Abstract
Context
The heating characteristics of a stationary device delivering sustained acoustic medicine with low-intensity therapeutic ultrasound (LITUS) are unknown.
Objective
To measure intramuscular (IM) heating produced by a LITUS device developed for long-duration treatment of musculoskeletal injuries.
Design
Controlled laboratory study.
Setting
University research laboratory.
Patients or Other Participants
A total of 26 healthy volunteers (16 men, 10 women; age = 23.0 ± 2.1 years, height = 1.74 ± 0.09 m, mass = 73.48 ± 14.65 kg).
Intervention(s)
Participants were assigned randomly to receive active (n = 20) or placebo (n = 6) LITUS at a frequency of 3 MHz and an energy intensity of 0.132 W/cm2 continuously for 3 hours with a single transducer or dual transducers on the triceps surae muscle. We measured IM temperature using thermocouples inserted at 1.5- and 3-cm depths into muscle. Temperatures were recorded throughout treatment and 30 minutes posttreatment.
Main Outcome Measure(s)
We used 2-sample t tests to determine the heating curve of the LITUS treatment and differences in final temperatures between depth and number of transducers.
Results
A mild IM temperature increase of 1°C was reached 10 ± 5 minutes into the treatment, and a more vigorous temperature increase of 4°C was reached 80 ± 10 minutes into the treatment. The maximal steady-state IM temperatures produced during the final 60 minutes of treatment at the 1.5-cm depth were 4.42°C ± 0.08°C and 3.92°C ± 0.06°C using 1 and 2 transducers, respectively. At the 3.0-cm depth, the maximal steady-state IM temperatures during the final 60 minutes of treatment were 3.05°C ± 0.09°C and 3.17°C ± 0.05°C using 1 and 2 transducers, respectively. We observed a difference between the temperatures measured at each depth (t78 = −2.45, P = .02), but the number of transducers used to generate heating was not different (t78 = 1.79, P = .08).
Conclusions
The LITUS device elicited tissue heating equivalent to traditional ultrasound but could be sustained for multiple hours. It is a safe and effective alternative tool for delivering therapeutic ultrasound and exploring dosimetry for desired physiologic responses.
Key Words: therapeutic modalities, tissue temperature, rehabilitation
Key Points
The low-intensity therapeutic ultrasound (LITUS) device heated tissues at 1.5 and 3.0 cm deep to approximately 3°C to 4°C after a 180-minute treatment using 1 and 2 transducers.
The intramuscular heating produced by the LITUS device was similar to that produced by traditional ultrasound devices that operate at the same frequency but a higher intensity over a shorter time.
This safe and effective alternative tool for delivering sustained ultrasound may prolong the beneficial physiologic response of therapeutic ultrasound by applying a greater amount of acoustic energy to the tissues.
Further exploration of the physiologic and clinical effects of LITUS is needed.
Therapeutic ultrasound for rehabilitation of musculoskeletal injuries is effective due to biomechanical effects of the acoustic wave and physiologic changes attributed to thermal heating of the treated tissues. Compression and rarefaction of cells caused by the mechanical-wave properties of ultrasound can modulate the cell membrane to increase protein synthesis,1 increase cellular migration,2 promote vascular regeneration,3 and modulate the inflammatory response.4 Thermal responses from ultrasonic-energy delivery include increased cellular metabolism,2,5 diminished pain perception,6 increased local circulation,1 reduced muscle spasm,7 decreased joint stiffness,8 reduced viscosity of fluid elements in tissues, and increased extensibility of collagen fibers.9
Temperature-based treatment goals are 1 way practitioners gauge the physiologic response and decide the settings of therapeutic ultrasound.10 Lehmann11 suggested a physiologic temperature paradigm that occurs when tissues are heated to a particular temperature. With mild heating (<40°C tissue temperature), the metabolic rate is accelerated, local blood flow increases, and the sensation of pain is decreased. With vigorous heating (>40°C tissue temperature), the stiffness of the tissue decreases, and the tissue elongates.
To control the application of therapeutic heating using traditional ultrasound devices, practitioners vary the intensity and frequency of the ultrasound system and the area and duration of manual application. The intensity of the ultrasound controls the extent of heating, whereas the frequency affects the depth of penetration. Typical therapeutic ultrasound uses frequencies of 1 or 3 MHz and energy intensity of 0.1 to 1.5 W/cm2. A 3-MHz frequency heats tissues up to 3.0 cm deep, whereas a 1-MHz frequency heats tissues up to 5.0 cm deep.12–14 Therapeutic ultrasound traditionally is delivered in 5- to 15-minute treatments and requires a trained clinician to manipulate the device during treatment.15,16
To prolong the duration of ultrasound therapy that a patient can receive, a low-intensity therapeutic ultrasound (LITUS) device is prescribed with a preset frequency (3 MHz) and intensity (0.132 W/cm2).17 This simplifies the operation for the clinician. Given the low intensity, LITUS devices can be prescribed for treatments lasting up to 4 hours.18 The bioeffects elicited when LITUS devices are used to treat musculoskeletal injuries for long durations are uncharacterized. Therefore, our purpose was to perform a controlled laboratory study using an established protocol to determine the extent of heating when the triceps surae muscle of human participants was treated continuously with LITUS using 1 or 2 stationary transducers for 3 hours.14 To our knowledge, we are the first to quantify the real-time heating effects of human tissue from long-duration ultrasound. We hypothesized the following: (1) A prolonged heating phase would occur due to the multihour application of LITUS. (2) After therapeutic tissue temperatures were reached, the LITUS device would maintain the temperature increase for the duration of the treatment, with the dual-transducer condition maintaining a steady-state temperature increase equal to that of the single transducer. (3) The total intramuscular heating produced by the device would create an optimal thermal dose calculated by the cumulative exposure minutes at 43°C (CEM43°C).19,20
METHODS
We used a placebo-controlled, repeated-measures crossover design to direct this study.
Participants
Twenty-six healthy participants (16 men, 10 women; age = 23.0 ± 2.1 years, height = 1.74 ± 0.09 m, mass = 73.48 ± 14.65 kg) were enrolled and completed the study. No dropouts or adverse events were associated with the study. Participants were recruited and screened per the sampling criteria. They were excluded if they presented with fever, lower leg infection or open wound, compromised circulation or sensation, injury to the lower leg within the 2 months before the study, or contraindication to ultrasound. Participants were instructed not to exercise within 24 hours of testing. All participants provided written informed consent, and the study was approved by the University of Utah Human Institutional Review Board.
Instrumentation
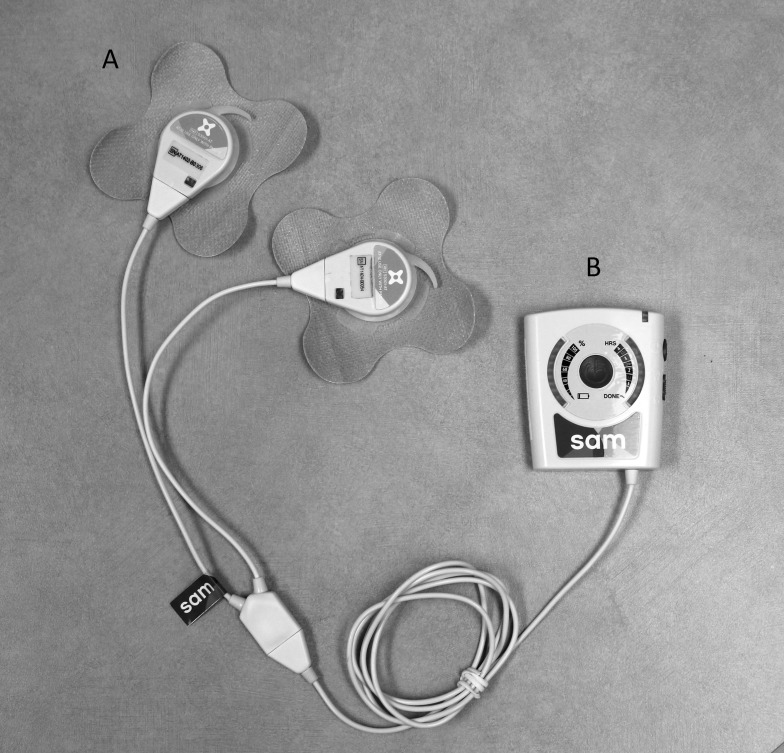
The LITUS devices (model sam-12; ZetrOZ Inc, Trumbull, CT) were manufactured using previously reported methods.21 The battery-operated device is about the size of a transcutaneous electrical nerve stimulation unit (Figure 1). It includes a power controller (Figure 1A) and 1 or 2 coin-sized ultrasound transducers (Figure 1B) that are applied to the treatment area and secured with ultrasound coupling bandages (Figure 1C).22 Two transducers can be applied simultaneously in dual-transducer mode to increase the treated area of tissue. The device delivers low-intensity continuous ultrasound at a 3-MHz frequency and 0.132-W/cm2 spatial average temporal intensity.17,23 Each transducer has an effective radiating area of 5 cm2; therefore, single-transducer mode delivers a total power of 0.65 W, and dual-transducer mode delivers 1.3 W. All 5 devices used in the study were calibrated individually during manufacture to account for any variance in output between transducers and remained within 2.5% ± 2.0% of their original calibration during the entire experiment, which we determined with an acoustic force balance (Onda Corporation, Sunnyvale, CA) using previously described methods.21,23
Figure 1. .
The low-intensity therapeutic ultrasound device includes, A, 1 or 2 coin-sized ultrasound transducers that are applied to the treatment area and secured with ultrasound coupling bandages and, B, a power controller. The device delivers low-intensity continuous ultrasound at a 3-MHz frequency and 0.132-W/cm2 spatial average temporal average intensity. The single-transducer mode has a total power of 0.65 W, and the dual-transducer mode delivers 1.3 W.
Procedures
We randomly assigned the 26 participants to placebo (n = 6) or active (n = 20) groups. The unbalanced groups were intentionally designed to assess intramuscular (IM) temperature changes produced by the LITUS device while also controlling for possible environmental variables with a placebo group. A cohort of 6 placebo participants was sufficient to measure environmental factors while reducing the unnecessary exposure of participants to muscle piercing with thermocouples. Participants completed 2 visits to the study site that were separated by at least 48 hours. On the first visit, participants received treatment with either 1 (7000 J) or 2 (14 000 J) ultrasound transducers for 3 hours. On the second visit, they received the alternate transducer condition. Each treatment occurred at the same time of day to limit diurnal variation. The placebo LITUS device appeared identical to the active device, except the placebo device was not powered on. If the participant received the placebo treatment on the first visit, he or she received the placebo treatment again on the second visit.
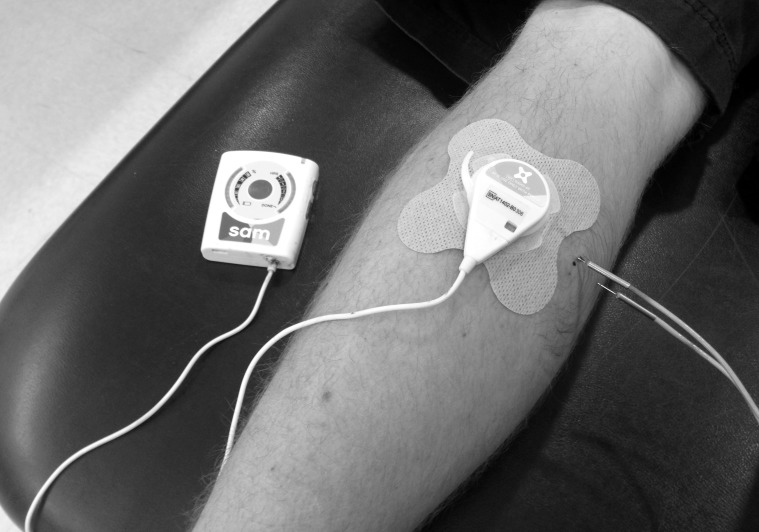
We selected a previously validated method for measuring the thermal effects of ultrasound in intramuscular tissue.14 During the experiment, participants lay prone on a treatment table (Figure 2). We shaved the left posteromedial calf to remove hair and sanitized the area with an iodine swab. The region of the posterior calf with the largest girth was noted visually, and distances of 1.5 and 3.0 cm down the medial side of the calf from the posterior surface of the skin were marked with a felt marker. At each point, we inserted 1 microprobe thermocouple (MT-23/5; Physitemp, Clifton, NJ) with an accuracy of 0.001°C into the posteromedial calf muscle at a direction parallel to the treatment table. Musculoskeletal imaging ultrasound (LogiQ 5P; General Electric Co, Fairfield, CT) was used to measure the exact depth from the skin surface (1.58 ± 0.15 and 2.91 ± 0.16 cm). A PT-6 thermocouple (Physitemp) was taped on the contralateral posterior calf to measure the temperature at the skin surface, and another PT-6 thermocouple recorded ambient temperature (average = 22.7°C). All thermocouples were interfaced with an electrothermometer (Iso-Thermex 256; Columbus Instruments, Columbus, OH) for temperature recording.
Figure 2. .
Participants lay prone on a treatment table with 2 thermocouples inserted horizontally into the left calf. The thermocouples were 1.5 and 3.0 cm from the posterior surface of the calf. The low-intensity therapeutic ultrasound device was placed directly over the thermocouples on the posterior calf.
We measured the reliability and validity of the MT-23/5 thermocouples directly before and after the study using previously described methods.24 Our MT-23/5 thermocouples had an intersession reliability of ± 0.16°C and a validity of −0.21°C against a mercury-calibrated thermometer (model 15-059-18; Fisher Scientific International, Inc, Hamptom, NH; National Institute of Standards and Technology traceable). Jutte et al25 reported that the Iso-Thermex electrothermometer was reliable (±0.03°C) between session measurements and valid within 0.06°C of a mercury-calibrated thermometer.
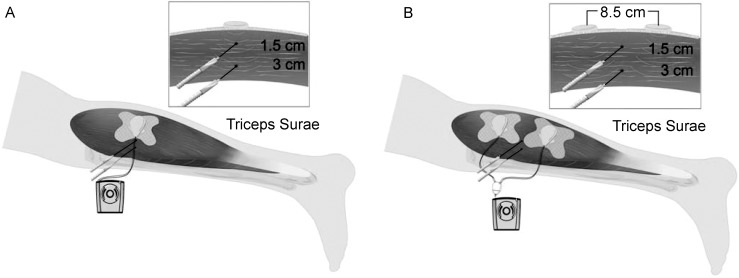
The appropriate treatment configuration was applied to each participant. We clipped the device to a medical-tape bandage and used ultrasound gel as a coupling medium. For single-transducer treatment, the bandage was applied to the skin and placed directly above the temperature probes within the tissue. For dual-transducer treatments, the devices were equally spaced above the temperature probes. The approximate distance between the centers of the 2 transducers was 8.5 cm. Therefore, the probes in the single-transducer condition were 1.5 and 3.0 cm from the acoustic source, whereas the probes in the dual-transducer condition were 4.5 and 5.2 cm from both acoustic sources (Figure 3).
Figure 3. .
Transducer placement of low-intensity therapeutic ultrasound device compared with thermocouple placement in the posterior calf. A, with 1 transducer, the thermocouples were placed directly beneath the transducer, but with 2 transducers, B, the thermocouples were centered between the transducers at depths of 1.5 and 3.0 cm. However, given the placement of the 2 transducers and thermocouples, the distances from the acoustic source and thermocouples were 4.5 and 5.2 cm, respectively.
After positioning the device, we instructed participants to lie still for the duration of the experiment. Thermocouple temperature readings were recorded once per minute. Initial tissue temperatures were measured for 5 minutes before the LITUS device was activated for 180 minutes. At completion of the treatment, the device shut off, and posttreatment temperature was recorded for 30 minutes.
Data Analysis
The temperature profile for each participant was recovered from the thermocouple logs. For the placebo-treatment participants, we observed a substantial cooling of the muscle. During inactivity, the blood flow through the muscle can be as little as 60% of what it is during even moderate or normal activity.26 As a result of inactivity and decreased blood flow, the muscle temperature decreases. To account for this temperature change, we used the temporal-average change observed in the placebo participants to normalize the intramuscular heating measurements of the active groups. The heating curves that are presented reflect the temperature change observed in the muscle tissue of human participants at rest relative to the placebo curves.
To quantitatively evaluate the first and second hypotheses, the time to reach thermal equilibrium (heating phase) was defined as the time when the temperature change was 90% of the final increase observed. To evaluate the therapeutic temperature maintenance across the remainder of the 3-hour treatment, the final heating level (mild, moderate, or vigorous) was considered. The temperature variation was examined over the last 60 minutes of treatment. If the standard deviation of IM temperature was less than 1°C, the temperature was considered to be maintained. If the standard deviation was less than 0.25°C, the temperature was considered well maintained. We selected these values because the therapeutic effect of IM heating has been described with a resolution of 1°C,11 so it is an effective cutoff for determining if the thermal effects will be consistent.
The maximal steady-state–temperature increase during treatment was calculated by considering the average temperature over the final 60 minutes of treatment. We determined differences in final normalized temperatures between depth and number of transducers by 2-sample t tests. We used JMP Pro 10 (SAS Inc, Cary, NC) for the 2-sample t tests and set the α level at .05.
To evaluate the thermal dosimetry of the device, we calculated the CEM43°C for the IM tissue.19 Cumulative exposure minutes at 43°C is an established formula for measuring and reporting thermal dose. The formula converts thermal exposure at a range of temperatures to a number that is more universal: CEM43°C = R(43−T). It is based on empirical data for thermal-dose limitations in tissue, and the constant R is equal to 0.25 when the temperature (T) is less than 43°C. General guidelines from a review of the literature suggest that a CEM43°C of 9 is generally acceptable for most tissues and a CEM43°C of 16 is acceptable for skin, muscle, and bone.20
RESULTS
During the protocol, tissue temperature decreased an average of 3.45°C ± 1.38°C at the 1.5-cm depth and 3.75°C ± 0.62°C at the 3.0-cm depth for placebo participants. During inactivity, physiologic cooling may also be attributed to heat loss to the surroundings, as the skin temperature decreased 2.49°C ± 1.28°C on average throughout 3.5 hours for both the placebo and active groups.
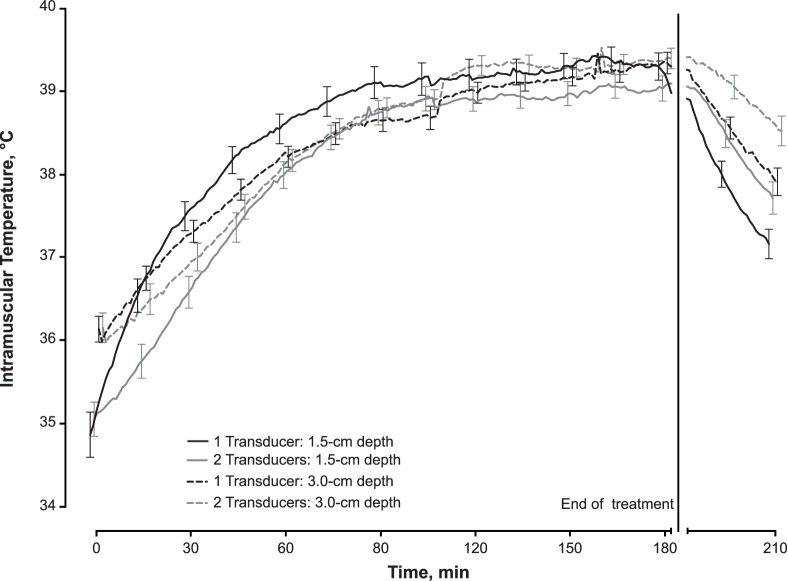
With normalization of the data to account for the physiologic cooling, an increase in tissue temperature was observed in the active ultrasound group. At the 1.5-cm depth, tissue temperatures increased 4.45°C ± 1.52°C from 1 transducer and 3.95°C ± 1.10°C from 2 transducers (Figure 4). At the 3-cm depth, tissue temperatures increased 3.18°C ± 0.90°C and 3.22°C ± 0.95°C from 1 and 2 transducers, respectively (Figure 4). A therapeutic temperature change (>1°C) was maintained for approximately 3 hours. The heating characteristics are described in the Table.
Figure 4. .
Intramuscular heating curves ± 1 standard error for the low-intensity therapeutic ultrasound treatments with 1 or 2 transducers at depths of 1.5- and 3.0-cm into the triceps surae muscle. The data were normalized to placebo participants due to a physiologic cooling that occurred with participants lying supine for 3.5 hours. Maximum heating from 1 transducer was 4.42°C ± 0.08°C at the 1.5-cm depth and 3.05°C ± 0.09°C at the 3-cm depth and from 2 transducers was 3.92°C ± 0.06°C at the 1.5-cm depth and 3.17°C ± 0.05°C at the 3-cm depth.
Table. .
Descriptive Statistics of Low-Intensity Therapeutic Ultrasound Heating Characteristics Using 1 or 2 Transducers at Depths of 1.5 cm and 3.0 cm into the Triceps Surae Musclea
| Depth, cm |
No. of Transducers |
Heating Rate, °C/minb |
Cooling Rate, °C/minb |
Initial Temperature, °C (Mean ± SD) |
Peak Temperature, °C (Mean ± SD) |
Change in Temperature, °C (Mean ± SD) |
| 1.5 | 1 | 4.45–4.42e−0.03t | 17.56–0.07t | 34.87 ± 1.22 | 39.32 ± 0.73 | 4.45 ± 1.52 |
| 1.5 | 2 | 3.96–4.54e−0.025t | 13.36–0.05t | 35.05 ± 0.93 | 39.01 ± 0.67 | 3.95 ± 1.10 |
| 3.0 | 1 | 3.36–3.51e−0.016t | 11.74–0.05t | 36.14 ± 0.70 | 39.32 ± 0.57 | 3.18 ± 0.90 |
| 3.0 | 2 | 3.53–4.12e−0.015t | 8.86–0.03t | 36.15 ± 0.80 | 39.37 ± 0.55 | 3.22 ± 0.95 |
Intramuscular heating for single-transducer treatments was measured directly beneath the transducer at the indicated depth. For dual-transducer treatments, intramuscular heating was measured midway between the 8.5-cm spaced transducers at the indicated depth, leading to greater distance away from the implanted thermocouples.
t = min.
The LITUS device produced a thermal response in tissues while the participants were resting. On average for all participants who received an active treatment, mild heating (temperature change > 1°C) was reached at 10 ± 5 minutes into the treatment, and vigorous heating (temperature change > 4°C) was reached at 80 ± 10 minutes.
The muscle tissue approached thermal equilibrium after 90 minutes at the 1.5-cm depth and after 120 minutes at the 3.0-cm depth. After that time at each depth, the temperatures increased at a minimal rate (<0.25°C/h). The standard deviation of the temperature increase over the last hour was less than 0.1°C in all cases and met the definition of sustained intramuscular heating. The maximal steady-state temperatures were 4.42°C ± 0.08°C at the 1.5-cm depth and 3.05°C ± 0.09°C at the 3.0-cm depth for 1 transducer and 3.92°C ± 0.06°C at the 1.5-cm depth and 3.17°C ± 0.05°C at the 3.0-cm depth for 2 transducers. We observed a difference between final normalized IM temperatures measured at each depth (t78 = −2.45, P = .02), but the number of transducers used to generate heating was not different (t78 = 1.79, P = .08).
The thermal dose, as measured by CEM43°C, was calculated for the temperature profile observed in each of the 80 tests. The distribution was right tailed, with a maximum CEM43°C of 5.92. The median thermal dose was 0.604, with first and third quartiles of 0.416 and 0.915, respectively. The thermal dose delivered was less than the CEM43°C of 9 for all tissues and nearly 70% lower than the CEM43°C limit of 16 suggested for skin, muscle, and bone. Therefore, the thermal dose that the device delivered was high enough to trigger the positive effects of ultrasound intramuscular heating but still within the recommended dosimetry limits for bone and soft tissue.
DISCUSSION
The physiologic heating associated with ultrasound exposure for more than 30 minutes has not been studied. Sports medicine practitioners use temperature-change targets to direct therapeutic ultrasound treatment settings.10 Lehmann11 suggested a physiologic temperature paradigm after studying the effect of various levels of heat on animal tissue.27 Lehmann11 proposed that mild heating correlated with an increase in cellular metabolism, increased circulation, and pain reduction at tissue temperatures of less than 40°C. Vigorous heating increased tissue extensibility at tissue temperatures equal to or greater than 40°C. Given the varying degrees of baseline temperature between deep and superficial tissues, other researchers14 have hypothesized that a relative increase in tissue temperature is needed for the associated mild to moderate heating (<4°C tissue temperature increase) and vigorous heating (≥4°C tissue temperature increase) effects to occur. Given the large cooling effect that we found with participants lying still for 3.5 hours, we reported our data as relative temperature change; however, future investigation is needed to truly understand the physiologic effects of the LITUS device at specific tissue temperatures.
Practitioners can use the presented LITUS heating curves to determine the optimal treatment duration required for the desired physiologically therapeutic thermal response. Mild heating with the LITUS device was achieved in 10 minutes, whereas vigorous heating required 80 minutes.
Ultrasound intensity varies greatly between the LITUS device and traditional ultrasound, but similarities exist between their respective heating curves. Both ultrasound devices created an overall temperature change of greater than 4°C in IM tissue.14 Given the lower ultrasound intensity, the LITUS device reached a 4°C temperature change after 90 minutes at the 1.5-cm depth and after 120 minutes at the 3.0-cm depth and maintained that heating level for the remainder of the 3-hour treatment. Traditional ultrasound at 3 MHz produces a vigorous temperature change approximately 8 to 10 minutes into the treatment when an intensity greater than 1.0-W/cm2 is used.28,29
Given the different experimental setups for the single-transducer and dual-transducer treatment conditions and total distances from the acoustic source or sources to the thermocouples, direct comparison of their reported values is not appropriate. We observed that the thermal effect created at the 3-cm depth was similar for both conditions, which suggests that being 3.0 cm from 1 transducer or 5.2 cm from 2 transducers results in similar acoustic energy delivery. This type of measurement provides insight into the overall shape of the thermal field in the tissue.
The LITUS heating curve is curvilinear, which is similar to that of traditional ultrasound.30 Ultrasound energy initially increases tissue temperature in a linear fashion, but thermoregulatory controls respond by increasing local circulation. Increased circulation combats tissue overheating by shunting heat away from the treatment area.31 After a thermoneutral condition occurs between the absorbed ultrasound energy and heat removal, a ceiling effect occurs, creating a steady-state IM heating response.30 During this steady-state heating, the treated tissue benefits from the increased circulation. The treatment time of the LITUS device that we used was prolonged, thus extending the benefits of the steady-state physiologic heating.
Therapeutic ultrasound applied for a longer duration, delivering more ultrasound energy per treatment, has produced better clinical outcomes than treatments applied for shorter periods. In a systematic review, Alexander et al16 observed that favorable patient outcomes for pathologic shoulder conditions occurred when at least 2500 J of energy were delivered per treatment session. Researchers using 720 J or fewer have not reported effects of ultrasound on patient outcomes, leading us to conclude that such low doses of ultrasound in effect deliver sham ultrasound. The use of suboptimal ultrasound treatment settings in current randomized clinical trials has limited the ability to conclude whether therapeutic ultrasound is an effective modality for musculoskeletal injury. We recommend that in future clinical studies, researchers use modern ultrasound devices capable of delivering long-duration treatments (>30 minutes) to determine whether therapeutic ultrasound using greater energy deposition is truly effective.15,16
In our study, 1 transducer delivered 7000 J, and 2 transducers delivered 14 000 J. A traditional ultrasound treatment (1.0 W/cm2 for 10 minutes) delivers 3000 J. The sustained acoustic-medicine approach provides practitioners and patients with an alternative method to successfully deliver established ultrasound therapy. In small clinical trials, researchers have reported positive patient outcomes over placebo groups when the LITUS device is used for muscle spasm,21 tendinopathy,32 and osteoarthritis.33 However, future studies are needed to optimize the therapeutic delivery of sustained acoustic medicine and to determine if clinical outcomes are improved with LITUS devices over traditional ultrasound treatments.
Measuring only the thermal change of the triceps surae muscle during LITUS treatments limits the correlation of the presented data to clinical outcomes, but it is a necessary preliminary step in understanding the efficacy of longer-duration LITUS treatments. Future authors should quantify the nonthermal effects of sustaining ultrasound delivery over multiple hours. Identifying the IM heating curves in healthy human participants allows us to infer our results of LITUS treatments to the suggested physiologic temperature paradigm of mild and vigorous heating.11,14 We would assume that similar heating curves would occur in an injured population. However, different tissue types may have different heating characteristics due to composition, depth, and proximity to bone and circulation. For example, tendon tissue is heated approximately 3 times faster than muscle during traditional ultrasound therapy.34 In addition, we hypothesize that different arrangements of 2 transducers may produce different heating characteristics. In our study, we positioned the 2 transducers for very little overlap between the ultrasound beams. However, if the transducers had been positioned closer or on opposite sides of the limb facing each other, the IM heating pattern might have been different.
Measuring the thermal characteristics under room-temperature ambient conditions created physiologic cooling with the participants lying at rest. The LITUS device is portable and meant to be worn while patients perform normal activities of daily living. Therefore, the heating response is assumed to change slightly with patient activity; however, the experimental model we used required participants to be at rest.
CONCLUSIONS
We determined that a new LITUS device for long-duration treatment heated tissues at 1.5 and 3.0 cm deep by approximately 3°C to 4°C after a 180-minute treatment using 1 and 2 transducers. The heating from the LITUS device was similar to that of other traditional ultrasound devices that operate at the same frequency but a higher intensity over a shorter period. The sustained application of ultrasound may prolong the beneficial physiologic response of therapeutic ultrasound by applying a greater amount of acoustic energy to the tissues, but further research is needed to truly understand the physiologic and clinical effects of this new device.
ACKNOWLEDGMENTS
Rebecca M. Taggart, BS; Kelly L. Stratton, BS; and George K. Lewis, Jr, PhD, were employed by ZetrOZ, Inc, which provided the ultrasound device.
REFERENCES
- 1.Naito K, Watari T, Muta T, et al. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res. 2010;28(3):361–369. doi: 10.1002/jor.20995. [DOI] [PubMed] [Google Scholar]
- 2.Roper J, Harrison A, Bass MD. Induction of adhesion-dependent signals using low-intensity ultrasound. J Vis Exp. 2012 doi: 10.3791/4024. (63):e4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garvin KA, Dalecki D, Hocking DC. Vascularization of three-dimensional collagen hydrogels using ultrasound standing wave fields. Ultrasound Med Biol. 2011;37(11):1853–1864. doi: 10.1016/j.ultrasmedbio.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns LD. Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis. J Athl Train. 2002;37(3):293–299. [PMC free article] [PubMed] [Google Scholar]
- 5.Samuels JA, Weingarten MS, Margolis DJ, et al. Low-frequency (<100 kHz), low-intensity (<100 mW/cm[2]) ultrasound to treat venous ulcers: a human study and in vitro experiments. J Acoust Soc Am. 2013;134(2):1541–1547. doi: 10.1121/1.4812875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003132.pub2. (1):CD003132. [DOI] [PubMed] [Google Scholar]
- 7.Draper DO, Mahaffey C, Kaiser D, Eggett D, Jarmin J. Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiother Theory Pract. 2010;26(3):167–172. doi: 10.3109/09593980903423079. [DOI] [PubMed] [Google Scholar]
- 8.Draper DO. Ultrasound and joint mobilizations for achieving normal wrist range of motion after injury or surgery: a case series. J Athl Train. 2010;45(5):486–491. doi: 10.4085/1062-6050-45.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung CK, Guo X, Ng YF. Pulsed ultrasound treatment accelerates the repair of Achilles tendon rupture in rats. J Orthop Res. 2006;24(2):193–201. doi: 10.1002/jor.20020. [DOI] [PubMed] [Google Scholar]
- 10.Draper DO, Wells AM, Vincent WJ, Rigby JH. Ultrasound treatment temperature goals: temperature dependent versus time dependent. Athl Train Sports Health Care. 2013;5(2):76–80. [Google Scholar]
- 11.Lehmann JF. Therapeutic Heat and Cold. 4th ed. Baltimore, MD: Williams & Wilkins;; 1990. pp. 207–213. [Google Scholar]
- 12.Franson J, Draper DO, Rigby JH, Johnson AW, Mitchell UH. Tissues at a 3-cm depth vigorously heat using 3-MHz ultrasound. Athl Train Sports Health Care. 2014;6(6):267–272. [Google Scholar]
- 13.Hayes BT, Merrick MA, Sandrey MA, Cordova ML. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J Athl Train. 2004;39(3):230–234. [PMC free article] [PubMed] [Google Scholar]
- 14.Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 15.Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Phys Ther. 2001;81(7):1339–1350. [PubMed] [Google Scholar]
- 16.Alexander LD, Gilman DRD, Brown DR, Brown JL, Houghton PE. Exposure to low amounts of ultrasound energy does not improve soft tissue shoulder pathology: a systematic review. Phys Ther. 2010;90(1):14–25. doi: 10.2522/ptj.20080272. [DOI] [PubMed] [Google Scholar]
- 17.Langer MD, Fleshman S, Lewis G., Jr . Paper presented at: Annual Conference of the American Institute of Ultrasound in Medicine; March 31. Las Vegas, NV: 2014. Bench and animal testing of a wearable long duration therapeutic ultrasound device. [Google Scholar]
- 18.Langer MD, Levine V, Taggart R, Ortiz R, Hernandez L, Lewis G., Jr . Paper presented at: 40th Annual Northeast Bioengineering Conference; April 25–27. Boston, MA.: 2014. Pilot clinical studies of long duration, low intensity therapeutic ultrasound for osteoarthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 20.van Rhoon G, Samaras T, Yarmolenko P, Dewhirst M, Neufeld E, Kuster N. CEM43°C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol. 2013;23(8):2215–2227. doi: 10.1007/s00330-013-2825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis GK, Langer MD, Henderson CR, Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med Biol. 2013;39(8):1429–1439. doi: 10.1016/j.ultrasmedbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GK, Jr, Guarino JL, Guffey B. Hydrogel ultrasound coupling device. US patent application 20130144193. June 6, 2013 inventors; ZETROZ, LLC, assignee. [Google Scholar]
- 23.Guo Y, Fleshman S, Lewis G, Lewis G., Jr . Paper presented at: 36th Annual International Conference of the Institute of Electrical and Electronics Engineers/Engineering in Medicine and Biology Society; August 26–30. Chicago, IL.: 2014. Ultrasonic modeling and hydrophone measurements of dual divergent transducers for wearable therapeutic ultrasound device. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long BC, Jutte LS, Knight KL. Response of thermocouples interfaced to electrothermometers when immersed in 5 water bath temperatures. J Athl Train. 2010;45(4):338–343. doi: 10.4085/1062-6050-45.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jutte LS, Knight KL, Long BC, Hawkins JR, Schulthies SS, Dalley EB. The uncertainty (validity and reliability) of three electrothermometers in therapeutic modality research. J Athl Train. 2005;40(3):207–210. [PMC free article] [PubMed] [Google Scholar]
- 26.Humphreys PW, Lind AR. The blood flow through active and inactive muscles of the forearm during sustained hand-grip contractions. J Physiol. 1963;166:120–135. doi: 10.1113/jphysiol.1963.sp007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehmann JF, Masock AJ, Warren CG, Koblanski JN. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil. 1970;51(8):481–487. [PubMed] [Google Scholar]
- 28.Holcomb WR, Joyce CJ. A comparison of temperature increases produced by 2 commonly used ultrasound units. J Athl Train. 2003;38(1):24–27. [PMC free article] [PubMed] [Google Scholar]
- 29.Merrick MA, Bernard KD, Devor ST, Williams MJ. Identical 3-MHz ultrasound treatments with different devices produce different intramuscular temperatures. J Orthop Sports Phys Ther. 2003;33(7):379–385. doi: 10.2519/jospt.2003.33.7.379. [DOI] [PubMed] [Google Scholar]
- 30.Demchak TJ, Straub SJ, Johns LD. Ultrasound heating is curvilinear in nature and varies between transducers from the same manufacturer. J Sport Rehabil. 2007;16(2):122–130. doi: 10.1123/jsr.16.2.122. [DOI] [PubMed] [Google Scholar]
- 31.Ducharme MB, Tikuisis P. Role of blood as heat source or sink in human limbs during local cooling and heating. J Appl Physiol (1985) 1994;76(5):2084–2094. doi: 10.1152/jappl.1994.76.5.2084. [DOI] [PubMed] [Google Scholar]
- 32.Lewis G, Hernandez L, Lewis GK, Jr, Ortiz R. Paper presented at: 166th Annual Meeting of the Acoustical Society of America; June 2–7. Montreal, Quebec, Canada.: 2013. Wearable long duration ultrasound therapy pilot study in rotator cuff tendinopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer MD, Levine V, Taggart R, Lewis GK., Jr . Paper presented at: Annual Conference of the American Institute of Ultrasound in Medicine; April 1, Las Vegas, NV.: 2014. Treatment of mild to moderate knee osteoarthritis with long duration, low intensity therapeutic ultrasound. [Google Scholar]
- 34.Chan AK, Myrer JW, Measom GJ, Draper DO. Temperature changes in human patellar tendon in response to therapeutic ultrasound. J Athl Train. 1998;33(2):130–135. [PMC free article] [PubMed] [Google Scholar]