Abstract
Cavernous malformations (CMs) arising from the optic nerve and chiasm are extremely rare. In large autopsy series, CMs were estimated to range from 0.02 to 0.13% in the general population. However, with introduction of MRI, these lesions were found more often than previously thought, ranging from 0.2% to 0.4%. Only 29 cases have been reported according to our knowledge. Most patients present with drop in visual acuity and visual field. Although MRI findings of cavernous malformations have been reported, they may not be diagnostic enough. Among the 29 reported, 16 underwent total resection with good results. In some, resection was complicated by damage to the surrounding neural tissue. Surgical removal is the recommended treatment to restore or preserve vision and to eliminate the risk of future hemorrhage. However, the anatomical location and eloquence of nearby neural structures can make these lesions difficult to access and remove. CMs appear to occur in every age group (range 4 months to 84 years mean-34.6 years) ith an approximately equal male to female ratio. They typically present with chiasmal apoplexy, characterized by sudden visual loss, acute headaches, retro orbital pain, and nausea
Keywords: Cavernous malformation, chiasmal apoplexy, MRI, neuro-endoscopy, optic chiasma
Introduction
Cavernous malformations (CMs) arising from the optic nerve and chiasm are extremely rare. In large autopsy series, CMs were estimated to range from 0.02 to 0.13% in the general population.[1,2] However, with the advent of MRI, they were detected more often than previously thought with an incidence of 0.2% to 0.4%[2]. A cavernous malformation (CM), also known as cavernous angioma or cavernoma, is a developmental vascular malformation characterized by the presence of sinusoid-like capillary vessels with very sluggish circulation.[3] CMs are berry-like collection of vascular spaces lined by thin walls devoid of smooth muscle. These are congenital lesions that develop in third to eighth week of gestation though they may grow “de novo” occasionally.
CMs have been reported to involve cranial nerves in the following locations: The optic nerve and chiasm, third nerve, seventh nerve within the temporal bone, and seventh and eighth nerves in the internal auditory canal.[4,5,6] CMs CMs are known to occur in every age group (range 4 months to 84 years mean-34.6 years) with an approximately equal male to female ratio.[2,5] We have recently treated chiasmal CM with neuro-endoscopic surgery
Case Report
A 27-year-old gentleman, presented with 3 months history of blurring of vision associated with headache. There was no history of loss of consciousness, seizures, or weakness in limbs. Visual examination showed acuity of 6/6 bilaterally with left temporal field defect.
MRI of brain, T1W, T2 axial, contrast and multiplanar sequences were performed which showed evidence of lobulated mixed signal intensity lesion seen in the optic chiasm, measuring 18 × 11 mm. Multiple hemorrhagic foci of varying sizes were seen within the lesion. No obvious enhancement was seen after IV contrast material injection. On the right side, the lesion was extending anteriorly up to the optic canal and superiorly abutting the hypothalamus. The features were suggestive of CM of the optic chiasm [Figures 1 and 2].
Figure 1.
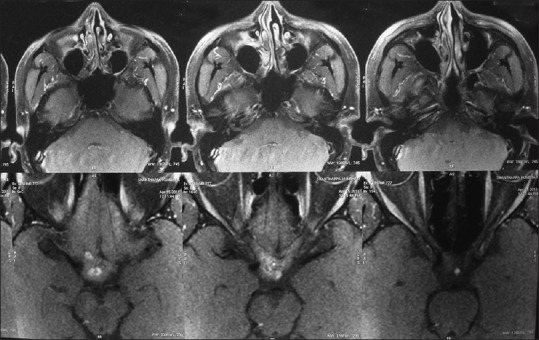
Post-contrast MRI brain axial section showing enhancing lesion in the chiasm
Figure 2.
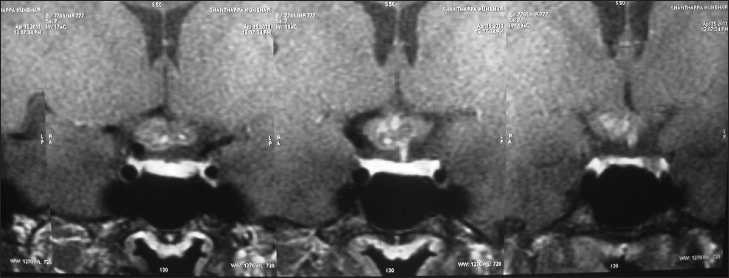
Post-contrast coronal section showing the same lesion
The lesion was removed neuro-endoscopically through the trans-nasal trans-sphenoidal route. After reaching the sella, dura opened and the pituitary gland was displaced. Under-surface of chiasm was visualized. The lesion was seen on the surface with brownish discoloration. A small incision was made and the lesion could be removed. Post–operatively, there was a transient worsening left temporal field defect. The histopathological examination and features are consistent with organizing hematoma.
Discussion
We report a rare case of a CM of the optic chiasma that was removed by the trans-nasal trans-sphenoid route using a neuro-endoscope. The current, well-established indications for surgical resection of CMs are recurrent hemorrhage, progressive neurologic deterioration, and intractable epilepsy, unless the location is associated with an unacceptably high surgical risk.[3,7] In some cases, resection was complicated by risk of damage to the surrounding neural tissue. As patients may suffer intratumoral hemorrhage after just biopsy or partial removal of the lesion, the role of surgical treatment of CMs of the optic nerve and chiasm must be considered carefully.[8,2] Despite its critical location, good surgical outcomes have been reported in the literature concerning chiasmal CMs. Thus, complete surgical removal should be attempted for chiasmal CMs. As demonstrated in our case and in previous reports, chiasmal CMs can be safely resected with preservation or even restoration of nerve function.
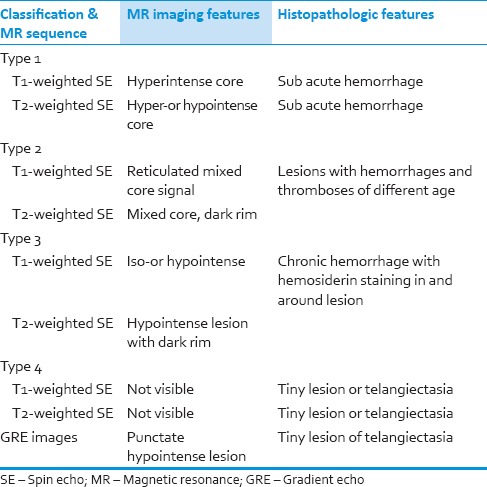
Based on MRI appearance and pathological correlation, Zabramski et al. have classified CMs into four types [Table 1] and also noted that symptomatic hemorrhage is more common in type I and type II.[9,10,11]
Table 1.
MR Findings in various stages of hemorrhage
Most cerebral CMs (CCMs) (50-80%) are apparently sporadic. A single CCM may be found in roughly 70% of patients with sporadic CCMs and in 8-19% of patients with familial disease. Multiple CCMs, indicative of familial forms,[3,4] are genetically heterogeneous, exhibiting an autosomal dominant inheritance with different preliminary estimates of disease penetrance at 3 loci:KRIT1/CCM1, CCM2, and PDCD10/CCM3, mapped to 7q, 7p, and 3q, respectively. These loci account for approximately 40%, 20%, and 40% of non-Hispanic familial cases, respectively.
Familial CCMs have been shown to have a 0.2-0.4% incidence per patient per year of de novo lesion formation.[3,4,9,10,12] For this phenomenon, two possible developmental mechanisms have been postulated: A Knudson 2-hit mechanism and a haploinsufficiency mode.[13,14,15,16] De novo formation has been associated with previous irradiation, viruses, hormonal influences in pregnancy, endothelial proliferation, and angiogenesis.[4,13,17] Notably, CCMs have the capacity for endothelial proliferation and neoangiogenesis, which may also explain the development of new CCMs along a biopsy tract.[18] A small amount of cavernous tissue transplanted to any point along the biopsy tract may induce the transformation of normal capillaries or the growth of new, fragile vessels or recanalization by nearby parenchymal vessels.[17] CMs present with site specific symptoms and require complex surgical techniques for resection. These lesions are frequently symptomatic, because of the eloquence of the tissue of origin. Characteristic clinical symptoms are three: Sudden headache, sudden change of visual acuity, and visual field. Lehner et al. 3 reported higher frequency of bleeding in chiasm out of his 30 chiasmal CMs. More than one-third had previous transient visual symptoms and headache 3,6,51. However, even subtle hemorrhage can be symptomatic if CM is located in the cranial nerves.[2,3,10] CMs within the anterior visual pathways can cause visual symptoms as well as headache, retro-orbital pain and nausea, and the so-called chiasmal apoplexy. The clinical symptoms of CMs are mostly the result of intrinsic and extrinsic bleeding.
Gross-total resection of these lesions is associated with favorable visual outcomes. Emergency surgery to prevent permanent damage to the visual pathway is an option to be kept in mind.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Maitland CG, Abiko S, Hoyt WF, Wilson CB, Okamura T. Chiasmal apoplexy. Report of four cases. J Neurosurg. 1982;56:118–22. doi: 10.3171/jns.1982.56.1.0118. [DOI] [PubMed] [Google Scholar]
- 2.Muta D, Nishi T, Koga K, Yamashiro S, Fujioka S, Kuratsu J. Cavernous malformation of the optic chiasm: Case report. Br J Neurosurg. 2006;20:312–5. doi: 10.1080/02688690601000238. [DOI] [PubMed] [Google Scholar]
- 3.Del Curling O, Jr, Kelly DL, Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702–8. doi: 10.3171/jns.1991.75.5.0702. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh VR, Albuquerque FC, Zabramski JM, Spetzler RF. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. 2003;53:352–7. doi: 10.1227/01.neu.0000073531.84342.c2. [DOI] [PubMed] [Google Scholar]
- 5.Matias-Guiu X, Alejo M, Sole T, Ferrer I, Noboa R, Bartumeus F. Cavernous angiomas of the cranial nerves. Report of two cases. J Neurosurg. 1990;73:620–2. doi: 10.3171/jns.1990.73.4.0620. [DOI] [PubMed] [Google Scholar]
- 6.Park DM, Kim DH. Cavernous angioma of the oculomotor nerve. J Korean Neurosurg Soc. 2005;38:147–50. [Google Scholar]
- 7.Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709–14. doi: 10.3171/jns.1991.75.5.0709. [DOI] [PubMed] [Google Scholar]
- 8.Lehner M, Fellner FA, Wurm G. Cavernous haemangiomas of the anterior visual pathways. Short review on occasion of an exceptional case. Acta Neurochir (Wien) 2006;148:571–8. doi: 10.1007/s00701-006-0751-3. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: A practical review of clinical and biological aspects. Surg Neurol. 2005;63:319–28. doi: 10.1016/j.surneu.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Surucu O, Sure U, Mittelbronn M, Meyermann R, Becker R. Cavernoma of the trochlear nerve. Clin Neurol Neurosurg. 2007;109:791–3. doi: 10.1016/j.clineuro.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Hempelmann RG, Mater E, Schröder F, Schön R. Complete resection of a cavernous haemangioma of the optic nerve, the chiasm, and the optic tract. Acta Neurochir (Wien) 2007;149:699–703. doi: 10.1007/s00701-007-1163-8. [DOI] [PubMed] [Google Scholar]
- 12.Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, et al. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988;319:343–7. doi: 10.1056/NEJM198808113190605. [DOI] [PubMed] [Google Scholar]
- 13.Boretius S, Gadjanski I, Demmer I, Bähr M, Diem R, Michaelis T, et al. MRI of optic neuritis in a rat model. Neuroimage. 2008;41:323–34. doi: 10.1016/j.neuroimage.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Miszkiel KA, MacManus DG, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127:2498–505. doi: 10.1093/brain/awh284. [DOI] [PubMed] [Google Scholar]
- 15.Hickman SJ, Miszkiel KA, Plant GT, Miller DH. The optic nerve sheath on MRI in acute optic neuritis. Neuroradiology. 2005;47:51–5. doi: 10.1007/s00234-004-1308-x. [DOI] [PubMed] [Google Scholar]
- 16.Miller NR. Primary tumors of the optic nerve and its sheath. Eye (Lond) 2004;18:1026–37. doi: 10.1038/sj.eye.6701592. [DOI] [PubMed] [Google Scholar]
- 17.Smoker WR, Gentry LR, Yee NK, Nerad JA. Vascular lesions of the orbit: More than meets the eye. Radiographics. 2008;28:185–204. doi: 10.1148/rg.281075040. [DOI] [PubMed] [Google Scholar]
- 18.Chung EM, Specht CS, Schroeder JW. From the archives of the AFIP: Pediatric orbit tumors and tumor like lesions: Neuroepithelial lesions of the ocular globe and optic nerve. Radiographics. 2007;27:1159–86. doi: 10.1148/rg.274075014. [DOI] [PubMed] [Google Scholar]


