Abstract
Canonical reporters such as green fluorescent protein (GFP) and luciferase have assisted researchers in probing cellular pathways and processes. Prior research in pathogenesis depended on sensitivity of biochemical and biophysical techniques to identify effectors and elucidate entry mechanisms. Recently, the β-lactamase (βlac) reporter system has advanced toxin and effector reporting by permitting measurement of βlac delivery into the cytosol or host βlac expression in intact cells. βlac measurement in cells was facilitated by the development of the fluorogenic substrate, CCF2-AM, to identify novel effectors, target cells, and domains involved in bacterial pathogenesis. The assay is also adaptable for high-throughput screening of small molecule inhibitors against toxins, providing information on mechanism and potential therapeutic agents. The versatility and limitations of the βlac reporter system as applied to toxins and effectors are discussed in this review.
Keywords: protein toxins, protein translocation, β-lactamase, fluorescent reporters
This review discusses the versatility and limitations of the βlac-CCF2 reporter system as applied to interactions of toxins and effectors with host cells.
Graphical Abstract Figure.
This review discusses the versatility and limitations of the βlac-CCF2 reporter system as applied to interactions of toxins and effectors with host cells.
INTRODUCTION
Recent advances in biochemical and biophysical techniques have resolved many properties of bacterial toxins including the identification of toxin receptors, how toxins enter endosomal compartments, toxin translocation through membranes and identification of post-translational modifications to host substrates. Distinctively, advances in bioimaging, facilitated by the generation of reagents and reporters, have allowed detection of toxins in live, intact cells (Zlokarnik et al. 1998). These reagents can assist in the quantification host cell signaling, gene expression, protein–protein interactions and localization of proteins of interest (Luo, Ho and Wilson 2008). A preferred reporter should mimic the properties of the protein of interest and should produce a stable, readily measurable signal under physiological conditions (Jiang et al. 2008). Many reporter genes are available in mammalian and bacterial vectors expressed directly within the host cell or for production of a purified reporter protein.
One early reporter is the Escherichia coli enzyme, β-galatosidase, a 116-kDa tetramer that hydrolyzes galatose-containing molecules such as lactose (Schenborn and Groskreutz 1999). The reporter has been utilized in molecular biology to screen for gene inserts that disrupt the expression of the holoenzyme β-galatosidase, which prevents cleavage of the colorimentric substrate, X-gal (Burn 2012). Chemiluminescent and fluorescent substrates are available to confirm transfection of plasmids containing the gene of interest fused to the reporter in mammalian cells. Since the reporter requires tetramerization for catalytic activity, is not optimal for use as an exogenous recombinant protein reporter. Additionally, mammalian host cells contain endogenous enzyme β-galatosidase in their lysosomal fractions, increasing background to the assays (Schenborn and Groskreutz 1999). The following section will discuss improved reporters applied to recombinant proteins, including effectors and toxins, in mammalian systems.
ADVANCES IN PROTEIN REPORTING
Perhaps the most recognized reporter is the green fluorescent protein (GFP) produced by the jellyfish Aequoria victoria. The gfp gene encodes a 26.9-kDa monomer, which when fused to another protein allows detection of protein localization and gene expression. GFP is a versatile reagent; variants generated include photostable and thermostable reporters with various fluorescent properties, along with variants that also report pH, protein–protein interactions and temporal expression (Tsien 1998; Lam et al. 2012). In two separate studies, the localization of botulinum neurotoxin A catalytic light chain (LC/A) was assessed in PC12 and Neuro-2a neuronal cell lines, utilizing a GFP for visualization. Vectors encoding GFP-LC/A variants transfected into these cells lines showed that the first eight N-terminal residues were required for plasma membrane localization of LC/A with substrate SNAP-25 (Fernandez-Salas et al. 2004; Chen and Barbieri 2011). Relative to full-length LC/A, the N-terminal deletion GFP-LC/A fusion proteins also failed to bind SNAP-25, as detected by Far-western analysis, providing a mechanism for the observed plasma membrane mislocalization (Chen and Barbieri 2011). Another study utilized GFP-fusion protein truncations of Pseudomonas aeruginosa type III secretion system (T3SS) effector ExoS to identify minimal membrane-interacting domains. Using HeLa cells, a leucine-containing domain comprising residues 51–77, termed the membrane localization domain (MLD), was shown to be required for perinuclear localization and ADP-ribosylation of substrate Ras by ExoS (Zhang and Barbieri 2005). The MLD is conserved in other type III effectors, suggesting a similar mechanism for membrane-localization following injection into the cytosol. In Vibrio cholerae multifunctional autoprocessing RTX toxins (MARTXVC), GFP-fusion proteins encoding various domains confirmed the presence of an MLD (Geissler, Tungekar and Satchell 2010). Primary sequence alignment of this domain identified MLDs in other putative toxins, including homologue MLD domains in Vibrio vulnificus. This study established that substitution of the MARTXVC MLD by homologues in V. vulnificus complemented intracellular localization (Geissler, Tungekar and Satchell 2010).
Luciferase reporters are oxidative enzymes isolated from organisms as diverse as fireflies and sea pansies. Luciferase is more sensitive than GFP at reporting gene expression and generates bioluminescence, which avoids cell autofluorescence (Thorne, Inglese and Auld 2010). This sensitivity makes luciferase a useful reporter for intact animal studies (Jiang et al. 2008). Luciferase catalyzes two sequential ATP-dependent reactions, which utilize a cation, oxygen and luciferin to emit a photon of light. In contrast to GFP, the luciferase system allows dual reporters to normalize signal from the protein of interest to a constitutively expressed standard. The current luciferase reporters range between 20 and 62 kDa, with varied half-lives, and different signal properties such as flashes or sustained emission (Thorne, Inglese and Auld 2010). More recently, secreted luciferases as well as membrane-permeable reagents have eliminated cell lysis steps to allow measurements in cells. For example, a reporter fusion of single-chain BoNT/D (Luc-scBoNT/D) was incubated with synaptosomes, and luciferase activity was quantified in cell fractions; low-level luciferase activity was measured in the cytosol, indicating delivery of refolded, luciferase cargo (Bade et al. 2004). Also, in a small molecule screen, cytosolic expression of luciferase in mammalian cells identified inhibitors of toxins such as ricin, shiga toxin, exotoxin A and diphtheria toxin that prevent host protein synthesis (Zhao and Haslam 2005).
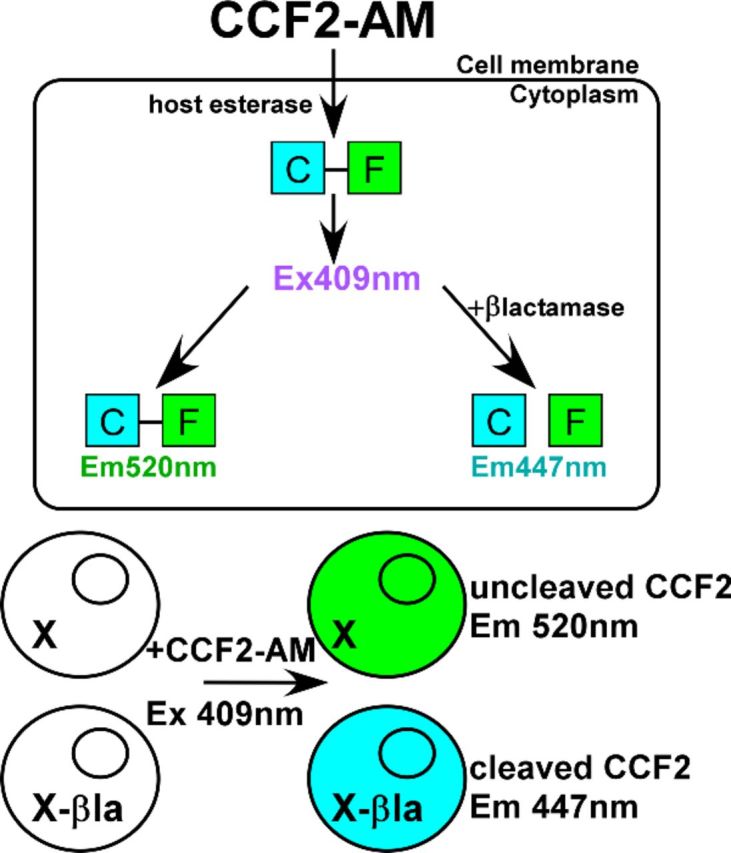
More recently, TEM-1 β-lactamase (βlac), a 28.6-kDa enzyme of E. coli which cleaves penicillin and cephalosporin family antibiotics, has been used as a reporter (Campbell 2004). Like the aforementioned reporters, βlac can be used to quantify gene expression, measure outputs of signal transduction and protein–protein interactions via complementation (Qureshi 2007). The utility of βlac as a reporter is partly due to the development of the fluorogenic substrate CCF2-AM and more recently, CCF4-AM (Zlokarnik et al. 1998). CCF2/4 substrates are composed of two fluorophores: a coumarin derivative linked to a fluorescein molecule by a cephalosporin backbone. When coumarin is excited at 409 nm, fluorescein undergoes fluorescence resonance energy transfer (FRET) and emits at 520 nm (Campbell 2004). βlac cleaves the cephalosporin backbone, uncoupling FRET and yielding coumarin emission at 447 nm as depicted in Fig. 1. The ratio of cleaved and intact CCF2 acts to normalize substrate loading. Table 1 highlights the benefits and limitations of each of the reporter systems described above.
Figure 1.
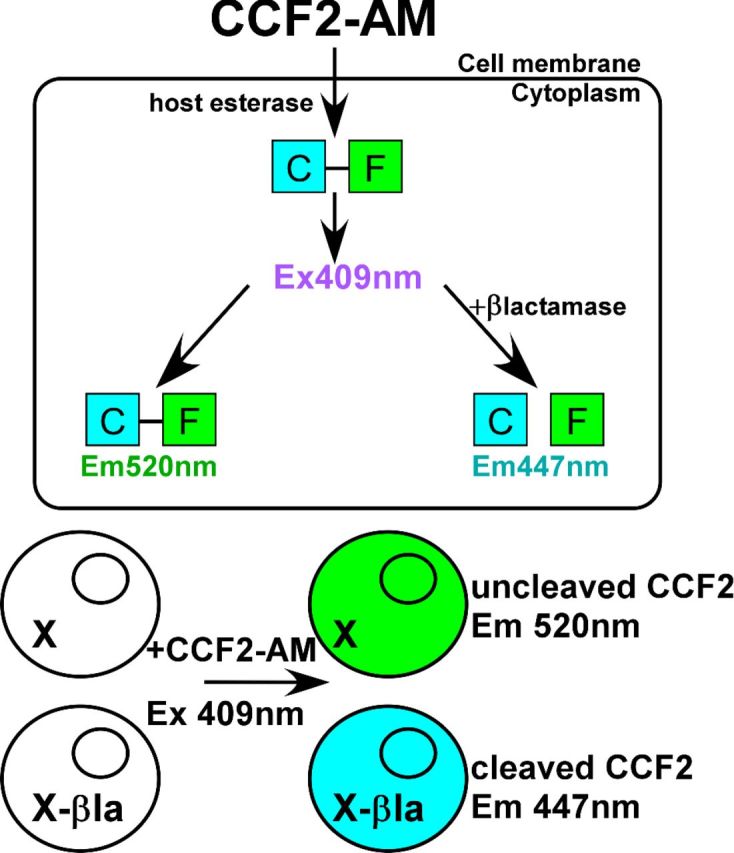
βlac/CCF2 assay. CCF2-AM substrate is membrane permeable until cleavage of the acetymoxymethyl (AM) tag by host esterases traps the substrate in the cytosol. In the absence of a β-lactamase, the substrate when excited at 409 nm undergoes FRET, emitting at 520 nm. In the presence of β-lactamase, the substrate is cleaved to uncouple FRET and emission is observed at 447 nm.
Table 1.
Cell reporter systems. The GFP, luciferase and β-lactamase reporter system known strengths and limitations.
| Reporter | Strength | Limitation | Reference |
|---|---|---|---|
| Green fluorescent protein | Intact animal reporting | Stoichiometric signal | Cubitt et al. (1995); Tsien (1998) |
| Localization information | Not membrane permeable | ||
| Detection by microscopy, microplate and FACS | Reporter always ‘on’ | ||
| Luciferase | Intact animal reporting | No localization information | Jiang et al. (2008); Thorne et al. (2010) |
| Sensitive bioluminescent signal | Signal transient | ||
| Microplate | Luminometer required | ||
| β-lactamase | Amplifying signal | No localization | Zlokarnik et al. (1998); Zlokarnik (2000); Campbell (2004) |
| CCF2 self-normalizing | Half-life ∼3–4 h | ||
| Detection by microscopy, microplate and FACS | In vivo limited |
The remainder of this review will present the utility of the βlac reporter system to characterize the intracellular action of protein toxins and effectors. In the following studies, βlac reporter is detected in bacteria expressing reporter fusions, as an exogenous reporter protein, or as a reporter measuring gene expression within a host cell. In a typical βlac/CCF2 assay, cells are loaded with CCF2-AM for 0.5–2 h in a calcium- and magnesium-free buffer containing a pump blocker such as probenecid (Zlokarnik et al. 1998). The substrate, CCF2-AM, is non-fluorescent and membrane permeable until host esterases cleave the acetymoxymethyl (AM) tag, trapping the substrate in the cytosol (Zlokarnik 2000). Following CCF2-AM incubation, cells may be fixed or analyzed by live microscopy. CCF2-AM is considered non-toxic and accumulates to ∼50-100 μM only in living cells (Zlokarnik et al. 1998). As few as 100 molecules of βlac can be detected in a 16-h incubation, while ∼15 000 molecules can be detected in 1-h incubation (Zlokarnik et al. 1998). The assay is commonly quantified by measuring either the ratio of cleaved to intact CCF2 or the number of cells exhibiting blueshifted emission as a percentage of total cells. Controls to verify signal due to CCF2-dependent cleavage include incubating substrate in the absence of a βlac-reporter and a catalytically inactive βlac (Ser70→Ala) reporter as depicted in Fig. 2. βlac-mediated CCF2 cleavage has been successfully used in several detection strategies to identify effectors, minimally functional domains, target cells, mechanisms for productive translocation and inhibitors through high-throughput screening (HTS), which are described below (Marketon et al. 2005; Zhu et al. 2009; Alam et al. 2011; Dolores et al. 2015; Zuverink et al. 2015).
Figure 2.
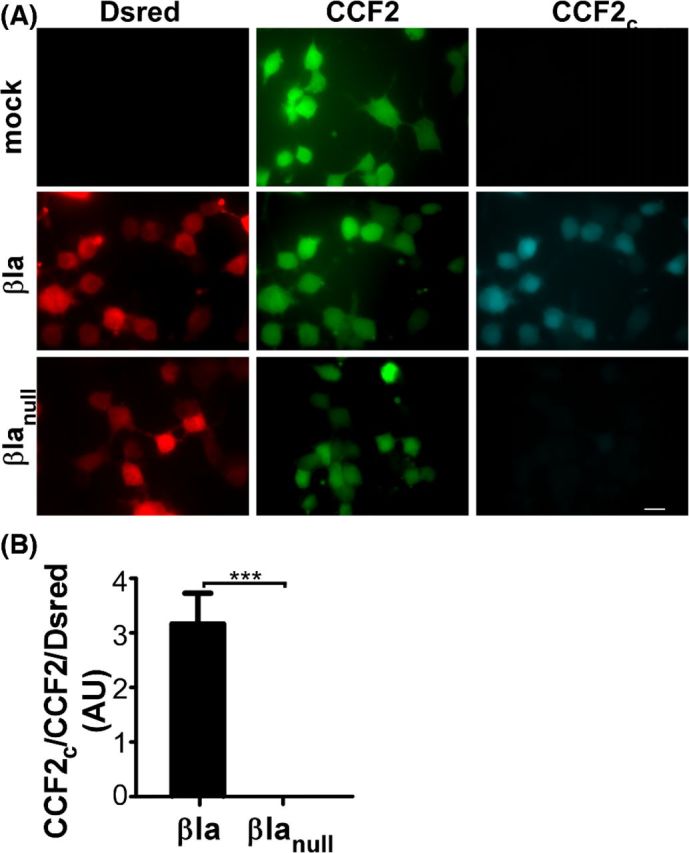
βlac/CCF2 assay controls. (A) Neuro-2a cells were mock-transfected or transfected with pCMVβla-Dsred and or catalytic null pCMVβlanull-Dsred (Ser70Ala) at 37°C for 24 hours before loading with 1 μM CCF2-AM at 25°C for 30 min. Intact, cytosolic CCF2 is shown in green and cleaved CCF2 (CCF2c) is shown in cyan. (B) CCF2 cleavage was quantified by taking the ratio of net average fluorescence intensity for cleaved CCF2 to intact CCF2, normalized to Dsred (CCF2c/CCF2/Dsred). This figure was reproduced from Chen et al. (2015).
Identification of effectors
Vibrio cholerae, P. aeruginosa, Yersinia pestis and Legionella pneumophila are Gram-negative pathogens that encode secretion systems such as the chaperone-assisted type I (T1SS), flagella-like type III (T3SS), conjugation-like type IV (T4SS) and the phage-like type VI (T6SS) that export effectors from bacteria into the host cytosol (Alfano and Collmer 2004; Costa et al. 2015). Among these pathogens, the secretion machinery is conserved, but the effectors vary in both number and function. Effectors are pre-synthesized and often associated with a chaperone in a primed orientation that is poised to respond to a signal for translocation; upon induction, effectors are secreted into the culture medium or host cell (Birtalan, Phillips and Ghosh 2002; Ghosh 2004). Recently, bioinformatics has predicted putative effectors near or within the operon encoding the respective secretion system with some success. βlac C-terminal fusions have identified novel secreted effectors in mammalian cells without cell lysis. The following studies verify that cells exhibiting blueshifted fluorescence are due to translocation of a βlac-effector and not bacterial expression alone by utilizing secretion and chaperone mutants, as well as non-effector fusions such as GST/MBP-fused βlac in parallel. If an effector has already been identified, a βlac fusion can be included as a positive control.
Vibrio cholerae strains -O1 and -O139 produce cholera toxin and factors required for colonization of the intestinal tract, which are associated with the disease cholera (Dziejman et al. 2005). Related pathologies are also observed with non-O1 and -O139 strains that contain a T3SS (Dziejman et al. 2005). In one study, bioinformatics identified potential T3SS effectors in these strains by selecting open reading frames (ORFs) with no homology towards other virulence factors (Alam et al. 2011). Selected putative effectors were overexpressed in yeast viability assays to assay cytopathic activity and confirmed to be T3SS substrates by V. cholerae transfer of βlac-fusion of effectors into mammalian cells (Alam et al. 2011). Identification of novel T3SS effectors may improve clinical outcomes by characterizing strains and providing therapeutic targets.
Legionella pneumophila is the causative agent of the respiratory disease Legionnaires’ disease which is spread by aerosol. Legionella contains the Dot/Icm T4SS that contributes to virulence, but has not been exhaustively characterized (Zhu et al. 2011). Previously, antibodies towards known effectors, purification of Legionella-containing vacuoles (LCV), or adenylate cyclase fusions were used to detect T4SS activity and identify substrates (Nagai et al. 2005; Urwyler, Brombacher and Hilbi 2009). In a large screen, around 800 ORFs >300 bp were subcloned into a plasmid as C-terminal βlac fusions which led to the identification of over 70 novel substrates (Zhu et al. 2011). The authors set up their multiplicity of infection (MOI) where their positive control, a known effector RalF-βlac, had 95% of cells with blueshifted emission and their negative control, FabI-βlac, an enzyme involved in fatty acid biosynthesis, resulted in 0% of cells with blueshifted emission. The authors further characterized the effectors based on translocation efficiency in U397 macrophages. One caveat to the βlac-effector fusions was that translocation efficiency of effectors was not correlated with reporter expression, which may indicate that the rate-limiting step is translocation into the host cytosol. The βlac-effector fusion screen provided an approach to screen a large quantity of candidate substrates. Most of the identified effectors did not contain classical motifs present in previously identified substrates, yet were observed to translocate. Legionella has also served as a T4SS surrogate to identify factors in the pathogen Coxiella burnetti, which does not have a robust genetic system (Chen et al. 2010). Legionella Dot/Icm can translocate C. burnetti effectors and has identified over 20 candidate substrates (Chen et al. 2010).
Identification of minimal, functional domains
The βlac reporter system has been used to identify and characterize the function of the minimal domains of toxins. In one study, the nuclear factor of activated T cells (NFAT) βlac reporter system, developed by Zloranik et al. was adapted to study minimal functional domains of Pasteurella multocida toxin (PMT) required for host signaling activation in Chinese Hamster Ovary (CHO) cells (Zlokarnik 2000; Luo, Ho and Wilson 2008). PMT deamidates Gln209 within the α-subunit of the heterotrimeric Gq protein, inhibiting GTPase activity (Orth et al. 2009). Constitutively activated Gq induces phospholipase Cβ1 activation and subsequent cleavage of phosphotydalinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphate (IP3). DAG and IP3 stimulate a series of phosphorylation events that ends with the transcription of βlac-NFAT. Earlier studies assayed ΔcAMP, phosphorylation state of pathway kinases or by visualizing cell morphological changes such as stress fibers as correlates for G protein targets (Luo, Ho and Wilson 2008).
The βlac/CCF2 assay identified exogenous and cytosol-expressed PMT domains that activate NFAT (Luo, Ho and Wilson 2008). CCF2 cleavage was compared under stimulated and unstimulated conditions to establish a range of detection. Additionally, a catalytically null PMT was used as a negative control for signaling and transfection of GFP alone confirmed that cytosolic overexpression did not stimulate βlac-NFAT expression. Pathway activation by exogenously incubated PMT was only observed when the catalytically active holotoxin was able to bind, enter and access the cytosol. Cytosolic expression of fragments containing the domain involved in substrate modification also directly activated βlac-NFAT expression. This βlac/CCF2 assay was adapted for a signaling readout amenable to HTS (see more about HTS below). Utilizing a known cell target for βlac expression allows screening of small molecule inhibitors of wild-type PMT and can potentially identify residues necessary for PMT action. This type of assay application could be utilized for diagnostics or to characterize effectors acting on well-established host pathways.
Another study dissected effector functions of the T3SS effector Cif, which is associated with zoonotic enteropathogenic and enterohemorrhagic E. coli (EPEC/EHEC) strains that cause diarrhea and hemorrhagic colitis, respectively. Cif arrests cells in G2/M phase and reorganizes actin. In this study, the authors reported that deletion of the N-terminal 16 residues of Cif inhibited intracellular delivery, as observed by absence of cells with blueshifted emission (Charpentier and Oswald 2004). Additional experiments confirmed that the C-terminal domain of Cif was solely responsible for host pathology. Substitution of the N-terminal T3SS targeting sequences of nearby encoded effectors rescued delivery of Cif-βlac into HeLa cells. This study provided an intact readout that confirmed other substrates contain a T3SS targeting sequence at their N terminus, which is separate from effector function.
A novel application in minimal domain characterization was employed for type 1 secreted V. cholerae MARTX toxin. MARTXVC inserts into the plasma membrane where a cysteine protease domain (CPD) becomes activated by host inositol hexakisphosphate (InsP6) and releases effector domains into the cytosol (Prochazkova et al. 2009). The activity of these effectors includes an actin-crosslinking domain (ACD), a Rho-inactivation domain (RID) and a putative alpha–beta hydrolase (ABH) (Dolores et al. 2015). To discriminate each effector's contribution to cell pathology, a series of MARTX fusions were created, which contained βlac alone or βlac with combinations of the effectors. The authors utilized negative controls such as secretion mutants, a CPD null and catalytically inactive effector domains. CCF2 cleavage (cells with blueshifted fluorescence) was observed with an active CPD, indicating restriction of βlac reduces CCF2 cleavage (Dolores et al. 2015). The βlac reporter system identified a cooperative effect between CPD cleavage and effector number as βlac translocation efficiency increased when one or more of the effector domains remained. Additionally, the assay confirmed presence of domain variants in the cytosol of an intact cell while dissecting effector function. The authors observed that the ACD domain had a dominant phenotype sufficient to inhibit macrophage phagocytosis. Unexpectedly, dissection of RID and ABH domains demonstrated each had opposing effects on host CDC42 activation levels.
Identification of target cells in vitro and ex vivo
The amenability of the βlac/CCF2 assay to fluorescence activated sorting (FACS) provides a novel method for identifying cells targeted by pathogens both in vivo and ex vivo. This reporting circumvents obstacles in target cell identification for cytotoxic effectors such as ExoU, which cleaves phospholipids, leading to rapid cell lysis (Sato and Frank 2004). Pseudomonas aeruginosa is a leading cause of ventilator-associated pneumonia and strains containing the T3SS are indicative of poor prognosis (Schulert et al. 2003). Pseudomonas aeruginosa strains may contain a combination of four known effectors ExoS, ExoT, ExoU and ExoY, with about one quarter of strains containing ExoU (Diaz and Hauser 2010). Pseudomonas aeruginosa expressing only atoxic ExoU-βlac was delivered intranasally into mice and their lungs were collected at different times post infection, incubated with CCF2-AM and immunostained to identify cell types (Diaz and Hauser 2010). The authors found resident alveolar macrophages were early targets for T3SS, and later into infection cells emitting blueshifted fluorescence numbers were representative of the responding immune cell composition, with a preference for neutrophils (Diaz and Hauser 2010). Interestingly, if the ExoU-βlac was expressed in strains containing other effectors such as ExoS or ExoT, cell targets were slightly altered (Diaz and Hauser 2010). To ensure identified cells emitting blueshifted fluorescence were due to the translocation of an effector, control strains harboring secretion and chaperone mutants or non-effector βlac-fusions were utilized, as in the effector identification studies.
In two separate studies, Y. enterocolitica, which causes gastrointestinal enterocolitis, and Y. pestis, the causative agent of plague, were engineered to inject βlac-fusions of Yersinia outer proteins (Yops) into cells. In the first study, Y. enterocolitica delivered YopE-βlac without cell preference into splenic suspensions, while delivery of the fusion was targeted to B cells, neutrophils, dendritic and macrophages in a mouse model (Koberle et al. 2009). Similarly, Y. pestis YopM-βlac differentially targeted cells in vivo compared to ex vivo, with a preference for phagocytic macrophages, neutrophils and dendritic cells. This suggests the two pathogens target cells of the innate and adaptive immune system differently (Marketon et al. 2005). The βlac assay provided insight on how these two pathogens target cells in vivo, important for treatment of these two infections.
Salmonella enterica serovar Typhimurium causes inflammation and gastroenteritis in mammals. Serovar Typhimurium encodes two T3SS systems on pathogenicity islands 1 and 2 (SPI-1 and SPI-2), used differentially to establish chronic persistence and later a systematic infection, respectively (Zhou and Galán 2001). Serovar Typhimurium is utilized in mice as a model for the human pathogen, serovar Typhi (Geddes, Cruz and Heffron 2007). Dissemination of the bacteria to the liver and splenic in mice following intraperitoneal injection is observed; however, the cells targeted by SPI-1 and SPI-2 were unknown (Geddes, Cruz and Heffron 2007). Assessment of individual C-terminal effector-βlac fusions confirmed substrate fidelity to a specific T3SS, and SPI-1 and SPI-2 secreted unique βlac-fusions (Geddes, Cruz and Heffron 2007). After 24 hpi, a preference for B cells, T cells, as well as phagocytic cells such as neutrophils were observed. When red fluorescently labeled Salmonella were used to deliver the fusions, blueshifted cells correlated with red expression only in SPI-2-expressing strains. The βlac reporter system did not identify mature macrophages as targets, which has potential to change how infection is treated. One caveat to this study was the bacterial burden required for visualization was within an order of magnitude below a burden that caused sepsis. Thus, the utility of the βlac-fusion analysis appears defined by the sensitivity of the CCF2 substrate.
Bacillus anthracis produces anthrax toxin (AT) upon sporulation in airways, the intestine or in wounds causing lethal infections (Young and Collier 2007). The heptameric binding domain protective antigen (PA) can oligomerize to bind and deliver a metalloprotease lethal factor (LF) or the adenylate cyclase known as edema factor (EF) (Young and Collier 2007). AT, like other toxins, is difficult to visualize due to potency, C-terminal LF-βlac fusion was engineered to identify target cells (Hu and Leppla 2009). In this study, LF-βlac fusion was incubated with PA in splenic suspensions or with cells sorted from these suspensions, and target cell populations compared (Hu and Leppla 2009). FACS identified cells exhibiting blueshifted emission, indicating that AT preferentially targeted macrophages, dendritic cells and B cells and to a lesser extent in CD4+ and CD8+ T cells (Hu and Leppla 2009). This preference was observed in both suspensions and purified spleen cells, and translocation efficiency did not correlate to receptor number.
Identifying mechanism for productive translocation
The previous studies above detected cytosolic translocation of βlac fusions by bacterial secretion system machinery or delivery by a pore-forming toxin. AB toxins, such as diphtheria toxin (DT), tetanus toxin (TeNT) and the botulinum toxins (BoNTs) are single polypeptide chains that contain catalytic (A) and binding (B) domains (Pellizzari et al. 1999). This protein is activated by proteolysis into a di-chain, which remains held together by an interchain disulfide bridge (Sagane et al. 1999). An AB toxin pH trigger allows insertion of a translocation domain into the endosome membrane, forming a channel to deliver the catalytic subunit into the cytosol (Pellizzari et al. 1999). Biophysical or electrophysiological techniques such as potassium ion or fluorescent dye release from liposomes and patch-clamp experiments that measure cation flow through pores have measured A domain translocation by assaying pore formation (Koriazova and Montal 2003; Puhar et al. 2004; Burns and Baldwin 2014). Cell-based studies measured delivery of the catalytic domain by analysis of substrate cleavage or modification, but require cell lysis (Pirazzini et al. 2013b).
The di-chain of TeNT is comprised of three domains; the N-terminal catalytic LC connected to the heavy chain (HC) containing a translocation domain (HCT) and a C-terminal receptor-binding domain (HCR) (Lacy et al. 1998). To study LC/T translocation in intact cells, a βlac was N-terminally fused to a catalytically null TeNT and cytosolic delivery of βlac was measured in cortical neurons (Zuverink et al. 2015). To confirm that CCF2 cleavage was due to translocation of the βlac-LC and not to the presence of an endosomal bound βlac, a fusion of βlac to the N terminus of the receptor-binding domain of TeNT (βlac-HCR/T) controlled for HCT-independent signal. Additionally, βlac activity of all reporters was confirmed by in vitro assay using a colorimetric substrate, fluorocillin green.
An intact interchain disulfide is required for neurotoxicity and productive translocation of the LC by the clostridial neurotoxins (CNTs) (Schiavo et al. 1990; Fischer and Montal 2007). More recently, inhibitors of host thioredoxin/thioredoxin reductase (Trx-TrxR) redox system were observed to be neuroprotective to CNTs in cerebellar granular neurons (Pirazzini et al. 2013a). Trx-TrxR has been previously demonstrated to reduce the interchain disulfide in vitro and was found associated with the cytosolic face of synaptic vesicles (Kistner, Sanders and Habermann 1993; Pirazzini et al. 2015). This finding along with inhibitor studies indicates a physiological role for Trx-TrxR in reducing the interchain disulfide of CNTs (Pirazzini et al. 2013a). To characterize the role of the interchain disulfide in translocation, a derivative of βlac-TeNT lacking the disulfide was generated by site-directed mutagenesis, and was not observed to deliver βlac-LC into the cytosol (Zuverink et al. 2015). βlac-TeNT with an intact interchain disulfide exhibited a dose-dependent decrease in translocation if cells were pre-treated with TrxR inhibitor auranofin. The use of βlac to report cytosolic delivery permitted interpretation that an intact disulfide was required to initiate translocation, but reduction of the disulfide in the cytosol was required for productive translocation and cytosolic refolding.
High-throughput screening
HTS automates and standardizes an assay to screen small molecule inhibitors against bacterial effectors or toxin targets, and can elucidate entry mechanisms through host protein inhibition. While most toxin action can be assayed by substrate modification or host signaling responses, many of these methods are not amenable to HTS. Like luciferase reporting, the βlac/CCF2 assay can be miniaturized and replicated, permitting screens for characterization and inhibition of toxin action. Controls for false positive molecules require verification that inhibitors do not affect substrate loading, inhibit βlac activity or quench the fluorophores.
Legionella pneumophila effector LepA-βlac was utilized to identify a potential trigger for secretion. LepA-βlac was expressed on an inducible plasmid in L. pneumophila as readout for T4SS translocation. Three small molecule libraries containing a sum of 2500+ annotated molecules were screened for inhibition of LepA-βlac delivery. The screen revealed that molecules with an IC50 for LepA-βlac targeted host receptors and proteins involved in cytoskeleton dynamics (Charpentier et al. 2009). The βlac reporter assay identified host phagocytosis as a signal for translocation of LepA, a discovery that will support further studies of this system, as T4SS is not inducible in culture.
The previously mentioned AT reporter LF-βlac screened inhibitors of translocation in the cervical carcinoma cell line, ME-180. Utilizing over 70 000 compounds, response curves were generated to select compounds that inhibited the delivery of LF-βlac into the cytosol (Zhu et al. 2009). Subsequent experiments validated inhibitor action on LF toxin chimeras delivered by PA in CHO and Raw264.7 cells, using cell viability as readout. The proposed mechanism for inhibitors identified was preventing the conversion of the PA pre-pore to pore by inhibiting endosomal acidification. Without reliable miniaturization, screens of this size would have been impossible.
CONCLUSIONS
The βlac reporter system has proved efficient in identifying pathogen effectors and physiological target cells. Furthermore, the βlac reporter shows promise in characterizing AB toxin translocation, at the cellular and molecular level. The reporter application in HTS should identify and confirm of how toxins and effectors exploit the host machinery to gain access to the cytosol, and may to identify inhibitors of toxin action.
Some constraints of the current βlac/CCF2 assay have emerged from implementation in toxin and effector studies. One issue in these applied assays involves the narrow ‘window’ of detection between established positive and negative controls. While overall detection of cleaved CCF2 substrate can be improved by increasing bacterial load, exogenous protein concentration or incubation length, this introduces variables such as decreased specificity of target cells or pathways, increased host burden and non-specific substrate cleavage. This emphasizes the importance of negative controls to avoid false positives. In addition, due to limits of detection and reporter turnover, the current assay may produce false negatives. With these limitations in mind, adaptation of this assay to individual systems is essential for interpretation.
Sensitivity also plays a role in the quantitative assessment of CCF2 readout. While ratiometric analysis can assist in identification of translocated effectors over negative controls, increased reporter expression or availability of receptors, as in the case of AT, is not always correlated with an increased ratio or increased translocation efficiency; therefore, rate-limiting steps such translocation or entry must be considered when comparing between different effectors or cell lines. The use of the ratiometric analysis alone does not give sufficient resolution in mixed cell populations, and cell markers or sorting may be required to discriminate phenotype. Finally, the utility of the βlac/CCF2 assay to resolve differential rates of activities in mutated toxins/effectors is not yet known.
Future applications of the βlac reporter depend on the availability of substrates with improved fidelity and sensitivity. Currently, two substrates hold promise to extend reporter imaging to in vivo whole animal models: cell-permeable near-infrared (CNIR), and the β-lactam and D-luciferin conjugate (BLUCO) (Yao, So and Rao 2007; Kong et al. 2010). CNIRs were used to visualize the activity of an endogenous plasma membrane-localized beta-lactamase, known as βlaC, during infection with Mycobacterium tuberculosis in mouse lungs, but require 24 hpi incubation for visualization (Kong et al. 2010). BLUCO requires cleavage by βlac to free D-luciferin, a substrate for luciferase-catalyzed bioluminescence. The generation of substrates recognized by dual reporter systems may increase precision and sensitivity when applied to detecting βlac-expressing pathogens or toxins intracellularly in luciferase-reporter mice. By resolving dissemination of pathogens or proteins in real-time, in vivo, progress in diagnosis and targeted treatment of infection will follow.
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI030162. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest. None declared.
REFERENCES
- Alam A, Miller KA, Chaand M, et al. Identification of Vibrio cholerae type III secretion system effector proteins. Infect Immun. 2011;79:1728–40. doi: 10.1128/IAI.01194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol. 2004;42:385–414. doi: 10.1146/annurev.phyto.42.040103.110731. [DOI] [PubMed] [Google Scholar]
- Bade S, Rummel A, Reisinger C, et al. Botulinum neurotoxin type D enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J Neurochem. 2004;91:1461–72. doi: 10.1111/j.1471-4159.2004.02844.x. [DOI] [PubMed] [Google Scholar]
- Birtalan SC, Phillips RM, Ghosh P. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol Cell. 2002;9:971–80. doi: 10.1016/s1097-2765(02)00529-4. [DOI] [PubMed] [Google Scholar]
- Burn SF. Detection of beta-galactosidase activity: X-gal staining. Methods Mol Biol. 2012;886:241–50. doi: 10.1007/978-1-61779-851-1_21. [DOI] [PubMed] [Google Scholar]
- Burns JR, Baldwin MR. Tetanus neurotoxin utilizes two sequential membrane interactions for channel formation. J Biol Chem. 2014;289:22450–8. doi: 10.1074/jbc.M114.559302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE. Realization of beta-lactamase as a versatile fluorogenic reporter. Trends Biotechnol. 2004;22:208–11. doi: 10.1016/j.tibtech.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Charpentier X, Gabay JE, Reyes M, et al. Chemical genetics reveals bacterial and host cell functions critical for type IV effector translocation by Legionella pneumophila. PLoS Pathog. 2009;5:e1000501. doi: 10.1371/journal.ppat.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004;186:5486–95. doi: 10.1128/JB.186.16.5486-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Banga S, Mertens K, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. P Natl Acad Sci USA. 2010;107:21755–60. doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Przedpelski A, Tepp WH, et al. Heat-labile enterotoxin IIa, a platform to deliver heterologous proteins into neurons. mBio. 2015;6:e00734. doi: 10.1128/mBio.00734-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Barbieri JT. Association of botulinum neurotoxin serotype A light chain with plasma membrane-bound SNAP-25. J Biol Chem. 2011;286:15067–72. doi: 10.1074/jbc.M111.224493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa TRD, Felisberto-Rodrigues C, Meir A, et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol. 2015;13:343–59. doi: 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- Cubitt AB, Heim R, Adams SR, et al. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995;20:448–55. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- Diaz MH, Hauser AR. Pseudomonas aeruginosa cytotoxin ExoU is injected into phagocytic cells during acute pneumonia. Infect Immun. 2010;78:1447–56. doi: 10.1128/IAI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolores JS, Agarwal S, Egerer M, et al. Vibrio cholerae MARTX toxin heterologous translocation of beta-lactamase and roles of individual effector domains on cytoskeleton dynamics. Mol Microbiol. 2015;95:590–604. doi: 10.1111/mmi.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziejman M, Serruto D, Tam VC, et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. P Natl Acad Sci USA. 2005;102:3465–70. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salas E, Steward LE, Ho H, et al. Plasma membrane localization signals in the light chain of botulinum neurotoxin. P Natl Acad Sci USA. 2004;101:3208–13. doi: 10.1073/pnas.0400229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Montal M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J Biol Chem. 2007;282:29604–11. doi: 10.1074/jbc.M703619200. [DOI] [PubMed] [Google Scholar]
- Geddes K, Cruz F, Heffron F. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 2007;3:e196. doi: 10.1371/journal.ppat.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Tungekar R, Satchell KJ. Identification of a conserved membrane localization domain within numerous large bacterial protein toxins. P Natl Acad Sci USA. 2010;107:5581–6. doi: 10.1073/pnas.0908700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. Process of protein transport by the type III secretion system. Microbiol Mol Biol R. 2004;68:771–95. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Leppla SH. Anthrax toxin uptake by primary immune cells as determined with a lethal factor-beta-lactamase fusion protein. PLoS One. 2009;4:e7946. doi: 10.1371/journal.pone.0007946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Xing B, Rao J. Recent developments of biological reporter technology for detecting gene expression. Biotechnol Genet Eng Rev. 2008;25:41–75. doi: 10.5661/bger-25-41. [DOI] [PubMed] [Google Scholar]
- Kistner A, Sanders D, Habermann E. Disulfide formation in reduced tetanus toxin by thioredoxin: the pharmacological role of interchain covalent and noncovalent bonds. Toxicon: Off J Int Soc Toxinol. 1993;31:1423–34. doi: 10.1016/0041-0101(93)90208-z. [DOI] [PubMed] [Google Scholar]
- Koberle M, Klein-Gunther A, Schutz M, et al. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 2009;5:e1000551. doi: 10.1371/journal.ppat.1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Yao H, Ren H, et al. Imaging tuberculosis with endogenous beta-lactamase reporter enzyme fluorescence in live mice. P Natl Acad Sci USA. 2010;107:12239–44. doi: 10.1073/pnas.1000643107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol. 2003;10:13–8. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- Lacy DB, Tepp W, Cohen AC, et al. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- Lam AJ, St-Pierre F, Gong Y, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–12. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ho M, Wilson BA. Application of intact cell-based NFAT-β-lactamase reporter assay for Pasteurella multocida toxin-mediated activation of calcium signaling pathway. Toxicon: Off J Int Soc Toxinol. 2008;51:597–605. doi: 10.1016/j.toxicon.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, et al. Plague bacteria target immune cells during infection. Science. 2005;309:1739–41. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Cambronne ED, Kagan JC, et al. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. P Natl Acad Sci USA. 2005;102:826–31. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JH, Preuss I, Fester I, et al. Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. P Natl Acad Sci USA. 2009;106:7179–84. doi: 10.1073/pnas.0900160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzari R, Rossetto O, Schiavo G, et al. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos T R Soc B. 1999;354:259–68. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzini M, Bordin F, Rossetto O, et al. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013a;587:150–5. doi: 10.1016/j.febslet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Pirazzini M, Rossetto O, Bertasio C, et al. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins C and D in neurons. Biochem Bioph Res Co. 2013b;430:38–42. doi: 10.1016/j.bbrc.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Pirazzini M, Tehran DA, Zanetti G, et al. The thioredoxin reductase - Thioredoxin redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon: Off J Int Soc Toxinol. 2015 doi: 10.1016/j.toxicon.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Prochazkova K, Shuvalova LA, Minasov G, et al. Structural and molecular mechanism for autoprocessing of MARTX toxin of Vibrio cholerae at multiple sites. J Biol Chem. 2009;284:26557–68. doi: 10.1074/jbc.M109.025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhar A, Johnson EA, Rossetto O, et al. Comparison of the pH-induced conformational change of different clostridial neurotoxins. Biochem Bioph Res Co. 2004;319:66–71. doi: 10.1016/j.bbrc.2004.04.140. [DOI] [PubMed] [Google Scholar]
- Qureshi SA. Beta-lactamase: an ideal reporter system for monitoring gene expression in live eukaryotic cells. Bio Techniques. 2007;42:91–6. doi: 10.2144/000112292. [DOI] [PubMed] [Google Scholar]
- Sagane Y, Watanabe T, Kouguchi H, et al. Dichain structure of botulinum neurotoxin: identification of cleavage sites in types C, D, and F neurotoxin molecules. J Protein Chem. 1999;18:885–92. doi: 10.1023/a:1020687430927. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–90. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Schenborn E, Groskreutz D. Reporter gene vectors and assays. Mol Biotechnol. 1999;13:29–44. doi: 10.1385/MB:13:1:29. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Papini E, Genna G, et al. An intact interchain disulfide bond is required for the neurotoxicity of tetanus toxin. Infect Immun. 1990;58:4136–41. doi: 10.1128/iai.58.12.4136-4141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- Thorne N, Inglese J, Auld DS. Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem Biol. 2010;17:646–57. doi: 10.1016/j.chembiol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–44. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Brombacher E, Hilbi H. Endosomal and secretory markers of the Legionella-containing vacuole. Commun Integr Biol. 2009;2:107–9. doi: 10.4161/cib.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, So MK, Rao J. A bioluminogenic substrate for in vivo imaging of beta-lactamase activity. Angew Chem. 2007;46:7031–4. doi: 10.1002/anie.200701931. [DOI] [PubMed] [Google Scholar]
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–65. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barbieri JT. A leucine-rich motif targets Pseudomonas aeruginosa ExoS within mammalian cells. Infect Immun. 2005;73:7938–45. doi: 10.1128/IAI.73.12.7938-7945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Haslam DB. A quantitative and highly sensitive luciferase-based assay for bacterial toxins that inhibit protein synthesis. J Med Microbiol. 2005;54:1023–30. doi: 10.1099/jmm.0.46143-0. [DOI] [PubMed] [Google Scholar]
- Zhou D, Galán J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 2001;3:1293–8. doi: 10.1016/s1286-4579(01)01489-7. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Hobson JP, Southall N, et al. Quantitative high-throughput screening identifies inhibitors of anthrax-induced cell death. Bioorg Med Chem. 2009;17:5139–45. doi: 10.1016/j.bmc.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Banga S, Tan Y, et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokarnik G. Fusions to beta-lactamase as a reporter for gene expression in live mammalian cells. Methods Enzymol. 2000;326:221–44. doi: 10.1016/s0076-6879(00)26057-6. [DOI] [PubMed] [Google Scholar]
- Zlokarnik G, Negulescu PA, Knapp TE, et al. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science. 1998;279:84–8. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- Zuverink M, Chen C, Przedpelski A, et al. A heterologous reporter defines the role of the tetanus toxin interchain disulfide in light-chain translocation. Infect Immun. 2015;83:2714–24. doi: 10.1128/IAI.00477-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


