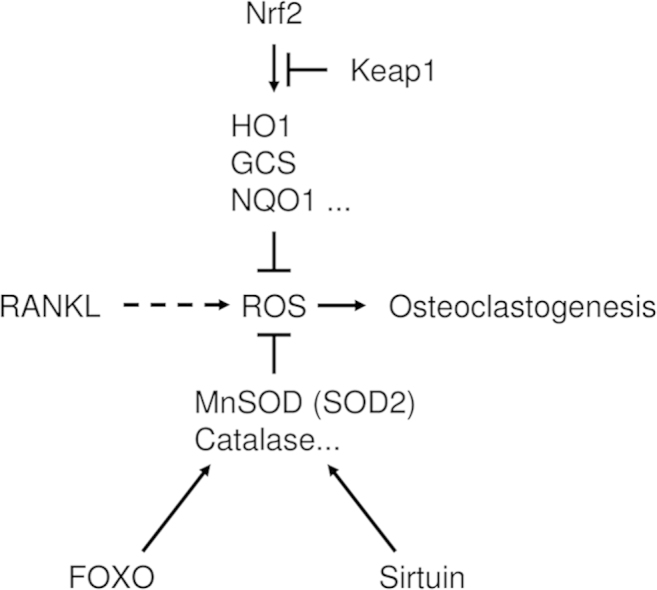
Abstract
It has been reported that reactive oxygen species (ROS), such as hydrogen peroxide and superoxide, take part in osteoclast differentiation as intra-cellular signaling molecules. The current assumed signaling cascade from RANK to ROS production is RANK, TRAF6, Rac1, and then Nox. The target molecules of ROS in RANKL signaling remain unclear; however, several reports support the theory that NF-κB signaling could be the crucial downstream signaling molecule of RANKL-mediated ROS signaling. Furthermore, ROS exert cytotoxic effects such as peroxidation of lipids and phospholipids and oxidative damage to proteins and DNA. Therefore, cells have several protective mechanisms against oxidative stressors that mainly induce cytoprotective enzymes and ROS scavenging. Three well-known mechanisms regulate cytoprotective enzymes including Nrf2-, FOXO-, and sirtuin-dependent mechanisms. Several reports have indicated a crosslink between FOXO- and sirtuin-dependent regulatory mechanisms. The agonists against the regulatory mechanisms are reported to induce these cytoprotective enzymes successfully. Some of them inhibit osteoclast differentiation and bone destruction via attenuation of intracellular ROS signaling. In this review article, we discuss the above topics and summarize the current information available on the relationship between cytoprotective enzymes and osteoclastogenesis.
Keywords: Osteoclast, Oxidative stress, Heme-oxygenase 1 (HO-1), Nrf2, FOXO, Sirtuin
Graphical abstract
Highlights
-
•
Three essential regulatory mechanism for cytoprotective enzymes were reported.
-
•
They are Nrf2, SIRT, and FOXO.
-
•
Intracellular ROS is used as signaling molecule during osteoclastogenesis.
-
•
Cytoprotective enzymes attenuates osteoclastogenesis by ROS scavenging.
-
•
Nrf2, SIRT, and FOXO could be therapeutic target for bone destructive disease.
1. Introduction
Osteoclasts are multi-nucleated cells that resorb bone tissue [1] and are differentiated from macrophage–monocyte cell lines [2]. Osteoclast differentiation, namely osteoclastogenesis, is strictly regulated by receptor activator of nuclear factor kappa-B ligand (RANKL), an osteoclastogenic signaling cytokine [3]. Reactive oxygen species (ROS), such as hydrogen peroxide and superoxide, work as intracellular signaling molecules following RANKL signaling during osteoclastogenesis [4], [5], [6]. However, apart from their role as intracellular signaling molecules, ROS exert cytotoxic effects such as peroxidation of lipids and phospholipids [7], and oxidative damage to proteins and DNA [8]. Therefore, cells have several protective mechanisms against these oxidative stressors [9], [10], [11] most of which induce cytoprotective enzymes [12], [13], [14], [15], [16], [17], [18], [19], [20] and ROS scavenging. Taken together, it is thought that cytoprotective mechanisms are attenuated during osteoclastogenesis to intensify intracellular ROS signaling.
In this review article, we have summarized the relationship between osteoclastogenesis and the protective mechanisms that work against oxidative stressors.
2. ROS work as intracellular signaling molecules during osteoclastogenesis
RANKL is an essential cytokine in osteoclastogenesis [1], [21], [22], [23], and various intracellular signaling molecules, such as nuclear factor of activated T-cells (NFAT) [24], mitogen-activated protein kinase (MAPK) [25], [26], tumor necrosis factor receptor-associated factor (TRAF) [27], [28], c-jun N-terminal kinase (JNK) [29], Akt [30], and ROS [4], [5] have been identified. ROS are interesting molecules because not only do they work as intracellular signaling molecules, but also they increase with age or with the onset of an inflammatory state, which subsequently leads to bone destruction [31], [32], [33], [34], [35], [36], [37]. In addition, exogenous hydrogen peroxide induces osteoclastogenesis [38], signifying that oxidative stress participates in the regulation of osteoclastogenesis from both within the cytoplasm and extracellularly.
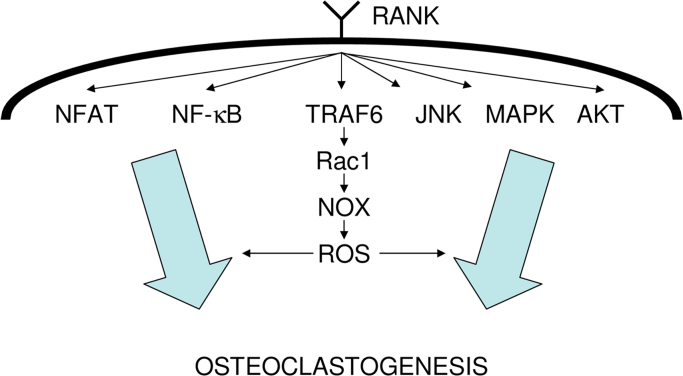
It is reported that TRAF6 plays a key linkage role in ROS production by RANKL [39]. We reported that dominant-interfering mutant form of TRAF6, significantly decreased ROS induction, although TRAF6 itself does not directly produce ROS. Rac, a functional downstream molecule and member of the Rho-GTPase subfamily, which is involved in the organization of the cytoskeleton, is a cytosolic component of NADPH oxidase (NOX) complex and responsible for the activation of NOXs [40]. The expression of a dominant-negative mutant of Rac1 blocks ROS production, signifying that Rac1 is responsible for regulating the generation of ROS during osteoclast differentiation [41]. In addition, NOXs have been reported as essential enzymes that produce ROS during osteoclast differentiation [42], [43], [44]. Taken together, the current assumed signaling cascade from RANK to ROS production is RANK, TRAF6, Rac1, and then NOX.
The target molecules of ROS in RANKL signaling remain unclear; however, several reports have suggested that MAPK, PI3K, and NF-kB activation are downstream events [45], [46]. Additionally, Bharti et al. reported that curcumin, which has ROS-scavenging properties, inhibits RANKL-induced NF-κB activation, which indicates that NF-κB signaling could be the crucial downstream signaling molecule of RANKL-mediated ROS signaling [47]. Current information about the intracellular signaling cascade of RANKL is summarized in Fig. 1.
Fig. 1.
Summary of the current information about the intracellular signaling cascade of RANKL. Intracellular signaling molecules after RANK were identified. The current assumed signaling cascade from RANK to ROS production is also described. Some reports suggest that NF-κB is the crucial downstream molecule of RANKL-mediated ROS signaling.
3. Defense mechanisms against ROS
As mentioned previously, ROS exhibit cytotoxicity [7], [8]; therefore, cells have several protective mechanisms against these oxidative stressors that mainly induce cytoprotective enzymes and ROS scavenging. The mechanisms regulating cytoprotective enzymes are summarized in Table 1.
Table 1.
Regulatory mechanisms of cytoprotective enzymes.
| Regulator | Cell type/experimental model | Tested function/findings | References |
|---|---|---|---|
| Nrf2 | L929 fibroblast, mutant Nrf2 expression | Mutant Nrf2 decreased HO-1 | [13] |
| Rat NQO-1 gene, promoter assay | Nrf2 regulated NQO-1 | [14] | |
| Human GCS gene, promoter assay | Nrf2 regulated GCS | [15] | |
| Nrf2 knockout mice | Nrf2 KO decreased NQO-1 and GCS | [16] | |
| FOXO | Breast cancer cells | FOXO3 regulates MnSOD | [49] |
| Primary mammalian neurons | MST-FOXO axis controls oxidative-stress response | [50] | |
| C. elegans and mice gene | pp66shc controls oxidative-stress response via FOXO3 (FKHRL1) | [51] | |
| Mutant mice | Insulin/IGF-1-FOXO pathway relates oxidative-stress Response | [52] | |
| Mammalian cells | Mammalian SIRT1 deacetylates FOXO3 and/or FOXO4 | [53] | |
| Mammalian cells | FOXO3 directly increase MnSOD | [54] | |
| Mouse NIH3T3 cells | ROS-Ral-JNK axis mediates FOXO4-dependent MnSOD upregulation | [55] | |
| SIRT | |||
| Human HEK293 cells | SIRT3 deacetylates and activates MnSOD | [60] | |
| Mammalian cells | SIRT3 activates MnSOD | [61] | |
| Renal tubular cells | SIRT1 activates catalase via FOXO3 | [62] | |
| Mammalian cells | SIRT1 activates MnSOD via FOXO4 | [64] | |
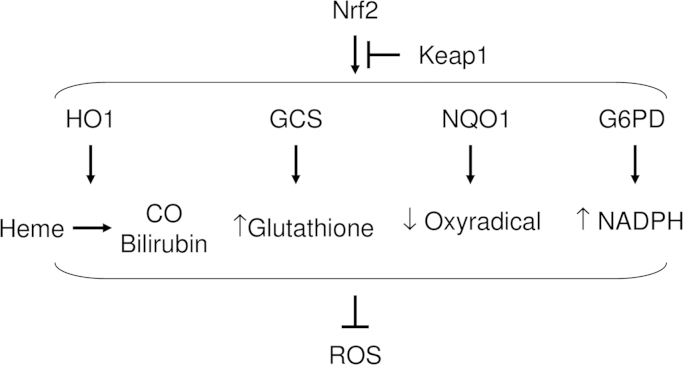
The most renowned regulator of cytoprotective enzymes is transcriptional factor nuclear factor E2-related factor 2 (Nrf2), which controls the gene expression of many cytoprotective enzymes, such as heme oxygenase-1 (HO-1) [13], NAD (P) H: quinone reductase (NQO1) [14], gamma-glutamylcysteine synthetase (GCS) [15], and the auxiliary cellular NADPH regenerating enzyme, glucose 6-phosphate dehydrogenase (G6PD) [16] (Fig. 2); all of these enzymes are ROS scavengers [17], [18], [19], [20]. However, kelch-like ECH-associated protein 1 (Keap1) negatively regulates Nrf2-dependent transcription of cytoprotective enzymes by inhibiting nuclear translocation, cytoplasmic ubiquitination, and degradation of Nrf2 [48].
Fig. 2.
Nrf2-mediated cytoprotective enzymes scavenge ROS. Nrf2 transcriptionally regulates the expressions of HO1, GCS, NQO1, and G6PD. HO1 convert heme into carbon oxide (CO) and bilirubin, and they scavenge ROS. GCS increases intracellular glutathione, which results in ROS scavenging. NQO1 reduces oxyradicals. G6PD increases intracellular NADPH, which augments ROS scavenging.
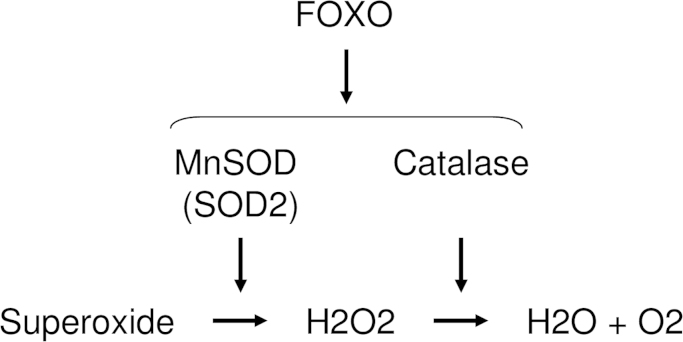
FOXO ubiquitous transcription factors, which are dephosphorylated and subsequently activated by oxidative stress, are involved in the regulation of redox balance [49], [50], [51], [52], [53]. It is reported that oxidative stress activates FOXO via mammalian Ste20-like kinases [50] and p66shc [51]. In addition, FOXO3 and 4 regulate the expression of superoxide dismutase (SOD) [51], [54] and catalase (CAT) [55], and SOD converts superoxide to hydrogen peroxide [56], which is subsequently detoxified by CAT (Fig. 3) [57]. Three isozymes of SOD—SOD1, 2, and 3—have been identified and characterized in mammals [58]. SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular. SOD1 and SOD3 are Cu-Zn-SOD types, whereas SOD2 is Mn-SOD.
Fig. 3.
FOXO-mediated cytoprotective enzymes scavenge ROS. FOXO regulates the expressions of MnSOD (SOD2) and catalase (CAT). MnSOD convert superoxide into H2O2, followed by the conversion into H2O and O2 by CAT.
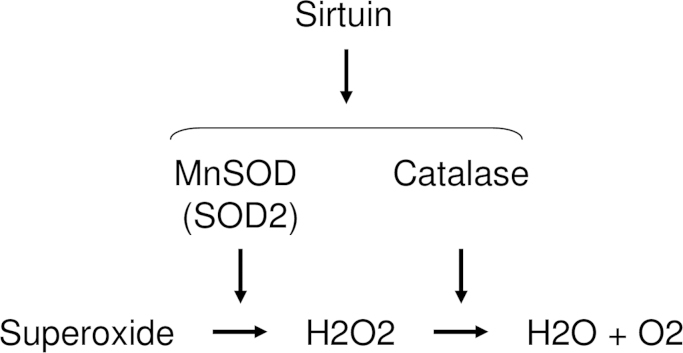
Sirtuin, which was originally identified as a protein deacetylase [59], is also a regulator of the expression of cytoprotective enzymes such as SOD [60], [61] and CAT (Fig. 4) [62]. Mammalian sirtuins consist of seven members (SIRT1–7), and have been implicated in various cellular responses including aging, transcription, apoptosis, and stress resistance [63]. Among them, the functions of Sirt1 and 3 in oxidative stress responses have been reported. SIRT1 deacetylates FOXO3 and 4 [53], which results in the upregulation of Mn-SOD [64]. Furthermore, Olmos et al. reported that SIRT1-dependent upregulation of cytoprotective enzymes depended on the formation of a FOXO3a/PGC-1α complex in vascular endothelial cells [65]. However, Chen et al. reported that SIRT3 directly upregulated SOD2 [60]. Regarding the crosslinking between SIRT and Nrf2, Huang et al. reported that SIRT1 upregulated HO-1 and SOD1 via induction of Nrf2 [66]. Overall, sirtuins, especially SIRT1 and 3, directly or indirectly regulate cytoprotective enzymes.
Fig. 4.
SIRT-mediated cytoprotective enzymes scavenge ROS. SIRT regulates the expressions of MnSOD (SOD2) and catalase (CAT). MnSOD convert superoxide into H2O2, followed by the conversion into H2O and O2 by CAT.
4. Cytoprotective enzymes and osteoclastogenesis
Since ROS operate as intracellular signaling molecules during osteoclastogenesis, a close relationship between osteoclastogenesis and cytoprotective enzymes is to be expected. Indeed, a well-known cytoprotective enzyme, HO-1, is a negative regulator of osteoclastogenesis [67], [68], [69]. Relationships between the mechanisms regulating cytoprotective enzymes and osteoclastogenesis have also been reported. Rana et al. reported that loss of Nrf2 accelerates ionizing radiation-induced bone loss in Nrf2 knockout mice [70]. Other groups have reported that Nrf2 negatively regulates osteoclastogenesis through attenuation of RANKL-mediated intracellular ROS signaling by cytoprotective enzymes [71], [72]. Furthermore, we previously reported that overexpression of Nrf2 induces the expression of cytoprotective enzymes, attenuates intracellular ROS signaling, and thereby inhibits osteoclastogenesis [71]. Both overexpression of Nrf2 and Nrf2 activation (induction of nuclear translocation) inhibit osteoclastogenesis [6], [73], [74]. These lines of evidence suggest that Nrf2 activation could be a therapeutic approach towards bone destructive diseases such as rheumatoid arthritis, osteoporosis, and periodontitis.
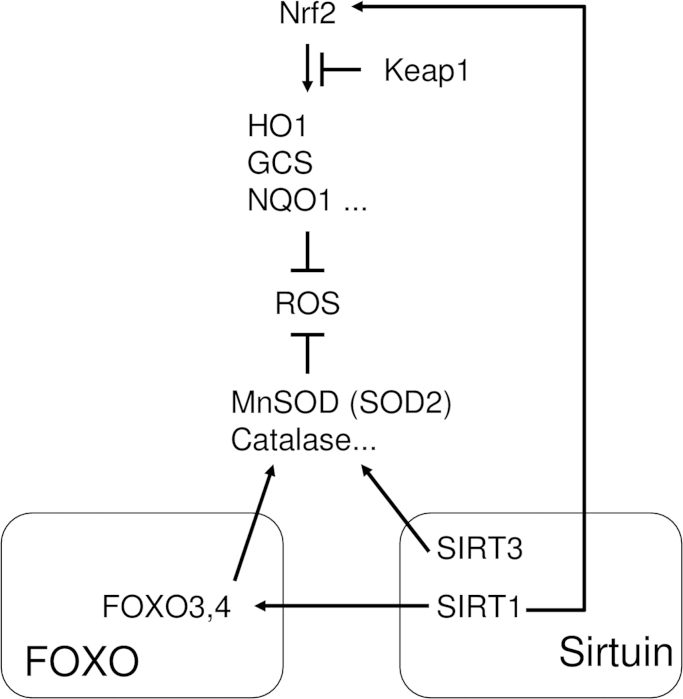
Another mechanism regulating cytoprotective enzyme FOXO contributes to the control of osteoclastogenesis. Bartell et al. reported that FOXO protein attenuates osteoclastogenesis via augmentation of cytoprotective enzymes [75]. Sirtuins, originally identified as protein deacetylases, have been reported as suppressors of osteoclastogenesis. SIRT1 suppresses osteoclastogenesis by the upregulation of cytoprotective enzymes via FOXO-mediated transcription and subsequent attenuation of intracellular ROS signaling [76]. Lee et al. reported that the overexpression of SIRT6, an NAD (+)-dependent deacetylase, suppresses bone destruction in a collagen-induced arthritis mouse model [77]. These lines of evidence suggest that the key molecule among the mechanisms regulating cytoprotective enzymes (Nrf2, FOXO, and sirtuin) negatively regulates osteoclastogenesis via attenuation of intracellular ROS signaling (Fig. 5).
Fig. 5.
Nrf2, FOXO, and sirtuin negatively regulate osteoclastogenesis via attenuation of intracellular ROS signaling. Nrf2 regulates the transcription of cytoprotective enzymes and ROS scavenging. However, Keap1 attenuates cytoprotective enzymes via degradation of Nrf2. Other mechanisms regulating cytoprotective enzymes have roles in ROS regulation: FOXO directly, and sirtuin directly and indirectly (via FOXO).
5. Regulatory mechanisms of potential pharmacological targets for bone destructive diseases
As discussed above, osteoclasts also possess mechanisms that regulate cytoprotective enzymes, which manage the intracellular ROS levels. Since intracellular ROS play a role in RANKL-mediated osteoclastogenesis, the mechanisms that regulate cytoprotective enzymes negatively control osteoclastogenesis via ROS scavenging mediated by cytoprotective enzymes. In other words, osteoclastogenesis is controlled via interference with the mechanisms regulating cytoprotective enzymes.
Indeed, some papers report that the activation of Nrf2 inhibits osteoclastogenesis [6], [73], [74]. The pharmacological activation of Nrf2 has been extensively explored in cancer research and chemical detoxification fields, thus potential Nrf2 activators such as sulforaphane [78], epigallocatechin gallate [79], curcumin [80], and N-acetylcysteine [81] are well-documented and known to inhibit bone destruction [72], [82], [83], [84]. Another regulatory molecule, FOXO, is also a potential therapeutic target for bone destructive diseases. Statins, HMG-CoA reductase inhibitors, induce FOXO phosphorylation [85] and exhibit osteoclastogenesis by ROS scavenging [86]. Regarding sirtuin-mediated regulatory mechanisms, resveratrol, an agonist of SIRT1 [87], inhibits osteoclastogenesis through the attenuation of ROS production [88], [89], [90]. Indeed, sirtuin activators such as resveratrol or other synthesized chemicals inhibit bone destruction [91], [92], [93], [94]. Some of the chemicals reported to activate cytoprotective enzymes and thereby inhibit bone destruction are summarized in Table 2.
Table 2.
Reported chemicals that can activate cytoprotective enzymes and thereby inhibit bone destruction.
6. Summary and perspective
In this review manuscript, we have summarized recent information about the relationship between osteoclastogenesis and the mechanisms regulating cytoprotective enzymes. Although some parts have been extensively explored, further investigations are necessary to gain a greater understanding. In particular, crosstalk among the mechanisms regulating cytoprotective enzymes and other signaling molecules should be elucidated.
Since some of the agonists that affect the mechanisms regulating cytoprotective enzymes have been reported as inhibitors of bone destruction, these chemicals could be potential drugs for the treatment for bone destructive diseases in the near future.
Acknowledgments
This research was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23689081, 25670841, and 15K11376), the Nestle Nutrition Council, Japan, the Translational Research Network Program from the Japanese Ministry of Education, Culture, Sports, Science and Technology (A-03), the Adaptable and Seamless Technology Transfer Program through Target-driven R&D from the Japan Science and Technology Agency (AS231Z01205G), and the Astellas Foundation for Research on Metabolic Disorders. The authors have no conflicts of interest.
Finally, the authors (HK and FS) give their heartfelt appreciation to the experimental reagent and instrument companies for their support during the rehabilitation process following the damage caused by the Tohoku Earthquake on March 11, 2011.
References
- 1.Roodman G.D. Advances in bone biology: the osteoclast. Endocr. Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum S.L., Tondravi M.M., Ross F.P. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J. Leukoc. Biol. 1997;61:381–388. doi: 10.1002/jlb.61.4.381. [DOI] [PubMed] [Google Scholar]
- 3.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y.X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W.J. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 4.Bax B.E., Alam A.S., Banerji B., Bax C.M., Bevis P.J., Stevens C.R., Moonga B.S., Blake D.R., Zaidi M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992;183:1153–1158. doi: 10.1016/s0006-291x(05)80311-0. [DOI] [PubMed] [Google Scholar]
- 5.Ha H., Kwak H.B., Lee S.W., Jin H.M., Kim H.M., Kim H.H., Lee Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004;301:119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Kanzaki H., Shinohara F., Kajiya M., Fukaya S., Miyamoto Y., Nakamura Y. Nuclear nrf2 induction by protein transduction attenuates osteoclastogenesis. Free Radic. Biol. Med. 2014;77:239–248. doi: 10.1016/j.freeradbiomed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Wells P.G., McCallum G.P., Chen C.S., Henderson J.T., Lee C.J.J., Perstin J., Preston T.J., Wiley M.J., Wong A.W. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- 9.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa-Hibi Y., Kobayashi Y., Chen C., Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid. Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C.P., Odewale I., Alcendor R.R., Sadoshima J. Sirt1 protects the heart from aging and stress. Biol. Chem. 2008;389:221–231. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 12.Mates J.M., Sanchez-Jimenez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front. Biosci. 1999;4:D339–D345. doi: 10.2741/mates. [DOI] [PubMed] [Google Scholar]
- 13.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Nrf2 Cook J.L. a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 14.Favreau L.V., Pickett C.B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 15.Wild A.C., Gipp J.J., Mulcahy T. Overlapping antioxidant response element and PMA response element sequences mediate basal and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase catalytic subunit gene. Biochem. J. 1998;332(2):373–381. doi: 10.1042/bj3320373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 17.Zhang S., Song C., Zhou J., Xie L., Meng X., Liu P., Cao J., Zhang X., Ding W.Q., Wu J. Amelioration of radiation-induced skin injury by adenovirus-mediated heme oxygenase-1 (HO-1) overexpression in rats. Radiat. Oncol. 2012;7:4. doi: 10.1186/1748-717X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pazdro R., Burgess J.R. The antioxidant 3H-1,2-dithiole-3-thione potentiates advanced glycation end-product-induced oxidative stress in SH-SY5Y cells. Exp. Diabetes Res. 2012;2012:137607. doi: 10.1155/2012/137607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akazawa S. Diabetic embryopathy: studies using a rat embryo culture system and an animal model. Congenit. Anom. 2005;45:73–79. doi: 10.1111/j.1741-4520.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- 20.Gurer-Orhan H., Orhan H., Suzen S., Puskullu M.O., Buyukbingol E. Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J. Enzyme Inhib. Med. Chem. 2006;21:241–247. doi: 10.1080/14756360600586031. [DOI] [PubMed] [Google Scholar]
- 21.Wong B.R., Rho J., Arron J., Robinson E., Orlinick J., Chao M., Kalachikov S., Cayani E., Bartlett F.S., 3rd, Frankel W.N., Lee S.Y., Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller K., Wong B., Fox S., Choi Y., Chambers T.J. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J. Exp. Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirotani H., Tuohy N.A., Woo J.T., Stern P.H., Clipstone N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 2004;279:13984–13992. doi: 10.1074/jbc.M213067200. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T., Katagiri H., Kanegae Y., Takayanagi H., Sawada Y., Yamamoto A., Pando M.P., Asano T., Verma I.M., Oda H., Nakamura K., Tanaka S. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J. Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.E., Woo K.M., Kim S.Y., Kim H.M., Kwack K., Lee Z.H., Kim H.H. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30:71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- 27.Wong B.R., Josien R., Lee S.Y., Vologodskaia M., Steinman R.M., Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 28.Darnay B.G., Haridas V., Ni J., Moore P.A., Aggarwal B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 29.Kim H.H., Lee D.E., Shin J.N., Lee Y.S., Jeon Y.M., Chung C.H., Ni J., Kwon B.S., Lee Z.H. Receptor activator of NF-kappaB recruits multiple TRAF family adaptors and activates c-Jun N-terminal kinase. FEBS Lett. 1999;443:297–302. doi: 10.1016/s0014-5793(98)01731-1. [DOI] [PubMed] [Google Scholar]
- 30.Wong B.R., Besser D., Kim N., Arron J.R., Vologodskaia M., Hanafusa H., Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 31.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Altindag O., Erel O., Soran N., Celik H., Selek S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2008;28:317–321. doi: 10.1007/s00296-007-0452-0. [DOI] [PubMed] [Google Scholar]
- 33.Almeida M., Han L., Martin-Millan M., Plotkin L.I., Stewart S.A., Roberson P.K., Kousteni S., O'Brien C.A., Bellido T., Parfitt A.M., Weinstein R.S., Jilka R.L., Manolagas S.C. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthusami S., Ramachandran I., Muthusamy B., Vasudevan G., Prabhu V., Subramaniam V., Jagadeesan A., Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Garrett I.R., Boyce B.F., Oreffo R.O., Bonewald L., Poser J., Mundy G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yalin S., Bagis S., Polat G., Dogruer N., Cenk Aksit S., Hatungil R., Erdogan C. Is there a role of free oxygen radicals in primary male osteoporosis? Clin. Exp. Rheumatol. 2005;23:689–692. [PubMed] [Google Scholar]
- 37.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 38.Suda N., Morita I., Kuroda T., Murota S. Participation of oxidative stress in the process of osteoclast differentiation. Biochim. Biophys. Acta. 1993;1157:318–323. doi: 10.1016/0304-4165(93)90116-p. [DOI] [PubMed] [Google Scholar]
- 39.Lee N.K., Choi Y.G., Baik J.Y., Han S.Y., Jeong D.W., Bae Y.S., Kim N., Lee S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 40.Abo A., Pick E., Hall A., Totty N., Teahan C.G., Segal A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Lebowitz D., Sun C., Thang H., Grynpas M.D., Glogauer M. Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J. Bone Miner. Res. 2008;23:260–270. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki H., Yamamoto H., Tominaga K., Masuda K., Kawai T., Teshima-Kondo S., Matsuno K., Yabe-Nishimura C., Rokutan K. Receptor activator of nuclear factor-kappaB ligand-induced mouse osteoclast differentiation is associated with switching between NADPH oxidase homologues. Free Radic. Biol. Med. 2009;47:189–199. doi: 10.1016/j.freeradbiomed.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki H., Yamamoto H., Tominaga K., Masuda K., Kawai T., Teshima-Kondo S., Rokutan K. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J. Med. Invest. 2009;56:33–41. doi: 10.2152/jmi.56.33. [DOI] [PubMed] [Google Scholar]
- 44.Callaway D.A., Jiang J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015;33:359–370. doi: 10.1007/s00774-015-0656-4. [DOI] [PubMed] [Google Scholar]
- 45.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 46.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 47.Bharti A.C., Takada Y., Aggarwal B.B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004;172:5940–5947. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 48.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J.W., Chandra D., Rudd M.D., Butler A.P., Pallotta V., Brown D., Coffer P.J., Tang D.G. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene. 2005;24:2020–2031. doi: 10.1038/sj.onc.1208385. [DOI] [PubMed] [Google Scholar]
- 50.Lehtinen M.K., Yuan Z., Boag P.R., Yang Y., Villen J., Becker E.B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T.K., Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 51.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 52.Murakami S. Stress resistance in long-lived mouse models. Exp. Gerontol. 2006;41:1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 53.Giannakou M.E., Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J., Huang T.T., Bos J.L., Medema R.H., Burgering B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 55.Essers M.A., Weijzen S., de Vries-Smits A.M., Saarloos I., de Ruiter N.D., Bos J.L., Burgering B.M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baehner R.L., Murrmann S.K., Davis J., Johnston R.B., Jr. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J. Clin. Invest. 1975;56:571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loew O.A. New enzyme of general occurrence in organisms. Science. 1900;11:701–702. doi: 10.1126/science.11.279.701. [DOI] [PubMed] [Google Scholar]
- 58.Grace S.C. Phylogenetic distribution of superoxide dismutase supports an endosymbiotic origin for chloroplasts and mitochondria. Life Sci. 1990;47:1875–1886. doi: 10.1016/0024-3205(90)90399-c. [DOI] [PubMed] [Google Scholar]
- 59.Gray S.G., Ekstrom T.J. The human histone deacetylase family. Exp. Cell Res. 2001;262:75–83. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S., Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozden O., Park S.H., Kim H.S., Jiang H., Coleman M.C., Spitz D.R., Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging. 2011;3:102–107. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasegawa K., Wakino S., Yoshioka K., Tatematsu S., Hara Y., Minakuchi H., Washida N., Tokuyama H., Hayashi K., Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 2008;372:51–56. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- 63.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Horst A., Tertoolen L.G.J., de Vries-Smits L.M.M., Frye R.A., Medema R.H., Burgering B.M.T. FOXO4 Is Acetylated upon Peroxide Stress and Deacetylated by the Longevity Protein hSir2SIRT1. J. Biol. Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 65.Olmos Y., Sanchez-Gomez F.J., Wild B., Garcia-Quintans N., Cabezudo S., Lamas S., Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid. Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang K., Chen C., Hao J., Huang J., Wang S., Liu P., Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol. Cell Endocrinol. 2015;399:178–189. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Zwerina J., Tzima S., Hayer S., Redlich K., Hoffmann O., Hanslik-Schnabel B., Smolen J.S., Kollias G., Schett G. Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone resorption. FASEB J. 2005;19:2011–2013. doi: 10.1096/fj.05-4278fje. [DOI] [PubMed] [Google Scholar]
- 68.Sakai E., Shimada-Sugawara M., Nishishita K., Fukuma Y., Naito M., Okamoto K., Nakayama K., Tsukuba T. Suppression of RANKL-dependent heme oxygenase-1 is required for high mobility group box 1 release and osteoclastogenesis. J. Cell Biochem. 2012;113:486–498. doi: 10.1002/jcb.23372. [DOI] [PubMed] [Google Scholar]
- 69.Ke K., Safder M.A., Sul O.J., Kim W.K., Suh J.H., Joe Y., Chung H.T., Choi H.S. Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol. Cell Endocrinol. 2015;409:11–20. doi: 10.1016/j.mce.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 70.Rana T., Schultz M.A., Freeman M.L., Biswas S. Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free Radic. Biol. Med. 2012;53:2298–2307. doi: 10.1016/j.freeradbiomed.2012.10.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanzaki H., Shinohara F., Kajiya M., Kodama T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J. Biol. Chem. 2013;288:23009–23020. doi: 10.1074/jbc.M113.478545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hyeon S., Lee H., Yang Y., Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Gambari L., Lisignoli G., Cattini L., Manferdini C., Facchini A., Grassi F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacol. Res. 2014;87:99–112. doi: 10.1016/j.phrs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Kanzaki H., Shinohara F., Itohiya-Kasuya K., Ishikawa M., Nakamura Y. Nrf2 activation attenuates both orthodontic tooth movement and relapse. J. Dent. Res. 2015;94:787–794. doi: 10.1177/0022034515577814. [DOI] [PubMed] [Google Scholar]
- 75.Bartell S.M., Kim H.N., Ambrogini E., Han L., Iyer S., Serra Ucer S., Rabinovitch P., Jilka R.L., Weinstein R.S., Zhao H., O'Brien C.A., Manolagas S.C., Almeida M. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 2014;5:3773. doi: 10.1038/ncomms4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H.N., Han L., Iyer S., de Cabo R., Zhao H., O'Brien C.A., Manolagas S.C., Almeida M. Sirtuin1 suppresses osteoclastogenesis by deacetylating FoxOs. Mol. Endocrinol. 2015;29(10):1498–1509. doi: 10.1210/me.2015-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H.S., Ka S.O., Lee S.M., Lee S.I., Park J.W., Park B.H. Overexpression of sirtuin 6 suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis. Arthritis Rheum. 2013;65:1776–1785. doi: 10.1002/art.37963. [DOI] [PubMed] [Google Scholar]
- 78.Kong A.N., Yu R., Hebbar V., Chen C., Owuor E., Hu R., Ee R., Mandlekar S. Signal transduction events elicited by cancer prevention compounds. Mutat. Res. 2001;480–481:231–241. doi: 10.1016/s0027-5107(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 79.Shen G., Xu C., Hu R., Jain M.R., Nair S., Lin W., Yang C.S., Chan J.Y., Kong A.N. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Pharm. Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 80.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekhar K.R., Spitz D.R., Harris S., Nguyen T.T., Meredith M.J., Holt J.T., Gius D., Marnett L.J., Summar M.L., Freeman M.L. Redox-sensitive interaction between KIAA0132 and Nrf2 mediates indomethacin-induced expression of gamma-glutamylcysteine synthetase. Free Radic. Biol. Med. 2002;32:650–662. doi: 10.1016/s0891-5849(02)00755-4. [DOI] [PubMed] [Google Scholar]
- 82.Morinobu A., Biao W., Tanaka S., Horiuchi M., Jun L., Tsuji G., Sakai Y., Kurosaka M., Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- 83.French D.L., Muir J.M., Webber C.E. The ovariectomized, mature rat model of postmenopausal osteoporosis: an assessment of the bone sparing effects of curcumin. Phytomedicine. 2008;15:1069–1078. doi: 10.1016/j.phymed.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Sanders K.M., Kotowicz M.A., Nicholson G.C. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: a pilot study. Transl. Res. 2007;150:215. doi: 10.1016/j.trsl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 85.Urbich C., Knau A., Fichtlscherer S., Walter D.H., Bruhl T., Potente M., Hofmann W.K., de Vos S., Zeiher A.M., Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 86.Moon H.J., Kim S.E., Yun Y.P., Hwang Y.S., Bang J.B., Park J.H., Kwon I.K. Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species. Exp. Mol. Med. 2011;43:605–612. doi: 10.3858/emm.2011.43.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pillai J.B., Chen M., Rajamohan S.B., Samant S., Pillai V.B., Gupta M., Gupta M.P. Activation of SIRT1, a class III histone deacetylase, contributes to fructose feeding-mediated induction of the alpha-myosin heavy chain expression. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1388–H1397. doi: 10.1152/ajpheart.01339.2007. [DOI] [PubMed] [Google Scholar]
- 88.Boissy P., Andersen T.L., Abdallah B.M., Kassem M., Plesner T., Delaisse J.M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–9952. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- 89.He X., Andersson G., Lindgren U., Li Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem. Biophys. Res. Commun. 2010;401:356–362. doi: 10.1016/j.bbrc.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 90.Shakibaei M., Buhrmann C., Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai C.Y., Yang J.Y., Rayalam S., Della-Fera M.A., Ambati S., Lewis R.D., Hamrick M.W., Hartzell D.L., Baile C.A. Preventing bone loss and weight gain with combinations of vitamin D and phytochemicals. J. Med. Food. 2011;14:1352–1362. doi: 10.1089/jmf.2010.0232. [DOI] [PubMed] [Google Scholar]
- 92.Artsi H., Cohen-Kfir E., Gurt I., Shahar R., Bajayo A., Kalish N., Bellido T.M., Gabet Y., Dresner-Pollak R. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology. 2014;155:3508–3515. doi: 10.1210/en.2014-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Momken I., Stevens L., Bergouignan A., Desplanches D., Rudwill F., Chery I., Zahariev A., Zahn S., Stein T.P., Sebedio J.L., Pujos-Guillot E., Falempin M., Simon C., Coxam V., Andrianjafiniony T., Gauquelin-Koch G., Picquet F., Blanc S. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 94.Mercken E.M., Mitchell S.J., Martin-Montalvo A., Minor R.K., Almeida M., Gomes A.P., Scheibye-Knudsen M., Palacios H.H., Licata J.J., Zhang Y., Becker K.G., Khraiwesh H., Gonzalez-Reyes J.A., Villalba J.M., Baur J.A., Elliott P., Westphal C., Vlasuk G.P., Ellis J.L., Sinclair D.A., Bernier M., de Cabo R. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]