Supplemental Digital Content is available in the text
Keywords: beta-palmitate, infant formula, palmitic acid, prebiotics, SN2-palmitate
ABSTRACT
Objectives:
Palmitic acid (PA) comprises 17% to 25% of human milk fatty acids, of which 70% to 75% are esterified to the SN2 position of the triglyceride (SN2-palmitate). In vegetable oils, which are commonly used in infant formulas, palmitate is primarily esterified to other positions, resulting in reduced calcium and fat absorption and hard stools. The aim of this study was to elucidate the effects of SN2-palmitate on nutrient excretion.
Methods:
In total, 171 Chinese infants were included (within 14 days of birth) in this multicenter study. Formula-fed infants were randomly assigned to receive either SN2-palmitate formula (INFAT, n = 57) or control formula (n = 57). The formulas (Biostime, China) differed only in their SN2 PA proportions. Stool was collected at 6 postnatal weeks.
Results:
The stool dry weight and fat content of the SN2-palmitate group were lower compared with the control group (dry weight 4.25 g vs 7.28 g, P < 0.05; fat 0.8 g vs 1.2 g, P < 0.05). The lipid component was also significantly lower for the SN2-palmitate group (0.79 g vs 1.19 g, P < 0.05). PA, representing ∼50% of the saponified fatty acids, was significantly lower in the SN2-palmitate group compared with the control group (0.3 g vs 0.7 g, P < 0.01). Breast-fed infants had a significantly lower stool dry weight, fat content, and saponified fat excretion compared with formula-fed infants (P < 0.01).
Conclusions:
Similar to breast milk, the SN2-palmitate infant formula primarily reduced calcium-saponified fat excretion. The results of this study further emphasize the nutritional importance of SN2-palmitate structured fat for infants.
What Is Known
Structured triglycerides, in which palmitic acid has been predominantly esterified to the SN2 position, developed to mimic human milk fat, have been demonstrated to benefit fat and calcium absorption, bone mineralization and strength, intestinal flora, and infant crying in healthy infants.
Prebiotics, such as galactooligosaccharides, have become common ingredients in infant formulas and have been demonstrated to benefit digestion and nutrient absorption.
What Is New
An SN2-palmitate infant formula reduced stool dry weight and fatty acid excretion in Chinese infants.
The beneficial effects of SN2-palmitate for formula-fed infants were maintained beyond those of galactooligosaccharides.
Human milk is the natural and optimal form of nutrition for infants up to 6 months of age (1). Despite the well-known advantages of breast-feeding, some mothers cannot or choose not to breast-feed and use infant formulas as a substitute.
Infants require bio-available sources of energy and nutrients to meet the requirements for their rapid growth, development, and expanding skeletal mass. An infant's typical nutritional profile involves a high-fat diet with frequent feedings; therefore, efficient fat absorption is required.
Efficient fat absorption is challenging because it is generally recognized that the exocrine pancreas has a poor secretory capacity during the neonatal period in comparison with adults, and the pancreatic lipase content is low.
In breast-fed (BF) infants, this problem is solved by the presence of the bile salt–stimulated lipase and by using fat globule lipids and triglycerides (TGs) with complex structures that enable efficient absorption and processing of a high-fat diet containing highly saturated fatty acids (FAs) without requiring much metabolic effort. For formula-fed infants, nutrient and energy availability depend on the formula composition (2).
Palmitic acid (PA) is the predominant saturated FA in human milk (3). Unlike other FAs, whose availability and quantity in human breast milk are affected by the mother's nutrition, PA abundance is conserved in all women regardless of ethnic origin or nutritional status (3). PA comprises 17% to 25% of the total FAs in mature human milk, 70% to 75% of which is esterified at the SN2 (β) position of the TG (β-palmitate) (3).
Lipid digestion occurs in both the stomach and small intestine (for a review, refer to (4)). Several enzymes contribute to lipid degradation, and their relative efficiencies reflect the species and age of the individual being considered and the substrate and effects of the particular physiological situation. The first step in triacylglycerol (TAG) digestion is partial hydrolysis into diacylglycerol and free fatty acids (FFAs), which occurs in the stomach and is performed by gastric lipases that preferentially hydrolyze the sn3-ester bond, resulting in the formation of sn1,2-diacylglycerols (5). This gastric predigestion facilitates lipid digestion in the duodenum. Gastric lipid digestion has a more prominent function in young infants compared with adults because it retains some activity in the small intestine in the former, broadening its contribution to lipid digestion. This activity can account for up to 60% of all dietary TAG hydrolysis in infants (6). The next step of TAG digestion is achieved by hydrolysis by pancreatic lipase, which is secreted by the pancreas from ∼30 weeks of gestation onward. In both term and preterm infants, however, this enzyme is present at a very low concentration until well into the first year of life (7). This lipase is position-specific and ideally catalyzes sn2-monoacylglycerol and FFA formation (8), each of which are absorbed separately. Absorption of unesterified FAs depends on their physical and chemical properties (9). Unsaturated FAs, such as oleic acid, linoleic acid, and linolenic acid, as well as short saturated FAs, which have melting points below intestinal temperature, are efficiently absorbed, whereas PA and stearic acid, which have melting points higher than intestinal temperature (63°C and 70°C, respectively), have an increased tendency to form insoluble FA soaps in the intestine that are subsequently excreted in the stool (9). PA in infant formula is digested and absorbed into tissues as sn2-monoacylglycerol (10), which is the preferred form of PA (11).
In contrast with human milk fat, most of the PA in vegetable oils is esterified to the sn1 and sn3 positions of the TG (12), whereas the sn2 position is usually occupied by an unsaturated FA. Infant formulas containing vegetable oils as a fat source provide infants with PA esterified to a different position of the TG compared with that found in human milk. Free PA molecules from vegetable oils, which were originally esterified to the sn1 and sn3 positions, tend to create complexes with dietary minerals, such as calcium (13). These PA-calcium soap complexes are insoluble and therefore indigestible, resulting in a loss of both calcium and fat and causing harder stools and discomfort to infants (14).
Structured TGs, in which PA has been predominantly esterified to the SN2 position (SN2-palmitate or β-palmitate), have been developed to imitate breast milk lipids. Regardless of the low concentration of the position-specific lipase in infants (7), the positioning of the PA affects significantly the absorption of this FA and of other FAs as well in formula-fed term (15–17) and preterm infants (18,19) (for a review, see (20)). This reduction in calcium and FA absorption is accompanied by increased calcium soaps and consequently by hard stools (14). Clinical studies have demonstrated the effects of SN2-palmitate formulas on infant bone mineralization and strength (16,21), intestinal flora composition (22), and crying (23) compared with low-SN2-palmitate infant formulas, that is, those containing high levels of sn1 and sn3 PA.
Prebiotics are added to infant formulas to promote optimum growth and development and to decrease infections (24). Prebiotics are nondigestible food ingredients that are found in human milk and benefit the host by selectively stimulating the growth and/or activity of one or a limited number of colonic bacteria, thereby improving the host's health (25).
In recent years, prebiotics such as galactooligosaccharides (GOS) and fructooligosaccharides have become common ingredients in infant formulas. These oligosaccharides have been widely investigated and have demonstrated potential beneficial effects on neonatal intestinal development, digestion, and nutrient absorption, as well as protection against infection.
Nutrient absorption by infants following formula feeding is of particular interest. The aim of this study was to assess the effects of SN2-palmitate formulas on lipid excretion. To the best of our knowledge, this is the first study to demonstrate these effects in the Chinese population using infant formula that already contains oligosaccharides known to affect nutrient absorption.
METHODS
Study Design and Participants
Healthy term infants (born at GA ≥ 37) who were appropriate for gestational age and <14 days of age were eligible for participation in this multicenter, randomized, double-blind study. Infants were excluded from the study if they suffered from a congenital or chromosomal disorder, neonatal morbidity, or metabolic illness.
Infants were enrolled by medical staff at 5 clinical centers located in 4 different cities in China (Beijing, Shanghai, Changsha, and Chengdu) between June 2011 and April 2012. Formula-fed infants were enrolled in the study after the mother decided unequivocally not to breast-feed. These infants were placed into groups according to a randomization process created by an external statistician based on blocks of 4 using sealed envelopes. BF infants served as a reference group. The formula-fed infants were randomly assigned to receive one of the following 2 formulas: an infant formula with SN2-palmitate (INFAT, Advanced Lipids AB, the SN2-palmitate group), in which 43% of the PA was esterified to the SN2 position of the glycerol backbone, or an infant formula containing a standard vegetable oil mixture, in which 13% of the PA was esterified to the SN2 position of the glycerol backbone (the control group). Both formulas (Biostime, Guangzhou, China) were produced under the same conditions using identical ingredients from the same batches, except for the lipid ingredients, which differed primarily in their FA structural distributions (supplementary Table 1).
This study was conducted according to the principles of the Declaration of Helsinki and good clinical practices. The protocol was approved by the ethics committees of each clinical center, and each parent gave written informed consent before inclusion.
Anthropometric Measurements
Growth parameters were measured at enrollment and at 6, 12, and 24 weeks. The following variables were measured: body weight, which was measured on an electronic infant scale, recorded to the nearest 10 g and repeated 3 times, with the average used for analysis, and with exclusion of any clearly erroneous value; body length, which was calculated as the mean of 2 measurements of the recumbent crown-heel length to the nearest 0.1 cm; frontooccipital head circumference, which was measured using a standard 1-cm-wide measuring tape to the nearest 0.1 cm.
Stool Collection and FA Analysis
Stool samples were collected by the parents during 24 hours when the infants were 6 weeks of age, with placement of rice paper into the diaper to ensure good recovery of stool. The samples were kept at 4°C until they arrived at the clinical centers. The fecal samples were then weighed, dried, stored at -80°C and analyzed as described elsewhere (14,16). Briefly, the freeze-dried samples were refluxed with petroleum ether to extract nonsaponified FAs. The residues were then refluxed again with petroleum ether (40–60)/acetic acid to recover soap fats. FAs, which were converted to methyl esters, were analyzed using a capillary gas chromatography apparatus (HP 5890, Hewlett Packard, Palo Alto, CA) that was equipped with a flame ionization detector and an autoinjector system. Separation was achieved on a 30-m J&W capillary column with an internal diameter of 0.25 mm and a film thickness of 1 μm (Agilent Technologies, Palo Alto, CA).
Statistical Analysis
The total saponified FA levels in the fecal samples were used as the key outcome and for sample size calculation. Assuming a multisite study and 20% dropout overhead correction, a sample size of 57 per group was determined to have 80% power to detect a difference with a 0.05 two-sided significance level (25a,25b). A block randomization procedure with blocks of 4 was used at all sites.
The baseline characteristics of the mothers and infants from the 2 formula groups were compared using the pairwise t-test for scale outcomes and the pairwise χ2 test for nominal outcomes. Stool FA analysis was performed using the nonparametric Mann-Whitney test.
BF infants served as the reference group; therefore, all parameters were evaluated compared with the BF infants. A P < 0.05 was considered to be statistically significant, with no correction for multiple testing.
RESULTS
Study Population
In total, 171 term infants were enrolled in the study. Of them, 57 were exclusively BF (the BF group), and 114 were randomly enrolled into either the SN2-palmitate group (n = 57) or the control formula-fed group (n = 57). Of the infants, 12 (7%) dropped out during the study period, only 3 of whom (<2%) were from the formula groups (Fig. 1).
FIGURE 1.
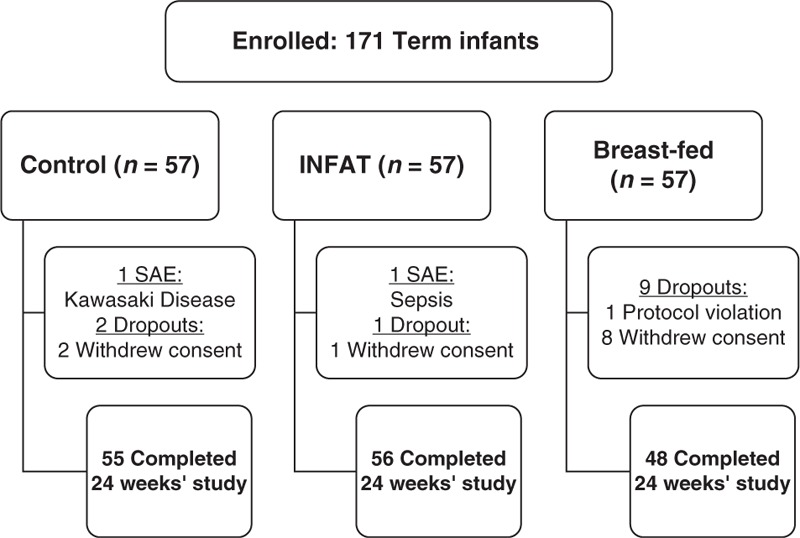
Flow diagram of study participants. SAE = serious adverse event.
Demographic Characteristics of Mothers and Infants
The maternal and infant characteristics are presented in Table 1. No significant differences in the maternal characteristics were observed between the formula-fed groups, and few of these characteristics differed between the BF and formula groups, including maternal education, which was higher compared with that of the SN2-palmitate group, and percentage of first pregnancy, which was higher compared with that of the control group.
TABLE 1.
Infants and maternal demographic characteristics
| Control (n = 57) | INFAT (n = 57) | BF (n = 57) | |
| Mother's age, y | 29.5 ± 4.2 | 29.3 ± 3.9 | 29.7 ± 3.1 |
| Primigravida, % | 86.0* | 91.2 | 96.5 |
| Maternal education >12 years, % | 77.2 | 68.4* | 89.5 |
| Maternal smoking (during pregnancy), % | 0 | 0 | 0 |
| Gestational age, wk | 39.2 ± 1.1 | 39.3 ± 1.1 | 39.3 ± 1.0 |
| Type of delivery, % vaginal | 36.8 | 31.6 | 29.8 |
| Sex, % male | 47.4 | 56.1 | 57.9 |
| Birth weight, g | 3311 ± 439 | 3451 ± 297† | 3375 ± 377 |
| Age at inclusion, days | 7.7 ± 4.5 | 8.0 ± 4.5 | 7.7 ± 4.9 |
Significance was calculated for 2 groups using t-test for continuous parameters and χ2 (df = 1) for categorical parameters. BF = breast-fed; INFAT = SN2-palmitate.
*Significantly different from BF (P < 0.05).
†Significantly different from control (P < 0.05)
No significant differences in the infant characteristics were observed between the formula-fed groups. Average birth weight was higher in the infants in the SN2-palmitate group compared with that of the infants in the control group (3.45 ± 0.3 vs 3.31 ± 0.4 kg, respectively, P < 0.05). This difference, however, disappeared at enrollment.
Anthropometric Data
The infants’ weights, lengths, and head circumferences at enrollment and at the ages 6, 12, and 24 weeks are summarized in supplementary Table 2. No significant differences were observed in any of the anthropometric measurements at baseline or at any visit during the study.
Formula Consumption
There were no significant differences in formula consumption between the control and SN2-palmitate groups at 6, 12, or 24 weeks (160.9 vs 163.9 mL · kg−1 · day−1, 144.0 vs 141.8 mL · kg−1 · day−1, and 110.6 vs 123.1 mL · kg−1 · day−1, respectively, P > 0.05). Fat intake was calculated based on formula consumption. Supplementary feeding was reported during the study period.
Stool Analysis
Stool samples were analyzed for water content by measuring their weights before and after freeze-drying. There were no differences in water content among the groups (73%, 72%, and 74% for the control, SN2-palmitate, and BF groups, respectively). Stool FA analysis was performed on a neutral fraction of free FAs and on a saponified fraction consisting of FAs bound to minerals, mainly calcium. The results were expressed as the absolute weights of each FA and of the total FAs excreted during 24 hours and as a percent of the weight of 100 mg of dry stool. At 6 postnatal weeks, the absolute 24-h stool dry weight for the infants in the SN2-palmitate group was significantly lower than that for the infants in the control group (4.25 g vs 7.28 g, P < 0.05). Unabsorbed nutrients contribute to stool dry weight. The lipid component of the absolute stool dry weight was also significantly lower for the SN2-palmitate group infants compared with the control infants (0.79 g vs 1.19 g, P < 0.05) (Fig. 2A).
FIGURE 2.
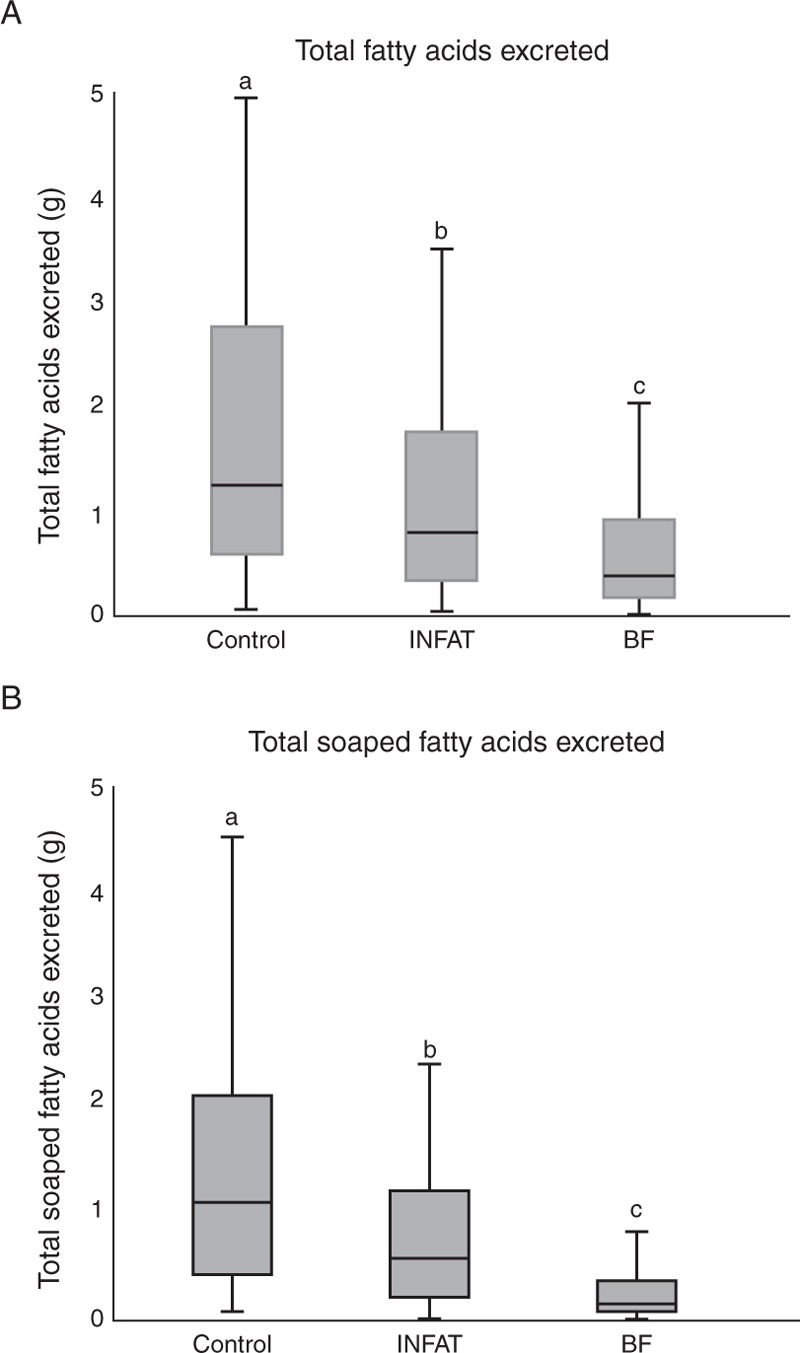
Stool FAs in 24-hour stool samples collected at 6 postnatal weeks. The bars represent the total FA soaps excreted, as measured in the stool samples. Significance was calculated for the 2 groups using the Mann-Whitney test. The different letters indicate statistical significance (P < 0.05) between the groups. BF = breast-fed; FA = fatty acid.
Most stool lipids were excreted as calcium soaps, which were present at a significantly lower level in the infants in the SN2-palmitate group than that in the control infants (0.56 g vs 1.03 g, P < 0.05) (Fig. 2B). Significant differences were observed in the levels of all saturated FAs in both the saponified FA and total FA fractions when individual FAs were analyzed; however, no significant differences were observed in the levels of unsaturated FAs (Table 2A). The main FA in the stool was PA, which comprised ∼50% of the saponified FAs. The absolute excreted PA in the stool was significantly lower in the SN2-palmitate group compared with that in the control group (0.32 g vs 0.72 g, P < 0.01; supplemental Table 3. Similar differences were observed when the FA weight was expressed as a percentage of the stool dry weight (mg/100 mg stool dry weight) (Table 2).
TABLE 2.
Stool biochemistry
| FA lost in stool (mg/100 mg stool dry weight) | |||
| Control (n = 56) | INFAT (n = 55) | BF (n = 50) | |
| Total C12:0 | 0.48 (0.23–0.73)a | 0.31 (0.14–0.41)b | 0.06 (0.03–0.11)c |
| Total C14:0 | 0.68 (0.34–0.93)a | 0.42 (0.24–0.58)b | 0.14 (0.08–0.29)c |
| Total C16:0 | 10.28 (5.29–15.06)a | 6.45 (4.54–9.35)b | 2.98 (1.63–5.80)c |
| Total C18:0 | 2.73 (2.06–3.54)a | 2.78 (2.29–3.69)a | 2.16 (1.32–3.72)a |
| Total C18:1 | 2.24 (1.43–3.16)a | 2.84 (1.76–4.28)b | 2.93 (1.24–5.22)a,b |
| Total C18:2 | 0.41 (0.24–0.69)a | 0.52 (0.31–0.91)a | 0.83 (0.34–1.46)b |
| Total FAs | 18.84 (10.95–25.47)a | 16.27 (11.62–21.87)b | 11.01 (5.43–17.23)c |
| Total soaped C12:0 | 0.38 (0.13–0.58)a | 0.17 (0.07–0.34)b | 0.02 (0.01–0.05)c |
| Total soaped C14:0 | 0.59 (0.27–0.85)a | 0.33 (0.17–0.45)b | 0.06 (0.03–0.18)c |
| Total soaped C16:0 | 9.36 (4.65–14.75)a | 5.95 (3.19–7.52)b | 1.60 (0.83–3.28)c |
| Total soaped C18:0 | 2.60 (1.86–3.37)a | 2.36 (1.78–3.44)a | 1.43 (0.67–2.77)b |
| Total soaped C18:1 | 1.20 (0.56–2.19)a | 1.30 (0.70–2.47)a | 0.53 (0.22–0.96)b |
| Total soaped C18:2 | 0.21 (0.08–0.33)a | 0.20 (0.09–0.35)a | 0.08 (0.05–0.25)b |
| Total soaped FAs | 15.19 (7.89–22.91)a | 12.17 (6.28–15.62)b | 4.05 (1.95–7.68)c |
| Data are expressed as median (25th percentile, 75th percentile) | |||
| Different letter represent significant difference (P < 0.05) | |||
| Calculated effect size | |||
| Control vs INFAT | Control vs BF | INFAT vs BF | |
| Total C12:0 | −0.236 | −0.561 | −0.490 |
| Total C14:0 | −0.260 | −0.514 | −0.445 |
| Total C16:0 | −0.256 | −0.494 | −0.369 |
| Total C18:0 | −0.006 | −0.149 | −0.129 |
| Total C18:1 | −0.158 | −0.088 | −0.039 |
| Total C18:2 | −0.156 | −0.211 | −0.097 |
| Total FAs | −0.120 | −0.290 | −0.218 |
| Total soaped C12:0 | −0.218 | −0.550 | −0.469 |
| Total soaped C14:0 | −0.272 | −0.530 | −0.444 |
| Total soaped C16:0 | −0.279 | −0.524 | −0.402 |
| Total soaped C18:0 | −0.057 | −0.302 | −0.234 |
| Total soaped C18:1 | −0.042 | −0.282 | −0.313 |
| Total soaped C18:2 | −0.014 | −0.190 | −0.205 |
| Total soaped FAs | −0.196 | −0.475 | −0.362 |
BF = breast-fed; FA = fatty acid; INFAT = SN2-palmitate.
The breast-fed infants exhibited the best nutrient absorption because they had a significantly lower stool dry weight, lower stool lipid levels, and reduced saponified fat excretion compared with both formula groups (P < 0.01) (Table 2 and Fig. 2).
Analysis of the stool dry weight without the lipid fraction also revealed significant differences between the formula groups, with a lower weight observed in the SN2-palmitate group compared with that in the control group (3.31 g vs 5.58 g, P < 0.05). The BF group presented a significantly lower stool dry weight compared with those of both the SN2-palmitate and control groups.
DISCUSSION
In the present study, we assessed the effects of formulas containing high SN2-palmitate levels in addition to prebiotics on FA excretion in infants at 6 postnatal weeks of age, revealing significant differences in stool dry weight and FA excretion between the SN2-palmitate and control groups.
This study demonstrated that infant formula containing SN2-palmitate in addition to prebiotics was well tolerated, and the infants in this group exhibited a similar growth rate to that of the infants in the breast-fed group. As hypothesized in this randomized, controlled, double-blind study, FA excretion of Chinese term newborns who were fed SN2-palmitate formula was significantly lower than that of newborns who were fed control formula. FA excretion of breast-fed term newborns was lower compared with that of newborns fed either of the formulas, emphasizing the superiority of human milk absorption. These data are consistent with those of previous publications that have demonstrated the beneficial effect of SN2-palmitate on FA excretion and absorption in Western infants (15–19). This study, however, assessed the effects of SN2-palmitate in addition to those of oligosaccharides included in 2 formulas on infants from a Chinese population. Prebiotics have been routinely added to infant formulas in recent years. A prebiotic is a nondigestible food ingredient that has several potential beneficial effects on neonatal intestinal development, including protection against infection, and facilitation of nutrient absorption (26). GOS are carbohydrates consisting of chains that include between 2 and 7 galactose units and a terminal glucose unit. They are enzymatically produced from lactose by the food industry and are widely used in infant nutritional formulations to mimic the biological functions of human milk oligosaccharides, including their effects on gut microbiota and the immune system. Studies have demonstrated that formulas containing prebiotics may have beneficial effects on neonatal intestinal development, digestion, and nutrient absorption, although contradicting data have been reported on calcium absorption and on whether these formulas protect against gastrointestinal infection (27–30). This study demonstrated that the improved FA absorption of the infants who were fed the SN2-palmitate formula was maintained even with the addition of prebiotics to the formula.
The infants in this study consumed ∼30 g of fat/day, which is in accordance with previous reports in the literature (9). The absorption of fat in term infants has been reported to be >90%, which was also demonstrated in this study. The absorption of FAs from vegetable oils by infants decreases with increasing FA chain length and with an increasing degree of unsaturation (9). Calculation of the percent of fat absorption based on the amount of formula consumed and the amount of fat excreted in the stool revealed only slight and not clinically significant differences between the formula groups (96% vs 97.4% in the control and SN2-palmitate groups, respectively); however, the difference in the percent of PA absorbed was more significant (88% vs 95% in the control and SN2-palmitate groups, respectively). Assuming that the breast-fed infants consumed a similar amount of fat, absorption was increased in those infants (99% of total fat and 98.5% of total PA). Thus, the differences in total fat excretion detected in this study did not result in a significant clinical effect, but the effect was more prominent for specific FAs.
The difference in the amount of excreted PA and other FAs because of the positioning of the PA on the TG is interesting especially in view of the relatively low presence of the pancreatic lipase, which is the 1,3 stereospecific lipase in infants. Roman et al (31) demonstrated the effect of the gastric lipase HGL, a lipase with a high stereospecificity for the hydrolysis of ester bonds at the sn3 position of glycerol, on the free FAs release in the stomach and revealed that palmitic (C16:0) and oleic (C18:1 n-9) were the main FFA released in the stomach. The release of the long chain FAs in the stomach might also enhance pancreatic secretion and pancreatic lipase activity (31). Furthermore, Johnson et al (32) demonstrated that the activity of pancreatic lipase–related protein 2 was enhanced by predigestion of both human milk lipids and infant formula lipids by gastric lipase, and the activity of carboxyl ester lipase was stimulated 11-fold following gastric lipase predigestion.
In line with previously published data (16), we also demonstrated that the majority of lipids were excreted in the form of calcium soaps. Because PA is the major saturated FA in infant formula and human milk and because it is primarily absorbed as 2-monoglycerol (10), it is not surprising that it was also found to be the major FA excreted in total fat and in the form of calcium soaps. The proportions of PA and stearic acid in excreted FAs were much higher compared with their amounts consumed in the infants’ diets, emphasizing the importance of their positions on the TG. Interestingly, infants who received the SN2-palmitate formula exhibited reduced excretion of saturated FAs, although only the PA TG position was significantly modified. The association between the prevention of palmitate soap formation and an improved absorption of other saturated FAs has been noted previously, and it has been suggested that the formation of palmitate soaps may reduce the absorptive capacity of the gut by binding bile salts or by acting as a solvent for other FAs (16). We found no significant effect of SN2-palmitate on the daily excretion of unsaturated FAs. The proportions of oleic (18:1) and linoleic (18:2n6) acids in the stool samples were the same for both formula groups. This finding is in accordance with a previous publication by Kennedy et al (16), who also demonstrated no significant difference in the excretion of these FAs.
Human milk is the natural and optimal form of nutrition for infants up to the age of 6 months (1). The results of this study further emphasize that nutrients are optimally absorbed from human milk, as expressed by the lower stool dry weight and stool total FA level of the breast-fed infants compared with those of the formula-fed infants.
Our results also revealed markedly reduced stool dry weights in the SN2-palmitate and breast-fed groups at 6 postnatal weeks. Differences in the stool dry weights between the treatment groups may indicate potential dissimilarities in nutrient absorption in general. This finding is of major interest because the existing studies of SN2-palmitate have primarily focused on fat and mineral excretion and to some extent also absorption and have not examined the possible effects of this compound on the absorption of other nutrients. In future studies, the effects of SN2-palmitate on the absorption of additional nutrients should be investigated.
In conclusion, the results of our study demonstrate that infant formula containing SN2-palmitate in addition to prebiotics reduces fat excretion during the first weeks of life. These findings emphasize the importance of the lipid component in infant formulas and suggest that optimal nutrient absorption from human milk may be achieved in part because of the structures of the specific lipids present in this milk.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Dr Z. Li from the Department of Pediatrics, Peking University Third Hospital, Beijing, China, Dr H. Cui from the Pediatric Ward, Beijing Friendship Hospital Affiliated with Capital University of Medical Science, Beijing, China, Dr A. Zhang from the Department of Pediatrics, The People's Hospital of Hunan Province, Changsha, China, Dr H. Yang from the Department of Child Health, West China Second University Hospital of Sichuan University, Chengdu, China, and Dr J. Wu from the Department of Pediatrics, Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China for acting as clinical investigators and for providing care to the study patients. We thank Prof Raanan Shamir from the Institute of Gastroenterology, Nutrition and Liver Diseases, Schneider Children's Medical Center of Israel, for scientific support. We also thank Diklah Geva from IntegriStat, Biostatistics Services, Tel Aviv, for statistical support.
On Pap
At the request of Louis XV, who was interested in the welfare of children, Parisian Joseph Raulin (1708–1784) wrote De la Conservation des Enfans in 1768. It was a compilation of extant wisdom on child care, and in a chapter entitled “The Nourishment of Infants Without Recourse to Milk,” he described the different methods used to prepare pap (a soft food for infants) in Europe:

Germans… have no difficulty in nourishing their children from their birth with pap composed of the milk of cows or sheep and of barley flour…It is much employed in Upper Germany and in Switzerland and even in France. They give the child this nourishment every four hours and make it drink between times. The most healthy drink, which these people serve is water in which they boil the rasping of a deer horn or ivory and anise seeds.∗ When the ordinary pap seems to incommode the children on account of its sourness and glutinosity, it is replaced by meat juice, the yelk [sic] of egg and bread or with toast reduced to powder…. Van Helmont∗∗ … advises to nourish infants from their birth, in place of milk, on a pap made of bread boiled in small beer, which he sweetens with sugar or honey and reduces to a jelly.… One sees it in Holland and Denmark … There is little difference between the paps made with beer and those made with water, as the volatile part of the beer is dissipated by the boiling and there rest in the pap only the aqueous part….
Frontispiece of De la Conservation des Infants. Note Athena, the Greek goddess of wisdom, hovering and cradling Knowledge (book) and Health (staff of Asclepius). Note also the mother on the right holding walking ribbons and the child wearing a pudding (a padded roll worn around a child's forehead to protect the head from a fall).
∗The raspings were most likely of horn velvet, believed to be rich in nutrients and promote rapid growth. Anise was used as an antispasmodic. Ivory and red coral were considered to prevent seizures.
∗∗Jan Baptist van Helmont (1580–1644) was an iatrochemist who believed digestion was a process of fermentation. He believed beer, a product of fermentation, provided a “predigested” substrate for infant nutrition.
—Contributed by Angel R. Colón, MD
Footnotes
Drs Bar-Yoseph and Lifshitz contributed equally to this work.
This study was supported by Enzymotec Ltd.
www.clinicaltrials.gov registration number: NCT01373541
The authors report no conflicts of interest.
REFERENCES
- 1.Agostoni C, Braegger C, Decsi T, et al. Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2009; 49:112–125. [DOI] [PubMed] [Google Scholar]
- 2.Giovannini M, Riva E, Agostoni C. Fatty acids in pediatric nutrition. Pediatr Clin North Am 1995; 42:861–877. [DOI] [PubMed] [Google Scholar]
- 3.Breckenridge WC, Marai L, Kuksis A. Triglyceride structure of human milk fat. Can J Biochem 1969; 47:761–769. [DOI] [PubMed] [Google Scholar]
- 4.Mu H, Hoy CE. The digestion of dietary triacylglycerols. Prog Lipid Res 2004; 43:105–133. [DOI] [PubMed] [Google Scholar]
- 5.Hamosh M, Scow RO. Lingual lipase and its role in the digestion of dietary lipid. J Clin Invest 1973; 52:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahamse E, Minekus M, van Aken GA, et al. Development of the digestive system-experimental challenges and approaches of infant lipid digestion. Food Dig 2012; 3:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manson WG, Weaver LT. Fat digestion in the neonate. Arch Dis Child Fetal Neonatal Ed 1997; 76:F206–F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattson FH, Volpenhein RA. The digestion and absorption of triglycerides. J Biol Chem 1964; 239:2772–2777. [PubMed] [Google Scholar]
- 9.Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr 2011; 2:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innis SM, Dyer R, Nelson CM. Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids 1994; 29:541–545. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CM, Innis SM. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am J Clin Nutr 1999; 70:62–69. [DOI] [PubMed] [Google Scholar]
- 12.Mattson FH, Volpenhein RA. The specific distribution of fatty acids in the glycerides of vegetable fats. J Biol Chem 1961; 236:1891–1894. [PubMed] [Google Scholar]
- 13.Small DM. The effects of glyceride structure on absorption and metabolism. Annu Rev Nutr 1991; 11:413–434. [DOI] [PubMed] [Google Scholar]
- 14.Quinlan PT, Lockton S, Irwin J, et al. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr 1995; 20:81–90. [DOI] [PubMed] [Google Scholar]
- 15.Carnielli VP, Luijendijk IH, Van Goudoever JB, et al. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J Pediatr Gastroenterol Nutr 1996; 23:553–560. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy K, Fewtrell MS, Morley R, et al. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr 1999; 70:920–927. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Lopez A, Castellote-Bargallo AI, Campoy-Folgoso C, et al. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum Dev 2001; 65 (suppl):S83–94. [DOI] [PubMed] [Google Scholar]
- 18.Carnielli VP, Luijendijk IH, van Goudoever JB, et al. Feeding premature newborn infants palmitic acid in amounts and stereoisomeric position similar to that of human milk: effects on fat and mineral balance. Am J Clin Nutr 1995; 61:1037–1042. [DOI] [PubMed] [Google Scholar]
- 19.Lucas A, Quinlan P, Abrams S, et al. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Arch Dis Child Fetal Neonatal Ed 1997; 77:F178–F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-Yoseph F, Lifshitz Y, Cohen T. Review of sn-2 palmitate oil implications for infant health. Prostaglandins Leukot Essent Fatty Acids 2013; 89:139–143. [DOI] [PubMed] [Google Scholar]
- 21.Litmanovitz I, Davidson K, Eliakim A, et al. High beta-palmitate formula and bone strength in term infants: a randomized, double-blind, controlled trial. Calcif Tissue Int 2013; 92:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaron S, Shachar D, Abramas L, et al. Effect of high beta-palmitate content in infant formula on the intestinal microbiota of term infants. J Pediatr Gastroenterol Nutr 2013; 56:376–381. [DOI] [PubMed] [Google Scholar]
- 23.Litmanovitz I, Bar-Yoseph F, Lifshitz Y, et al. Reduced crying in term infants fed high beta-palmitate formula: a double-blind randomized clinical trial. BMC Pediatr 2014; 14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugambi MN, Musekiwa A, Lombard M, et al. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J 2012; 11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 2008; 104:305–344. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Massey FJ. Introduction to Statistical Analysis. 4th ed. 1983; New York: McGraw-Hill, 281–4. [Google Scholar]
- O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics 1984; 40:1079–1087. [PubMed] [Google Scholar]
- 26.Calder PC, Etschmann SK, de Jong EC, et al. Early nutrition and immunity: progress and perspectives. Br J Nutr 2006; 96:774–790. [PubMed] [Google Scholar]
- 27.Fanaro S, Boehm G, Garssen J, et al. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr Suppl 2005; 94:22–26. [DOI] [PubMed] [Google Scholar]
- 28.Vandenplas Y. Oligosaccharides in infant formula. Br J Nutr 2002; 87 suppl 2:S293–S296. [DOI] [PubMed] [Google Scholar]
- 29.Overduin J, Schoterman MH, Calame W, et al. Dietary galacto-oligosaccharides and calcium: effects on energy intake, fat-pad weight and satiety-related, gastrointestinal hormones in rats. Br J Nutr 2013; 109:1338–1348. [DOI] [PubMed] [Google Scholar]
- 30.van den Heuvel EG, Schaafsma G, Muys T, et al. Nondigestible oligosaccharides do not interfere with calcium and nonheme-iron absorption in young, healthy men. Am J Clin Nutr 1998; 67:445–451. [DOI] [PubMed] [Google Scholar]
- 31.Roman C, Carriere F, Villeneuve P, et al. Quantitative and qualitative study of gastric lipolysis in premature infants: do MCT-enriched infant formulas improve fat digestion? Pediatr Res 2007; 61:83–88. [DOI] [PubMed] [Google Scholar]
- 32.Johnson K, Ross L, Miller R, et al. Pancreatic lipase-related protein 2 digests fats in human milk and formula in concert with gastric lipase and carboxyl ester lipase. Pediatr Res 2013; 74:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


