Abstract
BACKGROUND AND OBJECTIVES:
Complicated vascular anomalies have limited therapeutic options and cause significant morbidity and mortality. This Phase II trial enrolled patients with complicated vascular anomalies to determine the efficacy and safety of treatment with sirolimus for 12 courses; each course was defined as 28 days.
METHODS:
Treatment consisted of a continuous dosing schedule of oral sirolimus starting at 0.8 mg/m2 per dose twice daily, with pharmacokinetic-guided target serum trough levels of 10 to 15 ng/mL. The primary outcomes were responsiveness to sirolimus by the end of course 6 (evaluated according to functional impairment score, quality of life, and radiologic assessment) and the incidence of toxicities and/or infection-related deaths.
RESULTS:
Sixty-one patients were enrolled; 57 patients were evaluable for efficacy at the end of course 6, and 53 were evaluable at the end of course 12. No patient had a complete response at the end of course 6 or 12 as anticipated. At the end of course 6, a total of 47 patients had a partial response, 3 patients had stable disease, and 7 patients had progressive disease. Two patients were taken off of study medicine secondary to persistent adverse effects. Grade 3 and higher toxicities attributable to sirolimus included blood/bone marrow toxicity in 27% of patients, gastrointestinal toxicity in 3%, and metabolic/laboratory toxicity in 3%. No toxicity-related deaths occurred.
CONCLUSIONS:
Sirolimus was efficacious and well tolerated in these study patients with complicated vascular anomalies. Clinical activity was reported in the majority of the disorders.
What’s Known on This Subject:
Several case reports and retrospective case series have been published on the use of sirolimus for the treatment of vascular anomalies. Positive response with limited toxicity was obtained, but these reports have no standardization of response or toxicity criteria.
What This Study Adds:
This study is the first prospective trial for children and young adults with complicated vascular anomalies. These patients have limited medical options, and treatments have been based on surgical and interventional procedures. Sirolimus was proven effective and safe.
Vascular anomalies are a spectrum of rare diseases classified into vascular tumors and malformations.1,2 An updated classification system was adopted at the International Society for the Study of Vascular Anomalies (ISSVA) in April 2014 .3 Generally, vascular tumors are proliferative, and malformations enlarge through expansion of a developmental anomaly with no underlying proliferation. Growth and/or expansion of vascular anomalies can cause clinical problems such as disfigurement, chronic pain, recurrent infections, coagulopathies (thrombotic and hemorrhagic), organ dysfunction, and death. Individuals often experience progressive clinical symptoms with worsening quality of life. Limited treatment options are available, and the efficacy of these options has not been validated in prospective clinical trials.4 Historically, therapies have been mostly interventional and surgical for the palliation of symptoms. Ideal therapies for this diverse patient population would target cellular pathways important in abnormal vascular proliferation and growth.
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway is critical to cell growth and survival and has been shown to govern normal vascular development and angiogenesis.5 Sirolimus, a mammalian target of rapamycin (mTOR), integrates signals from the PI3K/AKT pathway to coordinate proper cell growth and proliferation by regulating ribosomal biogenesis and protein synthesis.6 Enhanced mTOR signaling increases expression of the vascular endothelial growth factor, a key regulator of angiogenesis and lymphangiogenesis.7 Disorders that lead to inappropriate activation of the PI3K/AKT/mTOR pathway have been shown to result in tissue overgrowth in association with vascular anomalies.
Overexpression of AKT/protein kinase B, TIE2 receptor–activating mutations, and the loss of function mutations in phosphatase and the tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor gene have been associated with the development of vascular anomalies in animals and humans.8–11 Furthermore, tuberous sclerosis and lymphangioleiomyomatosis are caused by inactivating mutations in the tuberous sclerosis complex tumor suppressor proteins TSC1 and TSC2, leading to increased activation of mTOR.12 Clinical trials of sirolimus in these diagnoses have produced promising results.12,13
Sirolimus was initially administered for compassionate use in a young patient with a Kaposiform hemangioendothelioma (KHE) with Kasabach-Merritt phenomenon (KMP) who had failed to respond to all standard treatment algorithms.14 Treatment rationale was based on the tumor’s significant lymphatic component and the activation of the PI3K/AKT/mTOR pathway in angiogenesis and lymphangiogenesis, as well as its use in tumors, lymphangioleiomyomatosis, tuberous sclerosis, and neurofibromas.14 Since the start of the study, several case reports and retrospective case series have been published, with positive results.15–24 This patient responded to sirolimus treatment, as did 5 additional high-risk patients. The discovery of somatic mutations in the PI3K/mTOR pathway (PIK3CA) and mutations, both somatic and germline, in complementary pathways (TIE2, RASA1, and PTEN) provide compelling evidence for the critical role of this pathway in the regulation of vascular growth and organization. These mutations provide the molecular rationale for mTOR inhibition in many of the disorders included in this study.25–28
Methods
The study was designed by the investigators and approved by the Data and Safety Monitoring Board of the Cancer and Blood Disease Institute at Cincinnati Children’s Hospital Medical Center and the institutional review boards at Cincinnati Children’s Hospital Medical Center and Boston Children’s Hospital. The protocol, including the statistical analysis plan, is available in the Supplemental Information.
Patients
Inclusion criteria included 1 of the 9 verified vascular anomaly diagnoses (initial enrolling diagnosis) (Table 1) and at least 1 of 6 predefined complications: coagulopathy, chronic pain, recurrent cellulitis (defined as >3 episodes per year), ulceration, visceral and/or bone involvement, and/or cardiac dysfunction. No patients were enrolled with multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia) or capillary lymphatic arterial venous malformations during the study period. The majority of patients had undergone some form of previous therapy for their vascular anomaly (Supplemental Table 10). Eligible patients were between 0 and 31 years of age with adequate organ function (liver, bone marrow, and renal), an adequate lipid panel, Karnofsky/Lansky performance status ≥50, and no concurrent use of cytochrome P450 3A4 enzyme inducers or inhibitors. Patients could not receive concurrent steroids (except for patients with KHE), chemotherapy, or radiation and 2 weeks must have elapsed since undergoing major surgery. Patients were prohibited from receiving myelosuppressive chemotherapy within 4 weeks of entry into the study. Patients could not have received hematopoietic growth factors, antineoplastic agents, enzyme-inducing anticonvulsants, or cytochrome P450 3A4 inhibitors or inducers within 7 to 14 days before entry to the study.
TABLE 1.
Enrolling Diagnosis
| Initial Enrolling Diagnosis | Updated Diagnosis Classification |
|---|---|
| Microcystic lymphatic malformation (n = 22) | Generalized lymphatic anomaly (n = 7) |
| Gorham syndrome (n = 3) | |
| Kaposiform lymphangiomatosis (n = 7) | |
| Microcystic lymphatic malformation (n = 5) | |
| KHE or TA with KMP (n = 10) | KHE with KMP (n = 10) |
| KHE or TA without KMP (n = 3) | KHE without KMP (n = 3) |
| Capillary venous lymphatic malformation (n = 13) | Capillary lymphatico/venous malformation (n = 13) |
| Lymphangiectasia (n = 3) | Abnormalities of the central conducting lymphatic channels (n = 3) |
| PTEN with vascular anomaly (n = 6) | PTEN/AVM (n = 2) |
| PTEN/overgrowth/VA (n = 4) | |
| Venous lymphatic malformation (n = 3) | Venous lymphatic malformation (n = 3) |
| Multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia (n = 0) | Multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia (n = 0) |
| Capillary lymphatic arterial venous malformation (n = 0) | Capillary lymphatic arterial venous malformation (n = 0) |
Diseases were initially stratified on the basis of the ISSVA classification reported in 1997; lesions were then recategorized based on the revised 2014 ISSVA classification.
Exclusion criteria included chronic steroid use, known HIV, chronic severe or uncontrolled medical disease, uncontrolled infection, and previous use of an mTOR inhibitor. Dental braces or prostheses were prohibited only if interfering with radiologic analysis of the vascular anomaly. Patients who had impairment of gastrointestinal function or disease that may decrease the absorption of sirolimus were excluded. Pregnant and breastfeeding women were excluded, and male and female subjects of reproductive potential were required to use effective contraceptive methods throughout the study and for 3 months after study end. Patients with uncontrolled infection and who were unwilling or unable to comply with the protocol were excluded from participation. All patients and/or guardians provided informed consent as approved by the local institutional review boards.
Treatment
Sirolimus was administered orally on a continuous dosing schedule at a starting dose of 0.8 mg/m2 per dose twice daily, with 1 course equivalent to 28 days. Using pharmacokinetically guided dosing, sirolimus levels were measured at appropriate times, and trough levels were maintained between 10 and 15 ng/mL. Planned treatment duration was 12 courses per patient. Continuation of treatment beyond 12 courses in patients with responsive or stable disease was permitted at the discretion of the treating institutions; data regarding treatment continuation beyond 12 courses were collected. Dose reductions or interruptions were permitted for clinically significant toxicities based on protocol outline. Patients with clinically significant grade 3 or 4 toxicities unresolved by dose adjustment were removed from the study. After completion of the protocol treatment, patients are followed up for 5 years to assess disease status (eg, growth of the vascular anomaly, complications) and therapy-related toxicity.
The primary outcome measure was the efficacy of sirolimus by the end of course (EOC) 6 defined by complete or partial response (CR/PR) and the incidence of toxicities and/or infection-related death. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (version 3.0). Laboratory testing to assess safety included hematologic, serum metabolic, and urine chemical tests.
Disease Evaluation
The optimal measure of disease response in patients with complex vascular anomalies has not been established. For this reason, determination of the efficacy of sirolimus incorporated 3 distinct methods: radiologic evaluation, functional impairment score (clinical measurement of disease), and health-related quality of life (HRQOL). The most common radiologic evaluation was an MRI performed according to a standardized protocol. Other entities deemed appropriate by the study radiologist included computed tomography scans and radiographs. HRQOL was assessed by using the Pediatric Quality of Life Inventory 4.0 (3–18 years) and Infant Scales (≤2 years) and the Functional Assessment of Chronic Illness system (>18 years). The functional impairment score was adopted from the measures of organ function that have been validated in the quantification of adverse event results from medical therapies and procedures. This instrument was piloted in the present study for use in vascular anomalies. Baseline assessment was performed before administration of study medication, and formal response was assessed after courses 3, 6, and 12. Response was established by change in at least 1 of these parameters (Table 2).
TABLE 2.
Evaluation of Disease Response
| Disease response will be established by changes in at least 1 parameter, coded by using the following criteria |
| Response by imaging |
| Assessment of other clinical measures (quality of life) |
| Clinical criteria and functional impairment |
| Response was established by change in at least 1 of these parameters |
| CR |
| No evidence of disease on radiologic imaging and |
| No evidence of organ dysfunction due to disease and |
| Normalization of quality of life criteria |
| PR |
| >20% reduction in size of target vascular lesion evident on radiologic imaging or |
| Improvement in target organ dysfunction by at least 1 grade or |
| Improvement of self-report PedsQL by >4.4 or proxy-report PedsQL by >4.5 compared with baseline; FACT-G by >3.99 |
| Progressive disease |
| >20% increase of target vascular lesion evident on radiologic imaging or |
| Worsening in target organ dysfunction by at least 1 grade or |
| Worsening of self-report PedsQL by >4.4 or proxy-report PedsQL by >4.5 compared with baseline; FACT-G by >3.99 |
| Stable disease |
| None of the above |
FACT-G, Functional Assessment of Cancer Therapy–General; PedsQL, Pediatric Quality of Life Inventory 4.0.
Statistical Design
The primary outcomes were overall responsiveness to sirolimus at EOC 6 defined as CR/PR (compilation of radiologic evaluation, HRQOL, and functional assessment) and the incidence of toxicities and/or infection-related deaths. Multinomial distributions were used to estimate the response and the incidence rates with their 95% confidence intervals (CIs) for overall response at EOC 6 and 12. Frequencies and percentages were used to express the breakdown of overall responses according to disease stratification.
This study comprised 2 stages to determine if there is an adequate level of disease responsiveness to sirolimus in children and young adults with vascular anomalies. The trial was written to disapprove the use of sirolimus in this population if the rate of CR/PR was ≤5%. Based on Simon’s optimal 2-stage design,29 an interim analysis was conducted on the first 23 patients. Futility and stoppage of the study were defined as zero or only 1 CR/PR. If the response rate was at least 18%, the planned 60 patients would provide a 90% power to show a significant response at a 0.05 level. The interim analysis results permitted trial continuation.
Results
The study was open to accrual from October 2009 through April 2013 and enrolled 61 patients. One patient was replaced after results of a biopsy revealed an ineligible diagnosis. Fifty-seven patients completed 6 courses of therapy and were evaluable for response; all 61 patients were evaluable for toxicity. Forty-six patients completed all 12 courses of protocol therapy. Fifteen patients did not complete treatment due to progressive disease (n = 8), drug-related toxicity (n = 2), physician decision to stop medication (n = 2), refusal or withdrawal of consent due to patient/family preference (n = 2), and protocol noncompliance (n = 1). Disease stratification and patient demographic characteristics are summarized in Tables 1 and 3, respectively.
TABLE 3.
Demographic Characteristics
| Characteristic | Value (N = 60) |
|---|---|
| Gender, n (%) | |
| Female | 35 (58) |
| Male | 25 (42) |
| Median age, y | 8.1 |
| Age range | 21 d–28.5 y |
| Age group, n (%) | |
| 0–9 y | 33 (55) |
| 10–19 y | 17 (28) |
| 20–29 y | 10 (17) |
Overall Clinical Outcome
Forty-seven (83%) of 57 patients who completed 6 courses of treatment had a PR at EOC 6, and 45 (85%) of 53 patients who completed 12 courses of treatment had a PR at EOC 12. Three patients (5%) had stable disease (SD) at EOC 6, and no patient had stable disease at EOC 12. Seven patients (12%) had progressive disease (PD) by EOC 6 and 8 (15%) by EOC 12 (Table 4). No patients experienced a CR.
TABLE 4.
Overall Response
| Overall Response | Course 6, (n = 57) | Course 12, (N = 53) |
|---|---|---|
| CR | 0 | 0 |
| PR | 47 (83) [70–95] | 45 (85) [73–97] |
| PD | 7 (12) [2–23] | 8 (15) [3–27] |
| SD | 3 (5) [0–13] | 0 |
Data are presented as n (%) [95% CI].
With reference to the updated ISSVA classification system, several disease entities had 100% PR at EOC 6 and 12: generalized lymphatic anomaly, KHE with KMP, capillary-lymphatic-venous malformation (CLVM), PTEN/arterial venous malformation, and venous lymphatic malformation. Gorham syndrome had 100% PR at the EOC 6. Only 1 disease stratification had 100% progressive disease: lymphangiectasia/abnormalities of the central conducting lymphatic channels (Table 5).
TABLE 5.
Disease Response at EOC 6 and 12
| Initial Enrolling Diagnosis | Updated Diagnosis Classification | Efficacy Presented According to Vascular Anomaly Diagnosis Following | |
|---|---|---|---|
| 6 Courses: Response (57 Evaluable) | 12 Courses: Response (53 Evaluable) | ||
| Microcystic lymphatic malformation (n = 22) | Generalized lymphatic anomaly | PR 7 (100%) | PR 7 (100%) |
| Gorham syndrome | PR 3 (100%) | PR 1 (50%), progressive disease 1 (50%) | |
| Kaposiform lymphangiomatosis | PR 5 (71%), stable disease 1 (14%), progressive disease 1 (14%) | PR 6 (86%), progressive disease 1 (14%) | |
| Microcystic lymphatic malformation | PR 2 (50%), progressive disease 2 (50%) (1 NE)a | PR 2 (50%), progressive disease 2 (50%) (1 NE)a | |
| KHE with KMP (n = 10) | PR 10 (100%) | PR 10 (100%) | |
| KHE without KMP (n = 3) | PR 1 (33%), stable disease 1 (33%), progressive disease 1 (33%) | PR 2 (66%), progressive disease 1 (33%) | |
| Capillary venous lymphatic malformation (n = 12) | Capillary lymphatico/venous malformation | PR 11 (100%) (1 NE) | PR 11 (100%) (1 NE) |
| Lymphangiectasia (n = 3) | Abnormalities of the central conducting lymphatic channels | Progressive disease 3 (100%) | Progressive disease 3 (100%) |
| PTEN with vascular anomaly (n = 6) | PTEN/AVM | PR 2 (100%) | PR 1 (100%) (1 LTFU)b |
| PTEN/overgrowth/VA | PR 3 (75%), stable disease 1 (25%) | PR 3 (100%) (1 NE) | |
| Venous lymphatic malformation (n = 3) | Venous lymphatic malformation | PR 3 (100%) | PR 2 (100%) |
| Overall | PR 47 (82%), stable disease 3 (5%), progressive disease 7 (12%) | PR 45 (85%), progressive disease 8 (15%) | |
AVM, arteriovenous malformation; LTFU, lost to follow-up; NE, not evaluable; VA, vascular anomalies.
Participants were removed from study treatments for reasons other than progressive disease.
Participant did not return for end of study visit at EOC 12.
Overall Outcomes Related to Response Criteria
Quality of Life
Overall, patients had significant improvement in quality of life measurements. Thirty-two percent (95% CI: 17–47) had normalization (CR) at EOC 6 and 39% (95% CI: 23–55) at EOC 12. At EOC 6, 52% (95% CI: 36–68) had a PR, with 50% (95% CI: 34–67) achieving a PR at EOC 12. Sixteen percent (95% CI: 4–28) of patients had stable disease at EOC 6 and 9% (95% CI: 0–19) at EOC 12. Only 1 patient at EOC 12 had progressive disease related to quality of life. This patient had improvement in functional impairment and a stable radiologic evaluation.
Functional Impairment Score
No patients had progression or CR. At EOC 6, 71% (95% CI: 56–85) had a PR, with 80% (95% CI: 67–94) at EOC 12. Twenty-nine percent (95% CI: 15–44) had stable disease at EOC 6 and 20% (95% CI: 6–33) at EOC 12.
Radiologic Evaluation
No patients had a CR at EOC 6 or 12. At EOC 6, only 1 patient (2%) had progressive disease (95% CI: 0–6), and no patients progressed at EOC 12. Thirty-five percent (95% CI: 20–51) had a PR at EOC 6, and 52% (95% CI: 36–69) had a PR at EOC 12. Stable disease was found in 63% (95% CI: 47–79) at EOC 6 and 48% (95% CI: 31–64) at EOC 12.
Toxicities
Toxicity data are summarized in Tables 6, 7, and 8. The most common toxicities (grades 3 or 4) attributed to sirolimus included blood/bone marrow at 27%, metabolic/laboratory at 3%, gastrointestinal at 3%, infection at 2%, lymphatic at 2%, and pulmonary/upper respiratory at 2%. Dose reduction was only required in 2 patients, 1 associated with possible laryngospasm and the second related to hypertriglyceridemia. Two patients were taken off study medicine secondary to toxicity. One patient was removed from the study secondary to persistent grade 2 nausea interfering with quality of life and the second for persistent grade 3 lymphedema. No toxicity-related deaths occurred during the study. Patients are being followed up for long-term toxicities every 6 months for 5 years; none has occurred thus far.
TABLE 6.
Adverse Events: Summary of All Grade 2 and Higher AEs According to Category Attributable to Sirolimus
| Toxicity Category | Possible | Probable | Definite |
|---|---|---|---|
| Blood/bone marrow, (%) | 17 (28) | 11 (18) | 2 (3) |
| Cardiac general, (%) | 0 | 1 (2) | 0 |
| Constitutional symptoms, (%) | 2 (3) | 2 (3) | 0 |
| Dermatology/skin, (%) | 3 (5) | 2 (3) | 0 |
| Gastrointestina, (%)l | 12 (20) | 18 (30) | 3 (5) |
| Infection, (%) | 9 (15) | 0 | 0 |
| Lymphatics, (%) | 3 (5) | 1 (2) | 0 |
| Metabolic/laboratory, (%) | 5 (8) | 6 (10) | 1 (2) |
| Musculoskeletal/soft tissue, (%) | 1 (2) | 0 | 0 |
| Pain, (%) | 5 (8) | 5 (8) | 1 (2) |
| Pulmonary/upper respiratory, (%) | 0 | 1 (2) | 0 |
Total participants = 60.
TABLE 7.
Summary of Adverse Events: Grade 3 and Higher Regardless of Attribution
| Toxicity Category | N | Incidence, % |
|---|---|---|
| Blood/bone marrow | 30 | 50 |
| Cardiac general | 1 | 2 |
| Coagulation | 5 | 8 |
| Constitutional symptoms | 5 | 8 |
| Gastrointestinal | 10 | 17 |
| Infection | 22 | 37 |
| Lymphatics | 2 | 3 |
| Metabolic/laboratory | 11 | 18 |
| Musculoskeletal/soft tissue | 1 | 2 |
| Neurology | 2 | 3 |
| Pain | 4 | 7 |
| Pulmonary/upper respiratory | 7 | 1 |
Total participants = 60.
TABLE 8.
Summary of AEs Grade 3 and Higher Attributable to Sirolimus
| Toxicity Category | N | Incidence, % |
|---|---|---|
| Blood/bone marrow | 16 | 27 |
| Gastrointestinal | 2 | 3 |
| Infection | 1 | 2 |
| Lymphatics | 1 | 2 |
| Metabolic/laboratory | 2 | 3 |
| Pulmonary/upper respiratory | 1 | 2 |
Total participants = 60.
One patient with a CLVM who completed therapy with PR died of presumed sepsis 1 year after completion. The patient was receiving a low daily dose of sirolimus (recent level <2 ng/mL). She had a febrile illness for which no medical therapy was sought despite a history of recurrent cellulitis and sepsis episodes.
Discussion
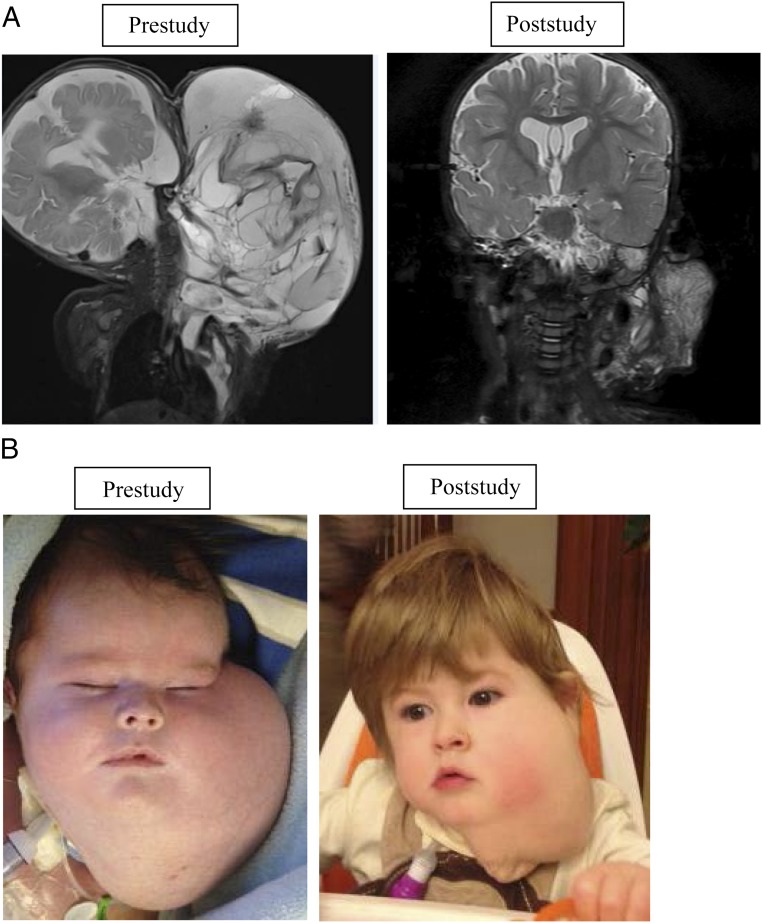
To the best our knowledge, this study is the only completed prospective clinical trial for patients with these complicated vascular diagnoses, and it confirms the overall efficacy of sirolimus in the treatment of vascular anomalies. Vascular anomalies have a variable natural history and often do not completely resolve, making assessment of response difficult. Assessment is further complicated by the classification of many different disease entities under the broad term “vascular anomaly,” with slightly different and variable phenotypes. In this prospective study, most enrolled patients with vascular anomalies exhibited beneficial overall responses. One patient with a microcytic and macrocytic lymphatic malformation with airway compromise had a remarkable response (Figs 1A and 1B).
FIGURE 1.
Macrocystic and microcystic lymphatic malformation with airway compromise. A, Coronal short-tau inversion-recovery magnetic resonance images of a young boy with a large focal macrocystic and microcystic lymphatic malformation; images were obtained before therapy initiation (left, at 6 weeks of age) and at the time of therapy cessation (right, 13 months later). The pretherapy image shows a large, infiltrating, multicystic mass of the left scalp, face, neck, and chest. The mass was causing airway compromise (not shown). With sirolimus therapy alone, the mass decreased markedly in size. B, Clinical examination revealed interval decrease in the size of the lesions, with decreased tumor bulk and firmness resulting in “saggy” tissue with less mass effect.
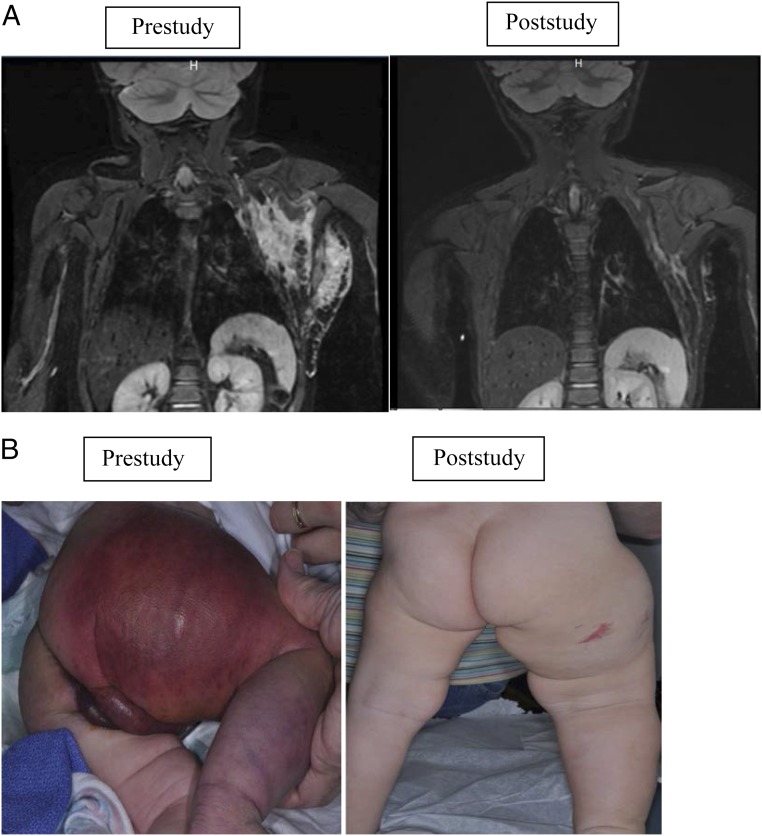
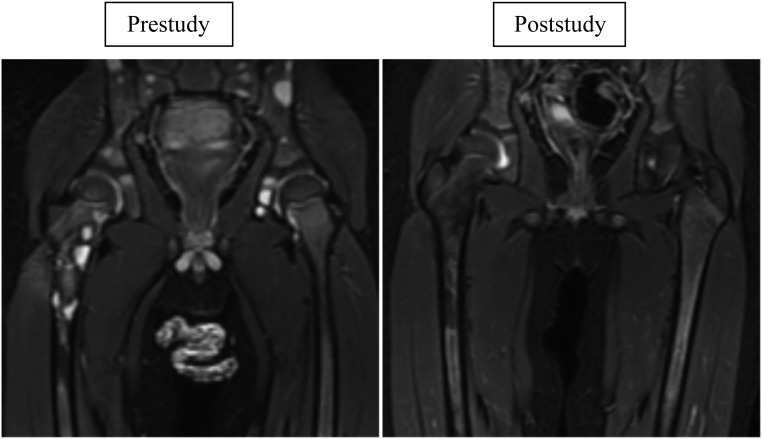
Impressive disease outcome was seen in patients with KHE and KMP, particularly in their hematologic response (Figs 2A and 2B). Previously, morbidity and mortality for these patients were most commonly secondary to KMP.30 Currently, a randomized Phase II study comparing vincristine therapy (expert consensus standard of care) versus sirolimus for the treatment of high-risk patients with KHE/KMP is accruing subjects.31 An adaptive study design will be used because this study is the first comparison for this population (ClinicalTrials.gov identifier NCT02110069, funded by the US Food and Drug Administration Office of Orphan Products Development 1R01FD004363 and Pfizer, Inc). Kaposiform lymphangiomatosis is a new entity with significant morbidity and a mortality rate as high as 66%.32 Of the 6 patients with Kaposiform lymphangiomatosis who responded to therapy, all have continued sirolimus treatment beyond the 12 courses without any disease progression. Figure 3 shows the bony improvement of 1 patient with generalized lymphatic anomaly according to results of an MRI. Other disease entities had life-altering improvement in coagulation parameters, pain, quality of life, and bleeding/leaking issues. Table 9 displays the remarkable decrease in cellulitis for a patient with CLVM. The effect was so significant that the majority of patients/families chose to continue sirolimus off-label at the end of the study (42 of 53 patients).
FIGURE 2.
KHE with KMP. A, Radiographic imaging of a patient with KHE and KMP. Coronal short-tau inversion-recovery magnetic resonance images of a young girl with a left chest wall KHE; images were obtained 18 days before therapy initiation and at the time of study conclusion. The pretherapy image shows a poorly defined, irregular mass of increased signal intensity infiltrating multiple tissue planes of the superficial and deep left lateral chest wall. The posttherapy image shows a marked interval decrease in the size of the mass. B, KHE with KMP functional impairment score/skin. Skin at prestudy and end of study. Physical examination at study conclusion revealed less purpura, petechiae, decreased warm, and softer cutaneous manifestations with improved range of motion.
FIGURE 3.
Generalize lymphatic anomaly with bone involvement. Coronal short-tau inversion-recovery magnetic resonance images of a young boy with generalized lymphatic anomaly obtained before therapy initiation (left, at 29 months of age) and at the time of therapy cessation (right, 11 months later). The pretherapy image shows numerous well-circumscribed, bright (fluid-signal intensity) lesions throughout the visualized bones of the pelvis and proximal right femur. The posttherapy image shows minimal residual osseous abnormality at these sites with no discrete cystic lesions.
TABLE 9.
One Patient’s Improvement in Recurrent Cellulitis
| Time Frame | No. of Infections | No. of Hospitalized Days |
|---|---|---|
| 6 months before study | 8 | 51 |
| Months 1 to 6 of study | 5 | 20 |
| Months 7 to 12 of study | 0 | 0 |
A 4-year-old patient with CLVM had a history of recurrent cellulitis, >8 episodes per year, and the first infection occurred at 2 days of life due to her lipomatous tumor. In the 6 months before initiation of the study, the patient was hospitalized for a total of 51 days. After 6 months of treatment, this number fell to 20 days; after another 6 months of sirolimus treatment, there were no cellulitis infections and no days of hospitalization.
Because of the inclusion of varied phenotypes, the largest study limitation was complicated data analysis. Because safety and efficacy in this population were unknown, only the patients with the most complicated condition were enrolled. All of these patients had failed to respond to previous therapies, including medication, interventional procedures, and/or surgery. Patients were excluded if these procedures were performed within 2 weeks of enrollment except for patients with KHE, who were allowed to waive the “washout” period. Due to small numbers, efficacy in each stratum cannot be determined, and further studies are indicated for disease strata using adaptive study designs. Furthermore, assessment based on the number of previous interventions cannot be determined and will need to be assessed in future trials with upfront medical therapy. All of these patients had extensive anomalies (as depicted in Supplemental Table 11). Numbers were too small to correlate anatomic site to response. Extent of disease and disease phenotype will be important to investigate in the future and how these factors relate to risk stratification.
Toxicity data were limited, and adverse effects were consistent with other studies.12,13 Long-term effects continue to be monitored as patients maintain sirolimus treatment secondary to its beneficial effects on these diseases. Our initial patient, treated before this study, has had no long-term issues >7 years from initiation of sirolimus. However, there are potential safety issues (eg, hypertriglyceridemia, hyperglycemia, hypercholesterolemia, potential risk of secondary malignancies) that should be monitored. Because the overall population is young and the diseases will not completely resolve, our present study will continue to follow up patients every 6 months for 5 years after study completion.
Although most patients chose to continue treatment with sirolimus after 1 year of treatment, some did not. Two patients, who came off study medicine because of parental/patient preference, restarted sirolimus off-study when efficacy was noted in retrospect after discontinuing the drug. Six patients discontinued treatment at EOC 12 but restarted off-label sirolimus treatment because of the recurrence of symptoms. Although the numbers are limited, all patients who restarted therapy had a CR. For diseases requiring ongoing sirolimus treatment, dose-minimizing strategies and long-term toxicities will be important to monitor because vascular anomalies can progress with puberty, active growth phases, infection, and trauma. Sirolimus may potentially be used selectively during these high-risk periods.
The age at initiation of sirolimus treatment may influence its efficacy. There were several patients of differing ages with the same diagnosis and phenotype in which the younger patients seemed to exhibit a more substantial response. This observation may be explained by physiologic changes of the lymphatic system over time that makes medical management less effective.
The numbers in this study were too small for phenotype/genotype correlation, but this correlation will be possible in the future and may guide treatment decisions, especially when new drugs become available for more molecularly targeted therapy. Biomarker studies are currently underway. Sixty of 61 study participants enrolled in the optional serum markers evaluation, and 52 of 61 participants enrolled in the optional tissue studies. Serum and tissue markers are currently being analyzed, and these findings may elucidate the mechanism(s) of action of sirolimus on vascular anomalies.
Conclusions
Sirolimus is an efficacious and safe treatment for the majority of patients with complicated vascular anomalies. Further study is needed to evaluate specific disease phenotypes, to understand mechanism of action, and to monitor for possible late effects and long-term treatment outcomes.
Glossary
- CI
confidence interval
- CLVM
capillary-lymphatic-venous malformation
- CR
complete response
- EOC
end of course
- HRQOL
health-related quality of life
- ISSVA
International Society for the Study of Vascular Anomalies
- KHE
Kaposiform hemangioendothelioma
- KMP
Kasabach-Merritt phenomenon
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PR
partial response
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
Footnotes
Dr Adams conceptualized and designed the study and drafted the initial manuscript; Drs Trenor, Hammill, Vinks, Patel, Chaudry, Gupta, Merrow, Fei, Dasgupta, Dickie, Elluru, Lucky, Weiss, Azizkhan, and Fei, as well as Mses Chute, Eile, and Hornung, conducted the initial analyses and reviewed and revised the manuscript; and Mses Wentzel, Mobberley-Schuman, Campbell, Brookbank, and McKenna designed the data collection instruments, coordinated and supervised data collection at the 2 sites, and critically reviewed the manuscript. All authors approved the final manuscript as submitted. All authors participated in the writing and in the decision to submit this manuscript for publication and thus vouch for the completeness and veracity of the data and data analysis.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00975819).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Office of Orphan Products (RO1FD003712-04) to Dr Adams at Cincinnati Children’s Hospital Medical Center. Pfizer Inc provided sirolimus but had no role in designing or conducting the study or in analyzing or reporting the data. This research was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422 [DOI] [PubMed] [Google Scholar]
- 2.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375–423 [PubMed] [Google Scholar]
- 3.Wassef M, Blei F, Adams DM, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015. Jul;136(1):e203-14. doi: 10.1542/peds.2014-3673. Epub 2015 Jun 8. [DOI] [PubMed]
- 4.Adams DM, Wentzel MS. The role of the hematologist/oncologist in the care of patients with vascular anomalies. Pediatr Clin North Am. 2008;55(2):339–355, viii [DOI] [PubMed] [Google Scholar]
- 5.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16(4):525–537 [DOI] [PubMed] [Google Scholar]
- 6.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16(1):29–37 [DOI] [PubMed] [Google Scholar]
- 7.Lee DF, Hung MC. All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle. 2007;6(24):3011–3014 [DOI] [PubMed] [Google Scholar]
- 8.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784(1):150–158 [DOI] [PubMed] [Google Scholar]
- 9.Perry B, Banyard J, McLaughlin ER, et al. AKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivo. Arch Dermatol. 2007;143(4):504–506 [DOI] [PubMed] [Google Scholar]
- 10.Morris PN, Dunmore BJ, Tadros A, et al. Functional analysis of a mutant form of the receptor tyrosine kinase Tie2 causing venous malformations. J Mol Med (Berl). 2005;83(1):58–63 [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Hampel H, Thiele H, et al. Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes. Lancet. 2001;358(9277):210–211 [DOI] [PubMed] [Google Scholar]
- 12.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormack FX, Inoue Y, Moss J, et al. ; National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group . Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024 [DOI] [PubMed] [Google Scholar]
- 15.Vlahovic AM, Vlahovic NS, Haxhija EQ. Sirolimus for the treatment of a massive capillary-lymphatico-venous malformation: a case report. Pediatrics. 2015;136(2). Available at: www.pediatrics.org/cgi/content/full/136/2/e513 [DOI] [PubMed] [Google Scholar]
- 16.Lackner H, Karastaneva A, Schwinger W, et al. Sirolimus for the treatment of children with various complication vascular anomalies. Eur J Pediatr 2015;174(12):1579–1584 [DOI] [PubMed] [Google Scholar]
- 17.Fogel AL, Hill S, Teng JM. Advances in the therapeutic use of mammalian target of rapamycin (mTOR) inhibitors in dermatology. J Am Acad Dermatol. 2015;72(5):879–889 [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Benjamin L, Wysong A, Hovsepian D, Teng J. Treatment of complex periorbital venolymphatic malformation in a neonate with a combination therapy of sirolimus and prednisolone. Dermatol Ther (Heidelb). 2015;28(4):218–221 [DOI] [PubMed] [Google Scholar]
- 19.Uno T, Ito S, Nakazawa A, Miyazaki O, Mori T, Terashima K. Successful treatment of Kaposiform hemangioendothelioma with everolimus. Pediatr Blood Cancer. 2015;62(3):536–538 [DOI] [PubMed] [Google Scholar]
- 20.Iacobas I, Simon ML, Amir T, et al. Decreased vascularization of retroperitoneal kaposiform hemangioendothelioma induced by treatment with sirolimus explains relief of symptoms. Clin Imaging. 2015;39(3):529–532 [DOI] [PubMed] [Google Scholar]
- 21.Rössler J, Braunschweiger F, Schill T. Medication-based therapy of infantile hemangioma and lymphatic malformations [in German]. HNO. 2014;62(1):12–18 [DOI] [PubMed] [Google Scholar]
- 22.Margolin JF, Soni HM, Pimpalwar S. Medical therapy for pediatric vascular anomalies. Semin Plast Surg. 2014;28(2):79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder U, Lauten M, Stichtenoth G, Gebhard MP, Buchholz M, Kaiser MM. Laryngomalacia and complicated, life-threatening mTOR-positive kaposiform hemangioendothelioma cured by supraglottoplasty and sirolimus. Klin Padiatr. 2014;226(6–7):362–368 [DOI] [PubMed] [Google Scholar]
- 24.Kai L, Wang Z, Yao W, Dong K, Xiao X. Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol. 2014;140(3):471–476 [DOI] [PubMed] [Google Scholar]
- 25.Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90(6):1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uebelhoer M, Nätynki M, Kangas J, et al. Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Hum Mol Genet. 2013;22(17):3438–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revencu N, Boon LM, Mulliken JB, et al. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29(7):959–965 [DOI] [PubMed] [Google Scholar]
- 28.Osborn AJ, Dickie P, Neilson DE, et al. Activating PIK3CA alleles and lymphangiogenic phenotype of lymphatic endothelial cells isolated from lymphatic malformations. Hum Mol Genet. 2015;24(4):926–938 [DOI] [PubMed] [Google Scholar]
- 29.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10 [DOI] [PubMed] [Google Scholar]
- 30.Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr. 2013;162(1):142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drolet BA, Trenor CC III, Brandão LR, et al. Consensus-derived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr. 2013;163(1):285–291 [DOI] [PubMed] [Google Scholar]
- 32.Croteau SE, Kozakewich HP, Perez-Atayde AR, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164(2):383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]