Abstract
Despite the frequency of labral tears in symptomatic developmental dysplasia of the hip, no consensus exists regarding the treatment of coexisting dysplasia of the hip and tearing of the acetabular labrum. The purpose of this prospective, MR arthrography (MRA) based 2-year follow-up study was to identify risk factors predicting the need for a hip arthroscopy (HA) after periacetabular osteotomy (PAO). Ninety-nine patients (104 hips) scheduled for PAO were evaluated preoperatively and at 2-year follow-up. MRA was performed in all patients prior to PAO. At follow-up, patients were divided into a non-arthroscopy and arthroscopy group. The two groups were compared clinical and radiological, and risk factors for HA after PAO were calculated. Patient reported outcome measures (WOMAC, Oxford Hip and SF36) were filled out before PAO and at follow-up. Ninety-five hips (91.3%) were evaluated. Twenty-six hips (27%) required an arthroscopy within 2 years of the PAO. Risk factors were preoperative borderline dysplasia, acetabular retroversion and complete labral detachment. Labral tearing, degeneration or hypertrophy did not negatively affect the outcome of PAO. Patients not requiring an arthroscopy had a statistically significant better outcome measured by patients reported outcome measures. After PAO, 27% of the hips needed intra-articular assessment. Conventional radiographs and MRA analysis can be used to identify predictors for patients requiring HA after PAO. At 2-year follow-up, the clinical outcome improved in all patients. However, those patients who had no need of a HA after their PAO had superior results.
INTRODUCTION
The Bernese periacetabular osteotomy (PAO) has become the preferred joint preserving treatment for symptomatic developmental dysplasia of the hip (DDH) [1]. Dorrell and Caterall [2] were among the first to report on the relationship between dysplastic osseous abnormalities and labrum pathology. Since then literature describing how the osseous abnormalities and the resulting pathological joint biomechanics in developmental DDH may frequently lead to damage of the acetabular labrum has been evolving [3–6]. Recently, Ross et al. found only 5 normal labrums in 73 dysplastic hips [7].
Despite the frequency of labral tears in symptomatic DDH and the increasing literature concerning labral pathology, no consensus exists regarding the treatment strategy for DDH with coexisting acetabular labral tear. Tearing of the labrum is recognized being involved in joint degeneration and this may untreated lead to osteoarthritis. This has lead to new concepts and treatment strategies regarding the treatment of labral tearing in DHH. Open arthrotomy during PAO was the first means of addressing intraarticular pathology during PAO surgery [8]. Later hip arthroscopy assisted PAO was introduced to assess and address any present intraarticular pathology [9]. There is no evidence that intraarticular assessment, open or arthroscopic, is superior to not assessing the joint during PAO. However, hip arthroscopy alone without addressing the bony abnormalities in DDH is in general not recommended, and studies have showed failure in DDH hips undergoing hip arthroscopy with debridement of the labrum [10], and resulted in high reoperation rates comparing mild DDH hips with normal hips [11]. Studies reporting the outcome of PAO performed without simultaneous assessment of the joint have shown high hip joint survival rates [12, 13]. However, femoroacetabular impingement after PAO has been observed with poor outcome, and some patients will require a subsequent hip arthroscopy, and it would be valuable to identify predictors for hip arthroscopy as well as assessment of the results after hip arthroscopy in PAO patients.
The purpose of this prospective, MR arthrography (MRA) based 2-year follow-up study was to identify risk factors predicting the frequency of the need for a hip arthroscopy after PAO, and finally to compare clinical and radiographic outcomes between patients require a subsequent arthroscopy and patients not requiring arthroscopy after PAO.
MATERIAL AND METHODS
Ninety-nine patients (104 hips) consecutively scheduled for PAO due to DDH were enrolled in the study. Patients were included from January 2010 to August 2011 and all surgeries were performed or assisted by the senior author in Aarhus, Denmark. Five patients were excluded from the study, because of multiple complaints from several joints and thus, were not considered being representative for this PAO cohort. Four patients failed to show up at 2-year follow-up. Hence, the study group consisted of 90 patients (95 hips, 79 females, 52 right hips). Mean age of the patients at the time of PAO surgery was 34.1 years (range 14.5–58.9 years). Before PAO eight hips had a hip arthroscopy (Table I) and one patient had had a combined femoral and pelvic osteotomy. Twenty-three patients underwent PAO surgery on the opposite hip within the 2-year study period, and three patients had screws removed following PAO. One complication among the 95 was observed: an obturator nerve affection resulting in pain and paralysis of the adductor muscles. Another hip developed osteoarthritis. Beside that no intra- or postoperative complications was observed. Bilateral dysplasia was seen in 78% of the patients. Indication for PAO were persisting hip pain, a center edge angle of Wiberg [14] <25o, pelvic bone maturity, internal rotation >15o, hip flexion <110o and Tönnis grade of osteoarthritis 0 or 1. The minimally invasive transsartorial approach was used in all cases [15]. Preoperatively and at 2-year follow-up, the clinical and radiographic outcome were evaluated. Follow-up was done primarily by one investigator (CHA), except for four patients seen by the senior author (KSO). For data analysis, the patients were divided into an arthroscopy group if a hip arthroscopy was required within the 2-year follow-up period and a non-arthroscopy group.
Table I.
Description of the eight hips undergoing hip arthroscopy (HA) prior to the PAO
| Hip | Time from HA to PAO | HA findings | HA procedures | HA after PAO |
|---|---|---|---|---|
| 12a | NA | NA | NA, but no effect of surgery | No |
| 13 | 1 year | Torn labrum | Labrum resection, short term effect | No |
| 15a | NA | NA | NA, but no effect of surgery | No |
| 38a | NA | Labrum tear | Reinsertion of labrum | No |
| 45 | 3 years | Intact labrum, cartilage pieces | Removal of several cartilage pieces | No |
| 46 | 6 years | Thin cartilage, loose pieces of cartilage, labrum tear | Resection of the damaged parts | Yes |
| 49 | 2.5 years | Labrum a little frayed | Resection of the frayed part of the labrum | Yes |
| 59 | 4 years | Labrum-cartilage separation, hypertrophic labrum, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy | Yes |
aInformation from patient, journal records not available.
HA findings, Findings during hip arthroscopy; HA procedures, Procedures performed during hip arthroscopy.
Clinical evaluation
At 2-year follow-up, patients were interviewed regarding continued mechanical symptoms (clicking, locking and instability) from the hip joint, dysesthesia of the dermatome innervated by the lateral femoral cutaneous nerve, any kind of surgical and non-surgical treatment since the PAO. Signs of trochanteric bursitis, internal and external snapping hip were noted. Any leg length discrepancy and range of motion was measured. Impingement test and FABER test [16] were performed; however, in three hips, the tests were left out due to recent hip arthroscopy, and in two hips due to severe hip pain. Preoperatively and at 2-year follow-up, patients were requested to fill out the Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) [17] , the Oxford hip score (OHS)[18] and the general health questionnaire short form 36, version 1 [19]. Each subscale of the WOMAC score was calculated. To enhance the comparability with other studies, the summarized WOMAC total score were normalized with 100 indicating the best possible score. The OHS score was given as a total score with 48 indicating the best possible score. From the SF36 data, the physical and mental component scores were subsequently calculated. Five patients failed to fill out the questionnaires preoperatively, hence only 90 hips were evaluated by questionnaires.
Radiographic evaluation
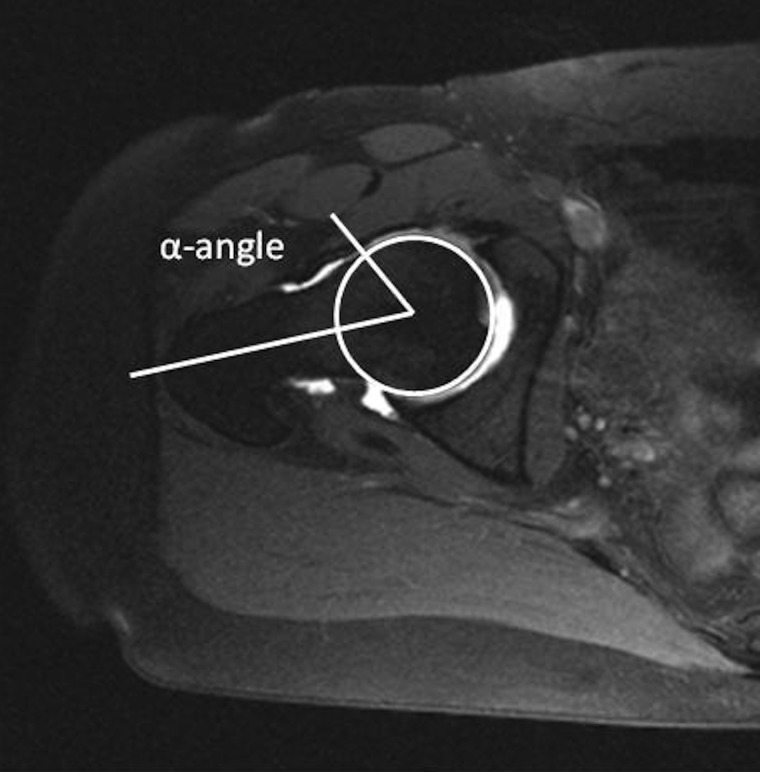
Conventional standing pelvic radiographs recorded preoperatively and at 2-year follow-up were analyzed. One investigator (CHA) assessed the following radiographic parameters: the center edge (CE) angle of Wiberg [14], the acetabular index (AI) angle [20], the presence of an os acetabuli [21], the Tönnis grade of osteoarthritis [20] and signs of retroversion (cross over sign) [21]. Hips were characterized dysplastic if the CE angle was between <25o. AI angles were considered normal if within 0o–10o. For the hip arthroscopy group, CE angles and AI angles after PAO were analyzed at the postoperative supine radiographs, since the arthroscopy may have changed the angles. Using supine exposures were justified by an earlier study showing no significant changes in these two angles when repositioning from the supine to the weight-bearing position [22]. The acetabulum was considered retroverted if the crossover sign [21, 23, 24] was present prior to PAO. All patients had a magnetic resonance arthrography (MRA) performed. The MRA were performed with a 1.5 Tesla Scanner (Siemens Magnetom Symphony) preceded by guided injection of 10 ml of diluted gadolinium contrast medium (Gd-DTPA, 2 mmol l−1) into the hip joint. The MRA was assessed for labral pathology in terms of degeneration, hypertrophic changes, tears and paralabral cysts. Labral lesions were graded according to the Czerny grading [25]. Czerny stages the labrum into groups according to shape, homogeneity and attachment to the acetabular rim. Cystic changes in the femoral head or in the acetabulum were noted. The α-angle was measured on oblique axial MRA images (Fig. 1) [26]. An α-angle ≥55o was considered pathological. One senior radiologist (J.G.) performed all intraarticular injections and analysis of MRA scans. Measurement of the α-angles was also performed by the C.H.A. In five hips, the α-angle could not be assessed due to imprecise oblique MRA images. Intra- and interobserver variability of the α-angle measurement was assessed by the C.H.A and the senior radiologist by doing rereadings of the MRA scan separated by 4 weeks. The mean of difference for intraobserver variability was 0.48o (SD ± 1.90o). The 95% limits of agreement (LOA) were −3.31o to 4.27o, and for the interobserver variability the mean difference was 1.52o (SD ± 3.14o), 95% LOA was −4.76o to 7.80o.
Fig. 1.
MRA measurement of the α-angle of Notzli on the oblique plane. After identification of the center of the femoral head, a line along the middle of the femoral neck and a line from the center to the point where the femoral head-neck junction ‘left’ the best fitted circle of the femoral head make up the α-angle.
Indication for hip arthroscopy
All patients with continuous symptoms in this study were primarily referred to the Sports Traumatology unit at Aarhus University Hospital, Denmark and evaluated by two experts in hip arthroscopy. Relevant patient history, continuous groin pain after PAO, a positive impingement or Faber test were indications for hip arthroscopy. Labral pathology diagnosed on MRA supported the diagnosis and indication. All patients referred in this study underwent hip arthroscopy.
Statistical analysis
Depending on distribution data was presented as means with 95% confidence intervals, or as medians with interquartile ranges. Odds ratios for hip arthroscopy were calculated using logistic regression. Non-parametric variables were evaluated with Wilcoxson sign rank test or Fisher’s exact test. Intra- and interobserver variability was assessed using the Bland–Altman approach [27–29], and data was presented as means of the difference with SD and 95% LOA.
RESULTS
Twenty-six of 95 hips (27%) had a hip arthroscopy within 2 years after PAO. One of these hips was converted to total hip arthroplasty two months after hip arthroscopy (7 months after PAO) (Table II).
Table II.
Description of the 26 hips undergoing hip arthroscopy (HA) after PAO
| Hip | Time from PAO to HA | MRA labrum diagnosis | MRA α-angle | HA findings | HA procedures |
|---|---|---|---|---|---|
| 9 | 9 months | Czerny 3A | 46o | Labrum damage anteriorly, mildly hypertrophic, pincer, CAMa | Rimtrim, labrum reinsertion, cheilectomy |
| 10b | 21 months | Czerny 3A, degeneration | 55o | Frayed labrum, pincer, minor CAM | Rimtrim, labrum reinsertion, minor cheilectomy |
| 22 | 13 months | Czerny 1A, degeneration | 51o | Labrum-cartilage separation, pincer, CAM, loose cartilage | Rimtrim, labrum reinsertion, cheilectomy, mircrofracture treatment |
| 26 | 6 months | Czerny 2B | 53o | Labrum-cartilage separation, pincer, minor CAM | Rimtrim, labrum reinsertion, minor cheilectomy |
| 31 | 16 months | Czerny 3A, degeneration | 44o | Labrum-cartilage separation, pincer, minor CAM, area with osteoarthritis | Rimtrim, minor cheilectomy |
| 33b | 15 months | Czerny 3A, degeneration | 58o | Labrum lesion with minor impact on the cartilage , pincer, bump on collum | Rimtrim, labrum reinsertion, minor cheilectomy |
| 40 | 7 months | Czerny 3B, degeneration | 44o | Labrum-cartilage separation, frayed labrum, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 46 | 4 months | No tears, mild hypertrophy | 43o | Labrum-cartilage separation, synovitis, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 49 | 15 months | Czerny 3A | 44o | Minor labrum-cartilage resection, minor pincer, CAM | Minor rimtrim, cheilectomy |
| 50 | 12 months | Czerny 3A, degeneration | 48o | Labrum-cartilage separation, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 52 | 8 months | Czerny 3A, degeneration | 69o | Labrum-cartilage separation, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 54b | 7 months | Czerny 3A, degeneration | 58o | Voluminous labrum, labrum- cartilage separation, minor CAM | Labrum reinsertion, minor cheilectomy |
| 58 | 24 months | Czerny 3A | 49o | Lesion of the cartilage at acetabulum and femur. Labrum attached. | Minor rimtrim, cheilectomy, synovectomy |
| 59 | 5 months | No tears, crushed and degeneration | 49o | Osteoarthritis acetabulum and caput femoris, labrum attached to the rim | Synovectomy |
| 60 | 12 months | Czerny 2A | 48o | Labrum-cartilage separation, minor pincer, minor CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 64 | 11 months | Czerny 3A, degeneration | 42o | Labrum-cartilage separation, pincer, minor CAM | Rimtrim, labrum reinsertion, minor cheilectomy |
| 65 | 11 months | Czerny 3A | 37o | Labrum not described, pincer minor CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 67 | 7 months | Czerny 3B, degeneration, hypertrophy | 61o | Labrum-cartilage separation, mild osteoarthritis, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 71b | 18 months | Czerny 3B | 66o | Degeneration of labrum, no tears, CAM osteoarthritis at acetabulum and femur | Cheilectomy |
| 72 | 5 months | Czerny 3A | 72o | Labrum-cartilage separation and influence of the cartilage, pincer, CAM | Rimtrim, labrum reinsertion, cheilectomy |
| 75b | 7 months | Czerny 3A | 57o | Not available | According to the patient ‘some bone work’. No effect. |
| 81 | 11 months | Czerny 3B | 54o | Rimtrim, labrum reinsertion, cheilectomy | |
| 82 | 9 months | Czerny 3A, degeneration | NA | Rimtrim, labrum reinsertion, cheilectomy | |
| 85b | 4 months | Normal | 50o | Labrum tear | Rimtrim, labrum reinsertion, cheilectomy |
| 95 | 11 months | Czerny 3A | 45o | Labrum tear | Labrum reinsertion |
| 100 | 16 months | Czerny 3A | 56 | Labrum tear, minimal pincer | Minimal rimtrim, labrum reinsertion |
aCAM term for the exostose on the femoral head–neck junction.
b(10) Repeat arthroscopy 11 months after first HA: refixation of labrum, minor rimtrim of the acetabulum and extended cheilectomy on femur. (33) Repeat arthroscopy 14 months after first HA: labrum healed, acetabular cartilage with wave-sign, minor rimtrim, minor cheilectomy, screw removal. (54) Repeat arthroscopy 8 months after first HA: labrum healed, minor pincer removed, minor cheilectomy, psoastenotomy. (71) Hip arthroplasty 6 months after HA. (75) Repeat arthroscopy 3 months after first HA. Labrum attached but anterior lesion. Pincer and minor CAM. Detachment of the labrum, rimtrim, reinsertion of labrum and minor cheilectomy. Psoastenotomy. (85) Repeat arthroscopy 11 months after first HA. (95) Repeat HA 9 months after HA: cheilectomy.
No significant differences in sex were found between the non-arthroscopy and arthroscopy group Significant adjusted predictors of need for hip arthroscopy after PAO were (i) mild dysplasia (OR 2.92); (ii) presence of the cross-over sign on preoperative radiographs (OR 3.30) and (iii) labrum detachment (Table III). The MRA analysis of the acetabular labrum revealed only six labrum (five in the non-arthroscopy group) without any signs of degeneration, hypertrophy or pathology according to the Czerny grading. No significant differences in labral pathology were found between the non-arthroscopy and arthroscopy group (Table IV). For the arthroscopy group, the median preoperative CE angle for was 20o (range 11o–24o) and the AI angle was 14o (range 8o–21o). The postoperative CE angle and the AI angle was 34o (range 17o–46o) and 3o (range −8o to 16o), respectively (Table V). At follow-up, the median CE angle and AI angle was 34o (range 25o–40o) and 1o (range −8o to 16o), respectively. For the hip arthroscopy group, both the CE angle and AI angle changed significantly after arthroscopy (Table VI). The median α-angle for the arthroscopy group was 50o (range 37o–72.o), with no significant difference between the non-arthroscopy and arthroscopy groups. About 18 of 95 hips were retroverted preoperatively, and four hips at 2-year follow-up, all in the non-arthroscopy group. Clinical testing after PAO for signs of impingement, trochanteric bursitis or persisting dysplasia revealed no significant difference between groups (Table VII).
Table III.
Odds ratios for predictors of clinical failure in terms of hip arthroscopy (n = 95a)
| Parameter | OR (95% CI) | P value | Adjusted OR (95% CI)b | P value |
|---|---|---|---|---|
| Borderline dysplasia (CE-angle ≥20° to <25°) | 2.82 (1.11–7.14) | 0.029 | 2.92 (1.13–7.52) | 0.026 |
| Postoperative AI angle <0° or >10° | 2.08 (0.77–5.65) | 0.151 | 2.48 (0.85–7.15) | 0.093 |
| Preoperatively cross over sign present | 3.52 (1.21–10.28) | 0.021 | 3.30 (1.09–9.95) | 0.035 |
| α-angle ≥55o | 1.47 (0.55–3.92) | 0.442 | 1.43 (0.52–3.94) | 0.493 |
| Labrum detachment | 2.28 (0.81–6.38) | 0.118 | 3.83 (1.18–12.44) | 0.025 |
| Labrum degeneration | 0.73 (0.30–1.79) | 0.486 | 0.88 (0.34–2.27) | 0.787 |
| Labrum hypertrophy | 3.62 (0.77–17.01) | 0.103 | 3.36 (0.69–16.42) | 0.134 |
| Presence of paralabral cyst | 2.31 (0.61–8.72) | 0.215 | 2.06 (0.53–7.98) | 0.295 |
aFive hips excluded from the analyses involving the α-angle.
bAdjusted for age (≤35 years)and borderline dysplasia.
Table IV.
Magnetic resonance arthrography characteristics (results for all hips and in groups, number of hips)
| Parameter | All hips | Arthroscopy group (n = 26) | Non-arthroscopy group (n = 69) |
|---|---|---|---|
| Degeneration of the labrum | |||
| Yes | 53 | 13 | 40 |
| No | 42 | 13 | 29 |
| Hypertrophied labrum | |||
| Yes | 18 | 2 | 16 |
| No | 77 | 24 | 53 |
| Paralabral cyst | |||
| Yes | 17 | 3 | 16 |
| No | 76 | 23 | 53 |
| Classification of labrum pathology | |||
| 0 | 12 | 3 | 9 |
| 1A | 3 | 1 | 2 |
| 1B | 2 | 1 | 2 |
| 2A | 14 | 1 | 13 |
| 2B | 3 | 1 | 2 |
| 3A | 44 | 16 | 28 |
| 3B | 17 | 4 | 13 |
aNo significant differences in labral pathology were found between the nonarthroscopy and arthroscopy group.
Table V.
Radiographic characteristics before and after PAO
| Parameter | Nonarthroscopy group (n = 69) | Arthroscopy group (n = 26) | P value |
|---|---|---|---|
| Before PAO | |||
| Center-edge angle | |||
| Median (interquartile range) | 17° (13° to 20°) | 20° (17° to 21°) | 0.055 |
| Range | −10° to 24° | 11° to 24° | |
| Acetabular index angle | |||
| Median (interquartile range) | 15° (12° to 20°) | 14° (12° to 18°) | 0.222 |
| Range | 0° to 33° | 8° to 21° | |
| After PAO | |||
| Center-edge angle | |||
| Median (interquartile range) | 34° (29° to 36°) | 34° (32° to 37°) | 0.317 |
| Range | 17° to 40° | 25° to 46° | |
| Acetabular index angle | |||
| Median (interquartile range) | 3° (1° to 6°) | 1o (−1° to 3°) | 0.010 |
| Range | −3° to 16° | −8° to 16° | |
| Crossover sign before PAOa | |||
| Before PAO | 9 | 9 | 0.036 |
| At 2-year followup | 4 | 0 | 0.572 |
aCrossover sign before arthroscopy were not possible to evaluate, since postoperative radiographs after PAO were supine taken.
Table VI.
Description of the changes in CE-angle in the arthroscopy group (n = 25a)
| Parameter | Before PAO | Before arthroscopy | After arthroscopy | P valueb |
|---|---|---|---|---|
| Center-edge angle | ||||
| Median (interquartile range) | 20° (17° to 21°) | 34° (32° to 37°) | 32° (29° to 36°) | 0.002 |
| Range | 11° to 24° | 25° to 46° | 22° to 40° | |
| Acetabular index angle | ||||
| Median (interquartile range) | 14° (12° to 18°) | 1° (−1° to 3°) | 4° (0° to 5°) | <0.001 |
| Range | 8° to 21° | −8v to 16° | −4° to 16° | |
aOne hip had only pre-arthroscopy radiographs and was left out for this analysis.
bStatistically significant difference between CE angles and AI angles before and after arthroscopy.
Table VII.
Clinical findings at 2-year follow-up
| Parameter | Arthroscopy group | Nonarthroscopy group | P value |
|---|---|---|---|
| Positive impingement | 8 | 25 | 0.796 |
| Positive FABER | 5 | 13 | 0.544 |
| Positive impingement and FABER | 3 | 12 | 0.753 |
| Trochanteric bursitis | 4 | 11 | 1.000 |
| Persisting dysesthesia | 16 | 35 | 0.367 |
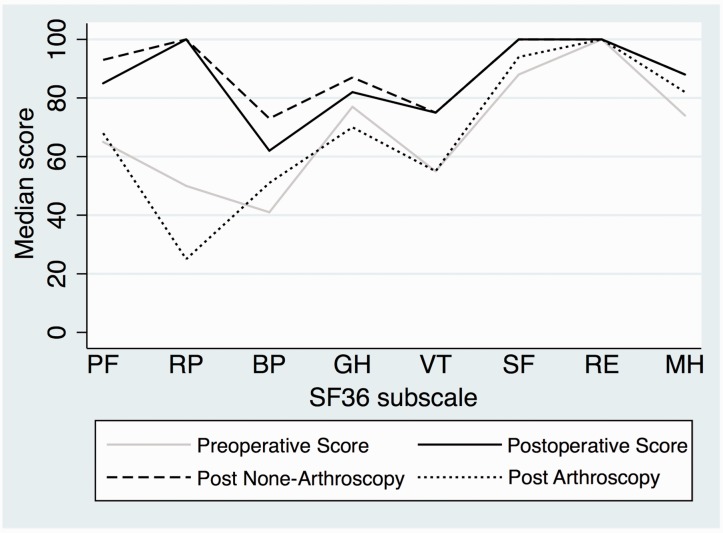
For both groups, the median normalized WOMAC total score increased from 66 (range 3–100) preoperatively to 89 (range 25–100) postoperatively and the median OHS increased from 28 (range 8–47) to 43 (range 12–48). The overall SF36 physical and mental component scores increased from 38 (range 16–55) to 48 (range 18–60) and from 54 (range 29–69) to 58 (range 27–78), respectively, (Table VIII). Improvements between the preoperative and 2-year follow-up assessment were observed in 7 of 8 subscales of the SF36 (Fig. 2). The total WOMAC score, the OHS and the physical component score of the SF36 differed statistically significant with superior results in the non-arthroscopy group compared to the arthroscopy group (P ≤ 0.001–0.013) (Table VIII). The preoperative scores for all patient reported outcome measures did not show any statistically significant differences between the arthroscopy and non-arthroscopy groups (P 0.067–0.810).
Table VIII.
Patient reported outcome measures for arthroscopy and nonarthroscopy group (n = 90 hips)
| Parameter | Preoperativea All (n = 90) | Postoperative All (n = 90) | Postoperative Arthroscopy (n = 26) | Postoperative nonarthroscopy (n = 64) | P value |
|---|---|---|---|---|---|
| WOMACb | |||||
| Pain | |||||
| Median (interquartile range) | 7 (5–10) | 2 (0–6) | 5 (1–9) | 1 (0–4) | <0.001 |
| Range | 0–20 | 0–14 | 0–14 | 0–12 | 0.013 |
| Stiff | |||||
| Median (interquartile range) | 3 (2–4) | 2 (0–2) | 2 (1–3) | 1 (0–2) | 0.001 |
| Range | 0–8 | 0–7 | 0–7 | 0–6 | <0.001 |
| Physical function | |||||
| Median (interquartile range) | 19 (11–29) | 4 (0–11) | 10 (3–24) | 2 (0–7) | |
| Range | 0–61 | 0–49 | 0–46 | 0–49 | <0.001 |
| Total scores | |||||
| Median (interquartile range) | 30 (17–41) | 8 (1–22) | 18 (8–36) | 5 (1–14) | |
| Range | 1–89 | 0–67 | 0–67 | 0–66 | |
| Normalized | |||||
| Median (interquartile range) | 66 (56–78) | 89 (73–98) | 78 (62–89) | 91 (82–100) | |
| Range | 3–100 | 25–100 | 25–100 | 33–100 | |
| Oxford hip scorec | |||||
| Total score (0–48) | |||||
| Median (interquartile range) | 28 (23–33) | 43 (35–47) | 36 (28–42) | 43 (37–47) | <0.001 |
| Range | 8–47 | 12–48 | 12–48 | 19–48 | |
| SF36 | |||||
| Physical component score (0–100) | |||||
| Median (interquartile range) | 38 (33–44) | 48 (38–55) | 39 (32–48) | 51 (44–56) | <0.001 |
| Range | 16–55 | 18–60 | 18–58 | 27–60 | |
| Mental component score (0–100) | |||||
| Median (interquartile range) | 54 (43–61) | 58 (52–61) | 56 (50–61) | 58 (52–61) | 0.243 |
| Range | 29–69 | 27–78 | 27–78 | 35–66 | |
aThe preoperative scores did not show any statistically significant differences between the arthroscopy and nonarthroscopy groups (P values 0.067–0.810).
bRaw scores with ‘0’ indicating best results. Normalized score with ‘100’ indicating best result.
cScore with 48 indicating best results.
Fig. 2.
Changes in SF36 subscale parameters for 90 patients before PAO and at 2-year follow-up after PAO. The postoperative subscale parameters are also illustrated separately for the nonarthroscopy group (dash) and the arthroscopy group (dot). SF36 consist of eight subscales with health-related parameters: physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH).
DISCUSSION
This study identifies radiographic predictors for the need of a hip arthroscopy 2 years after PAO. At 2-year follow-up, a statistical difference in patient reported outcome measures between the non-arthroscopy and arthroscopy group were found.
In mild dysplasia, only little reorientation is possible before overcorrection may occur, which could be the reason for the finding of a CE angle of 20o–25o being a significant predictor for subsequent arthroscopy. However, in this study a negative AI angle is not a significant factor similar to earlier findings reported by Steppacher [30]. Though, femoroacetabular impingement after PAO for hip dysplasia is well known [8].This could advocate for a thorough intraoperative assessment of femoroacetabular impingement. By restricting simultaneously intraarticular surgery only to patients with mild dysplasia the majority of the patients will avoid over-treatment and thereby the risk of unnecessary complications. However, a recent study of 26 patients with mild dysplasia undergoing arthroscopic treatment alone, demonstrates at 2-year follow-up significant improvement in patient reported outcome measures and VAS score [31].
In the present study, the non-arthroscopy group and the arthroscopy group showed improved WOMAC, OXFORD and the SF36 scores at 2-year follow-up. For all scores, the results for the non-arthroscopy group are superior to that in the arthroscopy group. However, when dichotomizing the WOMAC pain score into a no or low pain score group (WOMAC pain score <10) or a high pain score group (WOMAC pain score of 10 or more), 90% of patients (21 in the arthroscopy group and 60 patients in the non-arthroscopy group) had no or low pain. No statistical difference was found between groups. Finally, the intention to treat analysis of this study evaluated outcome at 2 years after PAO. The statistical difference found in patient reported outcome measures may be a result of the arthroscopy group only having mean 11.5 months (range 0–20.5 months) of follow-up between hip arthroscopy and the 2-year follow-up after PAO. Thus longer follow-up is needed to evaluate the final clinical result after delayed hip arthroscopy. However, excluding seven patients (seven hips) who had a hip arthroscopy within 6 months from 2-year follow-up did not change association. By including patients who previously underwent hip arthroscopy, the result of this study could possibly be biased. However, since this is a prospective cohort study illustrating daily clinical practice, these patients were not excluded.
Hip arthroscopy is offered to the patient by two experts at the Sports Traumatology unit, if the clinical findings suggest intraarticular pathology. However, the decision to offer a hip arthroscopy is multifactorial and it is difficult to apply narrow clinical indications regarding this end-point. MRA is considered the gold standard in imaging labral tears, but hip arthroscopy gives a direct view of the intra-articular status including any chondral damage. This means relying only on MRA findings and clinical tests, chondral damage may be overlooked. A study by Mechlenburg et al. showed unchanged status of cartilage thickness 2½ years after PAO assessed on MRI preoperatively and at follow-up indicating that osteoarthritis do not progress during follow-up even in the presence of a labral tear [32]. Fujii et al. [33] however, did find that advanced intra-articular lesions at the time of hip joint preserving surgery were a significant risk factor for high rate postoperative progression of osteoarthritis of the hip joint. Czerny’s classification of labral tears was in an earlier study found not to be prognostic for outcome [34] and Matheney et al. [35] found that a labral tear did not predict failure in terms of conversion to a THA after PAO. However, in this study a complete detachment of the acetabular labrum from the rim seen at MRA (Czerny 3A or 3B lesions), is a predictor for the need of a hip arthroscopy after PAO.
An interesting finding in this present study was that almost all patients with dysplasia had MRA verified pathology of the labrum and it is interesting to note that a great deal of the patients had a positive effect of PAO alone without addressing the labrum. We believe that redirection of the acetabulum results in significantly reduced load on the labrum which probably explains the good clinical result even when lesions of the labrum are present.
In conclusion, 27% (26 hips out of 95) of the hips underwent hip arthroscopy within the first 2 years after PAO. Predictors for hip arthroscopy were mild hip dysplasia, cross-over sign and a detached labrum evaluated on MRA. At follow-up 2 years after PAO, the clinical outcome in the non-arthroscopic group is superior to that in the arthroscopy group with statistically significant differences in patient reported outcome measures. In the majority of patients, a PAO without subsequent intraarticular assessment resulted in joint preservation with excellent clinical outcome, and currently we recommend PAO as first choice in patients with hip dysplasia. However, patient with mild dysplasia, cross over sign and detached labrum is at particular risk for the need of a hip arthroscopy and future studies must clarify treatment strategies for this patient group. We agree with Parvizi et al. [10] that hip arthroscopy alone without addressing the bony abnormalities in DDH is in general not recommended since a high reoperation rate has been observed.
The poor results in the arthroscopy group might be explained by the fact that the problem does not come from the hip joint itself, and that the need for hip arthroscopy after PAO might be lower. As a consequence of the present study, we do not refer our patients directly to arthroscopy if the present with pain at follow-up. Instead we focus on extra-articular reasons for pain. Our regimen at follow-up has changed in direction to ultrasound examination in order to address soft tissues around the hip, and we also refer the patients to iliopsoas exercises [36], since we often observe weakness and inflammation of iliopsoas. Currently, we are performing an ongoing prospective study focusing on soft tissue around the dysplastic hip. Which eventually might result in more knowledge about persisting pain in these patients since arthroscopy does not seem to be the answer.
Funding
Danish Rheumatism Association (R99-A1830) to C.H.A.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Ganz R, Klaue K, Vinh TS, et al. A new periacetabular osteotomy for the treatment of hip dysplasias. technique and preliminary results . Clin Orthop Relat Res 1988; 232: 26–36. [PubMed] [Google Scholar]
- 2.Dorrell JH, Catterall A. The torn acetabular labrum . J Bone Joint Surg Br 1986; 68: 400–3. [DOI] [PubMed] [Google Scholar]
- 3.Domb BG, Lareau JM, Baydoun H, et al. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res 2014; 472: 674–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip . J Bone Joint Surg Br 1991; 73: 423–9. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy JC, Lee JA. Acetabular dysplasia: A paradigm of arthroscopic examination of chondral injuries . Clin Orthop Relat Res 2002; 405: 122–8. [DOI] [PubMed] [Google Scholar]
- 6.Pitto RP, Klaue K, Ganz R, et al. Acetabular rim pathology secondary to congenital hip dysplasia in the adult. A radiographic study . Chir Organi Mov 1995; 80: 361–8. [PubMed] [Google Scholar]
- 7.Ross JR, Zaltz I, Nepple JJ, et al. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia . Am J Sports Med 2011; 39 (Suppl): 72S–8S. [DOI] [PubMed] [Google Scholar]
- 8.Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy . Clin Orthop Relat Res 1999; 363: 93–9. [PubMed] [Google Scholar]
- 9.Kim KI, Cho YJ, Ramteke AA, et al. Peri-acetabular rotational osteotomy with concomitant hip arthroscopy for treatment of hip dysplasia . J Bone Joint Surg Br 2011; 93: 732–7. [DOI] [PubMed] [Google Scholar]
- 10.Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: A cautionary note . J Arthroplasty 2009; 24: 110–3. [DOI] [PubMed] [Google Scholar]
- 11.Kalore NV, Jiranek WA. Save the torn labrum in hips with borderline acetabular coverage . Clin Orthop Relat Res 2012; 470: 3406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartig-Andreasen C, Troelsen A, Thillemann TM, et al. What factors predict failure 4 to 12 years after periacetabular osteotomy? . Clin Orthop Relat Res 2012; 470: 2978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troelsen A, Elmengaard B, Soballe K. Medium-term outcome of periacetabular osteotomy and predictors of conversion to total hip replacement . J Bone Joint Surg Am 2009; 91: 2169–79. [DOI] [PubMed] [Google Scholar]
- 14.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint with special reference to the complication of osteoarthritis . Acta Chir Scand 1939; 83 (Suppl): 5–135. [Google Scholar]
- 15.Troelsen A, Elmengaard B, Soballe K. A new minimally invasive transsartorial approach for periacetabular osteotomy . J Bone Joint Surg Am 2008; 90: 493–8. [DOI] [PubMed] [Google Scholar]
- 16.Troelsen A, Mechlenburg I, Gelineck J, et al. What is the role of clinical tests and ultrasound in acetabular labral tear diagnostics? . Acta Orthop 2009; 80: 314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee . J Rheumatol. 1988; 15: 1833–40. [PubMed] [Google Scholar]
- 18.Dawson J, Fitzpatrick R, Carr A, et al. Questionnaire on the perceptions of patients about total hip replacement . J Bone Joint Surg.Br 1996; 78: 185–90. [PubMed] [Google Scholar]
- 19.Bjorner JB, Thunedborg K, Kristensen TS, et al. The Danish SF-36 health survey: Translation and preliminary validity studies . J Clin Epidemiol 1998; 51: 991–9. [DOI] [PubMed] [Google Scholar]
- 20.Tönnis D. Congenital Dysplasia and Dislocation of the Hip in Children and Adults. New York: Springer, 1987. [Google Scholar]
- 21.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain . J Bone Joint Surg Br 1999; 81: 281–8. [DOI] [PubMed] [Google Scholar]
- 22.Troelsen A, Jacobsen S, Romer L, et al. Weightbearing anteroposterior pelvic radiographs are recommended in DDH assessment . Clin Orthop Relat Res 2008; 466: 813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamali AA, Mladenov K, Meyer DC, et al. Anteroposterior pelvic radiographs to assess acetabular retroversion: High validity of the “cross-over-sign” . J Orthop Res 2007; 25: 758–65. [DOI] [PubMed] [Google Scholar]
- 24.Troelsen A, Romer L, Jacobsen S, et al. Cranial acetabular retroversion is common in developmental dysplasia of the hip as assessed by the weight bearing position . Acta Orthop 2010; 81: 436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: Accuracy of MR imaging and MR arthrography in detection and staging . Radiology 1996; 200: 225–30. [DOI] [PubMed] [Google Scholar]
- 26.Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement . J Bone Joint Surg Br 2002; 84: 556–60. [DOI] [PubMed] [Google Scholar]
- 27.Altman RD, Bloch DA, Dougados M, et al. Measurement of structural progression in osteoarthritis of the hip: The Barcelona consensus group . Osteoarthr Cartilage 2004; 12: 515–24. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement . Lancet 1986; 1: 307–10. [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Applying the right statistics: Analyses of measurement studies . Ultrasound Obstet Gynecol 2003; 22: 85–93. [DOI] [PubMed] [Google Scholar]
- 30.Steppacher SD, Tannast M, Ganz R, et al. Mean 20-year followup of Bernese periacetabular osteotomy . Clin Orthop Relat Res 2008; 466: 1633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domb BG, Stake CE, Lindner D, et al. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: Two-year clinical outcomes of a surgical approach to a challenging problem . Am J Sports Med 2013; 41: 2591–98. [DOI] [PubMed] [Google Scholar]
- 32.Mechlenburg I, Nyengaard JR, Gelineck J, et al. Cartilage thickness in the hip measured by MRI and stereology before and after periacetabular osteotomy . Clin Orthop Relat Res 2010; 468: 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujii M, Nakashima Y, Noguchi Y, et al. Effect of intra-articular lesions on the outcome of periacetabular osteotomy in patients with symptomatic hip dysplasia . J Bone Joint Surg Br 2011; 93: 1449–56. [DOI] [PubMed] [Google Scholar]
- 34.Freedman BA, Potter BK, Dinauer PA, et al. Prognostic value of magnetic resonance arthrography for Czerny stage II and III acetabular labral tears . Arthroscopy 2006; 22: 742–47. [DOI] [PubMed] [Google Scholar]
- 35.Matheney T, Kim YJ, Zurakowski D, et al. Intermediate to long-term results following the bernese periacetabular osteotomy and predictors of clinical outcome . J Bone Joint Surg Am 2009; 91: 2113–23. [DOI] [PubMed] [Google Scholar]
- 36.Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: A prospective study of 207 patients . Br J Sports Med 2007; 41: 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]