Abstract
Objectives
Our goal was to evaluate changes in respiratory pattern among premature infants born at less than 29 weeks gestation who underwent a physiologic challenge at 36 weeks post-menstrual age with systematic reductions in supplemental oxygen and inspired airflow.
Study Design
Subjects were all infants enrolled in the Prematurity and Respiratory Outcomes Program at St. Louis Children’s Hospital and eligible for a physiologic challenge protocol because they were receiving supplemental oxygen or augmented airflow alone as part of their routine care. Continuous recording of rib cage and abdominal excursion and hemoglobin oxygen saturation (SpO2%) were made in the newborn intensive care unit.
Results
Thirty seven of 49 infants (75.5%) failed the challenge, with severe or sustained falls in SpO2%. And 16 of 37 infants (43.2%) who failed had marked increases in the amount of periodic breathing at the time of challenge failure.
Conclusions
An unstable respiratory pattern is unmasked with a decrease in inspired oxygen or airflow support in many premature infants. Although infants with significant chronic lung disease may also be predisposed to more periodic breathing, these data suggest that the classification of chronic lung disease of prematurity based solely on clinical requirements for supplemental oxygen or airflow do not account for multiple mechanisms that are likely contributing to the need for respiratory support.
Keywords: bronchopulmonary dysplasia, chronic lung disease, prematurity, hypoxemia, periodic breathing
INTRODUCTION
Clinical manifestations of chronic lung disease of prematurity (CLD), also known as bronchopulmonary dysplasia, have changed over the last 45 years.1,2 CLD has been defined by persistence of a supplemental oxygen requirement at 36 weeks post-menstrual age (PMA) among infants born before 32-weeks gestation who usually have persistent respiratory symptoms and chest radiograph changes. Historically, chronic lung disease of prematurity has been considered a consequence of lung immaturity and lung injury caused by immature surfactant production, incomplete alveolarization, oxygen toxicity, barotrauma, and infection.3 Despite important advances in treatment, many infants still require supplemental oxygen at 36 weeks PMA.
Infants with CLD have long-term respiratory morbidity with higher rates of re-hospitalization and are more likely to require respiratory medications during the first year of life. The cost for treatment of CLD in the United States in 2005 exceeded $2 billion.4,5
Although supplemental oxygen use has come to be synonymous with CLD,6 this conventional definition has limitations. While a persistent supplemental oxygen requirement may indicate that a newborn has only airway or alveolar disease, unstable ventilatory control can lead to hypoxemia that will also respond to supplemental oxygen support.7,8–11 In some studies, lengthy periods of spontaneous periodic breathing were recorded in more than half of premature subjects.12,13
This paper describes a single-center study within the Prematurity and Respiratory Outcomes Project (PROP), a multicenter study intended to identify genetic modifiers and biomarkers that will lead to targeted therapies for CLD. We examined breathing patterns during a systematic reduction in supplemental oxygen and flow in 49 consecutive preterm infants prescribed supplemental oxygen or airflow support for hypoxemia, and who met current clinical criteria for CLD. We assessed the prevalence of unstable ventilatory control to determine how often it might contribute to the child’s hypoxemia. These findings suggest a more complete phenotypic description of CLD is needed when gas exchange is altered, a step that is critical to identifying mechanistic factors and understanding the degree of involvement of alveolar and airway disease, or immature ventilatory control.
EXPERIMENTAL METHODS
PATIENTS
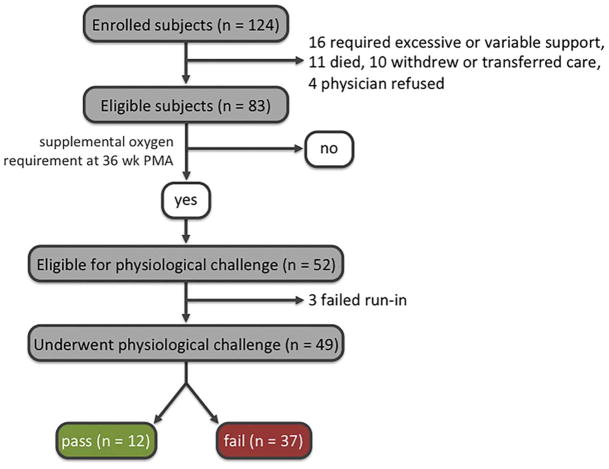
From August 12, 2011 to July 30, 2013, one hundred twenty-four (124) consecutive neonates born between 24 and 28-weeks gestation were enrolled prospectively at Saint Louis Children’s Hospital, one of the 7 centers participating in the National Institutes of Health-supported PROP. At 36 weeks PMA, the age at which the diagnosis of CLD is typically assigned, infants receiving supplemental oxygen or airflow support via nasal cannula were eligible to undergo a physiologic challenge, to confirm that without supplemental oxygen or augmented airflow their SpO2% would be unacceptably low and consistent with a diagnosis of CLD. Infants requiring mechanical ventilation or those deemed unstable by the clinical care team were excluded. The type and degree of support were chosen by the clinical care team without predetermined criteria. (Figure 1)
Figure 1.
Subject enrollment and outcomes.
Informed consent was obtained from the parents of each participant. The study was approved by the Washington University Human Research Protection Office and the PROP Observational Study Monitoring Board.
RESPIRATORY OUTCOME MEASURES
At 36 weeks PMA, infants receiving supplemental oxygen or airflow support via nasal cannula at fraction of inhaled oxygen (FiO2) of 0.21 underwent a physiologic challenge protocol according to the PROP Manual of Procedures.14
Infants were studied in the supine position, and the study was initiated with infants in behaviorally defined quiet sleep.15,16 During a 5-minute run-in period, the infant received the FiO2 and airflow rate prescribed by the clinicians supervising care. SpO2% was recorded continuously using a NONINR pulse oximeter (NONIN Medical, Inc., Minneapolis, MN). In response to a change in arterial oxyhemoglobin saturation (SaO2%), the oximeter has an effective averaging time in newborns of 1.5 to 3.0 sec, depending on the pulse rate.
Respiratory inductance plethysmography (RIP) was recorded (Biocapture, Cleveland Medical Devices, Cleveland, OH), by placing elastic bands around the infant at the nipple line and the umbilicus,17,18 during the run-in period, when the infant received prescribe FiO2 and flow, and during the challenge period. Data describing SpO2%, percent of quiet sleep time in periodic breathing (%PB), and minute ventilation during quiet sleep from QDC-calibrated RIP,18 were compared before and during the challenge.
RIP was also used to provide an index of the severity of respiratory system disease. The degree of Rib Cage vs. Abdomen Dyssynchrony over 60 breaths for each infant was described by a phase angle (ϕ) calculated from the plot of Rib cage vs. Abdominal excursion.17,19 Healthy term infants have ϕ near 12 degrees during quiet sleep.20
After the 5-minute run-in, FiO2 was reduced to 0.21 in steps by 0.20 or less, followed by wean of airflow provided by nasal cannula. Each step down in FiO2 was observed for 5 minutes, each step down in flow for 10 minutes. Failure at any step in the weaning protocol resulted in termination of the challenge with resumption of the prescribed support. If the infants tolerated all decrements of support during the weaning period, an observation period of up to 60 minutes ensued with the infant breathing only ambient room air. Physiologic challenge failure was defined as SpO2<90% for five consecutive minutes within any step in the challenge, or SpO2<80% for 15 consecutive seconds. If the infant did not worsen to meet criteria for failure during the 60-minute observation, he or she was deemed to have passed the room air challenge (RAC). In most instances we refer to the challenge as a “physiologic” challenge, rather than RAC, because many infants failed before breathing room air alone.
MEASUREMENTS DURING PHYSIOLOGIC CHALLENGE
Relative changes in minute ventilation were calculated from QDC-calibrated RIP.18 Periodic breathing (PB) was defined as at least 3 central apneas lasting at least 3 seconds separated by fewer than 20 seconds of regular breathing.21
Minute ventilation and percentage of time with PB for the entire run-in period were compared to the final minutes of the challenge. For minute ventilation, the 5-minute run in period was compared to the last minute of the challenge (failure, or completion). For PB, the percent of time in PB for the 5-minute run in was compared to the percent during the last 3 minutes before failure or completion of challenge. Some infants were still receiving caffeine as prescribed by the clinical team, and its potential impact on PB and challenge failure was also analyzed. Caffeine levels were not routinely measured in these infants or in our NICU, in general.
STATISTICAL MEASUREMENT AND COMPARISONS
Kruskal-Wallis test was used for comparison of caffeine effect among each of 3 groups. Categorical comparisons based on passing or failing the challenge were done by Chi square. Statistical significance was defined as p <0.05.
RESULTS
One hundred twenty-four infants were enrolled (Figure 1). Three infants failed during the run-in period when continuous recordings showed that the prescribed support did not maintain SpO2% in the passing range. Forty-nine infants underwent the challenge (Table 1). Twelve infants (24.5%) maintained oxygen saturations for 60 minutes of observation breathing room air alone and passed the RAC. Five infants had PB during the run-in period while on prescribed support before the challenge (range 5.1 to 102.0 seconds of PB, 1.7% – 34.0% of 5 minute period). Two of these did not maintain adequate SpO2% during the run-in period and were not tested further. Seventeen infants displayed more PB during the physiologic challenge, and 16 of these infants failed. (Figure 2) These 16 represented 43.2% of infants failing the challenge. The increase in percent of time with periodic breathing during the challenge, for those showing an increase, was 48.6 ± 21.6% (range 15.0% to 100.0%) That is, a hypothetical infant with 1 minute of PB during the 5 minute run in period (20% of recording time) and 2 minutes of PB during the last 3 minutes of the challenge (67% of the time) would have had an increase by 47 in the percent of recording time with PB.
TABLE 1.
Descriptive statistics for infants undergoing physiologic challenge: birth weight, gestational age, post-menstrual age, gender, FiO2, Flow rate for air and O2 delivered by nasal cannula, days receiving mechanical ventilation, and phase angle (ϕ) as a measure for rib cage-abdominal dyssynchrony. The use of 100% O2 via nasal cannula at flow < 1 liter per minute among infants at 36 weeks PMA is a common practice in St. Louis Children’s Hospital NICU
| Birth weight | 837 ± 168 grams (range, 400 to 1160] |
| Gestational age | 26 2/7 ± 12/7 weeks |
| Post-menstrual age | 36 2/7 ± 4/7 weeks |
| Females | 31 (63.3%) |
| FiO2 | 0.7 ± 0.4 |
| Supplemental O2 flow rate | 1.0 ± 1.1 lpm (median 1.0 lpm, range 0.06 to 5.0 lpm |
| Days on Mechanical Ventilation | Median 12.0 days, range 0 to 62 days |
| Phase Angle ϕ | 78.3 ±.42.1, range 12.2 to 157.8 degrees |
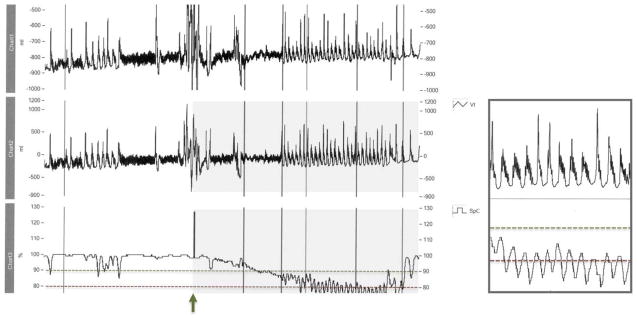
Figure 2.
Diagram showing a 5-minute recording from a patient who failed the challenge. The upper 2 tracings show respiratory excursions. The lowest tracing is SpO2% averaged every 3 to 5 sec. Note the stable SpO2% > 90% when respirations are regular. Note the intermittent hypoxemia and characteristic oscillations in SpO2% seen with the brief run of periodic breathing early in the tracings, and the continuous oscillations when PB is established. This pattern occurred in 43.2% of challenge failures. For this subject, continuous periodic pattern was associated with failure-range falls in SpO2% to < 80%.
First vertical mark: challenge begun, followed by brief run of periodic breathing. 2nd vertical mark: quiet sleep. 6th vertical mark: RAC failure was declared. The smaller box is an enlarged 30 sec excerpt from between vertical marks 5 and 6.
Because caffeine treatment may limit periodic breathing, we assessed the impact on PB of caffeine use, retrospectively. Six infants were treated with caffeine at the time of the challenge (12.2% of subjects); 4 of 6 passed the RAC without any PB; the 2 failing while on caffeine had 33% and 41% increases in the % of time with PB, but the caffeine concentrations were not measured. Thus, while only 12.2% of subjects were receiving caffeine at the time of the challenge, they accounted for 33.0% of those passing (4 of 12).
The number of days since caffeine had been discontinued was not different among infants failing without periodic breathing, passing the RAC, or failing with increased periodic breathing (Kruskal-Wallis, p = 0.73, medians, 11.2 days, 11.6 days, and 10.7 days without caffeine at the time of challenge).
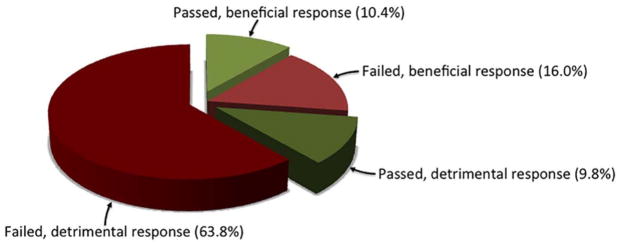
Seven of 37 infants failing the challenge had an increase in QDC-calibrated minute ventilation during challenge, with 3 of 12 infants passing the challenge also showing increased minute ventilation. (Table 2, figure 3)
TABLE 2.
Comparison of changes in minute ventilation and changes in percent of sleep time with periodic breathing between those passing and those failing the physiologic challenge
| Pass (n=12) | Fail (n=37) | |
|---|---|---|
| Minute volume increased | 3 | 7 |
| Periodic breathing | ||
| During run-in | 0 | 3 |
| Increased during Challenge | 1 | 16 |
| None or reduced during Challenge | 11 | 21 |
Figure 3.
Prevalence of changes in minute ventilation and respiratory pattern among infants receiving the physiologic challenge. The majority of infants (73.6%), whether they passed or failed, either developed PB or did not increase their minute ventilation, or both, in response to the challenge. Potentially beneficial response defined as increase in minute ventilation and no periodic breathing. Potentially detrimental, or dysfunctional, response is no change or reduction in minute ventilation, much periodic breathing, or combination of failure to increase minute ventilation while having periodic breathing.
Analysis of the impact of birth weight, gestational age, PMA, gender, and level of support on rates of passing and failing the challenge is best left to evaluation of the much larger sample from the multi-center PROP cohort. The size of our single center sample did not permit sufficient statistical power for subgroup comparisons. In our sample of only 49 subjects, however, those passing did not differ statistically from those failing by birth weight (p=0.09), gestational age (p=0.51), the diagnosis of intraventricular hemorrhage using ultrasonography (Chi square with Fisher exact test, p = 0.32), PMA (p = 0.60), gender (Chi square, p = 0.62) or FiO2 (p = 0.30). Those failing had higher flow rates prescribed before the challenge started (0.4 vs. 1.1 lpm, p = 0.01). Sixteen infants failed with increased periodic breathing; the subgroup failing at 36 weeks PMA with more PB had slightly lower mean gestational age than those 12 infants who passed the RAC, but this did not reach statistical significance (p = 0.10, 95% confidence limits of difference, −1.8 weeks to 0.2 weeks). To the extent that PB leads to clinically recognized apnea, these findings are consistent with a later resolution of apnea among infants born earlier.22
Although none of the infants was intubated or receiving mechanical ventilatory support at 36 weeks PMA, they are likely at considerable risk for CLD (Table 1). The median duration for mechanical ventilation was 12.0 days. Many also had marked RC-ABD dyssynchrony with a mean ϕ in the range described for preterms with BPD.20
DISCUSSION
In this study, we show that a large proportion of premature infants who meet clinical criteria for CLD, defined by supplemental oxygen or augmented airflow use at 36 weeks PMA, had unstable control of breathing that was unmasked during the physiologic challenge. Unstable ventilatory control may contribute to the inability of the clinical teams to further decrease the flow or FiO2. Specifically, we show that 32.7% of the infants studied treated with supplemental oxygen and airflow support at 36 weeks PMA need nasal cannula support at least in part due to periodic breathing and periodic apnea, and only 16.0% of infants who failed the challenge demonstrated a potentially beneficial response to hypoxemia by increasing minute ventilation. Furthermore, we show that 24.5% of infants receiving supplemental oxygen and airflow via nasal cannula at the time of the challenge were able to tolerate room air in the short term, using PROP criteria.14,23
These results have important implications for study design and subject selection for future investigations that attempt to define the pathophysiological bases of CLD phenotypes. Within PROP, which is designed to identify clinical risk factors and candidate biomarkers for CLD among infants born between 24 and 28-weeks PMA, we suspect that the current clinical definition of CLD will complicate our studies, because the need for supplemental oxygen support at 36 weeks PMA may be multifactorial and reflect an interaction between parenchymal dysfunction due to lung disease and immature respiratory control. Infants with significant CLD may also be particularly susceptible to developing PB when they become hypoxemic with even brief central apneas.24,25 Some of the infants studied had marked chest wall dyssynchrony (Table 1) in addition to requiring supplemental O2 suggesting that significant respiratory system compromise contributed to their propensity for desaturation during PB. Some infants meeting clinical criteria for CLD did in fact tolerate room air when evaluated using a formal protocol, while others showed an immature respiratory pattern such as PB that worsened during hypoxemia but that can be corrected with supplemental oxygen. A more physiologic definition of CLD is needed to ensure that mechanistic biomarkers being evaluated are actually linked to parenchymal lung disease, and to distinguish these infants from those that are otherwise well, or have hypoxemia for different reasons.2
Correction of respiratory pattern with supplemental O2 may be particularly applicable to infants born at the earliest gestational ages who otherwise would be expected to have higher rates of CLD.
Based on published reports,23 we expected that some subjects would pass RAC and thus not have CLD as currently defined, which was the case (24.5% passed RAC). However, the high frequency of challenge failures in infants who developed periodic apnea with characteristic oscillations in oxyhemoglobin saturations was not anticipated, even though supplemental oxygen therapy has been shown to stabilize breathing patterns in preterm infants who have PB.26,27 Thus, although the association of PB and hypoxemia is established, the frequency with which PB might contribute to the need for supplemental O2 among a group of infants at risk for CLD has not previously been quantified.
In this regard, it is likely (Figure 2 with oscillations) that observations made at the crib side, even by experienced nurses and clinicians, do not reliably identify infants that have altered breathing pattern causing hypoxemia, leading to under recognition of this phenomenon in the intensive care nursery. The frequent occurrence of intermittent hypoxemia during PB, but before physiologic challenge criteria for sustained hypoxemia were met, is demonstrated in the first minutes of the tracing in Figure 2. RIP scalar tracing allowed us to continuously capture respiratory patterns clearly over many minutes, and provided a record of respiratory pattern that can quickly be reviewed.
Five of our 52 subjects potentially eligible for the challenge study displayed PB while on their prescribed support, and 2 of these 5 infants failed to maintain passing SpO2% during the run-in period. A limitation of this study is that we did not record respiratory pattern for longer periods before reducing supplemental oxygen support, and it is possible that other infants in our cohort may have substantial PB on prescribed support that was not captured before the challenge.
We speculate that our findings may be relevant to the thus far unexplained observation from a large study showing that prior therapy with caffeine reduced the rate of BPD among treated premature infants.28,29 A plausible mechanism for the reduced need for supplemental oxygen in the group randomized to caffeine therapy before 36 weeks PMA would be that caffeine reduced rates of periodic breathing and apnea in these subjects, perhaps by accelerating the development of stable respiratory control.30–33
Other sites within the PROP collaborative did not routinely record respiratory pattern using RIP during the challenge, but it would be surprising if our findings were not generalizable and applicable to the larger cohort, given the clear propensity of premature infants to develop PB10–13,21,22
In conclusion, we want to emphasize that unstable control of breathing is common in premature infants who met clinical criteria for CLD. Unstable respiratory patterns may occur alone or in conjunction with airways and airspace diseases in many infants born between 24 and 28-weeks gestation, and contribute to the supplemental oxygen requirement at 36 weeks PMA. The role of PB and immature breathing patterns must be considered if we are to better understand the real contribution of lung disease to hypoxemia in premature infants.
What is known about this topic
Infants born prematurely are said to have chronic lung disease if they require supplemental oxygen at 36 weeks post-menstrual age. These infants also sometimes have unstable respiratory patterns that can cause hypoxemia and that can be made more stable by treating them with oxygen.
What this study adds
This study quantifies the prevalence, in a small cohort, of the potential contribution of an unstable respiratory pattern to the need for supplemental oxygen. It shows that a substantial minority of infants with presumed chronic lung disease who cannot be weaned to room air during a physiologic challenge fail the challenge after developing periodic breathing.
Acknowledgments
Financial support: The authors were supported by the National Institutes of Health (NIH) award HL101465, which supports the Prematurity and Respiratory Outcomes Project (PROP). The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. government.
Glossary
- List of Abbreviations
CLD, chronic lung disease
- NICU
Neonatal intensive care unit
- FiO2
fraction of inhaled oxygen
- lpm
liters per minute air flow rate
- PB
periodic breathing
- PMA
post-menstrual age
- PROP
Prematurity and Respiratory Outcomes Project
- RAC
room air challenge
- RIP
respiratory inductance plethysmography
Footnotes
Conflicts of interest: The authors have no other financial relationships or other conflicts of interest that compromise, or may be perceived to compromise, the integrity of the observations herein.
References
- 1.Northway WH. Prologue: Advances in Bronchopulmonary dysplasia [Editorial] Semin Fetal Neonatal Med. 2009;14:311. doi: 10.1016/j.siny.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–72. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 5.American Lung Association. Lung Disease Data. 2008 www.lungusa.org.
- 6.Walsh MC, Wilson-Costello D, Zadell A, et al. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 7.Bancalari EH, Jobe AH. The respiratory course of extremely preterm infants: a dilemma for diagnosis and terminology [Editorial] J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.05.054. 161-585-8. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43:937–44. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- 9.Al-Matary A, Kutbi I, Qurashi M, Alvaro R, et al. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol. 2004;28:264–72. doi: 10.1053/j.semperi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Waggener TB, Frantz ID, Cohlan BA, Stark AR. Mixed and obstructive apneas are related to ventilatory oscillations in premature infants. J Appl Physiol. 1989;66:218–26. doi: 10.1152/jappl.1989.66.6.2818. [DOI] [PubMed] [Google Scholar]
- 11.Simakajornboon N, Beckerman RC, Mack C, et al. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics. 2002;110:884–89. doi: 10.1542/peds.110.5.884. [DOI] [PubMed] [Google Scholar]
- 12.Hoppenbrouwers T, Hodgman JE, Harper RM, et al. Polygraphic studies of normal infants during the first six months of life. III Incidence of apnea and periodic breathing. Pediatrics. 1977;60:418–425. [PubMed] [Google Scholar]
- 13.Miller MJ, DiFiore JM. A comparison of swallowing during apnea and periodic breathing in premature infants. Pediatr Res. 1995;37:796–9. doi: 10.1203/00006450-199506000-00020. [DOI] [PubMed] [Google Scholar]
- 14.NIRA MOP: RAC protocol www.propstudy.org.
- 15.Prechtl HFR. The behavioural states of the newborn infant. Brain Research. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- 16.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–240. [PubMed] [Google Scholar]
- 17.Allen JL, Sivan Y. In: Measurements of chest wall function in Infant Respiratory Function Testing. Stocks J, Sly PD, Tepper RS, Morgan WJ, editors. John Wiley and Sons, Inc; New York: 1996. pp. 344–349. [Google Scholar]
- 18.Adams JA, Zabaleta IA, Stroh D, et al. Tidal volume measurements in newborns using respiratory inductance plethysmography. Am Rev Respir Dis. 1993;148:585–8. doi: 10.1164/ajrccm/148.3.585. [DOI] [PubMed] [Google Scholar]
- 19.Ulm LN, Hamvas A, Ferkol TW, et al. Sources of methodological variability of phase angle from respiratory inductance plethysmography among preterm infants. Ann Amer Thor Soc. 2014;11:753–60. doi: 10.1513/AnnalsATS.201310-363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren RH, Horan SM, Robertson PK. Chest wall motion in preterm infants using respiratory inductive plethysmography. Eur Respir J. 1997;10:2295–2300. doi: 10.1183/09031936.97.10102295. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus SM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012. p. 47. www.aasmnet.org. [Google Scholar]
- 22.Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354–9. doi: 10.1542/peds.100.3.354. [DOI] [PubMed] [Google Scholar]
- 23.Kaempf JW, Campbell B, et al. PCO2 and room air saturation values in premature infants at risk for bronchopulmonary dysplasia. J Perinatol. 2008;28:48–54. doi: 10.1038/sj.jp.7211859. [DOI] [PubMed] [Google Scholar]
- 24.Tourneux P, Leke A, Kongolo G, et al. Relationship between functional residual capacity and oxygen desaturation during short central apneic events during sleep in “late preterm” infants. Pediatr Res. 2008;64:171–6. doi: 10.1203/PDR.0b013e318179951d. [DOI] [PubMed] [Google Scholar]
- 25.Difiore JM, Martin RJ, Gauda EB. Apnea of prematurity – perfect storm. Respir Physiol and Neurobiol. 2013;189:213–22. doi: 10.1016/j.resp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Poets CF, Southall DP. Patterns of oxygenation during periodic breathing in preterm infants. Early Hum Dev. 1991;26:1–12. doi: 10.1016/0378-3782(91)90038-5. [DOI] [PubMed] [Google Scholar]
- 27.Weintraub Z, Alvaro R, Kwiatkowski K, et al. Effects of inhaled oxygen (up to 40%) on periodic breathing and apnea in preterm infants. J Appl Physiol. 1992;72:116–20. doi: 10.1152/jappl.1992.72.1.116. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 29.Bancalari E. Caffeine for apnea of prematurity [Editorial] N Engl J Med. 2006;354:2179–81. doi: 10.1056/NEJMe068028. [DOI] [PubMed] [Google Scholar]
- 30.Montandan G, Kinkead R, Bairam A. Adenosinergic modulation of respiratory activity-developmental plasticity induced by perinatal caffeine administration. Resp Physiol Neurobio. 2008;164:87–95. doi: 10.1016/j.resp.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Resp Physiol Neurobio. 2013;185:144–55. doi: 10.1016/j.resp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164:992–8. doi: 10.1016/j.jpeds.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhein LM, Dobson LR, Darnall RA, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014;168:250–7. doi: 10.1001/jamapediatrics.2013.4371. [DOI] [PubMed] [Google Scholar]