Abstract
Our objective was to develop state and metropolitan area-based surveillance projects to describe the characteristics of those with ALS and to assist with evaluating the completeness of the National ALS Registry. Because the literature suggested that ethnic/racial minorities have lower incidence of ALS, three state and eight metropolitan areas were selected to over-represent ethnic/racial minorities to have a sufficient number of minority patients. Project activities relied on reports from medical providers and medical records abstraction. The project areas represented approximately 27% of the U.S. population. The combined racial and ethnic distribution of these areas is 64.4% white, 16.0% African-American, 6.7% Asian, and 28.3% Hispanic. Most neurologists did not diagnose or provide care for ALS patients. The number of unique patients reported was close to expected (5883 vs. 6673). Age and gender distribution of patients was similar to the literature. The crude average annual incidence rate was 1.52 per 100,000 person-years, CI 1.44–1.61, and the 2009 prevalence rate was 3.84 per 100,000 population, CI 3.70–3.97. In conclusion, this study represents the largest number of clinically diagnosed ALS patients reported by neurologists in the U.S. Comparison of these data with those in the National ALS Registry will help evaluate the completeness of administrative databases.
Key words: Amyotrophic lateral sclerosis (ALS), incidence, prevalence, epidemiology, ALS surveillance
Introduction
Amyotrophic lateral sclerosis (ALS) is a rare progressive neurodegenerative disease that is diagnosed through a combination of signs and symptoms. Systematic reviews of worldwide literature estimate the yearly incidence of ALS to be 1.6–2.1 per 100,000 person-years (1,2). The prevalence has been changing over time and has moved from an estimate of 4.0/100,000 population (1) to more recently 5.9/100,000 population (2). Some studies suggest that ALS rates are higher among non-Hispanic Caucasians (whites) in western countries compared with those of African, Asian, and Hispanic descent (minorities) (3–5). There are limited data regarding the population based epidemiology of ALS in the United States (U.S.) with most studies having been conducted in limited geographic areas (6–8). Recent data from the National ALS Registry estimated the prevalence of ALS in the United States at 3.9 per 100,000 population (9).
The Agency for Toxic Substances and Disease Registry (ATSDR) initiated the congressionally mandated National ALS Registry in 2009 (9,10). Because ALS is a non-notifiable disease in the U.S., ATSDR conducted pilot projects to assess the feasibility of using existing data to identify persons with ALS. It was determined that it was feasible and the National ALS Registry was developed using existing national administrative data (11). In addition, a self-registration component was deployed in October 2010, which allows persons with ALS to self-register into the National ALS Registry (9,10).
Because of the non-traditional methodology used by the National ALS Registry to identify persons with ALS, ATSDR initiated surveillance projects in three states (Florida, New Jersey, and Texas) and eight metropolitan areas with large minority populations (Atlanta, Baltimore, Chicago, Detroit, Las Vegas, Los Angeles, Philadelphia, and the San Francisco Bay area) to evaluate the completeness of the National ALS Registry. Additional goals of the surveillance projects were to collect reliable and timely information regarding ALS incidence and demographic characteristics of persons with ALS in defined geographic areas. This paper describes the methodology used and the demographic characteristics of the patients identified.
Materials and methods
The catchment area was defined for the project sites based on residential status of potential ALS patients (Table I). Because the literature suggested that ALS affected minorities differently, the metropolitan areas were selected to over-represent racial minorities in order to have sufficient numbers of minorities with ALS.
Table I. Catchment areas for participating sites.
| Participating site | Catchment area | Populationa |
|---|---|---|
| Atlanta, Georgia | Cobb, Clayton, DeKalb, Fulton, and Gwinnett Counties | 3,365,297 |
| Baltimore, Maryland | City of Baltimore, Baltimore and Howard Counties | 1,713,075 |
| Chicago, Illinois | Cook (including City of Chicago) and DuPage Counties | 6,111,599 |
| Detroit, Michigan | Wayne County | 1,820,584 |
| Las Vegas, Nevada | Clark County | 1,951,269 |
| Los Angeles, California | Los Angeles County | 9,818,605 |
| Philadelphia, Pennsylvania | Philadelphia County | 1,526,006 |
| San Francisco, California | Alameda, Contra Costa, San Francisco, San Mateo, and Solano Counties | 4,496,326 |
| Florida | Entire State (67 Counties) | 18,801,310 |
| New Jersey | Entire State (21 Counties) | 8,791,894 |
| Texas | Entire State (254 Counties) | 25,738,765 |
a Population based on the 2010 U.S. Census, midyear of the three-year project period.14
All project sites identified neurologists in their catchment areas by using lists of neurologists from state medical licensing boards and mailing lists purchased from Medical Marketing Services. Those neurologists in specialties unlikely to diagnose or care for ALS patients, e.g. pediatric neurologists and neurosurgeons in urban areas, were removed. Neurologists were contacted to determine if they diagnosed and/or cared for ALS patients during the eligibility period. If they did, we explained the surveillance project, identified a contact, and requested that each eligible person with ALS be reported. A person with ALS was eligible to be reported if diagnosed and/or cared for from 1 January 2009 through 31 December 2011, and was a resident of one of the project's catchment areas.
Two reporting forms (the ALS Case Reporting Form (CRF) and the ALS Medical Records Verification Form (MRVF)) were developed with input from a consulting neurologist who specializes in the diagnosis and care of ALS patients. The CRF was used to collect information about each ALS case including personal identifiers, demographics, month and year of symptom onset, month and year of diagnosis, El Escorial criteria classification (12), family history of ALS, presence of dementia, and medical coverage. For this project, familial ALS was defined as an immediate family member (parent, sibling, child) having been diagnosed with ALS by a neurologist. The MRVF collected signs and symptoms, information needed to apply the El Escorial criteria, and electromyography (EMG) results (if available). For verification we selected a systematic sample of case reports based on the size of the practice and a targeted sample of unusual patients, e.g. less than 40 years of age at diagnosis. As a quality assurance activity, the consulting neurologist evaluated the MRVF and EMG results to confirm the ALS diagnosis.
We reviewed mortality data to identify additional possible ALS patients. State-specific death data were searched for the International Classification of Diseases (ICD)-10 code G12.2, the code for motor neuron disease (MND) (13), and/or text strings appropriate for ALS, in all of the cause of death fields, for the period 1 January 2009 through 31 December 2011. These data were compared with those patients reported. Either hardcopy death certificates were examined or text string searches were conducted on electronic records for decedents that had not been reported. If the cause of death was specifically ALS, attempts were made to identify the decedents’ treating physicians. Treating neurologists were then contacted and asked to complete an ALS CRF for eligible patients.
Each case report was examined as it was received and case reports for the same person were accepted if reported from different practices. Multiple reports for the same person were identified using a combination of first and last name, date of birth, last five numbers of the Social Security Number, gender, and city and state of residence. Upon completion of data collection, a composite record was created for patients reported more than one time.
Incidence rates were calculated for each project year using the project area specific populations from the 2010 U.S. Census (14) as the denominator and the number of new ALS patients reported by year as the numerator. The average annual incidence was calculated by adding the incidence for each year and dividing by three. Prevalence was calculated for 2009 by using the project area specific 2010 U.S. Census populations as the denominator and the number of ALS patients alive in 2009 who were diagnosed before 2010.
The project protocol was approved by the Centers for Disease Control and Prevention's Institutional Review Board. No patients were contacted. To offset the costs of participation, physicians received compensation for completing forms. Data were analyzed using Microsoft Excel®(15)and SPSS (16). A Poisson distribution was assumed in the calculation of the 95% confidence intervals (17).
Results
Neurologists
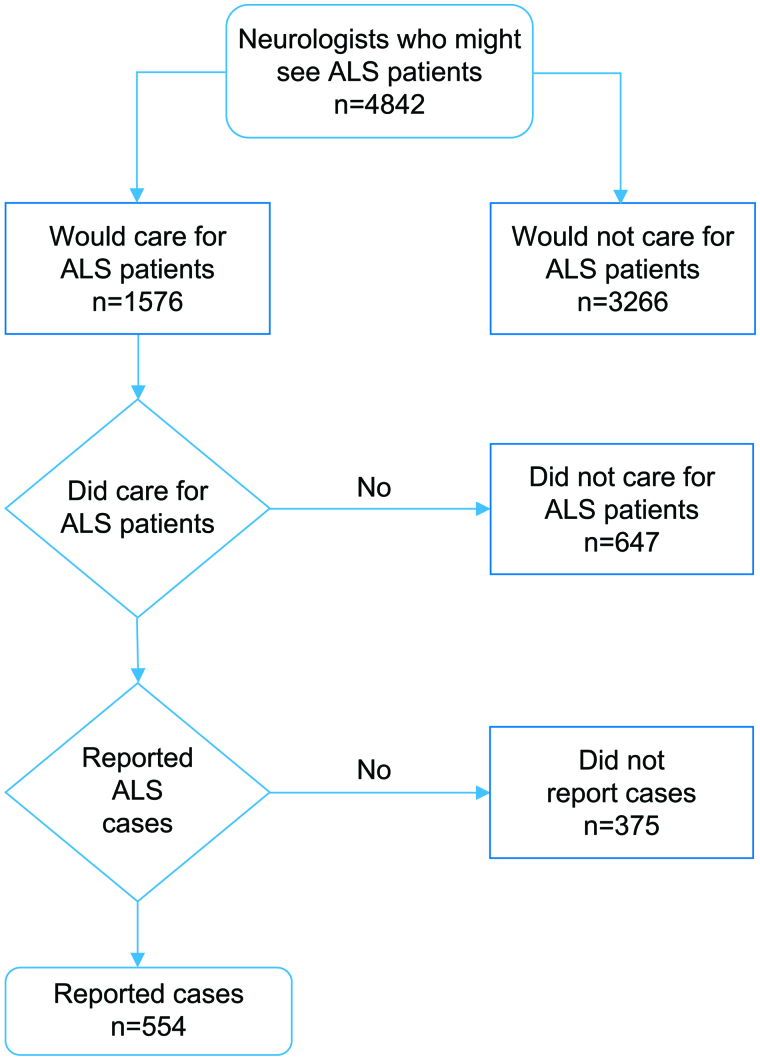
We identified 4842 neurologists who might diagnose and/or care for persons with ALS in the surveillance areas (Figure 1). Only 1576 (32.5%) of the neurologists contacted would diagnose or care for persons with ALS, and only 929 (19.2%) did so during the three-year project time-period. Of those neurologists who diagnosed and/or cared for ALS patients during the surveillance period, 554 (59.6%) reported patients. In the states, 26.5% of the neurologists contacted diagnosed and/or cared for ALS patients and 17.2% reported patients, compared with the metropolitan areas, where 12.2% of the neurologists contacted diagnosed and/or cared for ALS patients and 5.9% reported patients.
Figure 1. Flowchart showing neurologists reporting cases.
Case ascertainment and demographics
We received a total of 7062 case reports. The number of unique ALS patients reported was 5883 (3620 in states and 2263 in metropolitan areas). Based on previously published incidence and prevalence rates (1) and the total population in the project catchment area based on the 2010 U.S. Census (14), we expected 6673 patients. Therefore, we identified 88.2% of the expected patients.
Overall, 56.8% of reported patients were 60 years of age or older, and the gender distribution was the same in the states and metropolitan areas, with a ratio of 1.3 males to females. A larger percentage of minority patients was reported in the metropolitan areas (20.2%) than the states (8.9%). Seventy-seven percent of patients were non-Hispanic in both state and metropolitan areas. Reported patients were more likely to be white, male, non-Hispanic, and 50–79 years of age (Table II).
Table II. Demographic characteristics of ALS patients reported by project areas.
| Combined areas n = 5883 | States n = 3620 | Metropolitan areas n = 2263 | ||||
|---|---|---|---|---|---|---|
| # | % | # | % | # | % | |
| Age (in years) | ||||||
| < 30 | 75 | 1.3 | 37 | 1.0 | 38 | 1.7 |
| 30 – 39 | 262 | 4.4 | 159 | 4.4 | 103 | 4.6 |
| 40 – 49 | 748 | 12.7 | 452 | 12.5 | 296 | 13.1 |
| 50 – 59 | 1405 | 23.9 | 840 | 23.2 | 565 | 24.9 |
| 60 – 69 | 1698 | 28.9 | 1076 | 29.7 | 622 | 27.5 |
| 70 – 79 | 1238 | 21.0 | 794 | 21.9 | 444 | 19.6 |
| ≥ 80 | 403 | 6.9 | 237 | 6.6 | 166 | 7.3 |
| Unknown | 54 | 0.9 | 25 | 0.7 | 29 | 1.3 |
| Gender | ||||||
| Male | 3322 | 56.5 | 2047 | 56.5 | 1275 | 56.3 |
| Female | 2561 | 43.5 | 1573 | 43.5 | 988 | 43.7 |
| Race | ||||||
| White | 4401 | 74.8 | 2808 | 77.6 | 1593 | 70.4 |
| African American/black | 546 | 9.3 | 241 | 6.7 | 305 | 13.5 |
| Asian | 214 | 3.6 | 72 | 2.0 | 142 | 6.3 |
| Other | 18 | 0.3 | 8 | 0.2 | 10 | 0.4 |
| Unknown | 704 | 12.0 | 491 | 13.6 | 213 | 9.4 |
| Ethnicity | ||||||
| Hispanic | 634 | 10.8 | 410 | 11.3 | 224 | 9.9 |
| Non-Hispanic | 4562 | 77.5 | 2804 | 77.5 | 1758 | 77.7 |
| Unknown | 687 | 11.7 | 406 | 11.2 | 281 | 12.4 |
Of the 5883 unique patients reported, 3819 were diagnosed between 1 January 2009 and 31 December 2011. The overall incidence rate for the three-year period ranged from 1.42 to 1.62 per 100,000 person-years with an average annual incidence rate of 1.52 per 100,000 person-years, CI 1.44–1.61. Individual project incidence rates were only calculated for areas that collected at least 80% of the expected patients (nine of the 11 project areas). The individual project areas average annual incidence rate ranged from 0.98 to 1.98 per 100,000 person-years. In 2009, the prevalence rate was 3.58 per 100,000 population, CI 3.42–3.74 for the states, 4.28 per 100,000 population, CI 4.04–4.51 for metropolitan areas, and 3.84 per 100,000 population, CI 3.70–3.97 for both areas combined.
Diagnosis
Overall, 4846 (82.4%) of patients were reported as ‘definite’, ‘probable’, or ‘probable (lab supported)’ ALS according to the El Escorial criteria (12). Patients reported in the states were more likely to receive a classification of ‘definite’ ALS (55.7%) compared with metropolitan area reported patients (46.6%) (Table III).
Table III. El Escorial criteria of reported prevalent ALS patients – 1 January 2009 through 31 December 2011 by project areas.
| El Escorial criteria Classification | Combined areas | States | Metropolitan areas | |||
|---|---|---|---|---|---|---|
| # | % | # | % | # | % | |
| Definite | 3069 | 52.2 | 2016 | 55.7 | 1053 | 46.6 |
| Probable | 1295 | 22.0 | 750 | 20.7 | 545 | 24.1 |
| Probable (lab-supported) | 482 | 8.2 | 271 | 7.5 | 211 | 9.3 |
| Possible | 754 | 12.8 | 423 | 11.7 | 331 | 14.6 |
| Not classifiable | 283 | 4.8 | 160 | 4.4 | 123 | 5.4 |
| Total | 5883 | 100.0 | 3620 | 100.0 | 2263 | 100.0 |
Medical Record Verification Forms were requested from 967 (13.7%) of all reported case reports and 846 (87.5%) were received. Of those verified, approximately 80% were classified as El Escorial criteria ‘definite’, ‘probable’ or ‘probable (lab-supported)’ ALS. Only 15 (1.8%) were determined to not be ALS.
Time from symptom onset to diagnosis could be calculated for 5462 (92.8%) of the reported patients. Approximately 45% had symptoms for less than 12 months before diagnosis, and the time from symptom onset to diagnosis was similar in the states and metropolitan areas (Table IV).
Table IV. Time from symptom onset to diagnosis of reported ALS patients by project areas.
| Combined areas | States | Metropolitan areas | ||||
|---|---|---|---|---|---|---|
| # | % | # | % | # | % | |
| < 12 monthsa | 2588 | 44.0 | 1623 | 44.8 | 965 | 42.6 |
| 12-17 months | 1059 | 18.0 | 643 | 17.8 | 416 | 18.4 |
| ≥ 18 monthsb | 1815 | 30.9 | 1061 | 29.3 | 754 | 33.3 |
| Unknown | 421 | 7.1 | 293 | 8.1 | 128 | 5.7 |
| Total | 5883 | 100.0 | 3620 | 100.0 | 2263 | 100.0 |
aPatients with unknown month of diagnosis or month of symptom onset were included in the < 12 months category when the years were the same.
bPatients with missing month of onset symptom but year was present and were three years or more apart were placed in the ≥ 18-month category.
Familial ALS, dementia, and medical coverage
Data on family history of ALS were available for 5267 (89.5%) of the patients reported to the overall project. Approximately 4% (244) of the ALS patients were reported as having a family member with ALS. There was no difference in the percentage of patients reported with familial ALS between the states and metropolitan areas. A higher percentage of patients with unknown familial ALS status was reported from the states.
Information on the presence of dementia was available for 92.3% of the reported patients. Overall, dementia was reported for 413 (7.0%) patients. Patients reported from the states had a higher percentage of patients reported with dementia, whereas the percentage of patients reported with unknown dementia status was consistent between the states and metropolitan areas.
Of all reported patients, 3630 (61.7%) identified at least one federal payer (Medicare, Medicaid, or Veterans Health Administration), 2188 (37.2%) reported no federal payer (HMO, no insurance, or self-pay), and medical coverage was unknown for 1.1% of reported patients. Fewer patients reported from metropolitan areas had any federal payer compared with the patients reported from the states.
Discussion
This project collected data on the largest number of clinically reviewed ALS patients in the U.S. to date. The overall project areas represented more than one quarter (27.1%) of the U.S. population (14). Compared with the U.S., the overall project population had similar gender and age distributions and over-represented racial and ethnic minorities.
Most neurologists contacted did not diagnose or treat ALS patients. Almost twice as many neurologists saw ALS patients in states compared with metropolitan areas. In addition, in the metropolitan areas, a larger percentage (87.5%) of ALS patients were cared for at ALS specialty centers typically seeing 50 or more ALS patients per year compared with 70.1% in the states. This could be because ALS patients in metropolitan areas lived closer to ALS specialty centers.
Only 59.5% of neurologists who reported seeing an ALS patient during the surveillance period submitted cases. We do not know how many of the remaining neurologists actually had cases to report, how many cases they might have had, or if these were unique cases not already reported by another neurologist. Most of the neurologists who did not report cases were from three of the project areas and two of those areas received more than 90% of the expected case reports, and all the large specialty clinics in these areas participated. Those who did not report were most likely from small practices with few if any cases, therefore we do not believe this biased our results.
Reported patients were more likely to be older, white, and non-Hispanic. The states had a slightly higher percentage of white patients reported compared with the metropolitan areas, and the metropolitan areas had higher percentages of African-American/black and Asian patients reported. The selection of metropolitan areas to over-represent minority populations, and therefore having more minorities, might explain the racial differences between reported patients in the two areas. Age distribution was similar in the states and metropolitan areas, where the percentage of patients increased in each age category until ages 60–69 years. There was a male predominance and no major differences in the distribution of male and female patients between the states and metropolitan areas. The ratio of males to females was 1.3:1, which is consistent with the ratio found in current literature (2,18–20). Overall demographic characteristics were similar to those of previously published literature (1,2,6,21).
We found an average annual incidence rate of 1.52 per 100,000 person-years with a range of 1.42–1.62 per 100,000 person-years, which is consistent with the worldwide estimates of 1.6–2.5 per 100,000 person-years (1,2,7,20,22,23). Incidence estimates in the states were slightly higher than the metropolitan areas. This difference might be because of a larger minority population in the metropolitan areas who have been shown to have lower rates of ALS than whites (3–5). The prevalence rate in 2009 for the states and metropolitan areas was 3.58 and 4.28 per 100,000 population, respectively. This difference might be because of the larger minority population in the metropolitan areas who have been shown to have longer median survival time (24). The overall prevalence rate for the areas combined was 3.84 per 100,000 population which is consistent with current literature (6,7,9).
For persons in this study with known dates of symptom onset and diagnosis, the mean time from symptom onset to diagnosis was 18 months and the median time was 12 months. There was a slight difference in time from symptom onset to diagnosis for patients reported between the states and metropolitan areas. When comparing patients with symptom onset to diagnosis of 12 months or less and those with symptom onset to diagnosis of greater than 12 months, there was no difference between the groups related to age at diagnosis, gender, race, or ethnicity. One study reported 15.2 months as the mean duration of time from symptom onset to diagnosis (22). Several studies have reported a median duration as short as 10–11 months (23,25,26). While the reported time from symptom onset to diagnosis varies by study, it was never less than eight months and has remained stable (25). Time from symptom onset to diagnosis in this study might be related to the number of ALS patients who are diagnosed and/or seek diagnosis confirmation from ALS specialty centers.
The diagnosis of ALS is complex and the absence of a diagnostic test for the disease coupled with subtle symptom onset can delay diagnosis (25). More than 84% of reported patients were classified into ‘definite’, ‘probable’, or ‘probable (lab supported)’ El Escorial criteria categories and neurologists agree that these individuals have ALS. A greater proportion of patients was classified as ‘definite’ in the states compared with the metropolitan areas. It is possible that more general neurologists delayed diagnosis until symptoms progressed to ‘definite’. This may have resulted in those persons in the states being diagnosed at a later stage of disease allowing an El Escorial criteria classification of ‘definite’.
Multiple case reports for the same person with ALS were accepted from different practices and composite records were created before finalizing the data. It is possible that the records contained different patient information that, if merged differently, may have slightly impacted the categorical variables used to describe reported time from symptom onset to diagnosis and the reported El Escorial criteria classification. We do not believe the creation of composite records systematically biased the findings.
Familial ALS has generally been estimated at 5–10% of ALS patients (27,28). Our findings show an overall rate of familial ALS of 4.1%, although information was not available for 10.5%. These findings are consistent with more recent studies reporting the rate of familial ALS to be 3.7–5.1% among first degree relatives (29,30).
The scientific community has attempted to come to consensus regarding the definition of cognitive and behavioral impairments, including dementia, among ALS patients (31), making it difficult to estimate rates of comorbidities. In this project there was a difference in the rates of dementia reported in ALS patients between the states (8.3%) and metropolitan areas (5.0%). Overall, the rate was 7.0% for combined states and metropolitan areas, which is lower than previously published findings for dementia (range 10–15%) (32–34) and much lower than reported rates of cognitive impairment (35). This difference is most likely because information was taken from medical records review rather than clinical assessment.
Almost 62% of all the patients reported had at least one federal payer for insurance. This is consistent with the National ALS Registry, which estimated that nearly two-thirds of ALS patients will be captured through the federal health administrative data sets. In the National ALS Registry pilot project, the percentage of ALS patients covered by a federal payer ranged from 78% to 100% (11). This is significantly higher than the 39% of ALS patients eligible for the ALS COSMOS study having Medicare, Medicaid, or VA benefits (36). This difference might be due to changes in eligibility for Medicare and Veterans benefits for those diagnosed with ALS.
To address the concern that ALS is difficult to diagnose (37), and neurologists do not always agree, a sample of case reports was reviewed by the project consulting neurologist to verify diagnosis. Case reports selected for verification were weighted towards smaller practices. Completed MRVFs were obtained for approximately 12% of all case reports. The majority of case verifications requested were received and classified as ALS by both the reporting and consulting neurologists. The differences between the El Escorial criteria assigned by the reporting neurologist compared with the consulting neurologist could be because of being evaluated at different points in time, i.e. the person's ALS may not yet have progressed when first reported.
A very small percentage of patients was determined to be ‘Not ALS’, most likely the result of not enough documentation available to support an ALS diagnosis. This demonstrates that most patients determined to have ALS by reporting neurologists, regardless of specialty, were ALS patients.
Conclusions
This study represents the largest number of clinically diagnosed ALS patients reported by neurologists in the U.S. to date. In addition, the study was designed to over-represent minority populations. A few differences observed between state and metropolitan area results might be due to the large number of minorities in the metropolitan areas because minorities have been reported to have a lower rate of ALS compared with whites. Despite the over-sampling and differences between state and metropolitan area results, incidence, prevalence, and demographic characteristics of ALS patients are largely consistent with worldwide published literature.
This effort was time-consuming, labor-intensive, costly, and may not be feasible as an ongoing surveillance effort for other areas. These data will be used by ATSDR to identify data gaps in the National ALS Registry and help focus recruitment activities.
Acknowledgements
The authors thank the state and local health departments and other organizations that assisted with data.
This project was funded by the Agency for Toxic Substances and Disease Registry.
Declaration of interest: The authors declare no conflicts of interest. The conclusions of this article are those of the authors and do not necessarily represent the views of ATSDR, CDC, or the U.S. Department of Health and Human Services.
References
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the ‘common’ neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Chio A, Logrosino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41:118–30. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007;68:1002–7. doi: 10.1212/01.wnl.0000258551.96893.6f. [DOI] [PubMed] [Google Scholar]
- Gundogdu B, Al-Lahham T, Kadlubar F, Spencer H, Rudnicki SA. Racial differences in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:114–8. doi: 10.3109/21678421.2013.837930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O. Reduced frequency of ALS in an ethnically mixed population: a population based mortality study. Neurology. 2009;72:1640–5. doi: 10.1212/WNL.0b013e3181a55f7b. [DOI] [PubMed] [Google Scholar]
- Wagner L, Archer NP, Williamson D, Henry JP, Schiffer R, Jackson CE. Prevalence of amyotrophic lateral sclerosis in Texas, 1998–2003. Tex Med. 2012:108, e1. [PubMed] [Google Scholar]
- Turabelidze G, Zhu B, Schootman M, Malone J, Horowitz S, Weidinger J, et al. An epidemiological investigation of amyotrophic lateral sclerosis in Jefferson County, Missouri, 1998–2002. Neurotoxicology. 2008;29:81–6. doi: 10.1016/j.neuro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925–1998. Neurology. 2002;59:280–2. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis – United States, 2010–2011. MMWR Surveill Summ. 2014;63 ((Suppl 7)):1–14. [PubMed] [Google Scholar]
- Antao VC, Horton DK. The National Amyotrophic Lateral Sclerosis (ALS) Registry. J Environ Health. 2012;75:28–30. [PMC free article] [PubMed] [Google Scholar]
- Kaye WE, Sanchez M, Wu J. Feasibility of creating a National ALS Registry using administrative data in the United States. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:433–9. doi: 10.3109/21678421.2014.887119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: Revised criteria for the diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- World Health Organization International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) Geneva: World Health Organization. 1992 [Google Scholar]
- U.S Census Population estimates. http://www.census.gov/popest/data/national/totals/2013/index.html 2014
- Microsoft Excel [computer program] 2010 [Google Scholar]
- IBM SPSS Statistics for Windows 2010 [Google Scholar]
- Gardner MJ, Altman DG. Confidence intervals rather than p-values: estimation rather than hypothesis testing. Br Med J (Clin Res Ed) 1986;292:746–50. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwerse ES, Visser CE, Bossuyt PM, Weverling GJ. The Netherlands ALS Consortium Amyotrophic lateral sclerosis: mortality risk during the course of the disease and prognostic factors. J Neurol Sci. 1997;152((Suppl 1)):S10–7. doi: 10.1016/s0022-510x(97)00238-4. [DOI] [PubMed] [Google Scholar]
- Logrosino G, Traynor B, Hardiman O, Chio A, Mitchell D, Swingler R, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81:385–90. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire V, Longsreth WT, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington State. Neurology. 1996;47:571–3. doi: 10.1212/wnl.47.2.571. [DOI] [PubMed] [Google Scholar]
- Ragonese P, Cellura E, Aridon P, D'amelio M, Spartaro R, Taiello AC, et al. Incidence of amyotrophic lateral sclerosis in Sicily: a population based study. Amyotroph Lateral Scler. 2012;13:284–7. doi: 10.3109/17482968.2012.662689. [DOI] [PubMed] [Google Scholar]
- Murphy M, Quinn S, Young J, Parkin P, Taylor B. Increasing incidence of ALS in Canterbury, New Zealand: a 22-year study. Neurology. 2008;71:1889–95. doi: 10.1212/01.wnl.0000336653.65605.ac. [DOI] [PubMed] [Google Scholar]
- Jordan H, Fagliano J, Rechtman L, Lefkowitz D, Kaye W. Effects of Demographic Factors on Survival Time after a Diagnosis of Amyotrophic Lateral Sclerosis. Neuroepidemiology. 2015;44:114–20. doi: 10.1159/000380855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellura E, Spataro R, Taiello A, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clinical Neurology and Neurosurgery. 2012;114:550–4. doi: 10.1016/j.clineuro.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Khishchenko N, Allen K, Coffman C, Kasaeskis E, Lindquist J, Morgenlander J, et al. Time to diagnosis in the National Registry of Veterans with Amyotrophic Lateral Sclerosis. Amyotroph Lateral Scler. 2010;11:125–32. doi: 10.3109/17482960802572681. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population based study. Arch Neurol. 2000;57:1171–6. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- O’Toole O, Traynor BJ, Brennan P, Sheehan C, Frost E, Corr B, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008;79:30–2. doi: 10.1136/jnnp.2007.117788. [DOI] [PubMed] [Google Scholar]
- Byrne S, Walsh C, Lynch C, Bede P, Elamin M, Kenna K, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:623–7. doi: 10.1136/jnnp.2010.224501. [DOI] [PubMed] [Google Scholar]
- Gibson SB, Figueroa KP, Bromberg MB, Pulst SM, Cannon-Albright L. Familial clustering of ALS in a population based resource. Neurology. 2014;82:17–22. doi: 10.1212/01.wnl.0000438219.39061.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong M, Grace G, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein L, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–46. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Gordon P, Delgadillo D, Piquad A, Bruneteau G, Pradat P, Salachas F, et al. The range and clinical impact of cognitive impairment in French patients with ALS: a cross-sectional study of neuropsychological test performance. Amyotroph Lateral Scler. 2011;12:372–8. doi: 10.3109/17482968.2011.580847. [DOI] [PubMed] [Google Scholar]
- Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2012;83:102–8. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- Ringholz G, Appel S, Bradshaw M, Cooke N, Mosnik D, Schulz P. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65:586–90. doi: 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168–73. doi: 10.1136/jnnp-2013-307223. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Factor-Litvak P, Andrews H, Goetz RR, Andrews L, Rabkin JG, et al. ALS COSMOS Study Group ALS Multicenter Cohort Study of Oxidative Stress (ALS COSMOS): study methodology, recruitment, and baseline demographic and disease characteristics. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:192–203. doi: 10.3109/21678421.2013.864312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsh JM. Diagnostic challenges in ALS. Neurology. 1999;53((Suppl 5)):S26–30. [PubMed] [Google Scholar]