | 214.44±6.09 | 240.56±9.41a | 281.11±15.65a,b |
| HFD+STZ (n=7) | 228.00±5.17 | 251.00±13.51a | 250.00±15.15 |
| HFD+STZ+MLT (n=7) | 220.00±8.07 | 242.50±9.45a | 227.50±8.07b |
| HFD+STZ+LC (n=7) | 235.00±7.94 | 267.14±17.52 | 223.57±12.80b |
| HFD+STZ+GB (n=7) | 221.25±9.62 | 254.38±12.15a | 240.00±14.48 |
Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitin 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). Groups’ baseline weights and the body weights following 4-week HFD, one week after streptozotocin injection and at the end of the experiment are given. The results are shown as Values±SEM and n=shows the number of animals in the group,
p<0.05 statistical significance of the same group compared to baseline.
p<0.05 statistical significance of the same group compared to the 5th week values.
Table 2.
Fasting blood glucose values of the experiment animals
| Groups | Initial fasting blood glucose (mg/dl) | Fasting blood glucose (mg/dl after STZ/(i.p) (5th week)) | Final fasting blood glucose (mg/dl) |
|---|---|---|---|
| C (n=7) | 107.89±3.50 | 103.22±3.24 | 105.44±3.40 |
| HFD+STZ (n=7) | 101.00±2.73 | 271.80±37.27a | 289.70±40.21a |
| HFD+STZ+MLT (n=7) | 106.25±4.05 | 318.00±52.53a | 259.63±54.59a,b |
| HFD+STZ+LC (n=7) | 101.86±5.20 | 281.57±56.21a | 188.57±40.77b |
| HFD+STZ+GB (n=7) | 112.13±2.48 | 334.63±57.23a | 280.63±60.37a,b |
Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitin 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). Groups' baseline weights and the body weights following 4-week HFD, one week after streptozotocin injection and at the end of the experiment are given. The results are shown as Values±SEM and n=shows the number of animals in the group
p<0.05 statistical significance of the same group compared to baseline.
p<0.05 statistical significance of the same group compared to the 5th week value.
2.2. Vascular activity
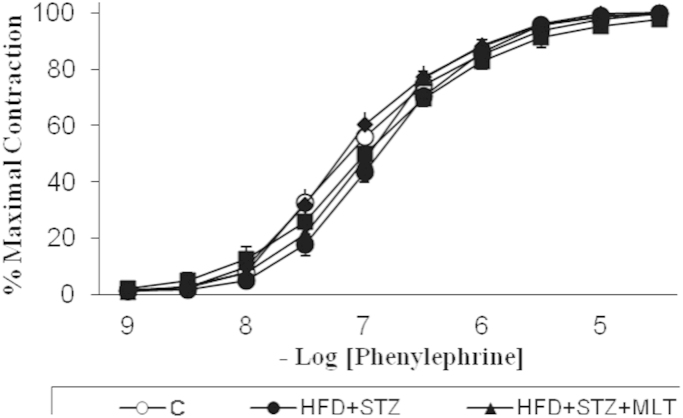
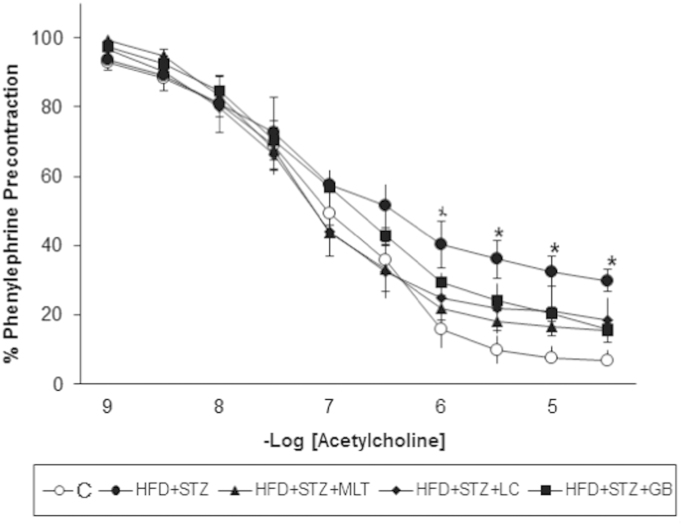
Phenylephrine induced contractile responses in control and diabetic aorta are shown in Fig. 1. There was no significant difference between groups in contractility and also sensitivity of phenylephrine. Acetylcholine induce relaxation was significantly decreased in untreated diabetic group compared with control group (p<0.05). L-carnitine and melatonin treatment significantly impaired the diabetes-induced decrease in acetylcholine relaxations (p<0.05; Fig. 2).
Fig. 1.
Contraction responses obtained with phenylephrine in vascular strips. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups.
Fig. 2.
Relaxation responses obtained with acetylcholine in vascular strips.Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups. *p<0.05 shows the significance compared to the controls.
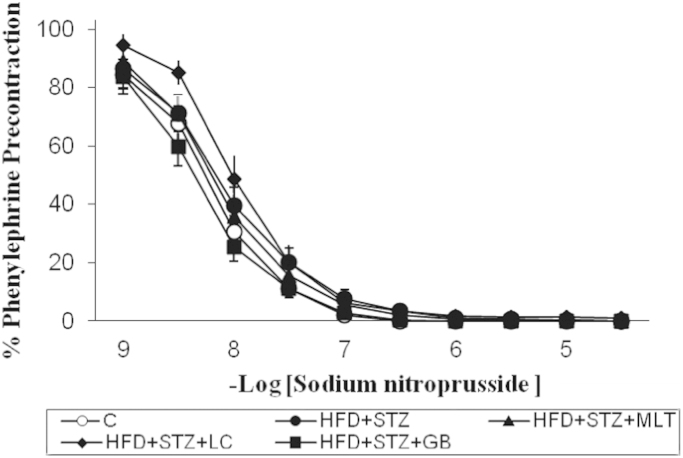
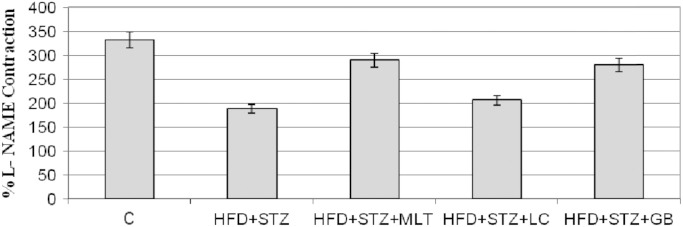
Relaxation responses of sodium nitroprusside showed no difference between groups. l-NAME contractions decreased in diabetic aortas compared to control group but there is no statistically significant difference between treated and untreated diabetic groups. Fig. 3 and Fig. 4
Fig. 3.
Relaxation responses obtained with sodium nitroprusside in vascular strips. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups.
Fig. 4.
Contractile responses to L-NAME (100 micro M) presented as the percentage of phenylephrine precontraction in endothelium intact thoracic aortic rings. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups.
2.3. Lipid peroxidation and antioxidant status
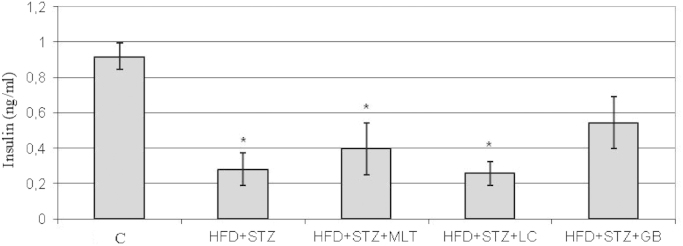
Blood lipid profile in all groups are shown in Table 3. Induction of diabetes significantly increased total cholesterol levels in diabetic group and this increase was significantly reversed by L-carnitine treatment (p<0.05) LDL cholesterol levels were significantly decreased in L-carnitine treated group compared with untreated diabetic group. Triglyceride and HDL cholesterol levels were slightly increased in untreated diabetic group, but this difference has no statistical significance. The plasma insulin levels significantly decreased in all diabetic groups compared to control group. (Fig. 5) Insulin levels were slightly increased in glibenclamide treated diabetic group but this difference has no statistical significance.
Table 3.
Lipid values obtained from blood samples
| Groups | Total cholesterol (mg/dl) | Triglyceride (mg/dl) | HDL-cholesterol (mg/dl) | LDL-cholesterol (mg/dl) |
|---|---|---|---|---|
| C | 27.71±2.62 | 36.43±3.45 | 9.00±.82 | 14.71±2.20 |
| HFD+STZ | 47.71±2.86a | 64.29±16.87 | 11.57±.95 | 20.14±2.43 |
| HFD+STZ+MLT | 37.71±5.23 | 58.29±13.11 | 10.88±2.22 | 15.00±3.19 |
| HFD+STZ+LC | 33.29±1.90b | 56.43±8.49 | 10.71±.48 | 10.57±1.76 b |
| HFD+STZ+GB | 39.43±2.75 | 55.00±7.61 | 11.00±1.07 | 15.14±1.62 |
Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitin 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups.
p<0.05 shows the statistical significance compared to the controls.
p<0.05 shows the statistical significance compared to the untreated diabetic group.
Fig. 5.
Serum Levels of Insulin. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups. *p<0.05 shows the significance compared to the controls.
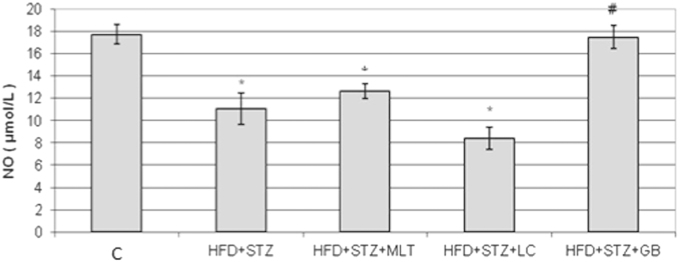
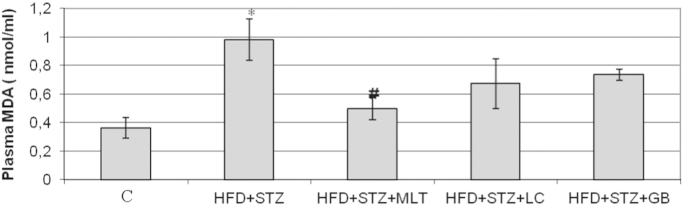
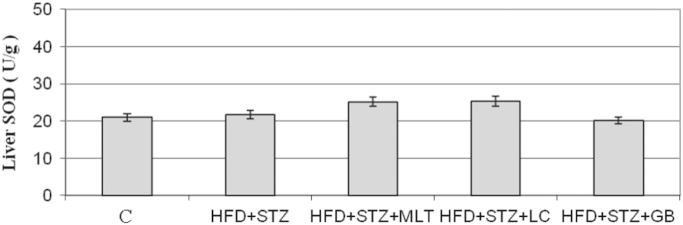
NO levels in diabetic rats were lower than other groups. L-carnitine and melatonin treatment reversed this decrease in plasma NO levels but only in glibenclamide treated group this is statistically significant (Fig. 6). The plasma MDA level in diabetic group was higher than control group and L-carnitine, melatonin and glibenclamide treated groups improved the diabetes-induced increase in plasma MDA levels (Fig. 7). However only in melatonin treated group treatment this increase found statistically significant (p<0.05). Neither diabetes induction nor treatment has no effect on the liver SOD levels (Fig. 8).
Fig. 6.
Serum Levels of NO. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups. *p<0.05 shows the statistical significance compared to the controls, #p<0.05 shows the statistical significance compared to the untreated diabetic group.
Fig. 7.
Serum Levels of MDA. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups. *p<0.05 shows the statistical significance compared to the controls, #p<0.05 shows the statistical significance compared to the untreated diabetic group.
Fig. 8.
Liver Levels of SOD. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups.
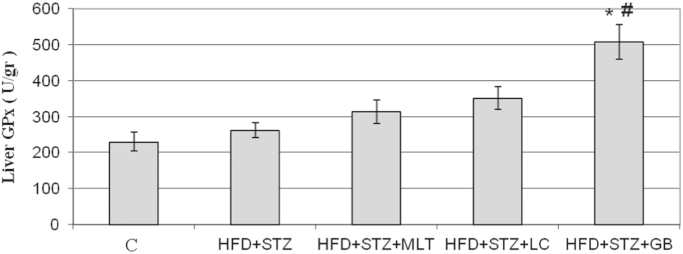
The liver glutathione peroxidase (GPx) activity was significantly decreased in diabetic group and increased in L-carnitine, melatonin and glibenclamide treated groups (Fig. 9). However only in glibenclamide treated group treatment this increase found statistically significant (p<0.05)
Fig. 9.
Liver Levels of GPx. Controls were abbreviated as (C), untreated diabetic group as (HFD+STZ), diabetic group treated with melatonin 10 mg/kg/day as (HFD+STZ+MLT), diabetic group treated with L-carnitine 0.6 g/kg/day as (HFD+STZ+LC) and diabetic group treated with glibenclamide 5 mg/kg/day was abbreviated as (HFD+STZ+GB). The results are shown as Values±SEM n=7 in all the groups. *p<0.05 shows the statistical significance compared to the controls, #p<0.05 shows the statistical significance compared to the untreated diabetic group.
3. Discussion
Objective of this study was to create an experimental model which was the closest to Type 2 DM characteristics and to investigate antioxidant features of melatonin which is known to have a potent antioxidant feature and L-carnitine which has not been well studies agent and, their effects on the vascular responses.
High-fat diet was used in Type 2 diabetes model in order to induce insulin resistance. Since in the case of this method was combined with multiple low dose STZ, beside insulin resistance insulin deficiency would also develop, this model was considered as a proper Type 2 diabetes animal model. Results of a recent rats fed with HFD to create experimental diabetes through various doses of STZ demonstrated that, administration of multiple low dose STZ (30 mg/kg i.p. weekly, for 2 weeks) created an obvious hyperglycemia with a high success rate.
Likewise, in this study 4-week HFD and STZ injection were observed to create an obvious hyperglycemia. In the conducted studies, a significant decrease has been observed in the plasma levels of carnitine in human and rats this decrease suggested that carnitine plays an important role in diabetic complications 10, 15. As it was demonstrated in this study, effects of L-carnitine on the blood levels of glucose in diabetic rats were consistent with the previous studies and showed its partial decreasing effect. There are also studies suggesting carnitine increases or does not change the blood glucose [16]. In the studies which demonstrated that melatonin failed to normalize hyperglycemia and body weight in STZ-induced diabetes, in this study also melatonin decreased but failed to normalize the blood glucose [17]. In addition, in this study serum levels of MDA was found to be lower in the treated compared to untreated diabetic group only decrease observed in the group treated with MLT was considered as statistically significant. There are conflicting studies in the literature reporting SOD levels increased, did not change or decreased in diabetes 18, 19.
In our study no significant change was observed in the levels of SOD which is an important marker for the oxidative stress. The levels of another oxidative stress marker, GPx levels were found to be higher than the controls, but glibenclamide therapy was observed to increase the level GPx by the highest rate. In addition, in this study triglyceride values of the untreated diabetic group were found to be significantly higher than the controls in terms of blood lipid levels. Triglyceride and LDL cholesterol levels were significantly decreased in the group administered L-carnitine therapy. Melatonin was found not to significantly affect blood lipid levels, consistently with the other studies [20]. In another study investigating effects of HFD and single low-dose STZ on rats, again low dose STZ administration was observed not to change plasma insulin values, feed with HFD alone to increase insulin levels and the combined administration to decrease insulin levels [21]. Whereas data obtained from this study demonstrated that insulin levels were significantly decreased in all the treated and untreated diabetic rats compared to the controls except glibenclamide group.
Endothelium dependent relaxation responses which occur with acetylcholine is impaired in various vascular beds in the Type 2 diabetes animal models and patients. Acetylcholine shows its relaxation affect by providing release of the relaxation factors such as NO and prostacyclin and endothelium-derived hyperpolarization factor (EDHF). Abnormal production or response of NO contributes to the vascular and endothelial dysfunction seen in diabetes.
Consistently with the previous studies, in this study it was seen that, Ach-induced endothelium dependent relaxing response was decreased, but no any change was observed in the sensitivity in aorta of the rats. Acetylcholine induced endothelium dependent relaxation is an effect under control of the muscarinic receptor and post receptor events are more potent in the impaired response rather than the receptor mechanisms in the endothelial cells. Possible mechanism of the decrease in acetylcholine induced endothelium dependent relaxation response might be due to highly increased of the free radical formation in the diabetic area might decrease effect of NO. It has been reported in the previous studies that, free radicals and partially superoxide and hydroxyl radicals interact with NO, resulting with formation of less potent vasodilators such as peroxynitrite NO2 and NO3. This products may be accounted for the impairment in endothelium dependent relaxation response.
In this study L-carnitine, melatonin and glibenclamide therapy partly normalized endothelium dependent relaxation response against acetylcholine in the diabetic aortas. Contraction responses against L-NAME were significantly decreased in the diabetic rat aortas. These results show that, impairment of endothelium dependent relaxation response in the aortas of STZ induced diabetic rats STZ fed with HFD is mainly a NO mediated event.
Sodium nitroprusside (SNP) directly affects vascular smooth muscle by increasing guanylate cyclase activity and creates a relaxation independently form endothelium. In our study, no any change was observed in the responses against SNP. This result indicated that response of the vascular smooth muscle against NO did not change in the diabetic aorta and actual harmful effect of diabetes was on the endothelium cells. Melatonin improved the impairment in endothelium dependent relaxation induced by acetylcholine, but did not create ant change in SNP induced endothelium independent relaxation in the aortas of diabetic rats.
In conclusion, melatonin therapy decreased oxidative stress and MDA levels that was increased in diabetes and increased the low NO levels. Whereas L-carnitine partly decreased blood glucose levels and lipid parameters in diabetic rats. L-carnitine therapy partly decreased the severity of hyperglycemia and this improvement was seen to be directly correlated with the partly improvement in endothelium dependent relaxation in the diabetic aorta. In light of these results, we believe that melatonin and L-carnitine could be helpful in prevention the complications related to Type 2 diabetes and its treatment.
Contributor Information
Derya Selcen Salmanoglu, Email: selcenderya@gmail.com.
Tugba Gurpinar, Email: tugbagurpinar@gmail.com.
Kamil Vural, Email: kfsvural@yahoo.com.
Nuran Ekerbicer, Email: nuranaladag@hotmail.com.
Ertan Darıverenli, Email: ertan6876@mynet.com.
Ahmet Var, Email: ahmet.var@hotmail.com.
References
- 1.Hartge M., Pharm B., Kintscher U. Endothelial dysfunction and its role in diabetic vascular disease. Endocrinol. Metab. Clin. N. Am. 2006;35:551–560. doi: 10.1016/j.ecl.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Montilla P.L., Vargas J.F., Túnez I.F. Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J. Pineal Res. 1998;25(2):94–100. doi: 10.1111/j.1600-079x.1998.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 3.Tamamoğulları N., Siliğ Y., Içağasıoğlu S. Carnitine deficiency in diabetes mellitus complications. J. Diabetes Complicat. 1999;13:251–253. doi: 10.1016/s1056-8727(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G., Greco A.V., Capristo E. L-carnitine improves glucose disposal in type 2 diabetic patients. J. Am. Coll. Nutr. 1999;18:77–82. doi: 10.1080/07315724.1999.10718830. [DOI] [PubMed] [Google Scholar]
- 5.Herrera M.D., Bueno R., De Sotomayor M.A. Endothelium-dependent vasorelaxation induced by L-carnitine in isolated aorta from normotensive and hypertensive rats. J. Pharm. Pharmacol. 2002;54:1423–1427. doi: 10.1211/002235702760345536. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Wahab M.H., Abdel-Allah A.R. Possible protective effect of melatonin and/or desferroxamine against streptozotocin-induced hyperglycemia in mice. Pharmacol. Res. 2000;41:533–537. doi: 10.1006/phrs.1999.0614. [DOI] [PubMed] [Google Scholar]
- 7.Anwar M.M., Meki A.R. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp. Biochem. Physiol.: A Mol. Integr. Physiol. 2003;135(4):539–547. doi: 10.1016/s1095-6433(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M., Lv X.Y., Li J. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008 doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C., Li J., Lv X. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high fat diet and streptozotocin. Eur. J. Pharmacol. 2009;12(620):131–137. doi: 10.1016/j.ejphar.2009.07.027. (1–3) [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues B., Xiang H., McNeill J.H. Effect of L-carnitine treatment on lipid metabolism and cardiac performance in chronically diabetic rats. Diabetes. 1988;37:1358–1364. doi: 10.2337/diab.37.10.1358. [DOI] [PubMed] [Google Scholar]
- 11.Gürpınar T., Ekerbiçer N., Uysal N. The histologic evaluation of atorvastatin and melatonin treatment on oxidative stress and apoptosis of diabetic rat. Pancreas. Kafkas Univ. Vet. Fak. Derg. 2010;16(4):547–552. [Google Scholar]
- 12.June C.C., Wen L.H., Sani H.A. Hypoglycemic effects of gynura procumbens fractions on streptozotocin-induced diabetic rats involved phosphorylation of GSK3β (Ser-9) in liver. Sains Malays. 2012;41(8):969–975. [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Methods Enzym. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Paulson D.J., Schmidt M.J., Traxler J.S. Improvement of myocardial function in diabetic rats after treatment with L-carnitine. Metabolism. 1984;33:358–363. doi: 10.1016/0026-0495(84)90199-9. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues B., Seccome D., Mc Neill J.H. Lack of effect of oral L-carnitine treatment on lipid metabolism and cardiac function in chronically diabetic rats. Can. J. Physiol. Pharmacol. 1990;68:1601–1608. doi: 10.1139/y90-244. [DOI] [PubMed] [Google Scholar]
- 17.Vural H., Sabuncu T., Arslan S.O. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J. Pineal Res. 2001;31:193–198. doi: 10.1034/j.1600-079x.2001.310301.x. [DOI] [PubMed] [Google Scholar]
- 18.Memisogullari R., Taysi S., Bakan E. Antioxidant status and lipid peroxidation in Type II. Diabetes Mellit. Cell. Biochem. Funct. 2003;21:291–296. doi: 10.1002/cbf.1025. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Seif M.A., Youssef A. Evaluation of some biochemical changes in diabetic patients. Clin. Chim. Acta. 2004;346:161–170. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Kedziora-Kornatowska K., Szewczyk-Golec K., Kozakiewicz M. Melatonin improves oxidative stress parameters measured in the blood of elderly type 2 diabetic patients. J. Pineal Res. 2009;46:333–337. doi: 10.1111/j.1600-079X.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan K., Viswanad B., Asrat L. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]