Abstract
The traditional Asian diet is rich in fruits, vegetables and soy, the latter representing a significant source of dietary isoflavones. The isoflavone prunetin was recently identified to improve intestinal epithelial barrier function in vitro and to ameliorate general survival and overall health state in vivo in male Drosophila melanogaster. However, the prunetin-mediated health benefits in the fruit fly were ascertained under standard living conditions. As the loss of intestinal integrity is closely related to a reduction in Drosophila lifespan and barrier dysfunction increases with age, effects on prunetin-modulated gut health under oxidative or pathogenic stress provocation remain to be elucidated. In this study, male adult D. melanogaster were administered either a prunetin or a control diet. Gut-derived junction protein expression and pathogen-induced antimicrobial peptide expressions as well as the stem cell proliferation in the gut were evaluated. Furthermore, survival following exposure to hydrogen peroxide was assessed. Prunetin ingestion did not attenuate bacterial infection and did not protect flies from oxidative stress. Intestinal mRNA expression levels of adherence and septate junction proteins as well as the stem cell proliferation were not altered by prunetin intake. Prunetin does not improve the resistance of flies against severe injuring, exogenous stress and therefore seems to function in a preventive rather than a therapeutic approach since the health-promoting benefits appear to be exclusively restricted to normal living circumstances.
Abbreviations: αTub84B, alpha-Tubulin at 84B; IMD, immune deficiency; NF-κB, nuclear factor κB; OD, optical density; P.carotovorum, Pectobacterium carotovorum subsp. carotovorum; Pe-GFP, Pseudomonas entomophila expressing the green fluorescent protein; P. entomophila, Pseudomonas entomophila; PH3, phospho-histone H3; prun, prunetin; qRT-PCR, quantitative RT-PCR
Keywords: Drosophila, Isoflavone, Infection, Resistance, Oxidative stress
Graphical abstract

Highlights
-
•
Gram-negative bacterial strains induce AMP-mediated defense in the fruit fly.
-
•
Prunetin improves life and health span in male fruit flies independent of gut health.
-
•
Prunetin fails to ameliorate resistance of the flies towards severe injury.
-
•
AMP expression, stem cell proliferation & oxidative stress resistance are unaffected.
1. Introduction
Diet plays a decisive role in the maintenance of health and in the prevention of chronic diseases [1]. Soy is a principal constituent in the traditional Asian diet which is generally rich in vegetables, fruits and legumes. Thereby soy is the most important dietary source of isoflavones. Prunetin is one representative of the isoflavone group which is synthesized via the isoflavonoid biosynthesis pathway from its precursor naringenin. Prunetin exhibits potent bioactivity [2] and modifies inflammatory processes [3], stress response [4] and intestinal epithelial barrier function [5]. Thereby, gut barrier function, inflammation and stress response are pivotal determinants of longevity [6], [7]. We have recently identified prunetin to significantly increase general survival in male Drosophila melanogaster w1118 and to coincidently ameliorate climbing activity, indicating an improved health state in senescent flies [8]. The prunetin-dependent increase in AMPK activation and an up-regulated expression of the longevity gene Sirtuin 1 seem to be responsible for prunetin-dependent health benefits. Moreover, the overall gut health is presumably improved by the consumption of prunetin in the male fruit fly, as gut-specific Relish (Rel) expression, a NF-κB family orthologue in the fruit fly, is upregulated by 49% compared with the control group [8]. This indicates a general advantage in gut integrity and defense capability, which in turn is positively associated with longevity. Drosophila is a suitable model for examining the effects of secondary plant compounds on gut integrity related to inflammation and aging because the fruit fly holds a complex and dynamic gut that is similar in structure and organization to the mammalian gut [8], [9]. The physiology and anatomy of mammalian and D. melanogaster intestinal tissues exhibit similar properties, each being composed of an enterocyte monolayer and enteroendocrine cells [9]. Additionally, the fruit fly is an appropriate model for investigating inherent immunity as insect immune function shares many similarities with the innate immune response of mammals [8], [10], [11]. The epithelial surfaces of the organs including the gut serve as first-line defenses against microorganisms by producing antimicrobial peptides (AMPs). Importantly, the loss of intestinal integrity is closely related to reductions in both the medium and maximum lifespan of D. melanogaster [6], [7]. Intestinal barrier dysfunction increases with age [6] and predicts age-onset mortality [7]. Furthermore, premature mortality has also been associated with increased AMP expression [7], which is related to changes in the intestinal immune response, possibly via alterations in the expression of Rel [12]. As prunetin significantly improves intestinal epithelial barrier function in CaCo-2 cells in vitro [5] and gut-specific Rel expression in vivo extending the lifespan of male D. melanogaster [8], we first-time investigated whether prunetin affects gut health in the male fruit fly under oxidative and infectious stress conditions and thereby possibly contributing to an increase in lifespan expectancy. This manuscript reveals for the first time that, although prunetin was recently identified as a plant bioactive improving the health and survival of male D. melanogaster under standard living conditions [8], prunetin fails to strengthen their gut health and resistance following exposure to oxidative stress or fruit fly pathogens.
2. Materials and methods
2.1. Fly strains and husbandry
The D. melanogaster strain w1118 (Bloomington Drosophila Stock Center #5905, Indiana University, Bloomington, IN, USA) was used for infection and oxidative stress resistance experiments, qRT-PCR and immunofluorescence analyses. The diptericin-green fluorescent protein reporter strain (Dpt-GFP), expressing GFP upstream of Dpt under the control of the dpt 2.2-kb promoter (kindly provided by Dr. C Wagner, Research Center Borstel, Germany), was used for infection experiments and is described elsewhere [13]. All Drosophila stocks were maintained on standard food at 25 °C and 60% humidity with a 12/12 h light/dark cycle; standard food and experimental food were prepared as described previously [8].
2.2. Test compounds
Prunetin (Sigma-Aldrich, Taufkirchen, Germany) was dissolved in dimethyl sulfoxide (DMSO; Carl Roth, Karlsruhe, Germany) and stored as a 50 mM stock solution at −80 °C. For experimental treatments, Drosophila standard food was supplemented with 25 µM prunetin as this concentration was used in a previous study and was proven to be effective [8]. Food supplemented with 0.05% DMSO (v/v) served as the vehicle control.
2.3. Experimental treatment of flies
To investigate how prunetin affects gut health, newly eclosed synchronized flies were permitted to mate for 2 days (as described previously [8]; according to [14]) and were separated according to sex. Male w1118 flies were transferred to experimental vials containing standard medium and supplemented either with prunetin (25 µM) or DMSO (control). The flies were treated for 10 day while transferred to fresh medium 3 times a week.
2.4. Bacterial strains and culture conditions
Pseudomonas entomophila (P. entomophila) and Pectobacterium carotovorum subsp. carotovorum (P. carotovorum) were obtained from the DSMZ (Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). P. entomophila and P. carotovorum were grown in Medium 1a consisting of 0.5% tryptone/peptone (Carl Roth) and 0.3% meat extract (Sigma-Aldrich), pH=7.0, at 29 °C according to the recommendations of the DSMZ. The GFP-expressing P. entomophila strain (Pe-GFP), carrying the plasmid pX2-GFP, was kindly provided by Dr. C Wagner (Research Center Borstel, Germany) and is described elsewhere [15]. Pe-GFP was grown in LB medium consisting of 1% tryptone/peptone, 0.5% yeast extract (Carl Roth) and 1% sodium chloride (Prolabo by VWR, Darmstadt, Germany), pH=7.5, supplemented with 100 µg/ml ampicillin (Sigma-Aldrich) at 29 °C.
2.5. Oral infection of D.melanogaster with P. carotovorum, P. entomophila and Pe-GFP
For the infection experiments, two-day-old male flies (w1118 or Dpt-GFP) were maintained on food containing either 25 µM prunetin or 0.05% DMSO (control) for 10 days as described. Bacteria were grown for 24 h and pelleted by centrifugation for 10 min at 3200 g. The bacteria were re-suspended in a 10% sucrose solution (w/v), and the OD600 was adjusted to 200, 5 (P. entomophila, Pe-GFP) and 100 (P. carotovorum). A 150-µl aliquot of contaminated sucrose solution was applied to a Whatman filter disk that completely covered the agar surface of a corresponding culture vial ([16] with modifications). For oral infection, flies were starved for 2 h in empty vials prior to their transfer to bacteria-containing vials ([17] with modifications). Thirty flies were flipped into the bacteria-containing vials and kept at 29 °C, which is the optimal growing temperature for the pathogens. Taking into account that fly metabolism and survival is possibly affected by increased ambient temperature [18], [19], additional control flies were treated in the same manner, receiving sucrose solution but without bacteria. To evaluate whole-fly mRNA expression levels, the flies were frozen at −80 °C 18 h post infection, which provided sufficient infection time [16]. The experiments were performed twice with 3 biological replicates per group. For fluorescence imaging and quantitative immunofluorescence analyses, the flies were treated as described above and then anesthetized, after which either their midguts were freshly dissected or whole flies were freshly homogenized in PBS/Triton-X100 (1% v/v). Green fluorescence was measured in a Tecan Infinite200 microplate reader (Tecan, Crailsheim, Germany) at an excitation wavelength of 485/20 nm and an emission wavelength of 535/25 nm. Fluorescent images of midguts were acquired with a Biozero BZ-8100 (Keyence, Neu-Isenburg, Germany) using a FITC filter system.
2.6. Bacterial infection efficiency
Flies were pre-fed, maintained and orally infected with Pe-GFP as described above. 4 h post infection, the flies were frozen at −80 °C until analysis [17]. The flies were homogenized in 10 µl PBS/Triton-X100 (1% v/v) per fly in a TissueLyser II (Qiagen, Hilden, Germany) and subsequently centrifuged. Green fluorescence was measured in the supernatants using a Tecan Infinite200 microplate reader at an excitation wavelength of 485/20 nm and an emission wavelength of 535/25 nm. Fluorescence in non-infected flies was considered as auto-fluorescence and subtracted from the values generated by the Pe-GFP-infected flies.
2.7. Dissection of midguts
Flies were dissected one after another as described previously [8]. The midgut was either preserved in TriFast reagent (peqlab Biotechnologie, Erlangen, Germany) and kept on ice (for RNA isolation) or fixed onto a chamber slide (Sarstedt, Nuembrecht, Germany) using a 4% paraformaldehyde solution (Carl Roth), pH=7.4 (for immunofluorescence staining).
2.8. Immunofluorescence
The detection of Phospho-Histone H3-positive cells is a suitable method for assessing intestinal stem cell proliferation [20]. Midguts were dissected as described and fixed in 4% paraformaldehyde in PBS for 15 min. After blocking for 60 min in PBS containing 5% goat serum and 0.3% Triton-X100, the guts were incubated with an anti-Phospho-Histone H3 antibody (PH3; Cell Signaling, Danvers, MA/USA) overnight at +4 °C. Following, guts were incubated with an Alexa Fluor 594-conjugated secondary antibody (Invitrogen by Life Technologies, Bleiswijk, the Netherlands), diluted in PBS (containing 1% BSA and 0.3% TritonX-100) and counterstained with DAPI (Sigma). Images were acquired with a Biozero BZ-8100 using TexasRed and DAPI filter systems. DAPI-positive and PH3-positive cells were automatically identified and counted with CellProfiler cell image analysis software [21]. The ratio of PH3/DAPI was calculated to quantify the intensity of stem cell proliferation.
2.9. qRT-PCR analysis
Total RNA was extracted with TriFast reagent (peqlab, Erlangen, Germany) from dissected midguts (≥25 per sample) and from whole flies (10 per sample) and qRT-PCR was performed using a one-step or two-step protocol as described previously [8]. Relative mRNA quantification was calculated using a standard curve. Target gene expression (Table 1) was normalized to the expression of the housekeeping gene alpha-Tubulin at 84B.
Table 1.
Primer sequences for real-time PCR in RNA samples of midguts and whole fly homogenates of male w1118D.melanogaster.
| Target gene | Full name |
Primer 5‘→3‘ |
|
|---|---|---|---|
| Forward | Reverse | ||
| Gut | |||
| α-Cat | α Catenin | GTACAGCTCGAGAAGCAATG | CCAGTGTCATCCCATTTAGC |
| arm | armadillo | CGTCATTGGACTCATACGC | GTGGTGGCTATCGAGGAAC |
| α-Spec | α Spectrin | CAGGAATACATCGCGTTCAT | CCTTGGTGAGGTTGCAGTAG |
| β-Spec | β Spectrin | CTGATGACGCTGAGCAATAG | GTCTCTGGCGAACTGGTACA |
| cora | coracle | GCTCGTCTCACTTCCAGGAG | CTTGTTCTTGATGGGACTGC |
| Pax | Paxillin | CGACTTCAAGGTTAGCAACG | GATCGTCTGGGTGAGATGTG |
| pyd | polychaetoid | CGATAGCAGTTAGCGATGTG | CGGTAGCATATTCCACGTTC |
| shg | shotgun | GCACCTTCAACGTTACCATC | AGTCACTGGCGCTGATAGTC |
| Whole fly | |||
| AttB | Attacin-B | CTCGGTTGAATCTCAGCAAG | CCATGACCAGCATTGTTGTA |
| AttC | Attacin-C | CAACACGCAGACCAAACC | GGAAGCTATCCCGCACAC |
| Dpt | Diptericin | GAGATGCAGTTCACCATTGC | CCCTGAAGATTGAGTGGGTA |
| Dro | Drosocin | GAGGATCACCTGACTCAAGC | ATGACTTCTCCGCGGTATG |
| Mtk | Metchnikowin | CTACATCAGTGCTGGCAGAG | TGGTTGGTTAGGATTGAAGG |
| αTub84B | alpha-Tubulin at 84B | TCAGACCTCGAAATCGTAGC | AGCCTGACCAACATGGATAG |
2.10. Survival following hydrogen peroxide (H2O2) treatment
As H2O2 generates hydroxyl radicals (·OH) in the presence of metal ions it was used to assess the resistance of male w1118 D.melanogaster against ·OH-induced oxidative stress. Adult male w1118 flies were fed either a prunetin- or DMSO-supplemented diet for 10 days as described. Subsequently, H2O2 treatment was performed according to [22] with slight modifications. Twenty flies per vial were starved for 2 h and subsequently transferred to new vials containing a Whatman filter paper soaked with a 10% H2O2/5% sucrose solution (w/v). The negative control group received a 5% sucrose solution only. Dead flies were counted every 4 h until all flies were dead. Survival rates were calculated by using the DLife program [14].
2.11. Statistical analyses
To calculate survival rates following H2O2 treatment the DLife software (Winchecker version 3.0; [14]) was used. Values are given as means and were statistically estimated via a Log-Rank Test based on R (i386 version 3.1.0). For qRT-PCR and calculation of relative fluorochrome quantities (GFP, PH3), values are given as means±SEM, except otherwise noted. The data were proven for normality of distribution (Kolmogorov-Smirnov and Shapiro-Wilk). Mean comparisons were carried out using Post Hoc multiple comparisons; the LSD test was applied in case of homogeneity of variances, the Games-Howell test in case of inhomogeneous variances. For qRT-PCR, mean comparisons were carried out using a 2 sided Student's t-test or a non-parametric Mann-Whitney-U test, respectively. Statistical analysis was performed by applying SPSS (version 19; SPSS Inc., Munich, Germany). Significance was accepted at p-values <0.05.
3. Results
3.1. Prunetin does not affect intestinal junction protein expression
Male w1118 flies were fed either a control or a prunetin-supplemented diet for 10 days. Subsequently, their midguts were prepared, and the mRNA-expression levels of various adherence and septate junction proteins were measured. The mRNA levels of the adherence and septate junction proteins alpha Catenin (α-Cat), armadillo (arm), alpha Spectrin (α-Spec), beta Spectrin (β-Spec), coracle (cora), Paxillin (Pax), polychaetoid (pyd) and shotgun (shg) [23] were assessed. The expression levels of all analyzed genes were not significantly altered in the midguts of the prunetin-treated male flies (Table 2).
Table 2.
mRNA expression levels in midguts of prunetin-treated male w1118D.melanogaster compared to controls.
| Target gene | Expression vs. control | ±SEM | p-Value |
|---|---|---|---|
| α-Cat | 1.001 | 0.067 | 0.992 |
| arm | 1.012 | 0.123 | 0.962 |
| α-Spec | 0.926 | 0.064 | 0.629 |
| β-Spec | 1.499 | 0.243 | 0.239 |
| cora | 0.935 | 0.013 | 0.666 |
| Pax | 0.982 | 0.079 | 0.927 |
| pyd | 0.845 | 0.179 | 0.480 |
| shg | 0.746 | 0.107 | 0.342 |
Male age-matched flies were fed a control diet (0.05% DMSO) or a prunetin supplemented diet (25 µM) for 10 days. mRNA expression levels were determined via two-step qRT-PCR and were normalized to the expression level of the housekeeping gene alpha-Tubulin at 84B. Values represent the mean±SEM of three independent experiments. n=3 (75 midguts per group in total, at least). Statistical evaluation: Student's t-test or Mann-Whitney-U.
We further examined effects of prunetin regarding the promotion of gut health in stressed male w1118 flies. Male flies were stressed via the oral administration of H2O2 or pathogenic bacteria to determine whether prunetin is equally capable of producing health and survival benefits in stressed flies as in flies reared under standard conditions. Prunetin did not affect gut integrity or function under conditions of infectious or oxidative stress as described hereafter.
3.2. Prunetin does not alter bacteria-induced antimicrobial peptides (AMPs) expression in male flies
Both P. carotovorum and P. entomophila can infect D. melanogaster, trigger immune responses and consequentially induce AMP expression in both the gut and the fat body [16], [24], [25], [26]. Oral infection of Drosophila with a high bacterial concentration is known to prevent intestinal stem cell proliferation due to irreversible damage to the gut, whereas infection with a low bacterial concentration can lead to increased stem cell proliferation [16]. The measurement of GFP-expressing bacteria is an accepted method of monitoring host infection [15]. Prunetin-fed and control male w1118 flies were orally infected with P. entomophila expressing green fluorescent protein (Pe-GFP) at an infectious dose of OD600=200. The infections were deemed successfully, as Pe-GFP-dependent green fluorescence measurements were significantly higher in whole fly lysates from infected vs. non-infected flies (p=0.001 compared with non-infected controls; LSD). Fluorescence intensity did not significantly differ between control flies (+38.8±4.1%) and prunetin-treated flies (+37.6±7.0% fluorescence intensity compared with non-infected controls).
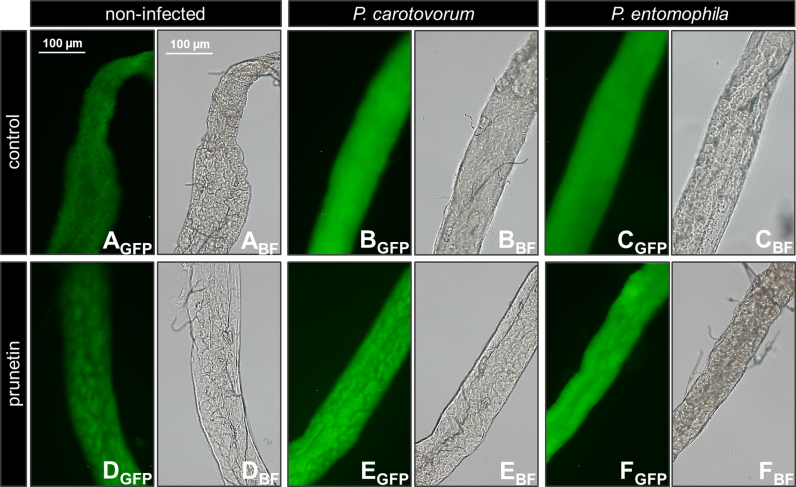
The following intestinal AMPs can be induced in the midgut by bacterial infection [20], [27], [28]: Mtk (Metchnikowin), Dro (Drosocin), AttB (AttacinB), AttC (AttacinC) and Dpt (Diptericin). Both P. carotovorum and P. entomophila significantly induced AMP expression in male w1118 flies following oral infections at infectious doses of OD600=100 and OD600=200 (Fig. 1A–E: Mtk at p<0.01 and p<0.05; Dro at p<0.01 and p<0.05; AttB at p<0.001, both; AttC at p<0.001 and p<0.05; Dpt at p<0.01 and p<0.001). Feeding flies with a prunetin-supplemented diet for 10 days did not significantly alter AMPs expression compared with control flies (Fig. 1A–E). Similar results were obtained using the Dpt-GFP reporter strain. This fly strain was chosen because Dpt expression is controlled by the transcription factor Rel [27] whose expression was shown to be modulated in the gut by prunetin ingestion [8]. Both P. carotovorum and P. entomophila significantly induced Dpt-GFP expression in male flies following infection at infectious doses of OD600=100 and OD600=200 for 26 h (p<0.05; Fig. 1F). Flies that were fed prunetin for 10 days did not exhibit significant different Dpt-GFP expression values compared with infected controls. Fluorescent imaging of the midguts of non-infected and bacteria-infected Dpt-GFP male flies revealed similar GFP expression intensities (Fig. 2A–F).
Fig. 1.
Relative expression levels of antimicrobial peptides (AMPs) in male w1118D.melanogaster (A-E) and Dpt-GFP reporter flies (F) following oral infection with fruit fly-pathogenic bacteria. Male w1118 and Dpt-GFP flies were fed a prunetin containing (25 µM) or a control diet for 10 days. Flies were subsequently starved for 2 h and orally infected with Pectobacterium carotovorum subsp. carotovorum (P. carotovorum) or Pseudomonas entomophila (P. entomophila) at infectious doses of OD600=100 or OD600=200. (A–E) Oral infection was conducted for 18 h and RNA was isolated from whole flies. mRNA expression levels of respective target genes were normalized to the expression of the housekeeping gene alpha-Tubulin at 84B. P. carotovorum and P. entomophila significantly induced mRNA expression of AMPs shown (A: Metchnikowin, B: Drosocin, C: Attacin B, D: Attacin C, E: Diptericin) at p-values <0.01, respectively (LSD and Games-Howell, if indicated). Prunetin treatment did not significantly alter AMP expression compared to respective infection controls. Bars represent the mean+SEM of two independent experiments. n=12, 120 flies per group in total; outliers were removed. (F) Whole body Green Fluorescent Protein (GFP)-expression values of orally infected Diptericin-GFP reporter flies (Dpt-GFP; infection for 26 h with P. carotovorum and P. entomophila at OD600=100 and OD600=200). P. carotovorum and P. entomophila significantly induced expression of Diptericin-GFP (Dpt-GFP; p<0.05; LSD) in both control and prunetin treated flies. Prunetin treatment did not significantly alter Dpt-GFP expression compared to the respective infection controls. Bars represent the mean+SEM of 7–10 biological replicates. n=7–10, 75–85 flies per group in total; outliers were eliminated.
Fig. 2.
Expression of Dpt-GFP in midguts of male Dpt-GFP reporter flies following oral infection with fruit fly-pathogenic bacteria. Representative fluorescence pictures of Dpt-GFP midguts (segments) referring to Fig. 1F. Both P. carotovorum and P. entomophila oral infections induced expression of gut-derived Dpt-GFP. Prunetin treatment did not alter Dpt-GFP expression in midguts compared to the respective infection controls. A, D: non-infected; B, E: P. carotovorum-infected (OD600=100); C, F: P. entomophila-infected (OD600=200). Fluorescence (GFP) or bright field (BF) pictures were acquired with equivalent exposure times, respectively.
3.3. Intestinal stem cell proliferation is not altered in prunetin-fed male flies compared with controls
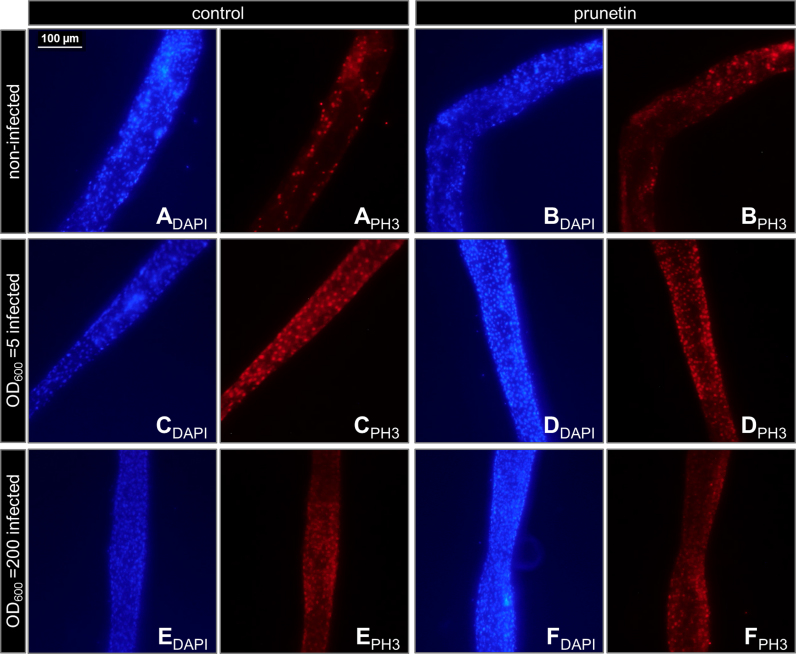
Stem cell proliferation did not significantly differ between guts isolated from prunetin-treated flies (Fig. 3B, D, F) and those isolated from control flies (Fig. 3A, C, E). The administration of infectious doses of Drosophila-pathogenic bacteria (P. entomophila) also did not lead to significant changes. PH3-positive cell counts in the midguts of prunetin-fed and control flies did not differ significantly from each other in the two infection groups. The PH3-positive cell count was altered by −4.9±5.2% (non-infected), +1.7±4.5% (OD600=5-infected) and +4.2±4.4% (OD600 =200-infected; ns) in midguts isolated from prunetin-treated flies compared to those isolated from control flies.
Fig. 3.
Representative immunofluorescence pictures of midgut segments prepared from male w1118D. melanogaster. Flies were fed with a control (A, C, E) or prunetin supplemented (B, D, F) diet for 10 days followed by the oral infection with P. entomophila at a low and high infectious dose of OD600=5 and OD600=200. Phospho-Histone H3 (PH3) is a marker for stem cell proliferation (chromophore: AlexaFluor594; red). Nuclei were counterstained with DAPI (blue). At least, six midguts per group and treatment were examined. DAPI- and PH3-positive cells were identified and counted with the CellProfiler cell image analysis software [21]. The ratio of PH3/DAPI was calculated to quantify the intensity of stem cell proliferation. Stem cell proliferation in the midguts of prunetin-fed and control flies did not differ significantly from each other in the two infection groups or in non-infected flies as quantified with the CellProfiler cell image analysis software.
3.4. Prunetin fails to decelerate mortality following H2O2 treatment
The treatment of male w1118 flies with a 10% H2O2 solution (diluted in a 5% sucrose solution) resulted in a significant decrease in the survival rate (blank circles, Fig. 4, p<0.001) compared with control flies receiving a 5% sucrose solution (triangles, Fig. 4). Pre-feeding flies with a prunetin-containing diet for 10 days did not affect survival compared to H2O2 treated flies (black circles, Fig. 4, p=0.665).
Fig. 4.
Prunetin treatment for 10 days does not prevent male w1118D.melanogaster from hydrogen peroxide (H2O2) induced stress. Age-matched flies were fed a prunetin containing diet (25 µM) or control food (DMSO 0.05%) for 10 days. Flies were starved for 2 h and subsequently treated with H2O2 (10% w/v) diluted in a 5% sucrose solution (w/v). Dead flies were counted every 4 h. Two biological replicates per treatment (n=2; 30 flies per group in total). Shown is one representative experiment out of three. Statistical comparison with Log-Rank.
4. Discussion
We have recently reported that prunetin significantly improves both survival and long-term health in male fruit flies of the w1118 strain [8]. The lifespan of D. melanogaster depends on the maintenance of intestinal integrity [6], [7], amongst others; however gut barrier dysfunction increases with age [6] causing age-onset mortality [7]. Furthermore, premature mortality has been associated with altered AMP expression [7], which contributes to changes in intestinal immune response, presumptively via alterations in the expression of the Drosophila NF-κB ortholog Rel [12]. The isoflavone prunetin is a potent enhancer of intestinal epithelial barrier integrity and down-regulates inflammation both in vitro and in vivo in mammals via its modulation of NF-κB activity [3], [5]. As previously shown, Rel expression was significantly increased in the midguts of male flies fed the prunetin-containing diet by 49% which contributed to an increased lifespan of male flies [8]. Although prunetin was identified to improve barrier integrity in vitro in a human enterocyte cell model (CaCo-2) by affecting junction protein integrity [5], junction-protein mRNA expression levels were not significantly regulated by prunetin-ingestion in the midguts of male w1118 flies (α-Cat, arm, α-Spec, β-Spec, cora, Pax, pyd, shg; Table 2). Therefore, alterations in junction protein levels in the gut do not seem to be primarily responsible for the modulation of gut barrier integrity and health following prunetin ingestion in male w1118 flies. Prunetin was identified to upregulate Rel expression in the midguts of male Drosophila, which at least partly contributed to increased survival under standard living conditions [8], indicating an ameliorated immune status as a reduction in Rel-response leads to diminished resistance to bacterial [29], [30] and fungal infections [12] and shortens the maximum lifespan of Drosophila [8], [31]. To examine if prunetin exhibits health benefits in the fruit fly also under severe exogenous stress, we orally infected prunetin-fed male fruit flies with Drosophila pathogens. P. entomophila and P. carotovorum are Gram-negative Drosophila-pathogenic bacterial strains [32], [33]. As Rel is part of the Drosophila immune deficiency (IMD) pathway, which regulates humoral defense against Gram-negative bacterial infections [11], [34], the prunetin-dependent upregulation of Rel strongly supported the assumption that flies receiving prunetin have improved defenses against infections caused by P. entomophila and P. carotovorum. Drosophila possesses a three-stage gut immune response cascade to oral infections, as illustrated by Buchon and colleagues [20]: 1.) immune response, 2.) stress response and 3.) epithelial renewal. Intestinal antioxidant defense is strongly involved in the protection of the host following oral bacterial infections in Drosophila [35]. However, we did not observe protection against orally provoked infections in prunetin-treated flies at any of the above-mentioned stages of the immune response. Although prunetin induces intestinal Rel expression, no significant differences in AMP expression patterns were apparent in bacterially infected, prunetin-fed flies compared with infected control flies (Fig. 1, Fig. 2). Similarly, the survival of flies exposed to H2O2 was not augmented by prunetin (Fig. 4). Additionally, intestinal stem cell proliferation was not altered by prunetin in flies infected with either a low or high bacterial load (Fig. 3). Chronic administration of prunetin has multidirectional effects in male D. melanogaster under standard living conditions improving their health state and survival as previously shown [8]. However, our new findings concerning prunetin-mediated effects on gut health and resistance to severe exogenous stressors in male fruit flies revealed no further improvement of prunetin-mediated health benefits. We conclude that prunetin is a potent secondary plant compound enhancing the health state of male D. melanogaster under normal living conditions but does not improve the resistance of the flies against severe injuring, exogenous stress. Hence, prunetin seems to be effective in a preventive rather than in a therapeutic approach as the health-promoting benefits of prunetin appear to be exclusively restricted to standard living conditions. Whether prunetin exhibits potential anti-oxidative and anti-inflammatory abilities in the fruit fly when orally applied at various, higher concentrations should be addressed in continuative experiments. These hypotheses should also be addressed in further in vivo studies in mammalian species.
Acknowledgments
We are grateful to N. J. Linford and S. D. Pletcher (Institute of Integrative Physiology, University of Michigan, Ann Arbor, MI, USA) and T. Roeder and C. Fink (Zoological Institute, Molecular Physiology, University of Kiel) for their support in fly husbandry and for providing fly stocks. We further thank C. Wagner (Research Center Borstel, Priority Area Allergy and Asthma, Borstel, Germany) for her donation of the mutant fly strain and bacteria. This work was funded by the DFG Cluster of Excellence “Inflammation at Interfaces” (EXC306 [MP VII]). S. P., G. R. and A. E. W. designed research; S. P. conducted research and analyzed data. All authors contributed to the writing of the paper and had primary responsibility for the final content. All authors have read and approved the final manuscript and declare no conflicts of interest.
References
- 1.Pallauf K., Giller K., Huebbe P., Rimbach G. Nutrition and healthy ageing: calorie restriction or polyphenol-rich “MediterrAsian” diet? Oxid. Med. Cell. Longev. 2013:707421. doi: 10.1155/2013/707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortensen A., Kulling S.E., Schwartz H., Rowland I., Ruefer C.E., Rimbach G., Cassidy A., Magee P., Millar J., Hall W.L., Kramer Birkved F., Sorensen I.K., Sontag G. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol. Nutr. Food Res. 2009;53(Suppl. 2):S266–S309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 3.Yang G., Ham I., Choi H.Y. Anti-inflammatory effect of prunetin via the suppression of NF-kappaB pathway. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;58:124–132. doi: 10.1016/j.fct.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Yun J.M., Im S.B., Roh M.K., Park S.H., Kwon H.A., Lee J.Y., Choi H.Y., Ham I.H., Kim Y.B., Lee J.M., Kim D.O., Park K.W., Kang H. Prunus yedoensis bark inhibits lipopolysaccharide-induced inflammatory cytokine synthesis by IkappaBalpha degradation and MAPK activation in macrophages. J. Med. Food. 2014;17:407–413. doi: 10.1089/jmf.2013.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piegholdt S., Pallauf K., Esatbeyoglu T., Speck N., Reiss K., Ruddigkeit L., Stocker A., Huebbe P., Rimbach G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-kappaB, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014;70:255–264. doi: 10.1016/j.freeradbiomed.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Ulgherait M., Rana A., Rera M., Graniel J., Walker D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piegholdt S., Rimbach G., Wagner A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2015 doi: 10.1096/fj.15-282061. [DOI] [PubMed] [Google Scholar]
- 9.Apidianakis Y., Rahme L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman N., Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann J.A., Reichhart J.M. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 12.Hedengren M., Asling B., Dushay M.S., Ando I., Ekengren S., Wihlborg M., Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 13.Leulier F., Rodriguez A., Khush R.S., Abrams J.M., Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linford N.J., Bilgir C., Ro J., Pletcher S.D. Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp.: JoVE. 2013;71:50068. doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vodovar N., Vinals M., Liehl P., Basset A., Degrouard J., Spellman P., Boccard F., Lemaitre B. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallet-Gely I., Opota O., Boniface A., Novikov A., Lemaitre B. A secondary metabolite acting as a signalling molecule controls Pseudomonas entomophila virulence. Cell. Microbiol. 2010;12:1666–1679. doi: 10.1111/j.1462-5822.2010.01501.x. [DOI] [PubMed] [Google Scholar]
- 17.Neyen C., Bretscher A.J., Binggeli O., Lemaitre B. Methods to study Drosophila immunity. Methods (San Diego, Calif.) 2014;68:116–128. doi: 10.1016/j.ymeth.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Cohet Y. Epigenetic influences on the lifespan of the Drosophila: existence of an optimal growth temperature for adult longevity. Exp. Gerontol. 1975;10:181–184. doi: 10.1016/0531-5565(75)90029-7. [DOI] [PubMed] [Google Scholar]
- 19.Simon A.F., Shih C., Mack A., Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 20.Buchon N., Broderick N.A., Poidevin M., Pradervand S., Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O., Guertin D.A., Chang J.H., Lindquist R.A., Moffat J., Golland P., Sabatini D.M. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Han S., Wang H., Wang T. Lutein extends the lifespan of Drosophila melanogaster. Arch. Gerontol. Geriatr. 2014;58:153–159. doi: 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Tepass U., Tanentzapf G., Ward R., Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 24.Basset A., Khush R.S., Braun A., Gardan L., Boccard F., Hoffmann J.A., Lemaitre B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liehl P., Blight M., Vodovar N., Boccard F., Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006;2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuraishi T., Binggeli O., Opota O., Buchon N., Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2011;108:15966–15971. doi: 10.1073/pnas.1105994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imler J.L., Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem. Immunol. Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- 28.Tzou P., Ohresser S., Ferrandon D., Capovilla M., Reichhart J.M., Lemaitre B., Hoffmann J.A., Imler J.L. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 29.Wu L.P., Choe K.M., Lu Y., Anderson K.V. Drosophila immunity: genes on the third chromosome required for the response to bacterial infection. Genetics. 2001;159:189–199. doi: 10.1093/genetics/159.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guntermann S., Primrose D.A., Foley E. Dnr1-dependent regulation of the Drosophila immune deficiency signaling pathway. Dev. Comp. Immunol. 2009;33:127–134. doi: 10.1016/j.dci.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Petersen A.J., Katzenberger R.J., Wassarman D.A. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics. 2013;194:133–142. doi: 10.1534/genetics.113.150854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieppois G., Opota O., Lalucat J., Lemaitre B. Pseudomonas entomophila: A Versatile Bacterium with Entomopathogenic Properties. In: Pseudomonas J.-L., Ramos J.B., editors. Vol. 7. 2014. pp. 25–49. (Goldberg und A Filloux (Hg.): New Aspects of Pseudomonas Biology). [Google Scholar]
- 33.Quevillon-Cheruel S., Leulliot N., Muniz C.A., Vincent M., Gallay J., Argentini M., Cornu D., Boccard F., Lemaitre B., van Tilbeurgh H. Evf, a virulence factor produced by the Drosophila pathogen Erwinia carotovora, is an S-palmitoylated protein with a new fold that binds to lipid vesicles. J. Biol. Chem. 2009;284:3552–3562. doi: 10.1074/jbc.M808334200. [DOI] [PubMed] [Google Scholar]
- 34.Ferrandon D., Imler J.L., Hetru C., Hoffmann J.A. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 35.Ha E.M., Oh C.T., Ryu J.H., Bae Y.S., Kang S.W., Jang I.H., Brey P.T., Lee W.J. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell. 2005;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]