Abstract
Introduction:
Previous unblinded trials have shown increased malaria among HIV-infected adults on antiretroviral therapy (ART) who stop cotrimoxazole (CTX) prophylaxis. We investigated the effect of stopping CTX on malaria in HIV-infected adults on ART in a double-blind, placebo-controlled trial.
Methods:
HIV-infected Ugandan adults stable on ART and CTX with CD4+ cell count at least 250 cells/μl were randomized (1 : 1) to continue CTX or stop CTX and receive matching placebo (COSTOP trial; ISRCTN44723643). Clinical malaria was defined as fever and a positive blood slide, and considered severe if a participant had at least one clinical or laboratory feature of severity or was admitted to hospital. Malaria incidence and rate ratios were estimated using random effects Poisson regression, accounting for multiple episodes.
Results:
A total of 2180 participants were enrolled and followed for a median of 2.5 years; 453 malaria episodes were recorded. Malaria incidence was 9.1/100 person-years (pyrs) [95% confidence interval (CI) = 8.2–10.1] and was higher on placebo (rate ratio 3.47; CI = 2.74–4.39). Malaria in the placebo arm decreased over time; although incidence remained higher than in the CTX arm, the difference between arms reduced slightly (interaction P value = 0.10). Fifteen participants experienced severe malaria (<1%); overall incidence was 0.30/100 pyrs (CI = 0.18–0.49). There was one malaria-related death (CTX arm).
Conclusion:
HIV-infected adults – who are stable on ART and stop prophylactic CTX – experience more malaria than those that continue, but this difference is less than has been reported in previous trials. Few participants had severe malaria. Further research might be useful in identifying groups that can safely stop CTX prophylaxis.
Keywords: antiretroviral therapy, cotrimoxazole, HIV, malaria, trimethoprim/sulfamethoxazole
Introduction
Adults with advanced HIV disease who are not on antiretroviral therapy (ART) are at an increased risk of opportunistic infections and malaria [1–3]. Cotrimoxazole (CTX), an antimicrobial agent containing trimethoprim and sulfamethoxazole, reduces the incidence of opportunistic infections, mortality and malaria [4–9]. However, CTX use is associated with increases in cost of care [10], risk of haematological toxicity [11], hypersensitivity skin reactions [12] and pill burden. Once started on ART, patients’ immune function improves and the risk of opportunistic infections reduces. Based on studies from developed countries it has been recommended to stop CTX once patients’ immune function has been restored [13,14]; however, this may not be advisable for sub-Saharan Africa where the prevalence of malaria and bacterial infections is often high. Of recent, WHO recommends that CTX may be discontinued in patients who are clinically stable with evidence of immune recovery and/or viral suppression on ART, but should be continued in countries with high endemicity of malaria and bacterial infections [15]. A systematic literature review found that patients who stop CTX prophylaxis experience an increase in malaria episodes, with the strongest evidence provided by randomized-controlled trials (RCTs); however, none of the reported RCTs was blinded so observational or reporting bias cannot be excluded [16]. We investigated the effect of CTX in a blinded, placebo-controlled trial on CTX cessation in HIV-infected adults who are stable on ART (COSTOP; ISRCTN44723643). The main results of this trial have been reported previously. In summary, the trial found that stopping CTX prophylaxis leads to a significant increase in CTX-preventable clinical events [mainly bacterial pneumonias; adjusted hazard ratio (aHR) = 1.57, 90% confidence interval (CI) = 1.12–2.21] and a significant decrease in grade 3/4 haematological adverse events (aHR = 0.70, 95%CI = 0.59–0.82), the co-primary outcomes of the trial. There was no effect on all-cause mortality. The estimated number needed to treat (NNT) for 1 year to prevent one infection (excluding malaria) was 113 [17]. In this article we provide a detailed account on the effect of CTX prophylaxis on malaria, a secondary outcome of the trial.
Methods
COSTOP was a randomized, double-blind, placebo-controlled noninferiority trial conducted in Uganda to determine whether long-term prophylaxis with CTX can be safely discontinued among HIV-infected adults on ART with sustained immune competence (defined as CD4+ cell counts ≥250 cells/μl) [17,18]. The trial had two co-primary outcomes: time to occurrence of the first CTX-preventable clinical event (according to a predefined list) or death, and time to the occurrence of the first grade 3 or 4 haematological adverse event [17]. COSTOP was conducted by the MRC/UVRI Uganda Research Unit on AIDS (MRC/UVRI) at its research clinics in Masaka and Entebbe in Uganda.
Study procedures
Detailed procedures have been described previously [18]. Briefly, participants were eligible for enrolment if they were HIV infected; aged 18–59 years; clinically asymptomatic; had been taking CTX and ART for at least 6 months; had two CD4+ cell counts not less than 250 cells/μl, the most recent within 4 weeks of enrolment; and were able to attend regular study appointments. Exclusion criteria included pregnancy, grade 3 or 4 anaemia, neutropenia or thrombocytopenia. At an initial screening visit, information was recorded on socio-demographics, medical history, current illness and medication and a physical examination was performed. Laboratory tests included a full blood count, malaria slide and CD4+ cell count.
At enrolment (2–4 weeks after screening), participants were randomized to receive either active CTX (960 mg) or matching placebo once daily in place of their regular CTX. Randomization was stratified by enrolment site and CD4+ cell count (≤250–499 and ≥500 cells/μl). Participants were provided with an insecticide-treated bed net (ITN) and educated about the importance of using it. Participants continued to receive ART from their usual providers, but trial staff monitored ART availability to ensure an uninterrupted supply. Enrolment started in January 2011 and was completed in March 2013.
Participants were seen every month for the first 3 months and three-monthly thereafter, and were followed for 12 months to 3.5 years, depending on date of enrolment. At each visit, adherence to trial drug, ART and ITN use was assessed, using a structured questionnaire and returned pill counts (trial drug only). Participants were seen by a doctor who assessed their health, treated any concurrent infections, and dispensed trial drug. Participants were issued with a supply of trial drug to last until their next scheduled visit, along with a 3 day/month buffer stock in case they were late. Blood samples were drawn at scheduled visits for a malaria slide, CD4+ cell count, and full blood count. Participants were encouraged to attend the study clinic if unwell. If the participant was suspected to have malaria, based on a history of malaria associated symptoms (reported fever, headache, chills and rigors, joint aches, muscles aches, vomiting or diarrhoea), a blood slide and other tests deemed necessary were done, and confirmed malaria was treated with arthemeter-lumefantrine according to national guidelines [19]. Participants who reported having been treated for malaria elsewhere (e.g. during a journey) were asked to present documentary evidence of diagnoses and test results. Participants were withdrawn from trial medication and started on open-label CTX if their CD4+ cell count fell below 250 cells/μl at any point during the trial.
Laboratory methods
A blood sample was used to prepare thick and thin films on a glass slide. Specimens were processed using Leishman's stain and examined by microscopy. Thick film specimens were used to record the number of parasites per 200 white blood cells and thin films to identify the plasmodium species.
For this study we considered two categories of malaria:
clinical malaria, defined by the presence or history (during the previous 2 weeks) of fever and microscopically confirmed malaria parasites;
severe malaria (based on WHO guidelines [20]), diagnosed if a patient had P. falciparum asexual parasitemia, no other obvious cause of symptoms and met any of the following criteria: convulsions, loss of consciousness, hypotension (systolic blood pressure <70 mmHg), admission to hospital due to malaria, laboratory evidence of liver or kidney damage, severe normocytic anaemia (haemoglobin <50 g/dl, PCV<15%), or hyperparasitaemia on blood slide (>5% or 250 000/μl).
Sample size
A total of 2180 participants were recruited and followed for up to 3.5 years. The sample size for the main trial was defined based on the power to demonstrate noninferiority of placebo for the primary efficacy outcome of time to the first CTX-preventable event or death [17,18]. For this study, assuming an incidence of 1.4 clinical malaria episodes/100 person-years (pyrs) in the control (CTX) arm, as observed in a previous study among HIV positive adults on ART in Uganda [21], and an average follow-up of 2 years, we estimated that the COSTOP trial would have more than 80% power to detect as significant (at the 5% level) a doubling of the incidence of clinical malaria in participants who stopped CTX compared with those who continued, assuming a 15% loss to follow-up at the end of the study.
Statistical analysis
Data were double-entered and verified in MS Access and analysed using Stata 12 (Stata Corp, College Station, Texas, USA). All analyses used an intention-to-treat (ITT) approach.
Baseline characteristics were compared between trial arms. Socioeconomic status (SES) was measured by combining data from all trial participants on housing construction and ownership of household items into an asset index score using principal component analysis [22].
Person years at risk were calculated from enrolment until the date last seen or end of trial. After each malaria episode, participants were considered to be not at risk for another episode until the episode resolved (as evidenced by resolution of symptoms and a negative repeat slide at the 14-day follow-up visit), or for 28 days, if a resolution date was not available. Time to first episode of clinical malaria was examined using Kaplan–Meier plots, and compared between trial arms using the log rank test. The incidence of all clinical malaria episodes, the rate ratio for the effect of trial arm, and 95%CI were estimated using random effects Poisson regression to account for multiple episodes within the same participant. The incidence of severe malaria was calculated and compared between arms.
In secondary analyses, follow-up time was divided into 12-month bands and analysis stratified by time to investigate possible effect modification of trial arm with time. Effect modification was assessed by comparing a model with fixed effects for treatment arm and timeband to one with treatment arm, timeband and their interaction, using the likelihood ratio test. In addition, effect modification by enrolment site was investigated.
The effect of trial drug adherence, and of ITN use, on malaria was assessed. The proportion of expected doses of trial drug taken, based on counts of returned pills at each scheduled visit, was calculated as (tablets dispensed – tablets returned)/(days elapsed since pills were dispensed). We defined ‘good’ adherence as taking 80–105% expected doses, allowing for adherence up to 105% due to possible imprecision in tablet counts. Each participant's overall adherence was categorized as being good at not less than 80% of visits or less than 80% of visits. At each visit, participants were asked if they had always slept under an ITN since their previous visit. Overall ITN use was characterized as having always used an ITN at not less than 90% of visits or less than 90% of visits.
Ethical approval
Approval for the study was obtained from the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council of Science and Technology, the Ugandan National Drug Authority, and the Ethics Committee of the London School of Hygiene and Tropical Medicine.
Results
A total of 2180 participants were enrolled into the COSTOP trial. Participants’ characteristics at baseline were balanced by trial arm (Table 1). Baseline characteristics have been described previously [17]. Overall, mean (SD) age was 41 [8] years; 74% of participants were female; 70% had primary education or less; and 52% had a CD4+ cell count below 500 cells/μl.
Table 1.
Baseline characteristics by trial arm and site.
| Overall (N = 2180) | Site | |||||||
| Entebbe | Masaka | |||||||
| CTX (N = 1089) | Placebo (N = 1091) | CTX (N = 501) | Placebo (N = 501) | Total (N = 1002) | CTX (N = 588) | Placebo (N = 590) | Total (N = 1178) | |
| Age | P = 0.002a | |||||||
| <30 | 85 (7.8) | 83 (7.6) | 47 (9.4) | 38 (7.6) | 85 (8.4) | 38 (6.5) | 45 (7.6) | 83 (7.0) |
| 30–34 | 148 (13.6) | 189 (17.3) | 75 (15.0) | 104 (20.8) | 179 (17.9) | 73 (12.4) | 85 (14.4) | 158 (13.4) |
| 35–39 | 232 (21.3) | 235 (21.5) | 112 (22.3) | 107 (21.3) | 219 (21.9) | 120 (20.4) | 128 (21.7) | 248 (21.1) |
| 40–44 | 262 (24.1) | 221 (20.3) | 116 (23.1) | 100 (20.0) | 216 (21.6) | 146 (24.8) | 121 (20.5) | 267 (22.7) |
| 45–49 | 198 (18.2) | 190 (17.4) | 87 (17.4) | 91 (18.1) | 178 (17.8) | 111 (18.9) | 99 (16.8) | 210 (17.8) |
| ≥50 | 164 (15.1) | 173 (15.9) | 64 (12.8) | 61 (12.2) | 125 (12.4) | 100 (17.0) | 112 (19.0) | 212 (18.0) |
| Mean age (SD) | 41.0 (8.0) | 40.7 (8.3) | 40.0 (8.0) | 41.5 (8.3) | ||||
| CD4+ cell count stratum | P = 0.001 | |||||||
| 250–<500 cells/μl | 563 (56.2) | 577 (49.0) | 282 (56.3) | 281 (56.1) | 563 (56.2) | 288 (49.0) | 289 (49.0) | 577 (49.0) |
| ≥500 cells/μl | 439 (43.8) | 601 (51.0) | 219 (43.7) | 220 (43.9) | 439 (43.8) | 300 (51.0) | 301 (51.0) | 601 (51.0) |
| Time on ART (years) | P < 0.001 | |||||||
| <1 | 73 (6.7) | 82 (7.5) | 46 (9.2) | 43 (8.6) | 89 (8.9) | 27 (4.6) | 39 (6.6) | 66 (5.6) |
| 1–2 | 144 (13.2) | 160 (14.7) | 71 (14.2) | 79 (15.8) | 150 (15.0) | 73 (12.4) | 81 (13.7) | 154 (13.1) |
| 2–5 | 508 (46.7) | 480 (44.0) | 261 (52.0) | 255 (50.9) | 516 (51.5) | 247 (42.0) | 225 (38.2) | 472 (40.1) |
| >5 | 364 (33.4) | 369 (33.8) | 123 (24.6) | 124 (24.7) | 247 (24.6) | 241 (41.0) | 245 (41.5) | 486 (41.3) |
| Sex | P = 0.520 | |||||||
| Man | 286 (26.3) | 283 (25.9) | 130 (26.0) | 125 (25.0) | 255 (25.5) | 156 (26.5) | 158 (26.8) | 314 (26.7) |
| Women | 803 (73.7) | 808 (74.1) | 371 (74.0) | 376 (75.0) | 747 (74.6) | 432 (73.5) | 432 (73.2) | 864 (73.3) |
| Education level | P < 0.001 | |||||||
| None | 111 (10.2) | 113 (10.4) | 46 (9.2) | 37 (7.4) | 83 (8.3) | 65 (11.2) | 76 (12.9) | 141 (11.9) |
| Primary | 649 (59.6) | 660 (60.5) | 259 (51.7) | 281 (56.1) | 540 (53.9) | 390 (66.3) | 379 (64.2) | 769 (65.3) |
| Secondary | 276 (25.3) | 268 (24.6) | 160 (31.9) | 157 (31.3) | 317 (31.6) | 116 (19.7) | 111 (18.8) | 227 (19.3) |
| Tertiary | 53 (4.9) | 50 (4.6) | 36 (7.2) | 26 (5.2) | 62 (6.2) | 17 (2.8) | 24 (4.1) | 41 (3.5) |
| Socio-economic status | P < 0.001 | |||||||
| Low | 486 (44.6) | 474 (43.4) | 197 (39.3) | 213 (42.5) | 410 (40.9) | 289 (49.1) | 261 (44.2) | 550 (46.7) |
| Medium | 318 (29.2) | 329 (30.2) | 117 (23.4) | 113 (22.6) | 230 (23.0) | 201 (34.2) | 216 (36.6) | 417 (35.4) |
| High | 285 (26.2) | 288 (26.4) | 187 (37.3) | 175 (33.9) | 362 (36.1) | 98 (16.7) | 113 (19.2) | 211 (17.9) |
| Marital status | P = 0.32 | |||||||
| Married/cohabiting | 473 (43.4) | 465 (42.6) | 213 (42.5) | 210 (41.9) | 423 (42.2) | 260 (44.2) | 255 (43.2) | 515 (43.7) |
| Divorced/separated/widowed | 577 (53.0) | 577 (52.9) | 271 (54.1) | 273 (54.5) | 544 (54.3) | 306 (52.0) | 304 (51.3) | 610 (51.8) |
| Single | 39 (3.6) | 49 (4.5) | 17 (3.4) | 18 (3.6) | 35 (3.5) | 22 (3.7) | 31 (5.3) | 53 (4.5) |
ART, antiretroviral therapy; CTX, cotrimoxazole.
aP value for comparison between sites, by χ2 test.
Although balanced by trial arm within each site, there were some differences in participant characteristics between the enrolment sites. Participants in Masaka were slightly older, had lower levels of education, and were of lower SES than those in Entebbe (Table 1). They also had higher CD4+ cell counts at enrolment, and had been on ART for longer.
Follow-up
Participants were followed for a median of 2.53 years (interquartile range = 1.86–2.76), with no evidence of a difference between arms (CTX median 2.53; interquartile range 1.96–2.76 years vs. placebo 2.53; 1.84–2.76 years; P = 0.66 by Wilcoxon rank-sum test). In total 1875 participants (943 CTX) completed follow-up (Fig. 1); 36 participants were withdrawn from trial drug (18 CTX) and started on open-label CTX; 232 participants were lost to follow-up (109 CTX); and 37 participants died (19 CTX). Only one death was malaria related (CTX) and was due to quinine toxicity. Overall, 13 043 scheduled visits were attended by participants on CTX and 12 913 by those on placebo (Fig. 1).
Fig. 1.
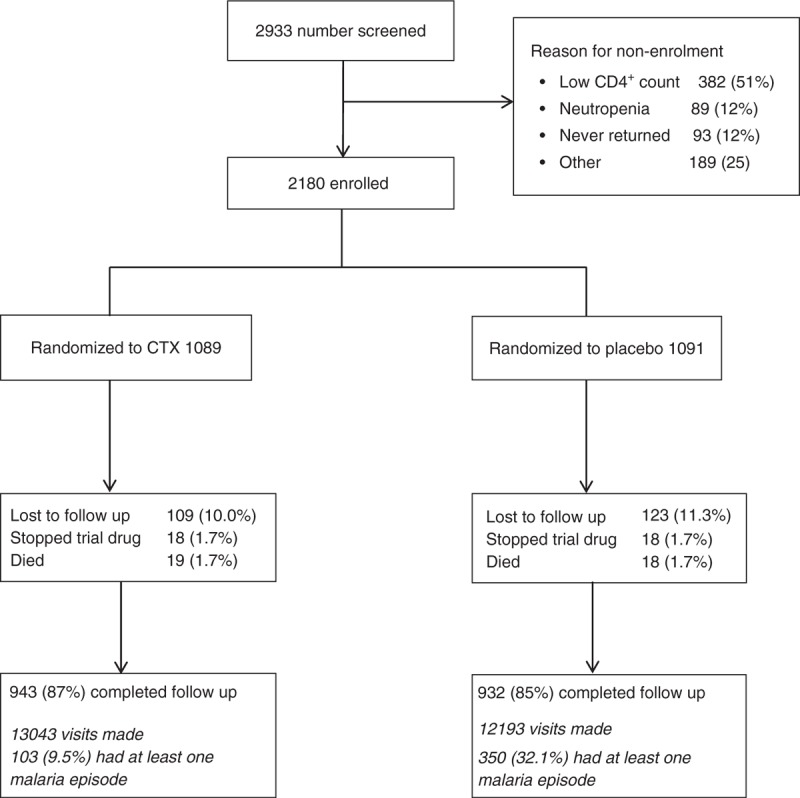
COSTOP trial profile.
Effect of stopping cotrimoxazole
There were 453 episodes of clinical malaria experienced by 362 participants (range 1–5) during follow-up; 9% of participants in the CTX arm and 24% of those in the placebo arm had at least one episode of clinical malaria. Time to the first clinical malaria event was significantly shorter in the placebo arm than in the CTX arm (log rank test P < 0.001) (Fig. 2).
Fig. 2.
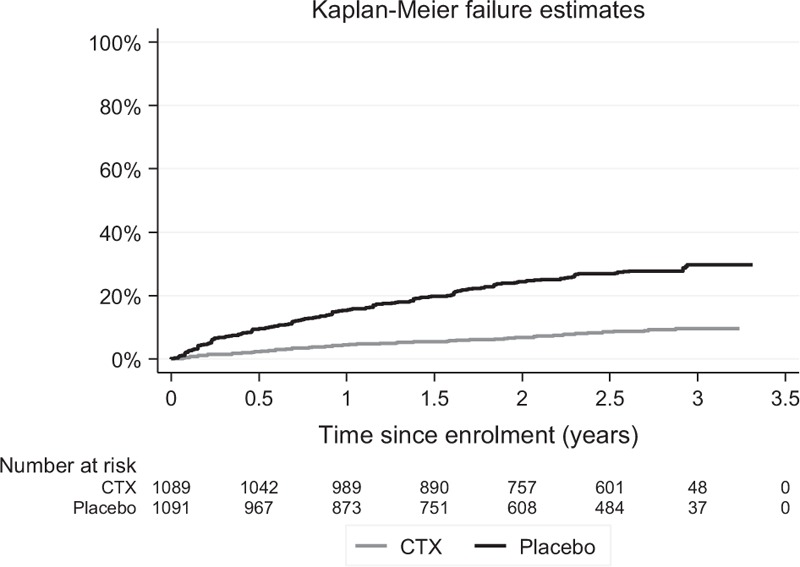
Time to occurrence of first malaria event by treatment arm.
The overall incidence of clinical malaria was 9.1 (95%CI = 8.2–10.1) episodes/100 pyrs. The incidence of malaria was 3.5 times higher in participants on placebo than on CTX (95%CI = 2.7–4.4; P < 0.001; Table 2).
Table 2.
Incidence of malaria by trial arm, follow-up time, trial site, adherence to trial drug and ITN use.
| Trial arm | Episodes | Person-years | Rate/100 person-years (95% CI)a | Rate ratioa | P value (CTX vs. placebo) | P value (for interaction)b | |
| Clinical malaria | |||||||
| CTX | 103 | 2540 | 4.1 (3.3–5.0) | 1 | <0.001 | ||
| Placebo | 350 | 2515 | 14.1 (12.5–15.0) | 3.5 (2.5–4.4) | |||
| Severe malariac,d | |||||||
| CTX | 2 | 2543 | 0.08 (0.02–0.31) | 1 | 0.004 | ||
| Placebo | 13 | 2524 | 0.52 (0.30–0.89) | 6.55 (1.48–29.01) | |||
| Stratified analyses | |||||||
| Stratum variable | |||||||
| Follow-up time | |||||||
| 1st year | CTX | 51 | 1065 | 4.8 (3.6–6.3) | 1 | <0.001 | 0.097 |
| Placebo | 183 | 1062 | 17.3 (14.8–20.2) | 3.6 (2.6–5.0) | |||
| 2nd year | CTX | 29 | 939 | 3.1 (2.1–4.5) | 1 | <0.001 | |
| Placebo | 120 | 924 | 13.1 (10.8–15.8) | 4.2 (2.8–6.4) | |||
| After 2nd year | CTX | 23 | 536 | 4.3 (2.8–6.5) | 1 | 0.004 | |
| Placebo | 47 | 528 | 9.0 (6.7–12.1) | 2.1 (1.3–3.5) | |||
| Site | |||||||
| Entebbe | CTX | 19 | 1215 | 1.6 (1.0–2.5) | 1 | <0.001 | <0.001 |
| Placebo | 127 | 1209 | 10.6 (8.8–12.8) | 6.8 (4.1–11.1) | |||
| Masaka | CTX | 84 | 1325 | 6.3 (5.1–7.9) | 1 | <0.001 | |
| Placebo | 223 | 1306 | 17.2 (14.8–20.0) | 2.7 (2.1–3.6) | |||
| Trial drug adherence | |||||||
| Good adherence at | |||||||
| ≥80% of visitse,f | CTX | 57 | 1724 | 3.3 (2.5–4.4) | 1 | 0.01 | 0.25 |
| <80% of visitse,f | CTX | 46 | 816 | 5.6 (4.1–7.6) | 1.7 (1.1–2.6) | ||
| ≥80% of visitse,g | Placebo | 205 | 1617 | 12.8 (12.0–14.9) | 1 | 0.05 | |
| <80% of visitse,g | Placebo | 145 | 897 | 16.4 (13.6–19.8) | 1.3 (1.0–1.6) | ||
| ITN use | |||||||
| Reported always using ITN at | |||||||
| ≥90% of visits | CTX | 86 | 2124 | 4.1 (3.3–5.1) | 1 | P = 0.98 | 0.39 |
| <90% off visits | CTX | 17 | 416 | 4.1 (2.5–6.7) | 1.0 (0.6–1.7) | ||
| ≥90% of visits | Placebo | 267 | 2038 | 13.3 (11.6–15.2) | 1 | P = 0.06 | |
| <90% off visits | Placebo | 83 | 476 | 17.5 (13.6–22.5) | 1.3 (1.0–1.8) | ||
CTX, cotrimoxazole; ITN, insecticide-treated bed net.
arates and rate ratios adjusted for clustering of multiple episodes within participant using random effects Poisson regression.
bP value for interaction between stratum variable with treatment arm.
cP. falciparum malaria with clinical or laboratory features of severity.
dRates and rate ratios from Poisson regression without adjustment for clustering since no participant had more than one episode.
e‘Good’ adherence defined as 80–105% of expected tablets taken, based on pill counts.
fRate ratio and P value for comparison in CTX arm.
gRate ratio and P value for comparison in placebo arm.
There was some evidence that the effect of stopping CTX on clinical malaria incidence decreased over time: although incidence remained higher in the placebo arm, the difference between arms was less in the third year (rate ratio = 2.1; 95%CI = 1.3–3.5) than in the first year (rate ratio = 3.6; 95%CI = 2.6–5.0; P for interaction = 0.10) (Table 2). In the placebo arm, clinical malaria incidence decreased from 17.3/100 pyrs in the first year to 9.0/100 pyrs after the second year (P for trend <0.001; Supplementary Figure). In the CTX arm, clinical malaria incidence remained similar over time (rate ratio for linear trend in incidence from one year to the next = 0.90, CI = 0.69–1.16, P = 0.40).
The relative effect of stopping CTX on clinical malaria was greater in Entebbe (rate ratio = 6.8; 95%CI = 4.1–11.1) than in Masaka (rate ratio = 2.7; 95%CI = 2.1–3.6; P for interaction <0.001) (Table 2). In both arms, the incidence of clinical malaria was higher in Masaka than in Entebbe (CTX: 6.3 vs. 1.6/100 pyrs, respectively; placebo 17.2 vs. 10.6/100 pyrs, respectively).
Overall, 15 (2 CTX, 13 placebo) episodes of severe malaria occurred among the 2180 participants (<1%) (Table 2). None of the participants had more than one episode of severe malaria. Reasons for classifying malaria as severe were: high parasitemia [2], loss of consciousness [1], mental confusion [1] and hospital admission [11]. The overall incidence of severe malaria was 0.30/100 pyrs (95%CI = 0.18–0.49); the incidence of severe malaria was 6.5 (95%CI = 1.5–29.0) times higher in the placebo arm than in the CTX arm. Only one participant (CTX arm) died of a malaria-related event.
The NNT with CTX for one year to prevent one malaria episode was 10, and was 233 for severe malaria.
Effect of adherence to trial drug and insecticide-treated bed net use on malaria
Among participants on CTX, the incidence of clinical malaria in those with good adherence at less than 80% of visits was 1.7 (95%CI = 1.1–2.6) times higher than in those with good adherence at not less than 80% of visits (P = 0.01) (Table 2). Among participants on placebo, malaria incidence was 1.3 (95%CI = 1.0–1.6) times higher in those with good adherence at less than 80% of visits (P = 0.05). The relative outcome of effect of stopping CTX was greater in participants with good adherence than in those with lower adherence (rate ratio = 3.9, 95%CI = 2.8–5.3 vs. rate ratio = 2.9, 95%CI = 2.0–4.2).
Malaria incidence did not differ by reported ITN use among participants on CTX (rate ratio = 1.0; 95%CI = 0.6–1.7; P = 0.98). However, malaria incidence was higher in participants on placebo who reported using an ITN at less than 90% of visits than in those who reported ITN use at not less than 90% of visits (rate ratio = 1.3, 95%CI = 1.0–1.8; P = 0.06), although there was no evidence of significant effect modification by treatment arm (P = 0.39).
Discussion
In this trial, participants on ART who stopped prophylactic CTX had a 3.5-fold higher probability to experience clinical malaria than those who continued. This is expected given the antimalarial properties of CTX [23] and is consistent with other studies in adults on ART who discontinued CTX [21,24–28]. However the rate ratio in our study was much smaller than reported by other randomized trials in adults. Campbell et al.[21] conducted an open-label, cluster (household) RCT in Uganda to investigate the effect of stopping CTX on the incidence of malaria and diarrhoea among HIV-infected adults on ART with CD4+ above 200 cells/μl. The trial enrolled 836 participants with median time on ART of 3.7 years and found that participants stopping CTX had a 32.5-fold (8.6–275.0) higher malaria incidence than those that continued. The trial was stopped after 4 months. Polyak et al.[28] conducted an open-label RCT comparing stopping versus continuing CTX prophylaxis among 500 HIV-infected adults in western Kenya who had been on ART for more than 18 months. After a year of follow-up participants stopping CTX had a 33.2-fold (95%CI = 4.5–241.0) higher malaria incidence than those who continued CTX.
The contrasting results between these trials and ours could have resulted from the shorter follow-up times (4 months and 1 year in the Campbell and Polyak studies, respectively) and from the smaller number of malaria episodes (55 and 34, respectively) compared with our study, in which 453 episodes of clinical malaria were documented over a median follow-up time of 2.5 years. Another explanation could be that participants in our trial may have used CTX from other sources outside the trial, which may have accounted for the smaller differences between arms. However, exit interviews conducted at the end of our trial did not find any evidence that participants had taken CTX from other sources. We used an ITT approach to the analysis; however, only 18 participants in the placebo arm were withdrawn from trial medication and started on open-label CTX, so this is unlikely to have had a large impact on our results. Importantly, the Campbell and Polyak studies were not blinded, so that investigators or participants might have been more likely to investigate or seek treatment if they felt that stopping CTX might be risky. This source of potential observer bias may have resulted in diagnosing malaria more frequently in participants who had stopped CTX.
Although the incidence of severe malaria was higher in the placebo arm, there was very little severe malaria in our trial (only 1.2% of participants in the placebo arm) and there was no statistical difference in overall number of deaths (due to any cause) between trial arms. This is consistent with findings in the study by Campbell et al.[21]. Only one malaria-related death was recorded, and this occurred in the CTX arm.
We found a marked decrease in malaria incidence over time in the placebo arm whereas the incidence in the CTX arm remained stable. This initially higher incidence among participants who had stopped CTX could be a consequence of the detrimental effects that HIV had caused on the innate immune response to malaria and other infections [29]. Whilst participants with low immunity were initially protected against malaria due to CTX, it is possible that the incidence of malaria increased when CTX was discontinued, until participants re-acquired some immunity against malaria. This has been described among children [30].
As expected, participants with good adherence to trial drug in the CTX arm had less malaria than those whose adherence was not as good. Unexpectedly, good adherence was also associated with reduced malaria incidence in the placebo arm. A possible explanation is that participants with suboptimal adherence to trial drug might also be less likely to adhere to other malaria prevention measures like ITN use. There was no association of reported ITN use with malaria in the CTX arm. In contrast, participants on placebo with high reported ITN use had less malaria than those with lower reported ITN use, suggesting that people who discontinue CTX would benefit from general malaria prevention measures.
There were fewer malaria episodes observed in participants at Entebbe than Masaka, in both trial arms and over time. Participants at Entebbe were generally younger, and had attained a higher education level and SES compared with those in Masaka. If participants in Entebbe were better able to take care of themselves, had better housing or could access treatment more easily this may have resulted in a lower incidence of malaria at the Entebbe site. Another possible explanation is that malaria endemicity and therefore exposure to malaria was higher in Masaka compared with Entebbe. Data on malaria endemicity by region in Uganda are limited; however, both areas are considered to have very high malaria endemicity [31]. Recent reports for the three quarters up to June 2015 showed an incidence of 101, 59, and 106 per 1000 population in Masaka district, and 82, 86 and 56 in Wakiso district, which includes Entebbe [32]. This seems to suggest that background exposure to malaria may be higher in Masaka.
Strengths and limitations
Strengths of our study include its design as a double-blind placebo-controlled randomized trial, its large sample size, and that participants were seen at frequent scheduled visits and sick visits at which screening for malaria was routinely performed and adherence to trial drug and ART assessed. Furthermore, participant retention was high, with more than 85% completing follow-up.
Unfortunately we do not have data on malaria incidence from HIV-negative individuals in our study area. We can therefore not determine the extent to which the incidence of malaria among HIV-infected participants on ART and CTX may have been reduced below normal levels.
Conclusion
In this blinded placebo-controlled trial in Uganda, participants who were stable on ART and stopped taking prophylactic CTX had malaria more frequently and severely than those who continued, but the difference was less than has been reported by earlier studies. Malaria incidence reduced in participants on placebo over time. Few participants experienced severe malaria. A potential decision to stop or continue CTX will have to take into account the main COSTOP trial results that have shown a clear benefit of continued CTX prophylaxis in preventing bacterial infections, but also showed an increase in neutropenia incidence and no reduction in overall mortality [17]. The NNT to prevent one CTX-preventable event or malaria is also worth considering. According to current WHO guidelines CTX may be discontinued in some situations, but should be continued in countries with high endemicity of malaria and bacterial infections [15]. Given the costs and toxicity of CTX and the potential development of wide spread of CTX resistance, further research will be useful in identifying groups and circumstances in which CTX prophylaxis could be safely stopped.
Acknowledgements
We are grateful to all the study participants and to the staff from the two COSTOP study sites, partner institutions, COSTOP Trial Monitors, the independent Trial Steering Committee, the independent Data Monitoring Committee, the independent Endpoint Review Committee, the MRC/UVRI social science team. We thank the UK Medical Research Council (MRC UK) and the UK Department for International Development (DFID) under the MRC/DFID concordant agreement through grant number G0902150 for funding the trial. K.B. receives support from the MRC UK and DFID (MRC grant number G0700837).
Contributions: R.K., P.M., J.L., H.G. conceived the study idea; R.K., Z.A., P.M., A.K., A.N. conducted the study; R.K., K.B. did the data analysis; R.K., K.B., H.G. developed the first draft. All authors contributed to the interpretation of the data, revised the article critically and approved the final version.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 2001; 15:899–906. [DOI] [PubMed] [Google Scholar]
- 2.Morgan D, Maude GH, Malamba SS, Okongo MJ, Wagner HU, Mulder DW, et al. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet 1997; 350:245–250. [DOI] [PubMed] [Google Scholar]
- 3.Vergis EN, Mellors JW. Natural history of HIV-1 infection. Infect Dis Clin North Am 2000; 14:809–825.v-vi. [DOI] [PubMed] [Google Scholar]
- 4.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A, et al. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ 2008; 337:a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeree MJ, Sauvageot D, Banda HT, Harries AD, Zijlstra EE. Efficacy and safety of two dosages of cotrimoxazole as preventive treatment for HIV-infected Malawian adults with new smear-positive tuberculosis. Trop Med Int Health 2005; 10:723–733. [DOI] [PubMed] [Google Scholar]
- 6.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999; 353:1463–1468. [DOI] [PubMed] [Google Scholar]
- 7.Watera C, Todd J, Muwonge R, Whitworth J, Nakiyingi-Miiro J, Brink A, et al. Feasibility and effectiveness of cotrimoxazole prophylaxis for HIV-1-infected adults attending an HIV/AIDS clinic in Uganda. J Acquir Immune Defic Syndr 2006; 42:373–378. [DOI] [PubMed] [Google Scholar]
- 8.Walker AS, Ford D, Gilks CF, Munderi P, Ssali F, Reid A, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet 2010; 375:1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manyando C, Njunju EM, D’Alessandro U, Van Geertruyden JP. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One 2013; 8:e56916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina Lara A, Kigozi J, Amurwon J, Muchabaiwa L, Nyanzi Wakaholi B, Mujica Mota RE, et al. Cost effectiveness analysis of clinically driven versus routine laboratory monitoring of antiretroviral therapy in Uganda and Zimbabwe. PLoS One 2012; 7:e33672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moh R, Danel C, Sorho S, Sauvageot D, Anzian A, Minga A, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d’Ivoire. Antivir Ther 2005; 10:615–624. [DOI] [PubMed] [Google Scholar]
- 12.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004; 364:1428–1434. [DOI] [PubMed] [Google Scholar]
- 13.Weverling GJ, Mocroft A, Ledergerber B, Kirk O, Gonzales-Lahoz J, d’Arminio Monforte A, et al. Discontinuation of Pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV-1 infection. EuroSIDA Study Group. Lancet 1999; 353:1293–1298. [DOI] [PubMed] [Google Scholar]
- 14.Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, et al. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med 1999; 340:1301–1306. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Guidelines on postexposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach; 2014. http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_dec2014/en/ [Accessed 12 June 2015]. [Google Scholar]
- 16.Kasirye R, Baisley K, Munderi P, Grosskurth H. Effect of cotrimoxazole prophylaxis on malaria occurrence in HIV-infected patients on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health 2015; 20:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munderi P LJ, Anywaine Z, Kasirye R, Kamali A, Nunn A, Grosskurth H. Is it safe to stop cotrimoxazole in adults on ART: COSTOP; a noninferiority RCT [abstract number 94]. Conference on Retroviruses and Opportunistic Infections (CROI); 23–26 February 2015. http://www.croiconference.org/sessions/it-safe-stop-cotrimoxazole-adults-art-costop-noninferiority-rct [Accessed 12 June 2015]. [Google Scholar]
- 18.Anywaine Z, Abaasa A, Levin J, Kasirye R, Kamali A, Grosskurth H, et al. Safety of discontinuing Cotrimoxazole prophylaxis among HIV infected adults on antiretroviral therapy in Uganda (COSTOP Trial): Design. Contemp Clin Trials 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MOH. National Policy on Malaria Treatment, Uganda 2006. http://library.health.go.ug/publications/leadership-and-governance-governance/policy-documents/national-policy-malaria-treatme-0 [Accessed 12 June 2015]. [Google Scholar]
- 20.WHO. Guidelines for treatment of malaria 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/ [Accessed 12 June 2015]. [Google Scholar]
- 21.Campbell JD, Moore D, Degerman R, Kaharuza F, Were W, Muramuzi E, et al. HIV-infected Ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/μL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis 2012; 54:1204–1211. [DOI] [PubMed] [Google Scholar]
- 22.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 2006; 21:459–468. [DOI] [PubMed] [Google Scholar]
- 23.Bushy HG., Sr Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother 1968; 33:72–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasasira AF, Kamya MR, Ochong EO, Vora N, Achan J, Charlebois E, et al. Effect of trimethoprim-sulphamethoxazole on the risk of malaria in HIV-infected Ugandan children living in an area of widespread antifolate resistance. Malar J 2010; 9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet 2006; 367:1256–1261. [DOI] [PubMed] [Google Scholar]
- 26.Skinner-Adams TS, Butterworth AS, Porter KA, D’Amico R, Sawe F, Shaffer D, et al. The frequency of malaria is similar among women receiving either lopinavir/ritonavir or nevirapine-based antiretroviral treatment. PLoS One 2012; 7:e34399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, Nahirya-Ntege P, Keishanyu R, Nathoo K, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med 2014; 370:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyak C YK, Singa B, Khaemba M, Walson J, Richardson B, John-Stewart G. CTX Prophylaxis Discontinuation Among ART-Treated Adults: A Randomized Non-Inferiority Trial. Boston; 2014. http://www.croiconference.org/sites/default/files/abstracts/98.pdf [Accessed 12 June 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis 2011; 11:541–556. [DOI] [PubMed] [Google Scholar]
- 30.Longwe H, Jambo KC, Phiri KS, Mbeye N, Gondwe T, Hall T, et al. The effect of daily co-trimoxazole prophylaxis on natural development of antibody-mediated immunity against P. falciparum malaria infection in HIV-exposed uninfected Malawian children. PLoS One 2015; 10:e0121643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 2012; 121:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MOH. Uganda Malaria Quarterly Bulletin 2015. http://www.health.go.ug/publications [Accessed 7 August 2015]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


