Abstract
The notion that electrophiles serve as messengers in cell signaling is now widely accepted. Nonetheless, major issues restrain acceptance of redox homeostasis and redox signaling as components of maintenance of a normal physiological steady state. The first is that redox signaling requires sudden switching on of oxidant production and bypassing of antioxidant mechanisms rather than a continuous process that, like other signaling mechanisms, can be smoothly turned up or down. The second is the misperception that reactions in redox signaling involve “reactive oxygen species” rather than reaction of specific electrophiles with specific protein thiolates. The third is that hormesis provides protection against oxidants by increasing cellular defense or repair mechanisms rather than by specifically addressing the offset of redox homeostasis. Instead, we propose that both oxidant and antioxidant signaling are main features of redox homeostasis. As the redox shift is rapidly reversed by feedback reactions, homeostasis is maintained by continuous signaling for production and elimination of electrophiles and nucleophiles. Redox homeostasis, which is the maintenance of nucleophilic tone, accounts for a healthy physiological steady state. Electrophiles and nucleophiles are not intrinsically harmful or protective, and redox homeostasis is an essential feature of both the response to challenges and subsequent feedback. While the balance between oxidants and nucleophiles is preserved in redox homeostasis, oxidative stress provokes the establishment of a new radically altered redox steady state. The popular belief that scavenging free radicals by antioxidants has a beneficial effect is wishful thinking. We propose, instead, that continuous feedback preserves nucleophilic tone and that this is supported by redox active nutritional phytochemicals. These nonessential compounds, by activating Nrf2, mimic the effect of endogenously produced electrophiles (parahormesis). In summary, while hormesis, although globally protective, results in setting up of a new phenotype, parahormesis contributes to health by favoring maintenance of homeostasis.
Keywords: Hormesis, Signaling, Kinetics, Thiols, Phytochemicals, Oxidative stress
Graphical abstract
Highlights
-
•
Redox homeostasis is the continuously challenged oxidative/nucleophilic balance.
-
•
Rheostatic redox signaling enzymes maintain oxidative/nucleophilic homeostasis.
-
•
Phytochemicals assist redox homeostasis through oxidative feedback (parahormesis).
-
•
Adaptation and hormesis while protective establish a new phenotype and set point.
1. The Golden Mean: the ethics of redox homeostasis
In the 19th Century Claude Bernard pointed out the relevance of the maintenance of the milieu intérieur we know today as homeostasis, as the crucial element of a healthy status. Consistently, the loss of homeostasis gives rise to a different status we recognize as disease. Maintaining or reestablishing homeostasis is therefore, the way of preventing disease and curing it.
Relevant evidence supporting the view of Claude Bernard emerges today from the modern view of inflammation and redox signaling. When presented with a challenge, an integrated biological system reacts to eliminate the challenge and prevent damage. For this purpose, oxidants that act as signaling species are increased from their steady state rate of production and elimination. The upturn of redox signaling increases both pro-inflammatory cytokines and pro-oxidant enzymes. Eventually, a feedback mechanism returns this “reactive” condition to the physiological range of oxidant production. However, when the overall attempt to reestablish the pre-challenge nucleophilic tone is inadequate, we recognize the new condition as an “inflammatory disease.”
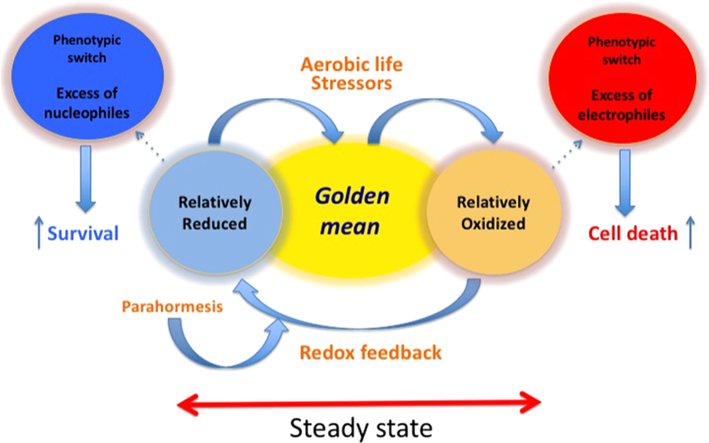
In philosophical terms, this pro/anti inflammation balance is reminiscent of the epicurean philosophy where the aurea mediocritas, the Golden Mean, is the best way to optimize the quality of life, maintaining the equilibrium among motivations pushing our behavior in opposite directions. The “good” is not necessarily toward a single direction. Therefore, the condition of tranquility (ἀταραξία) as described in the epicurean philosophy, is obtained by mitigating what would overwhelmingly push our behavior in either direction. This is not only the ethical way to reach an optimized quality of life, but also emerges as a major mechanism for protection of our health. We must be able to react to challenges, but never too much. Excess in either direction perturbs the homeostasis of the physiologists, which is the aurea mediocritas, the Golden Mean of the ethical philosophers. The concept of the Golden Mean in redox homeostasis, proposed and discussed in this article is summarized in Fig. 1.
Fig. 1.
The “Golden Mean” of redox homeostasis. A steady-state redox status of the ensemble of redox couples is maintained by metabolic fluxes and redox feedback where electrophiles produced by aerobic life stressors activate the mechanism reestablishing nucleophilic tone. Parahormesis refers to nonessential compounds that support the redox feedback loop activating the nucleophilic response. As long as homeostasis is maintained, there is not a phenotypic switch. In contrast, a phenotypic switch occurs in adaptation when a stable offset of homeostasis takes place. In the scheme two opposing examples of stable offset of redox homeostasis are illustrated; one refers to a more oxidizing environment (e.g. oxidative stress) facilitating cell death, while the other refers to a dramatically more reducing environment (e.g., constitutive activation of Nrf2), facilitating survival. Both conditions are part of pathological phenotypes.
2. When a challenge evolves to stress
The notion of stress was introduced in a biological context by Hans Selye to describe the inappropriate physiological response to a demand [1]. Under physiological conditions, different challenges (physical, chemical or biological) prime a response that pushes the cellular homeostasis toward the oxidative boundary of the steady state range [2] and is usually countered by a feedback reaction [3] addressed to diminish the response and restore homeostasis toward the mean. If the stimulus persists or the feedback response is inadequate (in quantitative or qualitative terms) the result is a permanent alteration of homeostasis. In the framework of redox biology, challenges that increase production of oxidants are part of innate immunity increasing elementary pro-inflammatory reactions, but not necessarily resulting in inflammation. Notably, these electrophiles also upregulate feedback nucleophilic responses to restore the original steady state. The notion of oxidative stress, as originally proposed, denoted a stable alteration of redox homeostasis [4]. Putting this in terms of what we define here as redox homeostasis, oxidative stress occurs only when the boundary of the physiologic redox steady state is breached.
Aerobic life, exposure to xenobiotics (food, drugs and poisons) and interaction with other living systems are potentially damaging events that directly or indirectly challenge redox homeostasis. The capacity to deal with these challenges is indispensable for life [5]. In the majority of reports on pathological conditions in which oxidative stress is seen as an underlying mechanism, it is proposed that when pro-oxidative/pro-inflammatory pathways are turned on, biological damage is produced only when antioxidant capacity is overwhelmed. This disregards the fact that electrophiles and nucleophiles are both primary players in redox signaling and that signal transduction takes place through the fine adjustment of a rheostat rather than by the flipping of an on–off switch. Organisms respond to different challenges by increasing both oxidative reactions and nucleophilic feedback in order to prevent a stable offset of redox homeostasis. Thus, while redox homeostasis is maintained by continuous signaling for the production and elimination of electrophiles and nucleophiles [6] a pathological status is generated either by: i) a permanent harmful challenge, or ii) an inappropriate response to injury, or iii) an inefficient nucleophilic feedback leading to stable displacement from the basal redox steady state.
As stress is the inability to deal with a challenge, oxidative stress describes the consequence of the failure to maintain the physiological redox steady state, which is the self-correcting physiological response to different challenges. Adaptive hormesis [7] is different from the maintenance of homeostasis. Typically, mild stimuli, potentially harmful when the exposure is larger in time and concentration, prime a phenotypic shift that brings protection against the same stimulus. Maintenance of redox homeostasis and adaptation may use some of the same signaling pathways; however, the crucial difference is that adaptation establishes a new homeostatic setting that provides some protection but is necessarily associated with a permanent modification of a function, which may be more or less severe. Adaptation to 100% O2, is a classic example of this. Exposure of mammals to 100% O2 kills within a few days by causing lung cell death and pulmonary edema [8], [9]. Young adult rats and some other rodents, when very young, can adapt to breathing 100% O2 by being exposed to 85–90% O2 for several days before exposure to 100% O2 [10]. The adaptive mechanisms (hormetic) include the induction of antioxidant and repair enzymes [9], [10]. But there is also significant alteration of lung structure so that animals returned to air resolve into permanently altered lungs, mimicking emphysema with its loss of alveoli and scarring [11]. Thus, the effect of 85% O2 allows exposure to 100% O2, but saying, according to the concept of hormesis, that the rodent is “stronger,” is debatable.
The redox state as defined from the redox potential of a specific redox couple, as in the popular Nernst equation for GSH and GSSG, is misleading. The equation provides a mathematical result related to the thermodynamics of the reaction, but not to the biological condition we aim to describe as redox state. What is relevant is indeed the concentration of reagents and products and by no means the ratio between the concentration of the reduced and the oxidized form. The actual concentrations of GSH and GSSG are generated from the rates of GSH oxidation by several hydroperoxides, GSSG reduction by NADPH, GSH conjugation to electrophiles, GSH and GSSG use in protein folding, export and import of GSH, GSSG and conjugates, synthesis of GSH, and import and synthesis of its constituent amino acids, particularly cys, as well as the scavenger pathway in which gamma-glutamyl transferase uses cystine to bypass glutamate cysteine ligase. Then there are the rates of synthesis, degradation and regulation of the activities of all the enzymes and transporters. Thus, the redox steady state is dependent on concentrations and kinetic rate constants for multiple reactions and is in constant flux. In other words, quantification of physiological redox homeostasis may entail determination of normal ranges of several major electrophiles and nucleophiles in a manner similar to the way we now determine normal physiological ranges for plasma glucose.
3. What drives redox homeostasis from the oxidizing side? Sources of electrophiles: aerobic life, generation of oxidizing molecules in water and lipid phase
Superoxide and H2O2 are continuously produced during aerobic metabolism. It was once thought that was only produced by the phagocytic cell NADPH oxidase (Nox2) in response against microbes [12], by leaks from the mitochondrial electron chain [13], [14], by some flavoprotein oxidases [15], [16], and the autoxidation of some small molecules and iron proteins [17], [18], [19]. With the exception of the phagocytic killing of microbes, the other production was considered an unfortunate consequence of living with oxygen. But, for the past two decades, intentional formation of H2O2 has been recognized as a component of intracellular signal transduction.
H2O2 production by cells varies markedly in terms of both amount and duration between what is required for cell signaling versus what is needed to kill a microbe. But, it must be noted that H2O2 production is never actually turned off and that its production is highly regulated as part of redox homeostasis. While superoxide is continuously produced, a variety of stimuli can provoke a marked increase in its generation. Following the discovery that Nox2 was one of several related Nox proteins that could generate superoxide [20], [21], [22], it was realized that signaling stimulated by a variety of agonists, challenges and stressors such as growth factors, hormones and shear forces, depended on increased superoxide production by Nox proteins [22], [23], [24], [25], [26].
Mitochondria are a continuous source of H2O2. The production of H2O2 is actually a result of the removal of by superoxide dismutase formed by the thermodynamically unfavorable oxidation of ubisemiquinone [27]. Functionally, H2O2 generation is accelerated when respiratory chain cytochromes are fully reduced (state 4 respiration) [28]. This is the condition of replenishment of metabolic energy (low ADP/ATP ratio) and overabundance of oxidizable substrates. This condition is well described by the concept of nutrient overload and mitochondrial gridlock typical of metabolic syndrome associated with type II diabetes [29].
Linked to the notion of potentially risky production of oxidants by the respiratory chain in state 4, mild uncoupling of respiratory chain can be seen as an indirect antioxidant mechanism as it increases the flow of electrons toward cytochrome oxidase while decreasing the electron transfer from ubisemiquinone to oxygen. Indeed, it appears that a minor uncoupling may be beneficial [30], [31]. All of this is physiologically relevant and recent work suggests that mitochondrial superoxide/H2O2 production is part of redox homeostasis as well as a response to stress [32], [33], [34]. This is a rather relevant area needing further in depth investigation.
Lipid peroxidation is a chain of reactions producing in membranes lipid hydroperoxides (LOOH) and their degradation products. It is initiated by the formation of a carbon centered radical in a polyunsaturated fatty acid of a complex lipid [35], [36]. Upon oxygen addition, the formed lipid hydroperoxyl radical initiates the propagation phase when carbon centered radicals and LOOH are continuously produced in the chain reactions. The length of the chain depends on nature of the membrane and the presence of the chain breaking antioxidant α-tocopherol. The most relevant mechanism of initiation is the hydrogen abstraction by a lipid alkoxy radical, in turn produced by breakdown of the O–O bond of a LOOH. This indicates that the crucial players of initiation are a transition metal and a preexisting LOOH. Notably, a unique and specific mechanism of the formation of initial traces of hydroperoxide derivatives of polyunsaturated fatty acids is not known with certainty. Nevertheless, there is no doubt that lipid peroxidation requires decomposition of LOOH. Consistently, the continuous reduction of LOOH by the selenoenzyme phospholipid hydroperoxide glutathione peroxidase (PHGPx, also known as GPx4) [37] is by far the most relevant anti-peroxidative mechanism.
In addition, lipoxygenases that catalyze production of LOOH require activation by hydroperoxides [38]. Indeed, in the presence of GPx4 and GSH, 15-lipoxygenase is inactive [39]. Lipophilic electrophiles are produced from hydroperoxides of polyunsaturated fatty acids (see [40] for recent review). The notion that all these species are produced only from free fatty acids released by a phospholipase has been recently challenged by the evidence that oxidized fatty acids can also be enzymatically produced in intact phospholipids [41], [42]. This concept specifically addresses the issue of redox signaling supported by lipid oxidation in the membrane compartment. It should also be noted that lipoxygenases as well as cyclooxygenases are probably never completely inactive, as suggested by the three decade-old “peroxide tone” concept of Lands [38], [43]. Indeed, peroxide tone is an integral part of redox homeostasis.
Among the major products of lipid peroxidation generated when LOOH are decomposed, some deserve special mention. The most relevant are α,β-unsaturated aldehydes, one of which 4-hydroxy-2-nonenal (HNE) is major product of decomposition of hydroperoxides of n-6 fatty acids. HNE apparently fulfills the requisites for being considered a signal transducing species [44], [45], [46]. While usually associated with oxidative stress, which dramatically elevates HNE production [47], [48], normal plasma contains 0.1–1.4 μM HNE [49], which is sufficient to maintain signaling for the production of steady state mRNA expression of enzymes active in maintaining the nucleophilic tone [50]. Notably, it has been recently reported that HNE is not only a major product of non-enzymatic lipid peroxidation [45], but can also be formed by a specific mechanism at the active site of a lipoxygenase [46]. Lipid peroxidation has been recently discovered as the underlying mechanism of a caspase independent cell death subroutine requiring free iron, named ferroptosis. Consistently, silencing of GPx4 or decreasing GSH invariably leads to cell death [51], [52].
Electrophiles, either soluble or lipophilic, act through post translational modifications (PTM) where the rate of conjugation with an amino acid residue depends largely on the nucleophilic reactivity of the target, with a cysteine in the thiolate (S-) form being markedly more reactive than lysine or histidine [53]. There is also a rank order of reactivity of different thiolate residues. The kinetic constraints for the oxidation/alkylation of different thiolate residues include having a low pKa, protonation of the leaving group and, obviously, the steric feasibility of the bimolecular interaction [54]. When disulfides are formed, the thermodynamics of the thiol disulfide redox couple also permits a distinction between low potential couples (relatively more resistant to reduction), usually having a structural role, and high potential couples (relatively easier to reduce), likely serving as the reversible redox coupling switches in signal transduction pathways [55].
In summary, the reduction of hydroperoxides is the crucial reaction controlling the steady state concentration of electrophiles continuously produced and, when appropriate, involved in signaling. This nicely fits the observation that silencing GPx1 or GPx4 produces an oxidizing environment specifically in the cytosol or membrane, respectively [56], and this deeply affects the phenotype.
4. What drives redox homeostasis from the reducing side? Sources of nucleophiles: Nrf2 and the nucleophilic tone
As outlined above, the formation of electrophiles is continuously countered by a feedback nucleophilic response, indispensable for maintaining redox homeostasis. Nrf2, also called NF-E2-related factor 2, is the master regulator for increasing genes that code for enzymes protecting cells from electrophilic stress. Thus, in non-stressed cells Nrf2 is relatively less active in coordination with the basal flow of endogenously generated electrophiles. The increased oxidative activation of Nrf2 accounts for both the enhanced synthesis of nucleophiles such as GSH and Trx and the enzymes catalyzing the redox transitions. Increased activity hey of the pentose phosphate shunt contributes to the increase of the nucleophilic tone by supplying the NADPH required for the continuous reduction of the disulfide forms of glutathione and Trx.
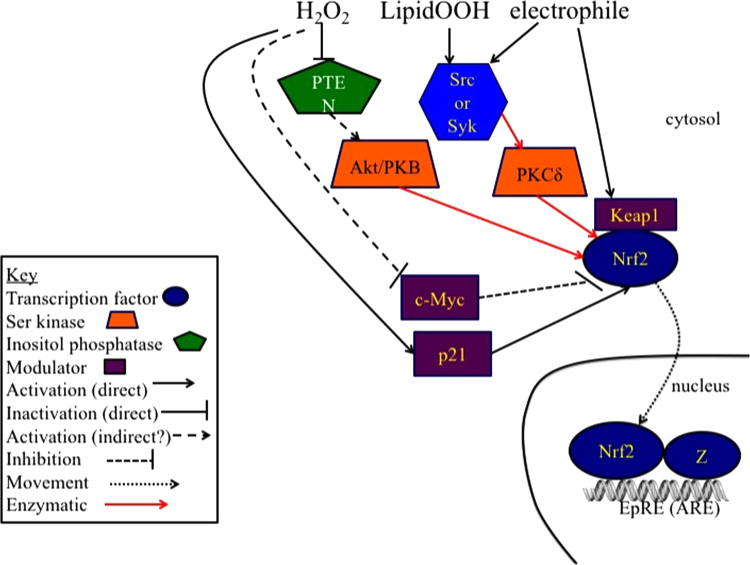
As we recently reviewed the history of the discovery of Nrf2 and Keap1, the regulator of Nrf2 and the actual sensor of electrophiles [6] and a whole volume on Nrf2 regulation has recently been co-edited by one of us [57], we will only very briefly describe the principal signaling pathways through which electrophiles activate Nrf2 (Fig. 2). It should however, be noted that H2O2 is a less efficient activator of Nrf2 in comparison with thiol-conjugating electrophiles (reviewed in [6]). Thus, redox homeostasis reflects more the state of electrophile production, seemingly produced by lipid peroxidation, than the flux of H2O2.
Fig. 2.
Electrophilic activation of Nrf2. When cysteines in Keap1 are modified by electrophiles, Nrf2 escapes Keap1 assisted ubiquitinylation and subsequent proteasomal degradation and can translocate to the nucleus [133], [134], [135]. Phosphorylation of Nrf2 by PKCδ and Akt assists in the nuclear translocation of Nrf2, where it increases transcription of genes through binding to the EpRE (also called ARE) element [136], [137]. PKCδ, and Akt are also activated by hydroperoxides and other electrophiles [138], [139], [140], [141], [142]. The proteins that are Nrf2 partners in binding to EpRE include Mafs G/F/K [143], c-Jun [144], c-Fos and Fra1 [145], c-Maf [146], JunD [147], [148], but little attention has been paid to whether any of their interactions with Nrf2 involve redox signaling. Similarly, little attention has been paid to the possibility of redox regulation of Bach1 and Nrf1 that compete with Nrf2 for binding to EpRE [149]. Furthermore, potential redox regulation of c-Myc inhibition of transcription through its binding to nuclear Nrf2 and acceleration of Nrf2 degradation [150] and the stabilizing influence of p21 [151] have yet to be explored in detail. All of these aspects may be part of the redox homeostasis involved with Nrf2. Additional redox-related activation of Nrf2 may occur during stress conditions through the sequestration of Keap1 by p62 such as occurs during dysregulation of autophagy [152].
During a long lasting inflammatory response to a persistent stressor, these same signaling pathways are used to attempt to restore redox homeostasis, but the adjustment results instead in the establishment of a new redox steady state. Notably, Nrf2 also activates two pathways eventually leading to an increased production of electrophiles. Heme oxygenase, expressed under control of Nrf2, releases iron [58]. Furthermore, the multidrug resistance protein, also under control of Nrf2, exports GSH [59]. This condition, in sensitive cancer, sensitizes cells to ferroptosis [60], the peculiar form of programmed cell death controlled by GPx4 [61] which relies on lipid peroxidation [62]. Activation of ferroptosis emerges today as a promising target for cancer chemotherapy and recent evidence indicates that p53 modulates cysteine availability for GSH synthesis and controls, seemingly through the same basic mechanism, oncosuppression and organismal homeostasis [63], [64]. Far from being paradoxical, these effects of Nrf2 leading to a decrease of the nucleophilic tone, highlight the relevance of the fine tuning of the redox steady state, operating by multiple enzymatic activities and feedback regulation. In addition, the Nrf2 related transcription factor, Nrf1, may have a role in redox homeostasis, which has not been generally recognized [65]. In transcriptional control of some genes, Nrf1 isoforms may competitively inhibit, substitute, or surpass Nrf2 activation of EpRE [66]. Intriguingly, Nrf1 is apparently essential during development, while Nrf2 is not [67], yet Nrf2 knockout is clearly the principle transcription factor that responds to increases in electrophiles [68].
5. NF-κB, oxidative signaling and inflammation
The increased in NF-κB activity is often considered as a sudden turning on of signaling for this essential transcription factor. But, as can be seen by simple observation in controls of nuclear NF-κB and positive bands in electrophoretic mobility shift assays, NF-κB is actually always “on” to some degree. Thus, turning up and down of NF-kB activity is an essential component of maintenance of redox homeostasis rather than a beginning and end of inflammation. Nonetheless, as for many cases in signaling, the tendency is to refer to upturn as activation. Schreck et al. [69] first demonstrated that NF-κB could be increased in cells by addition of exogenous H2O2. Increases in NF-κB activity caused by endogenous production of H2O2 was demonstrated a few years later [70], although it was thought at that time that only phagocytes produced H2O2 upon stimulation of their NADPH oxidase (now called Nox2).
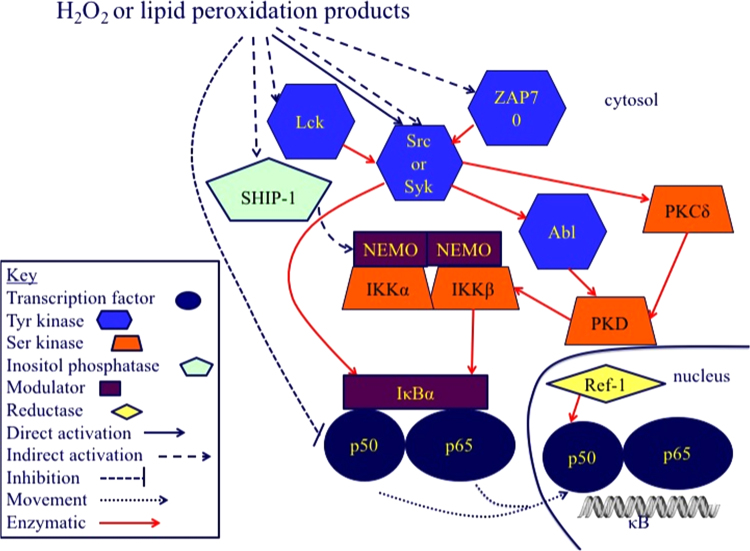
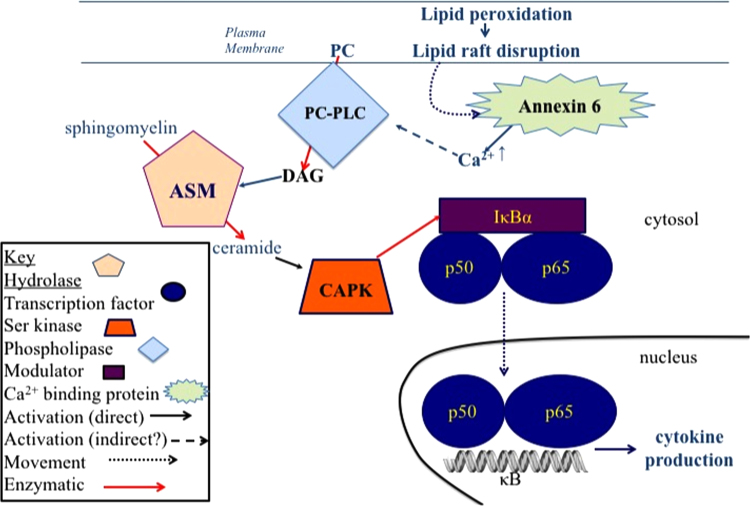
Multiple pathways (canonical (classical), non-canonical, and atypical) lead to NF-κB activation, but not all have been suggested to be redox regulated [71], [72]. We have not attempted to be comprehensive but rather focus on some potential targets and mechanism of action of oxidative regulation, particularly those that would likely occur during maintenance of redox homeostasis. Fig. 3 shows the activation of NF-κB by H2O2. This follows several pathways that differ among cell types [3] so that there is no one pathway that can be assumed to be involved in NF-κB activation without investigation. The mechanisms in Fig. 3 represent the likely signaling that occurs as part of redox homeostasis. In contrast, Fig. 4 represents the signaling pathway for NF-κB activation that occurs during stimulation of macrophages by iron-laden silica particles [73], [74]. The non-oxidative part of this pathway can also be stimulated by lipopolysaccharide [75], [76].
Fig. 3.
Hydroperoxide activation of NF-κB activation. NF-κB is composed of homo- and heterodimers of p50, p52, p65 (RelA), c-Rel, and RelB. Only p65, c-Rel, and RelB have transcriptional activation domains. IκB family members bind NF-κB dimers in the cytosol until IκB is phosphorylated and degraded. IκB isoforms differ among cell types in dependence upon the IKK complex for their phosphorylation. Two serine kinases, IKKα and IKKβ, and a modulator, NEMO (also called IKKγ) form the IKK complex that phosphorylates IκB. For an extensive description of NF-κB regulation not focusing on redox regulation, the reader is referred to some recent reviews [153], [154]. Early in the investigation of how H2O2 activated NF-κB, it became obvious that tyrosine phosphorylation was involved. H2O2, lipid hydroperoxides, and other electrophilic lipid peroxidation products, can directly activate members of the Src kinase family, Src, Syk, and Lyc and the closely related ZAP leading to activation of the tyrosine kinase Abl and serine kinase PKCδ that activate PKD. In an alternative pathway, Src or Syk directly phosphorylate IκB on a tyrosine residue, bypassing IKK activation. One of the more intriguing proposed targets in H2O2 activation of NF-κB is SHIP-1, an inositol-5-phosphatase. SHIP-1 activation by H2O2 has been shown to lead to the activation of the classical IKK-dependent pathway for NF-κB activation. SHIP-1 activity depends on its SH2 domain, which is of course, the classic target of tyrosine phosphorylation and in a study unrelated to NF-κB activation, SHIP-1 activity was demonstrated to be through its tyrosine phosphorylation by Lyn, another member of the Src kinase family [155]. Other possible pathways for NF-κB activation involving the Src kinases may exist; however, the activation of the Src kinases seems to be the key step through which most studies suggest H2O2 activates NF-κB. Although it is not clear exactly how and where NF-κB is oxidized, the redox chaperone activity of APE/Ref-1 is required to restore the ability of NF-κB to bind to DNA in the nucleus [156].
Fig. 4.
Activation of NF-κB-dependent cytokine production through lipid peroxidation. When non-cytotoxic levels of iron-laden particles interact with macrophages, the resulting lipid peroxidation in the area of the plasma membrane with which the particle interacts, produces a stress response that activates NF-κB and pro-inflammatory cytokine production. Lipid raft disruption caused by minor lipid peroxidation, results in the release of calcium from annexin 6 leading to the activation of phosphatidylcholine specific phospholipase C (PC-PLC) activation that produces diacylglycerol (DAG). DAG then activates acidic sphingomyelinase (ASM) that produces ceramide. Ceramide then activates ceramide-activated protein kinase (CAPK), which phosphorylates IκB allowing NF-κB to migrate to the nucleus. The PC-PLC-dependent pathway can also be stimulated by endotoxin, again representing a non-physiological stress response [75], [76].
While there are some cases in signal transduction where H2O2 is clearly the molecule involved, it must be stated that the actual molecule that interacts with the target in many other cases may not be H2O2 itself but rather a LOOH [77], [78]. There are also reports that hypochlorous acid and singlet oxygen can activate NF-κB and that peroxynitrite can either activate or inhibit NF-κB. Notably, dietary electrophilic isocyanates inhibit NF-κB activation [79], [80], [81] while HNE, an endogenous electrophile appears to directly inactivate NF-κB [82]. Thus, HNE does not appear to activate NF-κB under conditions where it forms adducts with other proteins leading to JNK activation [83], [84]. NF-κB activation therefore appears to discriminate between hydroperoxides and other electrophiles.
6. Oxidative signaling in JNK/AP-1 pathway
Similarly to NF-κB, activation of the mitogen activated protein kinases, Jun N-terminal kinase (JNK) was originally thought to be an on/off response, in this case to stress. Indeed, JNK was also called SAPK for stress activated protein kinase. But, JNK is now well established as both a key player in cell survival or death, and as essential to regulation of metabolism and cell proliferation [85]. Thus, as with NF-κB, its “activation” is really a turning up rather than a turning on.
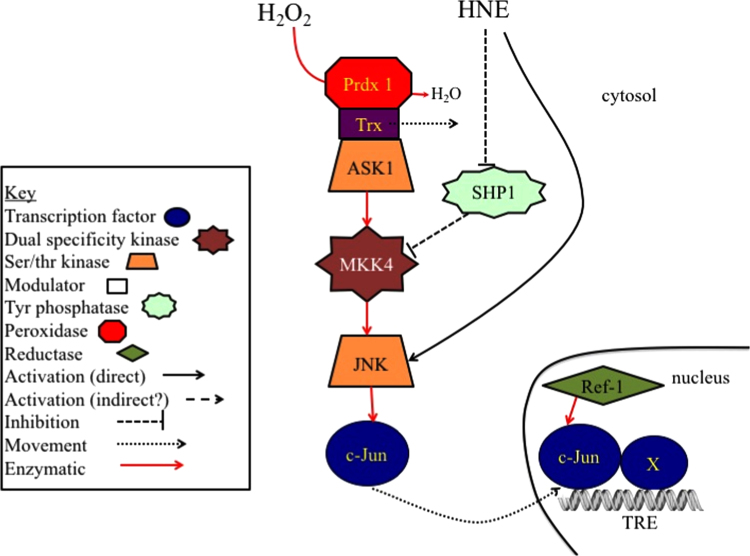
Increased JNK activity, increases phosphorylation and activity of the AP-1 transcription factor. This can be achieved by both hydroperoxides and HNE (Fig. 5). Ichijo and coworkers [86], [87] using exogenous H2O2, suggested that the mechanism of H2O2 activation of JNK was dissociation of thioredoxin (Trx) bound to the ASK1 (apoptosis signaling kinase 1) when that Trx was oxidized. Subsequently, the ability of endogenously generated H2O2 to activate the pathway from dissociation of Trx from ASK1 to c-Jun phosphorylation by JNK was demonstrated [88] and then shown to be dependent upon peroxiredoxin 1 (Prdx1) [89].
Fig. 5.
Redox activation of JNK/AP-1. H2O2 activates JNK through the Prdx1 catalyzed oxidation of Trx bound to ASK1. The Trx dissociates from ASK1, allowing the kinase to dimerize and phosphorylate the dual specificity (ser/thr and tyr phosphorylating) mitogen activated kinase kinase, MKK4. MKK4 in turn phosphorylates and activates JNK1 or JNK2. JNK then phosphorylates the c-Jun transcription factor, which pairs with another member (X) of the Jun/Fos family of transcription factors forming an AP-1 complex that binds to the TRE element of many AP-1-regulated genes. As with NF-κB (Fig. 3), AP-1 appears to require reduction in the nucleus by the redox chaperone activity of APE/Ref-1 to bind to DNA [156], [157].
An increase in JNK activity by HNE was shown initially to occur as a direct result of HNE binding to JNK [84]. JNK activity could be increased through inhibition of the upstream protein tyrosine phosphatase, SHP-1 [90] with pathways differing in cell types.
7. Oxidative signaling in PTEN/PI3K/Akt pathway
During physiological signaling stimulated by a variety of agonists, phosphatidylinositol phosphorylation in cell membranes is increased by PI3K (phosphatidylinositol 3 kinase) to increase phosphatidylinositol 3-phosphate (PI(3)P), phosphatidylinositol (3,4)-bisphosphate (PI(3,4)P2), and phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3 or PIP3). The increased formation of PIP3 allows several enzymes to increase their binding to the membrane where they are active. One of these PIP3-regulated enzymes is PDK1, the protein kinase that phosphorylates and activates Akt (also known as PKB), a key protein kinase that activates mTOR, IKKα, which activates NF-κB by phosphorylating IκB (Fig. 3), and a variety of other signaling kinases. Akt activity is essential in development and aberrations in it result in several pathologies [91].
PTEN (phosphatase and tensin homolog) is a phosphatase that removes a phosphate from PIP3 (phosphatidylinositol (3,4,5)-trisphosphate) to produce PIP2 (phosphatidylinositol (4,5)-bisphosphate), which does not bind and activate the PIP3-regulated enzymes. Importantly, PTEN is normally active in unstimulated cells. So, decreasing PTEN activity is critical in permitting increased activity of PIP3-dependent enzymes. One of the first signaling pathways to be demonstrated to have a direct interaction with H2O2 was the reversible inactivation of PTEN by Rhee and coworkers [92]. Later, it was shown that stimulation of endogenous transient H2O2 production could increase the resting level of PTEN glutathionylation and Akt activation [93]. Transient PTEN inactivation by glutathionylation is therefore a major player in regulating redox signaling that is homeostatic. In contrast, prolonged inactivation of PTEN allows signaling to go into the stress adaptive mode. As loss of PTEN activity is frequently found in cancers, this functional loss has become the target of recent therapeutic anticancer therapies [94]. Again, it should be noted that signaling through the PTEN/PI3K/Akt pathway does not usually involve a sudden turning on or off, but a rheostatic control of the activities of these important signaling enzymes.
8. Redox homeostasis and nutrition: from the postprandial oxidative stress to metabolic syndrome
To examine our proposal that everyday life generates challenges that increase oxidative pro-inflammatory pathways and evolve to stress only when not optimally counteracted by a feedback nucleophilic response, we will examine the impact of nutrition. The intake and oxidative metabolism of energetic substrates actually result in a transient oxidative challenge. The long lasting effect of chronic excessive caloric intake is obesity, often evolving to metabolic syndrome and eventually non-alcoholic fatty liver disease (NAFLD) [95], [96]. An alteration of redox homeostasis associated with chronic inflammation emerges today as a common motif of these pathological conditions.
Energy metabolism generates electrophiles that are expected to have an impact on cellular nucleophilic tone. Indeed, the response to glucose of physiological levels of insulin involves both Nox2 and Nox4. Notably, increased activation of Nox2 and increased transcription of Nox4 by insulin are similar to what is seen with transforming growth factor-β [25], [97] although the signaling pathway for insulin to activation of Nox proteins has not been fully elucidated [98], [99], [100], [101], [102].
Saturated free fatty acids trigger hepatic inflammation via Toll receptors [103] and NF-κB [104]. Increased availability of substrates of the respiratory chain primes production of superoxide/hydrogen peroxide in mitochondria. Finally, the excess of substrate availability in mitochondria eventually leads to synthesis of complex lipids and cholesterol, hyperlipidemia, fatty liver, and obesity [29]. Obesity, is associated with adipose tissue inflammation [2] contributing to glucose resistance [95] and NAFLD. In this respect, adipose tissue cytokines by activating NF-κB and an inflammatory response in the liver, contribute to the depression of the nucleophilic tone brought by oxidants produced by substrate-loaded mitochondria.
The stimulus for enhanced protein synthesis, primed by anabolic stimuli, evolves into ER stress when protein folding capacity is exhausted. ER stress [105] produces more oxidants and activates JNK, which in turn further exacerbates inflammation via NF-κB activation [106]. mTOR [107], activated by excess of nutrients, stimulates (via JNK) the Unfolded Protein Response (UPR) [108], which dampens cell sensitivity to insulin [109] and protein synthesis, concomitantly activating an antioxidant response [108]. In this respect, the UPR and decreased insulin signaling, due to IRS phosphorylation by JNK or 6SK1, can be seen as adaptive mechanisms that provide some immediate protection but contribute in the long run to stabilize a metabolic offset. This long-lasting activation is distinctly associated with an increased occurrence of liver and cardiovascular diseases [96], the leitmotif seemingly being the less nucleophilic cellular environment [110].
The mTOR pathway coordinates cell growth with availability of energetic nutrients. While its increased activity and downstream effects are primed by oxidation (seemingly descending from energy metabolism and insulin signaling) [111], reduction is expected to mimic the nutrient deprivation condition. The oxidative activation of mTOR is seen as an adaptive mechanism counteracting ER stress. This, increases UPR [112], but although that provides protection under emergency conditions, prolonged uncoupling eventually evolves toward metabolic disorder. The emerging leitmotif is that the excessive insulin signaling together with a large availability of energetic substrates decreases the steady state nucleophilic tone. This impacts, through electrophiles, on feedback nucleophilic reactions. Pathology seemingly emerges when homeostasis is compromised and a new more oxidized redox steady state is reached. Supporting the nucleophilic tone by enzymatic reactions producing an increase in nucleophiles and the supply of electrons from NADPH is seemingly a major mechanism for maintenance of physiological homeostasis and thus health.
The condition of increased global energetic substrate availability inhibits AMPK [113] and sirtuins [114], the regulators of metabolic control of the fasting condition, while decreased nucleophilic tone also activates mTOR [115] as a typical metabolic adaptive reaction. These regulatory mechanisms, and the preceding examples of redox regulation of normal metabolism and how their dysregulation leads to pathological disorders (obesity metabolic syndrome and NAFLD) demonstrate the importance of maintaining redox homeostasis controlled by the fine tuning of rheostats rather than the sudden switching on or off of signaling reactions. This also points out the physiological relevance of compounds we consume with nutrition, which are not nutritionally essential, but largely contribute to health by supporting nucleophilic tone in cooperation with the endogenous players of redox feedback, which we address further below.
9. Nucleophilic tone and cancer
A challenging paradox has recently appeared in publications concerning redox reactions and signaling in cancer. While a more oxidizing environment sustained by electrophiles increases the risk of carcinogenesis [116], [117], the uncontrolled activation of the pathways increasing the nucleophilic response favors malignancy and the metastatic spread of cancer cells [118]. The solution of this conundrum is the critical analysis of the context [119] to which our concept of homeostatic regulation of nucleophilic tone brings a substantial contribution. The major hallmarks of epithelial cancers (proliferation, survival, angiogenesis, EMT, escape from anoikis) are activated by oxidative redox transition [120]. In this respect, oxidative inflammation is a pro cancer event and the reestablishment of the nucleophilic tone is protective. On the other hand, in some malignancies Keap1 is mutated to a non-functional form so that Nrf2 is more longer lived and more abundant, producing a phenotype that provides a biological advantage for fast proliferating cells [121]. This phenotypic shift abolishes the normal rheostatic regulation of nucleophilic tone. In these cells, the addition of N-acetylcysteine and vitamin E further prevents the execution of cell death and leading to a more malignant invasive phenotype. Far from being paradoxical, this evidence further supports our notion of the importance of maintaining the “Golden Mean,” with alteration in either extreme favoring pathology (Fig. 1).
10. Redox signaling – the underlying pathways for redox homeostasis, stress and adaptation
Every form of life is constantly exposed to a myriad of potentially lethal stimuli. Therefore, cells, in order to survive, have to deal with these challenges by activating a complex series of defensive mechanisms. The acknowledged concertmaster organizing the amplitude and duration of responses is the nuclear factor NF-κB. For a comprehensive list of activators of NF-κB (physical, chemical and biological) see [122]. Notably, NF-κB is activated by oxidants (see above) but also produces, via activation of pro-inflammatory cytokines, increases in NADPH oxidases [123], [124], inducible nitric oxide synthetase [125], and electrophiles that decrease nucleophilic tone. The long lasting, poorly controlled or inappropriate activation of NF-κB produces the series of pathological condition cumulatively referred to as inflammation-dependent.
Nonetheless, feedback control of the NF-κB pathway results in reestablishment of nucleophilic tone. Oxidants produced by the NF-κB activated pro-inflammatory pathway increase Nrf2 activity, which in turn increases nucleophilic tone. This feedback loop is continuously active and responsible for much of redox homeostasis. Too long or too much of an upturn of NF-κB activity will cause the production of more electrophiles than can be handled by cellular homeostatic system.
Nucleophilic antioxidants are efficient antagonist of the NF-κB pathway and a myriad of botanical compounds sharing the chemical activity of “antioxidant” are listed as inhibitors of NF-κB activity, activation or function [126]. However, the actual nucleophilic nature of these “antioxidants,” which are almost invariably polyphenols, cannot directly supply the nucleophilic tone where the actual nucleophiles are GSH and Trx, nor can they play any reasonable physiological function by scavenging free radicals. The actual fate of these “antioxidants” is their oxidation. A common motif of these oxidized species is a α,β-unsaturated carbonyl, such as the essential functional component of HNE. HNE and other α,β-unsaturated aldehydes are among the most effective nucleophiles for activation of Nrf2 [127].
In summary, the nutritional antioxidants mimic signaling by endogenous electrophiles, including HNE. Indeed, studies of HNE have evolved from being a cytotoxic lipid peroxidation product [128] to a principal signaling electrophile in Nrf2 activation [44], [129]. The analysis of these events suggested a paradigm shift in which an increase in electrophiles normally leads to restored nucleophilic tone and only causes an offset from redox homeostasis when in excess. In principle, the redox pathways (production of oxidants and feedback nucleophilic response) are indispensable for healthy living and for maintaining redox homeostasis. An imbalance, even seemingly non-toxic, for too long can lead to an adaptive stress response; i.e., a phenotypic shift to a new homeostatic condition we often recognize as pathology.
11. Redox homeostasis, adaptation, hormesis and parahormesis
The broad concept of adaptation in biology, overlaps with the more specific notion of hormesis in toxicology. An integrated biological system (an organism or a cell) upon interaction with a potentially damaging challenge, if not severely injured, may undergo a phenotypic shift, acquiring resistance to the same or similar stressor. Hormesis is, therefore, the term describing the seemingly positive adaptive responses to minimal exposure to agents that produce a well-defined damage when the exposure is higher. Adaptation operates through the stable activation of different mechanisms including repair. Thus adaptive mechanisms establish a new homeostatic condition.
Prolonged exposure to a glucose concentration that is too low results in ketosis, while prolonged exposure to too high glucose results in depressed insulin sensitivity and diabetes. Such adaptations are positive as they contribute to prevent major damage while keeping the organism going for a while, but are definitely far from the normal physiological metabolic steady state and intrinsically harmful. The deviation from the physiological steady state in adaptation probably depends more on the extent of the stress than on the attempt to counter it.
Maintenance of redox homeostasis by feedback mechanisms instead, is quite different from adaptation, but it fits similar criteria. Redox homeostasis refers to the endogenous capacity of cells to continuously deal with challenges that generate electrophiles. Challenges brought by different stressors are the result of metabolic or environmentally non-injurious temporary movement toward the edge of the normal physiological range. Maintenance of redox homeostasis is achieved through feedback mechanisms operating at different level of complexity such as relief of the inhibition of an enzyme [130] or transcriptional up-regulation [131], [132].
Parahormesis is the mechanism by which exogenous agents (nutritional antioxidants) help maintain redox homeostasis by restoring nucleophilic tone. This mechanism is at a first glance a paradox as the molecular mechanism of these antioxidants is through oxidative alkylation of Keap1, the redox sensitive regulator of Nrf2. As described above, these molecules either have a chemical functional group, such as an isothiocyanate or α,β-unsaturated carbonyl, competent for Michael addition to sensitive cysteine residues of Keap1, or are oxidized to a product that does have such an electrophilic function. In terms of nutrition, these compounds, typically from the vegetable kingdom, have the function of contributing to the maintenance of the metabolic and redox steady state, but they are not essential as are vitamins. Their complementary function, to assist in maintaining redox homeostasis, becomes apparent only when a challenge exceeds the capacity of the endogenous nucleophilic feedback response (Fig. 1).
12. Conclusions
Reversible redox transitions of proteins are the modifiable elements of signal transduction pathways dynamically oscillating from the oxidized and the reduced form. The homeostatic setting depends upon the oxidation and reduction rates of independent reactions; thus, the concept of dynamic equilibrium is less appropriate than redox steady state. Kinetics controls the biological efficiency of reactions in both directions. Therefore, the concentration of electrophiles, nucleophiles and the activity of the enzymes catalyzing several of these reactions are the variable elements of the redox steady state. Nucleophilic tone is transiently depressed when the cell is challenged and it is feedback driven by electrophiles. An inadequate feedback response is the emerging cause of conditions where an excess of inflammatory reaction, or an excess or reaction to injury, or a deeply altered gene expression, leads to the altered homeostasis we recognize as pathology.
The evidence discussed here mostly comes from studying how pathological states evolve rather than how physiological redox homeostasis is maintained. We urge investigators to focus more effort on understanding the regulation of redox signaling during homeostasis with the expectation that comparison will reveal those redox signaling pathways that are the same in normal physiology and pathology and those that are actually novel to pathology. This could result in a greater rationale for focusing on some redox signaling pathways as contributing more to disease than others.
In terms of nutritional recommendations in the framework of translational medicine we can conclude that supporting nucleophilic tone by nutritional compounds competent for facilitating redox feedback (parahormetics) is the crucial nutraceutical function of these molecules. They are not as essential as vitamins, as there is not a specific deficiency syndrome, nor are they drugs, as there is not a specific effect suitable for quantitative evaluation; however, they facilitate the maintenance of the “Golden Mean” by helping maintain nucleophilic tone.
Acknowledgments
Supported by NIH, United States Grant ES023864 to HJF and Human Frontier Science Program, Italy Grant RGP0013/2014 to F.U.
References
- 1.Selye H. The Stress of Life. McGraw-Hill; New York: 1956. [Google Scholar]
- 2.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 3.Brigelius-Flohe R., Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadenas E., Sies H. Oxidative stress: excited oxygen species and enzyme activity. Adv. Enzym. Regul. 1985;23:217–237. doi: 10.1016/0065-2571(85)90049-4. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese E.J., Baldwin L.A. Defining hormesis. Hum. Exp. Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 8.Pagano A., Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann. N. Y. Acad. Sci. 2003;1010:405–416. doi: 10.1196/annals.1299.074. [DOI] [PubMed] [Google Scholar]
- 9.Frank L., Bucher J.R., Roberts R.J. Oxygen toxicity in neonatal and adult animals of various species. J. Appl. Physiol.: Respir. Environ. Excerc. Physiol. 1978;45:699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- 10.Crapo J.D., Tierney D.F. Superoxide dismutase and pulmonary oxygen toxicity. Am. J. Physiol. 1974;226:1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- 11.Jones R., Zapol W.M., Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am. J. Pathol. 1984;117:273–285. [PMC free article] [PubMed] [Google Scholar]
- 12.Babior B.M., Kipnes R.S., Curnutte J.T. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973;52:741. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman H.J., Kennedy J.A. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem. Biophys. Res. Commun. 1974;60:1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- 14.Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 15.Massey V., Strickland S., Mayhew S.G., Howell L.G., Engel P.C., Matthews R.G., Schuman M., Sullivan P.A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem. Biophys. Res. Commun. 1969;36:891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- 16.McCord J.M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 17.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170. [PubMed] [Google Scholar]
- 18.Misra H.P., Fridovich I. The generation of superoxide radical during autoxidation of ferredoxins. J. Biol. Chem. 1971;246:6886. [PubMed] [Google Scholar]
- 19.Misra H.P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972;247:6960. [PubMed] [Google Scholar]
- 20.Cheng G., Cao Z., Xu X., Meir E.G., Lambeth J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth J.D., Cheng G., Arnold R.S., Edens W.A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 22.Suh Y.A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 23.Lassegue B., Sorescu D., Szocs K., Yin Q., Akers M., Zhang Y., Grant S.L., Lambeth J.D., Griendling K.K. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 24.Colavitti R., Pani G., Bedogni B., Anzevino R., Borrello S., Waltenberger J., Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 25.Murillo M.M., Carmona-Cuenca I., Del Castillo G., Ortiz C., Roncero C., Sanchez A., Fernandez M., Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem. J. 2007;405:251–259. doi: 10.1042/BJ20061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manevich Y., Al-Mehdi A., Muzykantov V., Fisher A.B. Oxidative burst and NO generation as initial response to ischemia in flow-adapted endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2126–2135. doi: 10.1152/ajpheart.2001.280.5.H2126. [DOI] [PubMed] [Google Scholar]
- 27.Forman H.J., Azzi A. On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J. 1997;11:374–375. doi: 10.1096/fasebj.11.5.9141504. [DOI] [PubMed] [Google Scholar]
- 28.Forman H.J., Boveris A. Superoxide radical and hydrogen peroxide in mitochondria. In: Pryor W.A., editor. Free Radicals in Biology. Academic Press; New York: 1982. pp. 65–90. [Google Scholar]
- 29.Muoio D.M. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159:1253–1262. doi: 10.1016/j.cell.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 31.Perry R.J., Zhang D., Zhang X.M., Boyer J.L., Shulman G.I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy M.P. Modulating mitochondrial intracellular location as a redox signal. Sci. Signal. 2012;5:pe39. doi: 10.1126/scisignal.2003386. [DOI] [PubMed] [Google Scholar]
- 33.Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodson M., Darley-Usmar V., Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Church D.F., Pryor W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedard L., Young M.J., Hall D., Paul T., Ingold K.U. Quantitative studies on the peroxidation of human low-density lipoprotein initiated by superoxide and by charged and neutral alkylperoxyl radicals. J. Am. Chem. Soc. 2001;123:12439–12448. doi: 10.1021/ja011076d. [DOI] [PubMed] [Google Scholar]
- 37.Ursini F., Maiorino M., Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 38.Lands W.E. Interactions of lipid hydroperoxides with eicosanoid biosynthesis. J. Free Radic. Biol. Med. 1985;1:97–101. doi: 10.1016/0748-5514(85)90012-1. [DOI] [PubMed] [Google Scholar]
- 39.Schnurr K., Belkner J., Ursini F., Schewe T., Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 40.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov I., Kuhn H., Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15) Gene. 2015;573:1–32. doi: 10.1016/j.gene.2015.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pekarova M., Kuhn H., Bezakova L., Ufer C., Heydeck D. Mutagenesis of triad determinants of rat Alox15 alters the specificity of fatty acid and phospholipid oxygenation. Arch. Biochem. Biophys. 2015;571:50–57. doi: 10.1016/j.abb.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Kulmacz R.J., Lands W.E. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins. 1983;25:531–540. doi: 10.1016/0090-6980(83)90025-4. [DOI] [PubMed] [Google Scholar]
- 44.Forman H.J. Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction. Ann. N. Y. Acad. Sci. 2010;1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida K., Shiraishi M., Naito Y., Torii Y., Nakamura Y., Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-Hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J. Biol. Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 46.Poli G., Schaur R.J., Siems W.G., Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- 47.Kirichenko A., Li L., Morandi M.T., Holian A. 4-Hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol. Appl. Pharmacol. 1996;141:416–424. doi: 10.1006/taap.1996.0307. [DOI] [PubMed] [Google Scholar]
- 48.Rahman I., van Schadewijk A.A., Crowther A.J., Hiemstra P.S., Stolk J., MacNee W., De Boer W.I. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 49.Strohmaier H., Hinghofer-Szalkay H., Schaur R.J. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J. Lipid Mediat. Cell Signal. 1995;11:51–61. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Court N., Forman H.J. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiler A., Schneider M., Forster H., Roth S., Wirth E.K., Culmsee C., Plesnila N., Kremmer E., Radmark O., Wurst W., Bornkamm G.W., Schweizer U., Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doorn J.A., Petersen D.R. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- 54.Paulsen C.E., Carroll K.S. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem. Rev. 2013;113:4633–4679. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wouters M.A., Fan S.W., Haworth N.L. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid. Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 56.Yoo M.H., Gu X., Xu X.M., Kim J.Y., Carlson B.A., Patterson A.D., Cai H., Gladyshev V.N., Hatfield D.L. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer's disease. Antioxid. Redox Signal. 2010;12:819–827. doi: 10.1089/ars.2009.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mann G.E., Forman H.J., Yamamoto M., Kensler T.W., Hayes J.D. Special issue: Nrf2 regulated redox signaling and metabolism in physiology and medicine. Free Radic. Biol Med. 2015;88(Part B):91–480. doi: 10.1016/j.freeradbiomed.2015.08.002. Editors. [DOI] [PubMed] [Google Scholar]
- 58.Belcher J.D., Beckman J.D., Balla G., Balla J., Vercellotti G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franco R., Cidlowski J.A. Glutathione efflux and cell death. Antioxid. Redox Signal. 2012;17:1694–1713. doi: 10.1089/ars.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon M.Y., Park E., Lee S.J., Chung S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393–24403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galluzzi L., Bravo-San Pedro J.M., Kroemer G. Ferroptosis in p53-dependent oncosuppression and organismal homeostasis. Cell Death Differ. 2015;22:1237–1238. doi: 10.1038/cdd.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Hayes J.D. The membrane-topogenic vectorial behaviour of Nrf1 controls its post-translational modification and transactivation activity. Sci. Rep. 2013;3:2006. doi: 10.1038/srep02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biswas M., Chan J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L., Kwong M., Lu R., Ginzinger D., Lee C., Leung L., Chan J.Y. Nrf1 is critical for redox balance and survival of liver cells during development. Mol. Cell. Biol. 2003;23:4673–4686. doi: 10.1128/MCB.23.13.4673-4686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 69.Schreck R., Albermann K., Baeuerle P.A. Nuclear factor kB: an oxidative stress-responsive transcription factor of eukaryotic cells. Free Radic. Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 70.Kaul N., Forman H.J. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic. Biol. Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 71.Gloire G., Charlier E., Rahmouni S., Volanti C., Chariot A., Erneux C., Piette J. Restoration of SHIP-1 activity in human leukemic cells modifies NF-κB activation pathway and cellular survival upon oxidative stress. Oncogene. 2006;25:5485–5494. doi: 10.1038/sj.onc.1209542. [DOI] [PubMed] [Google Scholar]
- 72.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Premasekharan G., Nguyen K., Contreras J., Ramon V., Leppert V.J., Forman H.J. Iron-mediated lipid peroxidation and lipid raft disruption in low-dose silica-induced macrophage cytokine production. Free Radic. Biol. Med. 2011;51:1184–1194. doi: 10.1016/j.freeradbiomed.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Liu H., Zhang H., Forman H.J. Silica induces macrophage cytokines through phosphatidylcholine-specific phospholipase C with hydrogen peroxide. Am. J. Respir. Cell Mol. Biol. 2007;36:594–599. doi: 10.1165/rcmb.2006-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monick M.M., Carter A.B., Gudmundsson G., Mallampalli R., Powers L.S., Hunninghake G.W. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide- stimulated human alveolar macrophages. J. Immunol. 1999;162:3005–3012. [PubMed] [Google Scholar]
- 76.Carter A.B., Monick M.M., Hunninghake G.W. Lipopolysaccharide-induced NF-kappaB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am. J. Respir. Cell Mol. Biol. 1998;18:384–391. doi: 10.1165/ajrcmb.18.3.2972. [DOI] [PubMed] [Google Scholar]
- 77.Girotti A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 78.Imai H. Biological significance of lipid hydroperoxide and its reducing enzyme, phospholipid hydroperoxide glutathione peroxidase, in mammalian cells. Yakugaku Zasshi: J. Pharm. Soc. Jpn. 2004;124:937–957. doi: 10.1248/yakushi.124.937. [DOI] [PubMed] [Google Scholar]
- 79.Brunelli D., Tavecchio M., Falcioni C., Frapolli R., Erba E., Iori R., Rollin P., Barillari J., Manzotti C., Morazzoni P., D’Incalci M. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010;79:1141–1148. doi: 10.1016/j.bcp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Cavell B.E., Syed Alwi S.S., Donlevy A., Packham G. Anti-angiogenic effects of dietary isothiocyanates: mechanisms of action and implications for human health. Biochem. Pharmacol. 2011;81:327–336. doi: 10.1016/j.bcp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Kivela A.M., Makinen P.I., Jyrkkanen H.K., Mella-Aho E., Xia Y., Kansanen E., Leinonen H., Verma I.M., Yla-Herttuala S., Levonen A.L. Sulforaphane inhibits endothelial lipase expression through NF-kappaB in endothelial cells. Atherosclerosis. 2010;213:122–128. doi: 10.1016/j.atherosclerosis.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 83.Ishimura A., Ishige K., Taira T., Shimba S., Ono S., Ariga H., Tezuka M., Ito Y. Comparative study of hydrogen peroxide- and 4-hydroxy-2-nonenal-induced cell death in HT22 cells. Neurochem. Int. 2008;52:776–785. doi: 10.1016/j.neuint.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Parola M., Robino G., Marra F., Pinzani M., Bellomo G., Leonarduzzi G., Chiarugi P., Camandola S., Poli G., Waeg G., Gentilini P., Dianzani M.U. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J. Clin. Investig. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karin M., Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 86.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal- regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 88.Liu H., Zhang H., Iles K.E., Rinna A., Merrill G., Yodoi J., Torres M., Forman H.J. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic. Res. 2006;40:865–874. doi: 10.1080/10715760600758514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim S.Y., Kim T.J., Lee K.Y. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett. 2008;582:1913–1918. doi: 10.1016/j.febslet.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Rinna A., Forman H.J. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am. J. Respir. Cell Mol. Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dummler B., Hemmings B.A. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 92.Lee S.R., Yang K.S., Kwon J., Lee C., Jeong W., Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 93.Cruz C.M., Rinna A., Forman H.J., Ventura A.L., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dillon L.M., Miller T.W. Therapeutic targeting of cancers with loss of PTEN function. Curr. Drug Targets. 2014;15:65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornier M.A., Dabelea D., Hernandez T.L., Lindstrom R.C., Steig A.J., Stob N.R., Van Pelt R.E., Wang H., Eckel R.H. The metabolic syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Contreras-Ferrat A., Lavandero S., Jaimovich E., Klip A. Calcium signaling in insulin action on striated muscle. Cell Calcium. 2014;56:390–396. doi: 10.1016/j.ceca.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 99.Pina-Zentella G., de la Rosa-Cuevas G., Vazquez-Meza H., Pina E., de Pina M.Z. Taurine in adipocytes prevents insulin-mediated H2O2 generation and activates Pka and lipolysis. Amino Acids. 2012;42:1927–1935. doi: 10.1007/s00726-011-0919-x. [DOI] [PubMed] [Google Scholar]
- 100.Contreras-Ferrat A., Llanos P., Vasquez C., Espinosa A., Osorio-Fuentealba C., Arias-Calderon M., Lavandero S., Klip A., Hidalgo C., Jaimovich E. Insulin elicits a ROS-activated and an IP(3)-dependent Ca(2)(+) release, which both impinge on GLUT4 translocation. J. Cell Sci. 2014;127:1911–1923. doi: 10.1242/jcs.138982. [DOI] [PubMed] [Google Scholar]
- 101.Meng D., Mei A., Liu J., Kang X., Shi X., Qian R., Chen S. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS One. 2012;7:e48393. doi: 10.1371/journal.pone.0048393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Mouche S., Sajic T., Veyrat-Durebex C., Supale R., Pierroz D., Ferrari S., Negro F., Hasler U., Feraille E., Moll S., Meda P., Deffert C., Montet X., Krause K.H., Szanto I. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int. J. Obes. 2012;36:1503–1513. doi: 10.1038/ijo.2011.279. [DOI] [PubMed] [Google Scholar]
- 103.Lee J.Y., Sohn K.H., Rhee S.H., Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 104.Ajuwon K.M., Spurlock M.E. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J. Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 105.Hummasti S., Hotamisligil G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010;107:579–591. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 106.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 107.Blagosklonny M.V. Koschei the immortal and anti-aging drugs. Cell Death Dis. 2014;5:e1552. doi: 10.1038/cddis.2014.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 109.Johnson A.M., Olefsky J.M. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 110.Chartoumpekis D.V., Kensler T.W. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr. Diabetes Rev. 2013;9:137–145. doi: 10.2174/1573399811309020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida S., Hong S., Suzuki T., Nada S., Mannan A.M., Wang J., Okada M., Guan K.L., Inoki K. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J. Biol. Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Appenzeller-Herzog C., Hall M.N. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Shao D., Oka S., Liu T., Zhai P., Ago T., Sciarretta S., Li H., Sadoshima J. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab. 2014;19:232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hwang J.W., Yao H., Caito S., Sundar I.K., Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarbassov D.D., Sabatini D.M. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J. Biol. Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 116.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 117.Gao P., Zhang H., Dinavahi R., Li F., Xiang Y., Raman V., Bhujwalla Z.M., Felsher D.W., Cheng L., Pevsner J., Lee L.A., Semenza G.L., Dang C.V. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 119.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tobe R., Carlson B.A., Tsuji P.A., Lee B.J., Gladyshev V.N., Hatfield D.L. Differences in redox regulatory systems in human lung and liver tumors suggest different avenues for therapy. Cancers (Basel) 2015;7:2262–2276. doi: 10.3390/cancers7040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hayes J.D., McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 122.NF-kB Inducers. NF-kB Transcription Factors: Boston University Biology.
- 123.Anrather J., Racchumi G., Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 124.Hordijk P.L. Regulation of NADPH oxidases: the role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 125.Xie Q.W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 126.NF-kB Inhibitors. NF-kB Transcription Factors: Boston University Biology.
- 127.Zhang H., Forman H.J. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol. Ind. Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 129.Esterbauer H., Cheeseman K.H., Dianzani M.U., Poli G., Slater T. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by Fe2+ in rat liver microsomes. Biochem. J. 1982;208:129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Richman P.G., Meister A. Regulation of g-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- 131.Shi M.M., Iwamoto T., Forman H.J. g-Glutamylcysteine synthetase and glutathione increase in quinone-induced oxidative stress in bovine pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1994;267:L414–L421. doi: 10.1152/ajplung.1994.267.4.L414. [DOI] [PubMed] [Google Scholar]
- 132.Shi M.M., Kugelman A., Iwamoto T., Tian L., Forman H.J. Quinone-induced oxidative stress elevates glutathione and induces g-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J. Biol. Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- 133.Sekhar K.R., Spitz D.R., Harris S., Nguyen T.T., Meredith M.J., Holt J.T., Gius D., Marnett L.J., Summar M.L., Freeman M.L., Guis D. Redox-sensitive interaction between KIAA0132 and Nrf2 mediates indomethacin-induced expression of gamma-glutamylcysteine synthetase. Free Radic. Biol. Med. 2002;32:650–662. doi: 10.1016/s0891-5849(02)00755-4. [DOI] [PubMed] [Google Scholar]
- 134.Kwak M.K., Wakabayashi N., Itoh K., Motohashi H., Yamamoto M., Kensler T.W. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 135.McMahon M., Itoh K., Yamamoto M., Hayes J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 136.Huang H.C., Nguyen T., Pickett C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaiswal A.K. NRF2 signaling in coordinated activation of antioxidant genes expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 138.Kang K.W., Choi S.H., Kim S.G. Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: the role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric Oxide. 2002;7:244–253. doi: 10.1016/s1089-8603(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 139.Martin D., Rojo A.I., Salinas M., Diaz R., Gallardo G., Alam J., De Galarreta C.M., Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H., Forman H.J. Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- 141.Bloom D.A., Jaiswal A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 142.Niture S.K., Jain A.K., Jaiswal A.K. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Nguyen T., Huang H.C., Pickett C.B. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 144.Venugopal R., Jaiswal A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 145.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. J. Clin. Investig. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang H., Ramani K., Xia M., Ko K.S., Li T.W., Oh P., Li J., Lu S.C. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49:1982–1991. doi: 10.1002/hep.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dickinson D.A., Iles K.E., Zhang H., Blank V., Forman H.J. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 148.Wild A.C., Moinova H.R., Mulcahy R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 149.Dhakshinamoorthy S., Jain A.K., Bloom D.A., Jaiswal A.K. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J. Biol. Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 150.Levy S., Forman H.J. c-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen W., Sun Z., Wang X.J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang T., Harder B., Rojo de la Vega M., Wong P.K., Chapman E., Zhang D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 154.Perkins N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 155.Maxwell M.J., Yuan Y., Anderson K.E., Hibbs M.L., Salem H.H., Jackson S.P. SHIP1 and Lyn Kinase Negatively Regulate Integrin alpha IIb beta 3 signaling in platelets. J. Biol. Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 156.Ando K., Hirao S., Kabe Y., Ogura Y., Sato I., Yamaguchi Y., Wada T., Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flaherty D.M., Monick M.M., Carter A.B., Peterson M.W., Hunninghake G.W. GM-CSF increases AP-1 DNA binding and Ref-1 amounts in human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2001;25:254–259. doi: 10.1165/ajrcmb.25.2.4446. [DOI] [PubMed] [Google Scholar]