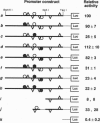
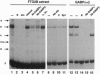
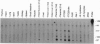Abstract
6-Phosphofructo-2-kinase (EC 2.7.1.105)/fructose-2,6-bis-phosphatase (EC 3.1.3.46) catalyzes the synthesis and degradation of fructose 2,6-bisphosphate, a ubiquitous stimulator of glycolysis. The liver (L-type) and muscle (M-type) mRNAs for this bifunctional enzyme arise from distinct promoters of the same gene. We have now characterized in rat hepatoma FTO2B cells another mRNA, which is transcribed from a third promoter of that gene. This F-type mRNA is present in fetal rat liver and muscle, in rat placenta, and in several established rat cell lines. The F promoter contains no TATA box but contains several binding sites for Sp1 and for members of the ets oncogene family. Transfection of FTO2B cells with constructs containing the intact or mutagenized F promoter showed that its activity depends mainly on one of these sites. This site bound a heteromeric FTO2B cell protein indistinguishable from the ets-related GA binding protein alpha/ankyrin-repeats GA binding protein beta transcription factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwig G. M., Bruder J. T., Hearing P. Different binding site requirements for binding and activation for the bipartite enhancer factor EF-1A. Nucleic Acids Res. 1992 Dec 25;20(24):6555–6564. doi: 10.1093/nar/20.24.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., McKnight S. L. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992 Dec;6(12B):2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Cifuentes M. E., Espinet C., Lange A. J., Pilkis S. J., Hod Y. Hormonal control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression in rat hepatoma cells. J Biol Chem. 1991 Jan 25;266(3):1557–1563. [PubMed] [Google Scholar]
- Crepin K. M., Darville M. I., Hue L., Rousseau G. G. Characterization of distinct mRNAs coding for putative isozymes of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Eur J Biochem. 1989 Aug 1;183(2):433–440. doi: 10.1111/j.1432-1033.1989.tb14946.x. [DOI] [PubMed] [Google Scholar]
- Crepin K. M., De Cloedt M., Vertommen D., Foret D., Michel A., Rider M. H., Rousseau G. G., Hue L. Molecular forms of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase expressed in rat skeletal muscle. J Biol Chem. 1992 Oct 25;267(30):21698–21704. [PubMed] [Google Scholar]
- Darville M. I., Antoine I. V., Rousseau G. G. Characterization of an enhancer upstream from the muscle-type promoter of a gene encoding 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Nucleic Acids Res. 1992 Jul 25;20(14):3575–3583. doi: 10.1093/nar/20.14.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville M. I., Crepin K. M., Hue L., Rousseau G. G. 5' flanking sequence and structure of a gene encoding rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6543–6547. doi: 10.1073/pnas.86.17.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin S. D., Bosselut R., Gégonne A., Ghysdael J., Brady J. N. Sequence-specific interaction of the Ets1 protein with the long terminal repeat of the human T-lymphotropic virus type I. J Virol. 1991 Oct;65(10):5513–5523. doi: 10.1128/jvi.65.10.5513-5523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Gégonne A., Bosselut R., Bailly R. A., Ghysdael J. Synergistic activation of the HTLV1 LTR Ets-responsive region by transcription factors Ets1 and Sp1. EMBO J. 1993 Mar;12(3):1169–1178. doi: 10.1002/j.1460-2075.1993.tb05758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Rousseau G. G. Fructose 2,6-bisphosphate and the control of glycolysis by growth factors, tumor promoters and oncogenes. Adv Enzyme Regul. 1993;33:97–110. doi: 10.1016/0065-2571(93)90011-2. [DOI] [PubMed] [Google Scholar]
- Lemaigre F. P., Durviaux S. M., Rousseau G. G. Identification of regulatory sequences and protein-binding sites in the liver-type promoter of a gene encoding 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Mol Cell Biol. 1991 Feb;11(2):1099–1106. doi: 10.1128/mcb.11.2.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau A. M., Rider M. H., Foret D., Rousseau G. G., Hue L. Rat hepatoma (HTC) cell 6-phosphofructo-2-kinase differs from that in liver and can be separated from fructose-2,6-bisphosphatase. Eur J Biochem. 1988 Jul 15;175(1):27–32. doi: 10.1111/j.1432-1033.1988.tb14161.x. [DOI] [PubMed] [Google Scholar]
- Luo X. C., Park K., Lopez-Casillas F., Kim K. H. Structural features of the acetyl-CoA carboxylase gene: mechanisms for the generation of mRNAs with 5' end heterogeneity. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4042–4046. doi: 10.1073/pnas.86.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau G. G., Hue L. Mammalian 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a bifunctional enzyme that controls glycolysis. Prog Nucleic Acid Res Mol Biol. 1993;45:99–127. doi: 10.1016/s0079-6603(08)60868-5. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Jackson S. P., Annarella M. B. Developmental expression of Sp1 in the mouse. Mol Cell Biol. 1991 Apr;11(4):2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Brown T. A., McKnight S. L. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Kerckaert J. P., Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992 Jun;6(6):965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- Weinhouse S. The Warburg hypothesis fifty years later. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976;87(2):115–126. doi: 10.1007/BF00284370. [DOI] [PubMed] [Google Scholar]
- Woods D. B., Ghysdael J., Owen M. J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992 Feb 25;20(4):699–704. doi: 10.1093/nar/20.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]