Abstract
Efficient and rhythmic cardiac contractions depend critically on the adequate and synchronized release of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptor Ca2+ release channels (RyR2) and its reuptake via sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a). It is well established that this orchestrated process becomes compromised in diabetes. What remain incompletely defined are the molecular mechanisms responsible for the dysregulation of RyR2 and SERCA2a in diabetes. Earlier, found elevated levels of carbonyl adducts on RyR2 and SERCA2a isolated from hearts of type 1 diabetic rats and showed the presence of these post-translational modifications compromised their functions. We also showed that these mono- and di-carbonyl reactive carbonyl species (RCS) do not indiscriminately react with all basic amino acid residues on RyR2 and SERCA2a; some residues are more susceptible to carbonylation (modification by RCS) than others. A key unresolved question in the field is which of the many RCS that are upregulated in the heart in diabetes chemically react with RyR2 and SERCA2a? This brief review introduces readers to the field of RCS and their roles in perturbing SR Ca2+ cycling in diabetes. It also provides new experimental evidence that not all RCS that are upregulated in the heart in diabetes chemically react with RyR2 and SERCA2a, methylglyoxal and glyoxal preferentially do.
Keywords: diabetes, methylglyoxal, glyoxal, malondialdehyde, 4-hydroxynonenal, sarcoplasmic reticulum Ca2+ cycling, ryanodine receptor, sarc(endo)plasmic reticulum Ca2+-ATPase, reactive carbonyl species, carbonylation
Introduction
Managing cardiovascular complications arising from the epidemic of diabetes mellitus (DM) that started more than forty years ago is undoubtedly one of the major health care challenges of 21st century. In the USA, 26 million individuals have diagnosed DM and ~2 million new cases are expected every year forward [1]. The current annual cost to diagnose and treat DM in the USA is in excess of $175 billion, with >$70 billion directed primarily towards to treating chronic complications including blindness, renal failure, heart failure and stroke [2]. Other developed countries also face similar challenges with about 8% of their population inflicted with DM [3–4]. In emerging economies (e.g., some Middle Eastern and south Asian countries) the problem is even more severe with ≥20% of their populations inflicted with DM [5]. Therapeutic strategies involving lifestyle changes (more exercise and food management), glucose and lipid lowering drugs, and anti-hypertensives are widely available to slow the development and/or progression of cardiovascular complications in individuals with DM [6–9]. Unfortunately, cardiovascular complications still persist. The ACCORD trial also reported that intensive glucose control using complex insulin strategies do not reduce mortality rates in patients with chronic/long term type 2 diabetes [10–11]. With the diabetic population aging, there is an urgent need to better understand fundamental mechanisms that initiate cardiovascular complications so as to expand the armamentarium of drugs available to reduce morbidity and mortality. Of interest in our laboratory is the roles reactive carbonyl species (RCS) play in the pathogenesis of diabetic cardiomyopathy. Specifically, we are interested in mechanisms by which RCS perturb sarcoplasmic reticulum (SR) Ca2+ cycling, trigger arrhythmias and impair cardiac contractility.
Sarcoplasmic reticulum Ca2+ cycling and cardiac contraction
Excitation-contraction coupling is the process whereby membrane depolarization brings about myocyte contraction. Following an action potential, voltage-gated L-type Ca2+ channels on the invaginated T-tubule membranes open and allow the influx of a small amount of extracellular Ca2+ inside the myocyte [12]. This influxed Ca2+ traverses the dyad junction space and bind to multiple, juxtaposed ryanodine receptor Ca2+ release channels (RyR2), causing the release of a much larger amount of Ca2+ from the SR and the generation of a global Ca2+ transient; a process known as calcium-induced calcium-release, (CICR). The near ten-fold rise in intracellular Ca2+ bind to troponin C, displacing it from actin thereby allowing the formation of strong actin-myosin cross-bridges needed for myocyte contraction. Contraction is terminated when Ca2+ release from the sarcoplasmic reticulum (SR) is returned via sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), and the influxed Ca2+ is extruded from the cell via the sodium Ca2+ exchanger (NCX) on the sarcolemmal membrane [13]. The integrity of Ca2+ cycling into and out of the SR dictates the strength of cardiac contraction and defects in this processes are diseases causing. For example, premature activation of RyR2 will trigger delayed-after-depolarization and arrhythmias and reduce the rate and force of cardiac contraction [14–15]. Reductions in activities of SERCA2a and NCX will elevate cytoplasmic Ca2+ which will prematurely activate RyR2 and trigger arrhythmias, reduce the amplitude of evoked Ca2+ transient and slow rate and force of cardiac contraction. Furthermore, defects in SERCA2a and NCX will elevate cytoplasmic Ca2+ that will lead to unregulated activation of signal transduction cascades [16]. A reduction in SERCA2a activity will also lower the Ca2+ content inside the SR which will activate the endoplasmic reticulum (ER) stress response [17–18].
Numerous studies have reported significant changes in SR Ca2+ cycling in the diabetic heart, including reductions in rate and amplitude of evoked Ca2+ release from the SR, and a slowing in rate of removal of Ca2+ from the cytoplasm [19–25]. More detailed analyses of the evoked Ca2+ transients also revealed non-uniform (dyssynchronous) Ca2+ release from SR and alterations in spontaneous Ca2+ release from the SR [21, 24–25]. Although it is accepted that these defects stem in part from changes in the activities of RyR2 and SERCA2a, specific molecular mechanisms responsible remain incompletely defined. General explanations for their altered activities include a reduction in steady-state level of protein and/or mRNA [24–33]. With respect to SERCA2a, changes in steady-state levels have been attributed to an increased O-GluNAcylation (O-linked N-acetylglucosamine) of its transcription factor Sp1, a reduction in thyroid hormone, and from an increased association with its intrinsic inhibitor protein, phospholamban (PLN) [34–37]. Recent data from the laboratories of Cohen and Schöneich [38] also suggest that increased oxidation at Cyst 674 (sulfonic acid adduct) may lead to enhanced degradation of SERCA2. Studies have also reported changes in the amount of phosphorylation of RyR2 at Ser2808 (human)/Ser2809 (rabbit and rodents) and Ser2814 (human)/Ser 2815 (rabbit and rodents) arising from altered activities of protein kinase A (PKA) and Ca2+-calmodulin kinase II, respectively, and phosphatases [24–25, 39, 40]. Another PKA-mediated phosphorylation site on RyR2, Ser2030, has been identified, but its role in the pathogenesis of diabetic cardiomyopathy remains undefined. We also reported increased disulfide bonds on RyR2 in DM, but the identities of the cysteine residues involved are not defined [42]. Recently, we purified RyR2 from hearts of rats with type 1 diabetes (T1D) under reducing and dephosphorylating conditions, and showed that its responsiveness to the intrinsic modulators (Ca2+, ATP, cADP-ribose, Mg2+) was altered [43]. These novel findings led us to conclude that mechanisms other than changes in expression, disulfide bond formation between adjacent cysteine residues, and phosphorylation at Ser2808(9) and Ser2814(5) are likely responsible for RyR2 dysregulation in diabetes. These data also indicate that RyR2 in diabetic animals was phenotypically distinct from RyR2 in control animals. The latter is especially important from a drug development viewpoint.
Cellular oxidants in diabetes
Hyperglycemia increases production of two groups of cellular oxidants, reactive oxygen species (ROS), and reactive carbonyl species (RCS). ROS which includes superoxide anion, O•−; hydroxyl radical, OH•; hypochlorous acid, HOCl; hydrogen peroxide, H2O2; and peroxynitrite, ONOO− are generated from NAD(P)H oxidases, nitric oxide synthases, xanthine oxidases and complex I and III of the mitochondrial electron transport chain [44–45]. RCS are small electrophilic, mono- and di-carbonyl species (Figure 1), that includes acrolein, Nεcarboxy(methyl)lysine, 3-deoxyglucosone, glyoxal (GO), 4-hydroxynonenal (4-HNE), malondialdehyde (MDA) and methylglyoxal (MGO). Unlike ROS that are generated mainly from the precursor superoxide anion (O2•−), RCS are diverse in chemical structures and are derived from multiple sources, including auto-oxidation of glucose and lipids, triose pathway fluxes and from enzymes such as semicarbazide–sensitive amine oxidases and methylglyoxal synthase [46–49]. RCS also have unique characteristic compared to ROS in that their half-lives are longer (minutes vs millisecond), and they are uncharged molecules, allowing them to migrate distance far from their site of production [50]. Cellular levels of RCS are tightly regulated by several RCS degrading enzymes including glutathione S-transferases (hGSTA4-4 and hGST5.8), aldose reductases, aldehyde dehydrogenases and glyoxalases [51–55].
Figure 1.
Chemical structures of mono- and di-carbonyl species and examples of adducts they form on basic amino acid residues of proteins.
The physiological roles for most RCS remain incompletely defined. What we know to date is that low concentrations (≤10 µM) of 4-HNE play important roles in regulate cell growth, proliferation, differentiation, and apoptosis [56–57]. Recent data suggest that MGO by acting as a GABAA agonist may help to lower anxiety [58–59]. What is clear is elevation in RCS is involved in pathogenesis of several disease, including neurodegeneration, cancer, and diabetic complications [47, 60–64]. When produced in amounts that exceed the capacity of its degrading enzyme(s), RCS will react with susceptible basic amino acid residues on proteins to form carbonyl adducts (Figure 1, right panel). Since there are no enzymes in mammalian cells that can break RCS adducts after they are formed on proteins, these adducts remain on the protein throughout its lifetime. For slowly turned over proteins, accumulated carbonyl adducts will likely compromise their functions. To date it is unclear if non-enzymatic carbonylation serves as a signal for protein degradation, akin to metal-catalyzed carbonylation [65].
Reactive carbonyl species and SR Ca2+ cycling defects
Earlier, we found higher levels of MGO adduct (argpyrimidine) on RyR2 and SERCA2a in hearts of rats with diabetic cardiomyopathy. We then showed using in vitro techniques that MGO can recapitulate in control myocytes, the alterations in SR Ca2+ cycling and alterations in contraction seen in diabetic myocytes [30, 66]. In lipid bilayer studies MGO altered the gating and conductance of RyR2. In Ca2+ uptake assays MGO impaired the ability of SERCA2a to translocate Ca2+ from the solution to the inside of SR vesicles. Using time-lapsed confocal imaging with primary rat ventricular mycoytes, MGO increased spontaneous Ca2+ release, induced dyssynchronous Ca2+ release from the SR and increased Ca2+ transient decay time. These findings are consistent with others showing that MGO play an important role in the pathogenesis of other diabetic complications, including hyperalgesia in diabetic nephropathy, in diabetic retinopathy and diabetic nephropathy [67–70].
The effects of MGO, GO and 4-HNE on RyR2
To date the role of other upregulated RCS in diabetes, including MDA, 4-HNE and GO on the functioning of RyR2 and SERCA2a and pathogenesis of diabetic cardiomyopathy remain unclear. In our laboratory, a series of in vitro experiments were initiated employing [3H]ryanodine binding, lipid bilayer and Ca2+ uptake assays, to establish rank-order potency for three of the major RCS, MGO, glyoxal GO, and 4-HNE. The procedures used for these assays are detailed elsewhere [30, 43, 66]. We also began using adduct-specific antibodies to determine relative levels of MGO, GO, 4-HNE and MDA adducts on RyR2 and SERCA2a in hearts from control, diabetic and drug-treated diabetic animals [71], to gain insights into the role these RCS are playing in the pathophysiology of the disease.
The underlying premise behind [3H]ryanodine binding assays is that at concentrations ≤10 nM, [3H]ryanodine binds inside the pore-forming region of the channel. The amount and/or rate of [3H]ryanodine binding would therefore be dependent on the degree of openness of channel, which could be readily regulated by varying the amount of Ca2+ in the binding buffer and the time for [3H]ryanodine binding to occur. At a fixed [Ca2+] in the binding buffer, a ligand can then be added to the buffer and if the amount of [3H]ryanodine bound to the RyR changes (increase or decrease), then this assay would provide a very robust measure of whether the ligand binds to and activates or deactivates the RyR. Figure 2 shows the effects of MGO, GO and 4-HNE on the binding of [3H]ryanodine to RyR2 after two hours of incubation. At low micromolar concentrations (≤ µM), MGO, GO and 4-HNE potentiated the binding of [3H]ryanodine to RyR2. However, at higher concentrations all three RCS dose-dependently displaced [3H]ryanodine binding to RyR2. The concentration of each of these RCS that inhibited 50% of [3H]ryanodine binding (IC50 inhibition) of were 310.7 ± 12.4 µM for MGO, 990.5 ± 18.8 µM for GO and 2250.5 ± 28.1 µM for 4-HNE. Using the Cheng-Prussoff equation [72] defined by Ki = IC50/(1 + (L/KL)) with L = 6.7 nM [3H]ryanodine) and KL of 1.2 nM for RyR2, the Ki of MGO was 47.2 ± 6.5 µM, 150.5 ± 7.3 µM for GO and 342.1 ± 18.0 µM for 4-HNE.
Figure 2. Effect of MGO, GO and 4-HNE on binding of [3H]ryanodine to RyR2.
Displacement [3H]ryanodine binding assays were used to determine relative affinities of MGO, GO and 4-HNE for RyR2. Briefly, SR membranes from control rat hearts (0.1 mg/ml) were incubated in binding buffer (500 mM KCl, 20 mM Tris·HCl, 0.3 mM Ca2+, 2 mM reduced glutathione, and 100 µM EGTA, 6.7 nM [3H]ryanodine, pH 7.4) with varying concentrations of MGO (0 – 300 µM), GO (0– 1000 µM), and 4HNE (0– 1000 µM) and for 2 hr at 37°C. After incubation, membranes were filtered, washed, and the amount of [3H]ryanodine remaining on the filter paper was determined using liquid scintillation counting. Non-specific binding was determined simultaneously by incubating vesicles with 1 µM unlabeled ryanodine. Data shown represent means ± S.E.M for four experiments performed using three different membrane preparations.
To assess the functional consequences, lipid bilayer assay was conducted, adding MGO, GO and 4-HNE to the cis chamber (equivalent to the cytoplasm) and assessing their effects on gating and conductance of RyR2. The procedures for this assay are detailed in a recent study [43, 66]. Earlier we showed that MGO increases the openness of an already opened RyR2 [66]. In this study, experiments conditions were manipulated to significantly reduce the Po of RyR2, i.e., make it near closed [43] and assess if MGO can also activate these closed channels. Figure 3 show that MGO can also significantly increase the Po of low activity RyR2. The increase in Po by low concentrations from MGO resulted from increases in the dwell time in the opened state (>3 fold) and the number of transitions from the closed to the opened state. MGO also reduced the conductance of RyR2 by about 20% (G = 667 ± 40 pS before and 540 ± 20 pS after MGO treatment, see Figure 3 (i), dotted lines). As shown earlier high concentrations of MGO also reduced Po of RyR2 [66].
Figure 3. Effects of MGO on activity of RyR2.
Panel (i) shows representative 2-sec recordings of RyR1 at +35 mV in the absence and presence of increasing amounts of MGO added to the cis chamber with 1.0 µM cis Ca2+. For this, phosphatidylethanolamine, phosphatidylserine and phosphatidylcholine in a ratio of 5:3:2 (35 mg/mL of lipid) in n-decane were painted across a 200 µm diameter hole of the bilayer cup. Single RyR2 channel was then fused to the bilayer and basal channel activity was recorded in symmetric KCl buffer solution (0.25 mM KCl, 20 mM K-Hepes, pH 7.4) with 1.0 µM cis (cytoplasmic) Ca2+. MGO (0 – 50 µM) was then added to the cis chamber (1:250 dilution) and the effects on gating and conductance of the channels were recorded. Panel (ii) shows mean ± S.E.M for n = 7 channels from three separate RyR2 preparations. * denotes significantly different from absence of drug (0 µM) at p < 0.05.
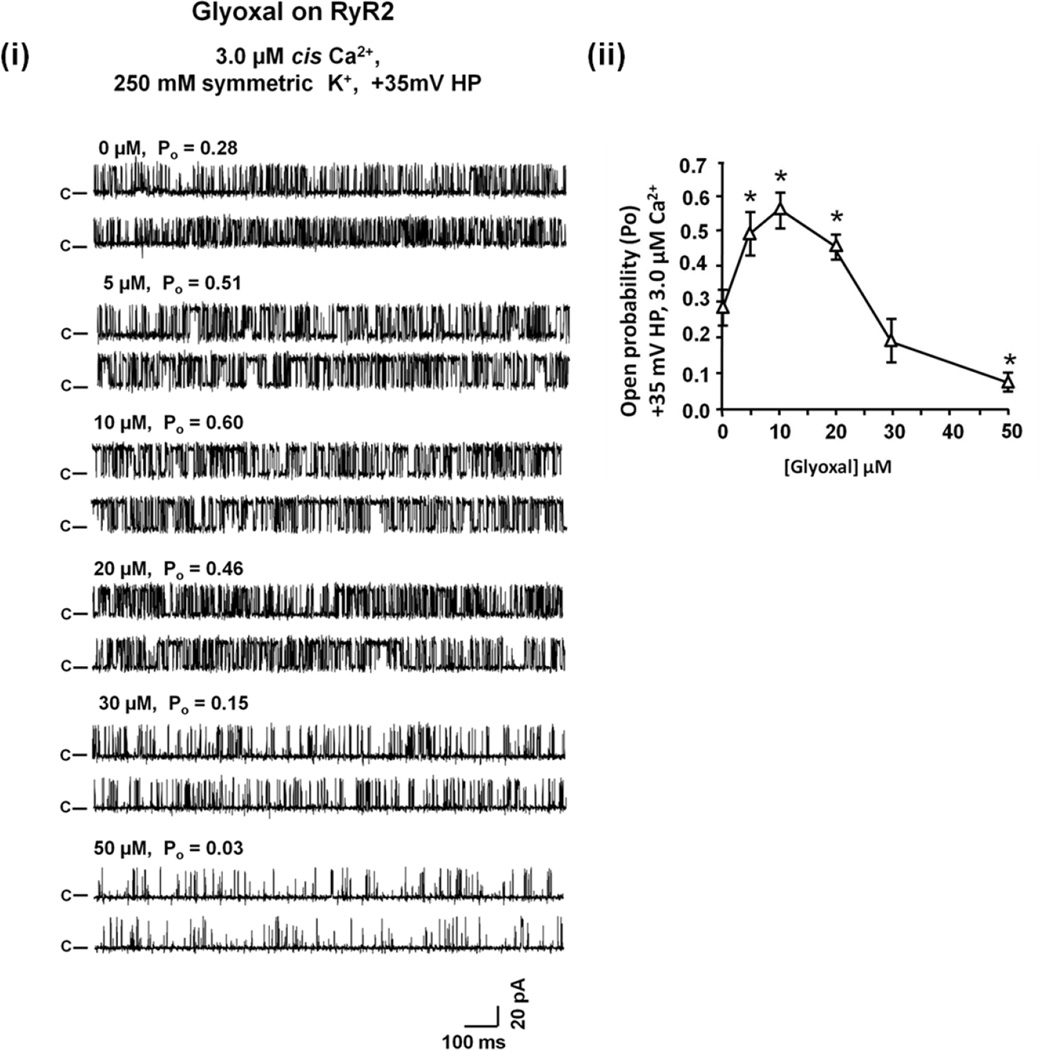
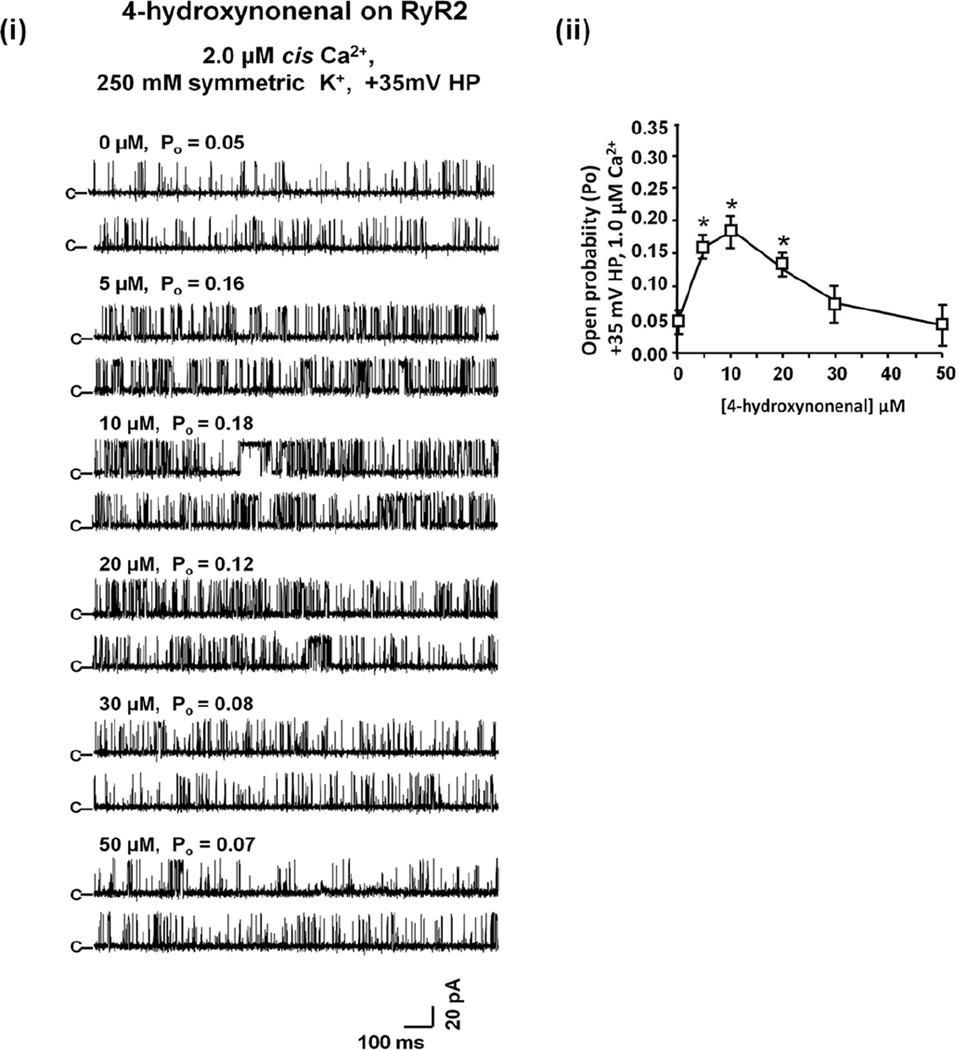
GO also dose-dependently increased and then decreased the Po of RyR2 (Figure 4). But unlike MGO, it did not alter the conductance of RyR2 (G = 662 ± 26 pS before and 645 ± 30 pS after GO treatment). Similarly to MGO and GO, low concentrations of 4-HNE (≤10 µM) increased the Po of RyR2 by modulating the dwell time in the opened state and the number of transitions from the closed to the opened state (Figure 5). Concentration of 4-HNE >than 10 µM, decreased the Po of RyR2. 4-HNE also did not alter the conductance of RyR2. Rank order potencies for channel opening and closing were MGO~GO>4HNE and MGO>GO>4HNE, respectively. It should be pointed out that although these are acute studies, they do provide valuable insights into the effects of these RCS on the activity of RyR2.
Figure 4. Effects of GO on activity of RyR2.
Panel (i) shows representative 2-sec recordings of RyR1 at +35 mV in the absence and presence of increasing amounts of GO added to the cis chamber containing 3.0 µM cis Ca2+. For this, phosphatidylethanolamine, phosphatidylserine and phosphatidylcholine in a ratio of 5:3:2 (35 mg/mL of lipid) in n-decane were painted across a 200 µm diameter hole of the bilayer cup. Single RyR2 channel was then fused to the bilayer and basal channel activity was recorded in symmetric KCl buffer solution (0.25 mM KCl, 20 mM K-Hepes, pH 7.4) with 1.0 µM cis (cytoplasmic) Ca2+. GO (0 – 50 µM) was then added to the cis chamber (1:250 dilution) and the effects on gating and conductance of the channels were recorded. Panel (ii) shows mean ± S.E.M for n = 6 channels from three separate RyR2 preparations. * denotes significantly different from absence of drug (0 µM) at p < 0.05.
Figure 5. Effects of 4-HNE on activity of RyR2.
Figure 3: Panel (i) shows representative 2-sec recordings of RyR1 at +35 mV in the absence and presence of increasing amounts of 4-HNE added to the cis chamber containing 2.0 µM cis Ca2+. For this, phosphatidylethanolamine, phosphatidylserine and phosphatidylcholine in a ratio of 5:3:2 (35 mg/mL of lipid) in n-decane were painted across a 200 µm diameter hole of the bilayer cup. Single RyR2 channel was then fused to the bilayer and basal channel activity was recorded in symmetric KCl buffer solution (0.25 mM KCl, 20 mM K-Hepes, pH 7.4) with 1.0 µM cis (cytoplasmic) Ca2+. 4-HNE (0 – 50 µM) was then added to the cis chamber (1:250 dilution) and the effects on gating and conductance of the channels were recorded. Panel (ii) shows mean ± S.E.M for n = 6 channels from three separate RyR2 preparations. * denotes significantly different from absence of drug (0 µM) at p < 0.05.
Effects of MGO, GO and 4-HNE SERCA2a activity
Pre-incubation of SERCA2a with a low dose of MGO (1 µM) potentiated its ability to transport Ca2+ while higher concentrations dose-dependently reduced its ability to transport Ca2+ (Figure 6). The concentration of MGO that inhibited SERCA2a activity by 50%, (EC50 inhibition) was 15.6 ± 7.4 µM. GO and 4-HNE also reduced the ability of SERCA2a to transport Ca2+, with EC50 inhibition of 55.6 ± 5.4 µM and 350.6 ± 9.6 µM, respectively (Figure 6). Rank order potency for inhibition of SERCA2a was MGO>GO>>4-HNE.
Figure 6. Functional effects of MGO, GO and 4-HNE on the ability of SERCA activity.
Ca2+ uptake assays were used to assess the effects of MGO, GO and 4-HNE on the ability of SERCA2a to transport Ca2+. Briefly, longitudinal vesicles from control rat hearts or HEK-293T cell membranes (SERCA2a) were resuspended in 1.5 ml buffer (30 mM Tris-HCl, pH 7.0, 100 mM KCl, 5 mM NaN3, 50 µM ryanodine, 5 mM MgCl2, 0.15 mM EGTA, 0.12 mM CaCl2, 1 µCi 45Ca2+ and 10 mM potassium oxalate) and divided into 3 × 500 µl aliquots. One aliquot was incubated with a SERCA inhibitor cocktail (10 µM thapsigargin, 100 µM ammonium molybdate, 0.05 µg bafilomycin) for 30 min at 37 °C, a second aliquot was incubated with either MGO, GO and 4-HNE (0 – 600 µM) for 30 min at 37 °C, and the third aliquot remained untreated but was also incubated for 30 min at 37 °C. Ca2+ uptake was initiated in each aliquot by the addition of 1 mM ATP (Na2ATP) and at various times, membranes were cold-filtered and washed. The amount of 45Ca2+ remaining on the filter papers was measured. Ca2+ uptake via SERCA was derived from subtracting uptake in the absence of drug from that in the presence of the SERCA inhibitor cocktail.
Amounts of MGO (argpyrimidine), GO, 4-HNE and MDA adducts on RyR2 and SERCA2a in diabetic rat heart
While in vitro assays can provide insights into the actions of RCS on the function of RyR2 and SERCA2a, they do not provide information of which of the RCS react with and impair the functions of these proteins in vivo. To address this, Western blot analyses employing adduct-specific antibodies were used to compare relative amounts of MGO GO, 4-HNE and MDA adducts on RyR2 and SERCA2 in hearts from control and diabetic rat hearts. In this study, RyR2 from diabetic hearts contained >2-fold higher levels of MGO (argpyrimidine) and GO adducts compared to RyR2 from controls (Figure 7A). MGO (argpyrimidine) adducts were also 2-fold higher on SERCA2a isolated from hearts with diabetic cardiomyopathy compared with SERCA2a from control hearts. 4-HNE and MDA adducts were not detected on RyR2 and SERCA2a from control or streptozotocin-induced diabetic rat hearts using two commercially available 4-HNE (EMD Millipore, Billerica, MA, catalogue#-AB5605 and Santa Cruz Biotechology Inc, Dallas TX, catalogue#-sc-130083) and MDA (EMD Millipore, Billerica, MA, catalogue#-442730-50UL and Santa Cruz Biotechology Inc, Dallas TX, catalogue#-sc-130087) antibodies. The lack of immuno-reactive MDA and 4-HNE adducts were not due to the absence of RyR2 and SERCA2a proteins in our SR preparations, since these proteins were readily detected using RyR2 and SERCA2a antibodies (Figure7A and 7B, 5th autoradiogram down). In a recent study, we showed that these antibodies detected MDA and 4-HNE adducts on proteins of Mw between 30 kDa and 80 kDa in our SR preparation [71].
Figure 7. Relative levels of MGO, GO, 4-HNE and MDA adducts on SERCA2a and RyR2.
SR membranes from control hearts and diabetic rat hearts with established cardiomyopathy (60 µg) were solubilized in gel dissociation medium and electrophoresed on Criterion 4–15% gradient Tris-glycine polyacrylamide gels (BioRad Technologies Inc Burlingame CA) for 210 min at 150V. At the end of the electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA) at 100 mA, 16 hr at 4°C using a semidry electroblotter (Owl Scientific Inc., Woburn, MA) using CAPS buffer (10 mM cyclohexylamino-1-propanesulfonic acid/0.01% SDS in 10% methanol, pH 9.0). Membranes were then blocked with 5% skinned milk and Western blots were performed using adduct-specific antibodies to determine relative levels of MGO (argpyrimidine), glyoxal, MDA, and 4HNE adducts on RyR2 and SERCA2a from control and diabetic rat hearts. Panel (i) shows representative autoradiograms showing relative levels of MGO, GO, 4-HNE and MDA adduct on RyR2 protein isolated from hearts of control (C) and diabetic rat with cardiomyopathy (D). β-actin is also shown. Graph below shows mean ± S.E.M from n≥ 4 experiments. * denotes significantly different control at p < 0.05. Panel (ii) shows epresentative autoradiograms showing relative levels of MGO, GO, 4-HNE and MDA adduct on SERCA2a protein isolated from hearts of control (C) and diabetic rat with cardiomyopathy (D). β-actin is also shown. Graph shows mean ± S.E.M from n≥ 4 experiments. * denotes significantly different control at p < 0.05.
Perspective
It is becoming clearer that therapeutic strategies beyond glucose lowering, lipid-lowering and anti-hypertensives are urgently needed to help reduce and/or reverse the incidence cardiovascular complications, including heart failure, in individuals with DM. Along these lines, two therapeutic approaches are receiving significant attention; reducing oxidative stress and inflammation [73]. As mentions above, hyperglycemia induces two groups of cellular oxidants, ROS and RCS. To date, ROS scavengers (vitamin E, vitamin C, and beta-carotene) have only minimally improved cardiovascular outcomes in patients with DM [74–75]. A possible reason for this is that the antioxidants used thus far in clinical trials may not have gain access to the sites where ROS are generated. Anti-oxidants and enzymes that degrade ROS are currently being nano-formulated in an attempt to target these agents into various cellular components [76]. Pharmacologic strategies to break carbonyl adducts after they are formed on proteins, such as Alagebrium (ALT-711), have also only minimally improved cardiovascular functions in patients with heart failure [77]. One reason why Alagebrium may have been less than successful in clinical trials is that it converts the charge on basic amino acid residues of proteins from positive to negative after removing the carbonyl adduct, the equivalent of genetic mutations [78].
Data from several laboratories including ours have recently shown that MGO is a potent inducer of ROS [66, 79]. Specifically, we showed that within seconds after exposure to MGO, spontaneous Ca2+ sparks increased in rat ventricular myocytes and this was followed by Ca2+ waves (~40–45 sec). About ten minutes thereafter, mitochondrial ROS production also increased. To date, mechanisms by which MGO increase mitochondrial ROS production is not well delineated. What we know to date using the mitochrondria-targeted Ca2+-sensitive dye, Rhod-2, and the mitochondria–targeted dye Mitotracker Green™, is that in addition to increasing cytoplasmic Ca2+, MGO also increases mitochondria Ca2+ in rat ventricular myocytes, ~45 sec after exposure (Figure 8). Since mitochondria are the principal source of ROS in cells, these new findings have suggest that removal and/or lowering of RCS would not prevent formation of adducts on proteins but also lower ROS production inside cells.
Figure 8.
Panel (i) shows representative time-lapsed confocal images showing changes in changes in mitochondrial Ca2+ in rat ventricular myocyte acutely exposed to 20 µM MGO and monitored for 65 sec. For this, myocytes were DMEM F12, with 1.8 mM CaCl2 were loaded with the mitochondria localizing probe MitoTracker® Green (100 nM, for 12 min) followed by the fluorogenic mitochondria-targeted Ca2+ probe Rho-2 (5 µM) for 30 min each. After loading, DMEM was replaced with Tyrode solution (140 mM NaCl, 5.4 mM KCl, 1 mM Na2HPO4, 10 mM HEPES, 5 mM glucose, 1.0 mM Ca2+, 1 mM MgCl2, pH 7.4) and cells were placed on the stage of a Ziess LSM 510 Meta laser-scanning microscope MitoTracker® Green was excited at 488 nM and emissions were monitored at 516 nm wavelength. Rhod-2 was excited at 576 nm and emission was at 552 nm. Panel (ii) shows average changes in mitochondria Ca2+ using Image J analysis software. Data are mean ± S.E.M for >12 cells. * denote significantly different from before addition of MGO.
The availability of high affinity antibodies against specific RCS adducts allowed us the opportunity to begin to address this question of which of the many RCS that are upregulated are responsible for perturbation in SR Ca2+ cycling and impairing cardiac contraction seen in chronic diabetes. The principal conclusion from the new data presented is that not all RCS that are upregulated in the heart in diabetes are likely to perturb SR Ca2+ cycling. Of the four RCS investigated, only two, MGO and GO chemically react with and form adducts on RyR2 and SERCA2a in the streptozotocin-induced rat model of type 1 diabetes. The reasons why glucose-generated MGO and glucose/lipid-generated GO preferentially react with RyR2 and SERCA2a and not lipid-derived 4-HNE and MDA is not clear at this time. However, several explanations are likely.
First, greater than 70% of the ATP needed for cardiac functioning is derived from beta-oxidation of free fatty acids (FFAs) [80–81]. This would suggest that the heart has an efficient detoxification system to rid itself of RCS derived from lipid metabolites. Whether organs, like liver and skeletal muscles that use glucose/pyruvate as its primary energy have a detoxification systems that are slanted towards removing glucose-derived RCS and would therefore be more susceptible to lipid-derived RCS are also not known at this time. Second, our data are consistent with the notion that some RCS are inherently more reactive (potent) than others. In this study we found that the di-carbonyl MGO and GO preferentially reacted with RyR2 and SERCA2 than the MDA and 4-NHE. Although MDA is di-carbonyl, one reason why it may not have reacted is that rapid enolizes at physiological pHs’ [82, 83] to the less reactive enol which is a mono-carbonyl like 4-HNE. A third reason may be related to where in the cell MGO, GO, 4-HNE and MDA are generated, and if the enzymes that degrade are up/down regulated. In myocytes, 4-HNE and MDA are generated from oxidation of polyunsaturated fatty acids/lipids on the plasmamembrane, within mitochrondria and in the cytoplasm [47, 84–85]. They are also generated from increased prostaglandin, thromboxane and arachadonic acid biosynthesis [86–87]. What is not well delineated is what fraction of the total 4-HNE and MDA generated inside the cell are derived from each pathway. Increases in expression and activities of aldose reductases and glutathione S-transferases (GSTA4-4 and GST5.8), major enzymes that degrade 4-HNE in diabetes, may also help explain the absence of 4-HNE adducts on RyR2 and SERCA2a [88]. Forth, MGO and GO are generated throughout the cytoplasm from variety of sources. Glyoxalase I (Glo-1), the principal enzyme that degrades MGO and GO [89] is also upregulated in diabetic myocytes [30] and its co-factor reduced glutathione is lowered [90]. This may help explain why MGO (argpyrimidine) and GO adducts are formed on RyR2 and SERCA2a in diabetes.
The new data presented in this short review is not without limitations. Only two commercially available antibodies against MDA and 4-HNE were used according to manufactures’ suggestions (1:000 dilution and 1:500 dilutions) employing 60 µg of SR membrane proteins from control and diabetic rat hearts per gel lane, incubated at 4°C for 16–20 hrs. In future studies other commercially available antibodies will be used along with other approaches such as mass spectrometry. The latter was not pursued earlier since we reasoned that if we could not detect 4-HNE and MDA adducts immunologically, it also be very challenging to detect them using mass spectrometry, especially for very large proteins like RyR2. Another major question that must be addressed is whether MGO, GO, 4-HNE and MDA adducts are formed on RyR2 and SERCA2a in other models of diabetes.
Conclusion
It is becoming clearer that RCS are covalently reacting with cellular proteins in DM and altering their functions. These RCS did not indiscriminately react with all available basic amino acid residues on proteins. Some residues are more susceptible to carbonylation than others, presumably because of the electronic environment of these residues. It is known that basic residues with low pKa’s are more likely to undergo carbonylation than residues with higher pKas, since a higher fraction of the low pKa residue will exist in a deprotonated state at a physiological pH of 7.4. New data present in this review show that not all RCS that are upregulated in diabetes covalently react with every protein inside cells. In the case of cardiac RyR2 and SERCA2a, they are preferentially modified by MGO and GO and not by MDA and 4-HNE. The reason for this selectivity is unclear at this time, but several reasons have been discussed.
New data are also emerging indicating that RCS, in particular MGO, perturbs intracellular Ca2+ homeostasis and increase oxidative stress by increasing mitochondrial ROS production inside cells. At this time it is not clear whether these events are preceding post-translational modifications, i.e., whether RCS are acting as agonist/antagonist at RyR2 and SERCA2 as well. What is clear at this time is that targeting RCS will not only prevent carbonylation/dysfunction of proteins inside cells, but will also minimize disturbance in intracellular Ca2+ homeostasis and reduce ROS production. Knowing this information is especially important since targeting only those RCS that are involved in disease pathogenesis would be advantageous over targeting all RCS in the diabetic heart.
Acknowledgments
This work was supported in part by grants from the Edna Ittner Research Foundation, American Diabetes Association (1-06-RA-11) and the National Institutes of Health (HL085061). The authors thank Janice A. Taylor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy. The authors apologize for relevant studies that have not been cited.
Footnotes
CONFLICT OF INTEREST
Chengju Tian - NONE
Fadhel Alomar - NONE
Caronda J Moore – NONE
Chun Hong Shao - NONE
Shelby Kutty - NONE
Jaipaul Singh - NONE
Keshore R. Bidasee - NONE
References
- 1.American Diabetes Association. Living with Diabetes: Complications. [accessed Nov 6th 2012]; http://www.diabetes.org/living-with-diabetes/complications/ [Google Scholar]
- 2.American Diabetes Association. Economic Costs of Diabetes, in the US in 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 3.Schernthaner G Diabetes and Cardiovascular Disease. Is intensive glucose control beneficial or deadly? Lessons from ACCORD, ADVANCE, VADT, UKPDS, PROactive, and NICE-SUGAR. Wien Med Wochenschr. 2010;160(1–2):8–19. doi: 10.1007/s10354-010-0748-7. [DOI] [PubMed] [Google Scholar]
- 4.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 5.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 9.Genuth S. The UKPDS and its global impact. Diabet Med. 2008;25(Suppl 2):57–62. doi: 10.1111/j.1464-5491.2008.02504.x. [DOI] [PubMed] [Google Scholar]
- 10.ACCORD Study Group. Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byingto RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 13.Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–159. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Knollmann BC. Mechanism underlying catecholaminergic polymorphic ventricular tachycardia and approaches to therapy. J Electrocardiol. 2011;44(6):650–655. doi: 10.1016/j.jelectrocard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Yano M, Yamamoto T, Kobayashi S, Matsuzaki M. Role of ryanodine receptor as a Ca2(+) regulatory center in normal and failing hearts. J Cardiol. 2009;53(1):1–7. doi: 10.1016/j.jjcc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Lehnart SE, Schillinger W, Pieske B, Prestle J, Just H, Hasenfuss G. Sarcoplasmic reticulum proteins in heart failure. Ann N Y Acad Sci. 1998;853:220–230. doi: 10.1111/j.1749-6632.1998.tb08270.x. [DOI] [PubMed] [Google Scholar]
- 17.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci USA. 2010;107(45):19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol. 1997;272(1 Pt 2):H148–H158. doi: 10.1152/ajpheart.1997.272.1.H148. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Y, Ahmed S, Grupp IL, Matlib MA. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol. 2001;281(3):H1137–H1147. doi: 10.1152/ajpheart.2001.281.3.H1137. [DOI] [PubMed] [Google Scholar]
- 21.Lacombe VA, Viatchenko-Karpinski S, Terentyev D, Sridhar A, Emani S, Bonagura JD, Feldman DS, Györke S, Carnes CA. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1787–R1797. doi: 10.1152/ajpregu.00059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligeti L, Szenczi O, Prestia CM, Szabó C, Horváth K, Marcsek ZL, van Stiphout RG, van Riel NA, Op den Buijs J, Van der Vusse GJ, Ivanics T. Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int J Mol Med. 2006;17(6):1035–1043. [PubMed] [Google Scholar]
- 23.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gómez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55(3):608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 24.Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes. 2005;54:3082–3088. doi: 10.2337/diabetes.54.11.3082. [DOI] [PubMed] [Google Scholar]
- 25.Shao CH, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol. 2007;42(1):234–246. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Penpargkul S, Fein F, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol. 1981;13(3):303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- 27.Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283(4):H1398–H1408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- 28.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53(12):3201–3208. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y-J, Elimban V, Takeda S, Ren B, Takeda N, Dhalla NS. Cardiac sarcoplasmic reticulum function and gene expression in chronic diabetes. Cardiovasc Pathol. 1996;1:89–96. [Google Scholar]
- 30.Shao CH, Capek HL, Patel KP, Wang M, Tang K, DeSouza C, Nagai R, Mayhan W, Periasamy M, Bidasee KR. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes. 2011;60(3):947–959. doi: 10.2337/db10-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuter H, Grönke S, Adam C, Ribati M, Brabender J, Zobel C, Frank KF, Wippermann J, Schwinger RH, Brixius K, Müller-Ehmsen J. Sarcoplasmic Ca2+ release is prolonged in nonfailing myocardium of diabetic patients. Mol Cell Biochem. 2008;308(1–2):141–149. doi: 10.1007/s11010-007-9622-3. [DOI] [PubMed] [Google Scholar]
- 32.Guner S, Arioglu E, Tay A, Tasdelen A, Aslamaci S, Bidasee KR, Dincer UD. Diabetes decreases mRNA levels of calcium-release channels in human atrial appendage. Mol Cell Biochem. 2004;263(1–2):143–150. doi: 10.1023/B:MCBI.0000041856.92497.0c. [DOI] [PubMed] [Google Scholar]
- 33.Bidasee KR, Nallani K, Henry B, Dincer UD, Besch HRJ. Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem. 2003;249(1–2):113–123. [PubMed] [Google Scholar]
- 34.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278(45):44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 35.Machackova J, Barta J, Dhalla NS. Molecular defects in cardiac myofibrillar proteins due to thyroid hormone imbalance and diabetes. Can J Physiol Pharmacol. 2005;83(12):1071–1091. doi: 10.1139/y05-121. [DOI] [PubMed] [Google Scholar]
- 36.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26(5):704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 37.Kim HW, Ch YS, Lee HR, Park SY, Kim YH. Diabetic alterations in cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban protein expression. Life Sci. 2001;70(4):367–379. doi: 10.1016/s0024-3205(01)01483-7. [DOI] [PubMed] [Google Scholar]
- 38.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schöneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008;45(6):756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rastogi S, Sentex E, Elimban V, Dhalla NS, Netticadan T. Elevated levels of protein phosphatase 1 and phosphatase 2A may contribute to cardiac dysfunction in diabetes. Biochim Biophys Acta. 2003;1638(3):273–277. doi: 10.1016/s0925-4439(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 40.Netticadan T, Temsah RM, Kent A, Elimban V, Dhalla NS. Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes. 2001;50(9):2133–2128. doi: 10.2337/diabetes.50.9.2133. [DOI] [PubMed] [Google Scholar]
- 41.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96(8):847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 42.Bidasee KR, Nallani K, Besch HR, Jr, Dincer UD. Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2) J Pharmacol Exp Ther. 2003;305(3):989–998. doi: 10.1124/jpet.102.046201. [DOI] [PubMed] [Google Scholar]
- 43.Tian C, Hong Shao C, Moore CJ, Kutty S, Walseth T, Desouza C, Bidasee KR. Gain of function of cardiac ryanodine receptor in a rat model of type 1 diabetes. Cardiovasc Res. 2011;91(2):300–309. doi: 10.1093/cvr/cvr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 45.Tousoulis D, Briasoulis A, Papageorgiou N, Tsioufis C, Tsiamis E, Toutouzas K, Stefanadis C. Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov. 2011;6:103–114. doi: 10.2174/157489011795933819. [DOI] [PubMed] [Google Scholar]
- 46.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28(12):1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 48.Vander Jagt DL. Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metabol Drug Interact. 2008;23(1–2):93–124. doi: 10.1515/dmdi.2008.23.1-2.93. [DOI] [PubMed] [Google Scholar]
- 49.Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007;115(1):13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim Biophys Acta. 2008;1777(10):1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3beta and mitochondrial function. BMC Med. 2012;10:40. doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Agadjanyan ZS, Dmitriev LF, Dugin SF. A new role of phosphoglucose isomerase. Involvement of the glycolytic enzyme in aldehyde metabolism. Biochemistry (Mosc) 2005;70:1251–1255. doi: 10.1007/s10541-005-0255-4. [DOI] [PubMed] [Google Scholar]
- 53.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwata K, Nishinaka T, Matsuno K, Kakehi T, Katsuyama M, Ibi M, Yabe-Nishimura C. The activity of aldose reductase is elevated in diabetic mouse heart. J Pharmacol Sci. 2007;103:408–416. doi: 10.1254/jphs.fp0070136. [DOI] [PubMed] [Google Scholar]
- 55.Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, Van Kuijk FJ, Ansari NH. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Barrera G, Pizzimenti S, Dianzani MU. 4-hydroxynonenal and regulation of cell cycle: effects on the pRb/E2F pathway. Free Radic Biol Med. 2004;37(5):597–606. doi: 10.1016/j.freeradbiomed.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Dianzani MU. 4-hydroxynonenal from pathology to physiology. Mol Aspects Med. 2003;24(4–5):263–272. doi: 10.1016/s0098-2997(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 58.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 59.Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest. 2012;122(6):2306–2315. doi: 10.1172/JCI61319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picklo MJ, Montine TJ, Amarnath V, Neely MD. Carbonyl toxicology and Alzheimer's disease. Toxicol Appl Pharmacol. 2002;184(3):187–197. doi: 10.1006/taap.2002.9506. [DOI] [PubMed] [Google Scholar]
- 61.Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24(4–5):293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 62.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28(6):385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10(2):389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goudeau J, Aguilaniu H. Carbonylated proteins are eliminated during reproduction in C. elegans. Aging Cell. 2010;9(6):991–1003. doi: 10.1111/j.1474-9726.2010.00625.x. [DOI] [PubMed] [Google Scholar]
- 66.Shao CH, Tian C, Ouyang S, Moore CJ, Alomar F, Nemet I, D'Souza A, Nagai R, Kutty S, Rozanski GJ, Ramanadham S, Singh J, Bidasee KR. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol Pharmacol. 2012;82(3):383–399. doi: 10.1124/mol.112.078352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, Van Kuijk FJ, Ansari NH. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 68.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18(6):926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 69.Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbank S, Curry JW, Miyata T, Brownlee M, Schlingemann RO, Schalkwijk C, Stitt AW. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia. 2012;55(3):845–854. doi: 10.1007/s00125-011-2393-0. [DOI] [PubMed] [Google Scholar]
- 70.Pedchenko VK, Chetyrkin SV, Chuang P, Ham AJ, Saleem MA, Mathieson PW, Hudson BG, Voziyan PA. Mechanism of perturbation of integrin-mediated cell-matrix interactions by reactive carbonyl compounds and its implication for pathogenesis of diabetic nephropathy. Diabetes. 2005;54(10):2952–2960. doi: 10.2337/diabetes.54.10.2952. [DOI] [PubMed] [Google Scholar]
- 71.Moore CJ, Shao CH, Nagai R, Kutty S, Singh J, Bidasee KR. Absence of malondialdehyde and 4-hydroxynonenal adducts on cardiac ryanodine receptor (RyR2) and sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2) in diabetes. Mol Cell Biochem. 2013 Jan 25; doi: 10.1007/s11010-013-1558-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 73.de Lemos ET, Oliveira J, Pinheiro JP, Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev. 2012;22012:741545. doi: 10.1155/2012/741545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 75.Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and pro-oxidants. J Cardiovasc Nurs. 2002;16(2):68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Rosenbaugh EG, Roat JW, Gao L, Yang RF, Manickam DS, Yin JX, Schultz HD, Bronich TK, Batrakova EV, Kabanov AV, Zucker IH, Zimmerman MC. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials. 2010;31(19):5218–5226. doi: 10.1016/j.biomaterials.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA BENEFICIAL investigators. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13(8):899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
- 78.Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382(6588):275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 79.Du J, Suzuki H, Nagase F, Akhand AA, Ma XY, Yokoyama T, Miyata T, Nakashima I. Superoxide-mediated early oxidation and activation of ASK1 are important for initiating methylglyoxal-induced apoptosis process. Free Radic Biol Med. 2001;31(4):469–478. doi: 10.1016/s0891-5849(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 80.Taha M, Lopaschuk GD. Alterations in energy metabolism in cardiomyopathies. Ann Med. 2007;39:594–607. doi: 10.1080/07853890701618305. [DOI] [PubMed] [Google Scholar]
- 81.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 82.Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986;261(29):13542–13545. [PubMed] [Google Scholar]
- 83.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43:550–557. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- 84.Marjani A. Lipid peroxidation alterations in type 2 diabetic patients. Pak J Biol Sci. 2010;13:723–730. doi: 10.3923/pjbs.2010.723.730. [DOI] [PubMed] [Google Scholar]
- 85.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–1149. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 86.Basu S. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic Res. 2004;38:105–122. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- 87.Dirkx E, Schwenk RW, Glatz JF, Luiken JJ, van Eys GJ. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot Essent Fatty Acids. 2011;85:219–225. doi: 10.1016/j.plefa.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Iwata K, Nishinaka T, Matsuno K, Kakehi T, Katsuyama M, Ibi M, Yabe-Nishimura C. The activity of aldose reductase is elevated in diabetic mouse heart. J Pharmacol Sci. 2007;103:408–416. doi: 10.1254/jphs.fp0070136. [DOI] [PubMed] [Google Scholar]
- 89.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li S, Li X, Li YL, Shao CH, Bidasee KR, Rozanski GJ. Insulin regulation of glutathione and contractile Phenotype in diabetic rat ventricular myocytes. Am J Physiol. 2008;292(3):H1619–H1629. doi: 10.1152/ajpheart.00140.2006. [DOI] [PubMed] [Google Scholar]