South-western Australia harbours a biodiversity hotspot on severely phosphorus-impoverished soils. Threats include eutrophication due to phosphorus enrichment, due to increased fire frequency and spraying with phosphite to reduce the impacts of the introduced pathogen Phytophthora cinnamomi. We propose a strategy to work towards alternatives to phosphite for pathogen management.
Keywords: Cluster roots, eutrophication, non-mycorrhizal plants, phosphite, Phytophthora cinnamomi, Proteaceae
Abstract
South-western Australia harbours a global biodiversity hotspot on the world's most phosphorus (P)-impoverished soils. The greatest biodiversity occurs on the most severely nutrient-impoverished soils, where non-mycorrhizal species are a prominent component of the flora. Mycorrhizal species dominate where soils contain slightly more phosphorus. In addition to habitat loss and dryland salinity, a major threat to plant biodiversity in this region is eutrophication due to enrichment with P. Many plant species in the south-western Australian biodiversity hotspot are extremely sensitive to P, due to a low capability to down-regulate their phosphate-uptake capacity. Species from the most P-impoverished soils are also very poor competitors at higher P availability, giving way to more competitive species when soil P concentrations are increased. Sources of increased soil P concentrations include increased fire frequency, run-off from agricultural land, and urban activities. Another P source is the P-fertilizing effect of spraying natural environments on a landscape scale with phosphite to reduce the impacts of the introduced plant pathogen Phytophthora cinnamomi, which itself is a serious threat to biodiversity. We argue that alternatives to phosphite for P. cinnamomi management are needed urgently, and propose a strategy to work towards such alternatives, based on a sound understanding of the physiological and molecular mechanisms of the action of phosphite in plants that are susceptible to P. cinnamomi. The threats we describe for the south-western Australian biodiversity hotspot are likely to be very similar for other P-impoverished environments, including the fynbos in South Africa and the cerrado in Brazil.
Introduction
South-western Australia harbours a global biodiversity hotspot, where exceptional numbers of endemic species are undergoing exceptional loss of habitat (Myers et al., 2000; Myers, 2001; Hopper and Gioia, 2004). Due to the old age of the Australian continent, the relative stability of its geology, and the fact that most of Australia was last glaciated over 250 million years ago and has been above sea level for 145 million years (Miller et al., 2005), many Australian soils are severely nutrient impoverished, and are especially low in phosphorus (P; Beadle, 1953). The most extremely P-impoverished soils occur in the region of the south-western biodiversity hotspot (Lambers et al., 2010, 2012; Laliberté et al., 2012; Fig. 1).
Figure 1:
Plants and plant communities in south-western Australia's global biodiversity hotspot. The photographs are described from top left to bottom right. Banksia grandis (Proteaceae), an iconic species in banksia woodlands and jarrah forest which is very sensitive to elevated soil phosphate levels and susceptible to Phytophthora cinnamomi. The tree provides a rich source of nectar for birds, honey possums, and insects. A plant community on sandy soil in Lesueur National Park, showing red-flowering Verticordia grandis (Myrtaceae) and yellow-flowering Stirlingia latifolia (Proteaceae), amongst numerous other species. Low shrub on rocky substrate in Lesueur National Park. Woodland, with Eucalyptus gomphocephala (Myrtaceae) on sandy soil lower in the landscape, in Lesueur National Park. Eucalyptus diversicolor (Myrtaceae) in karri forest in the high-rainfall region of the far south-west in Western Australia. Eucalyptus marginata (Myrtaceae) in jarrah forest with a species-rich understorey. In seasonally wet habitats, numerous carnivorous species are found, including Utricularia menziesii (Lentibulariaceae). The greatest biodiversity is found on very sandy soil; the surface soil may not reveal the substrate underneath, but activities of ants provide information of the nature of the soil immediately below the surface. Photographs by Marion Cambridge and Hans Lambers.
In Western Australia's biodiversity hotspot, the greatest plant diversity is found on the most severely P-impoverished, sandy soils (Lamont et al., 1977; Lambers et al., 2010; Fig. 2). A similar pattern of increasing plant diversity with lower soil P status has been found for nutrient-poor habitats in Royal National Park, in New South Wales, in eastern Australia, one of the first sites in the world set aside to become a national park in 1879 (Adam et al., 1989; Le Brocque and Buckney, 2003). This increase in plant diversity with lower soil fertility most probably reflects a global pattern (Huston, 1994; Grime, 2002; Wardle et al., 2004; Laliberté et al., 2013). For example, several long-term soil chronosequences worldwide show increases in local plant species richness with soil age, which is associated with lower soil [P] (Wardle et al., 2008; Laliberté et al., 2013). These patterns raise the possibility that P limitation per se might promote the coexistence of plant species (Olde Venterink, 2011). This may be related to the fact that P occurs in soil in many chemical forms and that several different P-acquisition strategies exist that can target specific forms (Lambers et al., 2006, 2008).
Figure 2:
Plant diversity and soil phosphorus (P) status in south-western Australia's global biodiversity hotspot. Note the relative abundance of non-mycorrhizal species on soils with the lowest phosphorus content. Modified after Lambers et al. (2010).
One important P-acquisition strategy is the symbiotic association with mycorrhizal fungi, which can enhance a plant's ability to acquire P from low-P soil (Lambers et al., 2008; Smith and Read, 2008). Although mycorrhizal plant species occur in P-impoverished soils from south-western Australia, non-mycorrhizal species are particularly successful on the most P-impoverished soils (Lambers et al., 2006, 2010). This is explained by the ‘mining’ strategy of the non-mycorrhizal species, as opposed to a ‘scavenging’ strategy of mycorrhizal species (Lambers et al., 2008), as detailed below. As a consequence, a significant proportion of south-western Australia's exceptionally rich plant biodiversity is represented by non-mycorrhizal species that show special adaptations to acquire P (Lambers et al., 2010). Most of these species are found nowhere else on Earth (Hopper and Gioia, 2004), and therefore deserve special conservation attention (Hopper, 2009). In order best to conserve this important element of the south-western Australian flora, it is necessary to understand the P nutrition of these species in order to evaluate better the threats that they face. In this review, we first describe the adaptations exhibited by non-mycorrhizal species that allow them to grow successfully in P-impoverished soils. We then describe how many of these species show high sensitivity to P, leading to P toxicity. Finally, we discuss two major threats faced by these species, namely eutrophication through P fertilization, and the disease caused by the introduced soil-borne plant pathogen Phytophthora cinnamomi (Coates and Atkins, 2001), which is managed by large-scale application of phosphite, a more reduced form of P that can be oxidized to phosphate (Table 1).
Table 1:
Two major threatening processes affect the biodiversity in the south-western Australian biodiversity hotspot and similar severely P-impoverished regions elsewhere in the world
| Threatening process | Cause(s) | Physiological studies | Management options |
|---|---|---|---|
| Eutrophication due to increased soil phosphorus concentrations | Increased fire frequency | Revealing the cause of extreme P sensitivity of some Australian species: low capacity to down-regulate P uptake | Reduce frequency of controlled burning |
| Competition among native species | Temporal monitoring of composition of the vegetation community | ||
| Weed invasion as dependent on soil nutrient availability | Vegetation monitoring and eradication strategies in sensitive areas | ||
| Fire suppressants | Effects on seed germination and persistence of P in soil | Replace P-containing suppressants by ones that do not contain P | |
| Storm water run-off from road verges and agricultural land | Seedling performance as dependent on P availability | Redirect storm water | |
| Dust from roads | Seedling performance as dependent on P availability | Use building material with minimal P content | |
| Application of phosphite to combat P. cinnamomi | Assess whether slow-growing P-efficient species are replaced by less P-efficient faster-growing ones | Replace P-containing fungicide (phosphite) by ones that do not contain P | |
| Phytophthora cinnamomi | Introduction of an alien pathogen with a very large host range, particularly in the largely P-sensitive Proteaceae | Assess life cycle and performance of P. cinnamomi | Containment/eradication: (i) management of water flows to prevent water movement across the landscape from infested areas to disease-free areas; (ii) use of soil fumigants to treat spot infestations; and (iii) use of herbicides and total plant removal (fallow soils) for 2–3 years to starve the pathogen of living host tissues |
| Spread of the pathogen by humans and feral animals | Mechanisms of phosphite action to boost the plant's immune system | Replace P-containing fungicide (phosphite) by ones that do not contain P | |
| Identification of most vulnerable plant communities; prioritization of research to those species and habitats | Use of measures to stop further spread of the pathogen |
Several factors can lead to eutrophication, some of which are discussed in detail. Physiological studies allow a better understanding of plant responses to threatening processes. These should ultimately guide management options with conservation outcomes. The physiological studies that are listed are discussed in the main text.
Adaptations to extremely phosphorus-impoverished soils
A plant family that is richly represented on severely P-impoverished soils in south-western Australia and similar landscapes in South Africa is the Proteaceae (Cowling and Lamont, 1998). Proteaceae species occur as major ecosystem components, often as keystone species. They are evergreen woody plants, ranging in size from prostrate shrubs to trees (Cavanagh and Pieroni, 2006; Collins et al., 2008). After fires, Proteaceae species regenerate either by sprouting epicormic shoots or by releasing seed from woody follicles (Lamont and Markey, 1995). Proteaceae species contribute prominently to ecosystem functioning, such as fixation of carbon, provision of an architectural structure or habitat, and sharing and recycling of resources to minimize net nutrient loss, especially, from the system (Shearer et al., 2009, 2012). Flowers provide food for birds, mammals, and insects (Hopper, 1980; Whelan and Burbidge, 1980; Ladd et al., 1996; Cavanagh and Pieroni, 2006; Collins, 2008).
Most of the species in the Proteaceae family produce proteoid roots (Purnell, 1960) and almost all of them are non-mycorrhizal (Shane and Lambers, 2005a). An exception is the cluster-rooted mycorrhizal species Hakea verrucosa, which grows on ultramafic rocks that are rich in nickel (Boulet and Lambers, 2005). The release of carboxylic acids from its cluster roots would increase the solubility of nickel and harm the plants; in addition, mycorrhizal associations may protect plants to some degree against metal toxicity (e.g. Schutzendubel and Polle, 2002; Christophersen et al., 2009). Proteoid roots are dense clusters of rootlets of determinate growth, which develop numerous root hairs (Lamont, 2003; Shane and Lambers, 2005a). Given that proteoid roots are not restricted to Proteaceae, the term ‘cluster roots’ is commonly used as an alternative (Shane and Lambers, 2005a). Cluster roots release vast amounts of carboxylates in an exudative burst (Watt and Evans, 1999; Shane et al., 2004a); the carboxylates mobilize P that is sorbed onto soil particles, making it available for uptake by plant roots (Lambers et al., 2006). Therefore, cluster roots effectively mine P that is unavailable for plants lacking this strategy. Outside the Proteaceae, cluster roots in Australia are found in Casuarinaceae (Reddell et al., 1997), Fabaceae (Lamont, 1972), and Restionaceae (Lambers et al., 2006). Cluster-root formation is suppressed in the presence of a high P supply (Watt and Evans, 1999; Shane and Lambers, 2005a), highlighting their important role for P uptake.
Dauciform (i.e. carrot-shaped) roots occur in some tribes of the non-mycorrhizal family Cyperaceae (Davies et al., 1973; Lamont, 1974; Shane et al., 2006b), another common family on nutrient-impoverished soils. Dauciform roots were probably first recognized for Schoenus ferrugineus (Cyperaceae) by Renner (1935), who thought they were a mycorrhizal structure (Pilzwurzel). Thereafter, they were described by Russian researchers (Selivanov and Utemova, 1969). Dauciform roots are morphologically very different from cluster roots, but functionally quite similar; they also release vast amounts of carboxylates in an exudative burst (Playsted et al., 2006; Shane et al., 2006a), and are suppressed in the presence of a high P supply (Shane et al., 2006a).
Large amounts of carboxylates can also be exuded in the absence of specialized structures such as cluster roots and dauciform roots, e.g. in a range of Kennedia species (Fabaceae) from south-western Australia (Ryan et al., 2012). These species without cluster roots differ vastly in the amount of carboxylates they release, but what they have in common is that their carboxylate exudation is reduced in the presence of arbuscular mycorrhizal fungi, which colonize the roots of some Kennedia species (Ryan et al., 2012). This suggests a trade-off in plant carbon allocation for P acquisition to either mycorrhizal fungi or carboxylate release.
Manganese (Mn) accumulation is common in Proteaceae (Jaffré, 1979; Shane and Lambers, 2005b; Rabier et al., 2007; Fernando et al., 2010), as well as in Fabaceae with cluster roots, e.g. Lupinus albus (Gardner et al., 1982) and Aspalathus linearis (Morton, 1983). This is explained by the ability of their cluster roots to mobilize Mn (Gardner et al., 1981; Grierson and Attiwill, 1989; Dinkelaker et al., 1995). Leaf [Mn] might therefore provide an indication of the extent to which roots depend on carboxylate release to acquire P (Shane and Lambers, 2005b). Following this reasoning, we found that cluster-rooted Proteaceae and dauciform-rooted Cyperaceae along a 2 million year chronosequence (Laliberté et al., 2012) have higher leaf [Mn] than their neighbours without any of the carboxylate-releasing strategies discussed above, regardless of soil age and P status (P. Hayes, E. Laliberté and H. Lambers, unpublished observations). We also found the same phenomena in non-mycorrhizal species that produce sand-binding roots. It is therefore likely that the functional significance of sand-binding roots (Shane et al., 2011; Smith et al., 2011) within an ecosystem is also, at least partly, that of mobilizing P through carboxylate release, but this requires further research.
The high abundance of Proteaceae in severely P-impoverished landscapes is not accounted for exclusively by their very efficient P-acquisition strategy. In addition, at least three other traits determine their P efficiency. First, their P-use efficiency in photosynthesis is amongst the highest ever recorded (Wright et al., 2004; Denton et al., 2007), which is partly accounted for by extensive replacement of phospholipids by lipids that do not contain P during leaf development (Lambers et al., 2012). However, the decline in leaf P concentration during leaf development must also involve other metabolic changes, because the replacement of phospholipids is too small to explain the decrease entirely. Second, their long-lived leaves are very efficient and proficient at remobilizing P during leaf senescence (Denton et al., 2007), which greatly increases the mean residence time of P within the plant. Third, their seeds, unlike their vegetative tissues, contain very high concentrations of P (Denton et al., 2007; Groom and Lamont, 2010), allowing seedlings to grow without an external P source for a prolonged period (Milberg and Lamont, 1997).
Phosphate sensitivity
Whilst Proteaceae and several other species that are endemic to south-western Australia and similar P-impoverished landscapes are very good at acquiring P and using it efficiently, many are extremely sensitive to P and readily display P-toxicity symptoms (Handreck, 1991; Parks et al., 2000; Shane et al., 2004b; Standish et al., 2007; Hawkins et al., 2008). Even a slight increase in the low P concentration that is common in their natural environment is enough to disturb their growth severely, and may cause death. However, whilst it is relatively common in P-impoverished landscapes, P sensitivity is by no means universal among species from P-impoverished habitats, and even some Proteaceae species are insensitive to elevated P supply, e.g. Grevillea crithmifolia (Shane and Lambers, 2006). The physiological mechanism accounting for P toxicity in higher plants is a low capacity to down-regulate their P uptake (Shane et al., 2004c; Shane and Lambers, 2006; de Campos et al., 2013). A low capacity to down-regulate P uptake is associated with a high capacity to remobilize P from senescing leaves, and vice versa (de Campos et al., 2013). Whether this association is based on a mechanistic link involving the regulation of P transporters has yet to be explored.
Phosphorus enters the plant as inorganic phosphate (Pi). Plants have both high-affinity and low-affinity mechanisms for the transport of Pi across the plasma membrane and into the cytosol against a steep electrochemical potential gradient (Cogliatti and Clarkson, 1983; Drew and Saker, 1984; Drew et al., 1984). The high-affinity transport is facilitated by Pi transport proteins of the PHT1 family (Muchhal et al., 1996). The proteins responsible for the low-affinity acquisition of Pi are unknown, but characterization mostly in heterologous systems suggests that they may also be PHT1 proteins (Harrison et al., 2002; Rae et al., 2003; Ai et al., 2009; Preuss et al., 2010). Caution, however, may be needed in drawing conclusions about Pi affinity determined in heterologous systems (Nussaume et al., 2011). Once inside the cell, Pi is transported into various compartments by proteins of the PHT2, PHT3, and PHT4 families. It is widely accepted that Pi is moved through the plant body by a variety of PHT1 proteins. While all PHT1 proteins that have been examined are able to transport Pi into cells in normal physiological conditions, only some may be able to release Pi from cells, and then only in specific conditions, such as during senescence (Preuss et al., 2010, 2011).
Every plant species that has been examined has multiple genes encoding high-affinity PHT1 proteins. Arabidopsis has nine PHT1 genes, while there are 13 PHT1 genes in rice. The exact number of PHT1 genes in the P-sensitive Australian Hakea prostrata (Proteaceae) is unknown, but it is not atypical compared with these model plants (R. Jost, A. B. Siddique, B. Mirfakhraei, R. Pontré, H. Lambers and P.M. Finnegan, unpublished observations). Each PHT1 gene has its own specific expression pattern (Karthikeyan et al., 2002; Mudge et al., 2002; Misson et al., 2004; Xiao et al., 2006; Morcuende et al., 2007; Nussaume et al., 2011). Most PHT1 genes are strongly expressed in roots, but are also expressed in other cell types, tissues, and organs, with overlapping expression patterns among the members of the family (Nussaume et al., 2011). The four PHT1 genes of H. prostrata that have been characterized so far appear to follow these general expression trends (R. Pontré, B. Mirfakhraei, R. Jost, H. Lambers and P. M. Finnegan, unpublished observations).
A hallmark of the expression of nearly all PHT1 genes is that their transcript abundance is repressed when tissues are exposed to high Pi and quickly derepressed when tissues are deprived of Pi (Karthikeyan et al., 2002; Mudge et al., 2002; Morcuende et al., 2007; Nussaume et al., 2011). This response to Pi availability provides an attractive mechanistic hypothesis for the low capacity of some native Australian plants to down-regulate their P uptake (Shane et al., 2004c; Shane and Lambers, 2006; de Campos et al., 2013). It may be that specific PHT1 genes are not repressed by a sudden influx of Pi or that Pi exerts a positive effect on PHT1 expression. Alternatively, PHT1 proteins present on the plasma membrane of specific cell types may not be removed from the membrane efficiently when Pi becomes more available. A putative repressor of PHT1 function, acting either directly or indirectly via PHO1, is the E2 ubiquitin conjugase PHO2 (Liu et al., 2012). Remarkably, pho2 mutants in Arabidopsis thaliana display a higher Pi uptake capacity than wild-type plants, leading to hyper-accumulation of Pi in leaves, resembling P-toxicity symptoms described for Proteaceae species (Dong et al., 1998). This raises the possibility that plants adapted to very low P availability may not need this rather expensive module governing the down-regulation of Pi uptake. The finding that PHO2 is still expressed in H. prostrata (R. Jost, P. M. Finnegan and H. Lambers, unpublished observations) does not necessarily contradict this statement, because PHO2 seems to have functions other than the repression of PHO1 and Pi transport (as well as PHT1 transporters; Liu et al., 2012). Further research is needed to understand the relative contribution of these processes to Pi toxicity in P-sensitive plants.
Each PHT1 gene may have a specific function. In Arabidopsis, AtPHT1;1 and AtPHT1;4 are responsible for the bulk of Pi acquisition from the soil solution (Misson et al., 2004; Shin et al., 2004). Interestingly, AtPHT1;4 transcript accumulation is not very responsive to changes in Pi availability (Woo et al., 2012). This is also the case for rice OsPHT1;1, which is constitutively expressed in both root and shoot tissues. This transporter is responsible for the bulk of Pi uptake and translocation in rice during P-replete conditions and is still under the control of OsPHO2, but not OsPHR2 (Sun et al., 2012). These findings indicate that there may be different regulatory pathways involved for inducible vs. bulk transport of Pi. In Proteaceae species, a similar uncoupling of PHT1 expression from the P-starvation response mediated via the MYB transcription factor PHR1 could have taken place. Other transporters, such as AtPHT1;5, and perhaps the low-affinity barley HvPHT1;6, have an important role in the source-to-sink mobilization of Pi from senescing leaves (Huang et al., 2011; Nagarajan et al., 2011). Some PHT1 genes are necessary for the rapid transport of Pi from the root to the shoot (H. Gaza, R. Jost and P. M. Finnegan, unpublished observations). In plants that form mycorrhizal associations, specific sets of PHT1 genes are induced to transport Pi from the mycorrhizal partner to the plant (Smith and Barker, 2002; Javot et al., 2007; Smith and Read, 2008; Pumplin and Harrison, 2009; Kobae and Hata, 2010; Loth-Pereda et al., 2011). At the same time, other PHT1 genes responsible for the acquisition of Pi from the bulk soil solution are repressed. This same response is observed during mycorrhization in the P-sensitive Australian Eucalyptus marginata (Myrtaceae; K. Kariman, R. Jost, S. J. Barker, P. M. Finnegan and M. Tibbett, unpublished observations).
Species adapted to moderately phosphorus-impoverished soils
Most species in Australia's biodiversity hotspot have the capacity to establish a mycorrhizal symbiosis (Brundrett, 2009), but mycorrhizal species do not become dominant on the most severely P-impoverished soils (Lambers et al., 2010). Their scavenging strategy allows them to acquire poorly mobile nutrients, such as P, that are at some distance from the root surface or in pores in the soil that are too small for root hairs to penetrate (Smith and Read, 2008), provided P is readily available and not strongly sorbed (Parfitt, 1979). Nevertheless, species that lack cluster roots or similar root specializations do co-occur with dominant Proteaceae (Lambers et al., 2006, 2010), which do not form mycorrhizal associations (Shane and Lambers, 2005a). This may highlight the advantage for a plant to invest carbon to produce cluster roots, rather than investing carbon in an arbuscular mycorrhizal (AM) symbiosis in low-P soils. Furthermore, plants do not produce their roots in isolation, but rather interact with each other through them. However, the nature and outcome of this interaction for P uptake are unknown. The greater efficiencies in acquiring soil P by cluster-rooted Proteaceae could ultimately lead to a competitive exclusion of neighbouring mycorrhizal species. In addition, cluster-rooted Proteaceae may release a range of compounds that inhibit microbial activity and hence restrict mycorrhization, as found in L. albus (reviewed by Lambers et al., 2013). However, as previously noted, total exclusion of mycorrhizal species does not occur on severely nutrient-impoverished soils, because many plant species co-occur without any obvious nutrient or growth deficiencies (Fig. 2).
While there are many possible explanations for why species of contrasting nutrient-acquisition strategies coexist in ancient, P-impoverished soils (Laliberté et al., 2013), one of these is fitness equalization through resource sharing. For example, facilitation of P uptake has been demonstrated between agricultural cluster-rooted and AM species. Indeed, both P uptake and biomass are increased when wheat (Triticum aestivum) is intercropped with white lupin, due to the ability of the latter species to mobilize P with citric acid exuded by its cluster roots (Horst and Waschkies, 1987; Cu et al., 2005). Phosphorus uptake from soil is also increased in AM wheat intercropped with chickpea (Cicer arietinum) growing on organic sources of P, and this is attributed to the release of acid phosphatases by chickpea (Li et al., 2004). With regard to plant species coexistence in south-western Australian P-impoverished soils, this raises the following question: is there a potential facilitation of Pi uptake between cluster roots and the roots of a neighbouring AM plant, through the release of carboxylates, ribonucleases, and/or phosphatases? If so, then facilitation by plants that release carboxylates, ribonucleases, and/or phosphatases may slow down competitive exclusion of mycorrhizal species. We note that such fitness equalization would act as an ‘equalizing mechanism’ (sensu Chesson, 2000), because it would not, by itself, be sufficient to lead to stable coexistence. However, other stabilizing mechanisms that reduce inter-specific over intra-specific competition (sensu Chesson, 2000), such as partitioning of soil P between species of different nutrient-acquisition strategies (Turner, 2008; Lambers et al., 2010), could also operate simultaneously and therefore lead to stable coexistence.
In pot experiments in a glasshouse study, Muler et al. (2013) found evidence for facilitation of the growth of ectomycorrhizal Scholtzia involucrata (Myrtaceae) by the cluster-rooted Banksia attenuata (Proteaceae). Interestingly, the positive effect of B. attenuata was not due to the mobilization of P, because addition of P did not enhance the growth of S. involucrata. Rather, the results showed facilitation of S. involucrata when grown with the cluster-rooted B. attenuata, due to enhanced acquisition of Mn and possibly other micronutrients. Leaf [Mn] of S. involucrata was increased in the presence of B. attenuata, and this increased [Mn] was associated with enhanced growth. It has yet to be explored how common the facilitation by carboxylate-releasing species is on severely P-impoverished soils. For example, facilitation does not appear to occur when Proteaceae plants have high levels of P in their tissue, possibly because this suppresses cluster-root activity. In a split-root experiment in a glasshouse, Wood (2011) found no evidence for facilitation of P uptake in the AM species S. involucrata or Hibbertia subvaginata in the presence of cluster roots of B. attenuata; neither biomass nor tissue [P] were positively related to the biomass of the neighbouring B. attenuata cluster roots. This result may have been due to the fact that the seedlings used by Wood (2011) had high initial P concentrations, especially in B. attenuata, with 1.2 ± 0.77 and 3.6 ± 0.77 mg g−1 dry weight in the young leaves and stems, respectively, i.e. 285% higher than plants growing in the wild (Lambers et al., 2012).
What determines the fact that Proteaceae are more or less excluded from soils with higher P levels (Lambers et al., 2010)? One possibility is that they might suffer P toxicity on such soils, as evidenced by pot trials with native species from P-impoverished locations on Hawkesbury Sandstone soils near Sydney, Australia (Thomson and Leishman, 2004). However, as not all Proteaceae are P sensitive (Shane and Lambers, 2006; Parks et al., 2007), this is unlikely to be the full explanation. Proteaceae in south-western Australia's biodiversity hotspot tend to have a very high leaf mass per unit leaf area (Groom et al., 1997; Lamont and Groom, 2002; Denton et al., 2007; Lambers et al., 2012), which tends to be associated with slow growth (Lambers and Poorter, 1992). Therefore, their inherently slow growth rate may lead to competitive exclusion of Proteaceae from less infertile sites. In the early 1950s, P fertilizers, as well as nitrogen and micronutrients, were added in various combinations to raise the fertility of an area of native heath vegetation in southern Australia. There was no significant response to the application of nitrogen or micronutrients, but a significant response to the combined application of nitrogen and P after 10 years. Fertilization with P showed major effects, which, collectively, gradually changed the heath vegetation, which initially had a significant component of Proteaceae species, towards an herbaceous sward over 22 years (Heddle and Specht, 1975). Below, we discuss how elevated soil P levels, for instance as a result of increased fire frequency, negatively impact most Proteaceae species.
Eutrophication: role of phosphorus
Given that Proteaceae species rarely occupy habitats that are not severely P impoverished, it is envisaged that P enrichment has a negative impact on the performance of these species (Heddle and Specht, 1975). Indeed, urban storm water run-off has a negative impact on the growth and survival of species naturally occurring on P-impoverished Hawkesbury Sandstone soils, including Banksia ericifolia (Leishman et al., 2004; Thomson and Leishman, 2004). Likewise, enrichment with P and other nutrients makes naturally P-impoverished habitats in urban bushland in Sydney, Australia, prone to invasion by exotic plants (King and Buckney, 2002).
In Bold Park, an area of urban bushland in Perth, Western Australia, increased fire frequency has led to elevated soil P levels and an abundance of exotic plants at the expense of species naturally occurring in P-impoverished soils, especially Proteaceae (Fisher et al., 2006, 2009). A comparison of species composition in another urban bushland area in Perth, Kings Park, also exposed to increased fire frequency and the associated elevation in soil P status, between 1939 and 1999, shows a decline in four species of Banksia (Proteaceae) and an increase in the ectomycorrhizal trees Eucalyptus gomphocephala, E. marginata and Corymbia calophylla (Myrtaceae; Crosti et al., 2007). Interestingly, there was an increase in the abundance of Banksia (formerly Dryandra) sessilis, showing that not all Proteaceae respond in the same manner to increased fire frequency. Similar changes have not been observed in pristine areas where fire frequencies have not increased.
One source of P in nutrient-poor areas is fire suppressants. Whilst not all contain P, those that do significantly enhance soil P, with an effect that persists for at least 12 months (Leach, 2013). In P-impoverished biodiverse areas, these P-containing fire suppressants should be avoided. Dust or run-off from roads, if they are constructed using nutrient-rich substrates, could also be a source of P, but this possibility has received very little attention.
In a study on the effects of water and nutrient incursion from agricultural land on adjoining native Banksia woodland in Western Australia, Grigg et al. (2000) found that numbers of species of native woody taxa and plant densities increased away from the agricultural area towards the pristine bushland. Total above- and below-ground biomass of woody species decreased away from the agricultural area, probably indicating a fertilization effect. Near the agricultural area, vegetation was appreciably depleted in leaf [Mn] compared with pristine bushland, suggesting that plants close to the boundary were relying less on carboxylate release for P acquisition. These data clearly show a negative impact of farming on nearby native bushland, with less P-sensitive species increasing in growth and more P-sensitive ones declining in abundance. Whilst the abundance of Proteaceae species in general was negatively impacted by incursion of nutrients and water from adjacent agricultural land, the abundance of Banksia prionotes increased (Grigg et al., 2000). Interestingly, both B. prionotes and B. sessilis, which we discussed above, are among very few Proteaceae species that inhabit the young and comparatively fertile coastal dunes (Dixon, 2011) in south-western Australia, which are relatively nutrient rich in comparison to the much older and strongly weathered and acidic dunes on the Swan coastal plain and areas further inland (Laliberté et al., 2012; Lambers et al., 2012).
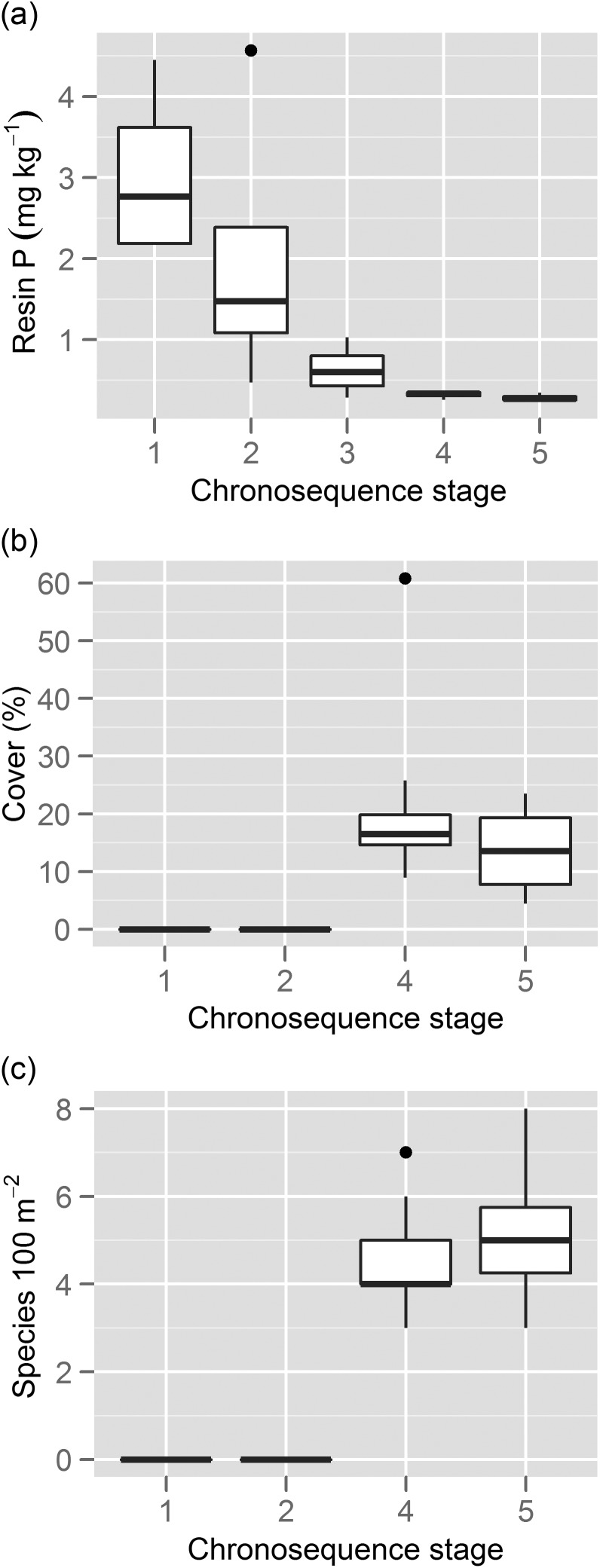
The Jurien Bay dune chronosequence in south-western Australia provides a natural P-availability gradient that is driven by differences in soil age (Laliberté et al., 2013). The sequence is formed of neighbouring coastal dune systems that were deposited at different periods during the last 2 million years (Laliberté et al., 2012, 2013), along which changes in the relative abundance and diversity of Proteaceae species can be quantified. The dune chronosequence follows the classical model of long-term soil development (Walker and Syers, 1976), in that total soil P is relatively high in young soils, and decreases gradually to become the limiting nutrient in old soils (Laliberté et al., 2012; Fig. 3a). Vegetation surveys along this dune chronosequence (G. Zemunik, unpublished data) clearly show that the relative abundance and species richness of Proteaceae species increase in older, more P-impoverished soils (Fig. 3b and c). Whether the lower relative abundance and diversity of Proteaceae species in younger dunes reflect their higher P availability or other soil conditions (e.g. high pH, high [calcium]) remains to be evaluated. What we can dismiss as a possible explanation for greater diversity on older dunes is that evolutionary processes continued for a longer period on the older dunes. Almost all of the species that occur on those older dunes also occur further inland, and they did not evolve locally on the older dunes themselves (Hopper and Gioia, 2004). Conversely, the habitat now provided by the younger dunes would be very similar to that of the older dunes when they were young (Laliberté et al., 2013).
Figure 3:
Changes in resin P (i.e. readily available P; a), percentage canopy cover of Proteaceae species (b), and species richness of Proteaceae (c) along five stages of the Jurien Bay 2 million year dune chronosequence in south-western Australia (Laliberté et al., 2012). Data for (a) are from Laliberté et al. (2012), whereas data for (b) and (c) are from 50 10 m × 10 m plots (i.e. five chronosequence stages × 10 replicate plots) of a recent vegetation survey (G. Zemunik; unpublished data). Stages 1–3 represent Holocene (<7000 years) dunes, whereas stages 4–5 represent Pleistocene dunes (>120 000 years; Laliberté et al., 2012).
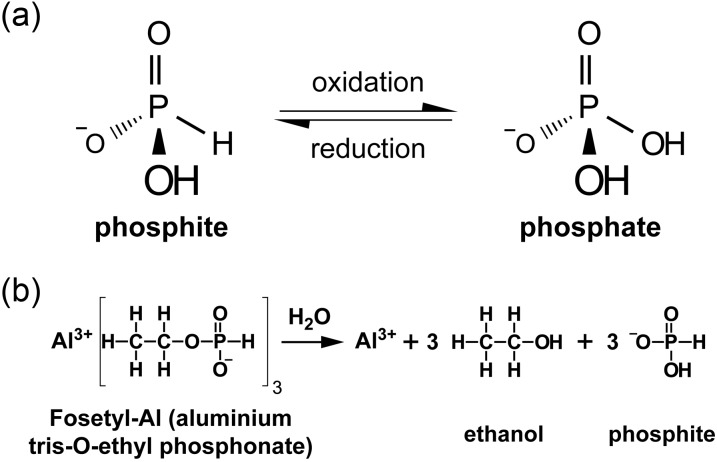
A special case of P enrichment of P-impoverished native bushland that arises as a result of spraying phosphite to reduce the impact of P. cinnamomi is discussed below. Phosphite is a more reduced form of P than phosphate (Fig. 4a); it is readily oxidized to phosphate by soil micro-organisms (White and Metcalf, 2007). Phosphite is an analogue of phosphate and is rapidly absorbed and translocated within the plant (Guest and Grant, 1991). It is mobile in the xylem and phloem (Ouimette and Coffey, 1989). However, in plants phosphite is not oxidized to phosphate, and there is no evidence that phosphite can be utilized by plants as a source of P (Guest and Grant, 1991; Förster et al., 1998). Therefore, it is likely that phosphite concentrations will increase within the plant with regular applications of phosphite; this in turn will eventually be converted to phosphate in the soil once plants senesce or following fire. Regular spraying with phosphite, at rates of 24 kg ha−1, is similar to annual P-fertilization rates used by wheat growers in south-western Australia (Lambers et al., 2010). Given that there is no export of P through cropping, applications of phosphite at these rates will inexorably enhance the total amount of P in the soil, leading to fertilization of plants. This situation will potentially have a negative impact on slow-growing species that do relatively better in P-impoverished habitats. That impact may not be due to their P sensitivity immediately, but may be through competitive interactions with faster-growing plants that lack strategies of extensive release of carboxylates and P-mobilizing enzymes (Heddle and Specht, 1975).
Figure 4:
Phosphite, phosphate, and organic phosphonate. (a) Phosphite is less oxidized than phosphate and is not a source of phosphorus for plants. In soil, phosphite is microbially oxidized to phosphate, which then becomes available for uptake by plant roots. (b) Fungicides such as Fosetyl-Al are organic phosphonates (characterized by a stable C–P bond) that releases phosphite upon hydrolysis.
Phytophthora cinnamomi as a major threat for Australia's biodiversity, and the use of phosphite to reduce the impact of plant disease
The introduced plant-pathogenic oomycete P. cinnamomi (Stramenopila, Oomycota) is a major threat to biodiversity in Australia's global biodiversity hotspot (Coates and Atkins, 2001; Shearer and Fairman, 2007; Fig. 5), as well as elsewhere in Australia (Weste and Marks, 1987; Cahill et al., 2008). It has been introduced into many of the global biodiversity hotspots, threatening susceptible rare flora and degrading plant communities, with severe consequences for fauna (Myers et al., 2000). Shearer et al. (2004a) estimated that 40% (2284 species) of the flora of the South-West Botanical Province of Western Australia are susceptible, and 14% (800 species) are highly susceptible. The pathogen is listed as a ‘key threatening process to Australia's biodiversity’ by the Environment Protection and Biodiversity Conservation Act 1999 (Anonymous, 1999).
Figure 5:
Devastating effects of P. cinnamomi occur throughout Western Australia's biodiversity hotspot, including national parks that exhibit the greatest biodiversity in the region and significant metropolitan bushland areas. Photographs by Chris Dunne, Department of Conservation, Western Australia.
At this stage, phosphite is the only available chemical tool to combat the disease in natural systems in Australia (Hardy et al., 2001; Shearer and Fairman, 2007). It has been marketed for several decades as the active ingredient released after hydrolysis of organic phosphonates (Fig. 4b; Cohen and Coffey, 1986) in fungicides such as Aliette® (i.e. Fosetyl-Al, aluminium tris-O-ethyl phosphonate; BayerCrop Science) and effectively applied against plant diseases (downy mildews, and root and crown rots) in crops such as stone fruits, pineapples, and vegetables, as well as turf grasses and ornamentals. Phosphite can protect individual plants (Hardy et al., 2001), but it does not stop disease progression in native vegetation (Shearer et al., 2004b, 2006; Dunne et al., 2010). Phosphite does reduce mortality due to P. cinnamomi (Pilbeam et al., 2000; Wilkinson et al., 2001; Barrett et al., 2003), and it can slow the rate of disease centre extension and reduce the rate of plant mortality (Shearer et al., 2004b). In the moderately resistant Lambertia formosa (Proteaceae) and the more susceptible Lambertia inermis, phosphite-induced protection is associated with increased superoxide release 8 h after inoculation, and increased phenylalanine ammonia lyase activity 24 h after inoculation (Suddaby et al., 2008). In E. marginata (Myrtaceae), the effect of phosphite in controlling the pathogen is probably determined by the phosphite concentration at the host–pathogen interface. When phosphite concentrations within the roots are low, it primes the tissue for a stronger and faster defence response upon pathogen infection (Jackson et al., 2000). Phosphite induces the host's defence against P. cinnamomi as well as a wide range of other pathogens, ranging from oomycetes to fungi and nematodes (Reuveni et al., 2003; Aguín et al., 2006; Oka et al., 2007; Percival et al., 2009; Lobato et al., 2010). Phosphite-induced resistance can protect treated plants for extended periods of months or even years before re-application becomes necessary (Tynan et al., 2001; Dunstan et al., 2010). High phosphite concentrations in the root tissue act directly on the pathogen to inhibit its growth before it can establish an association with the host, and the host defences remain unchanged (Jackson et al., 2000).
Although the term ‘fungicide’ is often used to describe the direct effect of phosphite on oomycete pathogens, it is in fact a biostat, because phosphite does not kill the pathogen, but only slows its growth. This is mainly explained by its impact on metabolism, most probably impairing phosphate homeostasis and metabolic reactions, at least partly through mimicking phosphate while not being metabolized (Griffith et al., 1990; Barchietto et al., 1992; Niere et al., 1994). Despite the wide and successful use of phosphite to prevent the spread of P. cinnamomi, our understanding of the molecular mechanisms underlying the protective effect of induced resistance in the plant is scant. Studies in the model plant Arabidopsis thaliana have enabled the mode of phosphite action to be analysed at the molecular level. The plant hormone salicylic acid, a well-known signalling molecule in defence responses, and the transcription co-activator NPR1 (non-expressor of PR1), involved in the salicylic acid-dependent signalling pathway, are important components in the mechanisms leading to increased resistance after phosphite application. Mutant plants impaired in the accumulation of salicylic acid or its signalling cascade show reduced phosphite-induced resistance against the oomycete pathogen Hyaloperonospora arabidopsidis (Molina et al., 1998; Friedrich et al., 2001). Moreover, phosphite application can lead to a stronger and faster defence response in A. thaliana subsequently inoculated with H. arabidopsidis (Massoud et al., 2012). This response involves the proteins EDS1 (enhanced disease susceptibility 1) and PAD4 (phytoalexin deficient 4), which are upstream of salicylic acid, and the mitogen activated protein kinase MPK4. This augmented activation of resistance mechanisms by pre-treatment with low levels of the inducing chemical, often leading to local and systemic immunity, has been known for a long time and termed ‘defence priming’ (Conrath et al., 2006). Interestingly, this acquired primed state can be passed on to the offspring by epigenetic modifications such as DNA methylation (Luna et al., 2012; Rasmann et al., 2012; Slaughter et al., 2012). This transgenerational induced resistance has the potential for an inheritable adaptation to pathogen pressure and might also explain the long-lasting effect of phosphite applications if similar epigenetic modifications are induced. Thus, understanding of the molecular mechanism of phosphite action has great potential. Current ‘omics technologies (i.e. genomics, proteomics, and metabolomics) allow determination of phosphite-induced changes in gene expression, and protein and metabolite abundances. Their application will allow a detailed description of the impact of phosphite on a whole-systems scale, and will help in the identification of key targets of phosphite, thus creating a knowledge base for the targeted development of alternative compounds. Although this type of approach is especially applicable to plant model species, such as A. thaliana, because of a highly developed genetic and molecular tool kit, similar approaches and translational knowledge transfer become increasingly applicable for non-model organisms (Lambers et al., 2012).
As discussed above, phosphite is oxidized to phosphate by soil microbes, potentially shifting the balance between highly P-efficient, non-mycorrhizal species and less P-efficient ones. Finding an alternative to phosphite would prevent the indirect increase of P availability in native ecosystems. The need for such alternatives is also apparent from reports of oomycete pathogens developing resistance to phosphite, as has been observed for lettuce downy mildew (Brown et al., 2004). Chemicals with similar defence-inducing properties, such as the salicylic acid analogue benzo-(1,2,3)-thiadiazole-7-carbothioic acid and the non-proteinogenic amino acid β-aminobutyric acid are available, and their efficacy has been studied (Conrath, 2009). So far, large-scale application, similar to phosphite, has not been successful, not even in agricultural systems that are easier to manage. Nevertheless, knowledge gained concerning these compounds will also improve our understanding of the mode of phosphite action and the identification of practical alternative compounds.
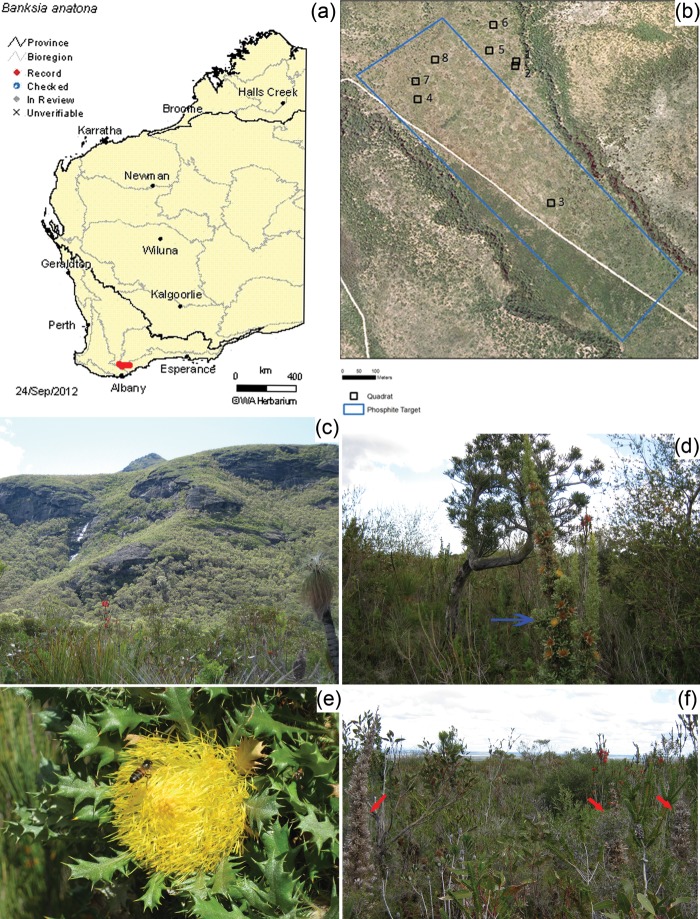
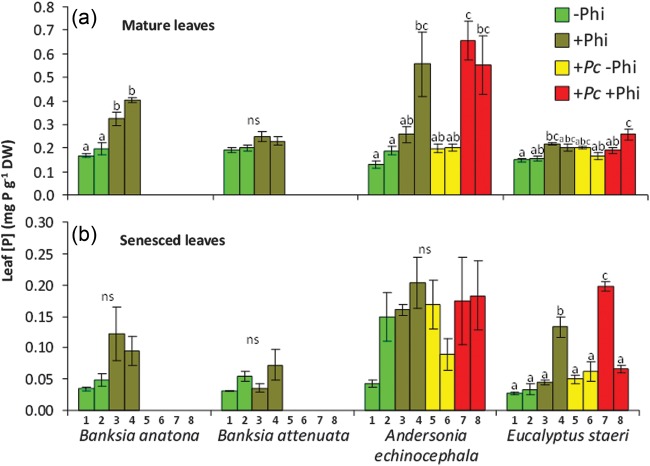
The elevation of leaf [P] resulting from phosphite application has scarcely been investigated, but is evidenced in nature. In Stirling Range National Park, an IUCN Red List Critically Threatened species, Banksia anatona (A.S.George) A.R.Mast & K.R.Thiele, is at extremely high risk of extinction because of P. cinnamomi infestations. Banksia anatona exists naturally only on the lower slopes of the Stirling Range (Fig. 6a,c,d and e). Phosphite has been applied by aerial spraying to a target area for 15 years to slow the loss of a B. anatona community due to P. cinnamomi infestation (Fig. 6b and f). Samples taken from monitoring quadrats inside and outside the phosphite spray target area show that the [P] in mature and senesced leaves of B. anatona where P. cinnamomi is absent has significantly increased compared with unsprayed areas (Fig. 7a and b). Evidence of elevated leaf [P] can also be seen in other species, including Banksia attenuata R.Br, Andersonia echinocephala (Stschegl.) Druce and Eucalyptus staeri (Maiden) Kessell & C.A.Gardner. Samples were taken in 2011 from B. anatona and B. attenuata only in areas where P. cinnamomi was absent, because irrespective of whether or not phosphite had been applied, over the time since monitoring quadrats were established, P. cinnamomi had already killed all individuals in the infested quadrats. This example highlights the urgent need for alternative, practical and effective compounds that do not contain P to be developed to manage P. cinnamomi infestations to prevent both species loss and the elevation of the P status of ecosystems. Whilst the biomass of major plants in the community has increased following phosphite application, to date there is no firm evidence of a change in plant community composition, possibly because of the low productivity of the system and the relatively long time it takes to achieve such a shift in community structure.
Figure 6:
Banksia anatona (A.S.George) A.R.Mast & K.R.Thiele (cactus banksia) is an IUCN Red List Critically Endangered ranked species at extremely high risk of extinction in the wild, because of P. cinnamomi infestations. (a) The distribution of B. anatona, found only on the lower slopes of the Stirling Ranges in south-western Australia (DEC, 2012). (b) The location of monitoring quadrats inside and outside of an annually sprayed phosphite treatment target area. The target area contains a P. cinnamomi infestation. (c) View of the Stirling Range to the north of the target treatment area. (d) Banksia anatona (blue arrow) is an upright, non-lignotuberous shrub, growing up to 5 m tall, found growing on grey sand over gravelly shale and rocky silty clay loam (DEC, 2012). (e) Close-up view of B. anatona leaves and a flower. (f) Banksia anatona is extremely susceptible to P. cinnamomi. Red arrows indicate dead B. anatona as a result of P. cinnamomi infection. Text and image used with permission.
Figure 7:
Leaf P concentration of mature (a) and senesced leaves (b) of B. anatona, B. attenuata, Andersonia echinocephala, and Eucalyptus staeri located on the lower slope of the Stirling Range, Western Australia; note the different scales for the two panels. The plants were located within quadrats (numbered 1–8; see Fig. 6b), growing naturally (–Phi), treated with phosphite (+Phi), infested with P. cinnamomi and not treated (+Pc –Phi), or infested and treated with phosphite (+Pc +Phi; n = 3). Error bars indicate SEM (n = 4); columns not sharing the same letter indicate significant differences between treatments within species according to Tukey's test (P = 0.05); ns, no significant difference. Different columns with the same colour refer to different quadrats (Fig. 6b) for the same treatment; three trees within each quadrat were sampled, and four leaves per plant were used. Missing bars are due to the fact that trees had succumbed to the pathogen before sampling had commenced.
With the exact molecular mode of phosphite action being unknown, it is important also to consider its impact on P-signalling networks in plants (Ticconi et al., 2001; Varadarajan et al., 2002). Disturbances in nutrient homeostasis have recently been shown to impact directly on plant–pathogen interactions (Pannell and Ewing, 2006; Van Damme et al., 2009; Stuttmann et al., 2011; Álvarez et al., 2012; Kruse et al., 2012). Therefore, phosphite may interact with Pi-signalling components that could trigger the suggested priming of defence responses. Phosphate-signalling networks can include both local and systemic signalling pathways, and it has been assumed that local responses are triggered upon the recognition of Pi itself, while systemic responses may be triggered by changes in hormone levels or in a yet to be identified organic phosphate ester pool (Liu et al., 2010; Thibaud et al., 2010; Chiou and Lin, 2011; Nagarajan and Smith, 2012). Given that phosphite cannot be metabolized by plants, it has been assumed only to interfere with the local sensing of Pi itself; therefore, it has long been considered an excellent tool to distinguish between different sensors (McDonald et al., 2001; Ticconi et al., 2004). Unfortunately, Pi is a strong inhibitor of methylphosphonate (an organic analogue of phosphite) transport with an apparent Ki of 5 µM (Pratt et al., 2009). Phosphite and methylphosphonate also show different accumulation patterns in subcellular compartments compared with Pi and are influenced by the presence of Pi according to detailed nuclear magnetic resonance studies using cell culture systems (Danova-Alt et al., 2008; Pratt et al., 2009). These findings suggest that any observed effects of phosphite need to be interpreted with caution. They also suggest that the P status of the plant is critically important for phosphite acquisition and allocation, and may explain the varying degree of success of plant protection in the field. Starving plants of Pi before the application of phosphite to circumvent some of these issues has been considered by Varadarajan and co-workers (2002); however, the strong overall growth inhibitory effects of phosphite on P-starved plants (Thao and Yamakawa, 2009) make it necessary to focus on short-term effects on Pi-signalling networks, and carefully monitor its tissue accumulation patterns to distinguish clearly between primary and secondary effects. This growth inhibition could also cause species loss in severely P-impoverished landscapes. At this time, it is also unclear whether different PHT transporters have different affinities for phosphite. As a first step, these kinetic properties would need to be determined and compared with the kinetics of Pi uptake in electrophysiological studies, as well as knock-out lines for individual transporters in Arabidopsis or other model plant species. As it is not clear whether any of the known PHT transporters also acts as a tranceptor that signals the availability of Pi, similar to the nitrate-sensing properties of the AtNTR1;1 transporter (Gojon et al., 2011), phosphite could directly interfere with this transceptor function, perturbing down-stream signalling pathways. Another possibility is that phosphite interferes with protein kinase/phosphatase regulatory units that control the phosphorylation status of PHT proteins, thereby regulating their incorporation into the plasma membrane (or their activity; Bayle et al., 2011).
Phosphite competitively inhibits phosphate uptake by cluster roots in Hakea sericea (Sousa et al., 2007). It also triggers cluster-root development alongside other P-starvation responses in P-sufficient white lupin (Gilbert et al., 2000). Given the above-mentioned observations in model plant species, phosphite has the potential to suppress cluster-root development in P-limited Proteaceae and other cluster-root producing plant species. Thus, there could be direct implications for the phosphate-mining strategy of native Proteaceae species upon long-term exposure to phosphite. The exact time frame over which these changes might occur is unknown, and detailed monitoring of treated flora and plant communities is therefore recommended. Given that phosphite has been used for only 15 years, the tipping point may not yet have been reached.
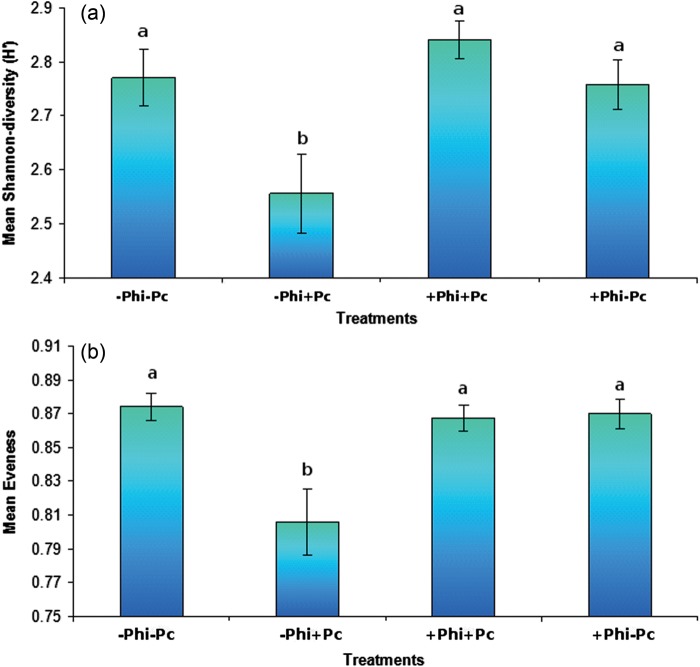
Even though phosphite applications benefit plant biodiversity in the shorter term by reducing dieback caused by P. cinnamomi, they may also lead to losses of P-sensitive or slow-growing plant species in the longer term. Very little information is available on the effects of long-term phosphite spraying in natural systems on species abundance and functioning. In a study in Gull Rock National Park, near Albany in Western Australia, a site exposed to phosphite application differs significantly from a control site without phosphite application in terms of species composition and diversity. The Shannon-diversity (Fig. 8a) index ranged from 0 (only one species is present, so no uncertainty to which species a randomly picked individual belongs) to 4.5 (where all the species are well represented, so there is high uncertainty; Hill, 1973; Keylock, 2005). The fewer the disturbances (i.e. P. cinnamomi infestation, phosphite spraying), the higher the index value. Evenness, on the other hand, represents how abundance is distributed among the different species within a community. An evenness index (Fig. 8b) closer to 0 indicates that most species are rare and only few are abundant (i.e. high dominance), while an index near to 1 means that all species have equal abundance (Hill, 1973; Keylock, 2005). The Shannon-diversity index suggests that the unsprayed and P. cinnamomi-infested site has the lowest species diversity when compared with the other three ‘treatments’, in accordance with other findings for the region (Bishop et al., 2010). This means that it is difficult to predict which species a randomly picked individual belongs to compared with the other three treatments, because the plant species present are rare and sparsely distributed. The most distinct treatments, in terms of plant species composition and abundance, were the unsprayed and P. cinnamomi-infested plots and the phosphite-sprayed and P. cinnamomi-free plots, followed by the phosphite-sprayed and P. cinnamomi-infested plots and the phosphite-sprayed and P. cinnamomi-free plots. Assuming that the plots at very close distance were similar before phosphite applications and P. cinnamomi infestation, we are faced with the conclusion that both P. cinnamomi and phosphite have negatively impacted the biodiversity in Gull Rock National Park, which is representative of a significant component of south-western Australia's global biodiversity hotspot.
Figure 8:
Mean Shannon-diversity (a) and evenness (b) for four ‘treatments’ (plus or minus P. cinnamomi and/or long-term phosphite spraying) at Gull Rock National Park, near Albany in Western Australia. The bars represent the SEM; n = 40. Mean Shannon-diversity index was significantly different between the four treatments (F(3,36) = 5.734, P = 0.003), as was the mean evenness (F(3, 36) = 8.111, P < 0.0001).
Concluding remarks
Given that the greatest plant diversity in south-western Australia occurs on its most severely P-impoverished soils, P enrichment of natural habitats is a major threat to biodiversity of this globally significant biodiversity hotspot. We surmise that this threat is very similar for other P-impoverished environments, including the fynbos in South Africa (Cowling et al., 1996; Richards et al., 1997; Linder, 2005) and the cerrado in Brazil (Eiten, 1972; Ratter et al., 1997; Lannes et al., 2012). To conserve the biodiversity of such habitats requires maintenance of low soil fertility, in particular a low soil P status (Heddle and Specht, 1975; Adam, 2012). Minimizing the impact of run-off, P-containing fire suppressants, and pesticides, and maintaining a low fire frequency are therefore essential (Table 1). In old, climatically buffered, infertile landscapes, P is the key nutrient that provides a threat to plant biodiversity, rather than nitrogen, which tends to be a key limiting factor in younger landscapes (Hopper, 2009; Lambers et al., 2010). Once these systems have been eutrophied, it is extremely difficult to restore their former glory (Standish et al., 2008; Standish and Hobbs, 2010).
Whilst P. cinnamomi is a major threat to Australia's biodiversity, combating it by using phosphite cannot be a viable long-term solution, because P enrichment of severely P-impoverished soils replaces one threat by another (Table 1). In the short term, in the absence of any alternative, phosphite applications are essential. However, what is urgently required is a much better understanding of how phosphite functions, so that we can work towards alternatives for phosphite to achieve the same immunizing effects that phosphite induces in plants in response to oomycete pathogens. Once we know which metabolites are affected by phosphite, we can target the metabolic pathways with which phosphite interacts. Based on that knowledge, we can screen alternative, non-P-containing compounds for their ability to induce the same defence mechanisms, and thus attempt to induce the same anti-Phytophthora effects as phosphite.
Acknowledgements
Field work in Gull Rock and Stirling Range national parks was funded by Australian Research Council Industry Linkage Project ‘Susceptibility to Phytophthora cinnamomi and sensitivity to phosphorus in native Australian plants: why are they linked?’ (LP0776252), the Department of Environment and Conservation (DEC) Western Australia, and a University of Western Australia Research Development Award. Thanks goes to Damien Rathbone (DEC) for support with Stirling Range field work and providing mapping of that treatment area.
References
- 1.Adam P, Stricker P, Anderson DJ. (1989) Species-richness and soil phosphorus in plant communities in coastal New South Wales. Austral Ecology 14: 189–198. [Google Scholar]
- 2.Adam P. (2012) Royal National Park – lessons for the future from the past. Proc Linn Soc NSW 134: B7–B24. [Google Scholar]
- 3.Aguín O, Mansilla JP, Sainz MJ. (2006) In vitro selection of an effective fungicide against Armillaria mellea and control of white root rot of grapevine in the field. Pest Manag Sci 62: 223–228. [DOI] [PubMed] [Google Scholar]
- 4.Ai P, Sun S, Zhao J, Fan X, Xin W, Guo Q, Yu L, Shen Q, Wu P, Miller AJ, et al. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809. [DOI] [PubMed] [Google Scholar]
- 5.Álvarez C, Ángeles Bermúdez M, Romero LC, Gotor C, García I. (2012) Cysteine homeostasis plays an essential role in plant immunity. New Phytol 193: 165–177. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous (1999) A national overview of Phytophthora cinnamomi in Australia. Supplementary information to accompany the draft National Threat Abatement Plan. Canberra. [Google Scholar]
- 7.Barchietto T, Saindrenan P, Bompeix G. (1992) Physiological responses of Phytophthora citrophthora to a subinhibitory concentration of phosphonate. Pestic Biochem Physiol 42: 151–166. [Google Scholar]
- 8.Barrett SR, Shearer BL, Hardy GESJ. (2003) The efficacy of phosphite applied after inoculation on the colonisation of Banksia brownii stems by Phytophthora cinnamomi. Australas Plant Pathol 32: 1–7. [Google Scholar]
- 9.Bayle V, Arrighi J-F, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L. (2011) Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beadle NCW. (1953) The edaphic factor in plant ecology with a special note on soil phosphates. Ecology 34: 426–428. [Google Scholar]
- 11.Bishop CL, Wardell-Johnson GW, Williams MR. (2010) Community-level changes in Banksia woodland following plant pathogen invasion in the Southwest Australian Floristic Region. J Veg Sci 21: 888–898. [Google Scholar]
- 12.Boulet F, Lambers H. (2005) Characterisation of arbuscular mycorrhizal fungi colonisation in cluster roots of shape Hakea verrucosa F. Muell (Proteaceae), and its effect on growth and nutrient acquisition in ultramafic soil. Plant Soil 269: 357–367. [Google Scholar]
- 13.Brown S, Koike ST, Ochoa OE, Laemmlen F, Michelmore RW. (2004) Insensitivity to the fungicide fosetyl-aluminum in California isolates of the lettuce downy mildew pathogen, Bremia lactucae. Plant Dis 88: 502–508. [DOI] [PubMed] [Google Scholar]
- 14.Brundrett MC. (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37–77. [Google Scholar]
- 15.Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL. (2008) Phytophthora cinnamomi and Australia's biodiversity: impacts, predictions and progress towards control. Aust J Bot 56: 279–310. [Google Scholar]
- 16.Cavanagh T, Pieroni M. (2006) The Dryandras. Australian Plant Society, Hawthorn, Wildflower Society of Western Australia, Nedlands. [Google Scholar]
- 17.Chesson P. (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31: 343–358. [Google Scholar]
- 18.Chiou T-J, Lin S-I. (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62: 185–206. [DOI] [PubMed] [Google Scholar]
- 19.Christophersen HM, Smith FA, Smith SE. (2009) Arbuscular mycorrhizal colonization reduces arsenate uptake in barley via downregulation of transporters in the direct epidermal phosphate uptake pathway. New Phytol 184: 962–974. [DOI] [PubMed] [Google Scholar]
- 20.Coates DJ, Atkins KA. (2001) Priority setting and the conservation of Western Australia's diverse and highly endemic flora. Biol Conserv 97: 251–263. [Google Scholar]
- 21.Cogliatti DH, Clarkson DT. (1983) Physiological changes in, and phosphate uptake by potato plants during development of, and recovery from phosphate deficiency. Physiol Plant 58: 287–294. [Google Scholar]
- 22.Cohen Y, Coffey MD. (1986) Systemic fungicides and the control of oomycetes. Annu Rev Phytopathol 24: 311–338. [Google Scholar]
- 23.Collins K, Collins K, George A. (2008) Banksias. Bloomings Books, Melbourne. [Google Scholar]
- 24.Collins MT. (2008) Factors affecting the recovery of orchids in a post-mining landscape. PhD thesis, University of Western Australia Crawley. [Google Scholar]
- 25.Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Newman M-A, Pieterse CMJ, Poinssot B, Pozo MJ, et al. (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 26.Conrath U. (2009) Priming of induced plant defense responses. Adv Bot Res 51: 361–395. [Google Scholar]
- 27.Cowling RM, MacDonald IAW, Simmons MT. (1996) The Cape Peninsula, South Africa: physiographical, biological and historical background to an extraordinary hot-spot of biodiversity. Biodivers Conserv 5: 527–550. [Google Scholar]
- 28.Cowling RM, Lamont BB. (1998) On the nature of Gondwanan species flocks: diversity of Proteaceae in Mediterranean south-western Australia and South Africa. Aust J Bot 46: 335–355. [Google Scholar]
- 29.Crosti R, Dixon KW, Ladd PG, Yates CJ. (2007) Changes in the structure and species dominance in vegetation over 60 years in an urban bushland remnant. Pac Conserv Biol 13: 158–170. [Google Scholar]
- 30.Cu STT, Hutson J, Schuller KA. (2005) Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant Soil 272: 143–151. [Google Scholar]
- 31.Danova-Alt R, Dijkema C, De Waard P, Köck M. (2008) Transport and compartmentation of phosphite in higher plant cells – kinetic and 31P nuclear magnetic resonance studies. Plant Cell Environ 31: 1510–1521. [DOI] [PubMed] [Google Scholar]
- 32.Davies J, Briarty LG, Rieley JO. (1973) Observations on the swollen lateral roots of the Cyperaceae. New Phytol 72: 167–174. [Google Scholar]
- 33.DEC (2012) Descriptions and mapping by the Western Australian Herbarium. Department of Environment and Conservation, Perth. [Google Scholar]
- 34.de Campos MCR, Pearse SJ, Oliveira RS, Lambers H. (2013) Down-regulation of net phosphorus uptake capacity is inversely related to leaf phosphorus resorption in four species from a phosphorus-impoverished environment. Ann Bot 111: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denton MD, Veneklaas EJ, Freimoser FM, Lambers H. (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30: 1557–1565. [DOI] [PubMed] [Google Scholar]
- 36.Dinkelaker B, Hengeler C, Marschner H. (1995) Distribution and function of proteoid roots and other root clusters. Bot Acta 108: 193–200. [Google Scholar]
- 37.Dixon KW. (2011) Coastal Plants: a Guide to the Identification and Restoration of Plants of the Perth Region. CSIRO Publishing, Melbourne. [Google Scholar]
- 38.Dong B, Rengel Z, Delhaize E. (1998) Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta 205: 251–256. [DOI] [PubMed] [Google Scholar]
- 39.Drew MC, Saker LR. (1984) Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley: evidence of non-allosteric regulation. Planta 160: 500–507. [DOI] [PubMed] [Google Scholar]
- 40.Drew MC, Saker LR, Barber SA, Jenkins W. (1984) Changes in the kinetics of phosphate and potassium absorption in nutrient-deficient barley roots measured by a solution-depletion technique. Planta 160: 490–499. [DOI] [PubMed] [Google Scholar]
- 41.Dunne CP, Crane CE, Shearer BL. (2010) Stem application of phosphite controls Phytophthora cinnamomi in native plant communities from south-west Western Australia. In. Fifth meeting of the International Union of Forest Research Organisations (IUFRO) Working Party. Rotorua, New Zealand, 7–12 March 2010, p. 51. [Google Scholar]
- 42.Dunstan WA, Rudman T, Shearer B, Moore NA, Paap T, Calver MC, Dell B, Hardy GSJ. (2010) Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biol Invasions 12: 913–925. [Google Scholar]
- 43.Eiten G. (1972) The Cerrado Vegetation of Brazil. Bot Rev 38: 201–341. [Google Scholar]
- 44.Fernando DR, Mizuno T, Woodrow IE, Baker AJM, Collins RN. (2010) Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol 188: 1014–1027. [DOI] [PubMed] [Google Scholar]
- 45.Fisher JL, Veneklaas EJ, Lambers H, Loneragan WA. (2006) Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum. Plant Soil 284: 253–264. [Google Scholar]
- 46.Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ. (2009) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biol Conserv 142: 2270–2281. [Google Scholar]
- 47.Förster H, Adaskaveg JE, Kim DH, Stanghellini ME. (1998) Effect of phosphite on tomato and pepper plants and on susceptibility of pepper to Phytophthora root and crown rot in hydroponic culture. Plant Dis 82: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 48.Friedrich L, Lawton K, Dietrich R, Willits M, Cade R, Ryals J. (2001) NIM1 overexpression in arabidopsis potentiates plant disease resistance and results in enhanced effectiveness of fungicides. Mol Plant Microbe Interact 14: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 49.Gardner WK, Parbery DG, Barber DA. (1981) Proteoid root morphology and function in Lupinus albus. Plant Soil 60: 143–147. [Google Scholar]
- 50.Gardner WK, Parbery DG, Barber DA. (1982) The acquisition of phosphorus by Lupinus albus L. II. The effect of varying phosphorus supply and soil type on some characteristics of the soil/root interface. Plant Soil 68: 33–41. [Google Scholar]
- 51.Gilbert GA, Knight JD, Vance CP, Allan DL. (2000) Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Ann Bot 85: 921–928. [Google Scholar]
- 52.Gojon A, Krouk G, Perrine-Walker F, Laugier E. (2011) Nitrate transceptor(s) in plants. J Exp Bot 62: 2299–2308. [DOI] [PubMed] [Google Scholar]
- 53.Grierson PF, Attiwill PM. (1989) Chemical characteristics of the proteoid root mat of Banksia integrifolia L. Aust J Bot 37: 137–143. [Google Scholar]
- 54.Griffith JM, Smillie RH, Grant BR. (1990) Alterations in nucleotide and pyrophosphate levels in Phytophthora plamivora following exposure to the antifungal agent potassium phosphonate (phosphite). J Gen Microbiol 136: 1285–1291. [Google Scholar]
- 55.Grigg AM, Pate JS, Unkovich MJ. (2000) Responses of native woody taxa in Banksia woodland to incursion of groundwater and nutrients from bordering agricultural land. Aust J Bot 48: 777–792. [Google Scholar]
- 56.Grime JP. (2002) Plant Strategies, Vegetation Processes, and Ecosystem Properties. Chicester, England: John Wiley & Sons. [Google Scholar]
- 57.Groom PK, Lamont BB, Markey AS. (1997) Influence of leaf type and plant age on leaf structure and sclerophylly in Hakea (Proteaceae). Aust J Bot 45: 827–838. [Google Scholar]
- 58.Groom PK, Lamont BB. (2010) Phosphorus accumulation in Proteaceae seeds: a synthesis. Plant Soil 334: 61–72. [Google Scholar]
- 59.Guest D, Grant B. (1991) The complex action of phosphonates as antifungal agents. Biol Rev Camb Philos Soc 66: 159–187. [Google Scholar]
- 60.Handreck K. (1991) Phosphorus and iron effects on the early growth of some Australian native plants. Proc Int Plant Prop Soc 40: 56–59. [Google Scholar]
- 61.Hardy GESJ, Barrett S, Shearer BL. (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australas Plant Pathol 30: 133–139. [Google Scholar]
- 62.Harrison MJ, Dewbre GR, Liu J. (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14: 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawkins H-J, Hettasch H, Mesjasz-Przybylowicz J, Przybylowicz W, Cramer MD. (2008) Phosphorus toxicity in the Proteaceae: a problem in post-agricultural lands. Sci Hortic 117: 357–365. [Google Scholar]
- 64.Heddle E, Specht R. (1975) Dark Island Heath (Ninety-Mile Plain, South Australia). VIII. The effect of fertilizers on composition and growth, 1950–1972. Aust J Bot 23: 151–164. [Google Scholar]
- 65.Hill MO. (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54: 427–432. [Google Scholar]
- 66.Hopper SD. (1980) Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Aust J Bot 28: 61–75. [Google Scholar]
- 67.Hopper SD, Gioia P. (2004) The Southwest Australian Floristic Region: evolution and conservation of a global hotspot of biodiversity. Annu Rev Ecol Evol Syst 35: 623–650. [Google Scholar]
- 68.Hopper SD. (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322: 49–86. [Google Scholar]
- 69.Horst WJ, Waschkies C. (1987) Phosphatversorgung von Sommerweizen (Triticum aestivum L.) in Mischkultur mit Weißer Lupine (Lupinus albus L.). Z Pflanzenernähr Bodenk 150: 1–8. [Google Scholar]
- 70.Huang CY, Shirley N, Genc Y, Shi B, Langridge P. (2011) Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol 156: 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huston MA. (1994) Biological Diversity. Cambridge University Press, Cambridge. [Google Scholar]
- 72.Jackson TJ, Burgess T, Colquhoun I, Hardy GESJ. (2000) Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathol 49: 147–154. [Google Scholar]
- 73.Jaffré T. (1979) Accumulation du manganèse par les Protéacées de Nouvelle Calédonie. Comptes Rendus de l'Académie des Sciences (Paris), D 289: 425–428. [Google Scholar]
- 74.Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ. (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104: 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG. (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keylock CJ. (2005) Simpson diversity and the Shannon–Wiener index as special cases of a generalized entropy. Oikos 109: 203–207. [Google Scholar]
- 77.King SA, Buckney RT. (2002) Invasion of exotic plants in nutrient-enriched urban bushland. Austral Ecology 27: 573–583. [Google Scholar]
- 78.Kobae Y, Hata S. (2010) Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol 51: 341–353. [DOI] [PubMed] [Google Scholar]
- 79.Kruse C, Haas FH, Jost R, Reiser B, Reichelt M, Wirtz M, Gershenzon J, Schnug E, Hell R. (2012) Improved sulfur nutrition provides the basis for enhanced production of sulfur-containing defense compounds in Arabidopsis thaliana upon inoculation with Alternaria brassicicola. J Plant Physiol 169: 740–743. [DOI] [PubMed] [Google Scholar]
- 80.Ladd PG, Alkema AJ, Thomson GJ. (1996) Pollen presenter morphology and anatomy in Banksia and Dryandra. Aust J Bot 44: 447–471. [Google Scholar]
- 81.Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwolll K-H, Zemunik G, Lambers H. (2012) Experimental assessment of nutrient limitation along a 2-million year dune chronosequence in the south-western Australia biodiversity hotspot. J Ecol 100: 631–642. [Google Scholar]
- 82.Laliberté E, Grace JB, Huston MA, Lambers H, Teste FP, Turner BL, Wardle DA. (2013) How does pedogenesis drive plant diversity? Trends Ecol Evol doi.org/10.1016/j.tree.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Lambers H, Poorter H. (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 22: 187–261. [Google Scholar]
- 84.Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambers H, Raven JA, Shaver GR, Smith SE. (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23: 95–103. [DOI] [PubMed] [Google Scholar]
- 86.Lambers H, Brundrett MC, Raven JA, Hopper SD. (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334: 11–31. [Google Scholar]
- 87.Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible W-R, Stitt M, Teste F, Turner BL. (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use efficiency. New Phytol 196: 1098–1108. [DOI] [PubMed] [Google Scholar]
- 88.Lambers H, Clements JC, Nelson MJ. (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100: 263–288. [DOI] [PubMed] [Google Scholar]
- 89.Lamont B. (1974) The biology of dauciform roots in the sedge Cyathochaete avenacea. New Phytol 73: 985–996. [Google Scholar]
- 90.Lamont BB. (1972) ‘Proteoid’ roots in the legume Viminaria juncea. Search 3: 90–91. [Google Scholar]
- 91.Lamont BB, Downes S, Fox JED. (1977) Importance-value curves and diversity indices applied to a species-rich heathland in Western Australia. Nature 265: 438–441. [Google Scholar]
- 92.Lamont BB, Markey A. (1995) Biogeography of fire-killed and resprouting Banksia species in south-western Australia. Aust J Bot 43: 283–303. [Google Scholar]
- 93.Lamont BB, Groom PK. (2002) Green cotyledons of two Hakea species control seedling mass and morphology by supplying mineral nutrients rather than organic compounds. New Phytol 153: 101–110. [Google Scholar]
- 94.Lamont BB. (2003) Structure, ecology and physiology of root clusters – a review. Plant Soil 248: 1–19. [Google Scholar]
- 95.Lannes LS, Bustamante MMC, Edwards PJ, Venterink HO. (2012) Alien and endangered plants in the Brazilian Cerrado exhibit contrasting relationships with vegetation biomass and N:P stoichiometry. New Phytol 196: 816–823. [DOI] [PubMed] [Google Scholar]
- 96.Leach DR. (2013) Fire Suppressant Impacts on Flora of the Swan Coastal Plain. PhD thesis, The University of Western Australia, Crawley (Perth). [Google Scholar]
- 97.Le Brocque AF, Buckney RT. (2003) Species richness–environment relationships within coastal sclerophyll and mesophyll vegetation in Ku-ring-gai Chase National Park, New South Wales, Australia. Austral Ecology 28: 404–412. [Google Scholar]
- 98.Leishman MR, Hughes MT, Gore DB. (2004) Soil phosphorus enhancement below stormwater outlets in urban bushland: spatial and temporal changes and the relationship with invasive plants. Soil Res 42: 197–202. [Google Scholar]
- 99.Li SM, Li L, Zhang FS, Tang C. (2004) Acid phosphatase role in chickpea/maize intercropping. Ann Bot 94: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Linder HP. (2005) Evolution of diversity: the Cape flora. Trends Plant Sci 10: 536–541. [DOI] [PubMed] [Google Scholar]
- 101.Liu J-Q, Allan DL, Vance CP. (2010) Systemic signaling and local sensing of phosphate in common bean: cross-talk between photosynthate and microRNA399. Mol Plant 3: 428–437. [DOI] [PubMed] [Google Scholar]
- 102.Liu T-Y, Huang T-K, Tseng C-Y, Lai Y-S, Lin S-I, Lin W-Y, Chen J-W, Chiou T-J. (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lobato MC, Olivieri FP, Daleo GR, Andreu AB. (2010) Antimicrobial activity of phosphites against different potato pathogens. J Plant Dis Prot 117: 102–109. [Google Scholar]
- 104.Loth-Pereda V, Orsini E, Courty P-E, Lota F, Kohler A, Diss L, Blaudez D, Chalot M, Nehls U, Bucher M, et al. (2011) Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiol 156: 2141–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. (2012) Next-generation systemic acquired resistance. Plant Physiol 158: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McDonald AE, Niere JO, Plaxton WC. (2001) Phosphite disrupts the acclimation of Saccharomyces cerevisiae to phosphate starvation. Can J Microbiol 47: 969–978. [DOI] [PubMed] [Google Scholar]
- 107.Massoud K, Barchietto T, Le Rudulier T, Pallandre L, Didierlaurent L, Garmier M, Ambard-Bretteville F, Seng J-M, Saindrenan P. (2012) Dissecting phosphite-induced priming in Arabidopsis infected with Hyaloperonospora arabidopsidis. Plant Physiol 159: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milberg P, Lamont BB. (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137: 665–672. [Google Scholar]
- 109.Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz ME, Sugarman PJ, Cramer BS, Christie-Blick N, Pekar SF. (2005) The Phanerozoic record of global sea-level change. Science 310: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 110.Misson J, Thibaud M-C, Bechtold N, Raghothama K, Nussaume L. (2004) Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol Biol 55: 727–741. [DOI] [PubMed] [Google Scholar]
- 111.Molina A, Hunt MD, Ryals JA. (1998) Impaired fungicide activity in plants blocked in disease resistance signal transduction. Plant Cell 10: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Blasing O, Usadel B, Czechowski T, Udvardi MK, Stitt M, et al. (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30: 85–112. [DOI] [PubMed] [Google Scholar]
- 113.Morton JF. (1983) Rooibos tea, Aspalathus linearis, a caffeineless, low-tannin beverage. Econ Bot 37: 164–173. [Google Scholar]
- 114.Muchhal US, Pardo JM, Raghothama KG. (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 10519–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mudge SR, Rae AL, Diatloff E, Smith FW. (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353. [DOI] [PubMed] [Google Scholar]
- 116.Muler AL, Oliveira RS, Lambers H, Veneklaas EJ. (2013) Does cluster-root activity of Banksia attenuata (Proteaceae) benefit phosphorus or micronutrient uptake and growth of neighbouring shrubs? Oecologia in press. [Google Scholar]
- 117.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- 118.Myers N. (2001) Mediterranean-climate regions: glowing hotspots of diversity. J Mediterr Ecol 2: 157–163. [Google Scholar]
- 119.Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. (2011) Arabidopsis pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol 156: 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagarajan VK, Smith AP. (2012) Ethylene's role in phosphate starvation signaling: more than just a root growth regulator. Plant Cell Physiol 53: 277–286. [DOI] [PubMed] [Google Scholar]
- 121.Niere JO, DeAngelis G, Grant BR. (1994) The effect of phosphonate on the acid-soluble phosphorus components in the genus Phytophthora. Microbiology 140: 1661–1670. [Google Scholar]
- 122.Nussaume L, Kanno S, Javot H, Marin E, Nakanishi TM, Thibaud M-C. (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 2 doi: 10.3389/fpls.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oka Y, Tkachi N, Mor M. (2007) Phosphite inhibits development of the nematodes Heterodera avenae and Meloidogyne marylandi in cereals. Phytopathology 97: 396–404. [DOI] [PubMed] [Google Scholar]
- 124.Olde Venterink H. (2011) Does phosphorus limitation promote species-rich plant communities? Plant Soil 345: 1–9. [Google Scholar]
- 125.Ouimette DG, Coffey MD. (1989) Phosphonate levels in avocado (Persea americana) seedlings and soil following treatment with fosetyl-Al or potassium phosphonate. Plant Dis 73: 212–215. [Google Scholar]
- 126.Pannell DJ, Ewing MA. (2006) Managing secondary dryland salinity: options and challenges. Agric Water Manage 80: 41–56. [Google Scholar]
- 127.Parfitt RL. (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 53: 55–65. [Google Scholar]
- 128.Parks SE, Haigh AM, Cresswell GC. (2000) Stem tissue phosphorus as an index of the phosphorus status of Banksia ericifolia L. f. Plant Soil 227: 59–65. [Google Scholar]
- 129.Parks SE, Haigh AM, Harris AM. (2007) Responses of six species of Proteaceae, in containers, to controlled-release fertilizer. Commun Soil Sci Plant Anal 38: 2227–2237. [Google Scholar]
- 130.Percival GC, Noviss K, Haynes I. (2009) Field evaluation of systemic inducing resistance chemicals at different growth stages for the control of apple (Venturia inaequalis) and pear (Venturia pirina) scab. Crop Prot 28: 629–633. [Google Scholar]
- 131.Pilbeam RA, Colquhoun IJ, Shearer B, Hardy GESJ. (2000) Phosphite concentration: its effect on phytotoxicity symptoms and colonisation by Phytophthora cinnamomi in three understorey species of Eucalyptus marginata forest. Australas Plant Pathol 29: 86–95. [Google Scholar]
- 132.Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H. (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170: 491–500. [DOI] [PubMed] [Google Scholar]
- 133.Pratt J, Boisson AM, Gout E, Bligny R, Douce R, Aubert S. (2009) Phosphate (Pi) starvation effect on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: an in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol 151: 1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Preuss CP, Huang CY, Gilliham M, Tyerman SD. (2010) Channel-like characteristics of the low-affinity barley phosphate transporter PHT1;6 when expressed in Xenopus oocytes. Plant Physiol 152: 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Preuss CP, Huang CY, Tyerman SD. (2011) Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell Environ 34: 681–689. [DOI] [PubMed] [Google Scholar]
- 136.Pumplin N, Harrison MJ. (2009) Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol 151: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Purnell H. (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust J Bot 8: 38–50. [Google Scholar]
- 138.Rabier J, Laffont-Schwob I, Bouraïma-Madjèbi S, Léon V, Prudent P, Viano J, Nabors MW, Pilon-Smits EAH. (2007) Characterization of metal tolerance and accumulation in Grevillea exul var exul. Int J Phytoremediation 9: 419–435. [DOI] [PubMed] [Google Scholar]
- 139.Rae AL, Cybinski DH, Jarmey JM, Smith FW. (2003) Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol Biol 53: 27–36. [DOI] [PubMed] [Google Scholar]
- 140.Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G. (2012) Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol 158: 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ratter JA, Ribeiro JF, Bridgewater S. (1997) The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot 80: 223–230. [Google Scholar]
- 142.Reddell P, Yun Y, Shipton WA. (1997) Cluster roots and mycorrhizae in Casuarina cunninghamiana: their occurrence and formation in relation to phosphorus supply. Aust J Bot 45: 41–51. [Google Scholar]
- 143.Renner S. (1935) Beitrag zur Kenntnis einiger Wurzelpilze. Eidgenössischen Technischen Hochschule Zürich, Switzerland. [Google Scholar]
- 144.Reuveni M, Sheglov D, Cohen Y. (2003) Control of moldy-core decay in apple fruits by beta-aminobutyric acids and potassium phosphites. Plant Dis 87: 933–936. [DOI] [PubMed] [Google Scholar]
- 145.Richards MB, Cowling RM, Stock WD. (1997) Soil nutrient dynamics and community boundaries in the Fynbos vegetation of South Africa. Plant Ecol 130: 143–153. [Google Scholar]
- 146.Ryan MH, Tibbett M, Edmonds-Tibbett T, Suriyagoda LDB, Lambers H, Cawthray GR, Pang J. (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35: 2061–2220. [DOI] [PubMed] [Google Scholar]
- 147.Schutzendubel A, Polle A. (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53: 1351–1365. [PubMed] [Google Scholar]
- 148.Selivanov IA, Utemova LD. (1969) Root anatomy of sedges in relation to their mycotrophy (in Russian). Transactions of Perm State Pedagogical Institute 68: 45–55. [Google Scholar]
- 149.Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H. (2004a) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shane MW, McCully ME, Lambers H. (2004b) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 151.Shane MW, Szota C, Lambers H. (2004c) A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant Cell Environ 27: 991–1004. [Google Scholar]
- 152.Shane MW, Lambers H. (2005a) Cluster roots: a curiosity in context. Plant Soil 274: 101–125. [Google Scholar]
- 153.Shane MW, Lambers H. (2005b) Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol Plant 124: 441–450. [Google Scholar]
- 154.Shane MW, Lambers H. (2006) Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. J Exp Bot 57: 413–423. [DOI] [PubMed] [Google Scholar]
- 155.Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H. (2006a) Specialized ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant Cell Environ 29: 1989–1999. [DOI] [PubMed] [Google Scholar]
- 156.Shane MW, Dixon KW, Lambers H. (2006b) The occurrence of dauciform roots amongst Western Australian reeds, rushes and sedges, and the impact of phosphorus supply on dauciform-root development in Schoenus unispiculatus (Cyperaceae). New Phytol 165: 887–898. [DOI] [PubMed] [Google Scholar]
- 157.Shane MW, McCully ME, Canny MJ, Pate JS, Lambers H. (2011) Development and persistence of sandsheaths of Lyginia barbata (Restionaceae): relation to root structural development and longevity. Ann Bot 108: 1307–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shearer BL, Crane CE, Cochrane A. (2004a) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Aust J Bot 52: 435–443. [Google Scholar]
- 159.Shearer BL, Crane CE, Fairman RG. (2004b) Phosphite reduces disease extension of a Phytophthora cinnamomi front in Banksia woodland, even after fire. Australas Plant Pathol 33: 249–254. [Google Scholar]
- 160.Shearer BL, Fairman RG, Grant M. (2006) Effective concentration of phosphite in controlling Phytophthora cinnamomi following stem injection of Banksia species and Eucalyptus marginata. Forest Pathology 36: 119–135. [Google Scholar]
- 161.Shearer BL, Fairman RG. (2007) Application of phosphite in a high-volume foliar spray delays and reduces the rate of mortality of four Banksia species infected with Phytophthora cinnamomi. Australas Plant Pathol 36: 358–368. [Google Scholar]
- 162.Shearer BL, Crane CE, Fairman RG, Dunne CP. (2009) Ecosystem dynamics altered by pathogen-mediated changes following invasion of Banksia woodland and Eucalyptus marginata forest biomes of south-western Australia by Phytophthora cinnamomi. Australas Plant Pathol 38: 417–436. [Google Scholar]
- 163.Shearer BL, Crane CE, Dunne CP. (2012) Variation in vegetation cover between shrubland, woodland and forest biomes invaded by Phytophthora cinnamomi. Australas Plant Pathol 41: 413–424. [Google Scholar]
- 164.Shin H, Shin H-S, Dewbre GR, Harrison MJ. (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642. [DOI] [PubMed] [Google Scholar]
- 165.Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. (2012) Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith RJ, Hopper SD, Shane MW. (2011) Sand-binding roots in Haemodoraceae: global survey and morphology in a phylogenetic context. Plant Soil 348: 453–470. [Google Scholar]
- 167.Smith SE, Barker SJ. (2002) Plant phosphate transporter genes help harness the nutritional benefits of arbuscular mycorrhizal symbiosis. Trends Plant Sci 7: 189–190. [DOI] [PubMed] [Google Scholar]
- 168.Smith SE, Read DJ. (2008) Mycorrhizal Symbiosis. Academic Press and Elsevier, London. [Google Scholar]
- 169.Sousa MF, Façanha AR, Tavares RM, Lino-Neto T, Gerós H. (2007) Phosphate transport by proteoid roots of Hakea sericea. Plant Sci 173: 550–558. [Google Scholar]
- 170.Standish RJ, Stokes BA, Tibbett M, Hobbs RJ. (2007) Seedling response to phosphate addition and inoculation with arbuscular mycorrhizas and the implications for old-field restoration in Western Australia. Environ Exp Bot 61: 58–65. [Google Scholar]
- 171.Standish RJ, Cramer VA, Hobbs RJ. (2008) Land-use legacy and the persistence of invasive Avena barbata on abandoned farmland. J Appl Ecol 45: 1576–1583. [Google Scholar]
- 172.Standish RJ, Hobbs RJ. (2010) Restoration of OCBILs in south-western Australia: response to Hopper. Plant Soil 330: 15–18. [Google Scholar]
- 173.Stuttmann J, Hubberten H-M, Rietz S, Kaur J, Muskett P, Guerois R, Bednarek P, Hoefgen R, Parker JE. (2011) Perturbation of Arabidopsis amino acid metabolism causes incompatibility with the adapted biotrophic pathogen Hyaloperonospora arabidopsidis. Plant Cell 23: 2788–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suddaby T, Alhussaen K, Daniel R, Guest D. (2008) Phosphonate alters the defence responses of Lambertia species challenged by Phytophthora cinnamomi. Aust J Bot 56: 550–556. [Google Scholar]
- 175.Sun S, Gu M, Cao Y, Huang X, Zhang X, Ai P, Zhao J, Fan X, Xu G. (2012) A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in Pi-replete rice. Plant Physiol 159: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thao HTB, Yamakawa T. (2009) Phosphite (phosphorous acid): fungicide, fertilizer or bio-stimulator? Soil Sci Plant Nutr 55: 228–234. [Google Scholar]
- 177.Thibaud M-C, Arrighi J-F, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L. (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 64: 775–789. [DOI] [PubMed] [Google Scholar]
- 178.Thomson VP, Leishman MR. (2004) Survival of native plants of Hawkesbury Sandstone communities with additional nutrients: effect of plant age and habitat. Aust J Bot 52: 141–147. [Google Scholar]
- 179.Ticconi CA, Delatorre CA, Abel S. (2001) Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol 127: 963–972. [PMC free article] [PubMed] [Google Scholar]
- 180.Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37: 801–814. [DOI] [PubMed] [Google Scholar]
- 181.Turner BL. (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96: 698–702. [Google Scholar]
- 182.Tynan KM, Wilkinson CJ, Holmes JM, Dell B, Colquhoun IJ, McComb JA, Hardy GESJ. (2001) The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Aust J Bot 49: 761–770. [Google Scholar]
- 183.Van Damme M, Zeilmaker T, Elberse J, Andel A, de Sain-van der Velden M, Van den Ackerveken G. (2009) Downy mildew resistance in Arabidopsis by mutation of HOMOSERINE KINASE. Plant Cell 21: 2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Varadarajan DK, Karthikeyan AS, Matilda PD, Raghothama KG. (2002) Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol 129: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walker TW, Syers JK. (1976) The fate of phosphorus during pedogenesis. Geoderma 15: 1–9. [Google Scholar]
- 186.Wardle DA, Walker LR, Bardgett RD. (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305: 509–513. [DOI] [PubMed] [Google Scholar]
- 187.Wardle DA, Bardgett RD, Walker LR, Peltzer DA, Lagerström A. (2008) The response of plant diversity to ecosystem retrogression: evidence from contrasting long-term chronosequences. Oikos 117: 93–103. [Google Scholar]
- 188.Watt M, Evans JR. (1999) Proteoid roots. Physiology and development. Plant Physiol 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weste G, Marks GC. (1987) The biology of Phytophthora cinnamomi in Australasian forests. Annu Rev Phytopathol 25: 207–229. [Google Scholar]
- 190.Whelan RJ, Burbidge AH. (1980) Flowering phenology, seed set and bird pollination of five Western Australian Banksia species. Aust J Ecol 5: 1–7. [Google Scholar]
- 191.White AK, Metcalf WW. (2007) Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61: 379–400. [DOI] [PubMed] [Google Scholar]
- 192.Wilkinson CJ, Holmes JM, Tynan KM, Colquhoun IJ, McComb JA, Hardy GESJ, Dell B. (2001) Ability of phosphite applied in a glasshouse trial to control Phytophthora cinnamomi in five plant species native to Western Australia. Australas Plant Pathol 30: 343–351. [Google Scholar]
- 193.Woo J, MacPherson CR, Liu J, Wang H, Kiba T, Hannah MA, Wang X-J, Bajic VB, Chua N-H. (2012) The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wood B. (2011) The effect of phosphorus addition on cluster-root formation of Banksia attenuata and its interaction with neighbouring arbuscular mycorrhizal species. Honours thesis, The University of Western Australia. [Google Scholar]
- 195.Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- 196.Xiao K, Liu J, Dewbre G, Harrison M, Wang ZY. (2006) Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula. Plant Biol 8: 439–449. [DOI] [PubMed] [Google Scholar]