Abstract
We report Aspergillus delacroxii (formerly Aspergillus nidulans var. echinulatus) causing recurrent prosthetic valve endocarditis. The fungus was the sole agent detected during replacement of a mechanical aortic valve conduit due to abscess formation. Despite extensive surgery and anti-fungal treatment, the patient had a cerebral hemorrhage 4 months post-surgery prompting a diagnosis of recurrent prosthetic valve endocarditis and fungemia.
Keywords: Endocarditis, Bioprosthesis, Surgical site infection, Aspergillus delacroxii, Aspergillus nidulans var. echinulatus
1. Introduction
Aspergillus species are rare causes of endocarditis with Aspergillus fumigatus being reported most frequently [1], [2]. However, the role of filamentous fungi in endocarditis may be underestimated because standard blood culture techniques offer unfavorable conditions for growth, and even when a blood culture isolate is obtained it may be misinterpreted as a contaminant if additional evidence is lacking.
We here present a case of recurrent prosthetic valve endocarditis caused by Aspergillus delacroxii (formerly Aspergillus nidulans var. echinulatus). The patient had a replacement of the entire aortic root and the aortic valve due an aneurysm of the ascending aorta and aortic valve regurgitation. Postoperatively, closure of the sternum split was delayed by bacterial infection, and after two months an aortic root abscess was diagnosed. A. delacroxii was an unexpected isolate from peroperative biopsies. Extensive revision surgery and implantation of a bioprosthesis was supplemented by anti-fungal therapy, a recurrence was diagnosed four months later by echocardiography and positive blood cultures.
2. Case
A 35-year old man in good general health was admitted to Aalborg University Hospital with a 1-month history of dyspnoea and a systolic murmur. Transoesophageal echocardiography (TEE) revealed an aneurysm of the ascending aorta and severe aortic valve regurgitation. There was no family record of Marfan's syndrome or other hereditary diseases. The entire aortic root and the aortic valve were replaced by a mechanical valve conduit (St. Jude, St. Jude Medical, St. Paul, MN, US) (day 0), and the patient was discharged day +5. He was readmitted with fever day +17, and a CT-scan revealed accumulation of pericardial fluid. A sternum split was performed, and 7 peroperative samples were obtained of which 3 revealed coagulase-negative staphylococci (CoNS) (pericardial fluid, pericardium (fibrin) and subcutis). Antibiotic therapy was commenced and directed by the antibiogram. Repeated TEE revealed no signs of endocarditis, but an echogenic structure was seen in the transversal pericardial recess between the left atrium and the aortic wall. The patient remained febrile during treatment with vacuum-assisted closure (VAC) of the sternum split, and antibiotic therapy was adjusted several times to account for changes in the antibiogram of CoNS. The patient was closely monitored for infection of the conduit using echocardiography, computed X-ray tomography (CT), and leukocyte scintigraphy. On day +75 a definitive diagnosis of endocarditis was established by TEE showing an aortic root abscess cavity communicating with the left outflow tract.
The patient was immediately referred to the Department of Cardiothoracic Surgery at Rigshospitalet, Copenhagen, and two days later (day +77) he underwent extensive revision and implantation of a Freestyle® bioprosthesis (Medtronic, Minneapolis MN, US) and a short Hemashield™ tube (Atrium Medical Corporation, Hudson NH, US). Peroperative samples were negative on bacteriological culture, but biopsies from the aorta graft and an unspecified site revealed growth of A. delacroxii; three additional samples (aorta, pericardium, and sternum) were negative on mycological culture. Voriconazole was instituted (maintenance dose 300 mg b.i.d.), and antibacterial therapy covering CoNS was maintained. The trough voriconazole plasma level (2.3 mg/L) was within the target range [3].
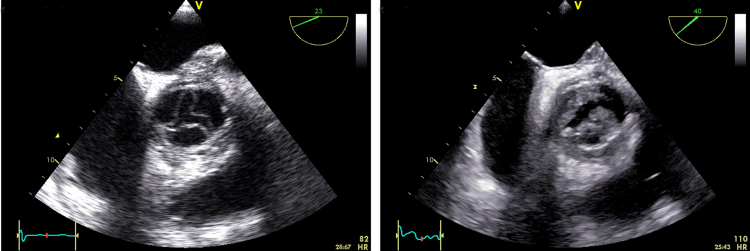
The patient had a rapid postoperative recovery, and voriconazole treatment was terminated day +119. TEE on the day before (Fig. 1, left) and transthoracic echocardiography (TTE) on days +136 and +153 left no suspicion of endocarditis.
Fig. 1.
Transoesophageal echocardiograms obtained on day +118 (left) and day +238 (right). The slender cusps of the aortic bioprosthetic valve were transformed by bulky vegetations during the 120 days' interval.
On day +212 (125 days after replacement surgery) the patient had a sudden cerebrovascular insult. An MR scan revealed an 8×8 cm hematoma within the right hemisphere communicating with the ventricular system and a mass effect. The condition deteriorated rapidly and a craniotomy was undertaken to evacuate the hematoma and alleviate intracranial pressure. Due to these circumstances no microbiological investigations were ordered, but two blood cultures drawn the next day were negative. The patient made a significant recovery, and between days +216 and +219 investigations included two additional blood cultures, bacteriological culture of cerebrospinal fluid (CSF), bacterial 16S rRNA gene amplification (CSF), and an aspergillus galactomannan assay (CSF), all being negative. Meropenem was administered for two weeks on suspicion of a nosocomial bacterial infection.
On days +230 and +234 (i.e. 18/24 days after the cerebrovascular insult) two blood cultures revealed growth of a filamentous fungus. The fungal isolates five months apart were confirmed to be A. delacroxii by classical macro- and micromorphologic examination [4], [5]. Further identification was performed by β-tubulin and calmodulin gene sequencing as previously described [5], [6]. Sequences of both genes matched the respective genes of A. delacroxii CBS strain no. 120.55 (341/342 bp (99.7%) for Genbank accession numbers: AY573553 (BTUB) and 440/440 bp (100%) for EF591677 (CMD): AY573553).
Susceptibility testing was done using Etest (AB bioMérieux, Herlev, Denmark) and RPMI 2% glucose agar buffered with MOPS (SSI Diagnostika, Hillerød, Denmark) for amphotericin B and caspofungin; the EUCAST EDEF9.1 reference method was followed for itraconazole, posaconazole, and voriconazole [7]. Susceptibility breakpoints have not yet been established for the A. nidulans complex (see Section 3) except for itraconazole (S:≤1 mg/L and R:>2 mg/L), hence EUCAST epidemiological cut off values (ECOFFs) were used to determine if the isolate was a wild type or not: itraconazole ECOFF 1 mg/L, posaconazole ECOFF 0.5 mg/L, and voriconazole ECOFF 1 mg/L. MICs (minimum effective concentration (MEC) for caspofungin) for the aorta/blood isolates, respectively, were for amphotericin B 0.5/0.5 mg/L, caspofungin ≤0.032/0.047 mg/L, itraconazole 1.0/0.5 mg/L, posaconazole 0.125/0.125 mg/L, and voriconazole 0.5/0.25 mg/L, respectively. Thus, the MICs and the MEC for caspofungin were within the wild type range for both isolates and all compounds, suggesting no presence of acquired resistance.
Concurrently with the relapse, TTE demonstrated vegetations on all 3 cusps (Fig. 1, right) and an aortic root abscess cavity. A serum sample was positive for aspergillus galactomannan.
Combination therapy with liposomal amphotericin B and voriconazole was instituted and thereafter maintained. Consultation with the Department of Cardiothoracic Surgery at Rigshospitalet concluded that surgical intervention would not be possible. Five blood cultures drawn after start of the combined treatment were negative. However, TTEs revealed progressive destruction of the aortic valve. Eighty two days after the diagnosis of endocarditis the patient (day +312) suffered a Pseudomonas aeruginosa bloodstream infection likely to originate from the urinary tract. The patient died a week later (day +317).
3. Discussion
Aspergillus taxonomy has changed significantly in recent years due to new tools for classification and identification [6]. Nonetheless, nomenclature has remained perplexing especially for clinicians, among others due to the use of separate names for the sexual and asexual stage. Fortunately, a single name principle, irrespective of the fungus' life history, has been adopted in a new code of nomenclature [8]. As a consequence the name A. delacroxii was given to the current species in 2014 while retaining the former names A. nidulans var. echinulatus (asexual form) and Emericella nidulans, var. echinulata (sexual form) as synonyms. The name honors a French physician, Édouard Georges Delacroix (1858-1907), who devoted his life to mycology and plant pathology.
Due to the novelty of the name, only the synonyms have been used in the few existing clinical and mycological reports [9], [10], [11]. A propensity for A. delacroxii infection has been recognized among patients with chronic granulomatous disease (CGD) [12], [13], but no other risk group has been delineated.
To our knowledge, only two cases of A. delacroxii infection have been reported in detail. A patient with CGD had a lung infection with direct spread to the spine and costae [14], and a patient with diabetes and bullous pemphigoid treated with high dose prednisolone had invasive pulmonary disease [15]. Both patients responded to antifungal therapy in combination with other relevant therapeutic measures.
Our patient's history did not suggest any congenital or acquired immunodeficiency, and there was no explanation for the sudden debut of aortic valve insufficiency. Aspergillus endocarditis is an acknowledged risk in patients undergoing cardiac surgery [16]. We have no definite source of the fungus, but the sternum split is a likely portal of entry because bacterial mediastinitis necessitated prolonged treatment with a VAC system. Other studies have previously drawn attention to dissemination of conidia through the air in operating theaters [17]. However, knowledge of the occurrence of the A. nidulans complex in hospital environments is fragmentary. A Portuguese study found the complex to account for 3 of 80 environmental Aspergillus isolates from air and surfaces in three wards with high-risk patients [11]. We are not aware of comparable Danish data, but a prospective multi-center survey of Aspergillus isolates from respiratory tract specimens identified a single patient colonized with the A. nidulans complex [18].
The unexpected finding of A. delacroxii as the sole microbial agent at the time of replacement surgery led immediately to initiation of voriconazole. Antifungal therapy was terminated after 6 weeks because surgical debridement was deemed to be radical, the prosthesis had been replaced in toto, and the patient was immunocompetent. Despite an interval of 18 days between the positive blood cultures and confirmation of endocarditis by TEE we find the causal role of A. delacroxii to be convincing; we suspect the cerebral hemorhage to result from a thromboembolic event, but fungal vasculitis cannot be ruled out. Of note, the cerebral hemorrhage happened after discontinuation of anticoagulation therapy.
Conclusion: This casuistic report demonstrates that A. delacroxii can cause a chronic surgical site infection involving prosthetic material, and the fungus may persist after extensive revision surgery and exchange of the prosthesis. Prolonged antifungal treatment may be warranted even in immunocompetent hosts.
Conflict of interest
None of the authors have conflicts of interest to declare.
Acknowledgments
We are indebted to the biotechnicians at Copenhagen University Hospital, Rigshospitalet and Aalborg University Hospital for preserving the fungal isolates for further studies. This study was conducted without external funding.
References
- 1.Pierrotti L.C., Baddour L.M. Fungal endocarditis, 1995–2000. Chest. 2002;122:302–310. doi: 10.1378/chest.122.1.302. [DOI] [PubMed] [Google Scholar]
- 2.Kalokhe A.S., Rouphael N., El Chami M.F., Workowski K.A., Ganesh G., Jacob J.T. Aspergillus endocarditis: a review of the literature. Int. J. Infect. Dis. 2010;14:e1040–e1047. doi: 10.1016/j.ijid.2010.08.005. doi: 0.1016/j.ijid.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Park W.B., Kim N.H., Kim K.H., Lee S.H., Nam W.S., Yoon S.H. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin. Infect. Dis. 2012;55:1080–1087. doi: 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 4.Christensen M., Raper K.B. Synoptic key to Aspergillus nidulans group species and related Emericella species. Trans. Br. Mycol. Soc. 1978;71:177–191. [Google Scholar]
- 5.Samson R.A., Visagie C.M., Houbraken J., Hong S.B., Hubka V., Klaassen C.H. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balajee S.A., Houbraken J., Verweij P.E., Hong S.B., Yaghuchi T., Varga J. Aspergillus species identification in the clinical setting. Stud. Mycol. 2007;59:39–46. doi: 10.3114/sim.2007.59.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 2008;14:982-4. 10.1111/j.1469-0691.2008.02086.x. Erratum in: Clin. Microbiol. Infect. 2009;15:103 [DOI] [PubMed]
- 8.J. McNeill, F.R. Barrie, W.R. Buck, V. Demoulin, W. Greuter, D.L. Hawksworth, et al., International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (Regnum Vegetabile, 154). Königstein im Taunus: Koeltz Scientific Books; 2012. Can be accessed at 〈〈http://www.iapt-taxon.org/nomen/main.php〉〉.
- 9.Verweij P.E., Varga J., Houbraken J., Rijs A.J., Verduynlunel F.M., Blijlevens N.M. Emericella quadrilineata as cause of invasive aspergillosis. Emerg. Infect. Dis. 2008;14:566–572. doi: 10.3201/eid1404.071157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubka V., Kubatova A., Mallatova N., Sedlacek P., Melichar J., Skorepova M. Rare and new etiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med. Mycol. 2012;50:601–610. doi: 10.3109/13693786.2012.667578. [DOI] [PubMed] [Google Scholar]
- 11.Sabino R., Veríssimo C., Parada H., Brandão J., Viegas C., Carolino E. Molecular screening of 246 Portuguese Aspergillus isolates among different clinical and environmental sources. Med. Mycol. 2014;52(5):519–529. doi: 10.1093/mmy/myu006. [DOI] [PubMed] [Google Scholar]
- 12.Segal B.H., DeCarlo E.S., Kwon-Chung K.J., Malech H.L., Gallin J.I., Holland S.M. Aspergillus nidulans infection in chronic granulomatous disease. Medicine. 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Henriet S.S., Verweij P.E., Warris A. Aspergillus nidulans and chronic granulomatous disease: a unique host-pathogen interaction. J. Infect. Dis. 2012;206:1128–1137. doi: 10.1093/infdis/jis473. [DOI] [PubMed] [Google Scholar]
- 14.White C.J., Kwon-Chung K.J., Gallin J.I. Chronic granulomatous disease of childhood. An unusual case of infection with Aspergillus nidulans var. echinulatus. Am. J. Clin. Pathol. 1988;90:312–316. doi: 10.1093/ajcp/90.3.312. [DOI] [PubMed] [Google Scholar]
- 15.Yu J., Mu X., Li R. Invasive pulmonary aspergillosis due to Emericella nidulans var. echinulata, successfully cured by voriconazole and micafungin. J. Clin. Microbiol. 2013;51:1327–1329. doi: 10.1128/JCM.02487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hamamsy I., Dürrleman N., Stevens L.M., Perrault L.P., Carrier M. Aspergillus endocarditis after cardiac surgery. Ann. Thorac. Surg. 2005;80:359–364. doi: 10.1016/j.athoracsur.2004.08.070. [DOI] [PubMed] [Google Scholar]
- 17.Jensen J., Guinea J., Torres-Narbona M., Muñoz P., Peláez T., Bouza E. Post-surgical invasive aspergillosis: an uncommon and under-appreciated entity. J. Infect. 2010;60:162–167. doi: 10.1016/j.jinf.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Mortensen K.L., Johansen H.K., Fuursted K., Knudsen J.D., Gahrn-Hansen B., Jensen R.H. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1355–1363. doi: 10.1007/s10096-011-1229-7. [DOI] [PubMed] [Google Scholar]