Abstract
Heterotopic ossification (HO) after hip arthroscopy is the abnormal formation of mature lamellar bone within extra skeletal soft tissues. HO may lead to pain, impaired range of motion and possibly revision surgery. There has been a substantial amount of recent research on the pathophysiology, prophylaxis and treatment of HO associated with open and arthroscopic hip surgery. This article reviews the literature on the aforementioned topics with a focus on their application in hip arthroscopy.
INTRODUCTION
Heterotopic ossification (HO) is the abnormal formation of mature lamellar bone within extraskeletal soft tissues. HO after hip arthroscopy may impede functional outcomes by causing pain, impingement and decreased range of motion. HO is one of the most common complications after hip arthroscopy with reported rates of 0–44% without prophylaxis [1–5]. Lesions range from small clinically insignificant foci of ossification to large deposits of bone that can cause stiffness and discomfort, ultimately compromising surgical outcomes. Revision surgery to excise HO may be required in patients who are experiencing refractory pain and/or restricted motion [4]. Despite the high incidence of HO and multiple clinical studies examining the phenomenon, the pathophysiology and etiology of HO remain unclear.
PATHOGENESIS AND MECHANISM OF HO
The pathological mechanism responsible for HO has been widely studied, yet a definitive mechanism has not been established. Current and historical research has illuminated the likely cellular linage responsible for HO and the cellular mechanism that most likely contributes to this pathologic ectopic tissue.
Candidate cells responsible for HO
Few studies evidence an ectodermal or endodermal cell origin for the mechanism and pathophysiology of HO [6–9]. Cells of ectodermal and endodermal origin may indirectly contribute to HO but it is unlikely that they directly give rise to heterotopic osteolineage cells [10]. Although endodermal cells may not be directly responsible for HO [11, 12], endodermal cells have been shown to affect HO through paracrine secretions and other cellular crosstalk mechanisms [13–18].
The most likely candidate cell responsible for HO is the mesenchymal stem cell (MSC) [10, 12, 19]. Wosczyna et al. [12], found that these cells consistently incorporated into areas of osteogenic and chondrogenic foci. Kan et al. [10] recently establish that the cells partaking in physiologic bone formation may not be implicated in the mechanism of HO.
Proposed mechanism
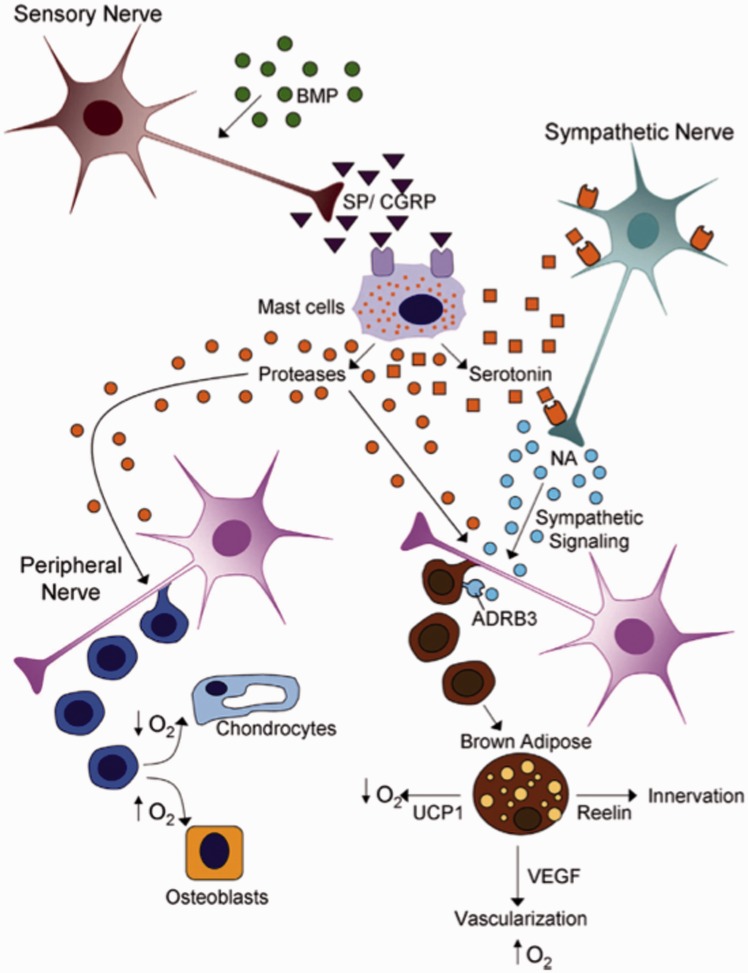
The definitive mechanism responsible for HO has not yet been established. However, inflammatory and traumatic processes have been implicated and scientifically substantiated as contributors to HO. The release of bone morphogenic protein 2 (BMP2) has been well established as an instigator of HO [19, 20] BMP2 is most likely released upon injury [21–23]and mediates an increase in inflammatory markers Substance P (SP) and calcitonin gene-related protein (CGRP) resulting in the recruitment of immune cells such as neutrophils, mast cells and platelets [20]. The resultant degranulation of mast cells, the increased activity of proteases and the presence of activated metaloproteases provoke local tissue disturbance. BPM2 has further been implicated to initiate the molecular pathway responsible for differentiation of peripheral nerve perineurium progenitor cells to brown adipose like cells, essential for nerve remodeling and vascularization vital to the formation of HO [20]. After perineurium type cells migrate to the site of inflammation and differentiate to brown adipose tissue type cells the increased oxygen requirements of the brown adipose tissue type cells establishes a hypoxic microenvironment favorable for chondrogenesis [24]. The microenvironment necessary for osteogenesis; however, requires a less hypoxic environment. Brown adipose type cells were also shown to express vascular endothelial growth factors, which contribute to new vasculature, oxygenating the hypoxic microenvironment and allowing for osteogenesis [25]. Figure 1 represents the molecular mechanism for HO.
Fig. 1.
Heterotopic ossification is initiated local tissue damage leading to increased BMP-2 targeting of sensory nerves. Subsequently, a cascade of molecular mechanisms including the binding of SP and CGRP to mast cell and sympathetic signaling induces the remodeling of peripheral nerves. This remodeling initiates the production of chondro-osseous, glial, vascular and neural progenitor cells. These new cells respond to signals from transient brown adipocytes that regulate local oxygen content, vascularization and innervation to produce HO. (Reproduced with permission from reference [9].)
Risk factors
Although risk factors predisposing the development of HO have been identified, only a handful of those identified have been validated in large studies. The majority of those risk factors evaluated where in patients after total hip arthroplasty (THA). The risk factors for HO in THA include patient related factors such as male gender [26], previous hip surgery [27] and history of HO [28]. Bone producing diseases such as ankylosing spondylitis [29], diffuse idiopathic skeletal hyperostosis and hypertrophic osteoarthritis [30] were also identified as risk factors for HO after THA. Additional considerations such as the surgical approach are important to HO formation, as it was shown that the lateral or anterolaeral approach carries greater risk for HO development than the posterior approach [31, 32]. The use of trochanteric or femoral osteotomy has also been shown to increase the risk for HO [33, 34]. Other non-arthroplasty related risk factors include brain injuries (e.g. head trauma, cerebrovascular accident), spinal cord or lower motor neuron injuries, soft tissue injuries (e.g. blunt trauma, joint dislocation), vascular diseases (e.g. atherosclerosis, valvular heart disease) and arthropathies [35].
Studies concerning the risk factor for HO formation in hip arthroscopy are scarce. To date, there are only two studies that examined factors (excluding pharmacologic prophylaxis) that may affect HO incidence after hip arthroscopy [36, 37]. Randelli et al. [3] suggested capsular incision to be a relevant predisposing factor. Amar et al. [37, p. 1124] showed that capsular repair failed to decrease the rate of HO after hip arthroscopy. Randelli et al. [3] noted that extensive rim trimming, anchor placement, and male gender to be the also relevant predisposing factors, and Bedi et al. [4, p. 685] noted that HO was more prevalent in male patients who underwent osteochondroplasty. Beckman et al. [36] retrospectively reviewed 357 consecutive cases of hip arthroscopy over a 3-year period. The authors identified both mixed-type femoroacetabular impingement (FAI) resection and the absence of non-steroidal anti-inflammatory drug (NSAID) prophylaxis as important predictors of HO development.
Classifications
The Brooker classification based on anteroposterior radiographs is commonly used to determine the level of HO [38]. Grade 1 represents small islands of bone within the soft tissues, grade 2 represents bone islands between the pelvis and femur with >1 cm between the bone surfaces, grade 3 represents bone islands that reduce the space between the pelvis and femur to <1 cm and grade 4 represents complete ankylosis of the hip. This classification has been criticized because bone that appears to be bridging may actually be located either anterior or posterior to the hip and thus does not cause significant reduction to the range of motion.
Clinical manifestations
The presence of small to medium size foci of HO is generally asymptomatic. However, large foci of HO may cause functional impairment in some patients. Beckman et al. [36] considered HO to be symptomatic for 9 of the 34 cases identified in his study. Bedi et al. [4] reported 7 cases of ectopic bone removal among 29 hips that developed HO at a mean duration of 11.6 months after hip arthroscopy.
The clinical manifestations of HO after hip arthroscopy are difficult to isolate from other sources of post-operative pain. Stiffness may result as part of scar formation with or without ectopic bone formation. A mechanical blockage can explain limited range of motion if the ectopic bone has been formed in the plane of motion—mainly anterior and lateral. Larson [1] reported one case of significant motion limitation resulting from ossification of the iliopsoas tendon. This motion deficit was nearly completely resolved at 1 year, and no further treatment was required at the most recent follow-up. The gain in range of motion after hip replacement surgery was significantly less in patients with class III or IV heterotopic bone formation than in those without heterotopic bone formation. However, heterotopic bone formation had no serious impact on hip muscle strength [26]. No difference with regard to functional outcomes after hip arthroscopy was found between those patients who developed HO and those who did not [5].
Imaging and laboratory findings
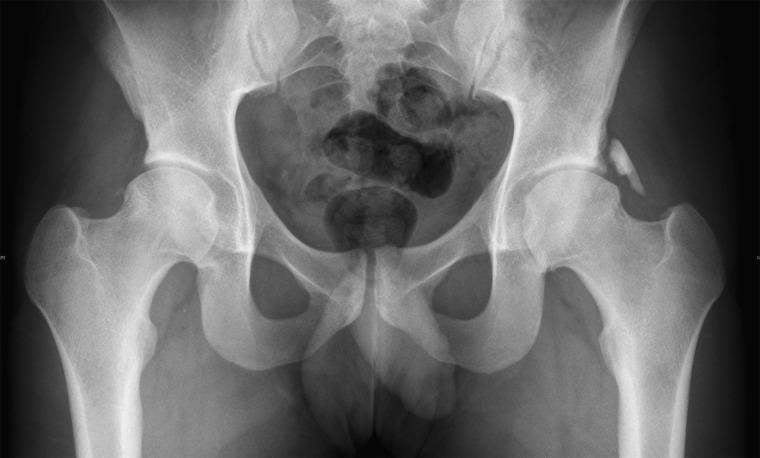
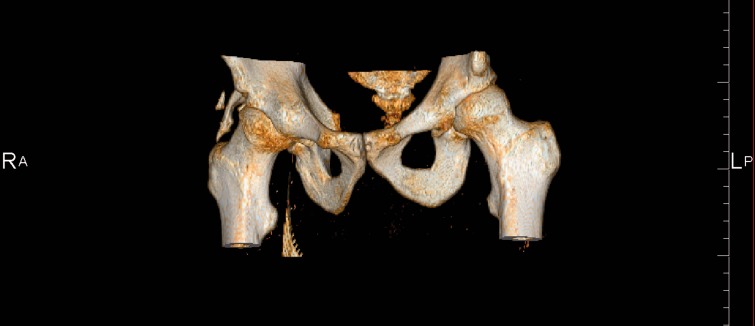
Imaging plays an important role in diagnosis and optimizing the timing of HO excision surgery. Plain radiographs and computed tomography (CT) imaging are the most reliable methods to evaluate advanced ossification foci. HO can present on post-operative radiographs as early as 2 weeks post-surgery. However, in a study looking at the incidence of HO after hip arthroscopy patients diagnosed with HO via X-ray at 9 weeks after surgery did not have radiographic evidence of HO on the 2-week post-operative radiograph [5]. Ossification observed by radiography typically appears as a cloud like hyper-density and gradually matures to solid bone by 3 months (Fig. 2). CT may show a soft tissue mass, followed by visualization of bone earlier than can be seen with conventional radiographs. During surgical planning, assessing the severity of ossification is crucial, as resection must be performed only when HO is mature. Although both plain radiographs and CT imaging are the standard references used to assess HO maturity, CT and 3D CT more accurately define the different stages of ossification than plain radiographs. In the case of revision surgery for the removal of HO, CT is essential for surgical planning and visualizing the shape and spatial location of the ectopic bone [39] (Fig. 3). Magnetic resonance imaging is typically not used in the diagnosis and assessment of HO. The signal intensity characteristics of pelvic HO change in various stages of HO maturation. With progressive maturity of HO, T2 signal intensity and contrast enhancement decrease but fat and cortical bone-equivalent signal intensity increase [40].
Fig. 2.
Follow up radiograph of a 20-year-old patient after bilateral hip arthroscopy. The radiograph was taken 9 months status post left and 3 months status post right hip arthroscopy. HO on the left side was evident on radiographs 10 weeks after the index procedure. NSAID prophylaxis using etodolac 600 mg once daily for 2 weeks was administered only after the operation on the right hip.
Fig. 3.
3D CT reconstruction of a 34-year-old triathlete showing grade 3 HO with acetabular origin and posterolateral location of the HO.
Ultrasonography is not generally employed to assess HO once the diagnosis is established. Ultrasound typically shows abnormalities in muscle as a chaotic disruption of the normal lamellar structure. Evidence of HO can be seen up to 10–14 days before radiographic evidence of HO appears [41]. Assessing HO using three-phase bone scintigraphy with technetium-99 m methylene diphosphonate may detect HO 2–6 weeks earlier than detection with standard X-ray radiography. Early in the course of HO, only the blood pool images may be positive whereas abnormal uptake during the soft tissue phase is diagnostic later in the course of the disease. Activity on delayed bone scans usually peaks a few months after injury, after which the intensity of activity on these scans progressively lessens, with a return toward normal at 6–12 months [42].
In the case of HO after trauma, blood alkaline phosphatase levels become abnormal ∼2 weeks post-injury and may reach 3.5 times the normal value 10-week after injury. These levels may return to normal values at ∼18 weeks after the injury [42].
Prophylaxis
Once an HO lesion is present, the continued formation and maturation of the lesion cannot be prevented by non-surgical measures. Consequently, either external beam radiation (EBR) or pharmacologic agent prophylaxis has gained acceptance to combat the initial formation of HO. Ionizing radiation exerts its influence on rapidly dividing cells by altering the structure of nuclear DNA. Thus, early post-operative radiation may prevent differentiation of the pluripotent mesenchymal cells into pathologic osteoblasts [43]. Both post- and pre-operative EBR are clinically effective in reducing the incidence of HO following hip surgery [43–46]. Commonly used protocols of EBR include post-operative, single-dose regimens of 600–800 Gy performed by post-operative day 3 as well as pre-operative single dose regimens of 800 Gy performed within 6 h prior to surgery [47]. Currently, there are no studies evaluating the efficacy of prophylactic irradiation for HO after hip arthroscopy.
Pharmacologic agents proposed for HO prophylaxis consists of non-selective cyclooxygenase (COX) inhibitors, selective COX-2 inhibitors, aspirin, BMP type 1 receptor inhibition and BMP antagonist, nuclear retinoic acid receptor γ agonists (RAR-γ), free radical (FR) scavengers and bisphosphonates. Excluding NSAIDs the abovementioned agents are not regularly used for HO prophylaxis regardless the index operation.
The prevention of HO using aspirin has demonstrated mixed results. One prospective study of 2649 patients deemed aspirin ineffective in HO prevention [48] while two other retrospective studies found aspirin to be superior to Coumadin in HO prevention after THA [49, 50]. BMP1 receptor inhibitors, BMP antagonist, RAR-γ agonists and FR scavengers have yet to be substantiated as efficacious for HO prophylaxis in human studies. Therefore, those measures currently offer no clinical use as post-operative HO prophylactic measures [51].
Biphosphonate (i.e. etidronate disodium and ethylhydroxydiphosphonate) treatment resulted in delay rather than prevention of osteoid mineralization and its use as a prophylactic measure was thus discontinued due to ineffectiveness [52, 53].
NSAIDs inhibit the conversion of arachidonic acid to prostaglandins by COX enzymes and thereby inhibit prostaglandin production. COX enzymes are found in two isoforms with differing distribution and expression. The COX-2 isoform, which is more frequently expressed in pro-inflammatory states, can be selectively targeted. Specifically targeting this isoform in prophylactic treatment of inflammatory conditions may be advantageous as it avoids many of the adverse side effects attributed to the simultaneous COX-1 and COX-2 inhibition by non-selective NSAIDs [54].
The rate of HO after hip arthroscopy without prophylaxis may be as high as 44% [5]. To date, only a limited number of studies have evaluated the role of NSAIDs (selective and non-selective) in HO prevention after hip arthroscopy [3, 4, 36]. Furthermore, the optimal NSAID agent as well as the optimal duration of treatment has yet to be determined. Randelli et al. [3] evaluated the efficacy of various NSAID agents as prophylaxis in patients post-hip arthroscopy. Five hips presented with HO, with overall prevalence of 1.6%. All five patients with HO belonged to the control group. The authors therefore concluded that NSAID prophylaxis for HO after arthroscopic FAI treatment proved to be an effective preventative measure (Table I).
Table I.
A literature review of relevant articles regarding the rates of HO after hip arthroscopy with and without prophylactic measures
| Author | Prophylaxis | HO (%) | Male/Female | Excision surgery |
|---|---|---|---|---|
| Larson et al. [1] | None | 6/96 (6.2%) | 54/42 | None of the patients required surgical excision. |
| Clohisy et al. [2] | None | 4/35(11.4%) | 28/7 | None of the patients required surgical excision |
| Randelli et al. [3] |
|
|
|
NA |
| Beddi et al. [4] |
|
|
|
|
| Rath et al. [5] | None | 22/50(44%) |
|
None of the patients required surgical excision |
| Beckman et al. [36] |
|
|
|
|
Bedi et al. [4] compared two HO prophylaxis protocols for hip arthroscopy patients. The majority of HO cases (72.4%) occurred in male patients and all cases occurred in the setting of osteoplasty performed for symptomatic FAI. The authors concluded that Indomethacin-based NSAID protocols for HO prophylaxis should be considered after hip arthroscopy in this patient population (Table I).
Beckman et al. [36] prospectively reviewed the role of Naproxen prophylaxis after hip arthroscopy. The rate of HO in the control (no prophylaxis) group was 25% (23/92) and in the study group the rate was 5.6% (11/196). The author further identified that patients undergoing acetabuloplasty along with osteochondroplasty were more likely to develop HO. In conclusion, the authors stated that routine NSAID prophylaxis reduces but does not eliminate the incidence of HO in patients undergoing hip arthroscopy (Table I).
Complications of prophylaxis treatment
Radiation for the prevention of HO carries a potential risk for malignant transformation. Reports of tumor induction by ionizing radiation in low doses are few. Even though one study found no evidence of tumor induction in any patient receiving <3000 cGy [55], other studies reported several cases of neural tumors arising after treatment of tinea capitis with doses of 1000–2000 cGy [56] and radiation-induced bone sarcomas after radiation exposure as low as 1200 cGy [57]. Moreover, doses of 1000–3000 cGy carry a relative risk for oncogenesis 10-fold greater than doses of <1000 cGy [58].
NSAID administration can potentially cause agent dependent adverse effects. Gastrointestinal tract intolerance, platelet inhibition and negative drug interaction with anticoagulation agents such as warfarin are contraindications and adverse effects that must be considered prior to indomethacin use [59]. The use of COX-2 selective inhibitors, especially rofecoxib, has potential adverse cardiovascular complications, particularly in patients with a history of cardiac disease and should be carried out with extra caution in these patients [60].
Surgical treatment—excision of HO
HO after hip arthroscopy is an asymptomatic incidental finding in most patients [4, 61]. However, it was suggested that revision surgery for ectopic bone removal may be indicated for one in four patients who develop HO [4, 36]. The decision to perform revision surgery is made on an individual basis and is based on the severity of the degree of functional impairment, pain and range of motion restriction after attempts of conservative non-operative rehabilitation. Surgery should be delayed until complete maturation of the HO process, as resection of immature HO leads to an increased complication rate and increased recurrence rate. Classifying the maturity of HO prior to surgical resection of HO foci is paramount and thus the following guidelines have been established. The radiographic appearance of the ossification should be consistent with dense cortical bone, levels of serum alkaline phosphatase should be within the normal range and bone scan findings should return to normal or near normal [62] which usually occurs within 6–12 months.
Bedi et al. [4] treated 7 of 29 patients who developed HO post-operatively with revision surgery to excise ectopic bone at a mean duration of 11.6 months after the index procedure. All of the revised hips had ectopic bone located anterior to the joint. The surgical approach for HO removal was arthroscopic for grade I–II HO and open excision of bone with capsulotomy for hips with grade III or IV HO. Beckman [36] reported arthroscopic ectopic bone resection in 9 of 34 patients who developed HO at >12 months post-operatively. This 12-month period was employed to ensure full maturation of the HO and to allow for adequate recovery from the index surgery.
In the case of revision surgery indicated for the removal of symptomatic HO, prophylaxis with either NSAIDS or radiation is mandatory. As previous HO is a significant risk factor for future occurrences appropriate prophylactic measures must be employed. Complications of the surgical removal of HO include hemorrhage, wound-healing issues, cellulitis, infection and possible recurrence of HO.
SUMMARY
HO is the abnormal formation of mature lamellar bone within extraskeletal soft tissues. HO is one of the most common complications after hip arthroscopy and may impede the functional outcome of surgery by causing pain, impingement and decreased range of motion. The most likely candidate cell responsible for HO is the MSC, which proliferates into pathologic tissue under the stresses of post-operative tissue damage. Risk factors pre-disposing to the development of HO include male gender, previous hip surgery and history of HO among others. The diagnosis of HO is routinely achieved with X-ray radiography although the optimal timing for X-ray diagnosis of HO is yet to be defined. The location of HO lesions can be further evaluated using CT radiography. There are many prophylactic measures currently available to prevent post-surgical HO, however the most commonly employed agents are NSAIDs. Finally, surgical excision of HO is indicated in patients with a severe degree of functional impairment, pain and range of motion restriction after attempts of conservative non-operative rehabilitation. Furthermore, HO lesions must be allowed to fully mature prior to surgical excision.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy 2008; 24: 540–6. [DOI] [PubMed] [Google Scholar]
- 2.Clohisy JC, Zebala LP, Nepple JJ, Pashos G. Combined hip arthroscopy and limited open osteochondroplasty for anterior femoroacetabular impingement. J Bone Joint Surg Am 2010; 92: 1697–706. [DOI] [PubMed] [Google Scholar]
- 3.Randelli F, Pierannunzii L, Banci L, et al. Heterotopic ossifications after arthroscopic management of femoroacetabular impingement: the role of NSAID prophylaxis. J Orthop Traumatol 2010; 11: 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedi A, Zbeda RM, Bueno VF, et al. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med 2012; 40: 854–63. [DOI] [PubMed] [Google Scholar]
- 5.Rath E, Sherman H, Sampson TG, et al. The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy 2013; 29: 427–33. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford RB, Racenis P, Fatherazi S, Izutsu K. Bone formation by BMP-7-transduced human gingival keratinocytes. J Dent Res 2003; 82: 293–7. [DOI] [PubMed] [Google Scholar]
- 7.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacic JC, Mercader N, Torres M, et al. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 2012; 125: 1795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichel LM, Salisbury E, Moustoukas MJ, et al. Molecular mechanisms of heterotopic ossification. J Hand Surg 2014; 39: 563–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan L, Kessler JA. Evaluation of the cellular origins of heterotopic ossification. Orthopedics 2014; 37: 329–40. [DOI] [PubMed] [Google Scholar]
- 11.Lounev VY, Ramachandran R, Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am 2009; 91: 652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res 2012; 27: 1004–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meury T, Verrier S, Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem 2006; 98: 992–1006. [DOI] [PubMed] [Google Scholar]
- 14.Trkov S, Eng G, Di Liddo R, et al. Micropatterned three-dimensional hydrogel system to study human endothelial–mesenchymal stem cell interactions. J Tissue Eng Regener Med 2010; 4: 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidarra SJ, Barrias CC, Barbosa MA, et al. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res 2011; 7: 186–97. [DOI] [PubMed] [Google Scholar]
- 16.Kolbe M, Xiang Z, Dohle E, et al. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A 2011; 17: 2199–212. [DOI] [PubMed] [Google Scholar]
- 17.Leszczynska J, Zyzynska-Granica B, Koziak K, et al. Contribution of endothelial cells to human bone-derived cells expansion in coculture. Tissue Eng Part A 2013; 19: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulnheim U, Muller P, Neumann HG, et al. Endothelial cells stimulate osteogenic differentiation of mesenchymal stem cells on calcium phosphate scaffolds. J Tissue Eng Regener Med 2014; 8: 831–40. [DOI] [PubMed] [Google Scholar]
- 19.Kan L, Kitterman J, Procissi D, et al. CNS demyelination in fibrodysplasia ossificans progressiva. J Neurol 2012; 259: 2644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salisbury EA, Lazard ZW, Ubogu EE, et al. Transient brown adipocyte-like cells derive from peripheral nerve progenitors in response to bone morphogenetic protein 2. Stem Cells Trans. Med 2012; 1: 874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sucosky P, Balachandran K, Elhammali A, et al. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol 2009; 29: 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong FN, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg 2008; 16: 619–25. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 2006; 99: 1044–59. [DOI] [PubMed] [Google Scholar]
- 24.Olmsted-Davis E, Gannon FH, Ozen M, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol 2007; 170: 620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilling CF, Wada AM, Lazard ZW, et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res 2010; 25: 1147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrengart L, Lindgren U. Functional significance of heterotopic bone formation after total hip arthroplasty. J Arthroplasty 1989; 4: 125–31. [DOI] [PubMed] [Google Scholar]
- 27.Sodemann B, Persson PE, Nilsson OS. Periarticular heterotopic ossification after total hip arthroplasty for primary coxarthrosis. Clin Orthop Relat Res 1988; 150–7. [PubMed] [Google Scholar]
- 28.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am 1977; 59: 345–51. [PubMed] [Google Scholar]
- 29.Sundaram NA, Murphy JC. Heterotopic bone formation following total hip arthroplasty in ankylosing spondylitis. Clin Orthop Relat Res 1986; 223–6. [PubMed] [Google Scholar]
- 30.Fahrer H, Koch P, Ballmer P, et al. Ectopic ossification following total hip arthroplasty: is diffuse idiopathic skeletal hyperostosis a risk factor? Br J Rheumatol 1988; 27: 187–90. [DOI] [PubMed] [Google Scholar]
- 31.Morrey BF, Adams RA, Cabanela ME. Comparison of heterotopic bone after anterolateral, transtrochanteric, and posterior approaches for total hip arthroplasty. Clin Orthop Relat Res 1984; 160–7. [PubMed] [Google Scholar]
- 32.Bischoff R, Dunlap J, Carpenter L, et al. Heterotopic ossification following uncemented total hip arthroplasty. Effect of the operative approach. J Arthroplasty 1994; 9: 641–4. [DOI] [PubMed] [Google Scholar]
- 33.Errico TJ, Fetto JF, Waugh TR. Heterotopic ossification. Incidence and relation to trochanteric osteotomy in 100 total hip arthroplasties. Clin Orthop Relat Res 1984; 138–41. [PubMed] [Google Scholar]
- 34.Eggli S, Woo A. Risk factors for heterotopic ossification in total hip arthroplasty. Arch Orthop Trauma Surg 2001; 121: 531–5. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg 2004; 12: 116–25. [DOI] [PubMed] [Google Scholar]
- 36.Beckmann JT, Wylie JD, Kapron AL, et al. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med 2014; 42: 1359–64. [DOI] [PubMed] [Google Scholar]
- 37.Amar E, Warschawski Y, Sampson TG, et al. Capsular closure does not affect development of heterotopic ossification after hip arthroscopy. Arthroscopy 2015; 31: 225–30. [DOI] [PubMed] [Google Scholar]
- 38.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 1973; 55: 1629–32. [PubMed] [Google Scholar]
- 39.Bressler EL, Marn CS, Gore RM, Hendrix RW. Evaluation of ectopic bone by CT. Am J Roentgenol 1987; 148: 931–5. [DOI] [PubMed] [Google Scholar]
- 40.Ledermann HP, Schweitzer ME, Morrison WB. Pelvic heterotopic ossification: MR imaging characteristics. Radiology 2002; 222: 189–95. [DOI] [PubMed] [Google Scholar]
- 41.Popken F, Konig DP, Tantow M, et al. Possibility of sonographic early diagnosis of heterotopic ossifications after total hip-replacement. Unfallchirurg 2003; 106: 28–31. [DOI] [PubMed] [Google Scholar]
- 42.Orzel JA, Rudd TG. Heterotopic bone formation: clinical, laboratory, and imaging correlation. J Nucl Med 1985; 26: 125–32. [PubMed] [Google Scholar]
- 43.Ayers DC, Evarts CM, Parkinson JR. The prevention of heterotopic ossification in high-risk patients by low-dose radiation therapy after total hip arthroplasty. J Bone Joint Surg Am 1986; 68: 1423–30. [PubMed] [Google Scholar]
- 44.Padgett DE, Holley KG, Cummings M, et al. The efficacy of 500 CentiGray radiation in the prevention of heterotopic ossification after total hip arthroplasty: a prospective, randomized, pilot study. J Arthroplasty 2003; 18: 677–86. [DOI] [PubMed] [Google Scholar]
- 45.Kienapfel H, Koller M, Wust A, et al. Prevention of heterotopic bone formation after total hip arthroplasty: a prospective randomised study comparing postoperative radiation therapy with indomethacin medication. Arch Orthop Trauma Surg 1999; 119: 296–302. [DOI] [PubMed] [Google Scholar]
- 46.Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am 2001; 83-A: 1783–8. [DOI] [PubMed] [Google Scholar]
- 47.Gross CE, Frank RM, Hsu AR, et al. External beam radiation therapy for orthopaedic pathology. J Am Acad Orthop Surg 2015; 23: 243–52. [DOI] [PubMed] [Google Scholar]
- 48.Neal BC, Rodgers A, Gray H, et al. No effect of low-dose aspirin for the prevention of heterotopic bone formation after total hip replacement: a randomized trial of 2,649 patients. Acta Orthop Scand 2000; 71: 129–34. [DOI] [PubMed] [Google Scholar]
- 49.Bek D, Beksac B, Della Valle AG, et al. Aspirin decreases the prevalence and severity of heterotopic ossification after 1-stage bilateral total hip arthroplasty for osteoarthrosis. J Arthroplasty 2009; 24: 226–32. [DOI] [PubMed] [Google Scholar]
- 50.Nunley RM, Zhu J, Clohisy JC, Barrack RL. Aspirin decreases heterotopic ossification after hip resurfacing. Clin Orthop Relat Res 2011; 469: 1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlou G, Kyrkos M, Tsialogiannis E, et al. Pharmacological treatment of heterotopic ossification following hip surgery: an update. Exp Opin Pharmacother 2012; 13: 619–22. [DOI] [PubMed] [Google Scholar]
- 52.Kjaersgaard-Andersen P, Ritter MA. Prevention of formation of heterotopic bone after total hip arthroplasty. J Bone Joint Surg Am 1991; 73: 942–7. [PubMed] [Google Scholar]
- 53.Haran MJ, Bhuta T, Lee BS. WITHDRAWN: pharmacological interventions for treating acute heterotopic ossification. Cochrane Database Syst Rev. 2010; CD003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borer JS, Simon LS. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: achieving a balance. Arthritis Res Ther 2005; 7(Suppl. 4): S14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JH, Chu FC, Woodard HQ, et al. Radiation-induced soft-tissue and bone sarcoma. Radiology 1978; 129: 501–8. [DOI] [PubMed] [Google Scholar]
- 56.Ron E, Modan B, Boice JD, Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988; 319: 1033–9. [DOI] [PubMed] [Google Scholar]
- 57.Arlen M, Higinbotham NL, Huvos AG, et al. Radiation-induced sarcoma of bone. Cancer 1971; 28: 1087–99. [DOI] [PubMed] [Google Scholar]
- 58.Tucker MA, D’Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 1987; 317: 588–93. [DOI] [PubMed] [Google Scholar]
- 59.Cella JP, Salvati EA, Sculco TP. Indomethacin for the prevention of heterotopic ossification following total hip arthroplasty. Effectiveness, contraindications, and adverse effects. J Arthroplasty 1988; 3: 229–34. [DOI] [PubMed] [Google Scholar]
- 60.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000; 343: 1520–8, 2 p following 28. [DOI] [PubMed] [Google Scholar]
- 61.Neal B, Gray H, MacMahon S, Dunn L. Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg 2002; 72: 808–21. [DOI] [PubMed] [Google Scholar]
- 62.Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med 2002; 43: 346–53. [PubMed] [Google Scholar]