Abstract
Purpose
To demonstrate, in a proof-of-concept study, whether potentiating ipsilesional higher motor areas (premotor cortex and supplementary motor area) augments and accelerates recovery associated with constraint induced movement.
Methods
In a randomized, double-blinded pilot clinical study, 12 patients with chronic stroke were assigned to receive anodal transcranial direct current stimulation (tDCS) (n = 6) or sham (n = 6) to the ipsilesional higher motor areas during constraint-induced movement therapy. We assessed functional and neurophysiologic outcomes before and after 5 weeks of therapy.
Results
Only patients receiving tDCS demonstrated gains in function and dexterity. Gains were accompanied by an increase in excitability of the contralesional rather than the ipsilesional hemisphere.
Conclusions
Our proof-of-concept study provides early evidence that stimulating higher motor areas can help recruit the contralesional hemisphere in an adaptive role in cases of greater ipsilesional injury. Whether this early evidence of promise translates to remarkable gains in functional recovery compared to existing approaches of stimulation remains to be confirmed in large-scale clinical studies that can reasonably dissociate stimulation of higher motor areas from that of the traditional primary motor cortices.
Keywords: Stroke rehabilitation, transcranial direct current stimulation, constraint-induced movement therapy, premotor cortex, motor recovery, transcranial magnetic stimulation
1. Introduction
More than 60% of patients with stroke experience chronic deficits of the paretic upper limb (Broeks, Lankhorst, Rumping, & Prevo, 1999). Deficits originate from damage to the motor cortices and their corticospinal tracts (CST) but are believed to persist due to an ensuing inter-hemispheric inhibitory imbalance (Cunningham et al., 2015; Murase, Duque, Mazzocchio, & Cohen, 2004). The excitability of the damaged ipsilesional motor cortices and their CST weakens because of an exaggerated, unopposed inhibition exerted by motor cortices of the contralesional hemisphere (Murase et al., 2004). This imbalance exaggerates as patients prefer to use the non-paretic limb in daily activities (Avanzino, Bassolino, Pozzo, & Bove, 2011). Therefore, to decrease the exaggerated inhibition, a focus in rehabilitation has been to force greater use of the paretic limb while discouraging use of the non-paretic limb (Murase et al., 2004). Even though impairments alleviate, gains with rehabilitative therapies still remain modest and variable (van der Lee, Beckerman, Knol, de Vet, & Bouter, 2004).
Stimulating the brain is a promising adjunct to maximize outcomes of rehabilitative therapies (Carey et al., 2008; Di Lazzaro et al., 2014; Halko et al., 2011; Hummel & Cohen, 2006a; Plautz et al., 2003; Plow, Carey, Nudo, & Pascual-Leone, 2009; Plow & Machado, 2014; Plow, Obretenova, Fregni, Pascual-Leone, & Merabet, 2012a; Plow et al., 2011; Plow, Obretenova, Jackson, & Merabet, 2012b; C. Rossi, Sallustio, Di Legge, Stanzione, & Koch, 2013; Sung et al., 2013; Talelli et al., 2012). To this end, stimulation is applied to facilitate the ipsilesional corticospinal axons and thus facilitating the ipsilesional primary motor cortex (M1) (Di Lazzaro et al., 2013) or indirectly facilitate it instead by suppressing its inhibitory homologue- the contralesional M1 (Fregni & Pascual-Leone, 2007; Nowak, Grefkes, Ameli, & Fink, 2009). Despite preliminary success, effectiveness of targeting M1 has been inconsistent –advantageous for rehabilitation in some trials (Bolognini, Pascual-Leone, & Fregni, 2009; Fregni & Pascual-Leone, 2007; Hummel & Cohen, 2006b) but inadequate in others (Bradnam, Stinear, Barber, & Byblow, 2012; Harvey, Winstein, & Everest Trial, 2009; Hesse et al., 2011; Malcolm et al., 2007; Nouri & Cramer, 2011; Plow et al., 2009; Plow, Cunningham, Varnerin, & Machado, 2015; Plow & Machado, 2014; C. Rossi et al., 2013; Seniow et al., 2012; Talelli et al., 2012). The inconsistencies are especially apparent when patients with more serious impairments are included (Hesse et al., 2011; Nouri & Cramer, 2011). However, there is evidence that the more impaired patients experience cortical plasticity in regions other than M1 (N. Ward, 2011), and thus, should be considered as an alternate cortical loci for stimulation.
One of most popular candidate includes the higher motor or the non-primary motor areas, such as the premotor cortex (PMC) and the supplementary motor area (SMA). These areas can assume the role of M1, and express plasticity in recovery especially with greater damage and impairment (Carey et al., 2002; Dancause & Nudo, 2011; Plow [nee (Bhatt)] et al., 2007; N. Ward, 2011; N. S. Ward, Brown, Thompson, & Frackowiak, 2003b; N. S. Ward et al., 2007). In fact, inactivation of these areas rather than of the ipsilesional M1 (Liu & Rouiller, 1999) reinstates motor deficits, suggesting their reorganization is causal, not epiphenomenal towards recovery (Fridman et al., 2004; Liu & Rouiller, 1999; Takeuchi, Tada, Chuma, Matsuo, & Ikoma, 2007; Zeiler et al., 2013). Their potential for plasticity emerges from key anatomic-physiologic abilities. 1) Higher motor areas are likely most spared in typical strokes affecting the middle cerebral artery because their medial regions are damaged primarily in anterior cerebral artery infarction, incidence of which is less than 3% (Gacs, Fox, Barnett, & Vinuela, 1983; Kang & Kim, 2008). In a recent report, Quinlan et al. 2015 show that damage to the PMC occurs in less than a third of patients, where M1 cortical damage occurs in greater than 50% of patients. 2) Higher motor areas constitute >60% of the frontal cortex and contribute 40%of the CST to the upper-limb, matching or exceeding contributions from M1. Thus, higher motor areas form direct, parallel contribution to control of the distal forelimb that is independent of M1 (Dum & Strick, 1991). Finally, 3) higher motor areas can be powerful at restoring the hemispheric balance since they share the most prominent callosal connections with M1 and homologues in the contralesional hemisphere (Marconi, Genovesio, Giannetti, Molinari,& Caminiti, 2003; Rouiller et al., 1994; Schubotz & von Cramon, 2003) by virtue of their role in complex, and bilateral tasks.
Given their potential to serve as alternate loci for plasticity, in a proof-of-concept study, we examined whether facilitating the ipsilesional higher motor areas with anodal transcranial direct current (tDCS) would confer any advantage to outcomes of constraint-induced movement therapy (CIMT) for patients with chronic stroke (Plow et al., 2015). In a sample that included mild as well as moderate- and severely-impaired patients, we examined advantages for function and patient-reported disability in using the paretic limb. To understand whether facilitating higher motor regions restores excitability of the ipsilesional hemisphere and reduces inhibition imposed by the contralesional hemisphere, weemployed transcranial magnetic stimulation (TMS).
2. Materials and methods
2.1. Subjects
We enrolled twelve patients (age: mean ± se: 61 ± 9 years) who were in the chronic phase of recovery (>6 months) following a first-ever ischemic or hemorrhagic stroke. Lesion locations were determined on T1-weighted images by a trained neurosurgeon (AM). Lesion volume was determined using MRIcro (http://www.mccauslandcenter.sc.edu/mricro) (Kalenine, Buxbaum, & Coslett, 2010; Zhu, Lindenberg, Alexander, & Schlaug, 2010). We included both, patients with even trace movement at fingers, thumb or wrist, as well as patients who were well recovered as long as they reported inadequate ability to use the paretic hand in daily life. We recruited patients with even severe impairments who generally do not qualify for CIMT so as to understand whether facilitating higher motor areas overrides detriments of serious damage. Exclusionary criteria related to contraindications of TMS (Nitsche et al., 2008; S. Rossi, Hallett, Rossini, & Pascual-Leone, 2009) and imaging (Plow (Bhatt) et al., 2007; N. S. Ward et al., 2007). Briefly, these included cardiac pacemaker, metallic implant in the head, seizures, and use of any neuro- or psychoactive medications (S. Rossi et al., 2009). The Institutional Review Board of the Cleveland Clinic approved the study. All subjects provided written informed consent.
2.2. Design
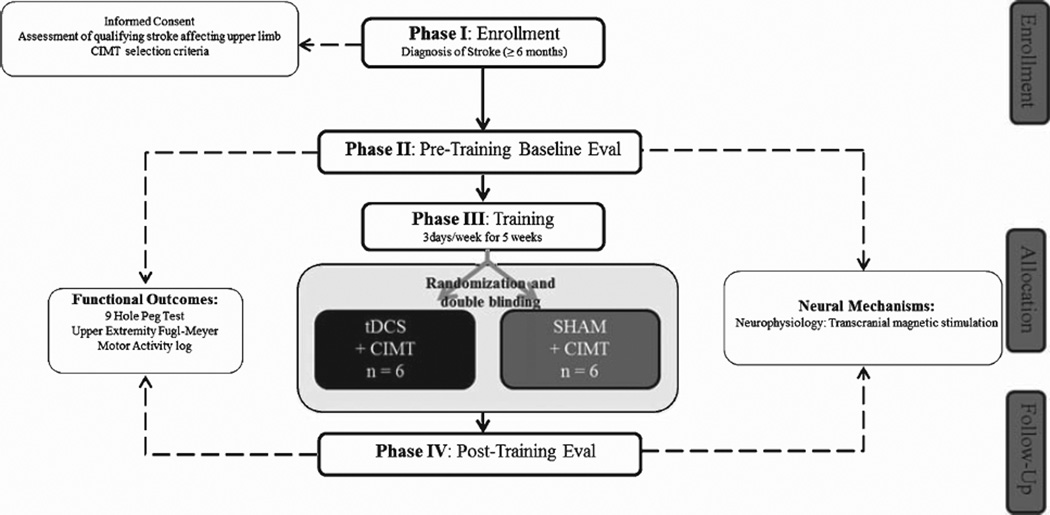
Using a single-center, randomized, double-blinded pilot clinical trial design (Plow et al., 2013) (http://clinicaltrials.gov/ct2/show/NCT01539096) (follow CONSORT diagram in Fig. 2), patients were assigned to receive tDCS to the ipsilesional higher motor areas in rehabilitation (tDCS+CIMT, n = 6) or sham tDCS in rehabilitation (sham+CIMT, n = 6). Outcomes and neural indices were collected at baseline and at posttest after 5 weeks of training. Rigor of blinding was confirmed at the end by asking both investigator and patients whether they believed they received tDCS or sham.
Fig. 2.
CONSORT diagram of patient flow through the study.
2.3. Rehabilitation
Both groups received CIMT (Taub, Uswatte, & Morris, 2003) for the paretic upper extremity with supervision from a physical therapist (EP). Intensive functional exercises were performed via a graded, regimented, feedback-driven approach. Patient-specific goals were emphasized. Rehabilitation was delivered for 30 min, twice a day, 3 times/week for 5 weeks (details (Plow et al., 2013)). A shortened protocol allowed us to deliver tDCS for duration considered safe in our previous work (Plow et al., 2012a). Although an even shorter protocol such as modified CIMT (Page, Levine, Leonard, Szaflarski, & Kissela, 2008) (30 min, 3 times/week for 10 weeks) would have sufficed, we anticipated compliance would be better if patients were required to come in for 15 sessions over 5 weeks rather than 30 sessions over ten. Patients were asked to restrain the non-paretic upper limb by placing it in a mitt for 2 hrs every weekday while performing home exercises. Exercise log was monitored at each session.
2.4. tDCS
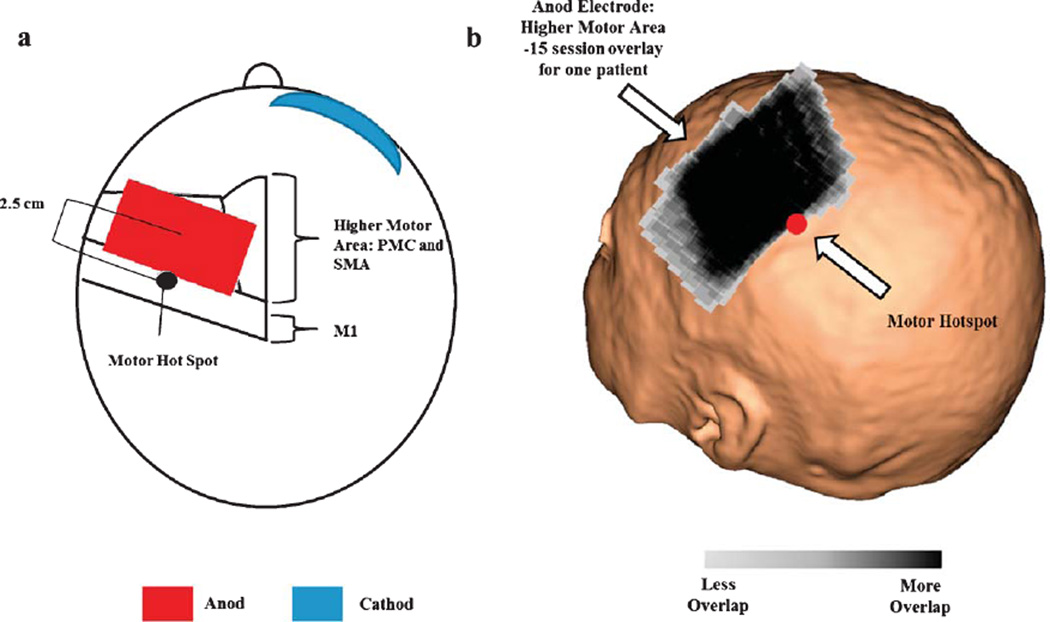
Details of tDCS are provided in Plow et al. 2013 Briefly, we applied tDCS using electrodes placed in saline-soaked sponges (5×7 sq. cm) of which the anodal electrode was applied to the scalp site corresponding to the higher motor cortices in the ipsilesional hemisphere, and the cathode was placed over the subraorbial area contralateral to the ipsilesional hemisphere (Soterix Medical Inc., NY) (Boros, Poreisz, Munchau, Paulus, & Nitsche, 2008). Higher motor areas were localized with stereotaxy utilizing patient’s MRI. Center of the anode was positioned ~2.5 cm anterior to a site in ipsilesional M1 that evoked the most optimal paretic hand movements with TMS (details below). In order to confirm that we were indeed placing the center of the electrode over the PMC, we transformed all patients MRI to Talairach space using AFNI (http://afni.nimh.nih.gov/afni/). Subsequently, we transformed the motor hotspot location as well as the center of the tDCS electrode to Talairach space. Across all patients, the motor hotspot resided in the primary motor cortex (x: 40.27 ± 4.1 y: 15.5 ± 4.1 z: 56 ± 4.6) and the center of the tDCS electrode resided in the premotor cortex (x: 37.1 ± 4.91 y: −6.91 ± 9.32 z: 52.6 ± 5.1). Previous work suggests this location targets dorsal PMC but is expected to affect SMA as well since it is only 1–1.5 cm medial (Boros et al., 2008). As such we label these areas together as ‘higher motor.’ Still, we emphasized localizing the dorsal PMC because it offers more CST (Dum & Strick, 1991). tDCS was delivered at a dose of 1mA for the entire duration of a rehabilitation session. For sham, current was turned on transiently (30–60 seconds) at outset to allow habituation to the sensation (Gandiga, Hummel, & Cohen, 2006).
2.5. Assessment of functional outcomes
Motor function was assessed using upper extremity Fugl-Meyer (UEFM), a measure of upper limb impairments (Page, Fulk, & Boyne, 2012) while dexterity was assessed using the nine-hole peg test (NHPT) (Zittel, Weiller, & Liepert, 2008). Motor Activity Log (MAL) recorded patient’s reported preferential use of the paretic limb over the non-paretic limb in daily life (details in Plow et al.) (van der Lee et al., 2004).
2.6. Assessment of neural indices
2.6.1. TMS
Single-pulse TMS (Magstim 2002, Wales, U.K) was delivered using a figure-of-eight coil (diameter 70 mm). Patient’s functional MRI activation (described in Cunningham et al., 2015) stereotactically guided TMS [BRAINSIGHT software (Rogue Research, Montreal, Canada)]. to M1. While patients rested their hands, TMS-evoked responses, called motor evoked potentials (MEP), were recorded using surface electromyography [Powerlab 4/25T (AD Instruments Inc. Colorado Springs, CO)] via bipolar electrodes (silver-silver chloride, 8 mm diameter) positioned over the contralateral first dorsal interosseous (FDI) muscle. A reference electrode was placed over the lateral epicondyle.
First, we identified the ‘hot spot’ i.e. the scalp site eliciting reliable criterion MEPs (≥50 µV in at least 3/5 trials) at the lowest stimulator intensity known as the resting motor threshold (rMT). We also defined active motor threshold (aMT); while patients contracted FDI at 15% of maximum voluntary contraction, we noted intensity required to evoke reliable criterion MEPs (≥200 µV in 3/5 trials). Map of corticospinal output devoted to the FDI was defined by delivering TMS pulses (5 each, @ 110% of rMT, randomized) to sites on a 5 × 7 grid (1 cm resolution centered on the hot spot). Finally, to study inter-hemispheric inhibition exerted mutually between both hemispheres, we examined ipsilateral silent period (iSP) exerted by each (Avanzino, Teo, & Rothwell, 2007; Harris-Love, Morton, Perez, & Cohen, 2011). While patients contracted their FDI muscle at 50% of maximum voluntary contraction [LabChart Pro (AD Instruments Inc. Colorado Springs, CO)], supra-threshold TMS pulses (15 at 150% rMT) were delivered to the hot spot in the hemisphere ipsilateral to the moving hand. Patients were provided with visual feedback to help maintain 50% contraction. Suppression of ongoing EMG was termed as iSP We used iSP instead of a commonly used bi-hemispheric TMS technique that labels suppression of an evoked MEP as inter-hemispheric inhibition. Our choice was guided by evidence and our experience that patients with stroke especially ones who are severely impaired do not reliably elicit MEPs in the paretic hand (Harris-Love et al., 2011). Since iSP instead captures inter-hemispheric inhibition as suppression of ongoing voluntary muscle activity, it is typically more feasible for patients to elicit.
2.7. Data analysis
Change in UEFM and NHPT indexed recovery of motor function, while change in MAL signified perceived disability in use of the paretic limb. We tested excitability of ipsilesional and contralesional M1 as rMT and aMT (Siebner & Rothwell, 2003). Maps were analyzed by including sites eliciting MEPs >30 µV (3 out of 5 trials). Map size was defined as the number of qualifying sites, denoting representation of CST devoted to the FDI. ISP was described as TMS-induced suppression of ongoing EMG of the contracting muscle (Chen, Yung, & Li, 2003; Harris-Love et al., 2011). Onset of iSP was defined when the EMG fell below 1 standard deviation of the pre-stimulus EMG for 5 ms, while offset was defined when it returned to within 1 standard deviation for 5ms (Chen et al., 2003). We computed inter-hemispheric inhibition as the percentage decrease in mean EMG relative to pre-stimulus EMG (Equation 1).
| Equation 1 |
3. Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (v18, SPSS Inc., Chicago, IL). The present study was meant to serve as a pilot exploratory study investigating the advantage of combining stimulation of higher motor areas with CIMT. As such, due to the small sample size of our groups we assessed between-group differences using Mann-Whitney U and within-group change (pre to post) using Wilcoxon signed rank test. Further, we determined the overall effect size of tDCS+CIMT and sham+CIMT using Cohen’s d (mean change divided by pooled standard deviation). According to Cohen’s criteria, d = 0.2 to 0.5 is small, 0.5 to 0.8 is moderate, and >0.8 is large. All correlation analyses were conducted using Spearman’s correlations. All tests were 2-tailed at α level of significance set at 0.05.
4. Results
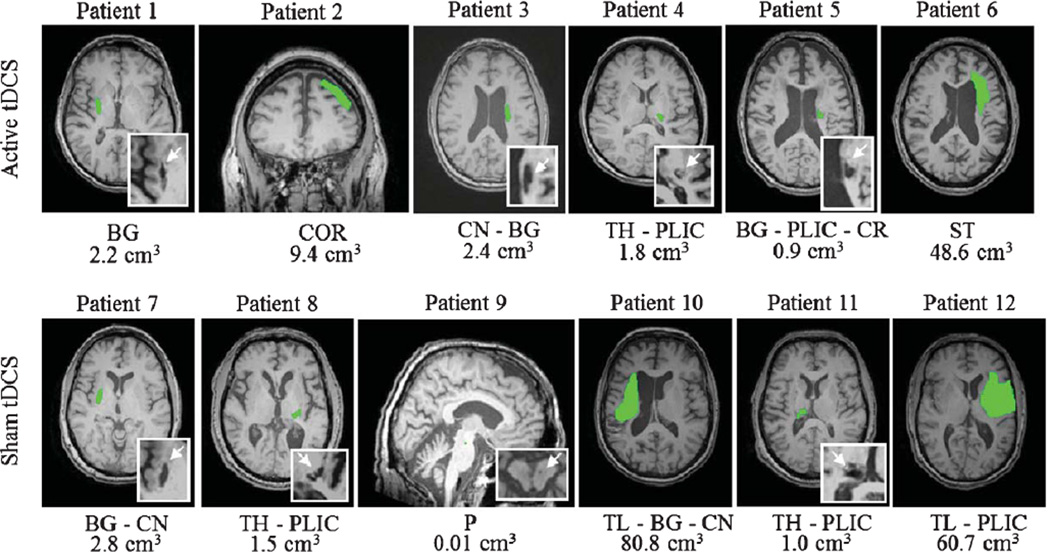
Neither investigator nor patients were able to correctly identify the assigned group of the patients. Patient characteristics are listed in Table 1. Each group comprised of 6 patients (tDCS+CIMT: age 64 ± 8; sham+CIMT: 59 ± 10). The mean baseline UEFM scores ranged from 15–53 for the active tDCS group and 26–59 for the sham tDCS group. There was no significant difference between age (z=−0.641, p= 0.589) and baseline UEFM between groups (z = −1.04, p = 0.297). Structural brain images are shown in Fig. 1. The lesion location of our patients were comprised of 8 with subcortical, 2 with subcortical-cortical, 1 with cortical and 1 with brainstem stroke. Using stereotactic navigation, the center of the anode electrode was on average placed 26.78 ± 8.6 mm from the hotspot across all patients. Figure 3 shows the anodal electrode overlay of 15 sessions for one patient. All patients were able to successfully participate and complete therapy.
Table 1.
Patient demographics
| Patient | Age/Gender/ Affected Side |
Time Since Stroke (Months) |
Lesion Etiology |
Lesion Location |
UEFM | |
|---|---|---|---|---|---|---|
| tDCS+CIMT | 1 | 68/Female/Left | 32 | I | BG | 35 |
| 2 | 66/Male/Left | 19 | I | COR | 50 | |
| 3 | 54/Male/Right | 29 | I | CN/BG | 44 | |
| 4 | 55/Female/Right | 48 | H | TH/PLIC | 47 | |
| 5 | 76/Male/Right | 24 | I | BG/CR/PLIC | 15 | |
| 6 | 63/Male/Right | 228 | H | ST | 53 | |
| Sham+CIMT | 7 | 58/Female/Left | 24 | I | BG/CN | 54 |
| 8 | 69/Male/Right | 23 | H | TH/PLIC | 46 | |
| 9 | 72/Male/Left | 84 | P | P | 59 | |
| 10 | 59/Female/Left | 23 | I | TL/BG/CN | 51 | |
| 11 | 50/Male/Left | 54 | H | TH/PLIC | 44 | |
| 12 | 45/Male/Right | 12 | I | TL/PLIC | 26 | |
| Sham+CIMT vs. tDCS+CIMT |
p = 0.589 | p = 0.470 | — | p = 0.3 | ||
| Z = −0.641 | Z = −0.723 | Z = −1.04 | ||||
| U= 14 | U=13.5 | U=11.5 |
Abbreviations: UEFM – Upper Limb Fugl-Meyer (max 66), I – Ischemic, H – Hemorrhagic, P – Pontine, BG – Basal Ganglia, COR – Cortical, CN – Caudate Nucleus, TH – Thalamus, PLIC – Posterior Limb of the Internal Capsule, CR – Corona Radiata, ST – Striatum, P – Pons, TL – Temporal Lobe.
Fig. 1.
Lesions are represented in green. Abbreviations: BG – Basal Ganglia, COR – Cortical, CN – Caudate Nucleus, TH – Thalamus, PLIC – Posterior Limb of the Internal Capsule, CR – Corona Radiata, ST – Striatum, P – Pons, TL – Temporal Lobe Mann-Whitney Test between Active and Sham Volume size: p = 1; Z = 0; U = 18.
Fig. 3.
a) figure adapted from Pavlova et al. 2014. Shows the location of the anod electrode relative to the motor hotspot. b) tDCS anod electrode placement overlay of 15 individual therapy session for one patient. Across all patients, the center of the anodal electrode was placed 26.78 ± 8.6 mm from the patients’ motor hotspot. The black region represents the most consistent placement of the tDCS electrode throughout the 15 sessions of therapy. The motor hotspot is represented as the red circle.
4.1. Functional outcomes
Results of functional outcomes are reported in Table 2.
Table 2.
Patient behavioral outcomes
| Pre M (SD) |
Post M (SD) |
d | P | |
|---|---|---|---|---|
| UEFM Total | ||||
| tDCS+CIMT | 40.67 (14) | 47.5 (12) | 0.53 | 0.028 |
| Sham+CIMT | 46.67 (11) | 49.33 (10) | 0.25 | 0.102 |
| P between group | 0.66 | 0.79 | ||
| MAL - Amount | ||||
| tDCS+CIMT | 1.3 (1.0) | 1.9 (1.3) | 0.52 | 0.173 |
| Sham+CIMT | 1.4 (0.85) | 2.4 (1.1) | 1 | 0.075 |
| P between group | 0.82 | 0.24 | ||
| MAL – How Well | ||||
| tDCS+CIMT | 1.4 (0.90) | 2.1 (1.4) | 0.61 | 0.166 |
| Sham+CIMT | 1.5 (0.58) | 2.4 (0.80) | 1.3 | 0.028 |
| P between group | 1 | 0.49 | ||
| 9 Hole Peg Test | ||||
| tDCS+CIMT | 3.7 (4.4) | 7.5 (3.7) | 0.94 | 0.102 |
| Sham+CIMT | 3.3 (4.5) | 4.2 (4.4) | 0.2 | 0.18 |
| P between group | 0.94 | 0.24 |
Abbreviations: UEFM – Upper Limb Fugle Meyer, MAL – Motor Activity Log, d – Cohen d score.
4.2. Upper extremity fugl- meyer
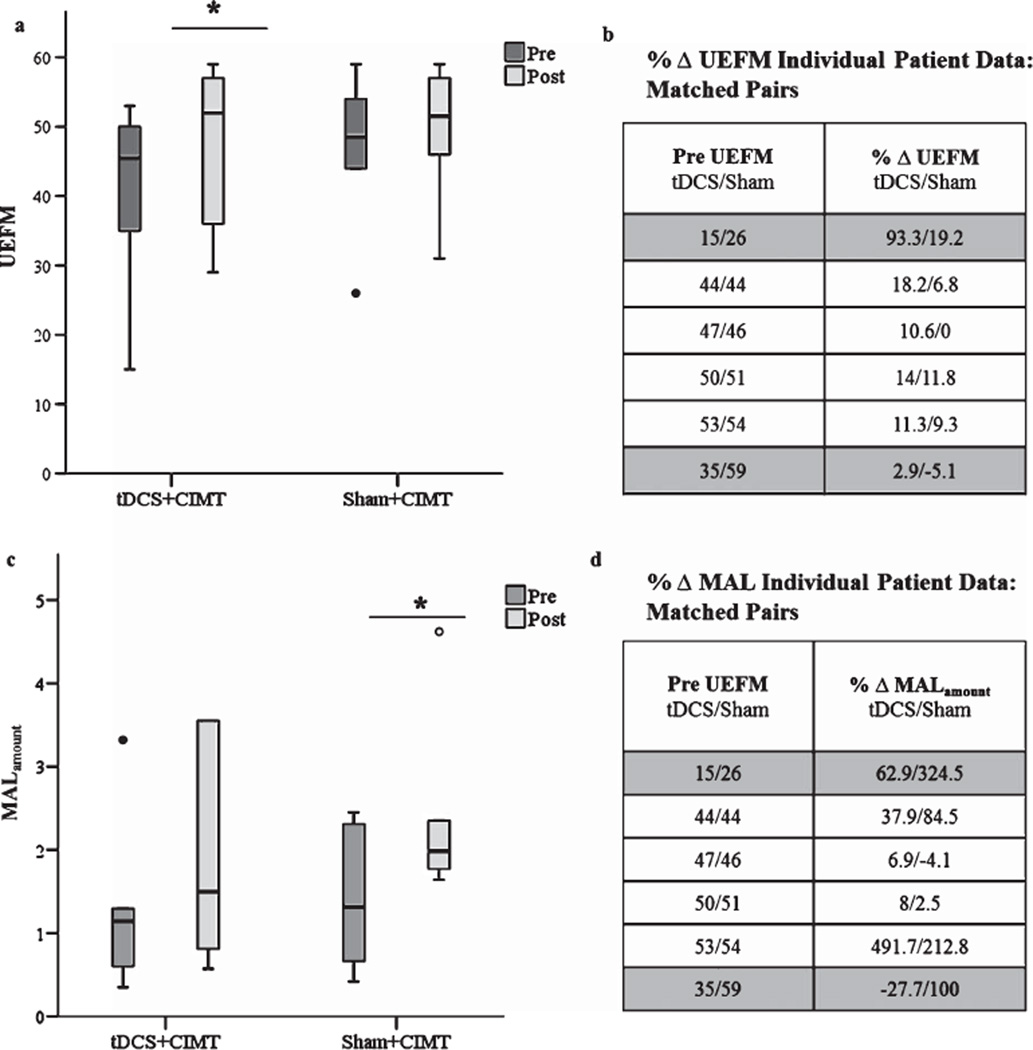
Wilcoxon-signed Ranks test indicated that UEFM only improved significantly in the tDCS+CIMT group (z=−2.2 p= 0.028), while the sham+CIMT group showed a non-significant change (z = 1.633 p = 0.102). The improvement in the tDCS+CIMT group had a moderate effect size of 0.53, where the effect size of the sham group was small (0.25). Patient #5 (Table 1) from the tDCS+CIMT group improved substantially on UEFM from 15 to 29 points. When we repeated the analysis without this individual, there was greater similarity in baseline UEFM scores between the two groups (tDCS: 45.8 ± 6.9; Sham: 46.7 ± 11.5); still, the tDCS+CIMT group demonstrates a significant increase in UEFM (z= −2.023 p = 0.043), though the effect size decreased to 0.33. Since there was no valid reason to exclude this individual, original analysis is retained in Fig. 4a. Some would argue that even though a significant difference in baseline UEFM does not exist, patients in the tDCS+CIMT group were still seemingly more impaired than patients in the sham + CIMT group, which may explain why they noted more recovery. To explore this possibility further, we closely studied differences in baseline UEFM. However, as mentioned above, the baseline discrepancy in the tDCS group is due to the most severely impaired patient. When the patients are matched according to their baseline UEFM every patient in the tDCS group had a greater percent increase in UEFM when compared to their matched pair in the sham+CIMT group (Fig. 4a and b). We were able to match (±1 point) 4 out of the 6 patients. We matched UEFM 15 with 26, as these two were the most impaired in our sample.
Fig. 4.
Individual behavioral outcomes for UEFM and MALamount pre and post therapy by group. a) Patients who received tDCS+CIMT showed greater increase in UEFM score when compared to the sham+tDCS group (p = 0.01 and p = 0.121, respectively). The tDCS+CIMT demonstrate 6.8 ± 4.2 point increase in UEFM compared to sham+tDCS 2.7 ± 3.5 point increase. b) When patients are matched based on pre UEFM score all patients in the tDCS+CIMT group showed a greater positive change in UEFM following therapy. c) The sham+tDCS demonstrated greater increase in MALamount compared to the tDCS+CIMT group (p= 0.075 and p= 0.173, respectively). The sham+tDCS group showed 1±1.2 point increase where the tDCS+CIMT showed 0.61 ± 0.9 point increase. Like-wise, the sham+tDCS group demonstrated a greater increase in MALhow-well when compared to the tDCS+CIMT group (p = 0.028 and p = 0.166, respectively) (figure not shown). d) When patients are matched based on pre UEFM score 4 out of the 6 patients in the sham+CIMT group showed a greater positive change in perceived use of the paretic hand in activities of daily living. Gray shading represents undesirable matching. We matched pre UEFM 15 with 26 because these patients are our most affected patients. We were unable to find adequate matching for pre UEFM 35 and 59. Abbreviations: UEFM: Upper Extremity Fugl-Meyer, MAL: Motor Activity Log, tDCS: Transcranial Direct Current Stimulation, CIMT: Constraint-Induced Movement Therapy.
4.3. Nine-hole peg test
Since we included several patients with severe impairments of the upper limb, many patients were not able to place all nine pegs in the allotted time. Therefore, we analyzed the number of pegs they were able to place. Wilcoxon signed rank test showed no significant difference from baseline to post-treatment for either group (tDCS+CIMT: z = −1.63, p= 0.1; sham+CIMT: z= −1.34, p= 0.18) and Mann-Whitney U tests showed no difference for the pre to post change between groups (z= −1.074 p = 0.283). However, the tDCS+CIMT group increased the number of pegs from 3.7 ± 4.4 pegs to 7.5 ± 3.7 with a large effect size of 0.94. Further, of the 3 patients in the tDCS+CIMT group that were unable to place any pegs at baseline, two were able to place all nine pegs at post-therapy. The sham+CIMT group had a small effect size of 0.2, where, of the three patients that were not able to place any pegs at baseline, only one was able to place one peg post-therapy.
4.4. Motor activity log
There was no difference in baseline scores between the 2 groups for either amount (p = 1) or quality of use (p= 0.937). The sham+CIMT group showed an increase in both perceived amount of use (z = −1.782 p = 0.075) and quality of use (z = −2.201 p = 0.028), and had a large effect size for each (1 & 1.3). Even though the tDCS+CIMT group did not significantly change in either perceived amount of use or quality of use, they still demonstrated a moderate effect of 0.52 and 0.61 (Fig. 4c and d). To explore the possibility that the sham+CIMT group may have perceived greater ability to use their paretic hand in daily living based on potentially greater availability of caregiver support at home, we conducted chi-square test for association between tDCS+CIMT and sham+CIMT. There was a statically significant association between group and available caregiver, χ2(1) = 6, p = 0.014. Only 2 patients in the tDCS+CIMT group had available care-giver in their homes, compared to all 6 patients in the sham+CIMT group.
4.5. Neural indices
Neither group showed an increase in excitability of the ipsilesional hemisphere nor the ipsilesional corticomotor maps of the FDI. In fact, the sham+CIMT group showed a significant decrease in the size of the map in the ipsilesional hemisphere (z= −1.9 p = 0.046). Recovery of motor function in neither group related to neurophysiologic changes within the ipsilesional hemisphere.
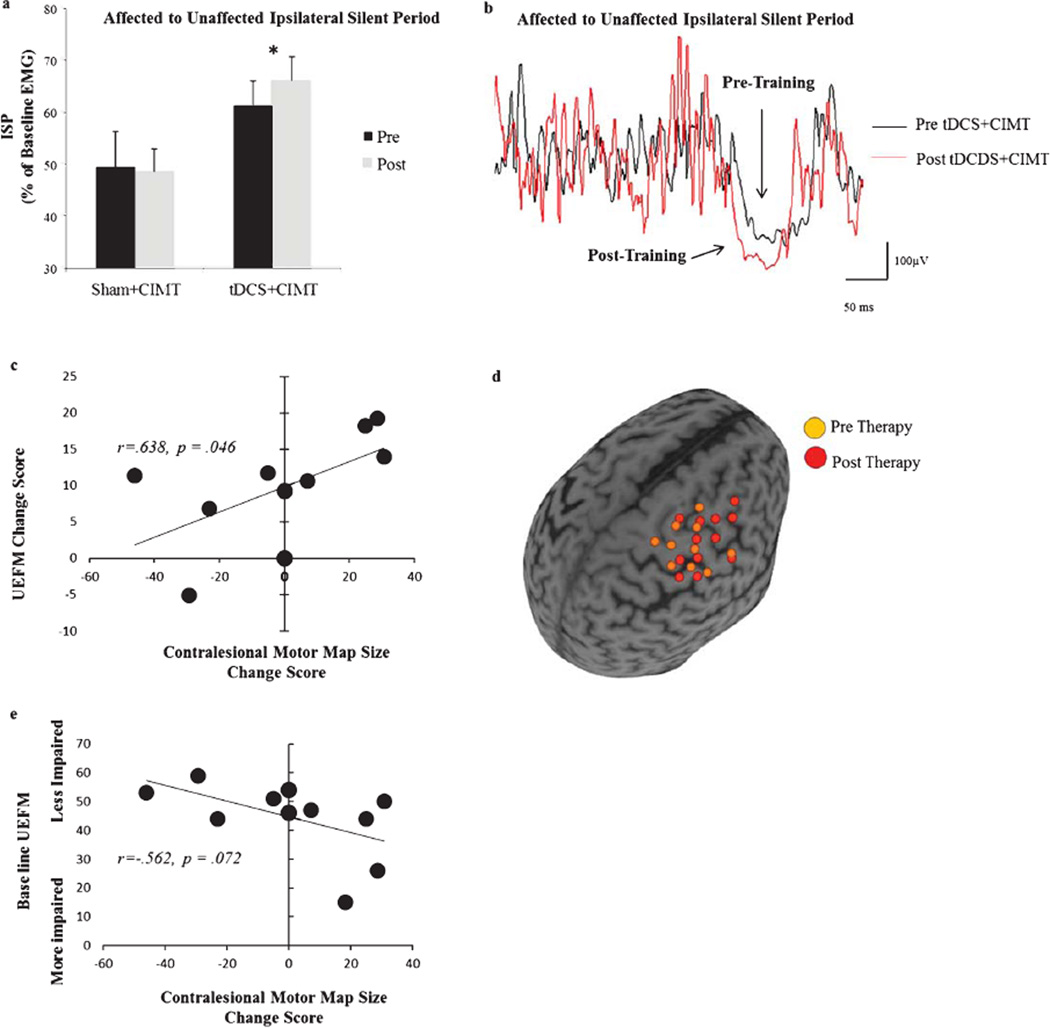
A more compelling result was that of inter-hemispheric inhibition as well as the contribution of the contralesional hemisphere. In the tDCS+CIMT group, the ability of the ipsilesional hemisphere to counter inhibition from the contralesional hemisphere improved (z = −2.0 p= 0.046) (Fig. 5a–b), while inhibition exerted by the contralesional hemisphere did not significantly change. There was no significant difference between groups at baseline (z = −1.281, p = 0.240). In addition, we witnessed a trend for significant increase in excitability of the contralesional hemisphere in the tDCS+CIMT group, marked by a decrease in rMT (z= −1.8p= 0.072). The tDCS+CIMT group also tended to show an overall increase in the map of the contralesional hemisphere, but because of an outlier, there was no significant change (z= −0.667 p = 0.498). Essentially, due to fatigue, mapping of the contralesional hemisphere could not be completed in one patient from the tDCS+CIMT group (Patient #1 (Table 1). Still, this patient was included in all other analysis. Of the five remaining patients, four showed a 20% increase in map area, while one showed a 46% decrease (Patient #6 (Table 1). In the sham+CIMT group, there was no change in inhibition from either hemisphere nor was there a change in the neurophysiologic outcomes of the contralesional hemisphere. Across all patients, not only did patients with the greatest motor improvement show the greatest increase in corticomotor maps in the contralesional hemisphere (r = 0.638 p = 0.047); we show a trend for significance that patients with the greatest motor impairment at baseline showed the greater increase in corticomotor maps in the contralesional hemisphere (r = −0.562, p = 0.072) (Fig. 5c – e). There was no correlation between any other outcomes.
Fig. 5.
Neurophysiologic predictors of recovery. a) Patients in the tDCS+CIMT group had greater inhibition imposed from the ipsilesional upon the contralesional hemisphere following therapy. There was no significant difference between groups at baseline (z = −1.281, p = 0.240). b) Single patient representative change in ISP. There was greater ISP inhibition was from before (black line) to after (red line) tDCS+CIMT therapy demonstrated in a single patient as a greater percent decrease in EMG relative to pre-stimulus EMG. c) Patients who showed a greater increase in cortical map size of the contralesional hemisphere demonstrated greater gains in UEFM following therapy. One patient was excluded from the analysis because they increased their UEFM score by 93%, compared to an average 8.9% in other patients and another was excluded because we were unable to complete the contralesional map due to fatigue. This patient was included in all other analysis. d) Single patient representative example of the change in map size before (orange sphere) and after (red sphere) therapy. e) Patients with greater motor impairment at baseline also showed the greatest increase in cortical map size od the contralesional hemisphere. Map size was the total number of active grid points (5×7 cm2) based on 3 out of 5 TMS trials that evoked an MEP >30 µV. Error bars are SE, and significance was p < 0.05.
5. Discussion
In this proof-of-concept, clinical study, we provide early evidence that suggests that facilitating the ipsilesional higher motor areas enhances outcomes of CIMT, specifically in the context of motor function and dexterity. A small sample size limits our ability to derive definitive conclusions, yet preliminarily, the advantage of our approach appears to be accelerating recovery where patients are able to show greater gains from a CIMT program that is contracted. Contrary to our hypothesis though, benefit of facilitating higher motor areas was associated with potentiated excitability of the contralesional rather than the ipsilesional hemisphere; but as hypothesized, the ipsilesional hemisphere countered inhibition from the contralesional hemisphere. Our early findings warrant larger clinical trials to confirm our hypothesis that facilitating higher motor areas potentially offers a consistent advantage for CIMT.
Our study has preliminarily demonstrated that patients who receive tDCS to the ipsilesional higher motor areas improve their overall motor function with a shortened CIMT protocol. Our motivation to shorten the protocol from the typical (6 hours/day for 2 weeks) or the modified version (30 hours for 10 weeks) to 15 hours over 5 weeks was to improve overall compliance, and utility of CIMT. With a shortened CIMT, patients that received sham failed to see any notable changes, but patients receiving facilitation of the ipsilesional higher motor areas experienced an average 7-point increase in UEFM. The gain is promising when considering that it exceeds clinically important difference ( = 4.25 points) (Page et al., 2012). Similar to our own, a recent study has reported no meaningful gain in UEFM following 2 weeks (total of 40 hours) of CIMT alone. The authors conclude that following a shortened protocol, functional improvements may be mediated by compensatory strategies rather than a decrease in motor impairment (Kitago et al., 2013). Still, it is early to conclude whether facilitating higher motor areas is accelerative; serial, interim assessments would be more pertinent to addressing the claim.
Previous studies that have combined facilitation of M1 with rehabilitation have shown inconsistent results. In 2008, a Phase III clinical trial (R. M. Levy et al., 2015) reported that stimulation of M1 provided no added benefit to rehabilitation despite positive Phase II results (R. Levy et al., 2008). The effect of stimulation was not apparent across individuals with severe impairments who suffered from greater damage to CST from M1. Since the completion of the trial, several groups have continued to report no benefit of the classical approach (Hesse et al., 2011; Seniow et al., 2012; Talelli et al., 2012) even as others have evidenced a promising 5–6-point advantage in UEFM (Bolognini et al., 2011; Lindenberg, Renga, Zhu, Nair, & Schlaug, 2010; Nair, Renga, Lindenberg, Zhu, & Schlaug, 2011). Although, preliminary, we show that 5 of the 6 patients receiving facilitation to the ipsilesional premotor areas achieved clinically important gains ranging between 5 to 14-point increase in UEFM. We are unable to discount that the sham group may have experienced a ceiling effect though there are several reasons to explain why this may not explain our results. Although the sham group had higher UEFM scores, their average impairment level is still comparable to that of samples in previous studies that show gains in UEFM; a moderate-to-mild score of 47 (Woodbury, Velozo, Richards, & Duncan, 2013) on UEFM at baseline should not preclude further gains in UEFM (Kim et al., 2010; Lindenberg, Zhu, & Schlaug, 2012). In addition, the difference in baseline was largely driven by one patient in the tDCS+CIMT group that had a baseline UEFM of 15. When this patient is removed, the baselines are comparable and the tDCS group still shows a significant increase in UEFM score, where no such change is observed in the sham. Furthermore, when we matched our patients based on baseline UEFM, we were still able to demonstrate a slight advantage of active tDCS when compared to sham stimulation (Fig. 4).
Contrary to our hypothesis that facilitating the ipsilesional higher motor areas would increase excitability of the ipsilesional hemisphere in line with the classical premise (Bolognini et al., 2011; Fregni & Pascual-Leone, 2007; Lindenberg et al., 2010; Nowak et al., 2009), here, we failed to witness any gains in the excitation of the ipsilesional hemisphere. Our inability to observe any changes in the ipsilesional hemisphere may be a result of our more impaired patients and the frequency of lesion location at the level of the posterior limb of the internal capsule. Instead, we have observed that facilitating premotor areas increases excitability of the contralesional hemisphere. In fact, across all patients, greater contralesional hemisphere excitation is associated with greater gains in motor improvement where patients with more severe motor impairment showed the greatest change in the contralesional hemisphere, indicating a positive role of the contralesional hemisphere in the more impaired patients. Although seemingly unexpected, our finding provides support for the adaptive role of the contralesional hemisphere, which to date, has been controversial (Di Pino et al., 2014).
Earlier studies have emphasized a competitive role of the contralesional hemisphere (Marshall et al., 2000; Murase et al., 2004; N. S. Ward, Brown, Thompson, & Frackowiak, 2003a), seeking to decrease its excitability, hence its ability to overly-inhibit the ipsilesional hemisphere (Nair et al., 2011). However, controversy surfaced when studies with fMRI showed a greater shift in cortical activation to the contralesional hemisphere with greater gains after rehabilitative therapy (Cramer et al., 1997; Johansen-Berg, Dawes, et al., 2002). Further, when the contralesional hemisphere is inactivated, animals with large infarcts lost the ability to perform a reaching task (Biernaskie, Szymanska, Windle, & Corbett, 2005), and severely impaired patients showed slower reaction time (Johansen-Berg, Rushworth, et al., 2002). The previous studies in support of the compensatory role of the contralesional hemisphere have largely been cross-sectional studies. This evidence coupled with our longitudinal results contradicts traditional model of inter-hemispheric inhibition, where greater excitability of the contralesional hemisphere is thought to invariably be abnormal and competitive. Instead, our evidence suggests its role may vary along a gradient, becoming adaptive/compensatory when damage/deficit exceeds a threshold (Di Pino et al., 2014; Stinear et al., 2007), as may be the case in our sample comprising patients with mild to significantly impairment. This concept has recently been described as the bimodal balance-recovery model (Di Pino et al., 2014).
Still, how can facilitating ipsilesional premotor areas recruit the contralesional cortices in recovery? Perhaps an increase in counter-inhibition from the ipsilesional hemisphere combined with an adaptive increase in excitability of the contralesional hemisphere suggests hemispheric cooperation via callosal connections between the higher motor areas. Previous studies have reported that the higher motor areas have greater transcallosal connections (Marconi et al., 2003; Rouiller et al., 1994; Schubotz & von Cramon, 2003) and are more functionally coupled (O’Shea, Johansen-Berg, Trief, Gobel, & Rushworth, 2007) when compared to the M1. In the present study, we show by facilitating the ipsilesional higher motor areas with tDCS, the ipsilesional hemisphere increased its ability to counter inhibition arising from the contralesional and facilitated excitability of the contralesional hemisphere. This facilitation of the contralesional hemisphere may be the indirect result of facilitating the premotor cortex of the ipsilesional hemisphere via dense transcallosal connections. The recovery related gains of the increased contralesional hemisphere excitability may be the result of uncrossed ipsilateral pathways originating from the PMC and SMA as they have the greatest proportion of ipsilateral contributions to the reticulospinal, rubrospinal and to propriospinal premotor neurons devoted to the proximal muscles as previously suggested (Bradnam, Stinear, & Byblow, 2013; Johansen-Berg, Rushworth, et al., 2002; Machado, Shoji, Ballester, & Marino, 2003; Netz, Lammers, & Homberg, 1997; Stinear et al., 2007; Zaaimi, Edgley, Soteropoulos, & Baker, 2012).
Recovery-related gains in neither group were accompanied by gains in recruitment of the ipsilesional hemisphere, but we did show a decrease in excitation of the ipsilesional hemisphere with sham. We believe such decrease may represent that the sham groupwas still in the early phases of recovery due to our short CIMT protocol. Although we did not see notable changes in UEFM, there is evidence that initial phases of recovery involve increased synaptic efficiency via trimming of redundant networks (Ramsey, Jansma, Jager, Van Raalten, & Kahn, 2004). The tDCS group, however, was potentially accelerated into late phases of recovery as demonstrated by their gains in motor function. To confirm this, future studies could emphasize serial TMS across groups receiving varying doses of CIMT.
Contrary to the reduced motor impairment for the active tDCS group, we found that patients receiving sham during CIMT reported greater overall use of their paretic hand during tasks of daily living as reported by MAL, when compared to patients who received active tDCS. While Bolognini. et al. 2011 similarly demonstrate a lack of advantage of stimulating M1 in affecting patient’s subjective perception of use of their paretic hand, the discrepancy here is the greater advantage noted in the sham group. The discrepancy in our results possibly speaks to the inadvertent difference in the level of caregiver support for their required at home constraint induced movement therapy. Coincidentally, the tDCS group only had 2 patients who had help from caregivers at home, where all 6 patients in the sham group had caregivers available at home. Since MAL tests the patient’s perceived ability to use the paretic hand in daily life and involvement of caregivers is a significant determinant of perception of upper limb function (Harris, Eng, Miller, & Dawson, 2010), we believe a difference in available caregiver support could explain the remarkably high improvement noted in the sham group on the MAL score.
The results of this preliminary study must be viewed carefully in the context of its scale, its sample and its target of stimulation. Although our intent was to stimulate the higher motor areas of the ipsilesional hemisphere, we do not suggest that we were able to isolate them from M1. The size of our electrode is in line with previous studies that targeted M1 (Bolognini et al., 2011) as well as a previous study targeting PMC (Nitsche et al., 2010). Still, we understand that we cannot discount the possibility that we stimulated the M1 inadvertently. However, we incorporated several methodological controls so that one could see that we were reliably targeting a region dorsal and antero-medial to M1 based on a reasonable first-pass approximation. It is in this context that we applied anodal tDCS to coordinates of higher motor regions established in previous studies (Nitsche et al., 2008); we used the patient’s own MRI and TMS-based neurophysiology to guide application of tDCS. Finally, with stereotactic navigation, we ensured that application from day-to-day was consistent within and across all patients (Fig. 3). However, future work should involve current density modeling to provide better confirmation. Further, this paper must be viewed specific to this study patient population as we cannot base inferences on the stroke population as a whole. We recognize the heterogeneity of our stroke population. Our interest was to include patients with impairment of the upper limb. Since impairment of the upper limb can result from a variety of stroke-related injury, we included patients with cortical-subcortical/cortical lesions as well as ischemic/hemorrhagic infarcts. Others have stated that inclusion of a heterogeneous population is certainly a limitation but serves to create evidence that is critical to test efficacy in later studies. Quinlan et al. 2015 discuss that there is often a trade off when recruiting patients, where they discuss that even though there is high statistical power when recruiting a homogenous population as a result of the low variance; including a heterogeneous population may eventually allow for more generalizability of the results. Still, as a result of our small sample size our results are by no means generalizable; but, they suggest an interesting effect of higher-motor stimulation that will be used to design larger clinical trials.
In summary, our study provides preliminary evidence that facilitating higher motor areas may be beneficial in rehabilitation of patients with stroke who suffer from mild to substantial damage and impairment. However, larger clinical trials are required for confirmation. Further, it is important that future work understands whether there are patients who would best respond to stimulation of one vs. another. Strategic comparisons of higher motor facilitation and M1 facilitation across diverse samples would yield information on individual responsiveness.
Acknowledgments
This work was supported by the National Institutes of Health (1K01HD069504) and American Heart Association (13BGIA17120055) to EBP as well as by the Clinical & Translational Science Collaborative (RPC2014-1067) to DAC. Conflicts of Interest: AM has the following conflicts of interest to disclose: ATI, Enspire and Cardionomics (distribution rights from intellectual property), Spinal Modulation and Functional Neurostimulation (consultant).
References
- Avanzino L, Bassolino M, Pozzo T, Bove M. Use-dependent hemispheric balance. J Neurosci. 2011;31(9):3423–3428. doi: 10.1523/JNEUROSCI.4893-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583(Pt 1):99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Szymanska A, Windle V, Corbett D. Bihemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21(4):989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–829. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27(5):1292–1300. doi: 10.1111/j.1460-9568.2008.06090.x. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22(11):2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: Implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. doi: 10.3389/fnhum.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: Results of a follow-up study. Disabil Rehabil. 1999;21(8):357–364. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77(1):132–145. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey [nee (Bhatt)] JR, Evans CD, Anderson DC, Plow E, Nagpal A, Kimberley TJ, et al. Safety of 6-Hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22(2):185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125(Pt 4):773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28(12):2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cunningham DA, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil. 2015;96(4 Suppl):S94–S103. doi: 10.1016/j.apmr.2014.07.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–295. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. 2014;7(6):841–848. doi: 10.1016/j.brs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ranieri F, Profice P, Pilato F, Mazzone P, Capone F, et al. Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stimul. 2013;6(4):641–643. doi: 10.1016/j.brs.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat Rev Neurol. 2014 doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: Non-invasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(Pt 4):747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Gacs G, Fox AJ, Barnett HJ, Vinuela F. Occurrence and mechanisms of occlusion of the anterior cerebral artery. Stroke. 1983;14(6):952–959. doi: 10.1161/01.str.14.6.952. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Halko MA, Datta A, Plow EB, Scaturro J, Bikson M, Mer-abet LB. Neuroplastic changes following rehabilitative training correlate with regional electrical field induced with tDCS. Neuroimage. 2011;57(3):885–891. doi: 10.1016/j.neuroimage.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: A TMS study. Neurorehabil Neural Repair. 2011;25(5):398–411. doi: 10.1177/1545968310395600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Eng JJ, Miller WC, Dawson AS. The role of caregiver involvement in upper-limb treatment in individuals with subacute stroke. Phys Ther. 2010;90(9):1302–1310. doi: 10.2522/ptj.20090349. [DOI] [PubMed] [Google Scholar]
- Harvey RL, Winstein CJ, Everest Trial G. Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23(1):32–44. doi: 10.1177/1545968308317532. [DOI] [PubMed] [Google Scholar]
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: An exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25(9):838–846. doi: 10.1177/1545968311413906. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation. Lancet Neurol. 2006a;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006b;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125(Pt 12):2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Kim JS. Anterior cerebral artery infarction: Stroke mechanism and clinical-imaging study in 100 patients. Neurology. 2008;70(24 Pt 2):2386–2393. doi: 10.1212/01.wnl.0000314686.94007.d0. [DOI] [PubMed] [Google Scholar]
- Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am JPhys Med Rehabil. 2010;89(11):879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- Kitago T, Liang J, Huang VS, Hayes S, Simon P, Tenteromano L, et al. Improvement after constraint-induced movement therapy: Recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013;27(2):99–109. doi: 10.1177/1545968312452631. [DOI] [PubMed] [Google Scholar]
- Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108(4):707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, et al. Epidural electrical stimulation for stroke rehabilitation: Results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968315575613. (Epub ahead of Print) [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: The effect of number of sessions on outcome. Neurore-habil Neural Repair. 2012;26(5):479–483. doi: 10.1177/1545968311427568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128(1–2):149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- Machado AG, Shoji A, Ballester G, Jr, Marino R. Mapping of the rat’s motor area after hemispherectomy: The hemispheres as potentially independent motor brains. Epilepsia. 2003;44(4):500–506. doi: 10.1046/j.1528-1157.2003.37602.x. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, Gonzalez Rothi LJ, Wu S, Reid K, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: An exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86(9):707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003;18(4):775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31(3):656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29(6):411–420. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jakoubkova M, Thirugnanasambandam N, Schmalfuss L, Hullemann S, Sonka K, et al. Contribution of the premotor cortex to consolidation of motor sequence learning in humans during sleep. J Neurophysiol. 2010;104(5):2603–2614. doi: 10.1152/jn.00611.2010. [DOI] [PubMed] [Google Scholar]
- Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77(11):1076–1083. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Inter-hemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23(7):641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human pre-motor cortex. Neuron. 2007;54(3):479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Phys Ther. 2008;88(3):333–340. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- Pavlova E, Kuo MF, Nitsche MA, Borg J. Transcranial direct current stimulation of the premotor cortex: Effects on hand dexterity. Brain Res. 2014;1576:52–62. doi: 10.1016/j.brainres.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: A feasibility study in primates. Neurol Res. 2003;25(8):801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Plow (Bhatt) E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182(4):435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- Plow [nee (Bhatt)] E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182(4):435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke. Stroke. 2009;40(5):1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Cunningham DA, Beall E, Jones S, Wyant A, Bonnett C, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: Study protocol for a randomized controlled trial. Trials. 2013;14:331. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Cunningham DA, Varnerin N, Machado A. Rethinking stimulation of the brain in stroke rehabilitation: Why higher motor areas might be better alternatives for patients with greater impairments. Neuroscientist. 2015;21(3):225–240. doi: 10.1177/1073858414537381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Machado A. Invasive neurostimulation in stroke rehabilitation. Neurotherapeutics. 2014;11(3):572–582. doi: 10.1007/s13311-013-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of visual field training for hemianopia with active versus sham transcranial direct cortical stimulation. Neurorehabil Neural Repair. 2012a;26(6):616–626. doi: 10.1177/1545968311431963. [DOI] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Halko MA, Kenkel S, Jackson ML, Pascual-Leone A, et al. Combining visual rehabilitative training and noninvasive brain stimulation to enhance visual function in patients with hemianopia: A comparative case study. PM R. 2011;3(9):825–835. doi: 10.1016/j.pmrj.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Jackson ML, Merabet LB. Temporal profile of functional visual rehabilitative outcomes modulated by transcranial direct current stimulation. Neuromodulation. 2012b;15(4):367–373. doi: 10.1111/j.1525-1403.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Jansma JM, Jager G, Van Raalten T, Kahn RS. Neurophysiological factors in human information processing capacity. Brain. 2004;127(Pt 3):517–525. doi: 10.1093/brain/awh060. [DOI] [PubMed] [Google Scholar]
- Rossi C, Sallustio F, Di Legge S, Stanzione P, Koch G. Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. Eur J Neurol. 2013;20(1):202–204. doi: 10.1111/j.1468-1331.2012.03703.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102(2):227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Functional-anatomical concepts of human premotor cortex: Evidence from fMRI and PET studies. Neuroimage. 2003;20(Suppl 1):S120–S131. doi: 10.1016/j.neuroimage.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Seniow J, Bilik M, Lesniak M, Waldowski K, Iwanski S, Czlonkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: A randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. 2012;26(9):1072–1079. doi: 10.1177/1545968312445635. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Sung WH, Wang CP, Chou CL, Chen YC, Chang YC, Tsai PY. Efficacy of coupling inhibitory and facili-tatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. 2013;44(5):1375–1382. doi: 10.1161/STROKEAHA.111.000522. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Chuma T, Matsuo Y, Ikoma K. Disinhibition of the premotor cortex contributes to a maladap-tive change in the affected hand after stroke. Stroke. 2007;38(5):1551–1556. doi: 10.1161/STROKEAHA.106.470187. [DOI] [PubMed] [Google Scholar]
- Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, et al. Theta burst stimulation in the rehabilitation of the upper limb: A semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26(8):976–987. doi: 10.1177/1545968312437940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following Constraint-Induced Movement therapy. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S77–S91. doi: 10.1016/s1047-9651(02)00052-9. ix. [DOI] [PubMed] [Google Scholar]
- van der Lee JH, Beckerman H, Knol DL, deVet HC, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410–1414. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol. 2011;589(Pt 23):5625–5632. doi: 10.1113/jphysiol.2011.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain. 2003a;126(Pt 11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain. 2003b;126(Pt 6):1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25(6):1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury ML, Velozo CA, Richards LG, Duncan PW. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil. 2013;94(8):1527–1533. doi: 10.1016/j.apmr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, et al. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013;44(2):483–489. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41(5):910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel S, Weiller C, Liepert J. Citalopram improves dexterity in chronic stroke patients. Neurorehabil Neural Repair. 2008;22(3):311–314. doi: 10.1177/1545968307312173. [DOI] [PubMed] [Google Scholar]