Abstract
Aim:
In this paper, we aim to present our experience with a series of patients with PMSAH. In addition, the clinical course of perimesencephalic subarachnoid hemorrgade (PMSAH) is discussed with an evaluation of etiologies, risk factors, and the necessity for a second angiogram on follow-up.
Materials and Methods:
The data for this study were obtained retrospectively from patients who were treated at the Uludag University, School of Medicine, Department of Neurosurgery, Division of Neurovascular Surgery's clinic with a diagnosis of PMSAH between January 1980 and March 2002.
Results:
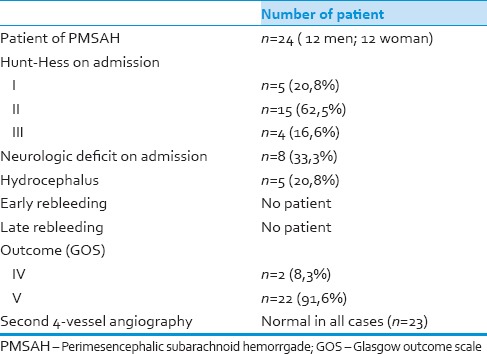
We identified a total of 24 patients, 12 male. The mean age at the time of hemorrhage was 53 ± 12 years. In all patients, the onset was typical with a sudden severe headache. Five of the patients were Hunt-Hess Grade I, 15 were Grade II, and 4 were Grade III. The initial 4-vessel angiography was normal in 23 cases. Twenty-two had a second 4-vessel angiography, and all were normal. We observed acute hydrocephalus in 5 patients (20.8%). We did not observe re-bleeding during the follow-up of our patients.
Conclusion:
Patients with PMSAH have a particularly excellent outcome, and there is no need to evaluate these patients with repeat angiography.
Keywords: Perimesencephalic hemorrhage, repeat cerebral angiography, subarachnoid hemorrhage
Introduction
In approximately, 15% of patients that had spontaneous subarachnoid hemorrhage (SAH), the cause of hemorrhage could not be detected despite detailed imaging studies.[1,2,3] It has been repeatedly shown that SAH of unknown origin is associated with a much better outcome than aneurysmal SAH.[2,3] However, a small number of patients suffer from re-bleeding and long-term disability because of delayed cerebral ischemia. Recently, van Gijn et al.[4] described a benign variant of SAH, the so-called perimesencephalic subarachnoid hemorrgade (PMSAH). The characteristic computed tomography (CT) pattern of hemorrhage is restricted to the perimesencephalic or prepontine cisterns in combination with a normal 4-vessel cerebral angiogram and has an excellent prognosis.[2,3,4,5] Despite the number of patients with PMSAH, many uncertainties remain regarding this distinct type of SAH, especially those relating to their etiology and evaluation.[5,6] The causes of PMSAH suggest a venous or capillary rupture at the level of the tentorial hiatus.[1,4,7] In this study, we describe our experience with a series of patients with PMSAH and our retrospective evaluation of etiologies, risk factors, and the need for a second angiogram on follow-up.
Materials and Methods
A retrospective review of the Uludag University, School of Medicine, Department of Neurosurgery, Division of Neurovascular Surgery's clinical records for all SAH patients admitted between 1980 and 2002 was performed. We found 602 relevant cases. In each, SAH was confirmed by a lumbar puncture and/or CT scan. Included in the series were patients with symptoms and signs suggesting SAH, and who received a CT within 24 h of the first clinical symptoms. SAHs associated with head trauma, blood dyscrasias, arteriovenous malformations, classical intracerebral hemorrhages, or brain tumors were not included in this study. During the study period, the policy in the department was to submit patients to repeat 4-vessel angiography after 2–4 weeks if this might aid in diagnosis. If an aneurysm was then verified, the patient was a candidate for surgery. The PMSAH CT patterns of SAH were defined according to the criteria described by Rinkel et al.:[8]
Center of the hemorrhage immediately anterior to the midbrain, with or without extension of blood to the anterior part of the ambient cistern or the basal part of the sylvian fissure
Incomplete filling of the anterior interhemispheric fissure and no extension to the lateral sylvian fissure, except for minute amounts of blood and
The absence of frank intraventricular hemorrhage.
Ventricular size was assessed on the basis of the CT score as first described by Vassilou this and Richardson. Hydrocephalus was diagnosed only when it was clinically significant and required ventriculoperitoneal shunting. The clinical condition on admission was graded according to the Hunt and Hess system, and any changes were noted. Angiographic vasospasm was evaluated in detail on the angiogram and graded as follows: Grade I - no angiographic vasospasm; Grade II - moderate angiographic vasospasm; and Grade III - severe angiographic vasospasm. All patients were treated symptomatically.
Results
Using a consecutive series of 602 patients with SAH and with a normal 4-vessel angiography, 24 fulfilled the criteria for PMSAH [Figure 1]. Twelve were male. The mean age at the time of hemorrhage was 53 ± 12 years. In 24 patients, the onset was typical with sudden severe headache with or without meningeal irritation. Five of these had loss of consciousness initially, one had double vision, one had dizziness, and one had facial paralysis. Five were in Hunt-Hess Grade I, 15 Grade II, and 4 Grade III [Figure 2]. We found four hypertensive patients, one with cardiac failure, and one with mitral valve replacement among our patients’ medical histories.
Figure 1.
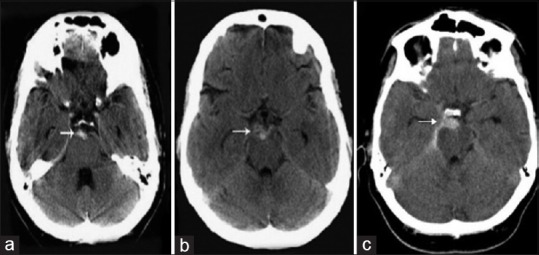
Axial computed tomographic scan obtained on admission (a and b) (white arrow) reveals a subarachnoid hemorrhage limited to the interpeduncular cistern (c) reveals a subarachnoid hemorrhage in the interpeduncular cistern and right ambient cistern (b and c) the tentorium may be more dense than normal
Figure 2.
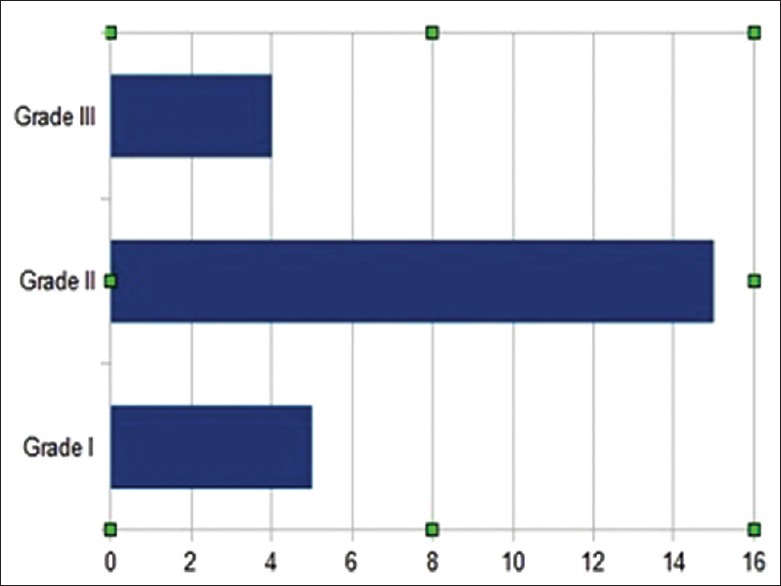
Chart showing number of patient according to Hunt and Hess system
Initial 4-vessel angiography was normal in 23 cases showing vasospasm in three. One patient had a fusiform dilatation of the basilar artery, which was treated using an endovascular approach. Twenty-two had a second 4-vessel angiography, and all were normal. There were 22 patients in Glasgow Outcome Scale (GOS) V and 2 in GOS IV. We observed acute hydrocephalus in 5 patients (20.8%). Only one subsequently deteriorated and required ventriculoperitoneal shunt placement. The level of consciousness did not change in any of the other 4 patients. Control CT scans during the 1st week after admission showed normalization of the size of the ventricles in these patients. We did not observe re-bleeding during the follow-up period [Table 1].
Table 1.
Characteristics of our patients
Discussion
Nonaneurysmal perimesencephalic hemorrhage has become well recognized as a distinct type of SAH and may account for up to two-thirds of all SAHs unknown cause.[3,5,6] A number of studies have previously reported that the outcome of patients with SAH, who had negative angiogram was better than that for patients with aneurysmal SAH. In 1985, van Gijn et al.[9,10] reported that among all patients with such an angiogram negative SAH, there is typically a propensity for blood to layer around the brain stem with minimal extension into the fissures and no intraventricular entry forming the so-called perimesencephalic nonaneurysmal SAH and recognized as a benign type H.[2,3,4,6]
Patients with PMSAH tend to be younger and less hypertensive as compared to those with aneurysmal SAH.[1,2] In our study, the mean age was 53 ± 12 years while it was 50.1 years in the literature.[1] Many of these studies reported that those who had negative angiograms were primarily men. We could not find disturbances related to sex in our series. Hypertension has been reported in 3–20% of the patients. We found 16.6%, which is consistent with the literature.[2]
The clinical presentation of patients with PMSAH is similar to those of patients with aneurysmal SAH: Sudden onset of headache, meningeal irritation, photophobia, nausea, and vomiting has been reported. The patients were commonly classified Hunt-Hess Grade I or II at the initial neurological examination, which contrasts with 70.8% of patients with nonaneurysmal. PMSAH reported that 93% of their patients were classified Hunt-Hess Grade I or II; 2 cases (6.8%) were classified Grade III at the initial neurological examination.[2] In our series, we found 20 patients (83.3%) were Grade I or II, and 4 (16.7%) were Grade III. However, Schwartz et al.[1] found no patients classified as Hunt-Hess Grade III at initial examination among their study group. There was a loss of consciousness in five patients (20.8%) with PMSAH, 1 with 7th nerve palsy, one with dizziness, and one with diplopia. The previous studies of angiogram negative SAHs have reported a rebleeding rate of 2–5%, depending on the study design, quality of angiogram, and length of follow-up. Schwartz, et al. reported that none of their patients with PMSAH had rebleeding after follow-ups ranging from 8 to 51 months. None of our patients had subsequent bleeding after a perimesencephalic pattern of SAH was found. One patient with PMSAH had aphasia and right hemiparesis at the time of the first angiogram as a direct complication of angiography.
A 0–15% incidence of hydrocephalus requiring surgical intervention has been reported for patients with angiogram negative SAH, but only 1% with PMSAH required shunt placement for acute hydrocephalus.[1] In our series, we determined acute hydrocephalus in 5 patients (20.8%) but only one subsequently deteriorated and required ventriculoperitoneal shunt placement. Recently Hop et al., studied 27 patients with PMSAH in which the occurrence of transient amnesia was associated with enlargement of the temporal horns, which could possibly be explained by temporary hippocampal dysfunction.[11] Clinical vasospasm is seen in 0–31% of patients with angiogram negative SAH.[1] By contrast, 3 patients were reported with clinical vasospasm after PMSAH, resulting in an observed incidence of 1–5%. In these 3 patients, the symptoms of cerebral ischemia and vasospasm on angiography developed immediately after angiography. In our study, we detected clinical vasospasm in 1 patient and angiographic vasospasm in 2. One patient with PMSAH had a clinical vasospasm at the time of the initial angiography. On the other hand, one published article suggested that severe and diffuse vasospasm does not exclude a diagnosis of pretruncal nonaneurysmal SAH.[1] Angiographic vasospasm has been reported more frequently than clinical vasospasm, which is mild and focal. If angiography was performed within 2 weeks after hemorrhage angiographic vasospasm, then it might have occurred in as many as 42% of patients with PMSAH.[12]
The pathogenesis of the PMSAH is controversial. van Gijn et al. concluded the perimesencephalic many types of SAHs originate from a nonarterial source such as a venous or capillary leak. They suggested that a tear might result from external forces such as torsion or friction against the tentorial margin or from abrupt swelling because of sudden onset of raised intrathorasic pressure. Although they called attention to tearing in the basal vein of Rosenthal or one of its tributaries, careful evaluation of venograms revealed no consistent abnormalities. On the other hand, recently Watanabe et al. determined more abnormalities in venous structures, especially in the basal vein of Rosenthal, and decided to compare the venous phase of patients with PMSAH with those of patients with aneurysmal SAH.[13] Nevertheless, some investigators have proposed other sources of hemorrhage such as anterior longitudinal pontine or interpeduncular and posterior communicating veins, ventriculostriate or thalamoperforating arteries, and/or vascular abnormalities. Previously, the three surgical explorations were performed but supported arterial sources of bleeding. Subsequent angiograms reveal positive findings in 2–24% of patients with angiogram negative SAH, depending on the quality of the first angiogram.[1]
If the first angiogram was of unsatisfactory quality showed vasospasm or allowed any doubt about a small aneurysm hiding in any particular area, or the presence of factors that predispose negative findings such as edema or a localized hematoma, we recommend a second angiogram. Pinto et al. reported that the risk of missing a posterior circulation aneurysm in a patient with a perimesencephalic type of SAH was about 5–6%.
Conclusion
We conclude that patients with PMSAH have a particularly excellent outcome and that it is not necessary to evaluate these patients with repeated angiography. When newer magnetic resonance techniques become available, any missing lesions causing concern may become more easily identified, and the questions will become less important. We do not recommend antifibrinolytic therapy, calcium canal blockers, anticonvulsant, or bed rest because of rebleeding, an uncomplicated course in all studies with venous or capillary leaks is absent. However, the type of hemorrhage remains unclear.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Schwartz TH, Yoon SS, Cutruzzola FW, Goodman RR. Third ventriculostomy: Post-operative ventricular size and outcome. Minim Invasive Neurosurg. 1996;39:122–9. doi: 10.1055/s-2008-1052231. [DOI] [PubMed] [Google Scholar]
- 2.Ildan F, Tuna M, Erman T, Göçer AI, Cetinalp E. Prognosis and prognostic factors in nonaneurysmal perimesencephalic hemorrhage: A follow-up study in 29 patients. Surg Neurol. 2002;57:160–5. doi: 10.1016/s0090-3019(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Wijdicks EF, Piepgras DG, Nichols DA, Ebersold MJ. Perimesencephalic subarachnoid hemorrhage. Additional perspectives from four cases. Stroke. 1994;25:1507–11. doi: 10.1161/01.str.25.7.1507. [DOI] [PubMed] [Google Scholar]
- 4.van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: A nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–7. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 5.Van Calenbergh F, Plets C, Goffin J, Velghe L. Nonaneurysmal subarachnoid hemorrhage: Prevalence of perimesencephalic hemorrhage in a consecutive series. Surg Neurol. 1993;39:320–3. doi: 10.1016/0090-3019(93)90014-r. [DOI] [PubMed] [Google Scholar]
- 6.Rinkel GJ, Wijdicks EF, Hasan D, Kienstra GE, Franke CL, Hageman LM, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet. 1991;338:964–8. doi: 10.1016/0140-6736(91)91836-j. [DOI] [PubMed] [Google Scholar]
- 7.Rinkel GJ, Wijdicks EF, Vermeulen M, Hageman LM, Tans JT, van Gijn J. Outcome in perimesencephalic (nonaneurysmal) subarachnoid hemorrhage: A follow-up study in 37 patients. Neurology. 1990;40:1130–2. doi: 10.1212/wnl.40.7.1130. [DOI] [PubMed] [Google Scholar]
- 8.Rinkel GJ, Wijdicks EF, Vermeulen M, Ramos LM, Tanghe HL, Hasan D, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol. 1991;12:829–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Giombini S, Bruzzone MG, Pluchino F. Subarachnoid hemorrhage of unexplained cause. Neurosurgery. 1988;2:313–6. doi: 10.1227/00006123-198802000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Brismar J, Sundbärg G. J Neurosurg. 1985. Subarachnoid hemorrhage of unknown origin: Prognosis and prognostic factors; pp. 349–54. [DOI] [PubMed] [Google Scholar]
- 11.Hop JW, Brilstra EH, Rinkel GJ. Transient amnesia after perimesencephalic haemorrhage: The role of enlarged temporal horns. J Neurol Neurosurg Psychiatry. 1998;65:590–3. doi: 10.1136/jnnp.65.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatter SB, Crowell RM, Ogilvy CS. Aneurysmal and microaneurysmal “angiogram-negative” subarachnoid hemorrhage. Neurosurgery. 1995;37:48–55. doi: 10.1227/00006123-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe A, Hirano K, Kamada M, Imamura K, Ishii N, Sekihara Y, et al. Perimesencephalic nonaneurysmal subarachnoid haemorrhage and variations in the veins. Neuroradiology. 2002;44:319–25. doi: 10.1007/s00234-001-0741-3. [DOI] [PubMed] [Google Scholar]


