ABSTRACT
Activation of leukemia inhibitor factor (LIF)–Stat3 or Wnt/β-catenin signaling promotes mouse embryonic stem cell (mESC) self-renewal. A myriad of downstream targets have been identified in the individual signal pathways, but their common targets remain largely elusive. In this study, we found that the LIF–Stat3 and Wnt/β-catenin signaling pathways converge on Sp5 to promote mESC self-renewal. Forced Sp5 expression can reproduce partial effects of Wnt/β-catenin signaling but mimics most features of LIF–Stat3 signaling to maintain undifferentiated mESCs. Moreover, Sp5 is able to convert mouse epiblast stem cells into a naïve pluripotent state. Thus, Sp5 is an important component of the regulatory network governing mESC naïve pluripotency.
KEY WORDS: Embryonic stem cell, Self-renewal, β-catenin, Stat3, Sp5
Summary: This study reveals a new function of Sp5 in mouse embryonic stem cell (ESC) self-renewal mediated by CHIR99021 and LIF, and reprogramming of EpiSCs into naїve ESCs.
INTRODUCTION
Mouse embryonic stem cells (mESCs) are pluripotent cell lines derived from pre-implantation embryos (Evans and Kaufman, 1981; Martin, 1981). They can be expanded in serum-containing medium with LIF, or serum-free N2B27 medium with 2i (hereafter N2B27/2i, see below) in vitro while retaining the capacity for multilineage differentiation (Smith, 2001; Williams et al., 1988; Ying et al., 2008). LIF binds to the LIF-receptor–gp130 heterodimer (gp130 is also known as IL6ST), leading to phosphorylation of Stat3 by JAK. Activated Stat3 then translocates into the nucleus and binds DNA (Niwa et al., 1998). Stat3 plays a key role in LIF-mediated mESC self-renewal. A chimeric Stat3 protein fused with the ligand-binding domain of the estrogen receptor (Stat3ER) maintains mESCs in an undifferentiated state in the presence of the synthetic ligand 4-hydroxytamoxifen (4-HT or 4-OHT) without LIF (Matsuda et al., 1999). By contrast, overexpressing a dominant-negative mutant of Stat3 in mESCs blocks LIF-induced activation of endogenous Stat3 and causes differentiation (Niwa et al., 1998). Furthermore, LIF fails to maintain Stat3-null mESC self-renewal (Ying et al., 2008). Many Stat3 targets have been identified recently, such as Klf4, Gbx2, Pim1, Pim3, Pramel7, Myc and Tfcp2l1 (Cartwright et al., 2005; Casanova et al., 2011; Hall et al., 2009; Martello et al., 2013; Tai and Ying, 2013; Ye et al., 2013). When overexpressed, these genes can bypass the LIF requirement for mESC maintenance. However, knockdown of any single gene does not abolish the self-renewal-promoting effect of LIF–Stat3 signaling, suggesting that LIF–Stat3 signaling triggers multiple downstream targets to promote mESC self-renewal.
2i contains two small molecules: CHIR99021 (CHIR) and PD0325901 (PD03), which inhibit glycogen synthase kinase 3 (GSK3) and mitogen-activated protein kinase (MAPK) kinases (MEK proteins), respectively (Huang et al., 2015; Ye et al., 2014; Ying et al., 2008). Inhibiting GSK3 results in the activation of the Wnt/β-catenin signaling pathway. Once in the nucleus, β-catenin liberates many Tcf3-repressed pluripotency genes (Wray et al., 2011), such as Esrrb, Nr0b1, Tfcp2l1 and Nanog (Martello et al., 2012). It is noteworthy that 2i can sustain Stat3-null mESC self-renewal (Ying et al., 2008), whereas the identity of β-catenin-null mESCs can be maintained in medium containing LIF and PD03 (Lyashenko et al., 2011). Taken together, these results suggest CHIR and LIF might share common or cross-compensatory downstream targets in promoting mESC self-renewal. Here, we identified Sp5 as one of common targets of CHIR and LIF, and revealed its new function in promoting mESC self-renewal and reprogramming.
RESULTS AND DISCUSSION
Identification of downstream targets of CHIR and LIF in mESCs
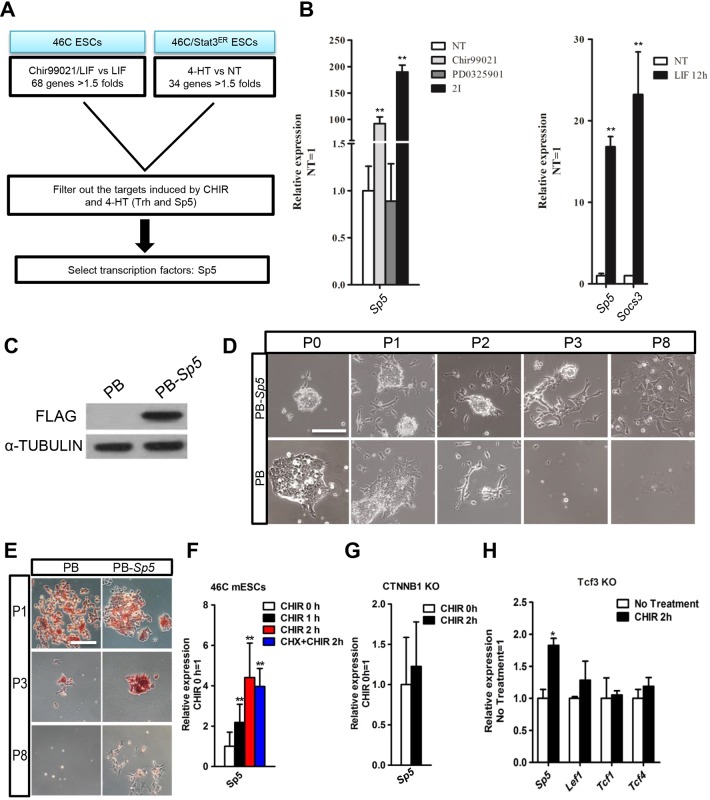
To screen the possible shared targets of the Wnt/β-catenin and LIF–Stat3 signaling pathways, we performed a DNA microarray analysis in mESCs treated with or without CHIR (GEO Number: GSE50393). We then looked for genes that were upregulated by 1.5 times or greater by CHIR treatment or by Stat3 stimulation (Bourillot et al., 2009). From this comparison, two common targets emerged: Trh and Sp5 (Fig. 1A). Trh, or thyrotropin-releasing hormone, is a secretory protein. Sp5 (trans-acting transcription factor 5) belongs to the Sp1 transcription factor family (el-Baradi and Pieler, 1991; Wimmer et al., 1993) and harbors three zinc fingers that are similar to those found in members of the KLF gene family (Suske et al., 2005; Treichel et al., 2001). KLF family members, such as Klf2, Klf4 and Klf5, play an important role in maintaining mESC self-renewal (Ema et al., 2008; Hall et al., 2009; Jiang et al., 2008; Qiu et al., 2015). We thus chose Sp5 as our candidate gene. Quantitative real-time PCR (qRT-PCR) was used to confirm that both CHIR and LIF treatments could induce the expression of Sp5 (Fig. 1B). Socs3, a direct target of LIF–Stat3 signaling, was used as a positive control and showed substantial induction upon LIF stimulation (Fig. 1B) (Niwa et al., 2009).
Fig. 1.
Overexpressing Sp5 partially mimics the effect of CHIR in medium with 2i. (A) Flow chart showing the method used to identify candidate genes downstream of CHIR and Stat3. (B) qRT-PCR analysis of Socs3 and Sp5 expression induced by CHIR, PD03 and 2i (left), or LIF (right) for 12 h. Data represent mean±s.d. of three biological replicates. **P<0.01 versus NT. NT, no treatment. (C) FLAG-tagged Sp5 was introduced into 46C mESCs and the protein level of FLAG-tagged SP5 was determined by western blotting. α-tubulin is used as a loading control. (D,E) Alkaline phosphatase staining images of PB-Sp5 mESCs cultured in N2B27 medium with PD03 for the indicated number of passages (P). Scale bars: 100 μm. (F–H) qRT-PCR analysis of the indicated gene expression after treatment with CHIR for the indicated time in 46C mESCs, CTNNB1-KO (β-catenin knockout) and Tcf3-KO mESCs. CHX, cycloheximide treatment. Data represent mean±s.d. of three biological replicates. **P<0.01 versus 0 h CHIR.
Overexpression of Sp5 promotes mESC self-renewal in the absence of CHIR
We then investigated the function of Sp5 in CHIR- and LIF-mediated self-renewal in mESCs. We generated an mESC line that overexpressed FLAG-tagged Sp5 using a PiggyBac vector (PB-Sp5) in which Sp5 expression was efficiently enhanced (Fig. 1C). Empty vector (PB) and PB-Sp5 mESCs grew robustly in N2B27/2i, and in serum-containing medium with LIF. We then withdrew CHIR to test the ability of Sp5 to replace the function of CHIR in N2B27/2i. Before passaging, PB-Sp5 mESCs maintained an undifferentiated state, whereas cells containing only PB began to die (Fig. 1D,E). Moreover, PB-Sp5 mESCs could be split for three more passages, but then also collapsed and lost alkaline phosphatase activity (Fig. 1D,E). Therefore, overexpressing Sp5 supports short-term mESC self-renewal when combined with PD03, indicating that Sp5 partially reproduces the effect of CHIR in 2i.
Sp5 is known to be a downstream target of the Wnt/β-catenin signaling pathway during vertebrate development and in cancer cells (Hoverter et al., 2012; Waaler et al., 2011; Weidinger et al., 2005). To determine whether Sp5 is regulated through the Wnt/β-catenin signaling pathway in mESCs, we examined the expression of Sp5 in 46C ESCs after CHIR treatment for 2 h. Sp5 exhibited significantly increased expression in response to CHIR, even in the presence of the protein synthesis inhibitor cycloheximide (Fig. 1F). Furthermore, CHIR failed to induce Sp5 expression in β-catenin-null ESCs (Fig. 1G), indicating that Sp5 is a direct target of β-catenin in mESCs. Notably, CHIR upregulated Sp5 in the absence of Tcf3 without a change in the expression of other members of the Tcf family, including Lef1, Tcf1 and Tcf4 (Fig. 1H), indicating that β-catenin can induce Sp5 expression through interaction with Tcf factors but not Tcf3.
Overexpression of Sp5 maintains mESC self-renewal in the absence of LIF
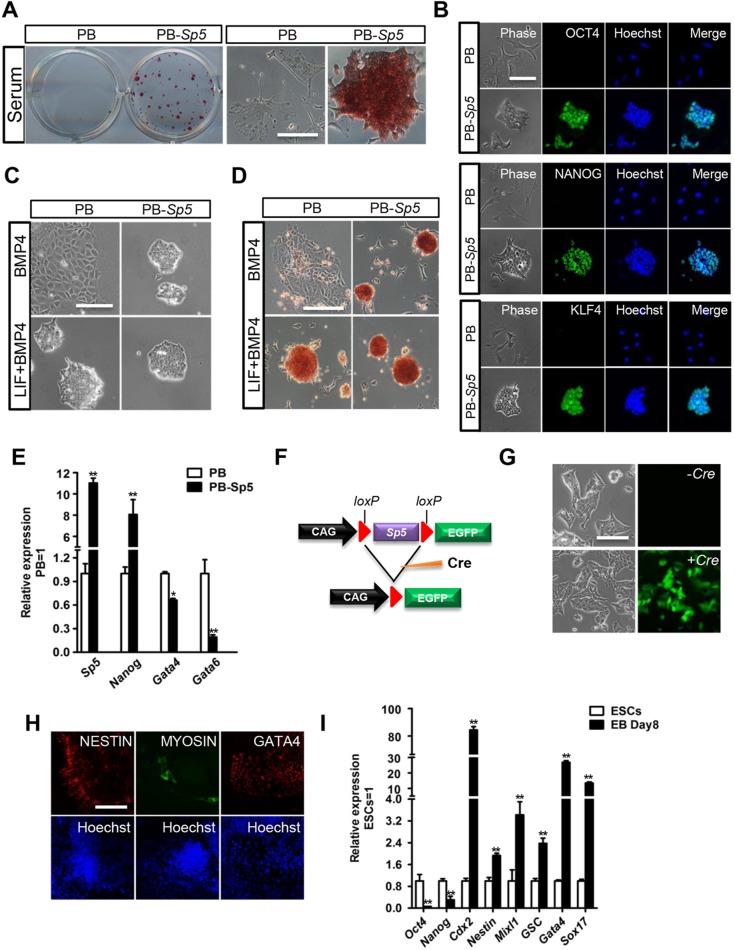
To determine the role of Sp5 in LIF–Stat3 signaling, we performed experiments in serum-containing medium without LIF. Surprisingly, PB-Sp5 mESCs could be continually passaged while retaining typical ESC morphology, positive alkaline phosphatase activity and high expression levels of the pluripotency markers OCT4, NANOG and KLF4, whereas empty vector (PB) control cells differentiated (Fig. 2A,B; Fig. S1A–C). The results were validated in OCRG9 mESCs harboring an Oct4–GFP pluripotency reporter (Fig. S1D,E). We then tried culturing PB and PB-Sp5 46C ESCs in N2B27 medium with BMP4, which maintains mESC self-renewal when combined with LIF (Ying et al., 2003). Similarly, PB-Sp5 mESCs maintained an undifferentiated state, whereas PB cells differentiated rapidly (Fig. 2C,D). We found that overexpression of Sp5 led to an upregulation in the level of Nanog, whereas it repressed the expression of the differentiation markers Gata4 and Gata6. Both of these markers are typically suppressed by Nanog in mESCs (Fig. 2E) (Chambers et al., 2003). These collective results suggest that elevated expression of Sp5 recapitulates the self-renewal-promoting effect of the LIF–Stat3 signaling pathway, probably through induction of Nanog expression.
Fig. 2.
Forced Sp5 expression promotes mESC self-renewal without LIF addition. (A) Alkaline phosphatase staining of PB and PB-Sp5 mESCs cultured in serum for five passages. Scale bar: 100 μm. (B) Immunofluorescence staining of PB and PB-Sp5 mESCs cultured in medium with serum for five passages. Scale bar: 100 μm. Hoechst, Hoechst 33342. (C,D) Phase contrast (C) and alkaline phosphatase staining (D) images of PB and PB-Sp5 mESCs cultured in N2B27 medium with BMP4 in the absence or presence of LIF for 8 days. Scale bars: 100 μm. (E) qRT-PCR analysis of Sp5, Nanog, Gata4 and Gata6 expression in PB and PB-Sp5 46C ESCs cultured in medium with LIF and serum. Data represent mean±s.d. of three biological replicates. *P<0.05, **P<0.01 versus PB. (F) Diagram showing the Cre-excisable construct used for Sp5 overexpression. (G) Phase contrast and GFP images of loxP-Sp5 mESCs after transfection with or without the Cre expression plasmid. ESCs were cultured in medium with LIF and serum. Scale bar: 100 μm. (H) Immunofluorescence staining for the neural marker nestin, the cardiomyocyte marker myosin, and the primitive endoderm marker GATA4 in differentiated cells derived from loxP-Sp5 mESCs in which the Sp5 transgene has been removed by Cre. Hoechst 33342 was used for nuclear staining. Scale bar: 100 μm. (I) qRT-PCR analysis of the indicated gene expression in ESCs and embryoid bodies (EB) derived from cells with an excised Sp5 transgene. Pluripotency genes, Oct4 and Nanog; trophoblast marker, Cdx2; ectoderm marker, nestin; mesoderm markers, Mixl1 and Gsc; endoderm makers, Gata4 and Sox17. *P<0.05, **P<0.01 versus ESCs.
To examine whether mESCs maintained by Sp5 overexpression retain pluripotency, we used a loxP-based excisable vector to overexpress Sp5. After expansion in the absence of LIF for five passages, the floxed-Sp5 mESCs were transiently transfected with a Cre expression vector to excise the floxed Sp5 transgene (Fig. 2F). After the excision of Sp5 transgene, the GFP transgene was activated, and their maintenance became dependent on LIF (Fig. 2G). The revertant cells maintained the ability to differentiate into cells from the three germ layers after forming embryoid bodies (Fig. 2H,I), suggesting that Sp5 overexpressing ESCs retain their pluripotency.
Overexpressing Sp5 sustains Stat3-null ESCs in an undifferentiated state
To determine whether Stat3 is dispensable for Sp5-promoted mESC self-renewal, we overexpressed Sp5 in Stat3-null mESCs and cultured them in serum-containing medium without LIF. These transfectants remained undifferentiated, whereas Stat3-null mESCs transfected with an empty vector died or differentiated after two passages (Fig. S2). These results suggest that the self-renewal-promoting effect of Sp5 is independent of Stat3.
Sp5 is a direct downstream target of the LIF–Stat3 signaling pathway
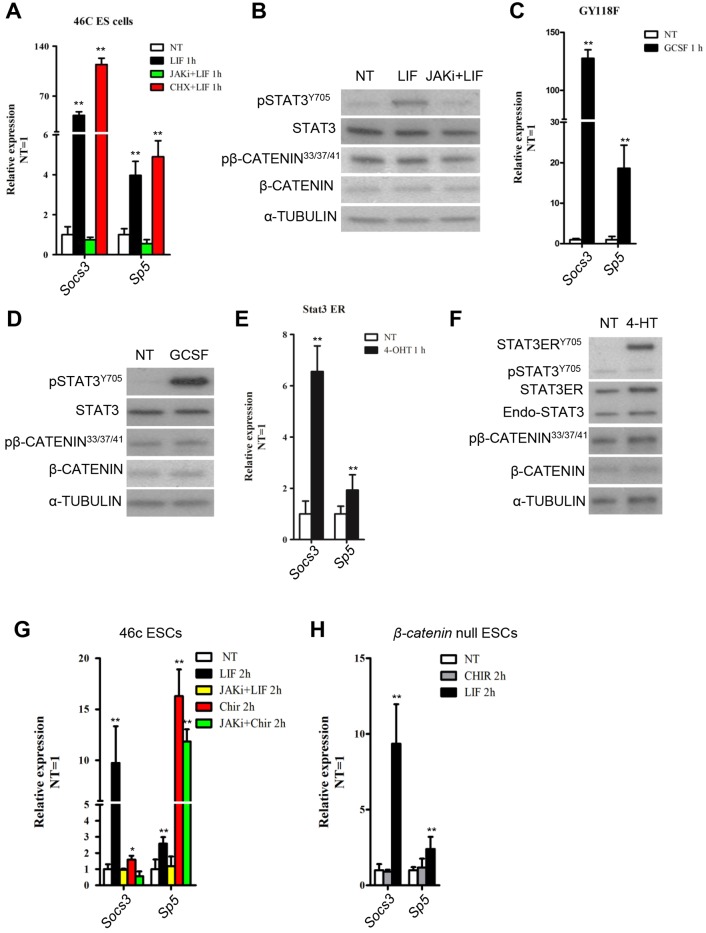
LIF mainly activates three intracellular signaling pathways: the JAK–Stat3, PI3K–AKT and MAPK pathways (Hirai et al., 2011). To examine whether Sp5 is a direct target of LIF–Stat3 pathway, we tested the responsiveness of Sp5 to the activation of Stat3. LIF stimulation for 1 h in 46C mESCs led to an accumulation of phosphorylated STAT3 and acute transcript induction of Socs3 and Sp5 (Fig. 3A,B). However, their expression was suppressed by JAK inhibitor (Fig. 3A). This phenomenon was also observed in the presence of the protein synthesis inhibitor cycloheximide (Fig. 3A,B). A similar expression pattern of Sp5 induction was observed in GY118F and Stat3ER transfectants (Fig. 3C–F). GY118F is a mutated chimeric receptor and only triggers JAK–Stat3 signaling in the presence of granulocyte colony stimulating factor (GCSF) (Burdon et al., 1999). Stat3ER stimulates Stat3 direct targets by translocating into the nucleus in the presence of 4-HT. This process occurs independently of endogenous STAT3, MAPK and AKT (Bourillot et al., 2009; Matsuda et al., 1999). Collectively, these data indicate that Sp5 is a direct target of the LIF–Stat3 signaling pathway. However, knockdown of Sp5 did not impair the self-renewal-promoting effect of LIF–Stat3 signaling (Fig. S3A,B). Interestingly, downregulation of Sp5 significantly induced Pim1 (a Stat3 direct target) expression (Fig. S3A) (Martello et al., 2013), suggesting that the self-renewal-promoting function of Sp5 is redundant with other Stat3 targets in mESCs.
Fig. 3.
Sp5 is a direct target of Stat3. (A) qRT-PCR analysis of Socs3 and Sp5 expression levels in 46C mESCs deprived of LIF overnight and stimulated with LIF for 1 h in the presence or absence of JAK inhibitor I (JAKi) or cycloheximide (CHX, 50 mg/ml). Data represent mean±s.d. of three biological replicates. **P<0.01 vesus 46C NT. NT, no treatment. (B,C) qRT-PCR analysis of Socs3 and Sp5 in GY118F and Stat3ER transfectants deprived of LIF overnight and stimulated with GCSF or 4-HT for 1 h. Data represent mean±s.d. of three biological replicates. **P<0.01 versus NT. (D–F) Western blot analysis of total and phosphorylated Stat3 and β-catenin in wild type, and GY118F- and Stat3ER-transfected mESCs. p, phosphorylated protein. (G,H) qRT-PCR analysis of Socs3 and Sp5 in β-catenin-null or 46C mESCs treated with LIF or CHIR for 2 h in the presence or absence of JAK inhibitor I. Data represent mean±s.d. of three biological replicates. *P<0.05, **P<0.01 versus NT.
LIF and CHIR upregulates Sp5 expression independently
Given that Sp5 is also a direct target of the Wnt/β-catenin signaling pathway (Dunty et al., 2014; Weidinger et al., 2005), we next wanted to examine whether LIF and CHIR depended on each other to exert their own upregulating effect. CHIR strongly induced Sp5 expression in 46C mESCs in the presence of JAK inhibitor whereas LIF failed (Fig. 3G). By contrast, LIF stimulated Sp5 expression in the absence of β-catenin, whereas CHIR-mediated induction of Sp5 was dependent on β-catenin (Fig. 3H), indicating that LIF and CHIR induce Sp5 transcription independently of one another.
Sp5 is able to reprogram EpiSCs to a naïve state
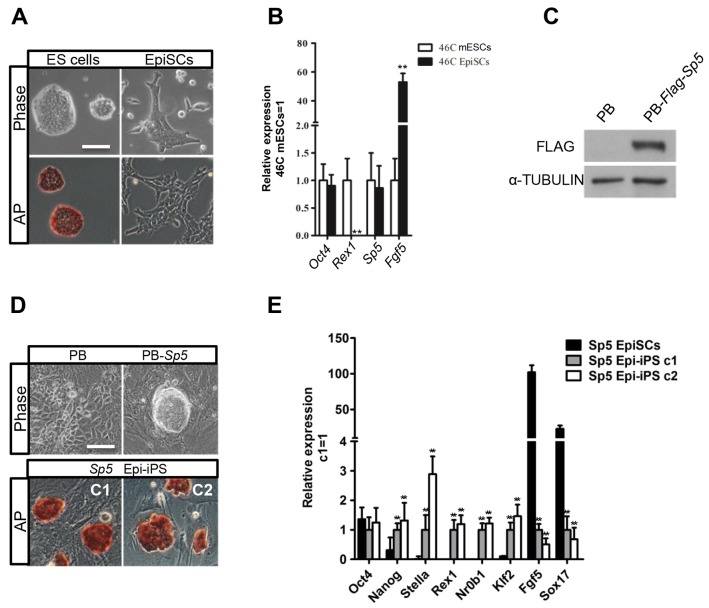
Another distinguishing feature of LIF–Stat3 signaling is its ability to convert mouse epiblast stem cells (EpiSCs) into cells with a naïve state of pluripotency (van Oosten et al., 2012; Yang et al., 2010). Mouse EpiSCs are isolated from epiblasts of post-implantation mouse embryos and share many features with human ESCs (Brons et al., 2007; Tesar et al., 2007). Stat3 and its multiple targets are expressed highly in mESCs, but are downregulated in EpiSCs. Importantly, their overexpression is sufficient to reprogram EpiSC into the mESC state (Guo et al., 2009; Tai and Ying, 2013; Yang et al., 2010). To test the role of Sp5 in EpiSC reprogramming, we first differentiated 46C mESCs into EpiSCs (Fig. 4A), but observed no obvious change in Sp5 expression (Fig. 4B). We then engineered 46C EpiSCs to overexpress Sp5 (Fig. 4C) and cultured 106 transfectants in medium containing LIF, serum and 2i. After 12 days, five ESC-like colonies emerged, whereas empty vector transfectants died or differentiated (Fig. 4D). We then picked two colonies and these colonies continuously expanded in medium containing serum and LIF. They expressed high levels of the naïve pluripotency markers Nanog, Stella, Nrob1, Klf2 and Rex1, but low levels of the EpiSC-specific markers Fgf5 and Sox17 (Fig. 4E). We confirmed this experiment in E3 EpiSCs carrying an Oct4–GFP pluripotency reporter (Greber et al., 2010). The Sp5-reprogrammed stem cells were expanded in medium containing serum and LIF over multiple passages, showing undifferentiated morphology and stable expression of the Oct4–GFP reporter (Fig. S4A,B). These results imply that forced expression of Sp5 is capable of converting EpiSCs into a naïve pluripotent state.
Fig. 4.
Forced expression of Sp5 promotes EpiSC reprogramming to naïve pluripotency. (A) Phase contrast and alkaline phosphatase (AP) staining images of 46C mESCs and 46C mESC-derived EpiSCs. Scale bar: 100 μm. (B) qRT-PCR analysis of expression patterns of Sp5, Oct4, Rex1 and Fgf5 in 46C mESCs and EpiSCs. Data represent mean±s.d. of three biological replicates. **P<0.01 versus 46C mESCs. (C) Western blot analysis for Sp5 in 46C EpiSCs transfected with vector expressing FLAG-tagged Sp5. (D) Phase contrast and alkaline phosphatase staining images of Sp5 EpiSC transfectants and Sp5 EpiSC transfectant-derived induced pluripotent stem cells (iPSCs). Scale bar: 100 μm. (E) qRT-PCR analysis of Oct4, Nanog, Stella, Nr0b1, Klf2, Rex1, Fgf5 and Sox17 expression in Sp5 EpiSCs and two Sp5 Epi-iPS colonies. Data represent mean±s.d. of three biological replicates. **P<0.01 versus Sp5 EpiSCs. C1 and C2 are the individual colonies of ESC-like cell. Epi-iPS, EpiSC-derived iPSCs.
Conclusions
CHIR and LIF have redundant function in mESC maintenance. We demonstrate that Sp5 is a convergent target of the Wnt/β-catenin and LIF–Stat3 signaling pathways. In support of this, we present evidence that Sp5 recapitulates features of Wnt/β-catenin and LIF–Stat3 signaling in promoting mESC self-renewal. Furthermore, overexpression of Sp5 is able to overcome the differentiation cues to convert EpiSCs into cells with naïve pluripotency. Our study therefore reveals a new function of Sp5 in mESC self-renewal, which is mediated by CHIR and LIF, and reprogramming of EpiSCs into naïve ESCs. In the future, understanding how Sp5 cooperates with other downstream targets of β-catenin and Stat3 to maintain the pluripotent ESC state might facilitate the development of new culture conditions for the derivation of authentic ESCs from various species.
MATERIALS AND METHODS
Cell culture
46C mESCs, kindly provided by Austin Smith (Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute, University of Cambridge, UK), were cultured on 0.1% gelatin-coated dishes. mESC medium contains Glasgow's minimum essential medium (GMEM; Sigma), 10% fetal calf serum (FCS; HyClone), 1% MEM nonessential amino acids (Invitrogen), 2 mM GlutaMax (Invitrogen), 0.1 mM β-mercaptoethanol (Invitrogen) and 100 units/ml LIF (prepared in-house). For serum-free culture, mESCs were maintained in N2B27 supplemented with 3 µM CHIR99021 and 1 µM PD0325901 (Sigma), or supplemented with 100 units/ml LIF and 10 ng/ml BMP4 (Peprotech).
Plasmid construction and cell transfection
The coding region of Sp5 was inserted into a PiggyBac vector and transduced into cells combined with 2 µg transposase using LTX (Invitrogen). Selection was continued for 1 week by adding 2 µg/ml puromycin. The short hairpin RNA (shRNA) plasmids were generated according to the Addgene PLKO.1 protocol. The target-specific sequence for Sp5 is 5′-GGATTCAAAGGATTTGCTTTC-3′.
Alkaline phosphatase activity assay and qRT-PCR
The alkaline phosphatase activity assay and qRT-PCR analyses were performed according to our previous report (Ye et al., 2013). The primers used for the qRT-PCR analyses are listed in Table S1.
Western blotting
Western blotting was performed according to a standard protocol. The primary antibodies used for probing were against α-tubulin (32-2500, Invitrogen; 1:2000), phosphorylated Stat3Y705 (9131S, Cell Signaling Technology; 1:1000), Stat3 (610190, BD Biosciences; 1:1000), phosphorylated β-CateninSer33/Ser37/Thr41 (9561S, Cell Signaling Technology; 1:1000) and β-catenin (610153, BD Biosciences; 1:1000).
Immunofluorescence staining
Immunostaining was performed with standard protocols. Primary antibodies used were against Oct4 (sc-5279, Santa Cruz Biotechnology; 1:200), Nanog (AF2729, R&D; 1:100), Klf4 (AF3158, R&D; 1:100), nestin (2Q178, Santa Cruz Biotechnology, 1:100), GATA4 (G4, Santa Cruz Biotechnology, 1:100) and myosin (MF-20, Developmental Studies Hybridoma Bank, 1:50).
mEpiSC derivation and reprogramming
For ESC-to-EpiSC differentiation, 5000 46C mESCs were plated into 0.1% gelatin-coated plates and cultured in serum medium supplemented with activin A (10 ng/ml, Peprotech), bFGF (10 ng/ml, Peprotech) and JW55 (20 µm, Tocris). Cells were used after 10 passages. For reprogramming, 106 transfectants were seeded onto a 3.5-cm dish and cultured in mESC medium supplemented with LIF and 2i. The number of alkaline-phosphatase-positive clones was counted under a microscope. ESC-like clones were picked and subsequently expanded in medium containing LIF and serum.
Accession numbers
Data and details of the method for the DNA microarray analysis are available in the Gene Expression Omnibus under accession number GSE50393.
Statistical analysis
All data are reported as the mean±s.d. A Student's t-test was used to determine the significance of differences in comparisons. Values of P<0.05 were considered as statistically significant.
Acknowledgements
We thank all members of the Ying laboratory for technical assistance, Edward Trope for critical reading of this manuscript, and Bradley J. Merrill (Department of Biochemistry and Molecular Genetics, University of Illinois at Chicago, IL) and Hans R. Schöler (Department of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, Germany) for providing Tcf3-null mESCs and Oct4–GFP EpiSCs, respectively.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.Y., D.Z. and F.C. designed the study and performed the experiments; D.W. and J.M. contributed reagents; F.L., S.H., K.H., Q.B. and D.L. analyzed data; S.Y. and Q.-L.Y. wrote the manuscript.
Funding
This work was supported by California Institute for Regenerative Medicine (CIRM) New Faculty Award II [grant number RN2-00938]; a CIRM Scientific Excellence through Exploration and Development (SEED) Grant [grant number RS1-00327]; the 211 Scientific Research Startup Fund of Anhui University [grant numbers 10117700027, 32030081, J10117700060]; the Student Research Training Program of Anhui University [grant number J10118515486]; and the Natural Science Foundation of Anhui province and China [grant numbers 1508085SQC204, 1508085MH189, 31501191]. Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.177675/-/DC1
References
- Bourillot P.-Y., Aksoy I., Schreiber V., Wianny F., Schulz H., Hummel O., Hubner N. and Savatier P. (2009). Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells 27, 1760-1771. 10.1002/stem.110 [DOI] [PubMed] [Google Scholar]
- Brons I. G. M., Smithers L. E., Trotter M. W. B., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A. et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191-195. 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- Burdon T., Chambers I., Stracey C., Niwa H. and Smith A. (1999). Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs 165, 131-143. 10.1159/000016693 [DOI] [PubMed] [Google Scholar]
- Cartwright P., McLean C., Sheppard A., Rivett D., Jones K. and Dalton S. (2005). LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132, 885-896. 10.1242/dev.01670 [DOI] [PubMed] [Google Scholar]
- Casanova E. A., Shakhova O., Patel S. S., Asner I. N., Pelczar P., Weber F. A., Graf U., Sommer L., Bürki K. and Cinelli P. (2011). Pramel7 mediates LIF/STAT3-dependent self-renewal in embryonic stem cells. Stem Cells 29, 474-485. 10.1002/stem.588 [DOI] [PubMed] [Google Scholar]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S. and Smith A. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643-655. 10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- Dunty W. C. Jr, Kennedy M. W. L., Chalamalasetty R. B., Campbell K. and Yamaguchi T. P. (2014). Transcriptional profiling of Wnt3a mutants identifies Sp transcription factors as essential effectors of the Wnt/beta-catenin pathway in neuromesodermal stem cells. PLoS ONE 9, e87018 10.1371/journal.pone.0087018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T. and Pieler T. (1991). Zinc finger proteins: what we know and what we would like to know. Mech. Dev. 35, 155-169. 10.1016/0925-4773(91)90015-X [DOI] [PubMed] [Google Scholar]
- Ema M., Mori D., Niwa H., Hasegawa Y., Yamanaka Y., Hitoshi S., Mimura J., Kawabe Y.-I., Hosoya T., Morita M. et al. (2008). Krüppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell 3, 555-567. 10.1016/j.stem.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Evans M. J. and Kaufman M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154-156. 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- Greber B., Wu G., Bernemann C., Joo J. Y., Han D. W., Ko K., Tapia N., Sabour D., Sterneckert J., Tesar P. et al. (2010). Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215-226. 10.1016/j.stem.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Guo G., Yang J., Nichols J., Hall J. S., Eyres I., Mansfield W. and Smith A. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063-1069. 10.1242/dev.030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Guo G., Wray J., Eyres I., Nichols J., Grotewold L., Morfopoulou S., Humphreys P., Mansfield W., Walker R. et al. (2009). Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 5, 597-609. 10.1016/j.stem.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Hirai H., Karian P. and Kikyo N. (2011). Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem. J. 438, 11-23. 10.1042/BJ20102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoverter N. P., Ting J.-H., Sundaresh S., Baldi P. and Waterman M. L. (2012). A WNT/p21 circuit directed by the C-clamp, a sequence-specific DNA binding domain in TCFs. Mol. Cell. Biol. 32, 3648-3662. 10.1128/MCB.06769-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Ye S., Zhou X., Liu D. and Ying Q.-L. (2015). Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network. Cell. Mol. Life Sci. 72, 1741-1757. 10.1007/s00018-015-1833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Chan Y.-S., Loh Y.-H., Cai J., Tong G.-Q., Lim C.-A., Robson P., Zhong S. and Ng H.-H. (2008). A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 10, 353-360. 10.1038/ncb1698 [DOI] [PubMed] [Google Scholar]
- Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R. T. and Hartmann C. (2011). Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 13, 753-761. 10.1038/ncb2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G., Sugimoto T., Diamanti E., Joshi A., Hannah R., Ohtsuka S., Göttgens B., Niwa H. and Smith A. (2012). Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11, 491-504. 10.1016/j.stem.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G., Bertone P. and Smith A. (2013). Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 32, 2561-2574. 10.1038/emboj.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634-7638. 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Nakamura T., Nakao K., Arai T., Katsuki M., Heike T. and Yokota T. (1999). STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18, 4261-4269. 10.1093/emboj/18.15.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I. and Smith A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048-2060. 10.1101/gad.12.13.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Ogawa K., Shimosato D. and Adachi K. (2009). A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460, 118-122. 10.1038/nature08113 [DOI] [PubMed] [Google Scholar]
- Qiu D., Ye S., Ruiz B., Zhou X., Liu D., Zhang Q. and Ying Q.-L. (2015). Klf2 and Tfcp2l1, two Wnt/beta-catenin targets, Act synergistically to induce and maintain naive pluripotency. Stem Cell Rep. 5, 314-322. 10.1016/j.stemcr.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G. (2001). Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435-462. 10.1146/annurev.cellbio.17.1.435 [DOI] [PubMed] [Google Scholar]
- Suske G., Bruford E. and Philipsen S. (2005). Mammalian SP/KLF transcription factors: bring in the family. Genomics 85, 551-556. 10.1016/j.ygeno.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Tai C.-I. and Ying Q.-L. (2013). Gbx2, a LIF/Stat3 target, promotes reprogramming to and retention of the pluripotent ground state. J. Cell Sci. 126, 1093-1098. 10.1242/jcs.118273 [DOI] [PubMed] [Google Scholar]
- Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L. and McKay R. D. G. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196-199. 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- Treichel D., Becker M.-B. and Gruss P. (2001). The novel transcription factor gene Sp5 exhibits a dynamic and highly restricted expression pattern during mouse embryogenesis. Mech. Dev. 101, 175-179. 10.1016/S0925-4773(00)00544-X [DOI] [PubMed] [Google Scholar]
- van Oosten A. L., Costa Y., Smith A. and Silva J. C. R. (2012). JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nat. Commun. 3, 817 10.1038/ncomms1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaler J., Machon O., von Kries J. P., Wilson S. R., Lundenes E., Wedlich D., Gradl D., Paulsen J. E., Machonova O., Dembinski J. L. et al. (2011). Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 71, 197-205. 10.1158/0008-5472.CAN-10-1282 [DOI] [PubMed] [Google Scholar]
- Weidinger G., Thorpe C. J., Wuennenberg-Stapleton K., Ngai J. and Moon R. T. (2005). The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr. Biol. 15, 489-500. 10.1016/j.cub.2005.01.041 [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A. and Gough N. M. (1988). Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684-687. 10.1038/336684a0 [DOI] [PubMed] [Google Scholar]
- Wimmer E. A., Jäckle H., Pfeifle C. and Cohen S. M. (1993). A Drosophila homologue of human Sp1 is a head-specific segmentation gene. Nature 366, 690-694. 10.1038/366690a0 [DOI] [PubMed] [Google Scholar]
- Wray J., Kalkan T., Gomez-Lopez S., Eckardt D., Cook A., Kemler R. and Smith A. (2011). Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat. Cell Biol. 13, 838-845. 10.1038/ncb2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., van Oosten A. L., Theunissen T. W., Guo G., Silva J. C. R. and Smith A. (2010). Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell 7, 319-328. 10.1016/j.stem.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Li P., Tong C. and Ying Q.-L. (2013). Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J. 32, 2548-2560. 10.1038/emboj.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Liu D. and Ying Q.-L. (2014). Signaling pathways in induced naive pluripotency. Curr. Opin. Genet. Dev. 28, 10-15. 10.1016/j.gde.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.-L., Nichols J., Chambers I. and Smith A. (2003). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281-292. 10.1016/S0092-8674(03)00847-X [DOI] [PubMed] [Google Scholar]
- Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P. and Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519-523. 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]