Abstract
Objective:
Gabapentin is commonly used off-label in the treatment of psychiatric disorders with success, failure, and controversy. A systematic review of the literature was performed to elucidate the evidence for clinical benefit of gabapentin in psychiatric disorders.
Data sources:
Bibliographic reference searches for gabapentin use in psychiatric disorders were performed in PubMed and Ovid MEDLINE search engines with no language restrictions from January 1, 1983, to October 1, 2014, excluding nonhuman studies. For psychiatric references, the keywords bipolar, depression, anxiety, mood, posttraumatic stress disorder (posttraumatic stress disorder and PTSD), obsessive-compulsive disorder (obsessive-compulsive disorder and OCD), alcohol (abuse, dependence, withdraw), drug (abuse, dependence, withdraw), opioid (abuse, dependence, withdraw), cocaine (abuse, dependence, withdraw), and amphetamine (abuse, dependence, withdraw) were crossed with gabapentin OR neurontin.
Study selection and data extraction:
The resulting 988 abstracts were read by 2 reviewers; references were excluded if gabapentin was not a study compound or psychiatric symptoms were not studied. The resulting references were subsequently read, reviewed, and analyzed; 219 pertinent to gabapentin use in psychiatric disorders were retained. Only 34 clinical trials investigating psychiatric disorders contained quality of evidence level II-2 or higher.
Results:
Gabapentin may have benefit for some anxiety disorders, although there are no studies for generalized anxiety disorder. Gabapentin has less likely benefit adjunctively for bipolar disorder. Gabapentin has clearer efficacy for alcohol craving and withdrawal symptoms and may have a role in adjunctive treatment of opioid dependence. There is no clear evidence for gabapentin therapy in depression, PTSD prevention, OCD, or other types of substance abuse. Limitations of available data include variation in dosing between studies, gabapentin as monotherapy or adjunctive treatment, and differing primary outcomes between trials.
Conclusions:
Further research is required to better clarify the benefit of gabapentin in psychiatric disorders.
Clinical Points
■ Gabapentin appears to have some benefit for anxiety disorders but failed to show benefit in bipolar disorder trials.
■ In the individual patient with a mixed psychiatric disorder, benefits are most likely due to anxiolytic effects.
■ Gabapentin has modest efficacy for alcohol craving and withdrawal symptoms and may have some benefit in opioid dependence as an adjunct therapy.
Gabapentin was originally approved by the US Food and Drug Administration (FDA) for the treatment of partial seizures in 1993,1,2 with subsequent approval for postherpetic neuralgia in 2002.3–5 Within a decade of initial FDA approval, gabapentin’s second most common use became off-label prescription for psychiatric disorders.6 Gabapentin’s use in psychiatric disorders has been shrouded in controversy, from the 1996 lawsuit against Warner-Lambert for promoting Neurontin for off-label indications, including psychiatric disorders,7,8 to more recent criticism of a number of industry-sponsored trials due to selective reporting and positive publication bias.9
Gabapentin was developed to create a γ-aminobutyric acid (GABA) neurotransmitter analog.2 However, it exerts no GABA agonist effects and does not inhibit GABA uptake or degradation.10–13 The most accepted molecular mechanism of gabapentin is binding at the α2δ1 subunit of Ca2+ channels affecting Ca2+ currents.14–16 The ubiquity of α2δ1 Ca2+ channels in the brain and spinal cord most likely explain the benefit of gabapentin in seizures, pain, and multiple disorders.16
Gabapentin has a limited, generally well-tolerated side effect profile, and since it is not hepatically metabolized, has minimal drug-drug interactions. With safety, efficacy, and a proposed mechanism well-established for treating neuropathic pain and seizure,2–5,10–13,16 numerous case reports and reviews suggest gabapentin’s potential efficacy as either monotherapy or adjunctive therapy in the treatment of bipolar disorder, depression, anxiety disorders, posttraumatic stress disorder (PTSD), alcohol dependence, and other types of drug abuse. The purpose of this review is to evaluate gabapentin use for psychiatric disorders with particular attention paid to randomized controlled trials.
METHOD
An initial bibliographic reference search for gabapentin use in psychiatric disorders was performed in PubMed and Ovid MEDLINE from January 1, 1983 (gabapentin’s appearance in medical research literature), to October 1, 2014 with no language restrictions. For psychiatric references, keywords bipolar, depression, anxiety, mood, posttraumatic stress disorder (posttraumatic stress disorder and PTSD), obsessive-compulsive disorder (obsessive-compulsive disorder and OCD), alcohol (abuse, dependence, withdraw), drug (abuse, dependence, withdraw), opioid (abuse, dependence, withdraw), cocaine (abuse, dependence, withdraw), and amphetamine (abuse, dependence, withdraw) were then crossed with gabapentin OR neurontin. Nonhuman studies were excluded.
The reference abstracts were read by 2 reviewers (M.D.P. and P.M.B. or M.D.P. and R.K.B.), and, based on the abstract, references were excluded if gabapentin was not a study compound or psychiatric symptoms were not studied. Nonblinded studies or case reports that did not describe a unique finding were eliminated. The resulting references were subsequently reviewed, analyzed, and discussed with special attention to clinical trials with quality of evidence level II-2 or higher.17,18
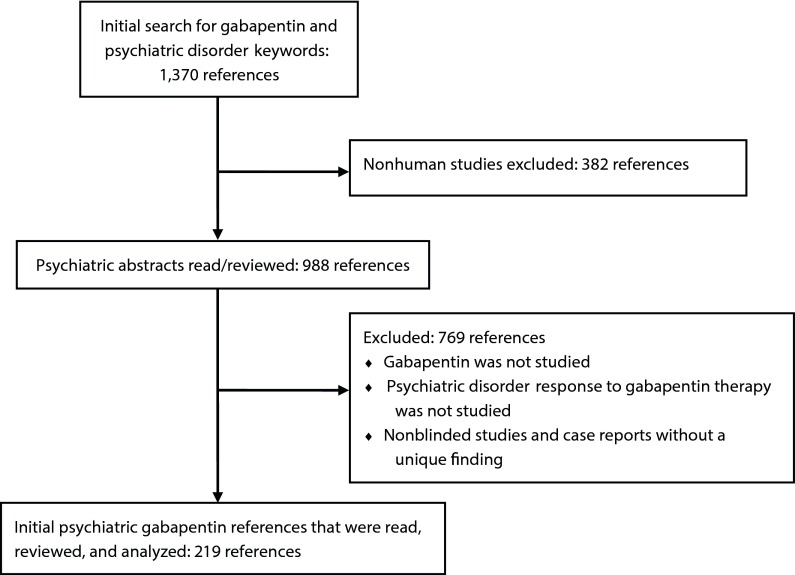
Initial keyword search for gabapentin use in psychiatric references resulted in 1,370 references. Eliminating nonhuman studies and based on the inclusion criteria, 219 articles pertinent to gabapentin use in the treatment of psychiatric disorders were extracted (Figure 1). Thirty-four clinical trials were quality of evidence level II-2 or higher (Table 1).
Figure 1.
Reference Search Design for Gabapentin Use in Psychiatric Disorders
Table 1.
Psychiatric Disorder Clinical Trials With Quality of Evidence at a Level of II-2 or Highera
| Condition | Study/Method | Participants | Gabapentin Dosing | Outcomes | Results | Conclusions | Funding |
| Bipolar disorder | Pande et al, 200019 Randomized, double-blind, placebo-controlled trial; gabapentin add-on Jadad18 score = 3 | 114 refractory bipolar 1 patients randomized: 59 placebo, 55 gabapentin | Gabapentin 600–3,600 mg/d in add-on group; all patients taking lithium, valproate, or both; 10-wk trial | YMRS, HDRS | YMRS and HDRS scores decreased in both groups; no significant difference after controlling for adjustments made to baseline medications | Gabapentin is not an effective add-on therapy to treat mania or depressive symptoms in bipolar I patients with persistent symptoms | Parke-Davis |
| Bipolar disorder | Frye et al, 200020 Randomized, double-blind, placebo-controlled crossover series; gabapentin monotherapy Jadad score = 5 | 38 refractory bipolar and unipolar patients randomized for three 6-wk medication trials | Gabapentin: mean = 3,987 mg/d (SD = 856); lamotrigine: mean = 274 mg/d (SD = 128); 6 wk per medication with 1-wk washout between | CGI-BP | Lamotrigine was superior to gabapentin and placebo in lowering CGI-BP scores; no difference between gabapentin and placebo | Gabapentin is not an effective monotherapy to treat mania or depressive symptoms in refractory bipolar/unipolar patients | Ted and Vada Stanley Foundation |
| Bipolar disorder | Obrocea et al, 200221 Randomized, double-blind, placebo-controlled crossover series; gabapentin monotherapy Jadad score = 3 | 45 patients with refractory bipolar (n = 35) and unipolar (n = 10) disorder randomized for three 6-wk medication trials (note: 38 patients from Frye et al, 200020) | Maximum gabapentin dose: 4,800 mg/d; maximum lamotrigine dose: 500 mg/d; 6 wk per medication with 1-wk washout between | CGI-BP | Response rates to lamotrigine were greater than rates from gabapentin and placebo; response to gabapentin predicted by younger age and lower weight | Gabapentin is not an effective monotherapy to treat mania or depressive symptoms in refractory bipolar/unipolar patients | Ted and Vada Stanley Foundation |
| Bipolar disorder | Vieta et al, 200632 Randomized, double-blind, placebo-controlled trial with add-on gabapentin Jadad score = 5 | 25 euthymic (in remission) bipolar I and II patients randomly assigned to gabapentin add-on or placebo for 1 y | Gabapentin 900–2,400 mg/d for 12-mo trial | CGI-BP modified, YMRS, HDRS, HARS, PSQI | Gabapentin vs placebo group showed higher drops in baseline to endpoint CGI-BP scores; no emerging manic or depressive symptoms in either group | While both placebo and gabapentin add-on groups did not experience manic or depressive episodes over 1 y, the gabapentin group had lower CGI-BP scores | Pfizer |
| Bipolar disorder | Astaneh and Rezaei, 201222 Case-control study Jadad score = 0 | 60 patients with bipolar disorder in acute phase of mania treated with lithium only or lithium and gabapentin 900 mg | Gabapentin 900 mg for 6 wk | YMRS | YMRS scores significantly improved in the case group | Adjunctive gabapentin is effective for treatment of acute mania | None reported |
| Anxiety disorder | Pande et al, 200055 Randomized, double-blind, placebo-controlled study for add-on gabapentin in panic disorder Jadad score = 3 | 103 patients with panic disorder randomized to treatment or placebo | Gabapentin 600–3,600 mg/d in add-on group for 8-wk trial | PAS | No difference in PAS scores by group; subset of patients with higher PAS scores experienced benefit from gabapentin | Gabapentin is no more effective than placebo in treating panic disorder symptoms | Parke-Davis |
| Anxiety disorder | Pande et al, 199954 Randomized, double-blind, placebo-controlled study for add-on gabapentin in social phobia Jadad score = 3 | 69 patients with social phobia were randomized to treatment or placebo | Gabapentin 900–3,600 mg/d in add-on group for 14-wk trial | LSAS, Brief Social Phobia Scale, Marks-Mathews’ Fear Questionnaire, Social Phobia Inventory, CGI, HDRS, HARS | LSAS scores showed a statistically significant decrease in the gabapentin group and similar reduction in other anxiety/clinical impression assays | Gabapentin is more effective than placebo in treating social phobia | Parke-Davis |
| Anxiety disorder | Ménigaux et al, 200559 Randomized, double-blind, placebo-controlled trial of gabapentin in preoperative patients Jadad score = 5 |
40 patients undergoing elective anterior cruciate ligament knee surgery randomized to receive gabapentin or placebo | Gabapentin 1,200 mg given 1–2 h presurgery | Anxiety from visual analog scales, pain scores, morphine use over 48 h, knee range of motion postoperative days 1 and 2 | Visual analog scale scores were lower in the gabapentin group; less morphine use and greater range of motion in the gabapentin group | Gabapentin 1,200 mg was more effective than placebo for preoperative anxiolysis, postoperative analgesia, and early knee mobilization | National Institutes of Health grant GM 61655, Gheens Foundation, Joseph Drown Foundation, and Commonwealth of Kentucky Research Challenge Trust Fund |
| Anxiety disorder | Clarke et al, 201061 Randomized, double-blind, placebo-controlled trial of gabapentin in preoperative patients Jadad score = 3 |
70 patients undergoing total hip arthroplasty randomized to receive gabapentin or placebo | Gabapentin 600 mg given 2 h presurgery | Visual analog scale anxiety, morphine use over 48 h, pain with movement | Visual analog scale scores did not differ by group; no difference in morphine use or in pain/movement | Gabapentin 600 mg is not effective to treat preoperative anxiety, decrease opiate use, or alter mobilization | Physician Services Incorporated of Ontario, Canada grant; Canada Research Chair in Health Psychology at York University |
| Anxiety disorder | Tirault et al, 201057 Randomized, double-blind, placebo-controlled trial of gabapentin in preoperative patients Jadad score = 5 |
210 patients undergoing general surgery randomized to receive preoperatively gabapentin, placebo, or hydroxyzine | Gabapentin 1,200 mg, hydroxyzine 75 mg, or placebo given presurgery | Visual analog scale scores | Gabapentin was more effective than placebo and hydroxyzine at reducing preoperative anxiety | Gabapentin 1,200 mg is effective at reducing preoperative anxiety | None reported |
| Anxiety disorder | Lavigne et al, 201256 Randomized, double-blind, placebo-controlled trial of gabapentin-treated breast cancer survivors Jadad score = 3 | 420 breast cancer patients who had completed chemotherapy cycles randomized to gabapentin or placebo | Gabapentin 300 mg/d, gabapentin 900 mg/d, or placebo | STAI | Gabapentin was more effective than placebo at decreasing anxiety at 8 wk | Gabapentin may provide a single effective treatment for both anxiety and hot flashes in breast cancer survivors | National Cancer Institute; gabapentin and placebo were provided by Pfizer; the secondary analysis was not supported by external funding |
| Anxiety disorder | Adam et al, 201260 Prospective, randomized, placebo-controlled study Jadad score = 5 | 64 surgical patients receiving general anesthesia randomized to gabapentin or placebo | Gabapentin 1,200 mg or placebo | STAI state and visual analog scale anxiety | STAI decreased significantly with gabapentin; visual analog scale scores did not decrease significantly | Gabapentin 1,200 mg provides preoperative anxiolysis without sedation or impairing preoperative memory | None reported |
| Anxiety disorder | Khezri et al, 201358 Randomized, double-blind, placebo-controlled study of melatonin and gabapentin in cataract surgery Jadad score = 5 | 130 patients scheduled for cataract surgery randomized to receive melatonin, gabapentin, or placebo | Gabapentin 600 mg, melatonin 6 mg, or placebo | Verbal pain score and verbal anxiety score | Anxiety scores were significantly decreased in both gabapentin and melatonin groups compared to placebo, with no significant difference between gabapentin and melatonin | Both gabapentin and melatonin significantly reduce anxiety compared to placebo when administered perioperatively; gabapentin decreases pain and increases sedation compared to placebo during retrobulbar block | Vice chancellor for Research, Qazvin University of Medical Science |
| PTSD | Stein et al, 200766 Randomized, double-blind, placebo-controlled trial for acute traumatic injury Jadad score = 5 | 48 acute trauma injury patients randomized to receive gabapentin, propranolol, or placebo | Gabapentin 900–1,200 mg/d, propranolol 60–120 mg/d, or placebo given within 48 h of trauma for 14 d | Acute Stress Disorder Scale PTSD, MDD, and panic disorder modules | No difference at 1-, 4-, or 8-mo follow-up in all PTSD and mood scales based on treatment group | Gabapentin or propranolol show no benefit over placebo at reducing depressive or PTSD symptoms in acute trauma patients | National Institute of Mental Health grants MH62037 (R21) and MH64122 (K24) |
| OCD | Onder et al, 200868 Randomized, open-label trial for add-on gabapentin in OCD Jadad score = 3 | 40 OCD patients randomized to receive fluoxetine or fluoxetine + gabapentin | Gabapentin 600–900 mg/d and/or fluoxetine 40–60 mg/d for 8 wk | Yale-Brown Obsessive-Compulsive Scale, CGI | No difference in either scale scores at 4, 6, and 8 wk by group; lower scores in gabapentin add-on group at wk 2 only | Marginal evidence that gabapentin potentiates the effects of fluoxetine at shorter time courses to improve OCD symptoms | None reported |
| Alcohol | Anton et al, 200969 Randomized, double-blind trial for flumazenil + gabapentin in alcohol withdrawal Jadad score = 3 | 60 alcohol-dependent patients randomized to treatment or placebo divided into high/low alcohol withdrawal symptoms | Up to 1,200 mg/d gabapentin for 39 d + 2 mg/d flumazenil for first 2 d | Percent days abstinent during treatment, time to first heavy drinking day | High withdrawal symptom patients had higher percent days abstinent and time to first heavy drinking day in flumazenil/gabapentin group; low withdrawal patients were better in the placebo group | Depending on pretreatment alcohol withdrawal status, high withdrawal symptom patients with high CIWA scores benefit from gabapentin + flumazenil treatment | Unrestricted grant from Hythiam Inc |
| Alcohol | Anton et al, 201170 Randomized, double-blind, placebo-controlled trial for naltrexone + gabapentin in alcohol withdrawal Jadad score = 4 | 150 alcohol-dependent patients randomized to 3 groups | Naltrexone 50 mg/d for 16 wk; naltrexone 50 mg/d with gabapentin up to 1,200 mg/d added for the first 6 wk; placebo | Interval to heavy drinking, number of drinking days | During first 6 wk, the gabapentin + naltrexone group was superior to naltrexone or placebo in interval to heavy drinking and number of drinking days; no difference at 16 wk | Gabapentin + naltrexone improves drinking outcomes in first 6 wk, but this effect does not endure after gabapentin is discontinued | NIAAA grants R01 AA009568 and K05 AA017435 |
| Alcohol | Myrick et al, 200781 Randomized, double-blind, placebo-controlled trial with gabapentin in alcohol use Jadad score = 3 | 35 non–treatment-seeking alcohol-dependent patients randomly assigned to gabapentin or placebo | 1,200 mg/d gabapentin for 8 d vs placebo | Tolerability of gabapentin; effects on drinking, craving, or intoxication | Patients tolerated gabapentin and placebo equally; no impact of gabapentin on alcohol effect, intoxication, or craving | Gabapentin is well tolerated and does not impact subjective experience of alcohol craving or consumption | Grants P50 AA010761 and K23 AA00314 |
| Alcohol | Myrick et al, 200973 Randomized, double-blind trial of gabapentin vs lorazepam in alcohol-dependent patients Jadad score = 5 | 100 treatment-seeking alcohol-dependent patients randomized to high- or low-dose gabapentin or lorazepam | 2 doses gabapentin 1,200 + 800 mg (high); 2 doses gabapentin 900 + 600 mg (low); 2 doses lorazepam 6 + 4 mg | CIWA-Ar, alcohol use | High-dose gabapentin and lorazepam were superior to low-dose gabapentin in decreasing CIWA-Ar scores; less craving, anxiety, sedation, and drinking after 10 d in gabapentin groups | Gabapentin (high dose) is as effective as lorazepam in lowering withdrawal symptoms in short term; gabapentin is better at diminishing symptoms and reducing probabilities of drinking | NIAAA grants AA10761 and AA00314 and VA Medical Research |
| Alcohol | Bonnet et al, 200376 Randomized, double-blind, placebo-controlled trial with clomethiazole + gabapentin add-on in withdrawal Jadad score = 4 | 61 alcohol-dependent patients assigned to clomethiazole + gabapentin or clomethiazole + placebo | Gabapentin 1,600 mg/d in add-on treatment group | CIWA, clomethiazole dose | Gabapentin add-on group was no different from placebo add-on in lessening CIWA scores or reducing clomethiazole dosing | Gabapentin is no more effective than placebo in treating alcohol withdrawal symptoms when clomethiazole is the primary medication for acute treatment | Gödecke/Parke-Davis |
| Alcohol | Furieri et al, 200784 Randomized, double-blind, placebo-controlled trial with gabapentin in alcohol withdrawal Jadad score = 5 | 60 male alcohol-dependent patients underwent 2-d in-patient detox and then randomized to receive gabapentin or placebo | Gabapentin 600 mg/d for 1 wk | Average percent of heavy drinking days, number of drinking days, number of abstinent days, alcohol craving | Gabapentin was superior to placebo in reducing drinks per day and average percent of heavy drinking days, decreasing cravings, and increasing days abstinent | Gabapentin is more effective than placebo in the first month after alcohol detoxification to decrease drinking behaviors | None reported |
| Alcohol | Mason et al, 200985 Randomized, double-blind, placebo-controlled trial of gabapentin in alcohol craving Jadad score = 3 | 33 alcohol-dependent patients randomly assigned to gabapentin or placebo | Gabapentin 1,200 mg/d for 1 wk | Subjective alcohol craving measures, affectively evoked craving | A significant attenuating effect of gabapentin vs placebo on several measures of subjective craving for alcohol and decreased cue reactivity to affectively evoked craving | Gabapentin may be effective in short-term treatment for promoting abstinence behaviors in alcohol-dependent patients | NIAAA grant R01AA012602 |
| Alcohol | Stock et al, 201374 Randomized, double-blind study of gabapentin vs chlordiazepoxide for outpatient alcohol detoxification Jadad score = 4 | 26 alcohol-dependent participants randomly assigned to gabapentin or chlordiazepoxide | Gabapentin 1,200 mg/d for 3 d, then 900 mg, 600 mg, and 300 mg for 1 d each; chlordiazepoxide 100 mg/d for 3 d, then 75 mg, 50 mg, and 25 mg for 1 d each | ESS, PACS, ataxia rating, CIWA-Ar | Mean ESS scores were lower at the late stage of treatment in the gabapentin group, but not earlier in treatment; PACS scores had a nonsignificant trend toward reduction by end of treatment; similar reduction of CIWA-Ar scores in both groups; no evidence of ataxia | Gabapentin may reduce alcohol craving and sedation by the end of detoxification in alcohol-dependent individuals | Unrestricted grants from the Western Institute for Biomedical Research; Pfizer supplied Neurontin (gabapentin) 300-mg capsules and matching placebo; support from University of Utah Study Design and Biostatistics Center, with funding in part from the Public Health Services research grant UL1-RR025764 and C06-RR11234 from the National Center for Research Resources |
| Alcohol | Mason et al, 201486 Randomized double-blind, placebo-controlled trial of gabapentin in outpatients with current alcohol dependence Jadad score = 5 | 150 participants with alcohol dependence with 3 d of abstinence assigned to placebo, gabapentin 900 mg, or gabapentin 1,800 mg | Gabapentin 600 mg/d, gabapentin 1,800 mg/d, or placebo with concomitant manual-guided counseling for 12 wk | Timeline Followback Interview validated by weekly breathalyzer determinations, monthly GGT levels, Craving Questionnaire Short Form, BDI-II, PSQI | Gabapentin significantly improved rates of abstinence and no heavy drinking, particularly in 1,800-mg/d group; similar results were observed with mood, craving, and insomnia | Gabapentin was effective in treating alcohol dependence in abstinent participants, as well as secondary measures of insomnia, mood, and craving | Funded by NIAAA grant. R37AA014028; gabapentin and matched placebo were provided by Pfizer |
| Cocaine | Bisaga et al, 200687 Randomized, double-blind, placebo-controlled trial of gabapentin vs placebo in cocaine dependence Jadad score = 4 | 99 cocaine-dependent patients randomized to gabapentin or placebo treatment group | Gabapentin 3,200 mg/d for 12 wk titrated up with 400 mg over 15 d and tapered off over 1 wk; all patients received relapse therapy | Cocaine use and proportion of days per week craving cocaine | No difference in cocaine use or craving by treatment group | Gabapentin is no more effective than placebo in treating cocaine dependence over the course of 12 wk of treatment | NIDA Center grant DA09236 and grants K23 DA00429, K23 DA16743, K02 DA00288, and K02 DA00465 |
| Cocaine | González et al, 200788 Randomized, double-blind, placebo-controlled trial of gabapentin vs tiagabine in cocaine dependence Jadad score = 3 | 76 treatment-seeking methadone-stabilized cocaine-dependent individuals randomized to gabapentin, tiagabine, or placebo | Gabapentin 2,400 mg/d titrated up over wk 1–5, continued for wk 6–10, and tapered during wk 11–12; tiagabine 24 mg/d titrated up and down similar to gabapentin in time course | Addiction Severity Index, SCID, Center for Epidemiologic Studies Depression Inventory, self-report drug use, urine samples | Cocaine-free urine samples were greater in the tiagabine group (22%) vs placebo (13%) or gabapentin (5%) groups; tiagabine reduced cocaine-seeking behaviors compared to gabapentin in placebo groups | Gabapentin is no more effective than placebo and is inferior to tiagabine in treating cocaine-dependent behavior in methadone-stabilized, treatment-seeking patients | NIDA grants K23DA14331, K05DA00454, R01-DA05626, and P50-DA12762 and the Veterans Administration Mental Illness Research, Education and Clinical Center |
| Cocaine | Berger et al, 200591 Randomized, placebo-controlled trial of gabapentin, reserpine, and lamotrigine for cocaine dependence Jadad = 1 | 60 cocaine-dependent patients randomly assigned to gabapentin, reserpine, lamotrigine, or placebo | Gabapentin 1,800 mg, reserpine 0.5 mg, lamotrigine 150 mg titrated over 2 wk | Urine benzoylecgonine level, cocaine CGI, observer and self-report of cocaine use with additional safety monitoring | Significant improvement of subjective measures of cocaine dependence in all groups, significant improvement in urine benzoylecgonine levels for reserpine but not for gabapentin | Gabapentin is likely ineffective in treating cocaine dependence | NIDA under interagency agreement Y 01 DA 50038–00; urine analyses were funded by NIDA contract N01DA-7–8074 |
| Cocaine | Mancino et al, 201489 Randomized, double-blind, placebo-controlled clinical trial of sertraline vs sertraline plus gabapentin Jadad = 5 | 99 depressed, cocaine-dependent patients randomly assigned to sertraline, sertraline plus gabapentin, or placebo | Gabapentin initial dose 200 mg twice daily titrated to 600 mg twice daily by day 11 plus sertraline titrated from 60 mg to 200 mg/d | HDRS (screening, intake, and then weekly); supervised urine samples 3 times/wk; self-report cocaine use | Sertraline alone (not with add-on gabapentin) showed significantly lower percentage cocaine-positive urine samples vs placebo | Sertraline plus gabapentin is not superior to sertraline alone for preventing relapse in cocaine-dependent individuals with depressive symptoms | NIDA grants P50-DA12762, K05-DA00454, and T32 DA022981 and National Institute of General Medical Services grant GM103425-09 |
| Methamphetamine | Heinzerling et al, 200695 Randomized, double-blind, placebo-controlled trial of gabapentin vs baclofen in methamphetamine dependence Jadad score = 3 | 88 methamphetamine-dependent patients assigned to gabapentin, baclofen, or placebo | Gabapentin 2,400 mg/d titrated up over 4 d continued for 16 wk and tapered over 3 d; baclofen 60 mg/d titrated up over 4 d for 16 wk and tapered for 3 d | Urine samples 3 times/wk for drug use; BDI; drug-craving measures on the visual analog scale | No difference in treatment groups in reducing methamphetamine use based on urine testing; patients reporting higher adherence to treatment regimens in baclofen vs gabapentin and placebo groups showed reduced drug use | Gabapentin is no more effective than placebo or baclofen in reducing methamphetamine use in drug-dependent patients over 16 wk of treatment | NIDA grant 1 P50 DA 18185 |
| Methamphetamine | Urschel et al, 201196 Randomized, double-blind, placebo-controlled trial of flumazenil plus gabapentin vs placebo in methamphetamine craving and use Jadad score = 3 |
135 methamphetamine-dependent participants randomized to flumazenil and hydroxyzine plus gabapentin or placebo treatment group | Flumazenil 2 mg administered intravenously on days 1, 2, 3, 21, and 22; hydroxyzine 50 mg for preinfusion and oral gabapentin titrated up to 1,200 mg/d for 30 d | A composite methamphetamine craving score was derived by combining 6 visual analog scales; urine drug testing and patient self-report of drug use was done daily during the study period | Craving and methamphetamine use was significantly reduced in the flumazenil plus gabapentin group | Flumazenil plus gabapentin is more effective than placebo in treating methamphetamine craving and use over a 30-d period | Unrestricted grant from Hythiam Inc |
| Methamphetamine | Ling et al, 201297Double-blind, placebo-controlled study of PROMETA protocol (flumazenil, gabapentin, and hydroxyzine) vs placeboJadad score = 5 | 120 treatment-seeking methamphetamine-dependent adults randomized to protocol or placebo | Flumazenil 2 mg administered intravenously on days 1, 2, 3, 22, and 23; hydroxyzine 50 mg for preinfusion and oral gabapentin titrated up to 1,200 mg/d for 40 d | Percentage of urine samples testing negative for methamphetamine during trial to 108 d | No significant difference between groups in urine drug test results, craving, treatment retention, or adverse events | PROMETA protocol is no more effective than placebo for decreasing methamphetamine use and craving or maintaining patients in treatment | Hythiam Inc for an investigator-initiated study; the funding agency played no role in study design or procedures except to provide specific information requested regarding the PROMETA protocol |
| Opioids | Kheirabadi et al, 2008100 Randomized, double-blind, placebo-controlled trial of gabapentin vs placebo in opioid dependence Jadad score = 5 | 40 patients with methadone-stabilized opioid dependence randomized to gabapentin or placebo | Gabapentin 900 mg/d titrated over 3 d and continued for 3 wk; methadone for all patients 20–65 mg/d and tapered 7.5% per day over 2 wk | SOWS | No difference between gabapentin and placebo add-on groups in controlling opiate withdrawal symptoms in patients receiving methadone | Gabapentin is no more effective than placebo as an adjunctive treatment in opioid withdrawal in patients stabilized with methadone | Research grant from the fluid research fund of the vice chancellor for research of Isfahan University of Medical Sciences |
| Opioids | Sanders et al, 2013103 Randomized, placebo-controlled pilot trial of gabapentin during detoxification with buprenorphine Jadad score = 4 | 24 participants with opioid dependence | Gabapentin titrated to 1,600 mg/d over 5 d, maintained until wk 5, then tapered to 200 mg/d over 4 d plus buprenorphine 12 mg/d through wk 2 then 10-d detox to 2 mg by wk 4 when it was discontinued | Objective Opiate Withdrawal Scale, Opiate Withdrawal Symptoms Checklist, self-report opioid use, physiologic signs 3 times/wk, supervised urine samples 3 times/wk | During buprenorphine taper, no significant treatment group differences with either objective or subjective measures of withdrawal symptoms; probability of opioid-positive urine was significantly decreased over time in the gabapentin group | Gabapentin may improve treatment outcomes in patients undergoing buprenorphine detoxification | NIDA grants DA10017 and 5T32DA022981-03, clinical and translational science award 1UL1RR029884, National Center for Research Resources grant 5P20RR020146-09, and National Institute of General Medical Sciences grant 8 P20 GM103425-09; these funding sources provided financial support only |
| Opioids | Moghadam and Alavinia, 2013102 Randomized, double-blind, placebo-controlled trial of methadone plus gabapentin vs placebo in opiate acute detoxification Jadad score = 2 | 60 patients using opium, opium extract, and heroin | Gabapentin 300 mg/d titrated to 300 mg 3 times daily with methadone 40–120 mg/d or methadone 40–120 mg/d with placebo | SOWS | Daily and cumulative doses of methadone were higher in the placebo group; more withdrawal symptoms were noted in the gabapentin group | Gabapentin was effective as add-on therapy for acute detoxification of opioids when added to methadone and lowers methadone consumption | None reported |
According to the US Preventive Services Task Force guidelines.17
Abbreviations: BDI = Beck Depression Inventory, CGI = Clinical Global Impressions scale, CGI-BP = Clinical Global Impressions Scale for Bipolar Illness, CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol–revised, ESS = Epworth Sleepiness Scale, GGT = gamma-glutamyltransferase, HARS = Hamilton Anxiety Rating Scale, HDRS = Hamilton Depression Rating Scale, LSAS = Liebowitz Social Anxiety Scale, MDD = major depressive disorder, NIAAA = National Institute on Alcohol Abuse and Alcoholism, NIDA = National Institute on Drug Abuse, OCD = obsessive-compulsive disorder, PACS = Penn Alcohol Craving Scale, PAS = Panic and Agoraphobia Scale, PSQI = Pittsburgh Sleep Quality Index, PTSD = posttraumatic stress disorder, SCID = Structured Clinical Interview for DSM-IV, SOWS = Subjective Opiate Withdrawal Scale, STAI = Speilberger Strait-Trait Anxiety Inventory, YMRS = Young Mania Rating Scale.
RESULTS
Bipolar Disorder
The randomized controlled trials19–21 investigating gabapentin for treating bipolar disorder indicate it is likely to be ineffective. Data interpretation is difficult: dosing varies by trial, gabapentin is used as both monotherapy and adjunctive therapy, patients have heterogeneous diagnoses, and primary outcomes differ between studies. Pande et al19 published the largest randomized controlled trial to date (N = 114) in which subjects were randomized to treatment with standard mood stabilizers or with adjunctive gabapentin. After receiving gabapentin 600–3,600 mg/d for 10 weeks, mood scale scores were no different between treatment groups.19 In a double-blind, randomized, crossover series (N = 31),20 patients with refractory bipolar and unipolar mood disorder received three 6-week monotherapy treatments of lamotrigine, gabapentin, or placebo. On the basis of the Clinical Global Impressions Scale for Bipolar Illness (CGI-BP), lamotrigine was superior in reducing symptoms versus gabapentin and placebo.20 Obrocea et al21 also found gabapentin and placebo inferior to lamotrigine in a crossover study of 35 patients with bipolar disorder and 10 patients with unipolar disorder for reducing depressive symptoms.
An abundance of open-label trials and case series exist on gabapentin’s use in bipolar disorder. While these data are less rigorous, they may be helpful with individual patient treatment (specific case comparison to similar specific clinical parameters), and review is warranted. Several case series22–25 on adjunctive gabapentin therapy in bipolar disorder suggest it may be effective. A case-control study22 of 60 patients in the acute phase of mania found that treatment with lithium and adjunctive gabapentin 900 mg significantly reduced symptoms. In 1 study,23 21 mixed-state patients refractory to mood stabilizers received concurrent gabapentin (300–2,000 mg/d) for 8 weeks. Ten patients showed significant improvement in CGI-BP scores, particularly with depressive symptoms.23 Erfurth et al24 published a case series on 14 patients with acute mania treated with gabapentin 1,200–4,800 mg/d. Six patients received gabapentin and valproic acid or lithium and 8 received gabapentin plus a benzodiazepine for sedation. On the basis of a mania assessment scale after 21 days, gabapentin appeared safe and efficacious, although 4 patients withdrew due to inadequate symptom management.24 Finally, in a case series of manic elderly patients (n = 7),25 gabapentin 900–1,200 mg/d with low-dose antipsychotics or valproate successfully resolved mania in 6 patients.
Additional studies address gabapentin as monotherapy or adjunctive therapy for acute mania in patients refractory to standard therapy and show equivocal results. A meta-analysis26 of 68 randomized controlled trials comparing the efficacy of antimanic drugs found gabapentin to be no more effective than placebo. In contrast, several case series and open-label trials suggest gabapentin efficacy for acute mania. Knoll et al27 examined 12 bipolar manic/hypomanic patients refractory to or intolerant of mood stabilizers and treated with gabapentin for 3–60 weeks with 900–3,300 mg/d. Half of the patients discontinued gabapentin due to side effects and half showed moderate improvement.27 Additional smaller studies28,29 showed manic/hypomanic patients experiencing a significant response to gabapentin. Some open-label studies30,31 of adjunctive gabapentin in bipolar mania have shown mixed benefit but suggest positive efficacy.
Investigating prophylaxis in euthymic bipolar patients, Vieta et al32 conducted a randomized, placebo-controlled trial to assess adjunctive gabapentin’s effect in treating and preventing bipolar symptoms. For 1 year, 13 patients received adjunctive gabapentin with standard mood stabilizers and 12 patients received adjunctive placebo. On the basis of the CGI-BP, gabapentin-treated patients showed significant improvement from baseline to month 12. However, other clinical measures assessing mania, depression, and sleep revealed no differences between treatment groups. Aside from small sample size, groups differed by baseline depressive episodes (19.3 and 8.3 mean episodes in gabapentin and placebo, respectively).32
In addition to alleged improvement in mania-associated symptoms, several reports33–37 suggest that gabapentin ameliorates other psychiatric symptoms as well. In an open-label trial (n = 22), Wang et al33 reported success in treating mild to moderate bipolar depression with adjunctive gabapentin (mean dose of 1,725 mg/d) for 12 weeks. In another study of 16 bipolar I and II patients receiving adjunctive gabapentin (mean dose of 1,310 mg/d), 8 showed improved depression, anxiety, and irritability symptoms at 12-week follow-up.34 Sokolski et al35 noted in an open-label add-on trial (n = 10) that gabapentin was effective, with improvement in depressive symptoms, mania ratings, and sleep disturbance persisting for 1 month posttreatment. Ghaemi et al36 retrospectively reviewed charts of 50 bipolar and unipolar mood spectrum disorder patients receiving adjuvant or monotherapy gabapentin. On the basis of the CGI-BP, 30% of patients showed significant improvement in mood.36 In a similar report, Ghaemi and Goodwin37 reviewed the charts of 21 patients with mood disorders treated with gabapentin (mean dose of 943 mg/d) either as monotherapy or adjunctive therapy for 2–52 weeks (mean of 17 weeks). On the basis of self-report mood scales, manic symptoms improved by 43.8% and depression scores by 27.6%. In the depressed subgroup of 10 patients, symptoms improved by 57.5%.37
Pharmaceutical marketing has greatly influenced gabapentin’s off-label use for bipolar disorder,38 and several uncontrolled case series22–25 using gabapentin in bipolar patients have contributed to the rise in off-label gabapentin prescriptions. A large number of peer-reviewed but noncomparative studies and reviews23–25,27–31,33–37,39,40 also support gabapentin’s role either as monotherapy after first-line treatment failure or as adjunctive therapy to mood stabilizers, antidepressants, or neuroleptics. Literature reviews41–48 referencing the off-label use of gabapentin in bipolar disorder reinforce the apparent efficacy of gabapentin for mood stabilization or augmentation. Despite arguments based on biological plausibility of gabapentin in treating mood disorders and disproportionate attention to less rigorous studies with positive findings, 4 randomized controlled trials have failed to support the claims.19–21,32
Depressive Disorders
To date, no controlled trials exist that investigate gabapentin’s effect in the treatment of major depression as monotherapy or adjunctive treatment, and according to several case reports and chart reviews,49–51 gabapentin use for depression is equivocal. In a chart review49 of 27 patients with depression refractory to standard antidepressant therapy, 10 patients responded to adjunctive gabapentin treatment (mean dose of 904 mg/d for 15 weeks). Maurer et al50 published a single case report of a 48-year-old woman with recurrent depression, somatization, and pain who responded to gabapentin 1,800 mg/d with improvement in both pain and depressive symptoms. Another narrative review51 regarding anticonvulsants in depression treatment concluded that there is insufficient evidence to support gabapentin’s use in depression.
Epilepsy patients are at increased risk for depression, most likely due to both psychosocial and neurologic factors.52 Harden et al53 randomized 40 epilepsy patients to receive adjunctive gabapentin or standard antiepileptic therapy. After 3 months of gabapentin treatment (mean dose of 1,615 mg/d), patients noted superior mood improvement compared to controls based on the Cornell Dysthymia Rating Scale. Groups were similar based on other mood scales, including the Hamilton Depression and Anxiety Rating Scales and the Beck Depression Inventory.53
Anxiety Disorders
Some evidence suggests that gabapentin possesses anxiolytic properties, though few data exist for patients with generalized anxiety disorder (GAD). Gabapentin has been examined as therapy for treating social phobia, panic and somatoform disorders, anxiety in breast cancer survivors, and surgery-associated anxiety with mixed results.
In a randomized, double-blind, placebo-controlled study, Pande et al54 randomized 69 patients with social phobia to receive gabapentin 900–3,600 mg/d or placebo for 14 weeks. Gabapentin was superior to placebo in treatment of symptoms associated with social phobia according to both patient- and clinician-rated scales.54 Another controlled trial55 of 103 patients with panic disorder found that based on Panic and Agoraphobia Scale scores, gabapentin 600–3,600 mg/d and placebo groups were similar. However, in a subset of patients with a Panic and Agoraphobia Scale score > 20, gabapentin was more effective than placebo in attenuating symptoms.55 A randomized, controlled, double-blind clinical trial56 found gabapentin 300 mg/d or 900 mg/d superior to placebo in reducing hot flashes and anxiety in breast cancer patients who had completed chemotherapy cycles.
Several studies report gabapentin as effective in reducing perisurgical anxiety in otherwise psychologically healthy patients. In 210 patients randomized to receive gabapentin 1,200 mg, hydroxyzine 75 mg, or placebo preoperatively, Tirault et al57 showed that gabapentin was superior to hydroxyzine or placebo in reducing anxiety. A randomized controlled trial58 of 130 patients undergoing cataract surgery found a single dose of gabapentin 600 mg to significantly decrease perioperative anxiety compared to placebo. However, there was no significant difference when gabapentin was compared to melatonin.58 Two additional randomized controlled studies59,60 found premedication with gabapentin to be effective in reducing presurgical anxiety. However, in a double-blind, randomized, placebo-controlled trial, Clarke et al61 reported no difference in pre- and post-medication anxiety between gabapentin (600 mg, n = 22) and placebo (n = 48) groups 2 hours postoperative.
Posttraumatic Stress Disorder
The available data suggest that gabapentin is a potentially effective adjuvant agent in the treatment of PTSD. In a retrospective study (n = 30),62 the majority of PTSD patients (77%) treated with adjunctive gabapentin (300–3,600 mg/d) demonstrated moderate improvement in sleep duration and a decrease in nightmares. Case reports63–65 suggest that gabapentin plus antidepressant therapy is useful in treating PTSD symptoms such as nightmares, flashbacks, anxiety, and fear. However, monotherapy gabapentin appears ineffective for prevention of PTSD. In patients admitted for surgical trauma, Stein et al66 examined gabapentin use in prevention of PTSD and depressive symptoms. Within 48 hours of the traumatic event, 48 patients were randomized to propranolol (60–120 mg/d), gabapentin (900–1,200 mg/d), or placebo for 14 days. Both treatments were similar to placebo in controlling depressive and PTSD-type symptoms.66 In a retrospective study, Fowler et al67 examined the effect of gabapentin and pregabalin on the development of PTSD in burned service members. In the study, 290 service members received gabapentin, pregabalin, or neither. There was no difference in incidence of PTSD between the groups.67
Obsessive-Compulsive Disorder
Only 1 study has evaluated gabapentin use for obsessive-compulsive disorder (OCD). Onder et al68 studied fluoxetine monotherapy versus fluoxetine with adjunctive gabapentin in controlling OCD symptoms. Forty patients were randomized (open-label) to fluoxetine 20 mg/d or fluoxetine 20 mg/d with gabapentin 600 mg/d. If patients were nonresponsive to either regimen at week 4, fluoxetine doses were increased to 40 or 60 mg/d and gabapentin to 900 mg/d. The gabapentin adjunctive treatment group showed significant reduction in OCD symptoms at 2 weeks, but the effect failed to persist past week 4. The authors speculate that gabapentin may accelerate fluoxetine’s potency in reducing OCD-type behaviors.68
Alcohol Dependence and Withdrawal
Gabapentin efficacy in alcohol dependence, abstinence, and acute alcohol withdrawal is suggested in studies by Anton et al.69,70 In 1 study,70 150 alcohol-dependent patients were randomized to placebo, naltrexone 50 mg/d for 16 weeks, or a protocol of naltrexone 50 mg/d for 16 weeks with gabapentin 1,200 mg/d added for the first 6 weeks. The 6-week combination of gabapentin and naltrexone showed improvement of interval to heavy drinking (∼20% less than patients not taking gabapentin) and number of drinking days (∼50% and ∼70% less, respectively) compared to placebo or naltrexone alone.70 While results were significant and promising, the first author had financial support from multiple pharmaceutical companies. Another study69 randomized 60 alcohol-dependent patients to placebo or a protocol of flumazenil 2 mg/d for 2 days and gabapentin 1,200 mg/d for 39 days. For patients with severe withdrawal symptoms, those who received the protocol (n = 7) spent more days abstinent compared to the placebo group (n = 9). No differences were observed between treatment and placebo groups in patients with mild or moderate withdrawal symptoms.69
In an open-label trial,71 patients with acute alcohol withdrawal (n = 37) received gabapentin 800 mg. Within 2 hours, 27 patients showed significant decrease on the Clinical Institute Withdrawal Assessment (CIWA). These early responders received gabapentin 2,400 mg/d for the next 2 days, during which 3 early responders worsened and 2 experienced withdrawal seizures. The 10 gabapentin nonresponders received standard therapy with benzodiazepine or clomethiazole. Similar CIWA scores were noted between the early responders versus nonresponders, suggesting that patients with moderate and mild withdrawal might benefit from gabapentin therapy.71 In another study,72 gabapentin was comparable to phenobarbital in treating acute alcohol withdrawal symptoms in 27 acutely withdrawing patients, with no outcome scores differing between the 2 drugs.
Myrick et al73 studied gabapentin versus lorazepam for treatment of acute alcohol withdrawal. They found that gabapentin 1,200 mg/d was superior to both gabapentin 900 mg/d and lorazepam 6 mg/d in decreasing alcohol withdrawal symptoms and lowering odds of drinking during and after treatment. Gabapentin patients reported less anxiety, less sedation, and decreased alcohol craving compared to the lorazepam group.73 In a small double-blind, randomized study of 26 veterans with alcohol dependence undergoing outpatient alcohol detoxification, Stock et al74 showed that gabapentin treatment reduced sedation and may decrease alcohol craving compared to chlordiazepoxide. No difference between CIWA-revised scores was found between treatment groups.74 In contrast, when Bonnet et al75 treated withdrawing patients (n = 46) with gabapentin 1,600 mg/d or placebo for 7 days, no difference in withdrawal symptoms or mood were noted. In a double-blind, randomized, placebo-controlled trial (n = 61) comparing gabapentin 1,600 mg/d versus clomethiazole and placebo,76 add-on gabapentin treatment was no more effective than placebo in reducing clomethiazole dosing or alleviating withdrawal symptoms.
While abuse of gabapentin itself (mixed with other agents) needs to be considered,77 gabapentin appears to be safe and well tolerated in individuals with alcohol dependence.78–83 Furieri et al84 assessed 60 Brazilian men with alcohol dependence after treatment for acute withdrawal and randomized them to either gabapentin 600 mg/d or placebo for 7 days. Gabapentin was more effective in reducing drinks per day, average percent of heavy drinking days, and increased number of days abstinent, while decreasing alcohol cravings.84 Mason et al85 randomized 33 untreated alcohol-dependent patients to 1,200 mg/d gabapentin or placebo for 1 week. Their results suggested that gabapentin was effective in attenuating subjective alcohol craving and craving associated with emotionally evocative stimuli compared to placebo.85 Most recently, Mason et al86 found that gabapentin, particularly at a dose of 1,800 mg/d, significantly improved rates of abstinence and no heavy drinking in a 12-week, double-blind, placebo-controlled trial of 150 participants with current alcohol dependence in the outpatient setting. In addition, a similar dose effect was seen in mood, insomnia, and craving.86
Drug Abuse, Dependence, and Withdrawal
Several placebo-controlled trials show that gabapentin is inappropriate therapy in preventing cocaine relapse. In a double-blind, randomized trial,87 patients with cocaine dependence (n = 99) were randomized to receive 3,200 mg/d of gabapentin or placebo, in addition to individual relapse prevention therapy. Primary outcome measures were days of cocaine use, self-reported cocaine craving, and treatment retention. There were no differences in treatment groups.87 Another double-blind, placebo-controlled trial88 involving methadone-treated cocaine-dependent patients affirmed no gabapentin benefit for cocaine abstinence. Mancino et al89 conducted an additional randomized controlled trial comparing sertraline alone to sertraline with gabapentin to treat cocaine-dependent patients with depressive symptoms. Sertraline alone showed a significantly lower percentage of cocaine-positive urine samples when compared to placebo, but gabapentin did not augment this effect.89 In a 48-day, double-blind crossover study (n = 7), Hart et al90 examined the effect of gabapentin maintenance (0, 600 mg/d, and 1,200 mg/d) on cocaine self-administration, cardiovascular, and subjective outcomes. Results showed that some cocaine-related subjective ratings were significantly decreased when participants were taking gabapentin. However, there was no effect on cocaine self-administration or cardiovascular effects.90 Berger et al91 found similar results but did not conduct a nonblinded study. A follow-up double-blind, crossover study by Hart and colleagues92 (n = 6) with a higher dose of gabapentin (0, 2,400 mg/d, and 3,200 mg/d) found that gabapentin did not decrease cocaine self-administration, cardiovascular effects, or subjective effects of cocaine. Despite benefit previously demonstrated in open-label non–placebo-controlled trials,93,94 the previously mentioned more rigorous placebo-controlled studies show that gabapentin is inappropriate pharmacotherapy in cocaine relapse prevention.
For treating methamphetamine dependence, gabapentin does not appear effective. In a 16-week randomized, double-blind, placebo-controlled trial (n = 88),95 patients with methamphetamine dependence were randomized to receive gabapentin 2,400 mg/d, baclofen 60 mg/d, or placebo for 4 months in addition to psychosocial counseling. On the basis of urine samples, the authors concluded that gabapentin was no more effective than placebo in reducing methamphetamine use.95 In a 1-month trial, Urschel et al96 showed that flumazenil and gabapentin were superior to placebo in decreasing methamphetamine craving and use. However, in a double-blind, placebo-controlled evaluation of the PROMETA protocol consisting of flumazenil, gabapentin, and hydroxyzine, Ling et al97 found the protocol to be no more effective than placebo in reducing methamphetamine use.
Although initial case reports and uncontrolled studies98,99 suggested a role for gabapentin in treating opioid dependence, cravings, and withdrawal symptoms, a randomized controlled trial contradicts such claims. Kheirabadi et al100 randomized 40 opiate-dependent patients to methadone-assisted detoxification with adjunctive gabapentin 900 mg/d or placebo. Gabapentin was no more effective than placebo in controlling opiate withdrawal symptoms. A 3-week, open-label study101 followed up the study by Kheirabadi et al100 to assess the use of adjunctive treatment with gabapentin 1,600 mg/d in 27 patients undergoing methadone-assisted detoxification. Compared to previous trials, there was no significant difference between groups treated with gabapentin 1,600 mg and 900 mg. Gabapentin 1,600 mg, however, was significantly superior in decreasing some symptoms of withdrawal.101Another randomized, placebo-controlled study (n = 60) by Moghadam and Alavinia102 found gabapentin to be an effective add-on therapy when added to methadone for acute detoxification of opioids, resulting in reduced methadone daily and cumulative doses and improved withdrawal symptoms. A small, randomized, placebo-controlled pilot trial of gabapentin use during buprenorphine-assisted detoxification procedure by Sanders et al103 found a significantly decreased probability of opioid-positive urine over time in patients treated with gabapentin versus placebo.
CONCLUSION
Since its clinical introduction in the early 1990s, gabapentin has been employed in a multitude of clinical disorders with increasing use in psychiatric disorders. Pharmaceutical companies with obvious financial interest have pushed gabapentin’s off-label use and crossed lines of ethics in publication results, culminating in the sentinel article by Vedula et al9 in 2009 criticizing industry-sponsored off-label gabapentin trials. In addition, interpretation of the current evidence is also complicated by the challenges of the variable dosing of gabapentin between trials, the heterogeneity of diagnoses, evaluating efficacy as monotherapy or adjunctive therapy, and differing primary outcomes.
Overall, gabapentin’s positive outcomes in off-label psychiatric use have been presented in a multitude of case series and open-label studies. However, these studies are biased toward positive results and are poorly controlled. Case series suggest benefit of adjunctive gabapentin for mood symptoms in bipolar disorder, though the existing randomized controlled trials do not support this finding. Gabapentin’s role in acute mania is equivocal, and limited data exist on its use as prophylaxis in bipolar disorder. One can argue the difficulty in trial design for bipolar disorder based on patient and treatment variability, but this is true for any bipolar disorder clinical therapeutic trial (and drugs have shown efficacy in double-blind, placebo-controlled trials).
Gabapentin does appear to provide benefit for some anxiety disorders, although randomized controlled trials have been limited to social phobia, anxiety in breast cancer, and perioperative anxiety. To date, no studies exist for gabapentin efficacy in generalized anxiety disorder. There is limited evidence to suggest the use of gabapentin in depression, PTSD, and OCD.
Multiple studies suggest gabapentin has some efficacy in alcohol dependence, withdrawal, and craving. Often examined as an alternative to benzodiazepines, gabapentin is not hepatically metabolized and thus may be preferred for patients with alcohol-associated liver disease or those who are taking other prescription or illicit drugs. Gabapentin appears to have potential in supporting abstinence. Its role as an alternative to benzodiazepines in acute alcohol withdrawal still requires more study.
As for gabapentin’s use in other types of substance dependence, there are no data to support its efficacy in cocaine or methamphetamine dependence. The clinical trials on the adjunctive use of gabapentin in opioid dependence have had equivocal results, but higher doses of gabapentin may be promising when coadministered with opioid replacement therapies. Further evaluation of gabapentin therapy in substance dependence should also account for more recent concerns over abuse of gabapentin itself, in the context of polysubstance abuse, and reports of withdrawal symptoms with abrupt cessation of gabapentin treatment.
Given its safety profile and generally well-tolerated side effects, further evidence-based research is needed to support expansion of gabapentin’s off-label use in psychiatric disorders. Future study should focus on elucidating gabapentin’s anxiolytic effects, as well as what true benefit it may provide in bipolar disorder as adjunctive therapy for mood stabilization. To achieve these goals, more rigorous randomized controlled trials are required with special attention paid to non–industry-sponsored studies. Moreover, particular consideration should be paid to primary outcomes, without the clouding effects of secondary outcomes.
Drug names:
buprenorphine (Subutex, Suboxone, and others), chlordiazepoxide (Librium and others), fluoxetine (Prozac and others), gabapentin (Neurontin, Gralise, and others), hydroxyzine (Visteril and others), lamotrigine (Lamictal and others), lorazepam (Ativan and others), methadone (Methadose and others), naltrexone (ReVia and others), pregabalin (Lyrica), propranolol (Inderal and others), sertraline (Zoloft and others), valproic acid (Depakene and others).
Potential conflicts of interest:
None reported.
Funding/support:
None reported.
References
- 1.McLean MJ. Gabapentin. Epilepsia. 1995;36(suppl 2):S73–S86. doi: 10.1111/j.1528-1157.1995.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 2.Maneuf YP, Luo ZD, Lee K. alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Semin Cell Dev Biol. 2006;17(5):565–570. doi: 10.1016/j.semcdb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Singh D, Kennedy DH. The use of gabapentin for the treatment of postherpetic neuralgia. Clin Ther. 2003;25(3):852–889. doi: 10.1016/s0149-2918(03)80111-x. [DOI] [PubMed] [Google Scholar]
- 4.Rice AS, Maton S. Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94(2):215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 5.Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 6.Steinman MA, Bero LA, Chren MM, et al. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145(4):284–293. doi: 10.7326/0003-4819-145-4-200608150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Petersen M. Court papers suggest scale of drug’s use: lawsuit says doctors were paid endorsers. N Y Times Web. 2003;C1:C2. [PubMed] [Google Scholar]
- 8.Larkin M. Warner-Lambert found guilty of promoting neurontin off label. Lancet Neurol. 2004;3(7):387. doi: 10.1016/s1474-4422(04)00792-6. [DOI] [PubMed] [Google Scholar]
- 9.Vedula SS, Bero L, Scherer RW, et al. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361(20):1963–1971. doi: 10.1056/NEJMsa0906126. [DOI] [PubMed] [Google Scholar]
- 10.Taylor CP, Gee NS, Su TZ, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29(3):233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 11.Su TZ, Lunney E, Campbell G, et al. Transport of gabapentin, a gamma-amino acid drug, by system l alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995;64(5):2125–2131. doi: 10.1046/j.1471-4159.1995.64052125.x. [DOI] [PubMed] [Google Scholar]
- 12.Lanneau C, Green A, Hirst WD, et al. Gabapentin is not a GABAB receptor agonist. Neuropharmacology. 2001;41(8):965–975. doi: 10.1016/s0028-3908(01)00140-x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JK, Lee SZ, Yang JR, et al. Does gabapentin act as an agonist at native GABA(B) receptors? J Biomed Sci. 2004;11(3):346–355. doi: 10.1007/BF02254439. [DOI] [PubMed] [Google Scholar]
- 14.Takasusuki T, Yaksh TL. The effects of intrathecal and systemic gabapentin on spinal substance P release. Anesth Analg. 2011;112(4):971–976. doi: 10.1213/ANE.0b013e31820f2a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrich J, Van Minh AT, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field MJ, Cox PJ, Stott E, et al. Identification of the α2-δ -1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Procedure manual. US Preventive Services Task Force Web site. http://www.uspreventiveservicestaskforce.org/Page/Name/procedure-manual—-section-4 . Updated August 2011. Accessed April 4, 2015.
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Pande AC, Crockatt JG, Janney CA, et al. Gabapentin Bipolar Disorder Study Group. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Bipolar Disord. 2000;2(3 pt 2):249–255. doi: 10.1034/j.1399-5618.2000.20305.x. [DOI] [PubMed] [Google Scholar]
- 20.Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20(6):607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biol Psychiatry. 2002;51(3):253–260. doi: 10.1016/s0006-3223(01)01206-9. [DOI] [PubMed] [Google Scholar]
- 22.Astaneh AN, Rezaei O. Adjunctive treatment with gabapentin in bipolar patients during acute mania. Int J Psychiatry Med. 2012;43(3):261–271. doi: 10.2190/PM.43.3.e. [DOI] [PubMed] [Google Scholar]
- 23.Perugi G, Toni C, Ruffolo G, et al. Clinical experience using adjunctive gabapentin in treatment-resistant bipolar mixed states. Pharmacopsychiatry. 1999;32(4):136–141. doi: 10.1055/s-2007-979219. [DOI] [PubMed] [Google Scholar]
- 24.Erfurth A, Kammerer C, Grunze H, et al. An open label study of gabapentin in the treatment of acute mania. J Psychiatr Res. 1998;32(5):261–264. doi: 10.1016/S0022-3956(98)00010-7. [DOI] [PubMed] [Google Scholar]
- 25.Sethi MA, Mehta R, Devanand DP. Gabapentin in geriatric mania. J Geriatr Psychiatry Neurol. 2003;16(2):117–120. doi: 10.1177/0891988703016002010. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- 27.Knoll J, Stegman K, Suppes T. Clinical experience using gabapentin adjunctively in patients with a history of mania or hypomania. J Affect Disord. 1998;49(3):229–233. doi: 10.1016/s0165-0327(98)00027-5. [DOI] [PubMed] [Google Scholar]
- 28.Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord. 1999;1(1):61–65. doi: 10.1034/j.1399-5618.1999.10113.x. [DOI] [PubMed] [Google Scholar]
- 29.Cabras PL, Hardoy MJ, Hardoy MC, et al. Clinical experience with gabapentin in patients with bipolar or schizoaffective disorder: results of an open-label study. J Clin Psychiatry. 1999;60(4):245–248. doi: 10.4088/jcp.v60n0408. [DOI] [PubMed] [Google Scholar]
- 30.McElroy SL, Soutullo CA, Keck PE, Jr, et al. A pilot trial of adjunctive gabapentin in the treatment of bipolar disorder. Ann Clin Psychiatry. 1997;9(2):99–103. doi: 10.1023/a:1026257303275. [DOI] [PubMed] [Google Scholar]
- 31.Schaffer CB, Schaffer LC. Open maintenance treatment of bipolar disorder spectrum patients who responded to gabapentin augmentation in the acute phase of treatment. J Affect Disord. 1999;55(2–3):237–240. doi: 10.1016/s0165-0327(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 32.Vieta E, Manuel Goikolea J, Martínez-Arán A, et al. A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. J Clin Psychiatry. 2006;67(3):473–477. doi: 10.4088/jcp.v67n0320. [DOI] [PubMed] [Google Scholar]
- 33.Wang PW, Santosa C, Schumacher M, et al. Gabapentin augmentation therapy in bipolar depression. Bipolar Disord. 2002;4(5):296–301. doi: 10.1034/j.1399-5618.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 34.Vieta E, Martinez-Arán A, Nieto E, et al. Adjunctive gabapentin treatment of bipolar disorder. Eur Psychiatry. 2000;15(7):433–437. doi: 10.1016/s0924-9338(00)00514-9. [DOI] [PubMed] [Google Scholar]
- 35.Sokolski KN, Green C, Maris DE, et al. Gabapentin as an adjunct to standard mood stabilizers in outpatients with mixed bipolar symptomatology. Ann Clin Psychiatry. 1999;11(4):217–222. doi: 10.1023/a:1022361412956. [DOI] [PubMed] [Google Scholar]
- 36.Ghaemi SN, Katzow JJ, Desai SP, et al. Gabapentin treatment of mood disorders: a preliminary study. J Clin Psychiatry. 1998;59(8):426–429. doi: 10.4088/jcp.v59n0805. [DOI] [PubMed] [Google Scholar]
- 37.Ghaemi SN, Goodwin FK. Gabapentin treatment of the non-refractory bipolar spectrum: an open case series. J Affect Disord. 2001;65(2):167–171. doi: 10.1016/s0165-0327(00)00218-4. [DOI] [PubMed] [Google Scholar]
- 38.Fullerton CA, Busch AB, Frank RG. The rise and fall of gabapentin for bipolar disorder: a case study on off-label pharmaceutical diffusion. Med Care. 2010;48(4):372–379. doi: 10.1097/MLR.0b013e3181ca404e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carta MG, Hardoy MC, Dessì I, et al. Adjunctive gabapentin in patients with intellectual disability and bipolar spectrum disorders. J Intellect Disabil Res. 2001;45(pt 2):139–145. doi: 10.1046/j.1365-2788.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- 40.Perugi G, Toni C, Frare F, et al. Effectiveness of adjunctive gabapentin in resistant bipolar disorder: is it due to anxious-alcohol abuse comorbidity? J Clin Psychopharmacol. 2002;22(6):584–591. doi: 10.1097/00004714-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347–364. doi: 10.1016/j.amjopharm.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Barrios C, Chaudhry TA, Goodnick PJ. Rapid cycling bipolar disorder. Expert Opin Pharmacother. 2001;2(12):1963–1973. doi: 10.1517/14656566.2.12.1963. [DOI] [PubMed] [Google Scholar]
- 43.Bhangoo RK, Lowe CH, Myers FS, et al. Medication use in children and adolescents treated in the community for bipolar disorder. J Child Adolesc Psychopharmacol. 2003;13(4):515–522. doi: 10.1089/104454603322724904. [DOI] [PubMed] [Google Scholar]
- 44.Botts SR, Raskind J. Gabapentin and lamotrigine in bipolar disorder. Am J Health Syst Pharm. 1999;56(19):1939–1944. doi: 10.1093/ajhp/56.19.1939. [DOI] [PubMed] [Google Scholar]
- 45.Brambilla P, Barale F, Soares JC. Perspectives on the use of anticonvulsants in the treatment of bipolar disorder. Int J Neuropsychopharmacol. 2001;4(4):421–446. doi: 10.1017/S1461145701002668. [DOI] [PubMed] [Google Scholar]
- 46.Carta MG, Hardoy MC, Hardoy MJ, et al. The clinical use of gabapentin in bipolar spectrum disorders. J Affect Disord. 2003;75(1):83–91. doi: 10.1016/s0165-0327(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 47.Evins AE. Efficacy of newer anticonvulsant medications in bipolar spectrum mood disorders. J Clin Psychiatry. 2003;64(suppl 8):9–14. [PubMed] [Google Scholar]
- 48.Williams JW, Jr, Ranney L, Morgan LC, et al. How reviews covered the unfolding scientific story of gabapentin for bipolar disorder. Gen Hosp Psychiatry. 2009;31(3):279–287. doi: 10.1016/j.genhosppsych.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Yasmin S, Carpenter LL, Leon Z, et al. Adjunctive gabapentin in treatment-resistant depression: a retrospective chart review. J Affect Disord. 2001;63(1–3):243–247. doi: 10.1016/s0165-0327(00)00187-7. [DOI] [PubMed] [Google Scholar]
- 50.Maurer I, Volz HP, Sauer H. Gabapentin leads to remission of somatoform pain disorder with major depression. Pharmacopsychiatry. 1999;32(6):255–257. doi: 10.1055/s-1999-7958. [DOI] [PubMed] [Google Scholar]
- 51.Vigo DV, Baldessarini RJ. Anticonvulsants in the treatment of major depressive disorder: an overview. Harv Rev Psychiatry. 2009;17(4):231–241. doi: 10.1080/10673220903129814. [DOI] [PubMed] [Google Scholar]
- 52.Harden CL, Goldstein MA. Mood disorders in patients with epilepsy: epidemiology and management. CNS Drugs. 2002;16(5):291–302. doi: 10.2165/00023210-200216050-00002. [DOI] [PubMed] [Google Scholar]
- 53.Harden CL, Lazar LM, Pick LH, et al. A beneficial effect on mood in partial epilepsy patients treated with gabapentin. Epilepsia. 1999;40(8):1129–1134. doi: 10.1111/j.1528-1157.1999.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 54.Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341–348. doi: 10.1097/00004714-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol. 2000;20(4):467–471. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):479–486. doi: 10.1007/s10549-012-2251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tirault M, Foucan L, Debaene B, et al. Gabapentin premedication: assessment of preoperative anxiolysis and postoperative patient satisfaction. Acta Anaesthesiol Belg. 2010;61(4):203–209. [PubMed] [Google Scholar]
- 58.Khezri MB, Oladi MR, Atlasbaf A. Effect of melatonin and gabapentin on anxiety and pain associated with retrobulbar eye block for cataract surgery: a randomized double-blind study. Indian J Pharmacol. 2013;45(6):581–586. doi: 10.4103/0253-7613.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ménigaux C, Adam F, Guignard B, et al. Preoperative gabapentin decreases anxiety and improves early functional recovery from knee surgery. Anesth Analg. 2005;100(5):1394–1399. doi: 10.1213/01.ANE.0000152010.74739.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adam F, Bordenave L, Sessler DI, et al. Effects of a single 1200-mg preoperative dose of gabapentin on anxiety and memory. Ann Fr Anesth Reanim. 2012;31(10):e223–e227. doi: 10.1016/j.annfar.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Clarke H, Kay J, Orser BA, et al. Gabapentin does not reduce preoperative anxiety when given prior to total hip arthroplasty. Pain Med. 2010;11(6):966–971. doi: 10.1111/j.1526-4637.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 62.Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141–146. doi: 10.1023/a:1012281424057. [DOI] [PubMed] [Google Scholar]
- 63.Berigan TR. Gabapentin in the treatment of posttraumatic stress disorder. Prim Care Companion J Clin Psychiatry. 2000;2(3):105. doi: 10.4088/pcc.v02n0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brannon N, Labbate L, Huber M. Gabapentin treatment for posttraumatic stress disorder. Can J Psychiatry. 2000;45(1):84. [PubMed] [Google Scholar]
- 65.Malek-Ahmadi P. Gabapentin and posttraumatic stress disorder. Ann Pharmacother. 2003;37(5):664–666. doi: 10.1345/aph.1C082. [DOI] [PubMed] [Google Scholar]
- 66.Stein MB, Kerridge C, Dimsdale JE, et al. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20(6):923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 67.Fowler M, Garza TH, Slater TM, et al. The relationship between gabapentin and pregabalin and posttraumatic stress disorder in burned servicemembers. J Burn Care Res. 2012;33(5):612–618. doi: 10.1097/BCR.0b013e31823dc710. [DOI] [PubMed] [Google Scholar]
- 68.Onder E, Tural U, Gökbakan M. Does gabapentin lead to early symptom improvement in obsessive-compulsive disorder? Eur Arch Psychiatry Clin Neurosci. 2008;258(6):319–323. doi: 10.1007/s00406-007-0798-z. [DOI] [PubMed] [Google Scholar]
- 69.Anton RF, Myrick H, Baros AM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334–342. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- 70.Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709–717. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonnet U, Hamzavi-Abedi R, Specka M, et al. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143–145. doi: 10.1093/alcalc/agp085. [DOI] [PubMed] [Google Scholar]
- 72.Mariani JJ, Rosenthal RN, Tross S, et al. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15(1):76–84. doi: 10.1080/10550490500419110. [DOI] [PubMed] [Google Scholar]
- 73.Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582–1588. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7–8):961–969. doi: 10.1345/aph.1R751. [DOI] [PubMed] [Google Scholar]
- 75.Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile—a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434–438. doi: 10.1016/j.pnpbp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514–519. doi: 10.1097/01.jcp.0000088905.24613.ad. [DOI] [PubMed] [Google Scholar]
- 77.Reeves RR, Burke RS. Abuse of combinations of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;(5):16. doi: 10.4088/PCC.14l01660. doi:10.4088/PCC.14l01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonnet U, Banger M, Leweke FM, et al. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32(3):107–109. doi: 10.1055/s-2007-979203. [DOI] [PubMed] [Google Scholar]
- 79.Bozikas V, Petrikis P, Gamvrula K, et al. Treatment of alcohol withdrawal with gabapentin. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):197–199. doi: 10.1016/s0278-5846(01)00234-2. [DOI] [PubMed] [Google Scholar]
- 80.Johnson BA, Swift RM, Addolorato G, et al. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29(2):248–254. doi: 10.1097/01.alc.0000153542.10188.b0. [DOI] [PubMed] [Google Scholar]
- 81.Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221–227. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 82.Rustembegovic A, Sofic E, Tahirović I, et al. A study of gabapentin in the treatment of tonic-clonic seizures of alcohol withdrawal syndrome. Med Arh. 2004;58(1):5–6. [PubMed] [Google Scholar]
- 83.Voris J, Smith NL, Rao SM, et al. Gabapentin for the treatment of ethanol withdrawal. Subst Abus. 2003;24(2):129–132. doi: 10.1080/08897070309511541. [DOI] [PubMed] [Google Scholar]
- 84.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691–1700. doi: 10.4088/jcp.v68n1108. [DOI] [PubMed] [Google Scholar]
- 85.Mason BJ, Light JM, Williams LD, et al. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14(1):73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81(3):267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 88.González G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87(1):1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Mancino MJ, McGaugh J, Chopra MP, et al. Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine-dependent patients with depressive symptoms. J Clin Psychopharmacol. 2014;34(2):234–239. doi: 10.1097/JCP.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 91.Berger SP, Winhusen TM, Somoza EC, et al. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 92.Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2–3):274–277. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 93.Haney M, Hart C, Collins ED, et al. Smoked cocaine discrimination in humans: effects of gabapentin. Drug Alcohol Depend. 2005;80(1):53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 94.Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62(1):19–23. doi: 10.4088/jcp.v62n0105. [DOI] [PubMed] [Google Scholar]
- 95.Heinzerling KG, Shoptaw S, Peck JA, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(3):177–184. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 96.Urschel HC, 3rd, Hanselka LL, Baron M. A controlled trial of flumazenil and gabapentin for initial treatment of methylamphetamine dependence. J Psychopharmacol. 2011;25(2):254–262. doi: 10.1177/0269881109349837. [DOI] [PubMed] [Google Scholar]
- 97.Ling W, Shoptaw S, Hillhouse M, et al. Double-blind placebo-controlled evaluation of the PROMETA protocol for methamphetamine dependence. Addiction. 2012;107(2):361–369. doi: 10.1111/j.1360-0443.2011.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martínez-Raga J, Sabater A, Perez-Galvez B, et al. Add-on gabapentin in the treatment of opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):599–601. doi: 10.1016/j.pnpbp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 99.Tay KH. Gabapentin and opioid craving. Pain Med. 2009;10(4):774. doi: 10.1111/j.1526-4637.2009.00617.x. [DOI] [PubMed] [Google Scholar]
- 100.Kheirabadi GR, Ranjkesh M, Maracy MR, et al. Effect of add-on gabapentin on opioid withdrawal symptoms in opium-dependent patients. Addiction. 2008;103(9):1495–1499. doi: 10.1111/j.1360-0443.2008.02248.x. [DOI] [PubMed] [Google Scholar]
- 101.Salehi M, Kheirabadi GR, Maracy MR, et al. Importance of gabapentin dose in treatment of opioid withdrawal. J Clin Psychopharmacol. 2011;31(5):593–596. doi: 10.1097/JCP.0b013e31822bb378. [DOI] [PubMed] [Google Scholar]
- 102.Moghadam MS, Alavinia M. The effects of gabapentin on methadone based addiction treatment: a randomized controlled trial. Pak J Pharm Sci. 2013;26(5):985–989. [PubMed] [Google Scholar]
- 103.Sanders NC, Mancino MJ, Gentry WB, et al. Randomized, placebo-controlled pilot trial of gabapentin during an outpatient, buprenorphine-assisted detoxification procedure. Exp Clin Psychopharmacol. 2013;21(4):294–302. doi: 10.1037/a0033724. [DOI] [PMC free article] [PubMed] [Google Scholar]