Abstract
Background
Patients with diabetic peripheral neuropathy (DPN) demonstrate gait alterations compared with their nonneuropathic counterparts, which may place them at increased risk for falling. However, it is uncertain whether patients with DPN also have a greater fear of falling.
Methods
A voluntary group of older adults with diabetes was asked to complete a validated fear of falling questionnaire (Falls Efficacy Scale International [FES-I]) and instructed to walk 20 m in their habitual shoes at their habitual speed. Spatiotemporal parameters of gait (eg, stride velocity and gait speed variability) were collected using a validated body-worn sensor technology. Balance during walking was also assessed using sacral motion in the mediolateral and anteroposterior directions. The level of DPN was quantified using vibration perception threshold from the great toe.
Results
Thirty-four diabetic patients (mean ± SD: age, 67.6 ± 9.2 years; body mass index, 30.9 ± 5.7; hemoglobin A1c, 7.9% ± 2.3%) with varying levels of neuropathy (mean ± SD vibration perception threshold, 34.6 ± 22.9 V) were recruited. Most participants (28 of 34, 82%) demonstrated moderate to high concern about falling based on their FES-I score. Age (r = 0.6), hemoglobin A1c level (r = 0.39), number of steps required to reach steady-state walking (ie, gait initiation) (r = 0.4), and duration of double support (r = 0.44) were each positively correlated with neuropathy severity (P < .05). Participants with a greater fear of falling also walked with slower stride velocities and shorter stride lengths (r = −0.3 for both, P < .05). However, no correlation was observed between level of DPN and the participant’s actual concern about falling.
Conclusions
Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy.
Recent work has demonstrated an association between diabetes mellitus and increased risk of falling.1 One of the best examples of this is the finding by Tilling et al2 that the annual incidence of falls in individuals older than 65 years with diabetes mellitus may be nearly 40%. Acquired neuromuscular deficits, foot and body pain, and use of psychotropic medications and specialty footwear are just a few of the reasons that individuals with diabetes mellitus are at increased risk for falling.1 However, there is no single factor believed to adversely affect fall risk in this population more than diabetic peripheral neuropathy (DPN)3–5; DPN allows sensory and motor deficits to occur in the feet and lower extremities.4,6 These deficits produce alterations in gait and balance performance, which, particularly in older and elderly adults, increases the likelihood of experiencing a fall event.5,7
In many respects, the psychological impairment associated with fear of falling can be just as burdensome as the physical harm experienced after an actual fall event. Concern about falling, for example, commonly leads to loss of confidence, higher levels of anxiety, social withdrawal, and restrictions in physical activity.8–13 Fear of falling may be widespread among older adults, affecting 26% to 61% of individuals, depending on the setting and type of population surveyed (ie, community-dwelling versus rehabilitation patients, previous fallers versus overall).8 However, it is unclear whether older adults with DPN have any greater fear of falling than their nonneuropathic counterparts.
There is limited information regarding how DPN influences concern about falling. Powell et al14 recently reported a reduced fear of falling in a sample of patients who had improved sensation in their feet after being treated for DPN with monochromatic near-infrared photoenergy. Similarly, Lalli et al15 also found that patients with painful DPN reported a greater fear of falling compared with those without neuropathy. However, neither study used a validated measure to assess their patients’ fear of falling. Therefore, the aim of the present study was to determine whether DPN is associated with greater concern about falling in older adults using a validated instrument and a cross-sectional study design. As a secondary aim, we assessed whether DPN and fear of falling might also be associated with increased fall risk in this population by studying their association with typical spatiotemporal parameters of gait.
Materials and Methods
Participants
Community-dwelling participants (45 years and older) were recruited between April 1, 2012, and July 31, 2012, from the Rosalind Franklin University Health System (North Chicago, Illinois) and from an 8-hour-long diabetes exposition (sponsored by the American Diabetes Association) held on April 14, 2012, at McCormick Place in Chicago, Illinois. Eligible participants had to have a medical diagnosis of type 1 or type 2 diabetes mellitus according to the American Diabetes Association criteria and be able to walk greater than 20 m without the assistance of a walking aid. Individuals were excluded if they had neurologic conditions other than DPN or established orthopedic conditions involving the lower extremity that might influence gait (eg, lower-extremity amputation and knee replacement surgery). Institutional review board approval was obtained for this work through Rosalind Franklin University of Medicine and Science, and all of the participants provided informed consent.
Measurement Protocol
Peripheral neuropathy was assessed via vibration perception threshold (VPT) testing as described by Young et al.16 The presence of moderate to severe neuropathy was determined by a VPT of at least 25 V, whereas those with a VPT less than 25 V were classified as having only mild or no neuropathy. With eyes closed in a seated position, participants were asked to identify when they perceived a vibratory sensation on the great toe (biothesiometer; Xilas Medical, San Antonio, Texas). The VPT values were recorded as continuous variables within a range of 1 to 100 V. An average of the values obtained at the right and left great toe was used for analysis. When VPT values were not available (one participant), results of the 10-g monofilament test were used to establish DPN.
Fear of falling was assessed using the Falls Efficacy Scale International (FES-I) questionnaire.17 This scale was developed and validated by the Prevention of Falls Network Europe and has become a widely accepted tool for assessing concern about falling.17,18 The instrument has also been validated across different cultures and languages.19 In this scale, scores are treated as continuous variables ranging from 16 to 64, where 16 indicates no concern and 64 indicates severe concern about falling. Participants in this study were further classified as having had low concern (score of 16–19), moderate concern (score of 20–27), or high concern (score ≥28) about falling according to the previous work of Delbaere et al.20
Glucose control was determined using hemoglobin A1c levels recorded within the past 3 months. Age, height, weight, and body mass index (calculated as weight in kilograms divided by height in meters squared) were also obtained for each participant. Finally, all of the participants underwent a structured foot examination that included 10-g monofilament testing, evaluation of peripheral pulses, and documentation of plantar calluses and structural deformities (eg, bunions and hammertoes).
Gait assessment was performed in the participant’s natural environment with validated21–23 wearable sensor technology (LEGSys; BioSensics LLC, Cambridge, Massachusetts). The inertial sensors were attached to the participant’s anterior shins, thighs, and lower back (Fig. 1). Each sensor measured the velocity of the angular rotation per segment around the coronal axis (flexion-extension). Participants were asked to walk at their habitual speed on a 20-m pathway during the shod condition. Multiple spatiotemporal gait parameters were assessed, including stride velocity, stride length, stride time, double stance, intercycle gait speed variability, and steps required to reach steady-state walking (ie, gait initiation).23,24 In addition, knee range of motion and balance during walking were also assessed; the latter was quantified using the range of sacral motion in the mediolateral and anteroposterior directions. Because parameters were collected from both legs, average values from the right and left extremities (eg, right and left knee range of motion) were used for statistical analysis. The beginning of steady-state gait was identified using the statistical intercycle fluctuation of gait velocity, as described in previous publications.24,25
Figure 1.

LEGSys portable wearable gait analyzer system with sensors attached via straps.
Statistical Analysis
The Kolmogorov-Smirnov test was used for testing normal distribution. For normally distributed parameters, between-group comparison was performed using independent-samples Kruskal-Wallis 1-way analysis of variance. Post hoc analyses for significance (P < .05) of main effects or interactions were performed using a Sidak adjustment when more than two values were compared, and an independent t test was administered when comparing only two values. For parameters that were not normally distributed, an independent-samples Mann-Whitney U test was used. Between-group comparison for categorical variables (eg, sex) was performed using the crosstab χ2 test. A linear regression model (stepwise) was applied to determine which gait parameters were independently associated with neuropathy severity (dependent variable: continuous VPT values). One participant with a missing VPT value was not included in this model. Only gait parameters with P ≤ .2 in bivariate testing were included in the multivariable analysis. A similar linear regression model was used to evaluate which variables were associated with concern about falling using continuous FES-I scores. Forward logistic regression was used to determine which parameters were most useful in discriminating between the DPN (VPT ≥25 V)16 and non-DPN groups. The Spearman correlation coefficient was used to assess the associations among neuropathy severity, FES-I scores, and the various gait parameters. Statistical analyses were performed with a statistical software program (SPSS, version 20; SPSS Inc, Chicago, Illinois). An α = 0.05 was used to determine significance.
Results
Study Demographics
Thirty-four patients (44% male) were recruited (mean ± SD: age, 67.6 ± 9.2 years; body mass index, 30.9 ± 5.7). All of the participants were diagnosed as having type 2 diabetes mellitus with a mean ± SD duration of 15 ± 12 years and a mean ± SD hemoglobin A1c level of 7.9% ± 2.3%. The mean ± SD VPT (n = 33, 1 missing) was 34.6 ± 22.9 V, and 47% of the patients could be classified as having had DPN (VPT ≥25 V) using previously described criteria.16
Results Stratified by DPN and Non-DPN Groups
Table 1 summarizes the demographic and gait characteristics of the DPN and non-DPN groups. In this sample, male participants were more likely to have DPN than were female participants (DPN versus non-DPN: 63% versus 28%, P = .04). On average, patients with DPN were 11.9 years (19%) older (P < .001, 95% confidence interval [CI], 7–17 years) and hemoglobin A1c values were 24% higher (P = .05; difference, 1.7%; 95% CI, 0%–3.4%) than in the non-DPN group. No other between-group differences were observed for demographic information.
Table 1.
Demographic and Gait Characteristics for Diabetic Participants with and Without Neuropathy
| Characteristic | Non-DPN Group (n = 18) |
DPN Group (n = 16) |
Difference (% Difference) |
95% CI | P Value |
|---|---|---|---|---|---|
| Age (years) | 62 ± 7 | 73 ± 8 | 11.9 (19) | 7 to 17 | <.001a |
| BMI | 31.2 ± 5.9 | 30.6 ± 5.7 | −0.7 (−2) | −5 to 3 | .73 |
| Sex (% male) | 28 | 63 | 35 (125) | NA | .04a |
| Duration of diabetes (years) | 13 ± 13 | 17 ± 11 | 3.5 (27) | −5 to 12 | .39 |
| Hemoglobin A1c (%) | 7.2 ± 1.6 | 8.9 ± 2.7 | 1.7 (24) | 0 to 3.4 | .05a |
| FES-I score | 31.7 ± 14.9 | 30.2 ± 11.4 | −1.5 (−5) | −11 to 8 | .74 |
| Concern about falling (%) | |||||
| Low | 17 | 19 | 2 (12) | NA | .6 |
| Moderate | 44 | 31 | −13 (−30) | NA | .3 |
| High | 39 | 50 | 11 (28) | NA | .3 |
| VPT (V) | 18.3 ± 4.5 | 49.7 ± 21.9 | 31 (169) | 23 to 45 | <.001a |
| Stride velocity (m/sec) | 0.92 ± 0.13 | 0.89 ± 0.23 | −0.03 (−3) | −0.2 to 0.1 | .63 |
| Stride length (m) | 1.09 ± 0.13 | 1.08 ± 0.22 | −0.01 (−1) | −.1 to 0.1 | .83 |
| Stride time (sec) | 1.20 ± 0.09 | 1.25 ± 0.16 | 0.05 (4) | −0.0 to 0.1 | .22 |
| Double stance (%) | 22.7 ± 4.2 | 27.6 ± 7.4 | 4.9 (22) | 0.7 to 9 | .02a |
| Knee range of motion (°) | 58 ± 8 | 55 ± 9 | −3 (5) | −9 to 3 | .34 |
| COMML (°) | 4.4 ± 1.4 | 4.6 ± 1.6 | 0.2 (5) | −0.8 to 1 | .74 |
| COMAP (°) | 4.7 ± 1.4 | 4.6 ± 1.4 | −0.1 (−2) | −1 to 1 | .83 |
| CV(SV) (%) | 5.9 ± 3.2 | 8.0 ± 4.8 | 2 (34) | 0.8 to 5 | .15 |
| Gait initiation (steps) | 2.7 ± 1.1 | 3.9 ± 2.1 | 1.2 (44) | 0.0 to 2.4 | .04a |
Note: Data are given as mean ± SD except where indicated otherwise.
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CI, confidence interval; COMML and COMAP, sacral motion in the mediolateral and anteroposterior directions; CV(SV), coefficient of variation in stride velocity (aka gait variability); DPN, diabetic peripheral neuropathy; FES-I, Falls Efficacy Scale International; NA, not applicable; VPT, vibration perception threshold.
Statistically significant.
Overall, gait performance was relatively worse in participants with DPN compared with nonneuropathic individuals. For example, participants with DPN showed nonsignificant trends toward greater intercycle gait speed variability (P = .15), longer stride times (P = .22), and less knee range of motion (P =.34). However, only steps taken during gait initiation and double-support percentage achieved statistical significance during bivariate testing. Patients with DPN demonstrated a 22% longer double-stance phase (P = .02; difference, 4.9%; 95% CI, 0.7%–9%) and a 44% increase in the number of steps needed to reach steady-state walking (P = .04; difference, 1.2 steps; 95% confidence interval, 0–2.4 steps) compared with patients without DPN. Logistic regression analysis also confirmed double-stance percentage and gait initiation steps were significantly related to DPN status (Cox & Snell r2 = 0.34), being the only two significant gait variables in the multivariable analysis. Finally, balance during walking assessed using sacral motion in the mediolateral and anteroposterior directions was not different in the DPN and non-DPN groups (P > .05).
When evaluating neuropathy on a continuous scale, age (r = 0.6, P > .001), hemoglobin A1c level (r = 0.39, P = .04), gait initiation steps (r = 0.4, P = .03), and double-support percentage (r = 0.44, P = .01) were each positively correlated with neuropathy severity (Fig. 2). However, only double-support percentage (β = 2.1, SE = 0.6, P < .001) was found to be associated with neuropathy severity (r2 = 0.58, SE = 19) during multivariable linear regression analysis.
Figure 2.
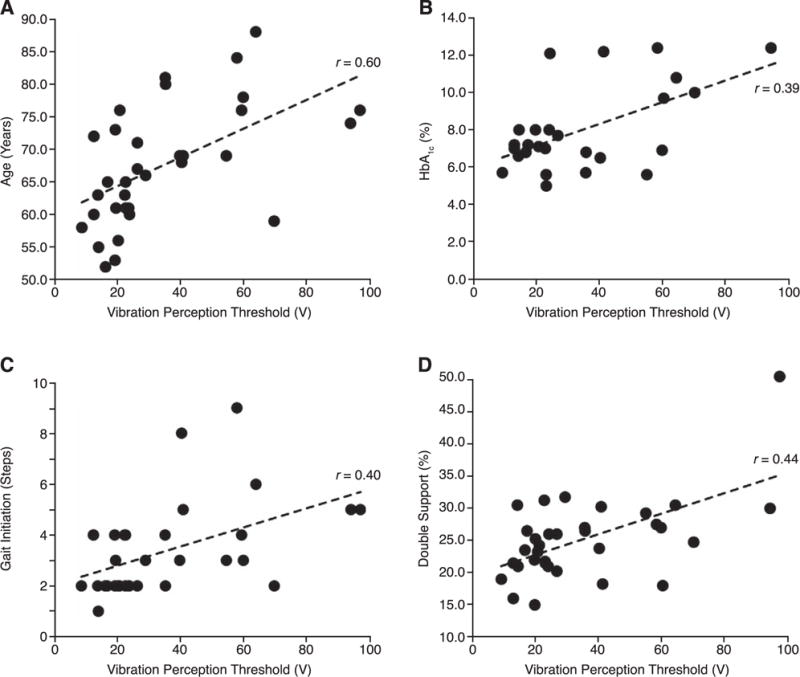
Associations between neuropathy severity quantified by the vibration perception threshold and age (A), hemoglobin A1c (HbA1c) level (B), number of steps required to achieve steady-state walking (C), and double support (D).
The DPN and non-DPN groups had almost the same level of concern about falling based on their mean ± SD FES-I scores (30.2 ± 11.4 versus 31.7 ± 14.9, P = .74). Furthermore, there was no association (P = .5) between VPT values and FES-I scores (Fig. 3).
Figure 3.
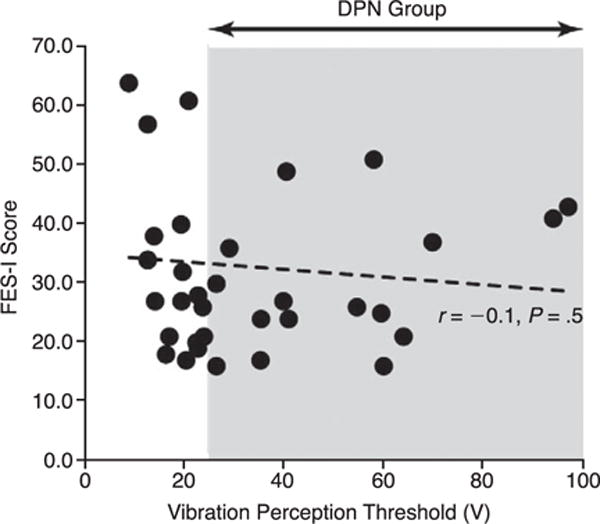
Scatterplot demonstrating the lack of association between fear of falling (Falls Efficacy Scale International [FES-I] scores) and vibration perception threshold values (level of neuropathy). The shaded area identifies participants in the diabetic peripheral neuropathy (DPN) group.
Results Stratified by Concern About Falling
Most participants in the study demonstrated moderate (38.2%) to high (44.2%) concern about falling. Table 2 summarizes the demographic and gait characteristics of participants stratified by low, moderate, and high concern about falling. There were no between-group differences observed for the measured demographic information.
Table 2.
Demographic and Gait Characteristics of Participants with Low, Moderate, and High Concern About Falling
| Characteristic | Concern About Falling
|
P Value | ||
|---|---|---|---|---|
| Low (n = 6) | Moderate (n = 13) | High (n = 15) | ||
| Age (years) | 66.5 ± 11.5 | 67.8 ± 9.9 | 66.8 ± 8.5 | .9 |
| BMI | 28.9 ± 5.5 | 30.6 ± 6.5 | 32.0 ± 5.2 | .5 |
| Sex (% male) | 33 | 46 | 47 | .8 |
| Duration of diabetes (years) | 15.3 ± 9.1 | 15.8 ± 13.3 | 13.9 ± 11.7 | .9 |
| Hemoglobin A1c (%) | 7.1 ± 1.5 | 8.1 ± 2.3 | 8.3 ± 2.7 | .6 |
| FES-I score (range, 16–64) | 17.2 ± 1.2 | 23.8 ± 2.7 | 42.7 ± 11.2 | <.001a |
| VPT (V) (range, 1–100 V) | 30.2 ± 15.9 | 34.6 ± 17.3 | 36.4 ± 29.4 | .8 |
| % DPN | 50 | 46 | 47 | .9 |
| Stride velocity (m/sec) | 1.02 ± 0.43 | 0.93 ± 0.16 | 0.83 ± 0.20 | .08 |
| Stride length (m) | 1.16 ± 0.11 | 1.11 ± 0.18 | 1.03 ± 0.18 | .2 |
| Stride time (sec) | 1.15 ± 0.11 | 1.20 ± 0.08 | 1.26 ± 0.13 | .2 |
| Double stance (%) | 22.3 ± 3.1 | 24.3 ± 5.0 | 26.7 ± 8.0 | .3 |
| Knee range of motion (°) | 59.5 ± 10.1 | 57.5 ± 9.1 | 55.1 ± 8.08 | .5 |
| COMML (°) | 4.4 ± 1.0 | 5.0 ± 1.4 | 4.1 ± 1.2 | .3 |
| COMAP (°) | 4.7 ± 1.0 | 4.7 ± 1.4 | 4.6 ± 1.5 | .9 |
| CV(SV) (%) | 5.5 ± 2.3 | 7.1 ± 6.4 | 8.0 ± 4.4 | .1 |
| Gait initiation (steps) | 2.5 ± 0.8 | 3.2 ± 1.5 | 3.7 ± 2.2 | .3 |
Note: Data are given as mean ± SD except where indicated otherwise.
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COMML and COMAP, sacral motion in the mediolateral and anteroposterior directions; CV(SV), coefficient of variation in stride velocity (aka gait variability); DPN, diabetic peripheral neuropathy; FES-I, Falls Efficacy Scale International; VPT, vibration perception threshold.
Statistically significant.
Nonsignificant trends were again observed across most gait parameters, suggesting poorer gait performance as the concern about falling increased. More specifically, participants with the greatest fear of falling generally exhibited slower stride velocity (P = .08), shorter stride length (P = .2), longer stride time (P = .2), longer double-support percentage (P = .3), and higher gait variability (P = .1) and required a greater number of steps to reach steady-state walking (P = .3). However, none of these observations achieved statistical significance during bivariate analysis. Multivariable logistic regression analysis, however, did identify stride velocity (β = −25, SE = 12, P = .03) as a significant correlate with fear of falling, with participants again in the higher concern about falling categories exhibiting slower stride velocities. Finally, balance during walking quantified by the sway of sacral motion in the mediolateral and anteroposterior directions did not differ significantly between the groups (P > .05).
When considering fear of falling on a continuous scale, stride velocity and stride length (both r = −0.3, P = .05) demonstrated a mild negative correlation with FES-I scores (Fig. 4). These variables were also retained in the mutivariable linear regression analysis (both P < .05). None of the participant demographic features (eg, age and body mass index) or clinical information (eg, hemoglobin A1c level and duration of diabetes) was found to be associated with fear of falling.
Figure 4.
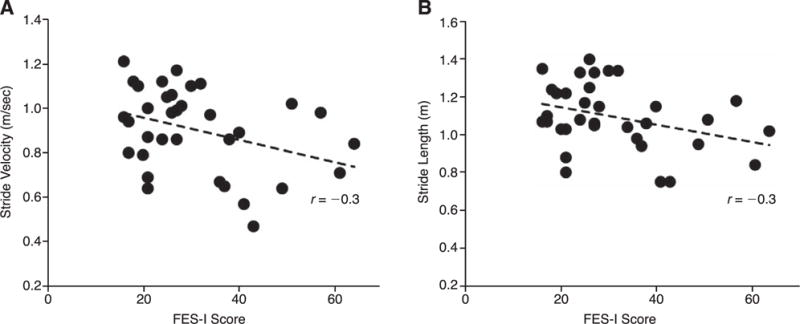
Associations between fear of falling (Falls Efficacy Scale International [FES-I] scores) and stride velocity (A) and stride length (B).
Discussion
We found that fear of falling was remarkably prevalent in this sample of older individuals with diabetes mellitus. In fact, 82% of those surveyed were classified as having had moderate to high concern about falling. Furthermore, we learned that individuals with DPN were not any more fearful of falling than their non-DPN counterparts and that level of peripheral neuropathy did not correlate well with concern about falling (Fig. 3).
These findings are important because, until now, it has not been clear whether diabetes confers any greater fear of falling in older adults. Previous work using the FES-I questionnaire in healthy older adult populations place most of their participants at the low end of moderate concern about falling (mean FES-I score of ~21), whereas the present sample, which consisted of older diabetic patients with largely mild to moderate neuropathy (average VPT of 35 V), demonstrated moderate to high concern about falling (mean FES-I score of 31). Health-care providers who are cognizant of this new information may be better equipped to help older adults with diabetes who present with unexplained anxiety, depression, and generalized withdrawal or lack of engagement. Moreover, these findings suggest that there is an apparent mismatch between perceived risk of falling and actual risk of falling in older adults with diabetes mellitus. This is important because gait performance declines with DPN26,27 and places individuals with DPN at greater risk for fall events.28–30 However, our work suggests, worsening gait performance is not always accompanied by a greater fear of falling. Therefore, health-care providers should not rely on older patients with diabetes, who are perhaps at the greatest risk for falling, to openly relate a concern about falling because they may not perceive it this way.
Previous studies have demonstrated that peripheral neuropathy and fear of falling are linked to changes in gait performance,4,26,31–34 and the present findings are in good agreement with these. Patients with DPN, for example, are known to adopt a conservative walking strategy, with a longer double-support phase and generally slower stride velocities compared with patients without DPN. In the present study, older participants with diabetes also spent significantly greater time in double support (bivariate association r = 0.44) as neuropathy severity increased. Also consistent with previous work, we found that patients with DPN demonstrated greater instability while walking, requiring more steps during gait initiation (P = .04) and exhibiting a tendency toward greater gait speed intercycle variability (P = .15), gait alterations that have recently been implicated in falls. Similarly, fearful participants in this study demonstrated a stabilizing adaptation to their gait, walking with slower stride velocities and shorter stride lengths. This is a characteristic gait pattern, first reported by Maki31 and again by Chamberlin and colleagues,32 that individuals who are fearful of falling often exhibit.
Powell et al14 and Lalli et al15 found that worsening DPN was associated with a greater fear of falling in their patients. At first glance, the present results seem to be inconsistent with these findings; however, neither study used a validated measure of fear of falling, and both authors described an association with painful peripheral neuropathy and increased fear of falling. The present study, on the other hand, looked exclusively at individuals with DPN, hypoesthesia, and lack of feeling in their feet. Therefore, it is possible that patients with DPN and neuritic foot pain (sometimes referred to as DPN with positive symptoms) are actually more fearful of falling than are patients with no DPN or insensitivity only, whereas those with varying levels of insensitivity demonstrate generally less overall variation in their concern about falling. These assumptions, however, need to be corroborated using validated measures of fear of falling in a population with the full spectrum of neuropathy severity and symptoms.
Although a patient’s level of apprehension may not dictate fall risk, it is still important to recognize when there are underlying psychological issues affecting our patients that can be addressed. Assuaging an individual’s concerns regarding their fear of falling can empower them to become more productive and engaged in basic and instrumental activities of daily living. Formal physical therapy is a viable option for those who demonstrate a lack of confidence with ambulation regardless of their actual risk of falls as weekly balance training has been shown to significantly increase satisfaction among diabetic patients.35
The results of this study should be interpreted in the context of several limitations. First, the patients with DPN were older than those without DPN, which may have accounted for some of the gait deterioration seen in the DPN group during bivariate analysis. Also, these results may have residual confounding as we looked at only a select number of demographic and clinical variables in our participants. Furthermore, many of the comparisons demonstrated nonsignificant trends, suggesting that the study may have been underpowered to detect important group differences. However, the main findings of this work, namely, the high prevalence of fearful walking in older patients with diabetes and the apparent lack of association between fear of falling and level of neuropathy, remain unaffected by these shortcomings.
Despite patients with DPN exhibiting worse gait and possibly being at increased risk for a fall event, we found that DPN did not translate into a greater fear of falling in older adults. Querying patients in this group regarding their fear of falling may do little to accurately assess fall risk. Instead, we need to be aware of the threat that faces this population and continually evaluate patients throughout the length of their encounter for signs of gait and balance deficits. When detected, health-care providers should then be available to work with patients and obtain the proper consultations to help mitigate the risk of falling once it has been identified.
Conclusions
We conclude that older adults with diabetes are generally fearful of falling. However, concern about falling in this population does not appear to be mediated via DPN. Therefore, fear of falling may be an unreliable indicator of actual fall risk. Future research should explore other candidate factors that may contribute to fall concern.
Acknowledgments
Financial Disclosure: This project was supported, in part, by grant 1T35DK074390-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, which had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest: Guest editor, Bijan Najafi, PhD, was not involved in the review and acceptance of this paper.
References
- 1.Crews RT, Yalla SV, Fleischer AE, et al. A growing troubling triad: diabetes, aging, and falls. J Aging Res. 2013;2013:342650. doi: 10.1155/2013/342650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006;20:158. doi: 10.1016/j.jdiacomp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Sawacha Z, Guarneri G, Avogaro A, et al. A new classification of diabetic gait pattern based on cluster analysis of biomechanical data. J Diabetes Sci Technol. 2010;4:1127. doi: 10.1177/193229681000400511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4:833. doi: 10.1177/193229681000400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najafi B, Bharara M, Talal TK, et al. Advances in balance assessment and balance training for diabetes. Diabetes Manage. 2012;2:293. [Google Scholar]
- 6.Giacomozzi C, D’Ambrogi E, Cesinaro S, et al. Muscle performance and ankle joint mobility in long-term patients with diabetes. BMC Musculoskelet Disord. 2008;9:99. doi: 10.1186/1471-2474-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allet L, Armand S, Golay A, et al. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24:173. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 8.Arfken CL, Lach HW, Birge SJ, et al. The prevalence and correlates of fear of falling in elderly persons living in the community. Am J Public Health. 1994;84:565. doi: 10.2105/ajph.84.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachman ME, Howland J, Tennstedt S, et al. Fear of falling and activity restriction: the survey of activities and fear of falling in the elderly (SAFE) J Gerontol B Psychol Sci Soc Sci. 1998;53:43. doi: 10.1093/geronb/53b.1.p43. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 11.Kressig RW, Wolf SL, Sattin RW, et al. Associations of demographic, functional, and behavioral characteristics with activity-related fear of falling among older adults transitioning to frailty. J Am Geriatr Soc. 2001;49:1456. doi: 10.1046/j.1532-5415.2001.4911237.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruce DG, Devine A, Prince RL. Recreational physical activity levels in healthy older women: the importance of fear of falling. J Am Geriatr Soc. 2002;50:84. doi: 10.1046/j.1532-5415.2002.50012.x. [DOI] [PubMed] [Google Scholar]
- 13.Rochat S, Martin E, Piot-Ziegler C, et al. Falls self-efficacy and gait performance after gait and balance training in older people. J Am Geriatr Soc. 2008;56:1154. doi: 10.1111/j.1532-5415.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 14.Powell MW, Carnegie DH, Burke TJ, et al. Reversal of diabetic peripheral neuropathy with phototherapy (MIRE) decreases falls and the fear of falling and improves activities of daily living in seniors. Age Ageing. 2006;35:11. doi: 10.1093/ageing/afi215. [DOI] [PubMed] [Google Scholar]
- 15.Lalli P, Chan A, Garven A, et al. Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complications. 2013;27:248. doi: 10.1016/j.jdiacomp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Young MJ, Every N, Boulton AJ. A comparison of the neurothesiometer and biothesiometer for measuring vibration perception in diabetic patients. Diabetes Res Clin Pract. 1993;20:129. doi: 10.1016/0168-8227(93)90006-q. [DOI] [PubMed] [Google Scholar]
- 17.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale-International (FES-I) Age Ageing. 2005;34:614. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 18.Kempen GI, Yardley L, van Haastregt JC, et al. The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37:45. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 19.Kempen GI, Todd CJ, Van Haastregt JC, et al. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in older people: results from Germany, the Netherlands and the UK were satisfactory. Disabil Rehabil. 2007;29:155. doi: 10.1080/09638280600747637. [DOI] [PubMed] [Google Scholar]
- 20.Delbaere K, Close JC, Mikolaizak AS, et al. The Falls Efficacy Scale International (FES-I): a comprehensive longitudinal validation study. Age Ageing. 2010;39:210. doi: 10.1093/ageing/afp225. [DOI] [PubMed] [Google Scholar]
- 21.Aminian K, Najafi B, Büla C, et al. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35:689. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 22.Aminian K, Trevisan C, Najafi B, et al. Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Posture. 2004;20:102. doi: 10.1016/S0966-6362(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 23.Najafi B, Helbostad JL, Moe-Nilssen R, et al. Does walking strategy in older people change as a function of walking distance? Gait Posture. 2009;29:261. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Lindemann U, Najafi B, Zijlstra W, et al. Distance to achieve steady state walking speed in frail elderly persons. Gait Posture. 2008;27:91. doi: 10.1016/j.gaitpost.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Najafi B, Miller D, Jarrett BD, et al. Does footwear type impact the number of steps required to reach gait steady state? an innovative look at the impact of foot orthoses on gait initiation. Gait Posture. 2010;32:29. doi: 10.1016/j.gaitpost.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Najafi B, Khan T, Fleischer A, et al. The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. JAPMA. 2013;103:165. doi: 10.7547/1030165. [DOI] [PubMed] [Google Scholar]
- 27.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women’s Health and Aging Study. Diabetes Care. 2000;23:1642. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 28.Macgilchrist C, Paul L, Ellis BM, et al. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010;27:162. doi: 10.1111/j.1464-5491.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 30.Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50:M211. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 31.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 32.Chamberlin ME, Fulwider BD, Sanders SL, et al. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005;60:1163. doi: 10.1093/gerona/60.9.1163. [DOI] [PubMed] [Google Scholar]
- 33.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382. doi: 10.2337/diacare.27.10.2382. [DOI] [PubMed] [Google Scholar]
- 34.Dinh T, Doupis J, Lyons TE, et al. Foot muscle energy reserves in diabetic patients without and with clinical peripheral neuropathy. Diabetes Care. 2009;32:1521. doi: 10.2337/dc09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavanagh PR, Derr JA, Ulbrecht JS, et al. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]


