Abstract
Benign prostatic hyperplasia (BPH) and associated lower urinary tract symptoms (LUTS) are common clinical problems in urology and affect the majority of men at some time during their lives. The development of BPH/LUTS is associated with an increased ratio of estrogen to androgen levels, and this ratio, when mimicked in a variety of animals, induces BPH and lower urinary tract dysfunction (LUTD). While the precise molecular etiology remains unclear, estrogens have been implicated in the development and maintenance of BPH. Numerous endogenous and exogenous estrogens exist in humans. These estrogens act via multiple estrogen receptors to promote or inhibit prostatic hyperplasia and other BPH-associated processes. The prostate is an estrogen target tissue, and estrogens directly and indirectly affect growth and differentiation of prostate. The precise role of estrogen action directly affecting prostate growth and differentiation in the context of BPH is an understudied area and remains to be elucidated. Estrogens and selective estrogen receptor modulators (SERMs) have been shown to promote or inhibit prostate proliferation illustrating their potential roles in the development of BPH as therapy. More work will be required to identify estrogen signaling pathways associated with LUTD in order to develop more efficacious drugs for BPH treatment and prevention.
Keywords: Benign prostatic hyperplasia, Selective estrogen receptor modulators, Lower urinary tract dysfunction, Treatment
Background
Benign prostatic hyperplasia (BPH) and the bothersome lower urinary tract symptoms (LUTS) that accompany it are some of the oldest clinical entities in urology. With advancing age, virtually all men will develop histologic evidence of BPH. A meta-analysis of autopsy studies demonstrated the ubiquity of BPH histology: while the prevalence in men 51–60 years old is 42±9.7%, this increases steadily with advancing decades to 88±10.9%in men 81–90 years [1]. LUTS are also common in men and typically worsen with age. Among men older than 65 years, one third with no or mild symptoms will develop clinically significant LUTS within 2 years [2]. For those men who already have moderate to severe symptoms, one quarter will experience symptom progression [2]. BPH-LUTS have a major impact on the lives of men and their families and represent a significant financial burden on the healthcare system, with direct costs estimated at 3.9 billion dollars annually [3].
Classically, BPH is thought of as new glandular and stromal growth in the transition zone, which leads to benign enlargement of the prostate and causes bladder outlet obstruction (BOO). As such, LUTS are often due to obstruction from the enlarged prostate. There is increasing recognition that age-related declines in detrusor function, neurologic control of micturition, prostatic fibrosis, smooth muscle contractility, and other unknown factors also contribute to BOO and LUTS, perhaps independently of prostatic hyperplasia [4]. Moreover, common comorbidities of BPH, including obesity, hypertension, diabetes, and metabolic syndrome, may also adversely affect voiding [5, 6]. Adding layers of complexity, there is likely a spectrum of BPH-LUTS disease and possibly distinct phenotypic and/or molecular subtypes of BPH. While not all male LUTS occur in the setting of BPH, benign enlargement of the prostate remains the most common cause of bladder outlet obstruction in men.
Modeling BPH
There has been substantial progress in medical and surgical management of BPH in the past century, but the underlying molecular etiology of this disease remains elusive. Our understanding of the critical and likely permissive role of androgens in the prostate has revolutionized the clinical approach to BPH. With contemporary medical management, including the use of 5-alpha reductase inhibitors (5ARI) [to block production of the potent androgen receptor ligand dihydrotestosterone (DHT) from testosterone (T)], for most men, BPH-LUTS is a chronic disease that can be managed medically. However, not all men with LUTS tolerate or improve with medical management, and some will progress despite treatment. In pioneering experiments, Walsh and Wilson demonstrated that estradiol (E2) acts in synergy with the DHT metabolite androstanediol to induce BPH in dogs [7]. Forty years of subsequent studies have confirmed the importance of estrogens in the development of BPH-LUTS, but the molecular basis for estrogen action remains unexplained. In part, detailed mechanistic studies of estrogen targets in BPH-LUTS have been limited by a lack of models that are both suitable for interrogating mechanisms and recreating the clinical aspects of BPH-LUTS. We recently developed a mouse model induced with a combination of testosterone (T) and E2 to mimic the increased estrogen to androgen ratio that develops with age in men. This model exhibits many clinical features of BPH, including urinary voiding dysfunction, bladder enlargement, new glandular prostatic growth, and BOO [8]. While this model is a logical extension of past BPH models, it utilizes a genetically tractable organism and offers a useful tool for investigating the underlying biology in BPH and is useful for testing novel treatment strategies. Since the publication of this work, several groups have presented different models for BPH. Cadmium, which may act via a steroid hormone receptor, induces a BPH-like state in the rat prostate [9]. Vignozzi et al. demonstrated that rabbits fed a high-fat diet to induce metabolic syndrome develop increased E2 to T ratio and lower urinary tract fibrosis, which is improved by exogenous T therapy [10]. Another mouse model of lower urinary tract dysfunction (LUTD) is the accelerated aging SAMP6 mouse fed a high-fat diet. These mice are diabetic and obese and, with time, develop LUTD [6]. Along with the male mouse treated with T+E2, these models are important tools for understanding the underlying biology of BPH, and for developing future treatment strategies.
A Contemporary Perspective on BPH
While estrogens may be important in BPH-LUTS, there is growing evidence that BPH-LUTS is a lower urinary tract manifestation of systemic metabolic derangement, which includes obesity, metabolic syndrome, and diabetes. In a recent epidemiologic study of 780 men, plasma E2 predicted progression of both storage and voiding symptoms as assessed by the American Urological Association Symptom Index [11]. A recent BPH case-control study showed that a gene polymorphism in the steriodogenic enzyme CYP17, which metabolizes and inactivates an estrogenic compound (3β-adiol), was associated with BPH [12•]. Greater abdominal fat and sleep apnea risk predicted progression of storage LUTS, underscoring the interaction of obesity with BPH-LUTS [11]. Obesity is a common comorbidity of BPH and also is an independent predictor of progression of LUTS [13]. Recent evidence shows that leptin, a hormone produced by adipocytes, stimulates proliferation of human BPH cells, and that this effect may be partially mediated by the direct effect of leptin on estrogen metabolism, as leptin induces aromatase expression [14•]. This provides yet another link of estrogens to BPH, suggesting that leptin might also be therapeutically targeted, perhaps with beneficial effects on obesity as well. Greater physical activity at baseline predicts improvement in storage and voiding symptoms in men, suggesting that lifestyle interventions might be a promising future strategy for BPH-LUTS and obesity [11]. The interaction of obesity, estrogens, and lower urinary tract dysfunction is an important area for future study.
More broadly, estrogens are important in a number of normal and pathologic processes in men. Estrogen deficiency, in addition to androgen deficiency, is an important contributor to age-related hypogonadism (declining T with age). Androgens regulate lean mass, muscle size, and strength; estrogen deficiency leads to fat accumulation and sexual function is regulated by both androgens and estrogens [15]. An important question for future research is which estrogen receptor (ER) mediates estrogen action in the maintenance of sexual function or adiposity.
Estrogens and Estrogen Receptors
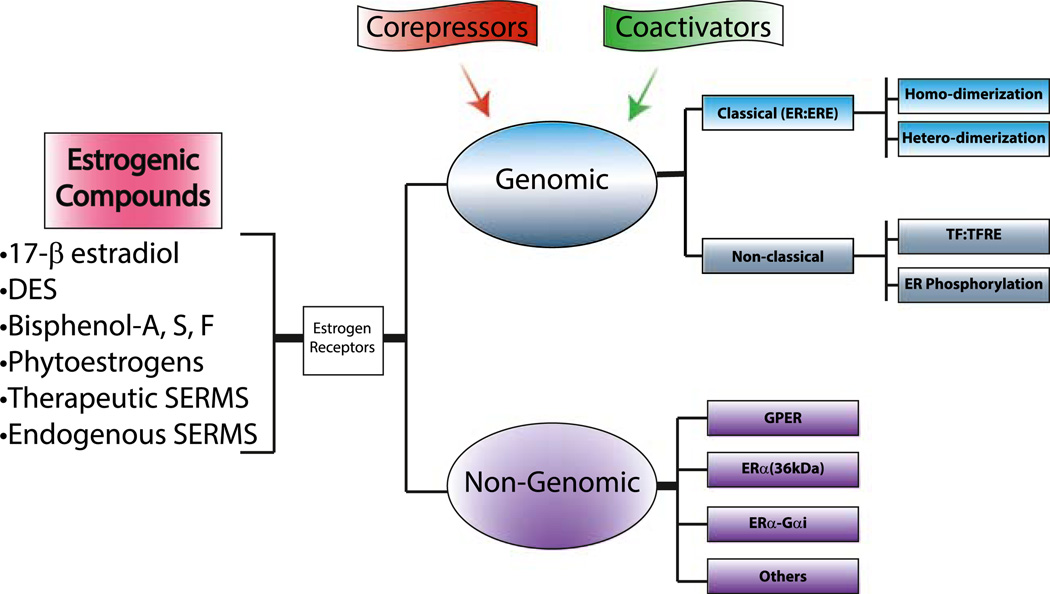
Estrogens are a broad term for compounds that activate receptors (i.e., the estrogen receptors [ERs]) for the major physiologic estrogen in higher organisms, 17β-estradiol (E2). However, in addition to E2, there are numerous estrogens found in the body (Fig. 1) including those that are endogenously produced, as well as those acquired from external sources. Estrogens from outside the body (xenoestrogens) can be derived from plant sources (e.g., phytoestrogens) or synthetic such as the many endocrine disruptors or environmental estrogens whose effects on human health have been widely debated (see below). Furthermore, a number of compounds can act either as agonists or antagonists for ERs depending upon the cell and tissue context. These selective ER modulators (SERMs) function by triggering unique conformations of ERs and thereby influence their interactions with specific partners including transcriptional coregulator proteins. Estrogens and SERMs have been shown to enhance or inhibit prostate proliferation and therefore could contribute to the pathogenesis of BPH [16].
Fig. 1.
Common estrogens and their receptors. Estrogens from a wide range of sources serve as ligands for a number of different estrogen receptors (ERs). Various estrogens can bind to ERs to facilitate genomic and non-genomic responses. Genomic ERs signaling can be enhanced or repressed through interactions with coactivators and corepresssors, respectively. Classical ER signaling occurs as a result of ERs binding directly to estrogen response elements (ERE) and nonclassical signaling results from ERs binding to other transcription factors (TF). Similar to classical ER signaling, these transcription factors bind to their own transcription factor response elements (TFRE). Non-genomic estrogen actions occur as a result of interactions with G protein-coupled estrogen receptor (GPER), ERα36, ERα-Gαi (ERα interactions with Gαi binding domain), or other unknown ER pathways. Whereas genomic signaling results in transcription which may take hours to observe biological responses, non-genomic generally causes rapid ER signaling within seconds to minutes
Environmental Estrogens
Estrogens can exhibit tumor promoter and/or suppressor activities, but their actions vary with the dose and the type of estrogenic agent used [17]. ERs have a reasonably high affinity for environmental estrogens such as bisphenol A (BPA), phthalates, pesticides, and polycyclic aromatic hydrocarbons [18]. These environmental estrogens are also known as endocrine disruptors because they can mimic and/or interfere with estrogen signaling in ER responsive tissues and organs. Bisphenol-A (BPA) has emerged as one of the most important and controversial environmental chemicals. It is well recognized as an endocrine disruptor, which is a substance with hormone-like action, or that affects the activity of naturally occurring hormones in the body. In 2010, Canada was the first country to formally designate BPA as a toxin. The European Union prohibited the use of BPA in baby bottles in 2011, and the US Food and Drug Administration (FDA) followed suit in July 2012. Despite these restrictions, BPA continues to be used in food packaging, certain plastics, and thermal ink paper products. Because the urinary and reproductive tract, and particularly the prostate, is acutely sensitive to BPA exposure during development [19], it is of great interest how BPA may influence prostate diseases in adulthood. Due to its estrogenic activity and ubiquitous exposure, BPA is likely an etiologic factor in BPH-LUTS, but more robust clinical and epidemiological studies are needed to establish this link [20, 21]. Understanding the role of BPA, as well as other estrogens, in the role of prostate diseases, may lead to regulatory changes regarding endocrine disruptor exposure and/or new treatment strategies targeting estrogen action.
While BPA is perhaps the most visible environmental estrogen, many other endocrine disruptors may influence BPH-LUTS. Bittner and colleagues have recently reported estrogenic activity in a range of plastic consumer products marketed to consumers as BPA-free [22]. Indeed, alternatives to BPA that are now used in polycarbonate plastic manufacture, bisphenol-S and bisphenol-F, also display in vitro estrogenic activity and affect T secretion by human fetal testes in a similar fashion to BPA [23]. Patients with BPH have higher levels of several endocrine disrupting organochloride pesticides, including pp-DDE and endosulfan-alpha compared to healthy age-matched controls [12•]. The potential risks to human health from BPA substitutes, and many other environmental endocrine disruptors, are largely unknown. BPA can bind to several kinds of receptors including the ER, AR, and aryl hydrocarbon receptor [24]. BPA has also been identified as a G protein-coupled estrogen receptor (GPER) agonist which suggests that BPA can signal through a non-genomic pathway to mobilize cellular responses to estrogen without initially affecting transcriptional responses of ERs [25]. BPA exposure has also been found to lower serum PSA levels in prostate cancer patients based on an assessment of urinary BPA levels. Bisphenol S (BPS), Tritan, polyethersulfone (PES), and polyethylene terephthalate (PET) were proposed as safer BPA alternatives [26]. Recent studies have stressed the importance of further analysis of these BPA alternatives as well as their metabolites based on the estrogenic potency associated with exposure to these compounds. BPS is considered by some an unacceptable BPA replacement because of its persistence in the environment compared to BPA [21]. In rodent studies, BPA-treated mice had a higher plasma E2/T ratio which is correlated with prostate disease [27]. Equally important BPA has been shown to increase the aromatase mRNA and protein levels in rodent prostates [27]. Phthalates have also been shown to have weak estrogenic activity and urinary excretions of metabolites are associated with adverse impact on the developing male reproductive system [28]. Not all environmental estrogens are associated with adverse effects in humans. For example, phytoestrogens derived from grains are presumed to have beneficial effects based on epidemiological studies. Phytoestrogens commonly act via the beta isoform of ERβ to elicit their effects [5]. Hence, ERβ-agonists may prove useful in the treatment of prostatic diseases.
Estrogen Receptors
For decades, ERα, a 66-kDa member of the nuclear receptor superfamily, was assumed to be the only ER [29] until 1995 when a second receptor for estrogens (i.e., ERβ) was discovered [29]. ERs (ERα and ERβ) have highly conserved sequence homology in their central DNA-binding domains (DBD) with less sequence conservation in their C-terminal ligand-binding domain (LBD). The NH2-terminal domains between the two receptors are variable. ERα and ERβ have similar affinities for E2 and they bind the same DNA response elements [18]. More recent genomic data show that ERα and ERβ can share many commonly regulated genes in a specific cell type but also regulate unique sets of target genes depending upon the ligand used to activate the receptor and the cell or tissue type [30].
ERs are expressed in the male lower urinary tract and reproductive tissues such as the bladder [31], prostate [31], urethra, and testis [32]. Human and rabbit bladders and prostates express ERα and ERβ [33, 31]. Classical estrogen signaling results from binding of an estrogen to an ER, dimerization of the ligand-bound receptors, followed by its association with target genes and recruitment of transcriptional coregulator proteins. The ensuing recruitment of many components of the transcription machinery, chromatin remodelers, and histone modifiers triggered by gene-specific binding of ERs impacts the efficiency of transcription from linked promoters (Fig. 1). ER dimers can also indirectly regulate transcription through interactions with other transcription factors such as AP1 and SP1, which bind to their own response elements but utilize the tethered ligand-bound to impart hormone-regulated transcription upon a subset of their direct target genes ultimately utilizing mechanisms outlined above.
Non-genomic ER Signaling
Non-genomic estrogen signaling involves rapid (i.e., within minutes), transcription-independent cellular responses including increased levels of intracellular calcium levels, production of cAMP, epidermal growth factor receptor (EGFR)-phosphorylation, and activation of MAPK/PI3K pathways [34]. Multiple studies have suggested the possibility that a specific 7-transmembrane G protein-coupled receptor GPER (aka: GPR30) is involved in one form of non-genomic estrogen signaling [35]. Currently, GPER function is implicated in reproductive, endocrine, urinary, nervous, immune, musculoskeletal, and cardiovascular systems [25, 35]. A generally accepted mechanism of non-genomic estrogen signaling includes E2 signaling through GPER to cause transactivation of EGFR after the protease-mediated release of tethered ligands [e.g., TGFα or heparin-bound EGF (HB-EGF)] promoting signal transduction activation of Erk-1/2 and MAPK pathways. Others have suggested GPER regulation via ERα-36, a 36-KDa-cell membrane associated form of ERα [36]. Finally, there is considerable evidence for a non-genomic role for intact ERs that localize to the plasma membrane [37, 38]. The role of non-genomic ER signaling in BPH/LUTS is largely unknown.
ER Action in BPH-LUTS
ERα and ERβ are detected in both the prostatic stroma and epithelium of BPH and normal prostate tissues. We previously investigated the tissue-based expression of ERα and AR in human BPH [39]. AR expression increased in BPH compared to normal prostate. ERα expression was also distinct in human BPH compared to normal prostate, with epithelial ERα increased and stromal ERα decreased. Moreover, cells expressing both receptors were more prevalent in human BPH, underscoring the increased hormone sensitivity of BPH. This work is important in establishing the relevance of investigating markers of interest in the human disease process prior to embarking on mechanistic studies with BPH-LUTD models. Using the male mouse treated with T+E2, we demonstrated that ERα, but not ERβ, is necessary for the development of urinary retention and bladder hypertrophy, common bladder complications of BPH [32]. In addition, ERα, but not ERβ, was necessary for hormonal induction of prostate growth in male mice treated with T+E2.
Many questions remain with regard to ERα action in BPH. For example, is stromal or epithelial ERα required for induction of BPH-LUTS in the mouse model? Using conditional ERαKO mice with selective loss of ERα in prostatic epithelium compared to selective loss of ERα in fibroblasts, it was demonstrated that epithelial, but not stromal, ERα is required for squamous metaplasia induced by diethylstilbestrol [40]. Although subtle, loss of stromal ERα had less branching morphogenesis compared to their WT littermates [40]. These results implicate that ERα expressed by stromal fibroblasts may play key role in prostate development, as well as in new glandular growth associated with BPH. In addition, it is also possible ERα expressed in other prostate stromal cell types (such as myocytes or myofibroblasts) could mediate the effects of estrogens in the induction of BPH. Collectively, these data suggest that targeting ERα may be an important aspect to treating BPH.
SERMs as Therapeutics for BPH-LUTS
Estrogens have long been implicated in BPH-LUTS, but no current therapies directly target estrogen action. We recently tested the SERMs raloxifene and tamoxifen for prevention of bladder complications in male mice treated with T+E2. While raloxifene prevented both bladder enlargement and prostate growth, an ERβ antagonist did not [32]. These results support that ERα is both a key mediator and therapeutic target in BPH. There are currently at least five FDA-approved SERMs in clinical use, tamoxifen, raloxifene, clomiphene, fulvestrant, and toremifene. All of these drugs are used effectively as either selective activators or inhibitors of ERα-mediated transcription depending on the cell type or target tissue. Common side effects of SERMs are related to estrogen withdrawal such as vasomotor events (hot flashes), and these drugs carry a black box warning for increased risk of thromboembolic events, including deep venous thrombosis and pulmonary embolism. Despite these risks, because these are already FDA-approved medications, these drugs are attractive candidates for translation into ERα antagonist therapies for BPH/LUTS.
Tamoxifen was the first SERM to reach the market and is FDA approved for adjuvant breast cancer therapy and risk reduction in pre- and postmenopausal high-risk women [41, 42]. It is also approved for ER-positive breast cancer treatment in men and appears to be well tolerated in this patient population. Tamoxifen has an estrogen agonist activity in the endometriumand therefore carries increased risk for endometrial hyperplasia and carcinoma, which is not the case for the second-generation SERM, raloxifene. Raloxifene is approved for treatment of osteoporosis and for reduction in the risk of invasive breast cancer in postmenopausal women [41]. In a small cohort of elderly men, raloxifene was well tolerated but did not affect markers of bone turnover or lipid levels [43]. While we did not compare efficacy of raloxifene and tamoxifen in BPH prevention directly, we demonstrated that raloxifene decreased both bladder and prostate mass in the male mouse treated with T+E2 [32]. It is unknown whether treatment with raloxifene will reverse BPH-LUTS, once established, in animal models. It also remains unknown whether raloxifene inhibits the growth of human BPH, and this is an important area for future investigation.
Clomiphene is a SERM approved for ovulatory dysfunction in women. Because it acts as an antagonist at hypothalamic and pituitary ERs, clomiphene is also used off-label to stimulate gonadotrophin release in men with hypogonadotrophic hypogonadism. In these men, clomiphene treatment induces increased serum T, E2, and luteinizing hormone; improves serum T to E2 ratio; increases bone mineral density; and improves symptoms of hypogonadism [44–46]. While clomiphene therapy achieves lower serum T compared to exogenous T supplementation via patches or gels, patients using both treatment strategies report similar levels of satisfaction [47]. While it is currently unknown whether clomiphene therapy improves spermatogenesis in this population, exogenous T should be avoided in men desiring future fertility due to suppression of the hypothalamic pituitary testis axis and consequent inhibition of spermatogenesis. A pure stereoisomer of clomiphene, enclomiphene, is currently in phase III trials for use in men [48]. In a small proof of concept study, enclomiphene improved sperm counts following 3 and 6 months of treatment [49••]. An unanticipated beneficial effect of enclomiphene in hypogonadal men is an improvement in fasting glucose. Given the demonstrated efficacy in improving serum T in hypogonadal men, clomiphene or enclomiphene would be especially attractive as personalized therapies for men with comorbid hypogonadism and BPH-LUTS. Much of the current literature on the use of clomiphene pertains to men of reproductive age; it remains unknown whether clomiphene or enclomiphene therapy in older men with hypogonadism and BPH-LUTS will also improve urinary function.
Toremifene is FDA-approved for use in metastatic breast cancer in women. It has also been evaluated for treatment of side effects of androgen deprivation therapy in men with metastatic prostate cancer (PrCa) and has been shown to decrease the incidence of pathologic fractures, improve vasomotor symptoms, prevent gynecomastia, and improve lipid profiles in this population [50]. More recently, toremifene was tested for the prevention of PrCa in men with high-grade prostatic intraepithelial neoplasia [51]. While this recent trial did not demonstrate efficacy in PrCa-free survival, few side effects were observed in this patient population, supporting the concept that SERMs, if used to treat BPH/LUTS, would be well tolerated by men.
We have previously demonstrated that raloxifene prevents bladder complications and prostate growth in male mice treated with T+E2 [32]. Because BPH is a disease process that likely begins in the fourth and fifth decades of life [1], potentially decades earlier than the development of LUTS, using a SERM as a preventative measure may prove challenging. Many natural compounds (so-called nutraceuticals) have estrogenic/SERM activity, and the use of herbal supplements for BPH has gained mass appeal. However, despite considerable promise, nutraceuticals have overall not been successful as BPH-LUTS therapies. A meta-analysis of saw palmetto (an extract of Serenoa repens) trials showed no benefit over placebo in reducing LUTS [52] or PSA [53], and beta-sitoserol has also proven to have limited benefits. Likewise, there is no demonstrated benefit of Serenoa repens, over placebo in improving LUTS [54]. Perhaps the most promising Bnutritional^ therapy for BPH consists of less animal protein, less simple carbohydrates, and more vegetables, which would likely also be helpful in mitigating obesity and diabetes, two common comorbid conditions [55].
Future Work and Questions in the Field
The era of SERMs as BPH-LUTS therapies has arrived, and these agents are being tested in clinical trials [56]. Given the role of ERβ as a Bbrake^ to prostatic proliferation, one therapeutic strategy that has been advanced for BPH is the use of an ERβ agonist. A 2014 trial tested the efficacy and safety of the ERβ agonist LY500307 in a phase II trial of patients with BPH-LUTS [57••]. The primary endpoint in this study was improvement in LUTS (measured with International Prostate Symptom Score), and it was powered to detect a 20 % decrease in total prostate volume. Secondary endpoints included total prostate volume, PSA, and peak urinary flow rate. The medication was well tolerated, with comparable adverse events, but the trial was terminated early due to failure to affect either primary endpoints or secondary efficacy measures. Questions still remain to be answered regarding this study (and similar types of clinical trials). Specifically, was the ERβ ligand dose at the prostate level optimal? In addition, was prostatic hyperplasia or apoptosis affected? Finally, was there an effect on prostatic fibrosis, smooth muscle contractility, or innervation? These questions are unattainable as the tissues are not commonly collected in this type of clinical trial. Moreover, numerous questions remain with selective ERβ agonists (i.e., SERβMs) and their potential ability to treat prostatic diseases like BPH. Should they be used in men with LUTS but have prostates that are small to average size (i.e., <30 ml)? Should they be used in combination with ERα antagonists? Furthermore, would SERβMs combined with 5ARI, PDE5a1-inhibitors, antihypertensive agents, or alphablockers synergize to ameliorate LUTS.
Our knowledge of estrogen biology in BPH-LUTS is still rather limited, with many fundamental questions remaining to be addressed. Is there an estrogen responsive subtype of BPH? Do estrogens directly impact bladder function, either via bladder ERs or innervation of the bladder, independent of the effects on prostate? Now that we have a better understanding of the critical roles of estrogens in sexual function and, potentially, dysfunction, is it a good idea to use SERMs as BPHLUTS therapeutics in men? Thesemedications are not without risks, and their long-term consequences are not yet understood.
Conclusions
Despite decades of scientific inquiry, we still remain confronted by one fundamental question: why does the prostate, which should remain growth quiescent after puberty, start growing again in middle age? Estrogens are undoubtedly important in a large number of normal and pathologic processes in men. We do not fully understand the potential role that estrogen-mimicking chemicals in our environment, such as bisphenol-A, may play in urologic diseases such as BPH. Taken with the findings of others, the present work implicates ERα as a key mediator, and potential therapeutic target in BPH while the role of non-genomic estrogen receptor signaling requires further investigation.
Acknowledgments
We would like to thank the NIH, NIDDK, NIGMS, and NIEHS for their financial support for these studies: U54DK104310, R01DK093690, RC2ESO18764 (W.A.R.). J.L.W. is a trainee in the University of Wisconsin Molecular and Environmental Toxicology Training Program NIEHS T32ES007015. T.M.N. is a trainee in the Medical Scientist Training Program at the University of Rochester funded by NIH T32 GM07356 and F30DK093173. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Jalissa L. Wynder, Tristan M. Nicholson, Donald B. DeFranco, and William A. Ricke each declare no potential conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JK, Wilt TJ, Wang PY, et al. Progression of lower urinary tract symptoms in older men: a community based study. J Urol. 2010;183:1915. doi: 10.1016/j.juro.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173:1309. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev. 2013;9:546. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebastiano C, Vincenzo F, Tommaso C, et al. Dietary patterns and prostatic diseases. Front Biosci (Elite Ed) 2012;4:195. doi: 10.2741/369. [DOI] [PubMed] [Google Scholar]
- 6.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, et al. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate. 2013;73:1123. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh PC, Wilson JD. The induction of prostatic hypertrophy in the dog with androstanediol. J Clin Invest. 1976;57:1093. doi: 10.1172/JCI108353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson TM, Ricke EA, Marker PC, et al. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 2012;153:5556. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prajapati A, Rao A, Patel J, et al. A single low dose of cadmium exposure induces benign prostate hyperplasia like condition in rat: a novel benign prostate hyperplasia rodent model. Exp Biol Med (Maywood) 2014;239:829. doi: 10.1177/1535370214536118. [DOI] [PubMed] [Google Scholar]
- 10.Vignozzi L, Morelli A, Sarchielli E, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012;212:71. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- 11.Martin S, Lange K, Haren MT, et al. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. J Urol. 2014;191:130. doi: 10.1016/j.juro.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 12. Kumar V, Banerjee BD, Datta SK, et al. Association of CYP1A1, CYP1B1 and CYP17 gene polymorphisms and organochlorine pesticides with benign prostatic hyperplasia. Chemosphere. 2014;108:40. doi: 10.1016/j.chemosphere.2014.02.081. This study provides an appealing link between genetic and environmental factors in BPH susceptibility. These finding show that polymorphisms in CYP17 is associated with a increased risk of developing BPH.
- 13.Maserejian NN, Chen S, Chiu GR, et al. Treatment status and progression or regression of lower urinary tract symptoms in a general adult population sample. J Urol. 2014;191:107. doi: 10.1016/j.juro.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Habib CN, Al-Abd AM, Tolba MF, et al. Leptin influences estrogen metabolism and accelerates prostate cell proliferation. Life Sci. 2015;121:10–15. doi: 10.1016/j.lfs.2014.11.007. This manuscript observed that leptin stimulates proliferation of human BPH cells through a direct effect on estrogen metabolism. These findings offer a possible therapeutic target in future BPH studies.
- 15.Malnick S, Somin M, Goland S. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:2456. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihua Z, Lathe R, Warner M, et al. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 19.Prins GS, Hu WY, Shi GB, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007– 2013. Environ Health Perspect. 2014;122:775. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hormann AM, Vom Saal FS, Nagel SC, et al. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA) PLoS One. 2014;9:e110509. doi: 10.1371/journal.pone.0110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bittner GD, Yang CZ, Stoner MA. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ Health. 2014;13:41. doi: 10.1186/1476-069X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eladak S, Grisin T, Moison D, et al. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103:11. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Michalowicz J. Bisphenol A—sources, toxicity and biotransformation. Environ Toxicol Pharmacol. 2014;37:738. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JS, Choi J-S, Kim W-K, Lee Y-J, Park J-W. Estrogenic potency of bisphenol S, polyethersulfone and their metabolites generated by the rat liver S9 fractions on a MVLN cell using a luciferase reporter gene assay. Reprod Biol Endocrinol. 2014;12:102. doi: 10.1186/1477-7827-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro B, Sanchez P, Torres JM, et al. Bisphenol A exposure during adulthood alters expression of aromatase and 5alpha-reductase isozymes in rat prostate. PLoS One. 2013;8:e55905. doi: 10.1371/journal.pone.0055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethylhexyl) phthalate exposure—findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson GR, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 30.Madak-Erdogan Z, Charn TH, Jiang Y, et al. Integrative genomics of gene and metabolic regulation by estrogen receptors alpha and beta, and their coregulators. Mol Syst Biol. 2013;9:676. doi: 10.1038/msb.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comeglio P, Morelli A, Cellai I, et al. Opposite effects of tamoxifen on metabolic syndrome-induced bladder and prostate alterations: a role for GPR30/GPER? Prostate. 2014;74:10. doi: 10.1002/pros.22723. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson TM, Moses MA, Uchtmann KS, et al. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol. 2015;193:722–729. doi: 10.1016/j.juro.2014.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignozzi L, Rastrelli G, Corona G, Gacci I, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease. J Endocrinol Investig. 2014;37:313. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 34.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 35.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:360. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SL, Yan LY, Liang XW, et al. A novel variant of ER-alpha, ERalpha36 mediates testosterone-stimulated ERK and Akt activation in endometrial cancer Hec1A cells. Reprod Biol Endocrinol. 2009;7:102. doi: 10.1186/1477-7827-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin ER. Extranuclear estrogen receptor’s roles in physiology: lessons from mouse models. Am J Physiol Endocrinol Metab. 2014;307:E133. doi: 10.1152/ajpendo.00626.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q, Chambliss K, Lee WR, et al. Point mutations in the ERalpha Galphai binding domain segregate nonnuclear from nuclear receptor function. Mol Endocrinol. 2013;27:2. doi: 10.1210/me.2011-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson TM, Sehgal PD, Drew SA, et al. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;85:140. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M, Yeh CR, Chang HC, et al. Loss of epithelial oestrogen receptor alpha inhibits oestrogen-stimulated prostate proliferation and squamous metaplasia via in vivo tissue selective knockout models. J Pathol. 2012;226:17. doi: 10.1002/path.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doran PM, Riggs BL, Atkinson EJ, et al. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res. 2001;16:2118. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- 44.Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 45.Moskovic DJ, Katz DJ, Akhavan A, et al. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524. doi: 10.1111/j.1464-410X.2012.10968.x. [DOI] [PubMed] [Google Scholar]
- 46.Shabsigh A, Kang Y, Shabsign R, et al. Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med. 2005;2:716. doi: 10.1111/j.1743-6109.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramasamy R, Scovell JM, Kovac JR, et al. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy. J Urol. 2014;192:875–879. doi: 10.1016/j.juro.2014.03.089. [DOI] [PubMed] [Google Scholar]
- 48.Hill S, Arutchelvam V, Quinton R. Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men. Drugs. 2009;12:109. [PubMed] [Google Scholar]
- 49. Kaminetsky J, Werner M, Fontenot G, et al. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone: gel. J Sex Med. 2013;10:1628. doi: 10.1111/jsm.12116. This clinical study elegantly showed that oral enclomiphene citrate provides beneficial effects in hypogonal men. Improved levels of serum testosterone in hypogonadal men makes enclomiphene an attractive drug for personalized BPH therapy.
- 50.Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2013;189:S45. doi: 10.1016/j.juro.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Taneja SS, Morton R, Barnette G, et al. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J Clin Oncol. 2013;31:523. doi: 10.1200/JCO.2012.41.7634. [DOI] [PubMed] [Google Scholar]
- 52.Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andriole GL, McCullum-Hill C, Sandhu GS, et al. The effect of increasing doses of saw palmetto fruit extract on serum prostate specific antigen: analysis of the CAMUS randomized trial. J Urol. 2013;189:486. doi: 10.1016/j.juro.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald R, Tacklind JW, Rutks I, et al. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): an updated Cochrane systematic review. BJU Int. 2012;109:1756. doi: 10.1111/j.1464-410X.2012.11172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Espinosa G. Nutrition and benign prostatic hyperplasia. Curr Opin Urol. 2013;23:38. doi: 10.1097/MOU.0b013e32835abd05. [DOI] [PubMed] [Google Scholar]
- 56.Garg M, Dalela D, Dalela D, et al. Selective estrogen receptor modulators for BPH: new factors on the ground. Prostate Cancer Prostatic Dis. 2013;16:226. doi: 10.1038/pcan.2013.17. [DOI] [PubMed] [Google Scholar]
- 57. Roehrborn CG, Spann ME, Myers SL, et al. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis. 2015;18:43–48. doi: 10.1038/pcan.2014.43. This clinical study found that estrogen receptor beta agonist(LY500307) was well tolerated in LUTD patients up to 24 weeks. Unfortunately this study was terminated early but can still serve as motivation for additonal therapeutic SERM trials.