Abstract
Objectives:
To analyse trends during 1980–2011 in hypertension prevalence and SBP/DBP by sex in Fiji Melanesian (i-Taukei) and Indian adults aged 25–64 years; and to assess effects of BMI.
Methods:
Unit record data from five population-based surveys were included (n = 14 191). Surveys were adjusted to the nearest previous census to improve national representativeness. Hypertension was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg and/or on medication for hypertension. Regression (Poisson and linear) was used to assess period trends.
Results:
Over 1980–2011 hypertension prevalence (%) and mean blood pressure (BP) (SBP/DBP mmHg) increased significantly (P < 0.001) in both sexes and ethnicities. Increases in hypertension were: from 16.2 to 41.3% in i-Taukei men (mean BP from 122/73 to 135/81); from 20.5 to 37.8% in Indian men (mean BP from 122/74 to 133/81); from 25.9 to 36.9% in i-Taukei women (mean BP from 126/76 to 132/81); and from 17.6 to 33.1% in Indian women (mean BP 117/71 to 130/81). The age-adjusted trend in hypertension and mean BP (over 32 years) declined after adjusting for BMI, with effects of obesity greater in women than men, and in Indians than i-Taukei. BMI explained 45% of the age-adjusted increase in DBP over the period in Indians (both sexes), and 16% (men) and 38% (women) in i-Taukei.
Conclusion:
Significant increases have occurred in hypertension prevalence and SBP/DBP in both sexes and ethnicities of Fiji during 1980–2011 with no indication of decline, contributing to significant premature mortality from cardiovascular disease.
Keywords: age, blood pressure, BMI, Fiji, hypertension, obesity, risk factors
INTRODUCTION
The Republic of Fiji Islands forms part of Melanesia in the South Pacific and is comprized of more than 330 islands. More than 96% of the population reside in the two main islands of Viti Levu and Vanua Levu [1]. After Papua New Guinea, Fiji has the largest population of the Pacific Island states with 837 000 at the 2007 census. The population is composed of 56.8% i-Taukei (formerly indigenous Fijians, Fiji Melanesians, or Fijians); 37.5% Fijians of Indian descent (formerly Indo–Fijians or Indians); and 5.7% ‘Others’, consisting of Chinese, European, part-European, Rotuman, other Pacific Islanders, and all other nationalities [1]. Since the mid-twentieth century, Fiji has experienced the demographic and epidemiological transitions, including declines in mortality, particularly infant and under-five deaths, and a change in major causes of death from infection/under-nutrition to noncommunicable disease [2,3]. Plateaux in life expectancy has occurred since the 1990s from increases in premature adult mortality [3–5], with cardiovascular disease (CVD) now the leading cause of death in Fiji [3]. Proportional mortality from CVD increased from around 20% in the 1960s to over 45% by 2010 [6].
Reduction in CVD mortality can be achieved through risk factor reduction in individuals and populations, including interventions targeting population groups identified at high-risk. A recent assessment of the Fiji health systems’ response to noncommunicable disease found limited evidence of health promotion targeting high-risk groups [7]. Reductions in premature adult mortality from decline in CVD occurred from the 1970s in Northern Europe, North America, Australia, and New Zealand [8], and led to significant increases in life expectancy in these populations. In Australia and New Zealand 80% of the CVD mortality decline has been associated with declines in population risk factors, including hypertension [9,10].
The evolution of risk factor prevalence underpinning the epidemiologic transition in Fiji has not been well documented. To that end, the present study analyses trends in hypertension prevalence, SBP and DBP in Fiji by sex and ethnicity from five population-based cross-sectional surveys conducted in Fiji over a 32-year period (1980–2011), using a standardized definition of hypertension and methodology for analysis. The present study enables assessment of population trends and evaluation of the effects of prevention and control interventions for blood pressure (BP) reduction over the past 32 years in Fiji. Population groups at increased risk of CVD because of increased hypertension prevalence, SBP, and DBP are also identified to assist policy and planning of targeted prevention and control interventions.
METHODS
Survey selection
Surveys that measured BP in adults aged 25–64 years in Fiji during 1950–2014 were identified through: a literature search of Medline, PubMed, and Global Health; an internet search; and direct contact with representatives from the Fiji Ministry of Health, the Fiji National Food and Nutrition Centre, the WHO, and the Secretariat for the Pacific Community. Surveys were included in this analysis if they were nationally representative at the time of the survey, or could be adjusted to the nearest previous census (by age, urban/rural residency, and ethnicity) to improve representativeness and minimize selection bias, as implemented in the WHO STEPS survey methodology [11]. Participants aged 25–64 years from the two major ethnicities in Fiji, i-Taukei, and Fijians of Indian descent (Indians), were included in the analysis. The ‘Other’ ethnicity was not included as it is a heterogeneous group consisting of other Pacific Islanders, Europeans, and Asians, and comprized only 5% of the total Fiji population at the 2007 census [1]. Surveys included in analyses are: the 1980 National Diabetes and Cardiovascular Disease Survey (n = 1,999) [12], the National Nutrition Surveys (NNS): 1993 (n = 1750) and 2004 (n = 3183) [13,14], and the WHO STEPS Surveys: 2002 (n = 4836) and 2011 (n = 2423) [11].
Several BP surveys undertaken in Fiji were not included in the current analysis because they measured BP in one sex only [15], in a specific and often narrow age group [15,16], one ethnicity only [15–20], in an exclusive or undefined urban/rural population [15,18–21], or survey results were only available as a broad summary [22]. These surveys were excluded because they were not nationally representative, and it was not possible to adjust their data to the nearest previous census (by age, ethnicity, and rural/urban distributions for each sex).
Data collection
The 1980 survey measured BP manually with random zero mercury sphygmomanometers; the 1993 NNS used manual aneroid sphygmomanometers; the 2002 STEPS, 2004 NNS, and 2011 STEPS surveys used digital aneroid automatic BP machines. All surveys took two BP measurements from participants who were seated for 10 minutes; the 1980 survey and 2002 and 2011 STEPS surveys took a third BP reading if the difference between measurements was at least 10 mmHg. The 1980 survey and the 2002 and 2011 STEPS surveys recorded all BP measurements, whereas the 1993 and 2004 NNS surveys recorded only the second BP measurement. The NNS BP measurements are adjusted so that analysis of all surveys is based on the average of the first and second BP reading. Adjustment of BP from the NNS involved using unit record data from the 2002 STEPS to derive predictive models from linear regression to estimate the first BP measurement (y variable) from the second BP measurement, accounting for age, BMI, urban-rural, and ethnicity (x variables), separately for each sex. The R-Square of the SBP model was 95% for men and 97% for women; the R-Square of the DBP model was 95% for men and women. These models were employed to predict the average of the first and second BP measurement for each patient in the 1993 and 2004 NNS data, and resulted in a predicted first SBP measurement ≈1–2 mmHg higher than the second measurement; and a predicted first DBP measurement ≈0–1 mmHg lower or higher than the second measurement (by sex and ethnicity). Hypertension was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg and/or taking medication for hypertension (self-reported).
Demographic adjustment and trend analyses
To improve national representativeness and minimize selection bias, each survey was adjusted to the most recent previous census for age group and urban–rural distributions by ethnicity and sex using case weights derived from the ratio of the proportions from the census and the survey for each stratum. This is the same methodology used in the WHO STEPS surveys [11]. Each survey was also age standardized to the 2007 census population to control for the effect of changes in the age structure of the population on BP means and prevalences during 1980–2011. Pregnant women were excluded from analyses. A combined unit record data set was constructed consisting of the five concatenated surveys (n = 14 191). Poisson regression (without offset) was used to analyse trends in hypertension prevalence over period, and linear regression was used to analyse mean SBP and DBP over period. Poisson regression (without offset) was used to investigate the effect of changes in BMI on hypertension prevalence over 1980 and 2011 (age-adjusted) by comparing the period trend in the relative risk (RR) of hypertension after adjusting for age, with that of a model adjusting for age, and BMI (as continuous variables). Increases in mean SBP and DBP over the period were analyzed in the same fashion using linear regression. SPSS version 22 was used for the adjustment of BP sequencing in 1993 and 2004 NNS (IBM Corp., New York, USA); SAS version 9.4 was used for the regression analysis (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Hypertension prevalence
Estimates of hypertension prevalence weighted to be nationally representative for age and urban/rural distributions (by ethnicity and sex) at the time of each survey indicate that from 1980 to 2011 there was a statistically significant increase (P < 0.001) in the period trend in hypertension prevalence in i-Taukei and Indian men and women (Table 1 and Fig. 1), and in men and women overall (both ethnicities combined) (Table 1 and Fig. 2).
TABLE 1.
Hypertension prevalence (%) by sex and ethnicity, Fiji, 1980–2011
| Hypertension prevalence % (95% confidence interval) | ||||||
| Year | N | Previous census | 2007 census | N | Previous census | 2007 census |
| i-Taukei men | i-Taukei women | |||||
| 1980 | 502 | 16.2 (13.0–19.4) | 18.6 (15.2–22.0) | 507 | 25.9 (22.1–29.7) | 27.8 (23.9–31.7) |
| 1993 | 399 | 22.3 (18.2–26.3) | 22.9 (18.8–27.0) | 445 | 21.5 (17.7–25.3) | 23.0 (19.1–26.9) |
| 2002 | 1212 | 25.8 (23.4–28.3) | 26.1 (23.7–28.6) | 1581 | 27.4 (25.2–29.6) | 28.0 (25.8–30.2) |
| 2004 | 829 | 39.9 (36.5–43.2) | 39.8 (36.5–43.1) | 981 | 39.3 (36.3–42.4) | 39.9 (36.9–43.0) |
| 2011 | 599 | 41.3 (37.3–45.2) | 41.2 (37.2–45.1) | 761 | 36.9 (33.4–40.3) | 36.2 (32.8–39.6) |
| 5-year changea | +3.83* (3.07–4.59) | +3.43* (2.64–4.22) | +2.06* (1.24–2.88) | +1.71* (0.86–2.56) | ||
| Indian men | Indian women | |||||
| 1980 | 469 | 20.5 (16.9–24.2) | 25.8 (21.8–29.8) | 521 | 17.6 (14.4–21.0) | 25.3 (21.5–29.0) |
| 1993 | 460 | 19.4 (15.8–23.0) | 24.4 (20.5–28.4) | 446 | 19.8 (16.1–23.5) | 25.9 (21.8–30.0) |
| 2002 | 759 | 21.7 (18.7–24.6) | 23.2 (20.2–26.2) | 1284 | 23.6 (21.2–25.9) | 25.4 (23.0–27.8) |
| 2004 | 652 | 33.2 (29.6–36.8) | 34.8 (31.1–38.4) | 721 | 32.5 (29.1–36.0) | 34.9 (31.4–38.4) |
| 2011 | 467 | 37.8 (33.4–42.2) | 39.1 (34.7–43.6) | 596 | 33.1 (29.3–36.8) | 33.6 (29.8–37.4) |
| 5-year changea | +2.34* (1.52–3.16) | +1.68* (0.78–2.59) | +2.42* (1.67–3.17) | +1.28* 0.04–2.14) | ||
| All men | All women | |||||
| 1980 | 971 | 18.4 (16.0–20.9) | 21.8 (19.2–24.4) | 1028 | 21.6 (19.1–24.1) | 26.7 (24.0–29.4) |
| 1993 | 859 | 20.7 (18.0–23.5) | 23.6 (20.8–26.4) | 891 | 20.6 (18.0–23.3) | 24.3 (21.5–27.1) |
| 2002 | 1971 | 23.8 (21.9–25.7) | 24.9 (23.0–26.8) | 2865 | 25.6 (24.0–27.2) | 26.8 (25.2–28.5) |
| 2004 | 1481 | 36.6 (34.2–39.1) | 37.6 (35.1–40.1) | 1702 | 36.0 (33.7–38.3) | 37.7 (35.4–40.0) |
| 2011 | 1066 | 39.7 (36.8–42.7) | 40.3 (37.3–43.2) | 1357 | 35.2 (32.6–37.7) | 35.1 (32.5–37.6) |
| 5-year changea | +3.08* (2.52–3.64) | +2.65* (2.05–3.24) | +2.28* (1.72–2.83) | +1.52* (0.91–2.12) | ||
Hypertension (SBP ≥ 140 and/or DBP ≥ 90 mmHg and/or taking medication for hypertension). N, number of participants in stratum; previous census, data adjusted to the most recent previous census for age group and urban–rural distributions by ethnicity and sex, to improve representativeness; 2007 census, data age-adjusted to the 2007 census to control for age confounding.
aRepresents the change in prevalences from Poisson regression (without offset) in each 5-year period.
*P < 0.001; 95% confidence interval.
FIGURE 1.
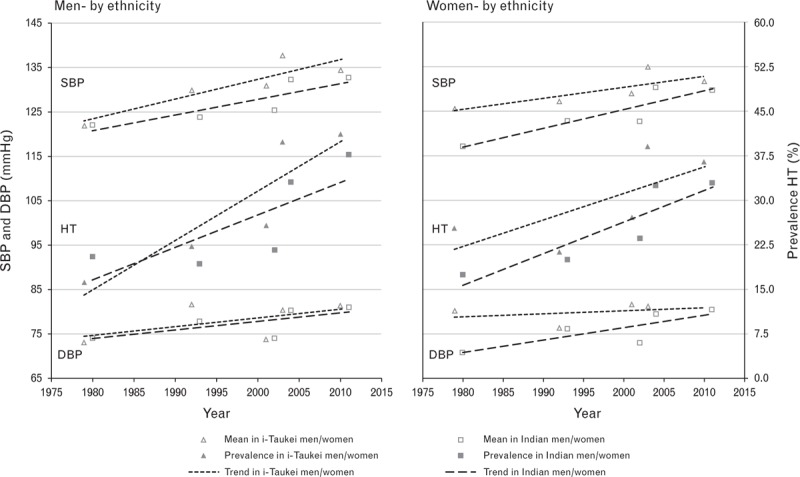
Mean SBP/DBP, and hypertension prevalence, by sex and ethnicity, Fiji, 1980–2011+. HT (hypertension = SBP ≥ 140 and/or DBP ≥90 mmHg and/or taking medication for HT); +data adjusted to the most recent previous census for age group and urban–rural distributions by ethnicity and sex.
FIGURE 2.
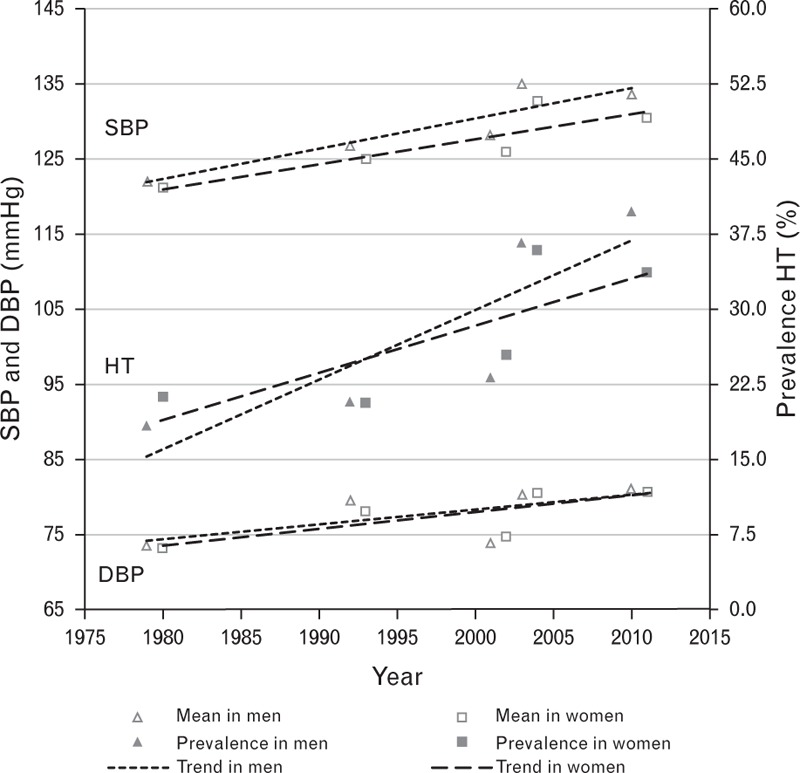
Mean SBP/DBP, and hypertension prevalence, by sex, Fiji, 1980–2011+. HT (hypertension = SBP ≥ 140 and/or DBP ≥ 90 mmHg and/or taking medication for HT); +data adjusted to the most recent previous census for age group, urban-rural distributions and ethnicity by sex.
The magnitude of the period trend in hypertension prevalence declined slightly after age-adjustment by 0.8% in i-Taukei men and by 0.6% in i-Taukei women, whereas the decline was larger in Indian men by 16.7% and in Indian women by 24.9%; all declines were statistically significant at P < 0.001 (Table 3). The increasing period trend in hypertension prevalence remained statistically significant (P < 0.001) in both sexes and ethnicities after age-adjustment.
TABLE 3.
Effects of age and BMI on trends in hypertension prevalence and mean blood pressure by sex and ethnicity, Fiji, 1980–2011a
| i-Taukei men | i-Taukei women | Indian men | Indian women | |
| RR for hypertension over 32 years (1980–2011) | ||||
| RR (95%CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 1. Period | 2.75 (2.17–3.50) | 1.59 (1.30–1.95) | 2.02 (1.57–2.60) | 2.02 (1.59–2.56) |
| 2. Period and age | 2.73 (2.15–3.46) | 1.58 (1.29–1.93) | 1.68 (1.31–2.16) | 1.51 (1.19–1.92) |
| Proportional decline in RR (1–2)a | 0.8% | 0.6% | 16.7% | 24.9% |
| 3. Period, age and BMI | 2.51 (1.97–3.18) | 1.44 (1.17–1.77) | 1.36 (1.06–1.74) | 1.17 (0.92–1.48) |
| Proportional decline in RR (2–3)a | 8.1% | 8.8% | 19.5% | 22.8% |
| Increase in mean SBP (mmHg) over 32 years (1980–2011) | ||||
| BP (95%CI) | BP (95% CI) | BP (95% CI) | BP (95% CI) | |
| 1. Period | 13.95 (12.09–15.82) | 7.17 (4.90–9.45) | 11.17 (9.15–13.19) | 12.70 (10.33–15.08) |
| 2. Period and age | 13.90 (12.07–15.72) | 7.11 (5.07–9.15) | 9.52 (7.58–11.46) | 9.02 (6.92–11.12) |
| Proportional decline in BP (1–2)a | 0.4% | 0.8% | 14.7% | 29.0% |
| 3. Period, age and BMI | 12.82 (11.04–14.59) | 5.33 (3.33–7.34) | 6.18 (4.91–8.71) | 5.51 (3.42–7.60) |
| Proportional decline in RR (2–3)a | 7.8% | 25.0% | 28.5% | 38.9% |
| Increase in mean DBP (mmHg) over 32 years (1980–2011) | ||||
| BP (95%CI) | BP (95% CI) | BP (95% CI) | BP (95% CI) | |
| 1. Period | 5.89 (4.58–7.20) | 4.23 (2.91–5.55) | 5.66 (4.23–7.08) | 8.37 (6.95–9.79) |
| 2. Period and age | 5.85 (4.57–7.14) | 4.21 (2.93–5.48) | 4.76 (3.37–6.16) | 6.83 (5.49–8.17) |
| Proportional decline in BP (1–2)a | 0.6% | 0.5% | 15.8% | 18.4% |
| 3. Period, age and BMI | 4.91 (3.68–6.14) | 2.63 (1.41–3.85) | 2.55 (1.20–3.90) | 3.83 (2.52–5.13) |
| Proportional decline in BP (2–3)a | 16.2% | 37.5% | 46.5% | 44.0% |
1–3, regression models; BP, blood pressure; RR, relative risk.
aProportional decline from previous model.
Mean SBP and DBP
Estimates of mean SBP and DBP weighted to be nationally representative for age and urban/rural distributions (by ethnicity and sex) at the time of each survey indicate that from 1980 to 2011 there was a statistically significant increase (P < 0.001) in the period trend in mean SBP and DBP in i-Taukei and Indian men and women (Table 2 and Fig. 1), and in men and women overall (both ethnicities combined) (Table 2 and Fig. 2).
TABLE 2.
Mean SBP and DBP (mmHg) by sex and ethnicity, Fiji, 1980–2011
| Mean SBP (95% CI) | Mean DBP (95% CI) | Mean SBP (95%CI) | Mean DBP (95%CI) | |||||||
| Year | N | Previous census | 2007 census | Previous census | 2007 census | N | Previous census | 2007 census | Previous census | 2007 Census |
| i-Taukei men | i-Taukei women | |||||||||
| 1980 | 502 | 121.9 (120.4–123.3) | 123.2 (121.7–124.7) | 73.0 (72.0–74.0) | 74.5 (73.4–75.5) | 507 | 126.0 (124.2–127.8) | 126.8 (124.9–128.7) | 76.2 (75.1–77.3) | 76.7 (75.5–77.8) |
| 1993 | 399 | 129.9 (128.5–131.2) | 130.2 (128.8–131.5) | 81.5 (80.6–82.4) | 81.6 (80.7–82.5) | 445 | 127.1 (125.6–128.6) | 127.6 (126.1–129.1) | 80.2 (79.4–81.0) | 80.5 (79.6–81.3) |
| 2002 | 1212 | 130.8 (129.9–131.8) | 130.9 (130.0–131.8) | 73.7 (73.0–74.3) | 73.8 (73.1–74.4) | 1581 | 129.1 (128.1–130.2) | 129.4 (128.4–130.4) | 76.6 (75.9–77.2) | 76.7 (76.1–77.3) |
| 2004 | 829 | 137.7 (136.5–138.9) | 137.7 (136.5–138.9) | 80.3 (79.5–81.2) | 80.1 (79.3–81.0) | 981 | 135.1 (133.7–136.6) | 135.5 (134.0–136.9) | 81.8 (80.9–82.6) | 81.8 (81.0–82.7) |
| 2011 | 598 | 134.5 (133.0–136.0) | 134.2 (132.8–135.7) | 81.3 (80.4–82.2) | 81.3 (80.4–82.2) | 761 | 131.9 (130.1–133.5) | 131.7 (130.0–133.4) | 81.4 (80.5–82.3) | 81.1 (80.2–82.0) |
| 5-year changea | +2.25* (1.95–2.55) | 2.01* (1.71–2.31) | +0.95* (0.74–1.16) | +0.70* (0.49–0.91) | +1.21* (0.85–1.57) | +1.06* (0.70–1.43) | +0.70* (0.49–0.92) | +0.60* (0.39–0.82) | ||
| Indian men | Indian women | |||||||||
| 1980 | 469 | 122.0 (120.2–123.8) | 124.4 (122.4–126.3) | 74.1 (72.8–75.4) | 75.9 (74.6–77.2) | 521 | 117.1 (115.3–119.0) | 120.4 (118.4–122.4) | 70.8 (69.7–71.9) | 72.2 (71.1–73.4) |
| 1993 | 460 | 123.8 (122.4–125.1) | 125.0 (123.5–126.4) | 77.8 (76.8–78.7) | 78.5 (77.5–79.5) | 446 | 122.7 (121.1–124.4) | 125.6 (123.8–127.4) | 76.1 (75.1–77.1) | 77.4 (76.4–78.5) |
| 2002 | 759 | 125.4 (124.2–126.5) | 125.8 (124.6–127.0) | 74.0 (73.1–74.8) | 74.2 (73.3–75.3) | 1284 | 122.7 (121.6–123.9) | 123.6 (122.4–124.8) | 73.1 (72.4–73.8) | 73.4 (72.7–74.1) |
| 2004 | 652 | 132.2 (130.8–133.6) | 132.8 (131.4–134.3) | 80.2 (79.3–81.2) | 80.6 (79.7–81.5) | 721 | 130.3 (128.5–132.0) | 131.5 (129.8–133.3) | 79.5 (78.5–80.5) | 80.1 (79.1–81.1) |
| 2011 | 466 | 132.7 (131.1–134.3) | 133.0 (131.4–134.7) | 80.9 (79.8–81.9) | 81.3 (80.3–82.4) | 596 | 130.0 (128.1–131.9) | 130.5 (128.6–132.4) | 80.6 (79.6–81.7) | 80.7 (79.7–81.7) |
| 5-year changea | +1.80* (1.46–2.16) | +1.45* (1.11–1.79) | +0.90* (0.67–1.13) | +0.64* (0.41–0.88) | +2.05* (1.67–2.43) | +1.55* (1.16–1.95) | +1.35* (1.13–1.58) | +1.11* (0.88–1.34) | ||
| All men | All women | |||||||||
| 1980 | 971 | 121.9 (120.8–123.1) | 123.7 (122.5–125.0) | 73.5 (72.7–74.4) | 75.2 (74.3–76.0) | 1028 | 121.4 (120.1–122.7) | 123.6 (122.2–125.0) | 73.4 (72.6–74.2) | 74.4 (73.6–75.2) |
| 1993 | 859 | 126.7 (125.7–127.7) | 127.4 (126.3–128.4) | 79.5 (78.9–80.2) | 79.9 (79.3–80.6) | 891 | 124.8 (123.7–125.9) | 126.6 (125.5–127.8) | 78.0 (77.4–78.7) | 78.9 (78.3–79.6) |
| 2002 | 1971 | 128.2 (127.5–128.9) | 128.9 (128.2–129.7) | 73.8 (73.3–74.3) | 73.9 (73.4–74.4) | 2865 | 126.0 (125.3–126.8) | 126.8 (126.0–127.6) | 74.8 (74.4–75.3) | 75.2 (74.7–75.7) |
| 2004 | 1481 | 135.0 (134.1–135.9) | 135.6 (134.6–136.5) | 80.3 (79.7–80.9) | 80.3 (79.7–80.9) | 1702 | 132.7 (131.6–133.9) | 133.8 (132.7–134.9) | 80.6 (80.0–81.3) | 81.1 (80.5–81.7) |
| 2011 | 1064 | 133.7 (132.6–134.8) | 133.7 (132.6–134.8) | 81.2 (80.5–81.8) | 81.3 (80.6–82.0) | 1357 | 131.0 (129.8–132.3) | 131.2 (129.9–132.4) | 81.0 (80.4–81.7) | 80.9 (80.3–81.6) |
| 5-year changea | +2.04* (1.82–2.26) | +1.79* (1.56–2.01) | +0.92* (0.76–1.07) | +0.67* (0.51–0.82) | +1.69* (1.42–1.95) | +1.36* (1.09–1.63) | +1.06* (0.91–1.22) | +0.89* (0.73–1.04) | ||
N, number of participants in stratum; previous census, data adjusted to the most recent previous census for age group and urban-rural distributions by ethnicity and sex to improve representativeness; 2007 census, data age-adjusted to the 2007 census to control for age confounding. CI, confidence interval.
aRepresents the change in means in each 5-year period from linear regression.
*P < 0.001
The magnitude of the period increase in mean SBP declined slightly (<1%) after adjusting for age in i-Taukei men and women, and by 14.7% in Indian men and 29.0% in Indian women. The period increase in mean DBP also declined slightly (<1%) in i-Taukei men and women, and by 15.8% in Indian men and 18.4% in Indian women (Tables 2 and 3). All declines after age-adjustment were statistically significant at P < 0.001. The increasing period trend in mean SBP and DBP remained statistically significant (P < 0.001) in both sexes and ethnicities after age-adjustment.
Effect of BMI on period (1980–2011) trends in hypertension prevalence
The RR of hypertension prevalence for the 32-year period (1980–2011), after adjustment for age and BMI, compared with adjusting for age alone (i.e. the age-adjusted effect of obesity), declined by 8–23% for hypertension prevalence. The mean BP for the 32-year period (1980–2011), after adjustment for age and BMI, compared with adjusting for age alone (i.e. the age-adjusted effect of obesity) decreased by 8–39% for SBP and 16–47% for DBP. Effects of obesity assessed in this way were generally greater in women than men, in Indians than i-Taukei and for mean DBP rather than SBP (Table 3). All declines in hypertension prevalence and mean SBP and DBP after adjustment for BMI were statistically significant at P < 0.001. The period trend in the RR for hypertension prevalence, after adjusting for age and BMI, remained statistically significant (P < 0.001) in i-Taukei men and women, but in Indian men the statistical significance declined (P = 0.017) and in Indian women the trend was no longer statistically significant (P = 0.201). The period trend in mean SBP and DBP, after adjusting for age and BMI, remained statistically significant (P < 0.001) in both sexes and ethnicities.
DISCUSSION
Empirical survey data for Fijians aged 25–64 years, adjusted to be nationally representative for age group, sex, ethnicity, and urban–rural residence, demonstrate a statistically significant continued increase in hypertension prevalence, SBP, and DBP in both sexes of the i-Taukei and Indian populations during 1980–2011. The most affected population group is i-Taukei men who had the highest level of hypertension prevalence, SBP, and DBP in 2011, and had the greatest increase over 1980–2011 in hypertension prevalence and SBP. Among women, the i-Taukei population had the highest level of hypertension prevalence, SBP, and DBP in 2011; however, Indian women had the greatest increase during 1980–2011 in these measures. Analysis of men and women overall (both ethnicities combined) indicates that hypertension prevalence was higher in women in 1980, but the increase over time was greater in men and by the mid-1990s hypertension prevalence in men exceeded that in women, and the sex differential widened to 2011. Men had higher SBP than women, and the sex differential progressively widened to 2011 because of a greater rate of increase in men. DBP remained similar between men and women throughout 1980–2011. The increasing period trends in hypertension prevalence and BP means remained highly statistically significant after age-adjustment to the nearest previous census, and to the 2007 census, with only a small decline in the increase in the i-Taukei ethnicity, but larger decline in Indians because of their greater change in age structure over the period compared with i-Taukei.
Data on trends over time in CVD risk factors in a population are more informative than analysis of levels from a single cross-sectional survey. Previous attempts at comparing BP means and prevalences from cross-sectional surveys have been hindered by differences in the definition of hypertension, the recording and computation of the average BP measurements used in analysis, and the aggregations (age/sex/ethnicity/urban–rural) used for the presentation of tabulated results. For example, all published results from the 1980 survey of hypertension prevalence used a definition of SBP at least 160 and/or DBP at least 95 mmHg, whereas more recent surveys define hypertension as SBP at least140 and/or DBP at least 90 mmHg and/or taking hypertension medication. These differences in definition have made it difficult to accurately establish trends over time. Through access to unit record data, we have been able to apply a standardized definition of hypertension to all surveys (SBP≥140 and/or DBP≥90 mmHg and/or taking hypertension medication), and develop a consistent methodology for computation using the average of the first and second BP measurements in analyses.
Previous population studies of BP in Fiji dating back to the 1950s have identified an association between increasing age and increases in BP means and hypertension prevalence in both sexes and ethnicities [15,16,21,23]. Increased BP with increasing age has also been found in other Pacific Islands, including Samoa [24] and Tonga [25], and elsewhere, including Australia [26]. However, some previous studies of least modernized populations indicate no significant increase in BP with age in the Pacific, including the northern Cook Islands (Pukapuka) [27], Tokelau [28], Wallis Island [29], and elsewhere, including Brazil and Kenya [30], highlighting that increased BP is not an inevitable consequence of aging.
Since BP in Fiji increased with age in the period under investigation, changing proportions of the population in age groups over time can confound the relationship of BP to period. Age-adjusted analyses (to the 2007 census) of the concatenated surveys, compared with adjustment to the most recent previous census, resulted in small reductions in the increase in mean BP and hypertension prevalence in the i-Taukei ethnicity over the period (1980–2011), but larger reductions in Indians. The increasing period trends, however, remained statistically significant in both sexes and ethnicities after adjusting for age in both ways. The greater decline in Indians is likely associated with changes in age structure because of out-migration of young adults over the period. Between the 1976 and 2007 censuses, the proportion of Indians aged 25–34 years (in the 25–64-year age group) decreased by around 10% from 43.5 to 34.5% in men and 44.6 to 32.0% in women, and the proportion of 55–64 years, increased by around 5–6% from 9.4 to 13.7% in men and 9.2 to 15.3% in women [1,31]. The overall population remains relatively young, however, with approximately 48% of Fijians below age 25 years and only 6.5% above age 60 years at the 2007 census [32].
The relationship between greater urbanization and higher hypertension prevalence and mean BP has been identified in population studies in Fiji since the 1950s [15,16,23]. Urbanization of the Fiji population over previous decades has proceeded apace: 37% of the population was classified as urban at the 1976 census [31], and 51% by the 2007 census [1]. Continuation of present trends indicates that 61% of the population will be urban by 2030 [1]. As urbanization continues to increase, BP and hypertension prevalence will be expected to rise. Urbanization is a distant (or ultimate) risk factor in the causal chain, and intermediary risk factors, including salt intake [30] and obesity [33] contribute significantly to increases in BP and hypertension prevalence. No surveys in Fiji have adequately measured population sodium intake. The 1980 survey assessed salt intake through urinary sodium concentration and found mean levels were higher in urban groups (both sexes and ethnicities) compared with their rural counterparts, and higher in Indians (by sex and urban/rural distribution) than in i-Taukei [23]. Comparison with other Pacific islands around that time showed that the more rural areas and outer islands, based to a significant extent on a subsistence economy, had lower mean urinary sodium concentration than the more modernized population of Fiji [34]. The prevalence of obesity in both sexes and ethnicities in Fiji has increased over recent decades, with higher levels in women compared with men, and in i-Taukei compared to Indians [14].
The effect of obesity on period increases in hypertension prevalence and mean SBP and DBP was assessed by estimating the additional effect of BMI on the age-adjusted period effect. Effects of obesity assessed in this way were generally greater in women than men, in Indians than i-Taukei, and for DBP rather than SBP. The apparent lesser effect of BMI in males, particularly i-Taukei, may be because of differing fat/muscle ratios compared to females, particularly Indian. BMI accounted for almost half of the increase in mean DBP in Indians.
Although in many developed countries the majority of deaths from CVD in the 21st century occur in older adults aged 65 years and above [35], in Fiji there is significant premature adult mortality from CVD with over half of deaths occurring in adults aged 40–59 years [32]. The prematurity of these deaths is not only a health concern, but also an economic and development issue for Fiji [32].
The present study is the first to identify trends in hypertension prevalence and mean SBP and DBP in Fiji by sex and ethnicity from large cross-sectional surveys over a 32-year period (1980–2011) using a standardized definition of hypertension and methodology for analysis. The findings indicate that preventive approaches relating to obesity from energy intake and inadequate physical activity, reduction in salt intake, and identification and treatment of cases of hypertension in the Fiji population [32] have not yet led to a decrease in hypertension prevalence or SBP and DBP. Rather, trends demonstrate a continued increase over the last 32 years. Reduction in CVD risk factors, including BP, has been achieved in recent decades in populations, such as Australia, through primary and secondary prevention associated with reduction in population risk factor levels [9]. The present study indicates that the most affected population groups in Fiji by sex and ethnicity are i-Taukei men who had the highest levels of hypertension prevalence and mean SBP and DBP in 2011, and the greatest rates of increase in these measures during 1980–2011. Among women, the i-Taukei population had the highest levels of hypertension prevalence and mean SBP and DBP in 2011; however, Indian women had the greatest rate of increase in these measures during 1980–2011. Although public health interventions aimed at CVD risk factor reduction are being expanded across the entire Fijian population [32], additional interventions targeting high-risk population groups may be necessary. CVD risk factor analyses in Fiji that consider the entire population mask divergent trends in the major ethnic groups.
ACKNOWLEDGEMENTS
No part of the work contained in this manuscript has been previously presented, either wholly or in part.
Funding for this work was provided under the Australian Government Department of Foreign Affairs and Trade Australian Development Research Awards Scheme (Grant no: 66886). We acknowledge the funding support from the National Institutes of Health (NIH) for the 1980 National Diabetes and Cardiovascular Disease Survey (Grant Number DK-25446).
Conflicts of interest
There are no conflicts of interest.
Reviewers’ Summary Evaluations
Reviewer 1
Linhart and colleagues analyzed 32-year trends in mean blood pressure and hypertension prevalence in Fiji Melanesian and Indian adults using population-based surveys. They identified significant increases in mean blood pressure levels and hypertension prevalence during this time period. Furthermore, the most current estimates indicate that roughly 40% of men and 35% of women in Fiji have hypertension. While these data identify hypertension as an important public health challenge, information on hypertension awareness, treatment and control is currently unavailable. Such data is needed for the development of effective policies for population-level prevention and control of this condition.
Reviewer 2
Arterial pressure data obtained in five cross-sectional studies were reanalyzed and summarized. The data show a strong increase in mean systolic and mean diastolic pressure as well as hypertension prevalence in adults of the two ethnicities that make up the majority of the Fiji population. Stratification according to ethnicity and sex identified i-Taukei men as having experienced the greatest rise in arterial pressure and hypertension prevalence during the last 30 years. The reasons for this specific finding remain to be identified. This study provides important data that will aid in developing cardiovascular disease prevention strategies for the Fiji population.
Footnotes
Abbreviations: BPn, blood pressure; CVD, cardiovascular disease; NNS, National Nutrition Survey; RR, relative risk
REFERENCES
- 1.Fiji Islands Bureau of Statistics. Census 2007 Results: Population Size, Growth, Structure and Distribution. Statistical News No 45. Suva: Government of Fiji; 2008. [Google Scholar]
- 2.Collins VR, Dowse GK, Cabealawa S, Ram P, Zimmet PZ. High mortality from cardiovascular disease and analysis of risk factors in Indian and Melanesian Fijians. Int J Epidemiol 1996; 25:59–69. [DOI] [PubMed] [Google Scholar]
- 3.Carter K, Cornelius M, Taylor R, Ali SS, Rao C, Lopez A, et al. Mortality trends in Fiji. Aust N Z J Public Health 2011; 35:412–420. [DOI] [PubMed] [Google Scholar]
- 4.Linhart C, Carter K, Taylor R, Rao C, Lopez A. Mortality trends in Pacific Island States. Noumea: Secretariat of the Pacific Community; 2014. [Google Scholar]
- 5.Taylor R, Carter K, Naidu S, Linhart C, Azim S, Rao C, Lopez A. Divergent mortality trends by ethnicity in Fiji. Aust N Z J Public Health 2013; 37:509–515. [DOI] [PubMed] [Google Scholar]
- 6.Carter K, Cornelius M, Taylor R, Ali SS, Rao C, Lopez A, et al. An assessment of mortality estimates for Fiji, 1949–2008: findings and life tables. Health Information Systems Knowledge Hub. Documentation note series. Number 12; November 2010. [Google Scholar]
- 7.Snowdon W, Raj A, Waqa G, Kanungo A, Robinson H. Noncommunicable diseases and health system responses in Fiji. The University of Melbourne. Working paper series Number 34, August 2013. [Google Scholar]
- 8.Mirzaei M, Truswell AS, Taylor R, Leeder SR. Coronary heart disease epidemics: not all the same. Heart 2009; 95:740–746. [DOI] [PubMed] [Google Scholar]
- 9.Taylor R, Dobson A, Mirzaei M. Contribution of changes in risk factors to the decline of coronary heart disease mortality in Australia over three decades. Eur J Cardiovasc Prev Rehabil 2006; 3:760–768. [DOI] [PubMed] [Google Scholar]
- 10.Tobias M, Taylor R, Huang K, Mann S, Sharpe N. Did it fall or was it pushed? The contribution of trends in established risk factors to the decline in premature coronary heart disease mortality in New Zealand. Aust N Z J Public Health 2008; 32:117–125. [DOI] [PubMed] [Google Scholar]
- 11.Cornelius M, Decourten M, Pryor J, Saketa S, Waganivalu TK, Laqeretabua A, Chung E. Fiji Non-Communicable Diseases (NCD) Steps Survey 2002 Geneva: Ministry of Health; World Health Organisation; Fiji School of Medicine; Menzies Centre for Public Health Research, University of Tasmania; 2002. www.who.int/chp/steps/FijiSTEPSReport.pdf [Accessed September 2010]. [Google Scholar]
- 12.Ram P, Collins V, Zimmet P, Taylor R, King H, Sloman G, Hunt D. Cardiovascular disease risk factors in Fiji: the results of the 1980 Survey. Fiji Med J 1983; 11:88–94. [Google Scholar]
- 13.National Food and Nutrition Centre (NFNC). 1993 National Nutrition Survey Main Report. Suva, Fiji: NFNC; 1995. March. [Google Scholar]
- 14.National Food and Nutrition Centre (NFNC). 2004 Fiji National Nutrition Survey Main Report. Suva, Fiji: NFNC; 2007. September. [Google Scholar]
- 15.Maddocks I. The influence of standard of living on blood pressure in Fiji. Circulation 1961; 24:1220–1223. [DOI] [PubMed] [Google Scholar]
- 16.Russell-Jones DL, Hoskins P, Kearney E, Morris R, Katoaga S, Slavin B, Turtle JR. Rural/urban differences of diabetes- impaired glucose tolerance, hypertension, obesity, glycosolated haemoglobin, nutritional proteins, fasting cholesterol and apolipoproteins in Fijian Melanesians over 40. Q J Med 1990; 74:75–81. [PubMed] [Google Scholar]
- 17.Nye ER, Bakani IR, Coverdale HA, Sutherland WHF, Spears GFS. Anthropometric characteristics, blood pressure and lipoprotein lipids in Fiji: comparison of an urban and rural population. Community Health Stud 1986; 10:19–30. [DOI] [PubMed] [Google Scholar]
- 18.Ram BP, Ram P. Hypertension and diabetes in Gau Island. Fiji Med J 1983; 11:35–38. [Google Scholar]
- 19.Ram BP, Ram P. Hypertension in Kadavu. Fiji Med J 1986; 14:134–140. [Google Scholar]
- 20.Munif H, Ram P. Hypertension in Lakeba. Fiji Med J 1986; 14:142–144. [Google Scholar]
- 21.Lovell RR, Maddocks I, Rogerson GW. The casual arterial pressure of Fijians and Indians in Fiji. Australas Ann Med 1960; 9:4–17. [DOI] [PubMed] [Google Scholar]
- 22.Hoskins P, Turtle JR, Handelsman DJ, Hannelly T. Diabetes mellitus, hypertension and obesity in Fiji. Diabetes 1984; 33 suppl 1: Abstract no. 467:120A. [Google Scholar]
- 23.Ram P, Banuve S, Zimmet P, Taylor R, King H, Sloman G, Hunt D. Hypertension and its correlates in Fiji: the results of the 1980 national survey. Fiji Med J 1982; 10:99–105. [Google Scholar]
- 24.Zimmet P, Taylor R, Jackson L, Faaivaso S, Ainuu J. Blood pressure studies in rural and urban Western Samoa. Med J Aust 1980; 2:202–205. [DOI] [PubMed] [Google Scholar]
- 25.Finau SA, Prior IA, Salmond CE. Hypertension among urban and rural Tongans. Med J Aust 1986; 144:16–20. [DOI] [PubMed] [Google Scholar]
- 26.Hodge RL. Risk factors in Australians: National heart foundations risk factor prevalence study 1980. Aust NZ J Med 1984; 14:395–399. [DOI] [PubMed] [Google Scholar]
- 27.Prior I, Grimly Evans J, Harvey H, Davidson F, Lindsey M. Sodium intake and blood pressure in two Polynesian populations. N Engl J Med 1968; 279:515–520. [DOI] [PubMed] [Google Scholar]
- 28.Prior I. Cardiovascular epidemiology in Polynesians in the Pacific. Singapore Med J 1973; 14:223–227. [PubMed] [Google Scholar]
- 29.Taylor R, Bennett PH, Zimmet P. Epidemiological studies of diabetes and cardiovascular disease in Wallis Polynesians – a comparison of residents of Wallis Island and first generation migrants to Nouméa, New Caledonia. South Pacific Commission Technical Paper, No. 181 Noumea: South pacific Commission; 1984. [DOI] [PubMed] [Google Scholar]
- 30.Mancilha-Carvalho JJ, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, et al. Blood pressure in four remote populations in the INTERSALT Study. Hypertension 1989; 14:238–246. [DOI] [PubMed] [Google Scholar]
- 31.Zwart F. Report on the Census of the Population 1976, Volume II, Demographic Characteristics. Parliamentary Paper No. 43. Suva: Parliament of Fiji; 1979. [Google Scholar]
- 32.Fiji Ministry of Health. Noncommunicable Diseases Prevention and Control: National Strategic Plan (2010–2014): From womb to tomb with a double edged sword, everyone's Business. Suva: Ministry of Health; 2009. [Google Scholar]
- 33.Shihab H, Meoni L, Chu A, Wang N, Ford D, Liang K, et al. Body mass index and risk of incident hypertension over the life course. The Johns Hopkins Precursors Study. Circulation 2012; 126:2983–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor R, Zimmet P, Levy S, et al. Group comparisons of blood pressure and indices of obesity and salt intake in Pacific populations. Med J Aust 1985; 142:499–501. [DOI] [PubMed] [Google Scholar]
- 35.Taylor R, Page A, Danquah J. The Australian epidemic of cardiovascular mortality 1935-2005: effects of period and birth cohort. J Epidemiol Community Health 2012; 66:e18. [DOI] [PubMed] [Google Scholar]


