Abstract
Hydrogen sulfide (H2S) is a gaseous signalling molecule involved in many physiological and pathological processes. There is increasing evidence that H2S is implicated in aging and lifespan control in the diet-induced longevity models. However, blood sulfide concentration of naturally long-lived species is not known. Here we measured blood sulfide in the long-lived naked mole-rat and five other mammalian species considerably differing in lifespan and found a negative correlation between blood sulfide and maximum longevity residual. In addition, we show that the naked mole-rat cystathionine β-synthase (CBS), an enzyme whose activity in the liver significantly contributes to systemic sulfide levels, has lower activity in the liver and is activated to a higher degree by S-adenosylmethionine compared to other species. These results add complexity to the understanding of the role of H2S in aging and call for detailed research on naked mole-rat transsulfuration.
Keywords: Transsulfuration, Hydrogen sulfide, Methionine, Longevity, Mole-rats, Low methionine diet
Graphical abstract
Highlights
-
•
Blood sulfide levels are low in long-lived species.
-
•
Naked mole-rat CBS harbours a mutation at an evolutionarily conserved cysteine C412L.
-
•
Naked mole-rat CBS is activated to an unusually high degree by SAM.
-
•
C431L, in contrast to C431S, in human CBS does not confer constitutive activation.
1. Introduction
Hydrogen sulfide (H2S) is a gasotransmitter playing a role in many physiological and pathological processes e.g. inflammation, apoptosis, cellular energetics, vascular contractility. Known molecular mechanisms underlying H2S effects include activation of ion channels, regulation of second messengers (cAMP, cGMP, free calcium) levels, and protein sulfhydration [1]. In mammals, H2S is produced mainly by two enzymes of the evolutionarily conserved transsulfuration pathway, cystathionine β-synthase (CBS, EC 4.2.1.22) and cystathionine γ-lyase (CSE, EC 4.4.1.1), as well as 3-mercaptopyruvate sulfurtransferase (MST, EC 2.8.1.2).
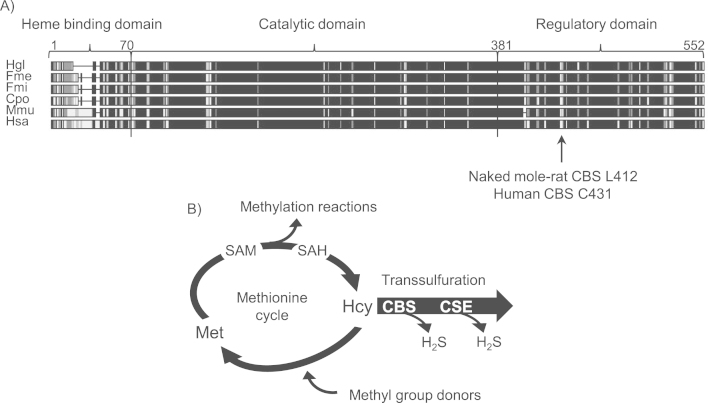
CBS is a key regulatory enzyme at the intersection of the transsulfuration pathway and methionine cycle, controlling the flux of methionine into transsulfuration (Fig. 1B). In the canonical reaction CBS catalyses condensation of homocysteine and serine to form cystathionine and water. However, when cysteine is used instead of serine, cystathionine and H2S are produced. CBS is a pyridoxal 5’-phosphate and heme dependent enzyme consisting of three structural domains: (i) N-terminal heme binding domain, (ii) catalytic core, and (iii) C-terminal regulatory domain with an autoinhibitory function (Fig. 1A). Binding of a universal methyl group donor S-adenosylmethionine (SAM) to the regulatory domain activates and stabilizes the enzyme [2], [3].
Fig. 1.
CBS – an H2S producing enzyme. (A) Six-species CBS sequence alignment. CBS consists of three domains. The mole-rat C412L substitution is located in the regulatory domain. Hgl – H. glaber, Fme – F. mechowii, Fmi - F. micklemi, Cpo – C. porcellus, Mmu – M. musculus, Hsa – H. sapiens (B) A simplified scheme showing the role of CBS in sulfur metabolism. Met – methionine, hcy – homocysteine.
Although there is increasing evidence that H2S is implicated in aging and lifespan control, its exact role in these processes is still not clear. Exogenous H2S increases lifespan in Caenorhabditis elegans [4]. Moreover, CBS is required for the life-prolonging effect of caloric restriction in Drosophila [5], and increased H2S production in models for diet-induced longevity was observed [6]. In contrast, a decrease in CBS protein levels and activity in response to methionine and isocaloric protein restriction, respectively, was shown [2], [7].
Importantly, sulfide concentration in naturally long-lived species remains unknown. A measure of longevity employed in this study is maximum longevity residual, which represents the relationship of the observed maximum lifespan of the species to its expected, body size-based lifespan calculated with the mammalian allometric equation [8]. Human and naked mole-rat belong to species with the highest maximum longevity residual. The naked mole-rat (Heterocephalus glaber) is a eusocial subterranean rodent native to East Africa. It has become the focus of increased attention in the field of aging and cancer research due to its extremely long life- and healthspan [9] as well as its resistance to cancer [10]. Here, we determine blood sulfide concentrations in six mammals (naked mole-rat, human, mouse, guinea pig, Fukomys mechowii, Fukomys micklemi) differing in their maximum longevity residual. In addition, since CBS activity in the liver significantly contributes to the circulating H2S levels [11], we comparatively analyse the naked mole-rat CBS gene.
2. Material and methods
2.1. Animals
Naked mole-rat colonies are maintained at Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany in an artificial burrow system with tunnels and plexiglass boxes. The system is heated to 26–29 °C with a constant high relative humidity of 60%–80%. The chambers contain wood bedding, twigs and unbleached paper tissue. Fresh food is given daily ad libitum and includes sweet potatoes, carrots, fennel, apples, a cereal supplement containing vitamins and minerals, and oat flakes. Sampling was approved by the local ethics committee of the “Landesamt für Gesundheit und Soziales”, Berlin, Germany (#ZH 156).
F. micklemi and F. mechowii are maintained at the animal facilities of the Department of General Zoology, University of Duisburg-Essen, Germany. They are housed as family groups in glass terraria on horticultural peat and fed ad libitum with carrots and potatoes every day, apples every second day, and grain and lettuce once a week. Room temperature and humidity is kept constant at 24±1 °C and 40±3%, respectively. Sampling was approved by Landesamt für Natur-, Umwelt- und Verbraucherschutz Nordrhein-Westfalen (Az. 84-02.04.2013.A164).
Guinea pigs (Cavia porcellus, Dunkin Hartley HsdDhl:DH) were purchased from Harlan Laboratories, AN Venray, Netherlands. Animals are maintained at Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany under room conditions in plastic cages with litter and hay as bedding. The range of the room temperature is 18–20 °C and of the humidity 40–50%. Fresh food is given daily and includes carrot, cucumber, salad, apples, and dry feed. Sampling was approved by the local ethics committee of the “Landesamt für Gesundheit und Soziales”, Berlin, Germany (G02217/12).
Mice (Mus musculus, C57BL/6) were maintained at the Center of Sepsis Control and Care (Jena University Hospital, Jena, Germany). They were maintained under artificial day-night conditions at room temperature, and received a standard diet and water ad libitum. Animals were randomly selected for each experiment. Sampling was approved by Thüringer Landesamt für Verbraucherschutz (02-035/12).
2.2. Human samples
Blood samples were obtained from healthy volunteers of European origin after written informed consent and approval by the Jena University Ethics Committee (3624-11/12).
2.3. Quantification of sulfide in whole blood
Sulfide was measured by GC/MS after extractive alkylation using a bis-pentafluorobenzyl derivative. The method and its calibration were described in detail in [12]. 25 µl blood was used and the volume of the reaction mixture was adjusted accordingly. Species and sampling information is listed in Table 1.
Table 1.
Species and sampling information for sulfide measurement in blood.
| Species | Mean age ±SD |
Number of females |
Number of males |
Source of blood | Anesthesia | ||
|---|---|---|---|---|---|---|---|
| Breeder | Non-breeder | Breeder | Non-breeder | ||||
| Hgl | 44 months ±5 | 3 | 2 | 2 | 3 | Heart puncture/vein | Isoflurane |
| Fme | 56 months ±15 | 9 | 7 | Vein | Ketamine and xylazine | ||
| Fmi | 31 months ±15 | 2 | 6 | 2 | 9 | Vein | Ketamine and xylazine |
| Mmu | 9 months±5 | 4 | 17 | Retro-orbital puncture | Isoflurane | ||
| Cpo | 12 months | 1 | 3 | Heart puncture | Medetomidine, midazolam, and fentanyl | ||
| Hsa | 45 years ±11 | 5 | 9 | Vein | – | ||
Hgl – H. glaber, Fme – F. mechowii, Fmi – F. micklemi, Cpo – C. porcellus, Mmu – M. musculus, Hsa – H. sapiens.
Blood sulfide level was correlated with maximum longevity residual obtained from the AnAge database (http://genomics.senescence.info/species accessed on 14.09.2015). Of note, there are no entries for F. mechowii and F. micklemi in the AnAge database. Therefore, maximum longevity residual for F. mechowii was calculated with the allometric equation provided by the database: tmax=4.88M0.153, tmax – maximum longevity, M – body weight. According to our records, the longest living individual of F. mechowii is 21yo breeding female, and the mean body weight of F. mechowii females is 250 g (unpublished data).
F. micklemi is a small bathyergid occurring in Western and Southern Zambia. The maximum lifespan of this species is not yet established because it has been assigned species status only relatively recently [13] and has been bred in laboratories only since 2008. However, F. micklemi is very closely related to the better studied F. anselli and F. kafuensis, with all three species belonging to the same “Fukomys micklemi” clade according to [14]. F. micklemi interbreeds with F. anselli in the lab (own unpublished data), and both species are nearly undistinguishable regarding their body measures and biology including mating and social system. We therefore used the maximum longevity residuals of F. anselli as the currently best available approximation for F. micklemi.
2.4. Cell culture
HCT116 cells were purchased from ECACC through Sigma (Sigma-Aldrich, St. Louis, MO, USA). Cells were grown at 37 °C in the presence of 5% CO2 in McCoy's Medium (Gibco, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 10% FBS.
HEK293-EBNA cells were a kind gift from Dr. Christoph Kaether (Leibniz Institute for Age Research-Fritz Lipmann Institute, Jena, Germany). Cells were grown at 37 °C in the presence of 5% CO2 in DMEM Medium (Gibco, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 10% FBS.
2.5. RT-PCR
RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. 900 ng total RNA was used for reverse transcription with QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). CBS and GAPDH were amplified with the use of primers listed in Table 2 and following PCR conditions: 95 °C 1 min, followed by 29 cycles with 95 °C 30 s, 59 °C 30 s, 72 °C 1 min, and final extension at 72 °C for 10 min. PCR products were analyzed by agarose (1% w/v) gel electrophoresis.
Table 2.
Primer sequences used for RT-PCR analysis, site-directed mutagenesis, and cloning.
| Gene | Primer names and sequences (5′-3′) |
|---|---|
| CBS | cbs_qPCRhum_1F: ATGCTGATCGCGCAAGAG; cbs_qPCRhum_1R: TCGCTCAGGAACTTGGTCAT |
| GAPDH | hs_GAPDH_1F: AACGGGAAGCTTGTCATCAATGGAAA; hs_GAPDH_1R: GCATCAGCAGAGGGGGCAGAG |
| Mmu CBS | mcbscDNA_EcoRI_1F: TCTAGGAATTCTCGACCCATCCTTGCTGAGTTTGT; mcbscDNA_PmeI_1R: CTAATGTTTAAACTCGATTGGGTGAGGAAGCTGGTAG |
| Hgl CBS CT1234TG | nmrCBSMut_1F: CCCACCGTCACCTGCGAGCACACCATC; nmrCBSMut_1R: GATGGTGTGCTCGCAGGTGACGGTGGG |
| Hgl CBS CT1234TC | nmrCBS_L412S_f: TGCTGCCCACCGTCACCTCCGAGCACACCATCGCCAT; nmrCBS_L412S_r: ATGGCGATGGTGTGCTCGGAGGTGACGGTGGGCAGCA |
| Fme CBS T355C T358G | CBSmechToAnsel_2F: GAGGACGCAGAGCGCGCCGGGATCCT; CBSmechToAnsel_2R: AGGATCCCGGCGCGCTCTGCGTCCTC |
Hgl – H. glaber, Fme – F. mechowii, Mmu – M. musculus.
2.6. RNA-seq
For library preparation 1 µg of total RNA was introduced into Illumina’s (Illumina, San Diego, CA, USA) TruSeq RNA sample prep kit v2 following the manufacturer’s instruction. Quality checking and quantification of the library was done using an Agilent Bioanalyzer 2100 in combination with an Agilent DNA 7400 kit (Agilent Technologies, Inc., CA, USA). The library was sequenced on a HiSeq2500 in high-output, 50 bp single-read mode. SBS sequencing chemistry v3 was used (Illumina, San Diego, CA, USA). Read information were extracted in FastQ format using bcl2fastq v1.8.4 (supported by Illumina). The sequencing approach resulted in 57,815,446 single-end reads. The reads were mapped to the human genome (hg19) taken the RefSeq [15] annotation (release 64) into account using tophat v.1.4.1 [16]. The mapping result was introduced into htseq-count [17] using the annotation as mentioned above to count reads per gene.
2.7. Test for positive selection
Orthologous genes were determined by best-bidirectional-blast-hits. Per species (Mus musculus, Rattus norvegicus, Mesocricetus auratus, Cricetulus griseus, Nannospalax galili, Chinchilla lanigera, Cavia porcellus, F. mechowii, F. micklemi, Canis lupus, Bos taurus, Pan troglodytes, Homo sapiens, Oryctolagus cuniculus) that splice variant was chosen that showed highest similarity to naked mole-rat transcript (XM_004885703). Codon alignments were conducted using prank [18]. The alignments were filtered by gBlocks [19]. Next, PAML's [20] branch-site test of positive selection was applied using naked mole-rat as foreground branch.
2.8. Plasmids and site-directed mutagenesis
The CBS CDS of naked mole-rat (XM_004885703), F. mechowii (KR028540), human (NM_000071), C431L and C431S variants, and human core with naked mole-rat regulatory domain were synthesized and cloned into pCMV6-AC plasmid by Blue Heron Biotechnology, Inc. (Bothell, WA, USA). The naked mole-rat L412C and L412S variants, and the F. mickelmi CDS (KR028541) were generated by site-directed mutagenesis using the naked mole-rat or F. mechowii pCMV6-AC constructs as a template. Primers containing desired mutations were designed with the primer-design program PrimerX (http://www.bioinformatics.org/primerx. Accessed 3 September 2014). The template replicated with Pfu/Psp DNA polymerase (GeneON, Ludwigshafen am Rhein, Germany) was digested with DpnI (NEB, Ipswich, MA, USA).
CDS with naked mole-rat core and human regulatory domain was created by exchanging regulatory domains between human and naked mole-rat pCMV6-AC constructs. Eco47III (Thermo Scientific, Waltham, MA, USA) (restriction site spanning nucleotides 1103 to 1108 of human CBS coding sequence) and PmeI (Thermo Scientific, Waltham, MA, USA) (restriction site in the multiple cloning site) were used to cut plasmids. The fragments were separated on a 1% agarose gel, purified with GeneJET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA), and ligated with the Quick Ligation Kit (NEB, Ipswich, MA, USA).
Mouse CDS (NM_178224) was amplified using mouse liver cDNA from MTC Multiple Tissue cDNA Panels (Clontech, Mountain View, CA, USA) as a template. Primers contained EcoRI and PmeI restriction sites. PCR product and pCMV6-AC plasmid were digested with EcoRI (NEB, Ipswich, MA, USA) and PmeI (Thermo Scientific Waltham, MA, USA) and purified from 1% agarose gel with GeneJET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA). PCR product was ligated into the plasmid with the Quick Ligation Kit (NEB, Ipswich, MA, USA).
All plasmids were amplified in E.coli TOP10 cells (Life technologies, Carlsbad, CA, USA) and purified with a Plasmid Maxi Prep kit (Qiagen, Valencia, CA, USA). Plasmid sequences were confirmed by Sanger sequencing using BigDye Terminator v3.1 Sequencing Standard Kit (Applied Biosystems, Foster City, USA) and ABI3730xl DNA Analyzer. All primer sequences are listed in Table 2.
2.9. CBS activity assay
HCT116 cells were transiently transfected with FugeneHD (Promega, Madison, WI, USA) according to manufacturer’s protocol using 4:1 reagent:DNA ratio. Cells were harvested 24 or 48 h after transfection and cell pellets were lysed in STEN buffer (50 mM Tris HCl, 150 mM NaCl, 2 mM EDTA, 0.2% NP40, pH 8) supplemented with Halt Protease Inhibitor Cocktail (Thermo Scientific, Waltham, MA, USA). Protein concentration was measured with Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA).
CBS activity was assayed by measurement of H2S production with an H2S specific probe 7-azido-4-methylcoumarin (AzMC) (Sigma-Aldrich, St. Louis, MO, USA). The assay was designed after [21]. The reaction mixture contained: 200 mM Tris HCl pH 8.0, 5 μM pyridoxal 5′-phosphate, 10 mM glutathione, 0.5 mg/mL BSA, 50 µM AzMC and cell lysate. Always the same amount of protein was used within one assay. The reaction mixture was incubated with or without SAM (0.5 mM) for 60 min at 37 °C. Always cell lysate from one transfection was divided and used for the measurement in the absence and the presence of SAM. The fluorescence at 450 nm (exc. 365 nm) was read with Infinite M1000 microplate reader (Tecan, Männedorf, Switzerland). Data were normalized to mock transfected cells. Fold activation was calculated by dividing CBS activity with SAM by CBS activity without SAM.
2.10. H2S producing CBS activity in the liver
Liver tissue from 8 non-breeding naked mole-rats and 8 mice (equal number of both sexes, mean age was 40 months±6 and 8 months±0.5, respectively) was cut into pieces and frozen immediately after collection and kept at −20 °C until usage. Liver pieces were disrupted in Tissue Lyser LT (Qiagen, Valencia, CA, USA) using stainless steel beads in 1 ml STEN buffer supplemented with proteinase inhibitor mix. Tissue lysate was incubated 30 min on ice and centrifuged at 13,000 rpm for 8 min. The supernatant was dialysed in Slide-A-Lyzer Dialysis Cassettes, 7 K MWCO (Thermo Scientific, Waltham, MA, USA) overnight at 4 °C in STEN buffer with one buffer change after 2 hours. Protein concentration measurement and CBS activity assay were performed as in CBS activation assay in cell lysates, except for the addition of 2.5 mM DL-propargylglycine (Sigma-Aldrich, St. Louis, MO, USA), an irreversible inhibitor of cystathionine gamma-lyase (CSE). 260 µg protein was used in the assay and the incubation time was 90 min. Data were normalized to the reaction mixture containing water instead of liver lysate.
2.11. Statistical analysis
Unpaired Student's t-test, one-way ANOVA followed by Tukey test, and Kruskal Wallies test followed by Nemenyi test were used to analyse differences in CBS activity in the liver, CBS activation, and sulfide levels in blood, respectively. A p-value of <0.05 was considered to indicate statistical significance.
3. Results
3.1. Low blood sulfide levels in long-lived species
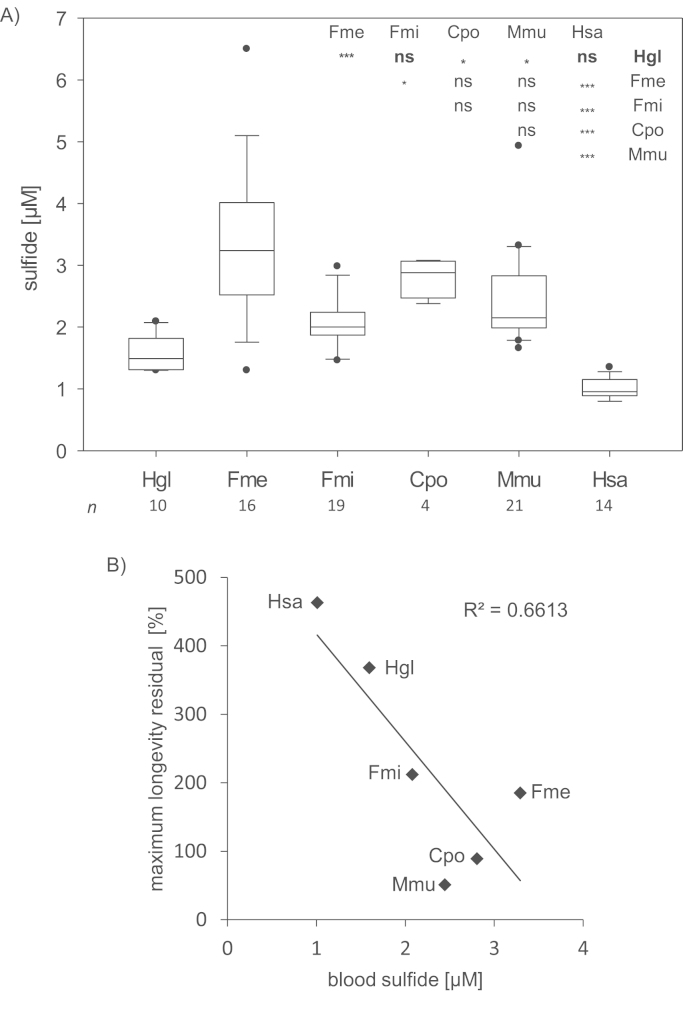
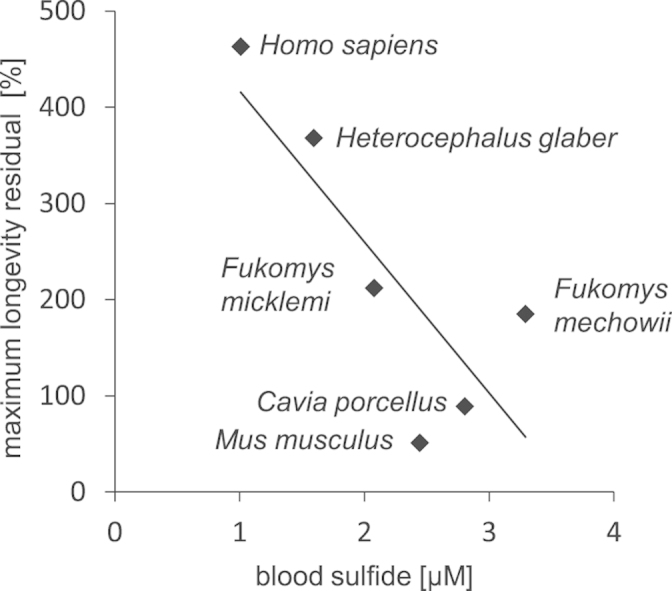
Using extractive alkylation with a bis-pentafluorobenzyl derivative we quantified sulfide concentration in whole blood of naked mole-rat, two other mole-rat species (F. mechowii, F. micklemi), mouse, guinea pig, and human. Sulfide levels differ significantly between species with human and naked mole-rat exhibiting the lowest values (Fig. 2A). Surprisingly, we found a negative correlation between maximum longevity residual and mean sulfide concentration in blood (Fig. 2B).
Fig. 2.
Sulfide concentration in blood. (A) Sulfide concentration in blood. p-value: *<0.05, **<0.01, ***<0.001, ns – not significant (Kruskal Wallies test followed by Nemenyi test). (B) Corellation between maximum longevity residual and mean sulfide concentration in blood. In (A) and (B) Hgl – H. glaber, Fmi – F. micklemi, Fme – F. mechowii, Cpo – C. porcellus, Mmu – M. musculus, Hsa – H. sapiens.
3.2. Low endogenous CBS activity in the naked mole-rat liver
Next, we compared endogenous CBS activity in the liver of naked mole-rat and mouse using H2S-specific probe 7-azido-4-methylcoumarin (AzMC). We observed that H2S producing activity of CBS is lower in naked mole-rat liver as compared to mouse (Fig. 3).
Fig. 3.
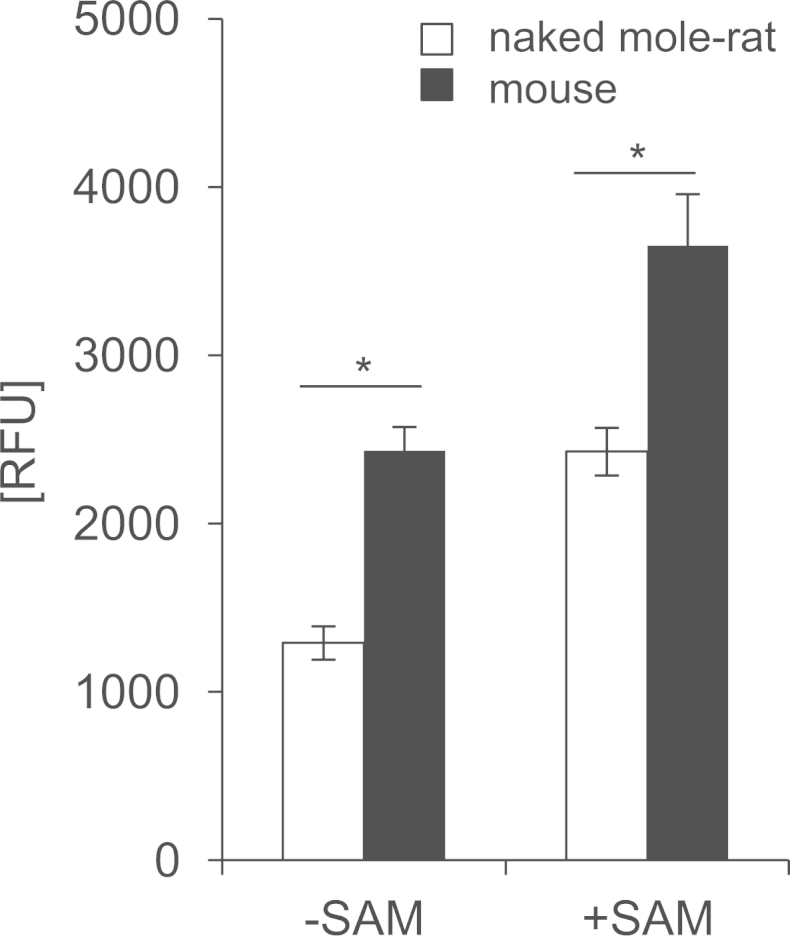
CBS activity in the liver. Data represent mean±SD of 8 animals. Data was normalized to water control (water instead of lysate in the reaction mixture), RFU – relative fluorescence unit. p-value: *<0.05 (unpaired Student's t-test).
3.3. Substitution of a conserved cysteine to leucine in naked mole-rat CBS does not affect activation
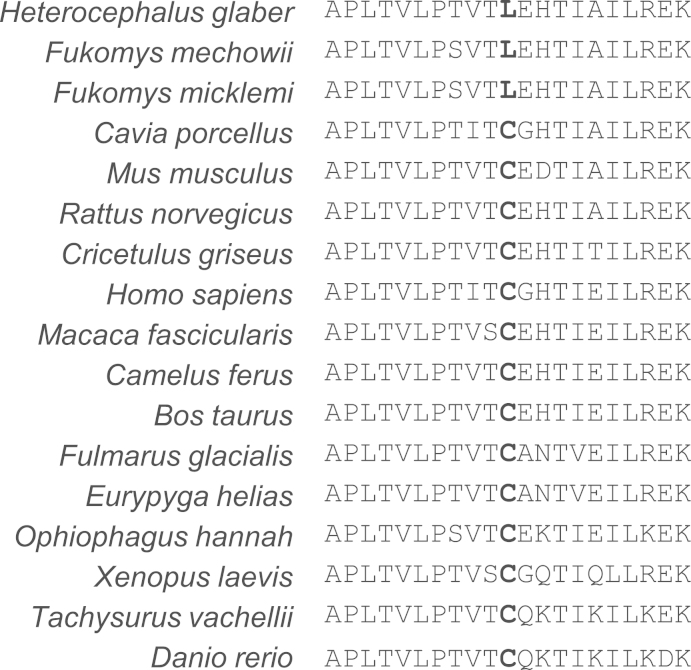
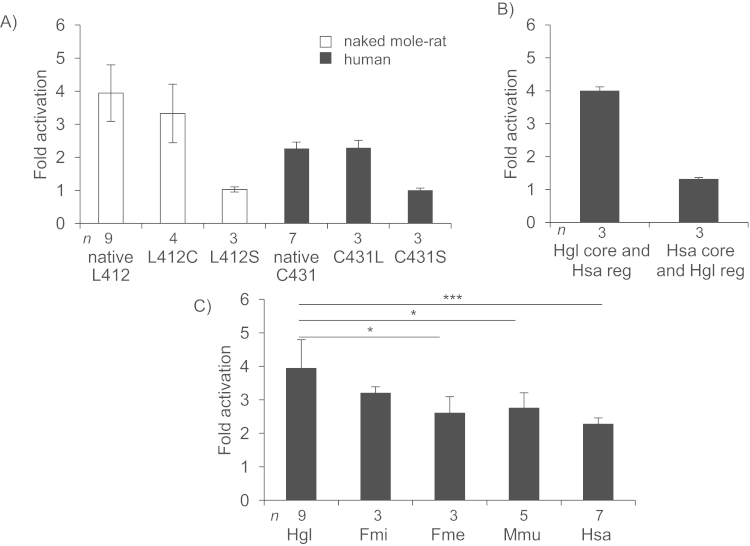
As CBS is a significant contributor to endogenous H2S production in mice [11] we screened the naked mole-rat CBS gene for signs of positive selection but failed (File S1). We noticed, however, in the regulatory domain at amino acid position 412 a cysteine>leucine substitution (C412L) present also in CBS from F. mechowii and F. micklemi. Cysteine at this position is conserved among all other analyzed vertebrates (Fig. 4). Notably in human CBS, the conversion of the corresponding cysteine to serine (C431S) creates a constitutively active form of the enzyme [22]. We, therefore, tested whether substitution of the conserved cysteine to leucine in the regulatory domain of naked mole-rat CBS affects the degree of activation of the enzyme in the presence of SAM. To this end, plasmids encoding either canonical or in vitro mutated human or naked mole-rat CBS were transfected into HCT116 human colorectal carcinoma cell line. One of the main criteria for the cell line to be used in this study was its lack of endogenous CBS expression, which – if present-would create a background signal in the assay. Zhang et al. described HCT116 cells as being CBS mRNA free [23]. In contrast, Yamamoto et al. [24] and Szabo et al. [25] reported low and high CBS expression, respectively. In light of this contradiction, we performed RT-PCR and RNA-seq which confirmed that the transsulfuration pathway in HCT116 cells is suppressed (no CBS and negligible CTH transcripts detectable) (File S2). H2S-producing CBS activity was tested in cell lysates with the use of AzMC in the presence and in the absence of SAM. Conversion of leucine at position 412 to cysteine in naked mole-rat CBS did not affect enzyme activation (Fig. 5A). Similarly, mutation of the corresponding cysteine to leucine in human CBS did not show any effect. Mutation to serine resulted in a constitutively active enzyme in both species, which is in agreement with published data [22].
Fig. 4.
Multi-species alignment of sequences flanking the conserved cysteine residue in CBS regulatory domain. The alignment region corresponds to position 426-446 of the alignment in File S1.
Fig. 5.
Activation of CBS by SAM. (A) Fold activation after HCT116 transfection with expression vectors containing either the naked mole-rat CDS with leucine, cysteine and serine at position 412 (native L412, L412C and L412S, respectively) or the human CDS with cysteine, leucine and serine at position 431 (native C431, C431L and C431S, respectively). (B) Fold activation after HCT116 transfection with expression vectors coding for chimeric CBS: naked mole-rat N-terminal heme binding and catalytic domain, and human regulatory domain (Hgl core and Hsa reg) and vice versa (Hsa core and Hgl reg). (C) Fold activation of CBS from different species. Hgl – H. glaber, Fmi-F. micklemi, Fme – F. mechowii, Mmu – M. musculus, Hsa – H. sapiens. In (A) (B) (C), data represent mean±SD of n biological replicates, p-value: *<0.05, **<0.01, ***<0.001 (one-way ANOVA followed by Tukey test). Data were normalized to the lysate from mock transfected cells.
3.4. Strong activation of naked mole-rat CBS by SAM
The described experiment, however, revealed that the SAM activation level of naked mole-rat CBS (4-fold) is nearly doubled compared to human CBS (2.3-fold). CBS from F. mechowii, F. micklemi, and mouse show intermediate degree of activation (Fig. 5C).
To test whether the regulatory domain is responsible for the high activation of naked mole-rat CBS we exchanged regulatory domains between human and naked mole-rat CBS. While naked mole-rat CBS activation is not influenced by the presence of human regulatory domain, human CBS core with naked mole-rat regulatory domain shows a 42% decrease in activation (Fig. 5B). This suggests that the cause for a high degree of activation of naked mole-rat CBS lies outside of the regulatory domain.
4. Discussion
The data presented in this study reveal an intriguing negative correlation between blood sulfide levels and maximum longevity residual. In the light of reported increase of transsulfuration activity and high H2S levels in diet-induced longevity models [5], [6], this observation is unexpected and suggests that the role of H2S in natural and diet-induced longevity is different.
Given the plethora of reported H2S effects, this finding is difficult to interpret. H2S is elevated in cardiovascular [26] and rheumatic disease [27], which suggests that the low sulfide levels may be beneficial. In addition, low CBS activity and low sulfide levels in naked mole-rat may contribute to cancer resistance in this species, as it has been shown that H2S is promoting both tumor growth and vascularisation [28]. However, the vast majority of studies report beneficial effects of increased H2S levels and most translational approaches aim to develop H2S-delivering therapeutics [1].
The low sulfide concentration in naked mole-rat blood is consistent with our findings that H2S producing activity of CBS is lower in naked mole-rat as compared to mouse. Naked mole-rats feed on the underground parts of plants. Roots and tubers show very low methionine content [29]. Therefore we hypothesize that naked mole-rat diet is low in methionine. This was also pointed out by Buffenstein [30]. Methionine restriction is one of the most powerful dietary regimens resulting in longer lifespan [31]. Interestingly, it has been reported that methionine restriction leads to a decrease in CBS protein levels [2]. Hence, low CBS activity in the naked mole-rat liver may be an adaptation to a low methionine diet. The described features of naked mole-rat CBS may be beneficial under conditions of limited methionine availability. Namely, low basal CBS activity allows for remethylation of homocysteine in order to maintain sufficient methionine levels. However, in case of methionine surplus, a high degree of CBS activation by SAM efficiently directs toxic homocysteine to degradation by the transsulfuration pathway. Consistently with our hypothesis, isocaloric protein restriction in rats results in increased remethylation of homocysteine to methionine and decrease in CBS activity [7]. Because of unknown specificity and affinity of commercially available anti-CBS antibodies against naked mole-rat CBS, we were not able to compare basal activity and the amount of CBS in the liver across species. This must be acknowledged as a limitation of the study.
Naked mole-rat single amino acid changes are used to explain its phenotype [32], [33]. In the interspecies CBS sequence comparison, we found a mole-rat specific substitution of a conserved cysteine with leucine in the CBS regulatory domain. The amino acid change was potentially interesting given the results of mutagenesis of a corresponding cysteine in the human CBS [22]. Since examples of misleading conclusions on the role of naked mole-rat-specific amino acid changes based on sequence comparisons were already elucidated [34], we experimentally studied the functional consequences of the observed substitution and found that it has no effect on the CBS activation.
However, this experiment revealed a strong activation of naked mole-rat CBS in response to SAM. While in our experiments human CBS show an activation of 2.3 fold, in the literature values ranging from 2 to 5-fold can be found [2], [35]. This discrepancy can be explained by the fact that SAM binding to CBS is considerably affected by surface electrostatics [35]. Nevertheless, under normalized conditions used in this study (the same cell line transfected with CBS from different species under the control of identical promoters) naked mole-rat CBS shows consistently a higher degree of activation by SAM than human, mouse, and F. mechowii CBS.
CBS activity substantially affects plasma homocysteine levels [36], [37]. Since hyperhomocysteinemia is observed in many age-related diseases [38] and is a strong predictor of mortality among individuals with coronary artery disease [39], further characterization and kinetic studies of naked mole-rat CBS may shed new light on the mechanisms underlying the extremely long healthspan of this species.
In summary, we found a negative correlation between blood sulfide concentration and maximum longevity residual and provide the first insights into naked mole-rat transsulfuration pathway. We report low H2S producing CBS activity in the naked mole-rat liver and a high activation of naked mole-rat CBS by SAM. In addition, we determined that the substitution of the conserved cysteine (C412L) in the regulatory domain of naked mole-rat CBS does not affect the degree of activation of the enzyme. The described features of naked mole-rat CBS call for detailed research on naked mole-rat transsulfuration pathway.
Acknowledgments
We thank Ralf A. Claus and Marcel Kramer for providing mouse samples and Jacqueline Fischer for excellent technical assistance. We would also like to thank Paul Van Daele for providing F. micklemi used in this study. This work was supported by grants from the Leibniz Association to TH and MP (WGL, SAW-2012-FLI-2) and the German Research Foundation DFG to PD (DA 992/3-1) and MP (PL 173/8-1).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.01.008.
Contributor Information
Maja Dziegelewska, Email: maja.dziegelewska@leibniz-fli.de.
Susanne Holtze, Email: holtze@izw-berlin.de.
Christiane Vole, Email: christiane.vole@uni-due.de.
Ulrich Wachter, Email: ulrich.wachter@uni-ulm.de.
Uwe Menzel, Email: Uwe.Menzel@hki-jena.de.
Michaela Morhart, Email: morhart@izw-berlin.de.
Marco Groth, Email: marco.groth@leibniz-fli.de.
Karol Szafranski, Email: karol.szafranski@leibniz-fli.de.
Arne Sahm, Email: arne.sahm@leibniz-fli.de.
Christoph Sponholz, Email: Christoph.Sponholz@med.uni-jena.de.
Philip Dammann, Email: Philip.Dammann@uk-essen.de.
Klaus Huse, Email: klaus.huse@leibniz-fli.de.
Thomas Hildebrandt, Email: HILDEBRANDT@izw-berlin.de.
Matthias Platzer, Email: matthias.platzer@leibniz-fli.de.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug. Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 2.Prudova A., Bauman Z., Braun A., Vitvitsky V., Lu S.C. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein J.D., Kyle W.E., Martin J.L., Pick A.M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1975;66:81–87. doi: 10.1016/s0006-291x(75)80297-x. [DOI] [PubMed] [Google Scholar]
- 4.Miller D.L., Roth M.B. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabil H., Kabil O., Banerjee R., Harshman L.G., Pletcher S.D. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl. Acad. Sci. USA. 2011;108:16831–16836. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalhan S.C., Uppal S.O., Moorman J.L., Bennett C., Gruca L.L. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J. Biol. Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Magalhaes J.P., Costa J., Church G.M. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 10.Liang S., Mele J., Wu Y., Buffenstein R., Hornsby P.J. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9:626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen K.K., Geoghagen N.S., Jin L., Holt T.G., Luo Q. Pharmacological activation and genetic manipulation of cystathionine beta-synthase alter circulating levels of homocysteine and hydrogen sulfide in mice. Eur. J. Pharmacol. 2011;650:86–93. doi: 10.1016/j.ejphar.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 12.McCook O., Radermacher P., Volani C., Asfar P., Ignatius A. H2S during circulatory shock: some unresolved questions. Nitric Oxide. 2014;41:48–61. doi: 10.1016/j.niox.2014.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Daele P.A.A.G., Dammann P., Meier J.L., Kawalika M., Van De Woestijne C. Chromosomal diversity in mole-rats of the genus Cryptomys (Rodentia: Bathyergidae) from the Zambezian region: with descriptions of new karyotypes. J. Zool. Lond. 2004;264:317–326. [Google Scholar]
- 14.Van Daele P.A., Verheyen E., Brunain M., Adriaens D. Cytochrome b sequence analysis reveals differential molecular evolution in African mole-rats of the chromosomally hyperdiverse genus Fukomys (Bathyergidae, Rodentia) from the Zambezian region. Mol. Phylogenet. Evol. 2007;45:142–157. doi: 10.1016/j.ympev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Pruitt K., Brown G., Tatusova T., Maglott D. Chapter 18 the reference sequence (RefSeq) database. In: McEntyre J., Ostell J., editors. The NCBI Handbook [Internet] National Center for Biotechnology Information (US); Bethesda (MD): 2002. [Google Scholar]
- 16.Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders S., Pyl P.T., Huber W. HTSeq – a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loytynoja A., Goldman N. Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science. 2008;320:1632–1635. doi: 10.1126/science.1158395. [DOI] [PubMed] [Google Scholar]
- 19.Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 21.Thorson M.K., Majtan T., Kraus J.P., Barrios A.M. Identification of cystathionine beta-synthase inhibitors using a hydrogen sulfide selective probe. Angew. Chem. Int. Ed. Engl. 2013;52:4641–4644. doi: 10.1002/anie.201300841. [DOI] [PubMed] [Google Scholar]
- 22.Frank N., Kery V., Maclean K.N., Kraus J.P. Solvent-accessible cysteines in human cystathionine beta-synthase: crucial role of cysteine 431 in S-adenosyl-L-methionine binding. Biochemistry. 2006;45:11021–11029. doi: 10.1021/bi060737m. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W., Braun A., Bauman Z., Olteanu H., Madzelan P. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 2005;65:1554–1560. doi: 10.1158/0008-5472.CAN-04-1554. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T., Takano N., Ishiwata K., Ohmura M., Nagahata Y. Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nat. Commun. 2014;5:3480. doi: 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo C., Coletta C., Chao C., Modis K., Szczesny B. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013;2:e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muniraj N., Stamp L.K., Badiei A., Hegde A., Cameron V. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int. J. Rheum. Dis. 2014 doi: 10.1111/1756-185X.12472. [DOI] [PubMed] [Google Scholar]
- 28.Hellmich M.R., Coletta C., Chao C., Szabo C. The therapeutic potential of cystathionine beta-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingenbleek Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J. Nutr. 2006;136:1641S–1651S. doi: 10.1093/jn/136.6.1641S. [DOI] [PubMed] [Google Scholar]
- 30.Ables G.P., Brown-Borg H.M., Buffenstein R., Church C.D., Elshorbagy A.K. The first international mini-symposium on methionine restriction and lifespan. Front. Genet. 2014;5:122. doi: 10.3389/fgene.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orentreich N., Matias J.R., DeFelice A., Zimmerman J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z., Zhang Y., Chen L. Single amino acid changes in naked mole rat may reveal new anti-cancer mechanisms in mammals. Gene. 2015;572:101–107. doi: 10.1016/j.gene.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim E.B., Fang X., Fushan A.A., Huang Z., Lobanov A.V. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delsuc F., Tilak M.K. Naked but not Hairless: the pitfalls of analyses of molecular adaptation based on few genome sequence comparisons. Genome Biol. Evol. 2015;7:768–774. doi: 10.1093/gbe/evv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pey A.L., Majtan T., Kraus J.P. The role of surface electrostatics on the stability, function and regulation of human cystathionine beta-synthase, a complex multidomain and oligomeric protein. Biochim. Biophys. Acta. 2014;1844:1453–1462. doi: 10.1016/j.bbapap.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L., Jhee K.H., Hua X., DiBello P.M., Jacobsen D.W. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ. Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo H.K., Sorond F.A., Chen J.H., Hashmi A., Milberg W.P. The role of homocysteine in multisystem age-related problems: a systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1190–1201. doi: 10.1093/gerona/60.9.1190. [DOI] [PubMed] [Google Scholar]
- 39.Nygard O., Nordrehaug J.E., Refsum H., Ueland P.M., Farstad M. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl. J. Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material