We evaluated the usefulness of long-term plant carbon economy of a xerophyte shrub as a tool in conservation. Reserves manipulation through defoliation decreased reproduction in the long-term but not growth. Root and shoot reserves can be used as indicators of how much biomass can be harvested without threatening future reproduction
Keywords: Climate change, critical oxygen saturation, elasmobranch, Leucoraja erinacea, metabolism, performance
Abstract
Although fish population size is strongly affected by survival during embryonic stages, our understanding of physiological responses to environmental stressors is based primarily on studies of post-hatch fishes. Embryonic responses to acute exposure to changes in abiotic conditions, including increase in hypoxia, could be particularly important in species exhibiting long developmental time, as embryos are unable to select a different environment behaviourally. Given that oxygen is key to metabolic processes in fishes and aquatic hypoxia is becoming more severe and frequent worldwide, organisms are expected to reduce their aerobic performance. Here, we examined the metabolic and behavioural responses of embryos of a benthic elasmobranch fish, the little skate (Leucoraja erinacea), to acute progressive hypoxia, by measuring oxygen consumption and movement (tail-beat) rates inside the egg case. Oxygen consumption rates were not significantly affected by ambient oxygen levels until reaching 45% air saturation (critical oxygen saturation, Scrit). Below Scrit, oxygen consumption rates declined rapidly, revealing an oxygen conformity response. Surprisingly, we observed a decoupling of aerobic performance and activity, as tail-beat rates increased, rather than matching the declining metabolic rates, at air saturation levels of 55% and below. These results suggest a significantly divergent response at the physiological and behavioural levels. While skate embryos depressed their metabolic rates in response to progressive hypoxia, they increased water circulation inside the egg case, presumably to restore normoxic conditions, until activity ceased abruptly around 9.8% air saturation.
Introduction
Many marine ecosystems are expected to experience physicochemical changes associated with increasing anthropogenic greenhouse gas emissions (Intergovernmental Panel on Climate Change, 2013). Such changes extend beyond global warming and include reduction in pH (i.e. ocean acidification), alterations in circulation patterns, increased stratification and more frequent and prolonged hypoxic (i.e. low-oxygen) events (Perry et al., 2005; Cheung et al., 2013; Intergovernmental Panel on Climate Change, 2013). In fact, increased stratification and reduced mixing are expected to result in significant declines in dissolved oxygen, especially in semi-enclosed bodies of water, such as bays (Bakun et al., 2015). As oxygen is key to sustain every aerobic activity, it is important to understand whether and how marine organisms might tune their metabolic performance to cope with more frequent and intense hypoxic events. This is particularly crucial for benthic organisms inhabiting near-coastal waters as these are expected to be much more affected by hypoxia than highly mobile, pelagic species that are likely to possess the locomotory capacity required to sustain migrations to refugia (Lauder and Di Santo, 2015). At the same time, perhaps reflecting adaptations to typically low-oxygen environments, benthic organisms are generally thought to be able to tolerate moderate-to-severe levels of hypoxia (Seibel, 2011; Heinrich et al., 2014). Despite this fact, populations of several benthic species have already declined during the last decades, and low oxygen levels are often implicated in the impairment of vital activities, including foraging and reproduction (Schurmann and Steffensen, 1997; Behrens and Steffensen, 2007; Svendsen et al., 2012).
When faced with low oxygen levels, fishes can respond either by decreasing oxygen consumption rates (i.e. oxyconformity response) or by maintaining the same oxygen consumption rates despite the decrease in available dissolved oxygen in the water (i.e. oxyregulatory response). One simple and commonly adopted metric to identify species-specific sensitivity and tolerance to low oxygen levels is to quantify the oxygen saturation in the ambient water that triggers the switch from oxyregulation to oxyconformity response (Speers-Roesch et al., 2012). The progressive drop in metabolic rates seen during severe hypoxia is caused by oxygen deficiency and is associated with the transition to anaerobic metabolism (Pörtner, 2010). The oxygen saturation (or pressure) point at which quiescent aquatic ectotherms are unable to maintain the normoxic metabolic rate is termed ‘critical oxygen saturation’ (Scrit). The capacity of a fish to extract oxygen from the ambient water and survive acute hypoxic events is reflected in its specific Scrit, with high Scrit indicating a high sensitivity (and low tolerance) to low oxygen levels (Speers-Roesch et al., 2012). Correspondingly, Scrit represents an important ecological tipping point to understand the resilience of populations to declining levels of oxygen (Mueller et al., 2011).
Knowing at which point species conform to oxygen levels in the water is not only important to understand the capacity of fishes to respond to challenges of hypoxia (Urbina et al., 2012), but also to assess assumptions for disparate metabolic theories in ecology (Kearney and White, 2012). Although it is becoming clear that the vast majority of adult and juvenile fishes are capable of coping physiologically with a moderate decrease in ambient oxygen (Ultsch et al., 1981; Virani and Rees, 2000; Perry et al., 2009; Kearney and White 2012; Urbina et al., 2012; Svendsen et al., 2014), the metabolic responses of embryos remain uncertain. This could be particularly important in fishes that have a long developmental time, as they are most likely to experience hypoxic events at some point during embryogenesis. Most juvenile and adult fishes can respond to acute hypoxia by using a series of tactics, including moving to a different area, increasing ventilation rates, reducing swimming speed, performing aquatic surface respiration and fuelling metabolic processes through anaerobic pathways (Pollock et al. 2007; Chapman and McKenzie 2009; Poulsen et al., 2011; Svendsen et al., 2012; Genz et al., 2013). In contrast, embryonic fishes have a reduced array of choices when facing hypoxia. Given that embryos are spatially constrained inside the egg case and cannot select a different habitat until they hatch, they are most likely to respond to acute hypoxia by increasing ventilation rate or by depressing aerobic metabolism. Additionally, it might be adaptively advantageous to hatch precociously in order to locate a better habitat if the ontogenetic development is sufficiently advanced to ensure survival outside of the egg case (Petranka et al., 1982; Czerkies et al., 2001).
The little skate, Leucoraja erinacea (Mitchill, 1825), is a benthic oviparous elasmobranch that inhabits near-coastal water (down to 90 m depth) in the northwestern Atlantic, from the Gulf of Maine to Cape Hatteras (Bigelow et al., 1953). According to the International Union for Conservation of Nature (IUCN), L. erinacea is near threatened because of declining population, and the species is likely to become overfished by commercial fisheries in the near future. Female L. erinacea is reproductive year-round and produces large embryos individually surrounded by jelly inside leathery protective egg cases deposited on sandy or muddy flats (Leonard et al., 1999; Di Santo, 2015). The egg case has horns with tendrils that secure it to the substrate (Bigelow et al., 1953). After elasmobranch embryos develop external gill filaments, the jelly plug inside the egg case dissolves and the embryo starts to whip its tail to facilitate the passage of oxygenated clean water inside and through the case (see https://youtu.be/eziPYb1INA0; Thomason et al., 1996; Tullis and Peterson, 2000; Hoff, 2009; Di Santo, 2015). This tail-beat activity, vital for survival and development of the embryo, is associated with an increase in metabolism (Leonard et al., 1999) and it is strongly affected by climate-related stressors, including temperature and acidification (Di Santo, 2015). The combination of long developmental time (5–12 months; Di Santo, 2015) and the low likelihood to relocate to more favourable habitats given the high philopatry observed in the species (although no study to date has quantified the capacity of this fish to sustain migrations; Lauder and Di Santo, 2015) could have crucial consequences for the resilience of L. erinacea in possible near-future hypoxic scenarios.
In the present study, we quantified the oxygen consumption and tail-beat rates of embryonic L. erinacea during normoxia and progressive hypoxia. We hypothesized that skate embryos experiencing hypoxia might respond by using physiological and behavioural tactics in the following order: (i) increasing tail-beat rates as an attempt to circulate water and restore normoxia inside the egg case; (ii) switching from oxygen regulation to oxygen conformity; and (iii) hatching prematurely to escape the egg case. To quantify these responses, we used intermittent flow respirometry to measure oxygen consumption while the embryos were continuously whipping their tail in normoxic conditions and continued the measurements during incremental reductions in oxygen.
Materials and methods
Experimental animals
Newly laid (<1-week-old) little skate embryos (n = 6) were obtained from the Marine Biological Laboratory, Woods Hole, MA, USA. Embryos were transported to Boston University using a well-aerated, thermally controlled container. Once at Boston University, they were held each in independently filtered tanks, at constant salinity (33 ppt, obtained by mixing Instant Ocean® and deionized water), temperature (15°C), pH (8.1) and photoperiod (14 h light–10 h dark) in a cold environmental room (Harris Environmental Systems, Inc., Andover, MA, USA). Throughout the development, skate embryos were supplied with normoxic water (∼95% air saturation). Skate embryos were used for experimentation when the egg case was open and the yolk sac diameter was ≤1 mm to ensure that the embryos were close to hatching (∼6 months at 15°C; Di Santo, 2015). The yolk size was estimated visually by shining a red light through the semi-opaque egg case and measuring the yolk diameter with digital callipers. This late developmental stage was selected to examine the possibility that hypoxia could trigger hatching and allow the skate to search for a more favourable habitat.
Hypoxia experimental set-up
To decrease oxygen content in water, we employed a steady oxygen depletion system based on nitrogen displacement (Bennett and Beitinger, 1995; Svendsen et al., 2014). Briefly, nitrogen gas from a cylinder tank was bubbled into a covered holding tank to remove oxygen. The flow of nitrogen into the tank was controlled with a Fisher Scientific nitrogen regulator, a one-way valve and a gas bubbler. While maintaining constant temperature (15°C), salinity (33 ppt) and pH (8.1), dissolved oxygen was decreased by 5% in a stepwise fashion to examine the responses to different oxygen levels (n = 18) between 95 and 10% air saturation.
Oxygen consumption and tail-whip frequency
Embryos were placed and oriented ventrally in the experimental tank 4 h prior to data collection to ensure that measurements were taken in unstressed individuals (Leonard et al., 1999; Di Santo, 2015). Measurements of the oxygen consumption rate (; in milligrams of O2 per kilogram per hour) while the embryos were continuously beating their tail in the egg case [here defined as active metabolic rate (AMR) in accordance with previous studies; see Leonard et al., 1999; Di Santo, 2015] were conducted using a custom-made Plexiglas® intermittent flow respirometer connected with a flush and a recirculating pump similar to previous studies (Svendsen et al., 2012; Tirsgaard et al., 2015). The total volume of the empty respirometer was 0.701 l but was adjusted for the water displacement caused by each skate egg case and the oxygen probe. The temperature was maintained constant (15 ± 0.1°C) throughout the experiment by submerging the respirometer chamber into a water bath connected to a digitally controlled Aqua Logic chiller.
For each measurement of AMR, oxygen saturation was adjusted to match the desired level in the chamber while the flushing and the circulation pumps were actuated. Embryos experienced each saturation condition for 30 min. Following this period, the flushing pump was turned off and oxygen decline in the respirometer was measured for 20 min. Oxygen concentration was measured using a YSI oxygen meter (model 550A) calibrated daily. Measurements of AMR continued until skate embryos ceased whipping their tail for at least 1 min. Activity rates (tail beats per minute) were recorded for 1 min at 5 min intervals at each tested oxygen level by shining a red light through the semi-opaque egg case. After each trial, skates were returned to normoxic conditions and gently extracted from the egg case using blunt forceps inserted into the anterior slit. Once extracted, skates were weighed to record wet mass to the nearest 0.01 g. Oxygen consumption rates were calculated based on the slope of a linear regression of the decline in oxygen concentration inside the chamber during the 20 min measurement phase. Measurements were adjusted by mass using the coefficient (0.67) previously tested and applied for this species and other elasmobranchs (Di Santo and Bennett, 2011a; Di Santo, 2015) using the following equation:
Blank respirometry trials were conducted with the empty egg cases for 1 h, but background respiration was never detected (n = 6).
Statistical analysis
Analyses of oxygen consumption and tail-beat rates in relationship to O2 saturation levels were conducted using a repeated-measures ANOVA, followed by Dunnett’s test (control: air saturation of 95%) to estimate the oxygen levels that triggered a change in metabolic and activity rates. All statistical analyses were based on α = 0.05 and were undertaken in JMP Pro, version 11 (SAS).
Results
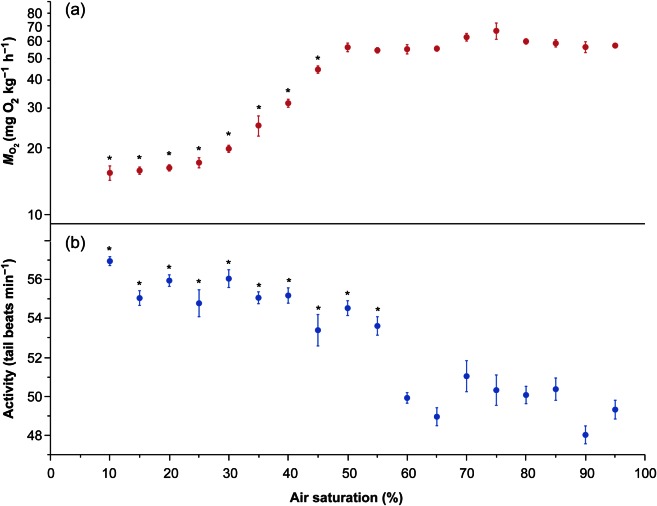
Hypoxic conditions (at and below 45% air saturation; Scrit) significantly reduced AMR in little skate embryos, indicating an oxygen conformity response (repeated-measures ANOVA, F85,17 = 102.45; P < 0.000001; Fig. 1a). However, a stepwise decrease in oxygen did not significantly affect AMR between 95 and 50% air saturation (Dunnett’s test, α = 0.05), indicating an oxygen regulating response. Skate embryos maintained similar tail-beat rates across oxygen saturations down to 55%, after which the rate increased (repeated-measures ANOVA, F85,17 = 30.81; P < 0.000001; Dunnett’s test, α = 0.05; Fig. 1b). Surprisingly, there was no gradual decrease in tail-beat frequency as a function of progressive hypoxia. Instead, we observed an abrupt complete cessation of all movement at 9.8 ± 0.05% air saturation (i.e. tail-beat collapse). All skates recovered and survived the procedure, but hypoxia did not trigger hatching, despite the fact that aerobic metabolism was depressed and activity ceased completely at the end of the experiment.
Figure 1:
Near-hatch embryonic little skate (Leucoraja erinacea; n = 6) oxygen consumption (; a) and activity (b), assessed as tail-beat rate (means ± SEM) as a function of decreasing oxygen in the environment (air saturation, expressed as a percentage). Asterisks show statistically significant difference in mean and activity rates from normoxic levels, 95% air saturation (repeated-measures ANOVA, followed by Dunnett’s test; α = 0.05).
Discussion
Over recent decades, hypoxic events have increased in frequency, severity and geographical extent and it is therefore crucial to know the response of fish performance at different levels of hypoxia (Herbert et al., 2011). A reduction in AMR at oxygen levels below Scrit forces fishes to use anaerobic pathways and has detrimental effects on activity and resilience of species (Schurmann and Steffensen, 1997; Claireaux et al., 2000; Chabot and Claireaux, 2008). The present study indicates that embryos of L. erinacea are highly sensitive to hypoxia, because they decrease oxygen consumption at air saturation levels of 45%, while activity in the egg case increases, possibly to enhance ventilation and exchange of water with the surrounding environment. Combining metabolic and behavioural measurements, our results show that embryos of L. erinacea maintained constant metabolic rates during activity down to 45% (Scrit), revealing an oxygen regulating response to changing oxygen levels. However, at oxygen saturations below Scrit, metabolism declined abruptly and significantly with increasing levels of hypoxia, suggesting an oxygen conformity response and, possibly, a progressive reliance on anaerobic metabolism. Interestingly, activity was more sensitive to decreasing oxygen than metabolic rates, because tail-beat rates increased at higher levels of oxygen in the water (55%). Thus, this behavioural switch occurred well above Scrit, indicating that the first response to oxygen decline in the environment is behavioural rather than metabolic. Furthermore, activity did not decrease before completely collapsing at the end of the trial. This result is in contrast to previous studies, because complete cessation of hyperventilation in hypoxic conditions is uncommon in fishes (Perry et al., 2009).
It is widely assumed that when the aerobic scope for activity is limited by the reduction in oxygen in the ambient water, fishes will attempt to reduce energetic expenditure by decreasing movement (Chabot and Claireaux, 2008). Although this assumption has been substantiated by studies on relatively sluggish species of the genera Gadus (Schurmann and Steffensen, 1997), Carassius (Nilsson et al., 1993) and Solea (Dalla Via et al., 1998), it was not confirmed in more active species of the genera Tunnus (Bushnell and Brill, 1991), Oncorhynchus (Poulsen et al., 2011) and Cynoscion (Brady et al., 2009). Based on these few studies, there seems to be an apparent correlation between ‘physiotype’ (e.g. active vs. inactive fishes) and activity regulation (Burggren and Randall, 1978; Metcalfe and Butler, 1984; Cook et al., 2011; Di Santo and Bennett, 2011b). In fact, active fishes might upregulate their locomotory performance to escape hypoxic environments, whereas more sluggish physiotypes might downregulate activity in order to reduce energy costs during a temporary oxygen crisis, and thus decrease the chance of incurring lethal metabolic deficits. Although it might be expected that benthic fishes, such as skates, would respond to a decrease in oxygen levels by reducing both activity and metabolic rates, embryos seem to exploit a different tactic, i.e. they significantly increase tail-beat rate down to a point where activities can no longer be sustained. During severe hypoxia, activity was elevated by ∼10% from normoxic conditions, but metabolic rates were depressed up to ∼70%. This suggests a decoupling of activity and aerobic metabolism, as embryos were possibly becoming progressively more reliant on anaerobic pathways, a strategy that could not be sustained for a prolonged period of time (Pörtner and Farrell, 2008).
Studies on the effect of hypoxia on fish embryos are scarce but they suggest that both acute and chronic exposure to hypoxia may result in a decrease in survival and growth and an increase in developmental time and malformations (Podrabsky et al., 2001; Shang and Wu, 2004; Breitburg et al., 2009). A few studies on the effect of hypoxia on elasmobranchs suggest that some species in this group may even be sensitive to a brief acute exposure to low oxygen levels. For example, the shovelnose ray (Aptychotrema rostrata) cannot tolerate oxygen tensions of 2 kPa for 30 min (Speers-Roesch, 2012). On the contrary, the epaulette shark (Hemiscyllium ocellatum) exhibits an extraordinary tolerance to hypoxia, which may be given by the capacity to minimize changes in fluid acidosis and cardiac function (Speers-Roesch, 2012). In the present study, all L. erinacea embryos survived the acute exposure to hypoxia but did not hatch spontaneously and needed to be extracted from the egg cases to be weighed and measured. In response to hypoxia, some fishes may hatch prematurely, whereas others may in fact delay hatching (Hassell et al., 2008). For instance, precocious hatching has been observed in fishes of the genus Coregonus (Czerkies et al., 2001), Oncorhynchus (Ciuhandu et al., 2005) and Acanthopagrus (Hassell et al., 2008). However, these same studies observed delayed hatching as well. It is plausible that a similar phenomenon occurred in L. erinacea, where most fish did not respond to low ambient oxygen by hatching (or escaping the egg case) even though anecdotal evidence suggests that early hatching could occur (V.D.S., personal observation). In fact, during preliminary studies in which skate embryos were maintained in a closed respirometer and allowed to consume oxygen without flushing, they exited the egg case at ∼2 mg O2 l−1 (at 15°C; V.D.S., personal observation). However, other abiotic factors, such as CO2 and ammonia, were not controlled, and skates might have hatched in response to these stressors rather than low oxygen levels.
Leucoraja erinacea embryos are enclosed in a capsule throughout the relatively long development (up to 1 year), and it is therefore critical for the survival of this species that embryos possess the capacity to surmount hypoxic events in their habitat using various mechanisms. If hypoxic events continue to increase in frequency and to expand geographically in the northwestern Atlantic (Fulweiler et al., 2012), these could potentially affect fitness and resilience of populations of L. erinacea. As reproduction in the fish is not timed with seasons but occurs year-round (Palm et al., 2011) and the species shows strong philopatry (Frisk and Miller, 2006, 2009), female L. erinacea are not expected to modify reproductive timing or spawning location (Di Santo, 2015; Lauder and Di Santo, 2015). Moreover, warming could further exacerbate the effect of hypoxia because metabolic processes require more oxygen at higher temperatures (Perry et al., 2005). However, in the present study, temperature and other abiotic factors (pH and salinity) were kept constant, so the shift between oxyregulation and oxyconformity should be attributed to oxygen levels alone.
It is clear that for large embryos, stuck in the egg case for several months, tail-beat activity is the only means by which water is circulated and moved though the egg case and, ultimately, normoxia is maintained. As other tactics, such as precocious hatching, are only observed occasionally, prolonged aquatic hypoxia could severely impair development of embryonic oviparous elasmobranchs, with a possible increase in pre-hatching mortality. Nevertheless, an approach whereby physiological and behavioural responses to climatic stressors are combined with spatial and temporal data on abiotic stressors (i.e. hypoxia, warming and acidification) could help to identify vulnerable areas and potential refugia, thus improving conservation outcomes for oviparous sharks and skates.
Funding
This project was funded by grants from the American Fisheries Society, the American Society of Ichthyologists and Herpetologists, the American Elasmobranch Society, Flying Sharks, The Oceanário de Lisboa, and the Portuguese Association for the Study and Conservation of Elasmobranchs to V.D.S.; while conducting the experiments and writing the manuscript, V.D.S. was supported by the Warren-McLeod Research, Dana Wright and Ryan Kelley Fellowships; A.H.T. was supported by the UROP program at Boston University; and J.C.S. was supported by a grant (SFRH/BPD/89473/2012) from the Foundation for Science and Technology (FCT) in Portugal.
Acknowledgements
The authors would like to thank the Marine Biological Laboratory at Woods Hole, MA, USA for providing skate embryos, and the Boston University Marine Program and Phil Lobel for sharing laboratory space and equipment. Three anonymous reviewers provided helpful comments on a previous version of this manuscript. The research was conducted under the approved Institutional Animal Care and Use protocol no. 11-041 at Boston University.
References
- Bakun A, Black BA, Bograd SJ, García-Reyes M, Miller AJ, Rykaczewski RR, Sydeman WJ (2015) Anticipated effects of climate change on coastal upwelling ecosystems. Curr Clim Change Rep 1: 85–93. [Google Scholar]
- Behrens JW, Steffensen JF (2007) The effect of hypoxia on behavioural and physiological aspects of lesser sandeel, Ammodytes tobianus (Linnaeus, 1785). Mar Biol 150: 1365–1377. [Google Scholar]
- Bennett WA, Beitinger TL (1995) Technical notes: Overview of techniques for removing oxygen from water and a description of a new oxygen depletion system. Progress Fish-Cult 57: 84–87. [Google Scholar]
- Bigelow HB, Schroeder WC, Hole W (1953) Fishes of the Gulf of Maine. US Government Printing Office, Washington, DC. [Google Scholar]
- Brady DC, Targett TE, Tuzzolino DM (2009) Behavioral responses of juvenile weakfish (Cynoscion regalis) to diel-cycling hypoxia: swimming speed, angular correlation, expected displacement, and effects of hypoxia acclimation. Can J Fish Aquat Sci 66: 415–424. [Google Scholar]
- Breitburg DL, Hondorp DW, Davias LA, Diaz RJ (2009) Hypoxia, nitrogen, and fisheries: integrating effects across local and global landscapes. Ann Rev Mar Sci 1: 329–349. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Randall DJ (1978) Oxygen uptake and transport during hypoxic exposure in the sturgeon Acipenser transmontanus. Respir Physiol 34: 171–183. [DOI] [PubMed] [Google Scholar]
- Bushnell PG, Brill RW (1991) Responses of swimming skipjack (Katsuwonus pelamis) and yellowfin (Thunnus albacares) tunas to acute hypoxia, and a model of their cardiorespiratory function. Physiol Zool 64: 787–811. [Google Scholar]
- Chabot D, Claireaux G (2008) Environmental hypoxia as a metabolic constraint on fish: the case of Atlantic cod, Gadus morhua. Mar Poll Bull 57: 287–294. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, McKenzie DJ (2009) Behavioral responses and ecological consequences. Fish Physiol 27: 25–77. [Google Scholar]
- Cheung WWL, Sarmiento JL, Dunne J, Frölicher TL, Lam VWY, Deng Palomares ML, Watson R, Pauly D (2013) Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat Clim Change 3: 254–258. [Google Scholar]
- Ciuhandu CS, Stevens ED, Wright PA (2005) The effect of oxygen on the growth of Oncorhynchus mykiss embryos with and without a chorion. J Fish Biol 67: 1544–1551. [Google Scholar]
- Claireaux G, Webber DM, Lagardère JP, Kerr SR (2000) Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J Sea Res 44: 257–265. [Google Scholar]
- Cook DG, Wells RM, Herbert NA (2011) Anaemia adjusts the aerobic physiology of snapper (Pagrus auratus) and modulates hypoxia avoidance behaviour during oxygen choice presentations. J Exp Biol 214: 2927–2934. [DOI] [PubMed] [Google Scholar]
- Czerkies P, Brzuzan P, Kordalski K, Luczynski M (2001) Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 196: 151–158. [Google Scholar]
- Dalla Via J, Van den Thillart G, Cattani O, Cortesi P (1998) Behavioural responses and biochemical correlates in Solea solea to gradual hypoxic exposure. Can J Zool 76: 2108–2113. [Google Scholar]
- Di Santo V. (2015) Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J Exp Mar Biol Ecol 463: 72–78. [Google Scholar]
- Di Santo V, Bennett WA (2011a) Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish Physiol Biochem 37: 1–6. [DOI] [PubMed] [Google Scholar]
- Di Santo V, Bennett WA (2011b) Is post-feeding thermotaxis advantageous in elasmobranch fishes? J Fish Biol 78: 195–207. [DOI] [PubMed] [Google Scholar]
- Frisk MG, Miller TJ (2006) Age, growth, and latitudinal patterns of two Rajidae species in the northwestern Atlantic: little skate (Leucoraja erinacea) and winter skate (Leucoraja ocellata). Can J Fish Aquat Sci 63: 1078–1091. [Google Scholar]
- Frisk MG, Miller TJ (2009) Maturation of little skate and winter skate in the western Atlantic from Cape Hatteras to Georges Bank. Mar Coast Fish 1: 1–11. [Google Scholar]
- Fulweiler RW, Rabalais NN, Heiskanen AS (2012) The eutrophication commandments. Mar Pollut Bull 64: 1997–1999. [DOI] [PubMed] [Google Scholar]
- Genz J, Jyde MB, Svendsen JC, Steffensen JF, Ramløv H (2013) Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp Biochem Physiol A Mol Integr Physiol 165: 54–60. [DOI] [PubMed] [Google Scholar]
- Hassell KL, Coutin PC, Nugegoda D (2008) Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri). Mar Poll Bull 57: 302–306. [DOI] [PubMed] [Google Scholar]
- Heinrich DDU, Rummer JL, Morash AJ, Watson S-A, Simpfendorfer CA, Heupel MR, Munday PL (2014) A product of its environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv Physiol 2: doi:10.1093/conphys/cou047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert NA, Skjæraasen JE, Nilsen T, Salvanes AGV, Steffensen JF (2011) The hypoxia avoidance behaviour of juvenile Atlantic cod (Gadus morhua L.) depends on the provision and pressure level of an O2 refuge. Mar Biol 158: 737–746. [Google Scholar]
- Hoff G. (2009) Embryo developmental events and the egg case of the Aleutian skate Bathyraja aleutica (Gilbert) and the Alaska skate Bathyraja parmifera (Bean). J Fish Biol 74: 483–501. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (2013) Climate Change: the Assessment Reports of the Intergovernmental Panel on Climate Change. In The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, USA.
- Kearney MR, White CR (2012) Testing metabolic theories. Am Nat 180: 546–565. [DOI] [PubMed] [Google Scholar]
- Lauder GV, Di Santo V (2015) Swimming mechanics and energetics of elasmobranch fishes. In Shadwick RE, Farrell AP, Brauner CJ, eds, Fish Physiology, Vol 34A, Physiology of Elasmobranch Fishes: Structure and Interaction with Environment. Academic Press, New York, pp 219–253. [Google Scholar]
- Leonard JBK, Summers AP, Koob TJ (1999) Metabolic rate of embryonic little skate, Raja erinacea (Chondrichthyes: Batoidea): the cost of active pumping. J Exp Zool 283: 13–18. [Google Scholar]
- Metcalfe JD, Butler PJ (1984) Changes in activity and ventilation in response to hypoxia in unrestrained, unoperated dogfish (Scyliorhinus canicula L.). J Exp Biol 108: 411–418. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Joss JMP, Seymour RS (2011) Effects of environmental oxygen on development and respiration of Australian lungfish (Neoceratodus forsteri) embryos. J Comp Physiol B 181: 941–952. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Rosen PR, Johansson D (1993) Anoxic depression of spontaneous locomotor activity in crucian carp quantified by a computerized imaging technique. J Exp Biol 180: 153–162. [Google Scholar]
- Palm BD, Koester DK, Driggers WB, Sulikowski JA (2011) Seasonal variation in fecundity, egg case viability, gestation, and neonate size for little skates, Leucoraja erinacea, in the Gulf of Maine. Environ Biol Fishes 92: 585–589. [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915. [DOI] [PubMed] [Google Scholar]
- Perry SF, Jonz MG, Gilmour KM (2009) Oxygen sensing and the hypoxic ventilatory response. In Richards J, Farrell AP, Brauner C, eds, Fish Physiology, Vol 27, Hypoxia. Academic Press, San Diego, CA, pp 193–253. [Google Scholar]
- Petranka JW, Just JJ, Crawford EC (1982) Hatching of amphibian embryos: the physiological trigger. Science 217: 257–259. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Carpenter JF, Hand SC (2001) Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol Regul Integr Comp Physiol 280: R123–R131. [DOI] [PubMed] [Google Scholar]
- Pollock MS, Clarke LMJ, Dubé MG (2007) The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ Rev 15: 1–14. [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Poulsen SB, Jensen LF, Nielsen KS, Malte H, Aarestrup K, Svendsen JC (2011) Behaviour of rainbow trout Oncorhynchus mykiss presented with a choice of normoxia and stepwise progressive hypoxia. J Fish Biol 79: 969–979. [DOI] [PubMed] [Google Scholar]
- Schurmann H, Steffensen JF (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- Seibel BA. (2011) Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J Exp Biol 214: 326–336. [DOI] [PubMed] [Google Scholar]
- Shang EH, Wu RS (2004) Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ Sci Technol 38: 4763–4767. [DOI] [PubMed] [Google Scholar]
- Speers-Roesch B, Richards JG, Brauner CJ, Farrell AP, Hickey AJ, Wang YS, Renshaw GM (2012) Hypoxia tolerance in elasmobranchs. I. Critical oxygen tension as a measure of blood oxygen transport during hypoxia exposure. J Exp Biol 215: 93–102. [DOI] [PubMed] [Google Scholar]
- Svendsen JC, Steffensen JF, Aarestrup K, Frisk M, Etzerodt A, Jyde M (2012) Excess posthypoxic oxygen consumption in rainbow trout (Oncorhynchus mykiss): recovery in normoxia and hypoxia. Can J Zool 90: 1–11. [Google Scholar]
- Svendsen JC, Genz J, Anderson WG, Stol JA, Watkinson DA, Enders EC (2014) Evidence of circadian rhythm, oxygen regulation capacity, metabolic repeatability and positive correlations between forced and spontaneous maximal metabolic rates in lake sturgeon Acipenser fulvescens. PLoS ONE 9: e94693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason JC, Davenport J, Le Comte E (1996) Ventilatory mechanisms and the effect of hypoxia and temperature on the embryonic lesser spotted dogfish. J Fish Biol 49: 965–972. [Google Scholar]
- Tirsgaard B, Svendsen JC, Steffensen JF (2015) Effect of temperature on specific dynamic action in the Atlantic cod Gadus morhua. Fish Physiol Biochem 41: 41–50. [DOI] [PubMed] [Google Scholar]
- Tullis A, Peterson G (2000) Growth and metabolism in the embryonic white-spotted bamboo shark, Chiloscyllium plagiosum: comparison with embryonic birds and reptiles. Physiol Biochem Zool 73: 271–282. [DOI] [PubMed] [Google Scholar]
- Ultsch GR, Jackson DC, Moalli R (1981) Metabolic oxygen conformity among lower vertebrates: the toadfish revisited. J Comp Physiol B 142: 439–443. [Google Scholar]
- Urbina MA, Glover CN, Forster ME (2012) A novel oxyconforming response in the freshwater fish Galaxias maculatus. Comp Biochem Physiol A Mol Integr Physiol 161: 301–306. [DOI] [PubMed] [Google Scholar]
- Virani NA, Rees BB (2000) Oxygen consumption, blood lactate and inter-individual variation in the gulf killifish, Fundulus grandis, during hypoxia and recovery. Comp Biochem Physiol A Mol Integr Physiol 126: 397–405. [DOI] [PubMed] [Google Scholar]