Abstract
The insula has been implicated in many sequelae of stroke. It is the area most commonly infarcted in people with post-stroke arrhythmias, loss of thermal sensation, hospital acquired pneumonia, and apraxia of speech. We hypothesized that some of these results reflect the fact that: (1) ischemic strokes that involve the insula are larger than strokes that exclude the insula (and therefore are associated with more common and persistent deficits); and (2) insular involvement is a marker of middle cerebral artery (MCA) occlusion. We analyzed MRI scans of 861 patients with acute ischemic hemispheric strokes unselected for functional deficits, and compared infarcts involving the insula to infarcts not involving the insula using t-tests for continuous variables and chi square tests for dichotomous variables. Mean infarct volume was larger for infarcts including the insula (n = 232) versus excluding the insula (n = 629): 65.8 ± 78.8 versus 10.2 ± 15.9 cm3 (p < 0.00001). Even when we removed lacunar infarcts, mean volume of non-lacunar infarcts that included insula (n = 775) were larger than non-lacunar infarcts (n = 227) that excluded insula: 67.0 cm3 ± 79.2 versus 11.5 cm3 ± 16.7 (p < 0.00001). Of infarcts in the 90th percentile for volume, 87% included the insula (χ2 = 181.8; p < 0.00001). Furthermore, 79.0% infarcts due to MCA occlusion included the insula; 78.5% of infarcts without MCA occlusion excluded the insula (χ2 = 93.1; p < 0.0001). The association between insular damage and acute or chronic sequelae likely often reflects the fact that insular infarct is a marker of large infarcts caused by occlusion of the MCA more than a specific role of the insula in a range of functions. Particularly in acute stroke, some deficits may also be due to ischemia of the MCA or ICA territory caused by large vessel occlusion.
Keywords: Stroke, Infarct volume, Outcomes, Insula
Highlights
-
•
The insula is the most commonly infarcted area in patients with a wide range of deficits.
-
•
In 861 acute ischemic hemispheric strokes, mean infarct volume was much larger when infarct included the insula (p < 0.00001).
-
•
Of infarcts in the 90th percentile for volume, 87% included the insula (χ2 = 181.8; p < 0.00001).
-
•
Nearly 80% of infarcts due to MCA occlusion included the insula
-
•
Identified associations between insular infarct and deficits should control for lesion volume.
1. Introduction
The insula, also known as the insular cortex, is the small part of the brain that lies deep within the lateral sulcus. It is a triangular region, covered by the fronto-parietal and temporal opercula (Varnavas and Grand, 1999). The insula has bidirectional connections with most of the brain, including the frontal, temporal, parietal, cingulate, subcortical structures (basal ganglia and thalamus), and limbic structures (amygdala, periamygdaloid areas, and entorhinal cortex) (Augustine, 1996). The arterial supply of the insula is complex: arteries supplying the insula arise from the middle cerebral artery (MCA), with predominance from the M2 segment and a small contribution from the insular branches of the M1 segment (Türe et al., 2000). Because it receives blood supply from both M1 and M2, complete MCA occlusion may be necessary to produce a substantial insular infarct.
The insular cortex has been implicated in viscerosensory, visceromotor, and interoceptive functions (Augustine, 1985), and also in complex processes such as emotions, music, and language (Shura et al., 2014). It has been reported that stroke-induced insular damage is associated with a variety of deficits, including disrupted autonomic nervous system (Augustine, 1985, De Raedt et al., 2015, Inamasu et al., 2013), emotion (Calder, 1996, Ibanez et al., 2010), pain functions (Cattaneo et al., 2007, Cereda et al., 2002), apraxia of speech (Dronkers, 1996), other aspects of speech production such as respiration (Ackermann and Riecker, 2010), auditory processing (Bamiou et al., 2003), gustatory functions (Flynn, 1999, Small, 2010), and somatosensory function (Stephani et al., 2011). Insular stroke has been associated with cardiac arrhythmias and cardiac death after stroke (Oppenheimer, 2006, Seifert et al., 2015, Tokgozoglu et al., 1999) as well as hospital acquired pneumonia (Kemmling et al., 2013). It is possible that the frequent association between insular damage and at least some of the post-stroke sequelae that have been attributed to the insula can be explained by the fact that patients with strokes involving the insula have larger volume strokes (and are therefore less likely to recover from any sequelae), compared to patients with stroke with no insular involvement (Hillis et al., 2004). Previous studies have shown that the insula is frequently involved in any large MCA territory infarct (Nakano et al., 2001, Truwit et al., 1990). However, it has not been shown that strokes involving the insula are larger than other hemispheric strokes. The goal of this study is to test the hypotheses that: (1) ischemic strokes including the insula are larger in volume than other hemispheric strokes; and (2) insular infarct is a marker of MCA occlusion. We tested these hypotheses in a series of 861 patients with acute ischemic hemispheric stroke, unselected for clinical deficits. This study was a retrospective analysis of prospectively collected data.
2. Methods
2.1. Participants
Patients were identified from a prospective database of patients who presented with acute ischemic hemispheric stroke to the Johns Hopkins Medical Institutions stroke service (East Baltimore campus or Johns Hopkins Bayview Medical Center) and consented for a study of language or cognitive deficits associated with areas of acute ischemia. All participants gave informed consent (if they demonstrated intact comprehension), or their closest relative or legal representative consented (if the patient had impaired comprehension) to the study according to according to the Declaration of Helsinki. The study was approved by the Johns Hopkins Medicine Institutional Review Board
Inclusion criteria were: (1) symptoms of acute ischemic hemispheric stroke; (2) age 18 or older; (3) able to complete testing within 48 h from onset of symptoms. Exclusion criteria included: (1) contraindication for MRI; (2) premorbid dementia or other neurological disease; (3) impaired level of consciousness or need for ongoing sedation; (3) hemorrhage; and (4) stroke limited to the brainstem or cerebellum. For this study we also excluded patients who were found to have: (1) chronic stroke; (2) no lesion on diffusion-weighted image (DWI); (3) other (non-stroke) lesions on MRI, such as tumor; and (4) poor quality DWI, preventing reliable measurement of lesion volume. From the database we identified 861 patients with acute strokes unselected for functional deficits. The mean age of participants was 61.1 years (SD of 15.5 years).
2.2. Imaging
MRI scans were obtained within 48 h of symptom onset. Participants underwent the following imaging: T2, fluid attenuation inversion recovery (FLAIR; to evaluate for old lesions), susceptibility weighted images (to evaluate for hemorrhage), PWI (to evaluate for areas of hypoperfusion), and DWI (to evaluate for acute ischemia). DWI scans were 5 mm in thickness and provided whole-brain coverage. MR/CT angiography (to evaluate for stenosis or occlusion) was obtained for 620 patients. Acute DWI scans were evaluated by a technician blinded to clinical data (including MRA/CTA) for presence or absence of involvement of the insula. A neurologist, also blinded to clinical data evaluated a subset of 50 scans for inter-rater reliability. For those 50 scans there was 96% point-to-point percent agreement on involvement of the insula. Stroke laterality was determined based on the DWI image and was categorized as left hemisphere, right hemisphere or bilateral. Volumes of tissue dysfunction on DWI were calculated using Image J (http://imagej.nih.gov/ij/;(Schneider et al., 2012).
2.3. Data analysis
Volume of infarct and demographics were compared for lesions involving the insula and those not involving the insula, using unpaired t-tests (STATA, version 16). The association between lesions involving the insula and (1) MCA occlusion and (2) MCA stenosis or occlusion (and other dichotomous variables) were evaluated by chi-square tests.
3. Results
3.1. Analysis of all strokes
A total of 861 patients were included in the study. Mean age of the study group was 61.1(± SD 15.5); 49.6% were men (see Table 1 for additional demographics). Analysis of the DWI images revealed that 414 (48.1%) had acute right hemisphere infarcts, 417 (48.4%) had acute left hemisphere infarcts and 30 (3.5%) patients had bilateral acute infarcts. Among the 861 patients, 232 (27%) had insular involvement and 629 (73%) had no involvement of the insula. Mean infarct volume was larger for infarcts including the insula versus excluding the insula: 65.8 ± 78.8 cm3 versus 10.2 ± 15.9 cm3; t = − 16.8; p < 0.0001. Moreover, among infarcts in the 90th percentile for volume, 87% included the insula (χ2 = 181.8; p < 0.0001). Not surprisingly, because larger strokes are associated with more severe neurological deficits, patients with insular involvement had higher National Institutes of Health Stroke Scale (NIHSS) scores than those without insular involvement (7.1 ± 4.3 versus 2.8 ± 2.2; t = − 14.9; p < 0.0001).
Table 1.
Demographic information (mean age 61.1(± SD 15.5)).
| Sex |
Race |
Hemisphere involved |
||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | African Americans | Caucasians | Other | Left | Right | Bilateral | |
| All infarcts | 49.6% | 50.4% | 49.2% | 47.9% | 2.9% | 48.4% | 48.1% | 3.5% |
| Lacunar infarcts | 56.5% | 36.5% | 55.3% | 35.3% | 2.3% | 48.2% | 51.8% | 0 |
3.2. Subanalysis excluding lacunar infarcts
We repeated analyses after excluding lacunar infarcts, defined as, “round or ovoid lesion of increased signal relative to white or deep gray matter on DWI and hypointense on the apparent diffusion coefficient map, in the cerebral hemispheric white matter or basal ganglia or in the brain stem with greatest diameter < 20 mm” (from Potter et al., 2011, but without T2, FLAIR, or CT criteria). There were 85 patients with lacunar infarcts, who did not differ significantly from the total group with respect to age (mean age 61.4 ± 15.9) or other demographics, although there was a trend for lacunar infarcts to be more common among men (χ2 = 3.2; ns) and African Americans (χ2 = 3.9; ns; see Table 1). Of the 775 patients with non-lacunar stroke, 227 (29.3%) had insular involvement and 548 (70.7%) had no insular involvement. Mean infarct volume was larger for non-lacunar infarcts including the insula versus excluding the insula: 67.0 cm3 ± 79.2 versus 11.5 cm3 ± 16.7; t = − 15.8; p < 0.00001. Still, among non-lacunar infarcts in the 90th percentile for volume, 87% included the insula (χ2 = 137.5; p < 0.0001).
3.3. Subanalysis of patients with vessel imaging
Data from the subset of 620 participants with magnetic resonance angiogram (MRA) or computed tomography angiogram (CTA) revealed that 62 (10.0%) patients had MCA occlusion and 63 (10.2%) had MCA stenosis. Among the 62 patients with occlusion of the MCA, 49 (79%) had insular infarcts and 13 (21%) had infarcts not involving the insula (χ2 = 93.1, df1, p < 0.0001; Table 2). Among the 63 patients with MCA stenosis, 29 (46.0%) had infarcts involving the insula and 34 (54.0%) had infarcts not involving the insula. Insular cortex involvement was also strongly associated with the presence of either MCA occlusion or stenosis (χ2 = 114.7, df2, p < 0.0001) (Table 3, Fig. 1).
Table 2.
MCA occlusion and insular infarct (χ2 = 93.1, df1, p < 0.0001).
| MCA patent | MCA occluded | Difference in volume between occluded vs non-occluded MCA |
Total | |
|---|---|---|---|---|
| No insular infarct | N = 438 9.1 ± 14.6 cm3 | N = 13 9.8 ± 13.1 cm3 | ns | 451 9.1 ± 14.5 cm3 |
| Insular infarct | N = 120 47.5 ± 54.8 cm3 | N = 49 110.6 ± 111.2 cm3 | t = − 4.93 p < 0.00001 | 169 65.8 ± 80.5 cm3 |
| Difference in volume for insular vs no insular infarct | t = − 8.71 p < 0.00001 | t = − 3.24 p = 0.0019 | ||
| 558 | 62 | 620 |
Table 3.
MCA occlusion/stenosis and insular infarct (χ2 = 114.7, df2, p < 0.0001).
| MCA patent | MCA occluded | MCA stenosed | Total | |
|---|---|---|---|---|
| No insular infarct | 404 | 13 | 34 | 451 |
| Insular infarct | 91 | 49 | 29 | 169 |
| 495 | 62 | 63 | 620 |
Fig. 1.
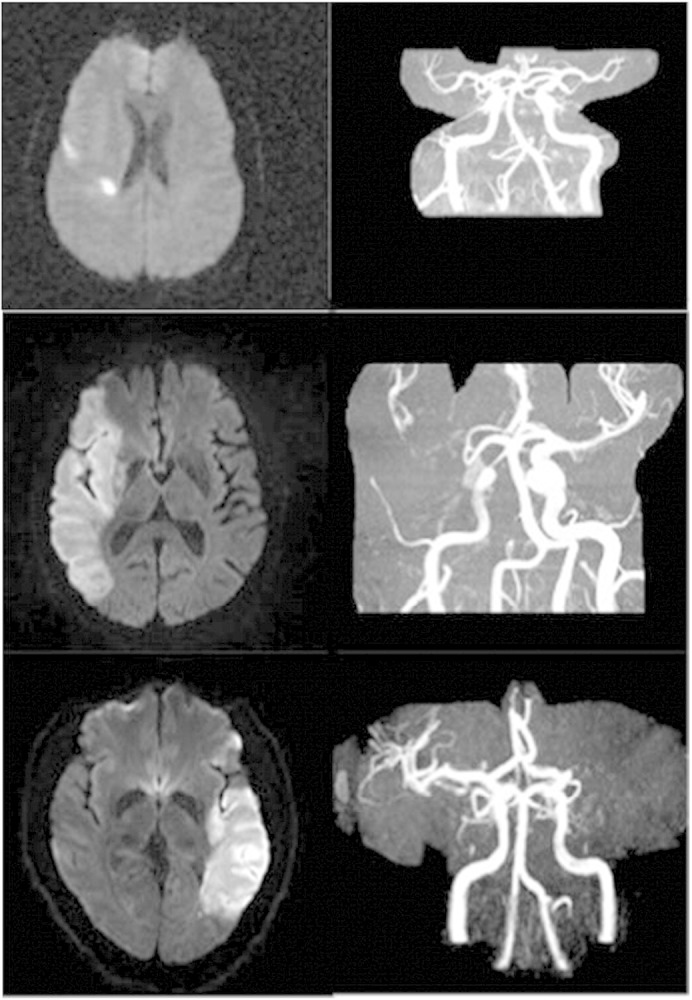
Top panel. Infarct not involving the insula with patent MCA. Lower panel. Strokes involving the insula with MCA occlusion.
Interestingly, the volume of infarcts was larger for patients with MCA occlusion compared to those without MCA occlusion, but only in patients with insular infarcts (Table 3). This finding should be considered preliminarily because there were only a small number of patients without insular infarcts who had MCA occlusion. But there was no trend for their infarcts to be larger than those of other patients without insular involvement. We can speculate that cases of MCA occlusion without acute insular stroke are those cases in which the occlusion has been gradual, and thus does not cause a large stroke.
We also evaluated whether or not there was any difference in the relationship between volume of infarct and MCA occlusion in left versus right hemisphere stroke, because earlier research has demonstrated a higher frequency of left MCA occlusion relative to right MCA occlusion (Hedna et al., 2013). Among left hemisphere strokes, 26/308 (8.4%) had MCA occlusion; not surprisingly, those with MCA occlusion had larger infarcts than those without (86.8 ± 107.2 vs 15.8 ± 24.4 cm3; t = − 6.6; p < 0.00001). Among right hemisphere strokes, 32/286 (11.1%) had MCA occlusion; those with MCA occlusion had larger infarcts than those without (78.4 ± 85.4 vs 19.6 ± 40.2 cm3; t = − 6.6; p < 0.00001). Even in those with bilateral strokes, the 4/26 with MCA occlusion on one side had larger strokes than those without occlusion (195.1 ± 2.2 vs. 11.3 ± 20.2; t = − 4.3; p = 0.0002). There was not a significant difference between the frequency of insular involvement between left and right hemisphere stroke (36.0 vs. 37.6%). The association between MCA occlusion and insular involvement was strong for both left hemisphere stroke (χ2 = 24.1; p < 0.0001) and right hemisphere stroke (χ2 = 68.5; p < 0.0001).
4. Discussion
In a relatively large cohort of patients with acute ischemic hemispheric stroke, we demonstrate that the mean volume of stroke with insular involvement is significantly larger than the mean volume of strokes without insular involvement. Moreover, the largest strokes, and those due to MCA occlusion, usually involved the insula. The large lesion volume in strokes affecting the insula could explain poor clinical outcomes and persistent deficits compared to strokes sparing the insula. Previous studies that indicate acute infarction measuring > 70 cm3 predicts poor outcome in acute stroke setting (Yoo et al., 2009, Yoo et al., 2010).
These data illustrate a pitfall in lesion-overlap studies of patients with post-stroke deficits; the area of greatest overlap in any deficit due to MCA stroke is likely to be in the insula. Simply overlapping lesions to determine if the same structure is involved in all the strokes in patients with a particular deficit is not a valid method of determining structure–function relationships (Rorden and Karnath, 2004). Furthermore, these data also call for caution in interpreting other lesion-deficit association studies of acute or chronic stroke (e.g. using voxel-symptom lesion mapping), because there will nearly always be greatest power to detect an association between the deficit and the lesion in the insula. For example, one study found that hospital acquired pneumonia (HAP) was associated with damage to the insula (Kemmling et al., 2013); this result may reflect the strong association between damage to the insula (large MCA volume strokes) and persistent deficits in mobility and other known risk factors for HAP. Patients with and without HAP in that study were matched for NIHSS scores, but there was a significantly greater percentage of left hemisphere strokes among those without HAP; the equivalent NIHSS score is associated with smaller volume of lesion in left hemisphere compared to right hemisphere stroke (Fink et al., 2002, Gottesman et al., 2009, Menezes et al., 2007, Woo et al., 1999). This bias can account for the fact that only the right insula (included in the largest strokes) was associated with HAP. Insular infarct has also been found to be associated with a wide range of chronic motor, sensory and cognitive sequelae (Ibanez et al., 2010). But in studies that show the overlap of lesions of all participants, the insula is often the area with most lesions (e.g. (Hope et al., 2013, Wu et al., 2015), and thus the greatest power to detect the association. Therefore, it is essential to control for volume of infarct as well as demographic factors that may influence the outcome (e.g. (Hope et al., 2013, Wu et al., 2015).
Isolated infarcts of the insula generally produce minor and transient deficits in somatosensory, gustatory, vestibular, or cardiovascular functions (Boucher et al., 2015, Cereda et al., 2002, Frontzek et al., 2014, von Brevern et al., 2014). Transient neuropsychological disorders, including aphasia (left posterior insula), dysarthria, and somatoparaphrenia have also been reported in acute insular stroke (Cereda et al., 2002). However, some reported deficits observed in association with acute insular infarct may be due to hypoperfusion of the MCA territory, as illustrated in Fig. 2. Further investigation of deficits associated with isolated insular lesions (without cortical hypoperfusion or damage to surrounding areas) is needed to determine if the insula itself is essential for many of the functions that have been found to be impaired when it is damaged. Outcome in strokes restricted to the insula appears to be uniformly good, given the transient nature of the reported deficits; but long term outcome studies of patients with such lesions (particularly with detailed evaluation of sensory, emotional, vestibulatory, and gustatory functions) are lacking. Given that the insula is highly connected to many vestibular, somatosensory, viscerosensory, gustatory areas, as well as the limbic system and other networks critical for complex social, emotional, and cognitive functions (involving amygdala, temporopolar, parahippocampal, orbitofrontal, and anterior cingulate cortices) (see (Shura et al., 2014) for review) it may indeed serve as a critical node for a variety of disparate functions, including at least some those in which it has been implicated in previous studies.
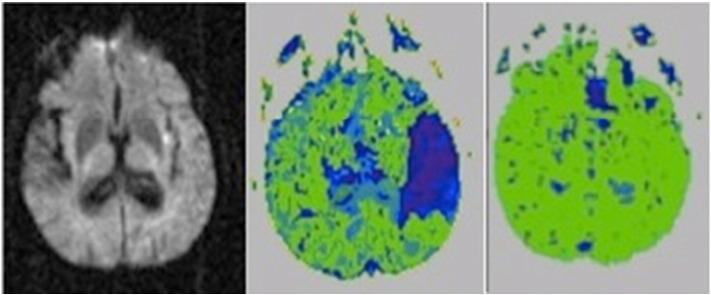
Fig. 2.
Pre-treatment DWI at Day 1 (left panel), PWI (middle panel) of a patient with acute Wernicke's aphasia associated with acute infarct in the insula and hypoperfusion of the entire left temporal cortex. His aphasia resolved when the left temporal cortex was reperfused with intervention (right panel).
We did not replicate the interesting finding of a previous study (Hedna et al., 2013) that left MCA occlusion is more common than right MCA occlusion, and associated with more severe stroke. The lack of a hemispheric difference in our study might be due to an ascertainment bias in our sample. We included only patients who gave consent for participation in a study of stroke recovery or whose identified decision-maker provided informed consent. Patients who are severely aphasic (often due to left MCA occlusion) cannot provide informed consent; and it is often difficult to obtain timely consent from a legal decision-maker. Therefore, it is possible that we enrolled disproportionately fewer patients with left MCA occlusion. Nevertheless, there was a very strong association between insular damage and MCA occlusion in both left and right hemisphere stroke.
In summary, some deficits that have been ascribed to insular lesions might be better explained as a consequence of large lesions or hypoperfusion due to MCA occlusion or stenosis (especially in the acute stage). That is not to say that the insula is not critical for some of the functions for which it has been implicated. Indeed, there is independent evidence that it plays a role in corticolimbic networks underlying social, emotional, and autonomic functions. Functional neuroimaging studies show activation of the insula associated with many tasks (e.g. (Brown et al., 2011, Gu et al., 2012, Jabbi et al., 2007, Lamm et al., 2011, Moser et al., 2009, Phillips et al., 1997); but functional imaging can only show that a region is engaged in a task, not that it is essential for the task (Fellows et al., 2005, Müller and Knight, 2006). Neuroimaging studies also show that the insula is functionally connected, as well as structurally connected, with many regions (Cerliani et al., 2012, Cloutman et al., 2012, Dennis et al., 2014). Lesions to the insula, or to its connections, could plausibly disrupt a variety of functions. Lesion-deficit studies complement functional imaging studies, but need to adequately control for volume of lesion and consider the potential influence of hypoperfusion beyond the infarct.
Previous studies have shown a frequent involvement of the insula in MCA territory infarction (Fink et al., 2005, Nakano et al., 2001, Truwit et al., 1990), but ours may show the clearest association with infarct volume. Fink et al. (2005) reported that insular infarct was associated with lenticulostriate territory infarction, infarct of more than one-third of MCA territory infarction; higher NIHSS score, and proximal vascular occlusion. They also showed that anterior insula infarct was associated with infarct in the superior division MCA territory, while posterior insula infarct was associated with infarct in the inferior division. Our results confirm that involvement of the insula on early DWI is a marker of probable MCA occlusion. Furthermore, in our study, only patients with insular stroke showed larger strokes in association with MCA occlusion. These findings have important clinical significance because the presence of insular involvement on early DWI could signal the need for urgent vessel imaging to consider rapid intervention such as thrombolysis or embolectomy to prevent progression to large volume stroke.
Acknowledgments
The research reported in this paper was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders and National Institute of Neurological Disorders and Stroke) through awards R01 NS047691, R01 DC05375, R01 DC03681, and P41 EB015909. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
References
- Ackermann H., Riecker A. The contribution (s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct. Funct. 2010;214(5–6):419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Augustine J.R. The insular lobe in primates including humans. Neurol. Res. 1985;7(1):2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Augustine J.R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bamiou D., Musiek F.E., Luxon L.M. The insula (Island of Reil) and its role in auditory processing: literature review. Brain Res. Rev. 2003;42(2):143–154. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Boucher O., Turgeon C., Champoux S., Ménard L., Rouleau I., Lassonde M.…Nguyen D.K. Hyperacusis following unilateral damage to the insular cortex: a three-case report. Brain Res. 2015;1606:102–112. doi: 10.1016/j.brainres.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Brown S., Gao X., Tisdelle L., Eickhoff S.B., Liotti M. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. NeuroImage. 2011;58(1):250–258. doi: 10.1016/j.neuroimage.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder A.J. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn. Neuropsychol. 1996;13(5):699–745. [Google Scholar]
- Cattaneo L., Chierici E., Cucurachi L., Cobelli R., Pavesi G. Posterior insular stroke causing selective loss of contralateral nonpainful thermal sensation. Neurology. 2007;68(3):237. doi: 10.1212/01.wnl.0000251310.71452.83. (doi:68/3/237 [pii]) [DOI] [PubMed] [Google Scholar]
- Cereda C., Ghika J., Maeder P., Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59(12):1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- Cerliani L., Thomas R.M., Jbabdi S., Siero J.C., Nanetti L., Crippa A.…Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum. Brain Mapp. 2012;33(9):2005–2034. doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman L.L., Binney R.J., Drakesmith M., Parker G.J., Ralph M.A.L. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. NeuroImage. 2012;59(4):3514–3521. doi: 10.1016/j.neuroimage.2011.11.016. [DOI] [PubMed] [Google Scholar]
- De Raedt S., De Vos A., De Keyser J. Autonomic dysfunction in acute ischemic stroke: an underexplored therapeutic area? J. Neurol. Sci. 2015;348(1):24–34. doi: 10.1016/j.jns.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Dennis E.L., Jahanshad N., McMahon K.L., Zubicaray G.I., Martin N.G., Hickie I.B.…Thompson P.M. Development of insula connectivity between ages 12 and 30 revealed by high angular resolution diffusion imaging. Hum. Brain Mapp. 2014;35(4):1790–1800. doi: 10.1002/hbm.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384:14. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Heberlein A.S., Morales D.A., Shivde G., Waller S., Wu D.H. Method matters: an empirical study of impact in cognitive neuroscience. J. Cogn. Neurosci. 2005;17(6):850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Fink J.N., Selim M.H., Kumar S., Silver B., Linfante I., Caplan L.R., Schlaug G. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke; J. Cerebral Circ. 2002;33(4):954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- Fink J.N., Selim M.H., Kumar S., Voetsch B., Fong W.C., Caplan L.R. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch. Neurol. 2005;62(7):1081–1085. doi: 10.1001/archneur.62.7.1081. [DOI] [PubMed] [Google Scholar]
- Flynn F.G. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13(1):55–78. [Google Scholar]
- Frontzek K., Fluri F., Siemerkus J., Müller B., Gass A., Christ-Crain M., Katan M. Isolated insular strokes and plasma MR-proANP levels are associated with newly diagnosed atrial fibrillation: a pilot study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman R.F., Kleinman J.T., Davis C., Heidler-Gary J., Newhart M., Hillis A.E. The NIHSS-plus: improving cognitive assessment with the NIHSS. Behav. Neurol. 2009;22(1):11–15. doi: 10.3233/BEN-2009-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Gao Z., Wang X., Liu X., Knight R.T., Hof P.R., Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(Pt 9):2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedna V.S., Bodhit A.N., Ansari S., Falchook A.D., Stead L., Heilman K.M., Waters M.F. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J. Clin. Neurol. 2013;9(2):97–102. doi: 10.3988/jcn.2013.9.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hope T.M., Seghier M.L., Leff A.P., Price C.J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clin. 2013;2:424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A., Gleichgerrcht E., Manes F. Clinical effects of insular damage in humans. Brain Struct. Funct. 2010;214(5–6):397–410. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- Inamasu J., Sugimoto K., Watanabe E., Kato Y., Hirose Y. Effect of insular injury on autonomic functions in patients with ruptured middle cerebral artery aneurysms. Stroke; J. Cerebral Circ. 2013;44(12):3550–3552. doi: 10.1161/STROKEAHA.113.003099. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Swart M., Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Kemmling A., Lev M.H., Payabvash S., Betensky R.A., Qian J., Masrur S., Schwamm L.H. Hospital acquired pneumonia is linked to right hemispheric peri-insular stroke. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Menezes N.M., Ay H., Wang Zhu M., Lopez C.J., Singhal A.B., Karonen J.O.…Sorensen A.G. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke; J. Cerebral Circ. 2007;38(1):194–197. doi: 10.1161/01.STR.0000251792.76080.45. doi:01.STR.0000251792.76080.45 [pii] [DOI] [PubMed] [Google Scholar]
- Moser D., Fridriksson J., Bonilha L., Healy E.W., Baylis G., Baker J.M., Rorden C. Neural recruitment for the production of native and novel speech sounds. NeuroImage. 2009;46(2):549–557. doi: 10.1016/j.neuroimage.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N., Knight R. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nakano S., Iseda T., Kawano H., Yoneyama T., Ikeda T., Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories with angiographically confirmed site of arterial occlusion. AJNR Am. J. Neuroradiol. 2001;22(4):654–659. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance. Clin. Auton. Res.: Off. J. Clin. Auton. Res. Soc. 2006;16(1):6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Senior C., Brammer M., Andrew C., Calder A.J.…Williams S. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Potter G.M., Marlborough F.J., Wardlaw J.M. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke. 2011;42(2):359–366. doi: 10.1161/STROKEAHA.110.594754. [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci. 2004;5(10):812–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert F., Kallmünzer B., Gutjahr I., Breuer L., Winder K., Kaschka I.…Schwab S. Neuroanatomical correlates of severe cardiac arrhythmias in acute ischemic stroke. J. Neurol. 2015;1-9 doi: 10.1007/s00415-015-7684-9. [DOI] [PubMed] [Google Scholar]
- Shura R.D., Hurley R.A., Taber K.H. Insular cortex: structural and functional neuroanatomy. J. Neuropsychiatry Clin. Neurosci. 2014 doi: 10.1176/appi.neuropsych.260401. [DOI] [PubMed] [Google Scholar]
- Small D.M. Taste representation in the human insula. Brain Struct. Funct. 2010;214(5–6):551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Stephani C., Vaca G.F., Maciunas R., Koubeissi M., Lüders H. Functional neuroanatomy of the insular lobe. Brain Struct. Funct. 2011;216(2):137–149. doi: 10.1007/s00429-010-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokgozoglu S.L., Batur M.K., Top uoglu M.A., Saribas O., Kes S., Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke; J. Cerebral Circ. 1999;30(7):1307–1311. doi: 10.1161/01.str.30.7.1307. [DOI] [PubMed] [Google Scholar]
- Truwit C.L., Barkovich A.J., Gean-Marton A., Hibri N., Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. 1990;176(3):801–806. doi: 10.1148/radiology.176.3.2389039. [DOI] [PubMed] [Google Scholar]
- Türe U., Yasargil M.G., Al-Mefty O., Yasargil D.C. Arteries of the insula. J. Neurosurg. 2000;92(4):676–687. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- Varnavas G.G., Grand W. The insular cortex: morphological and vascular anatomic characteristics. Neurosurgery. 1999;44(1):127–136. doi: 10.1097/00006123-199901000-00079. [DOI] [PubMed] [Google Scholar]
- von Brevern M., Süßmilch S., Zeise D. Acute vertigo due to hemispheric stroke: a case report and comprehensive review of the literature. J. Neurol. Sci. 2014;339(1):153–156. doi: 10.1016/j.jns.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Woo D., Broderick J.P., Kothari R.U., Lu M., Brott T., Lyden P.D.…Grotta J.C. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30(11):2355–2359. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- Wu O., Cloonan L., Mocking S.J., Bouts M.J., Copen W.A., Cougo-Pinto P.T.…Rost N.S. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke; J. Cerebral Circ. 2015 doi: 10.1161/STROKEAHA.115.009643. doi:STROKEAHA.115.009643 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A.J., Verduzco L.A., Schaefer P.W., Hirsch J.A., Rabinov J.D., Gonzalez R.G. MRI-based selection for intra-arterial stroke therapy: Value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke; J. Cerebral Circ. 2009;40(6):2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A.J., Barak E.R., Copen W.A., Kamalian S., Gharai L.R., Pervez M.A.…Schaefer P.W. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with national institutes of health stroke scale score improves the prediction of acute stroke outcome. Stroke; J. Cerebral Circ. 2010;41(8):1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]