Abstract
Context
The superimposed-burst (SIB) technique is commonly used to quantify central activation failure after knee-joint injury, but its reliability has not been established in pathologic cohorts.
Objective
To assess within-session and between-sessions reliability of the SIB technique in patients with patellofemoral pain.
Design
Descriptive laboratory study.
Setting
University laboratory.
Patients or Other Participants
A total of 10 patients with self-reported patellofemoral pain (1 man, 9 women; age = 24.1 ± 3.8 years, height = 167.8 ± 15.2 cm, mass = 71.6 ± 17.5 kg) and 10 healthy control participants (3 men, 7 women; age = 27.4 ± 5.0 years, height = 173.5 ± 9.9 cm, mass = 78.2 ± 16.5 kg) volunteered.
Intervention(s)
Participants were assessed at 6 intervals spanning 21 days. Intraclass correlation coefficients (ICCs [3,3]) were used to assess reliability.
Main Outcome Measure(s)
Quadriceps central activation ratio, knee-extension maximal voluntary isometric contraction force, and SIB force.
Results
The quadriceps central activation ratio was highly reliable within session (ICC [3,3] = 0.97) and between sessions through day 21 (ICC [3,3] = 0.90–0.95). Acceptable reliability of knee extension (ICC [3,3] = 0.75–0.91) and SIB force (ICC [3,3] = 0.77–0.89) was observed through day 21.
Conclusions
The SIB technique was reliable for clinical research up to 21 days in patients with patellofemoral pain.
Key Words: central activation ratio, knee, quadriceps muscles
Key Points
The superimposed-burst technique was reliable for clinical research in patients with patellofemoral pain.
Quadriceps central activation ratio and maximal voluntary isometric contraction force appeared to be reliable outcome measures through day 21 and could be improved with a familiarization period.
Central motor drive to motor neurons is necessary to produce force about a given muscle.1 A variety of intrinsic factors, including fatigue,1 suboptimal voluntary effort,2 and peripheral injury,3 may influence the central motor drive to a muscle, thereby reducing force production. A reduction in central motor drive has been termed central activation failure and results from the inability to voluntarily recruit all motor neurons in a motor-neuron pool.1 The superimposed-burst (SIB) technique has been used to quantify central activation failure in the quadriceps musculature2 and to assess outcomes after knee-joint injury in clinical and intervention research. Theoretically, this technique allows clinicians to estimate complete motor-neuron activity via calculation of the central activation ratio (CAR); however, it relies on the patient's achieving a maximal voluntary isometric contraction (MVIC), which may be difficult in the presence of pathologic conditions. Whereas the central nervous system modulates neuromuscular function differently after joint injury, reliable MVICs have been established among pathologic cohorts. However, the reliability of the SIB technique to estimate quadriceps activation has not been assessed in these populations and is limited to short-term follow-up in healthy individuals.4
To study the effects of interventions on reduced neuromuscular capacity, we must establish the ability of this technique to reliably assess quadriceps activation over time in pathologic cohorts. Persistent muscle weakness has been well described in patients with patellofemoral pain (PFP),3 presenting a clinical dilemma for health care practitioners in the progression of rehabilitation. To our knowledge, the reliability of the SIB technique has not been examined in individuals with PFP. Therefore, the purpose of this study was to assess within-session and between-sessions reliability of the SIB technique for measuring quadriceps muscle activation in patients with self-reported PFP and in healthy individuals.
METHODS
We used a 2 × 6 mixed-model design with repeated measures to compare groups (PFP, healthy control) over time (baseline, 1 hour, 24 hours, day 7, day 14, day 21) for dependent variables in the involved limb. Dependent variables were quadriceps CAR, knee-extension MVIC force (FMVIC), and SIB force (FSIB).
Participants
A total of 10 patients with self-reported PFP (1 man, 9 women; age = 24.1 ± 3.8 years, height = 167.8 ± 15.2 cm, mass = 71.6 ± 17.5 kg) and 10 participants with no self-reported history of knee injury (3 men, 7 women; age = 27.4 ± 5.0 years, height = 173.5 ± 9.9 cm, mass = 78.2 ± 16.5 kg) volunteered. Patients with a history of lower extremity surgery or knee injury other than PFP within 6 months of the study were excluded.
Participants were deemed symptomatic if they reported pain in the anterior aspect of their knees. They had to report at least 2 of the following: pain when ascending or descending stairs, pain during running, pain after sitting for long periods, or a previous physician diagnosis of PFP.5 Participants were evaluated to rule out the probability of extra-articular pathologic conditions. All participants provided written informed consent, and the University of Virginia Institutional Review Board for Health Sciences Research approved this study.
Procedures
Each participant completed 5 testing sessions. A baseline measurement was obtained during the first session and a second measurement 1 hour later. Follow-up measurements were obtained 24 hours, 7 days, 14 days, and 21 days after the baseline measurement. Participants were screened subjectively for soreness and fatigue at each subsequent visit to ensure complete recovery before testing.
Superimposed-Burst Technique
We measured quadriceps force with a strain gauge (model 41; Sensotec, Columbus, OH) that has a range of 1 to 1000 lb (0.45 to 450 kg) and was mounted onto the frame of a custom-fabricated chair.6 A load cell was connected to the participant's lower leg via a cable and hook-and-loop strap secured to the distal shank (Figure). The height of the load cell was adjusted so that the line of pull from the leg was perpendicular to the load cell. The strain gauge was connected to a Data Acquisition System (model MP150; BIOPAC Systems, Inc, Santa Barbara, CA). Electrical stimulation for the SIB technique was produced using previously described procedures.6
Figure.
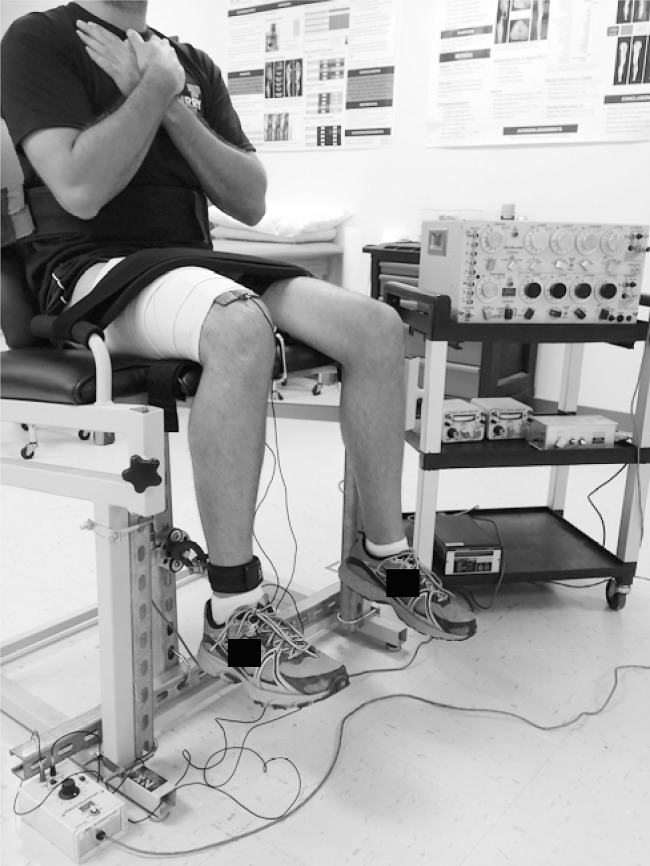
Superimposed-burst configuration. The torso and thigh were secured with hook-and-loop straps, and the distal shank was attached to the load cell via a cable and hook-and-loop cuff positioned 2 cm proximal to the lateral malleolus. Two rectangular electrodes were placed centrally over the thigh, with the proximal electrode positioned at the height of the greater trochanter. The distal electrode was positioned in line with the proximal electrode approximately 2 cm proximal to the superior pole of the patella. The load cell was securely fixed to a vertically oriented immovable beam.
Two nonadhesive, carbon-impregnated 8.9 × 10.2-cm rubber electrodes were coated with aqueous conductive gel, placed centrally over the participant's thigh, and secured with an elastic wrap as previously described.6 Participants were seated with their hips and knees flexed to 90° and were instructed to keep their upper extremities across their chests. The trunk, waist, and thigh were secured to the chair using hook-and-loop straps to eliminate aberrant motion. The clinician (J.L.F.) provided oral encouragement during testing to ensure maximal effort.
Quadriceps Activation Testing
Participants were acclimated through a series of submaximal isometric knee-extension contractions at 25%, 50%, and 75% of their perceived maximal efforts before data collection. They performed 5 maximal contractions separated by 2 minutes of rest to determine the FMVIC. After acclimatization, participants rested for 5 minutes and then performed 3 separate MVICs. When the isometric force plateaued, an electrical stimulus was applied manually to the quadriceps musculature, causing an immediate and transient increase in FSIB.
Data Analysis
Quadriceps CAR was calculated as previously described.1 Pilot data suggested that averaging the MVIC from multiple trials produces a reliable estimate; therefore, we obtained all force values from a mean of the 5 test trials. We calculated the FMVIC by taking the mean of a 100-millisecond epoch immediately before the electrical stimulus.6 Force data were filtered with a 50-Hz low-pass filter.
Statistical Analysis
We computed separate mixed-model intraclass correlation coefficients (ICCs [3,3]) to report the reliability of each outcome measure, using the baseline and 24-hour measures as reference values for comparison. The 24-hour measure was included to assess a learning effect, given the novelty of the task, and was intended as a secondary, exploratory analysis. The strength of reliability coefficients was interpreted based on ranges of poor (<0.69), fair (0.70–0.79), good (0.80–0.89), and high (0.90–1.00).7
The standard error of measurement (SEM) was calculated as SD × √(1 − ICC) to determine the random systematic measurement error associated with each outcome measure. The minimal detectable change (MDC95) score was calculated as 1.96 × √2 × SEM to identify the 95% confidence level for a physiologic change occurring beyond that which could be associated with measurement error.8
A 2-way analysis of variance with repeated measures for time was conducted to determine overall group differences for each dependent variable. Post hoc procedures were performed if appropriate. We used SPSS (version 20.0; IBM Corporation, Armonk, NY) for all statistical analyses. The α level was set a priori at or less than .05.
RESULTS
Within-Session and Between-Sessions Reliability
The ICC values, SEM, mean difference, and MDC95 using the baseline measures as a reference are presented in Table 1. High within-session reliability was observed for CAR, whereas good reliability was detected for FMVIC and FSIB in the PFP group. Between-sessions reliability of CAR measures was high through day 21, but FMVIC and FSIB ranged from fair to high for the PFP group (Table 1). The ICC values, mean differences, SEM, and MDC95 using day 2 as a reference are presented in Table 2. The CAR, FMVIC, and FSIB demonstrated high reliability through day 21 for the PFP group.
Table 1. .
Reliability Data for SIB Measures Using the Day 1 Prebaseline Measure as a Reference for All Comparisonsa
| Reliability |
Patellofemoral Pain Group |
Healthy Group |
||||||
| ICC [3,3] (95% CI)b |
SEM |
Mean Difference |
Minimal Detectable Change at 95% Confidence Level |
ICC [3,3] (95% CI)b |
SEM |
Mean Difference |
Minimal Detectable Change at 95% Confidence Level |
|
| Within session | ||||||||
| Day 1 (prebaseline– postbaseline) | ||||||||
| CAR, %c | 0.97 (0.89, 0.99) | 2.41 | 0.64 ± 4.96 | 6.68 | 0.87 (0.47, 0.97) | 2.65 | −1.84 ± 5.29 | 7.35 |
| MVIC, N | 0.87 (0.46, 0.97) | 42.28 | 44.84 ± 84.08 | 117.18 | 0.99 (0.96, 1.00) | 15.61 | 9.81 ± 32.35 | 43.26 |
| SIB, N | 0.89 (0.55, 0.97) | 41.26 | 54.27 ± 82.00 | 114.37 | 0.99 (0.98, 1.00) | 11.36 | 24.19 ± 22.26 | 31.48 |
| Between sessions | ||||||||
| Day 1–day 2 | ||||||||
| CAR, %c | 0.92 (0.68, 0.98) | 3.89 | −0.87 ± 7.86 | 10.77 | 0.76 (0.01, 0.94) | 3.01 | −1.21 ± 5.64 | 8.34 |
| MVIC, N | 0.78 (0.12, 0.95) | 57.56 | 34.42 ± 106.31 | 159.56 | 0.98 (0.91, 1.00) | 20.31 | −10.57 ± 43.00 | 56.29 |
| SIB, N | 0.81 (0.22, 0.95) | 58.96 | 43.84 ± 106.25 | 163.42 | 0.99 (0.96, 1.00) | 16.64 | −3.67 ± 35.32 | 46.13 |
| Day 1–day 7 | ||||||||
| CAR, %c | 0.90 (0.60, 0.98) | 4.63 | −2.62 ± 8.91 | 12.84 | 0.78 (0.12, 0.95) | 2.97 | −3.07 ± 5.70 | 8.23 |
| MVIC, N | 0.75 (0.00, 0.94) | 64.20 | 38.04 ± 119.03 | 177.95 | 0.96 (0.83, 0.99) | 28.86 | −14.49 ± 59.87 | 80.00 |
| SIB, N | 0.77 (0.08, 0.94) | 65.87 | 62.46 ± 116.08 | 182.58 | 0.96 (0.84, 0.99) | 35.30 | 1.55 ± 73.21 | 97.86 |
| Day 1–day 14 | ||||||||
| CAR, %c | 0.95 (0.81, 0.99) | 3.39 | −3.60 ± 6.85 | 9.39 | 0.48 (−1.08, 0.87) | 3.86 | −7.30 ± .54 | 10.70 |
| MVIC, N | 0.91 (0.66, 0.98) | 39.48 | −2.52 ± 71.73 | 109.44 | 0.95 (0.81, 0.99) | 29.15 | −9.40 ± 49.08 | 80.79 |
| SIB, N | 0.89 (0.54, 0.97) | 44.23 | 19.95 ± 73.78 | 122.60 | 0.92 (0.68, 0.98) | 45.87 | 34.48 ± 81.67 | 127.15 |
| Day 1–day 21 | ||||||||
| CAR, %c | 0.92 (0.67, 0.98) | 3.92 | −5.58 ± 7.52 | 10.87 | 0.43 (−1.30, 0.86) | 4.53 | −6.35 ± 7.60 | 12.55 |
| MVIC, N | 0.86 (0.37, 0.97) | 45.19 | 13.92 ± 85.09 | 125.25 | 0.94 (0.77, 0.99) | 31.67 | 12.38 ± 59.94 | 87.80 |
| SIB, N | 0.84 (0.28, 0.96) | 48.78 | 48.85 ± 92.60 | 135.21 | 0.94 (0.76, 0.99) | 39.60 | 53.53 ± 73.42 | 109.77 |
Abbreviations: CAR, central activation ratio; CI, confidence interval; ICC, intraclass correlation coefficient; MVIC, maximal voluntary isometric contraction; SEM, standard error of measurement; SIB, superimposed burst.
Positive values indicate day 1 values were larger than comparison.
Intraclass correlation coefficient using a 2-way fixed model with consistency from average measures.
100% indicates complete activation.
Table 2. .
Between-Sessions Reliability Data for SIB Measures Using Day 2 as a Reference For All Comparisonsa
| Between-Sessions Reliability |
Patellofemoral Pain Group |
Healthy Group |
||||||
| ICC [3,3] (95% CI) |
SEM |
Mean Difference |
Minimal Detectable Change at 95% Confidence Level |
ICC [3,3] (95% CI) |
SEM |
Mean Difference |
Minimal Detectable Change at 95% Confidence Level |
|
| Day 2–day 7 | ||||||||
| CAR, % | 0.97 (0.79, 0.99) | 2.36 | −1.75 ± 4.72 | 6.55 | 0.93 (0.71, 0.98) | 1.52 | −1.86 ± 2.67 | 4.21 |
| MVIC, N | 0.98 (0.92, 1.00) | 16.92 | 6.93 ± 34.09 | 46.90 | 0.96 (0.85, 0.99) | 25.92 | −4.01 ± 36.81 | 71.84 |
| SIB, N | 0.95 (0.78, 0.99) | 20.69 | 16.58 ± 39.81 | 57.34 | 0.93 (0.66, 0.98) | 26.99 | 3.82 ± 56.22 | 74.82 |
| Day 2–day 14 | ||||||||
| CAR, % | 0.94 (0.61, 0.97) | 3.65 | −2.73 ± 7.35 | 10.13 | 0.66 (−0.23, 0.92) | 2.79 | −6.09 ± 2.36 | 7.74 |
| MVIC, N | 0.92 (0.66, 0.98) | 35.58 | −36.97 ± 62.44 | 98.62 | 0.96 (0.84, 0.99) | 25.85 | −9.42 ± 6.38 | 71.66 |
| SIB, N | 0.95 (0.79, 0.99) | 25.85 | −25.94 ± 2.34 | 76.69 | 0.94 (0.77, 0.98) | 37.32 | 23.10 ± 72.07 | 103.44 |
| Day 2–day 21 | ||||||||
| CAR, % | 0.92 (0.54, 0.98) | 3.80 | −4.72 ± 6.22 | 10.54 | 0.69 (−0.20, 0.93) | 3.03 | −5.14 ± 4.05 | 8.40 |
| MVIC, N | 0.94 (0.77, 0.99) | 25.75 | −18.50 ± 45.65 | 71.37 | 0.96 (0.84, 0.99) | 25.77 | 15.24 ± 35.63 | 71.43 |
| SIB, N | 0.98 (0.90, 0.99) | 24.82 | 0.07 ± 48.58 | 68.80 | 0.97 (0.89, 0.99) | 37.99 | 45.37 ± 62.65 | 105.29 |
Abbreviations: CAR, central activation ratio; CI, confidence interval; ICC, intraclass correlation coefficient; MVIC, maximal voluntary isometric contraction; SEM, standard error of measurement; SIB, superimposed burst.
Positive values indicated day 2 values were larger than comparison.
Group Comparisons
Quadriceps CAR, FMVIC, and FSIB means for each time interval and group are presented in Table 3. We observed a group main effect for CAR (F1,17 = 5.48, P = .03) and FMVIC (F1,17 = 8.35, P = .01), indicating less quadriceps activation and strength in PFP patients. We noted a main effect of time for CAR (P = .04) in the healthy group only, indicating less quadriceps activation at 24 hours than on day 14. Participant demographics were not different between groups (P ≥ .05).
Table 3. .
Quadriceps Central Activation Ratio, Knee-Extension Maximal Voluntary Isometric Contraction Force, and Superimposed-Burst Force (Group Mean ± SD)
| Time |
Patellofemoral Pain Group |
Healthy Group |
||||
| Central Activation Ratio, % |
Maximal Voluntary Isometric Contraction, N |
Superimposed Burst Force, N |
Central Activation Ratio, % |
Maximal Voluntary Isometric Contraction, N |
Superimposed Burst Force, N |
|
| Day 1 prebaseline | 75.8 ± 14.9 | 326.0 ± 132.3 | 424.4 ± 140.8 | 83.8 ± 6.6 | 444.1 ± 147.6 | 532.5 ± 187.0 |
| Day 1 postbaseline | 74.9 ± 13.4 | 282.8 ± 94.7 | 373.8 ± 101.5 | 85.7 ± 8.0 | 436.3 ± 149.9 | 510.8 ± 171.9 |
| Day 2 | 76.3 ± 12.7 | 302.8 ± 113.6 | 393.2 ± 126.5 | 85.3 ± 5.6a | 454.6 ± 132.2 | 534.9 ± 163.1 |
| Day 7 | 77.5 ± 14.6 | 295.9 ± 125.4 | 376.6 ± 135.0 | 86.9 ± 6.1 | 458.6 ± 133.7 | 531.1 ± 165.4 |
| Day 14 | 78.9 ± 16.0 | 339.2 ± 136.9 | 419.1 ± 120.4 | 90.7 ± 3.8a | 464.0 ± 119.8 | 511.8 ± 135.3 |
| Day 21 | 79.8 ± 13.5 | 300.9 ± 103.2 | 369.9 ± 93.3 | 89.8 ± 5.4 | 439.3 ± 115.8 | 489.5 ± 128.3 |
Indicates difference between day 2 and day 14 measures within group (P ≤ .05).
DISCUSSION
The SIB technique has been used widely in clinical outcomes research to assess quadriceps muscle function.3 To our knowledge, no researchers have evaluated the reliability of this technique in pathologic cohorts. Our results suggested that the quadriceps CAR and its constituents demonstrated good to high within-session reliability. The highly reliable CAR measures in the PFP patients were consistent with previous reports of healthy individuals4 yet higher than in our healthy control participants. The observed within-session CAR reliability among healthy individuals was lower than several previous researchers4,9 have reported. Good within-session reliability in MVIC was detected among PFP patients, but confidence intervals were considerably larger than for CAR measures. In contrast, healthy individuals had highly reliable MVIC measures with narrow confidence intervals.
We find it interesting that high between-sessions reliability in CAR was observed up to day 21 for PFP patients, whereas healthy individuals demonstrated fair reliability in CAR only through day 7. However, confidence intervals within each group demonstrated wide variability, which may highlight the need for additional instructional techniques, such as visual feedback, to reduce variability in maximal-effort contractions. In contrast, MVIC remained highly reliable through day 21 in healthy individuals. Both within-session and between-sessions comparisons revealed that PFP patients consistently showed higher reliability in CAR and lower reliability in MVIC through day 21 (Table 1). Clinically, this may indicate that patients with PFP displayed greater variability during a knee-extension MVIC but decreased variability within the available motor-neuron pool to accomplish the task. From an intervention perspective, this suggests that changes in muscle activation, and not FMVIC, may be easier to detect in these patients, whereas the opposite may be true in healthy individuals.
A clear distinction in between-sessions reliability for CAR was observed between groups when using the baseline measure as a reference for comparison, with healthy participants demonstrating less reliability at each measurement interval. This discrepancy may be attributed partially to the novelty of the task and the instructions provided by the clinician, in which case we would expect to see enhanced reliability when participants had experience with the task. To support this hypothesis, we assessed reliability as an exploratory analysis, using day 2 as a reference (Table 2). It is notable that CAR reliability was better in each group at day 7 and in healthy participants at days 14 and 21. In addition, MVIC reliability was better in both groups at days 7, 14, and 21. This observed improvement in reliability suggests that clinical researchers evaluating quadriceps strength and activation using a potentially novel technique may need to include a thorough practice session to ensure the most precise measurement. Including a familiarization period immediately before assessments of neuromuscular function has been described, but it remains unclear if practice with such novel tasks will be retained in subsequent testing sessions.10
The SEM and MDC95 provide an estimate of measurement precision.8 According to these findings, within-session changes in CAR and MVIC of greater than 7% and 117 N, respectively, would be necessary to represent treatment effects among PFP patients. Whereas both groups demonstrated similar MDCs in CAR, a change of only 43 N would indicate a treatment effect in MVIC among healthy individuals. Similarly, between-sessions MDC values in CAR were comparable between groups, ranging from 8.23% to 12.84% and much higher for MVIC in PFP patients (109.44–177.95 N) than for healthy individuals (56.29–87.80 N). These values may be due to considerably larger SEM values, supporting the hypothesis of greater variability among patients with PFP.
Our study had several limitations and potential sources of measurement error. In previous studies, researchers9 using the SIB technique have secured participants in a stationary dynamometer during a maximal contraction, but we used a custom-made chair. Although this may influence movement during testing, the described technique presents a more clinically based method of assessment. Accessory motion has been reported to alter estimates of quadriceps activation,10 but we do not know whether this arrangement affected the proportion of quadriceps activation relative to the surrounding musculature. In addition, Roberts et al10 reported that oral instruction reduces aberrant motion, as indicated by a reduction in surface EMG activity of the surrounding musculature. Even though we did not directly measure these factors, CAR values in healthy individuals were consistent with previous data from our laboratory10 but were lower than values reported in previous studies.4,9 Similarly, PFP patients demonstrated lower CAR values than previously reported,3 which may be attributed to the aforementioned factors. Given that PFP is multifactorial, it is plausible that varied neuromuscular strategies developed within this patient population, allowing them to exhibit different contractile patterns from those of healthy counterparts. Alterations in voluntary force production have been shown to directly influence electrically induced measures of muscle activation,2 making the assessment of pathologic cohorts necessary to evaluate the use of this technique in clinical research. Last, we did not use visual feedback or an automated trigger system, which have been suggested to enhance the reliability of CAR measures obtained via the SIB technique. However, Park and Hopkins4 reported high reliability in MVIC and CAR using comparable procedures. To draw valid comparisons among studies, it may be important to begin with established procedures.
CONCLUSIONS
The SIB technique was reliable for clinical research in patients with PFP. Quadriceps CAR and FMVIC appeared to be reliable outcome measures through day 21 and can be improved with a familiarization period.
REFERENCES
- 1.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Stackhouse SK, Dean JC, Lee SC, Binder-MacLeod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23(11):1706–1712. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J, Hopkins JT. Within- and between-session reliability of the maximal voluntary knee extension torque and activation. Int J Neurosci. 2013;123(1):55–59. doi: 10.3109/00207454.2012.725117. [DOI] [PubMed] [Google Scholar]
- 5.Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–163. doi: 10.1016/s0749-8063(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 6.Hart JM, Fritz JM, Kerrigan DC, Saliba EN, Gansneder BM, Ingersoll CD. Reduced quadriceps activation after lumbar paraspinal fatiguing exercise. J Athl Train. 2006;41(1):79–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Myers CR, Blesh TE. Measurement in Physical Education. New York, NY: Ronald Press;; 1962. eds. [Google Scholar]
- 8.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Hopkins JT. Quadriceps activation normative values and the affect of subcutaneous tissue thickness. J Electromyogr Kinesiol. 2011;21(1):136–140. doi: 10.1016/j.jelekin.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Roberts D, Kuenze C, Saliba S, Hart JM. Accessory muscle activation during the superimposed burst technique. J Electromyogr Kinesiol. 2012;22(4):540–545. doi: 10.1016/j.jelekin.2012.01.008. [DOI] [PubMed] [Google Scholar]


