We tested the impacts of temperature and variable food availability on the development and metabolic rate of the larvae of a coral reef damselfish, Amphiprion percula. Our results suggest that larval fishes will be severely impacted, both independently and synergistically, by climate-change related elevated temperatures and reductions in food supply.
Keywords: Connectivity, developmental rate, population viability, thermal reaction norm
Abstract
Climate-change models predict that tropical ocean temperatures will increase by 2–3°C this century and affect plankton communities that are food for marine fish larvae. Both temperature and food supply can influence development time, growth, and metabolism of marine fishes, particularly during larval stages. However, little is known of the relative importance and potential interacting effects of ocean warming and changes to food supply on the performance of larval fishes. We tested this for larvae of the coral reef anemonefish, Amphiprion percula, in an orthogonal experiment comprising three temperatures and three feeding schedules. Temperatures were chosen to represent present-day summer averages (29.2°C) and end-of-century climate change projections of +1.5°C (30.7°C) and +3°C (32.2°C). Feeding schedules were chosen to represent a reduction in access to food (fed daily, every 2 days, or every 3 days). Overall, larvae took longer to settle at higher temperatures and with less frequent feeding, and there was a significant interaction between these factors. Time to metamorphosis was fastest in the 30.7oC and high food availability treatment (10.5 ± 0.2 days) and slowest in the 32.2oC and low food availability treatment (15.6 ± 0.5 days; i.e. 50% faster). Fish from the lower feeding regimens had a lower body condition and decreased survivorship to metamorphosis. Routine oxygen consumption rates were highest for fish raised at 32.2°C and fed every third day (162 ± 107 mg O2 kg−1 h−1) and lowest for fish raised at 29.2°C and fed daily (122 ± 101 mg O2 kg−1 h−1; i.e. 35% lower). The elevated routine oxygen consumption rate, and therefore greater energy use at higher temperatures, may leave less energy available for growth and development, resulting in the longer time to metamorphosis. Overall, these results suggest that larval fishes will be severely impacted by climate-change scenarios that predict both elevated temperatures and reduced food supply.
Introduction
Climate-change models predict that tropical sea surface temperatures will increase by up to 3°C this century (Ganachaud et al., 2011; Lough, 2007; Meehl et al., 2007). Although global warming is projected to occur more slowly in tropical than in temperate regions (Meehl et al., 2007), tropical ectothermic species may be especially vulnerable to rising temperatures because many have a narrower thermal tolerance range than equivalent temperate species (Pörtner and Farrell, 2008; Sunday et al., 2011) and tend to live closer to their thermal optimum; therefore, even relatively small increases in temperature could lead to declines in individual performance (Stillman, 2003; Tewksbury et al., 2008). Furthermore, many biotic effects of warming are mediated through metabolic rate, which is a fundamental measure of physiological activity. In ectotherms, metabolic rate increases exponentially rather than linearly with temperature (Gillooly et al., 2001; Dillon et al., 2010). Consequently, an increase in metabolic rate caused by warming would require organisms to increase food intake in order to gain enough energy to cover basic functions, including growth. However, food is rarely unlimited in the natural environment; therefore, understanding the interaction between warming and food supply is important for predicting climate-change impacts on ectotherms in the tropics.
Coral reef ecosystems may be especially sensitive to ocean warming because coral bleaching and subsequent mortality is often linked to high temperatures (Glynn, 1991; Hoegh-Guldberg, 1999). Degradation of coral reef habitat has negative consequences for many coral-associated organisms, such as coral reef fish (Wilson et al., 2006; Munday et al., 2008a; Pratchett et al., 2008). Direct physiological consequences of climate change on reef fishes will further compound these effects. Recent laboratory experiments have shown that higher temperatures, in the range predicted for the end of this century, (i.e. up to 3°C higher than current summer averages), lead to reductions in aerobic scope (Nilsson et al., 2009), critical swimming speeds (Johansen and Jones, 2011), somatic growth (Munday et al., 2008b), and reproductive output (Donelson et al., 2010) of adult reef fishes.
Sensitivity to temperature may be magnified during early life stages (e.g. eggs, larvae), which are the key stages for mortality, dispersal, and connectivity (Houde, 1989; Wood and McDonald, 1997). Larval coral reef fishes typically exhibit increased growth rates and shorter pelagic larval durations (PLDs) with increasing temperatures within their natural temperature range (McCormick and Moloney, 1995; Wilson and Meekan, 2002; Green and Fisher, 2004; Sponaugle et al., 2006; Takahashi et al., 2012). However, little is known about the effects that climate-change-associated elevated temperatures, above the normal range, will have on these traits (Pankhurst and Munday, 2011). While some studies have predicted that increased temperatures will lead to increased growth, shorter PLDs, and higher survivorship (e.g. O'Connor et al., 2007), others have suggested that more variable survival is likely due to a higher starvation risk associated with higher metabolic rate (Houde, 1989; Munday et al., 2012), and that connectivity patterns may be altered due to shorter PLDs (Munday et al., 2009).
The impact of climate change on the availability of food for larval coral reef fishes is uncertain. Elevated ocean temperatures are predicted to affect the structure of plankton communities that are a food source for larvae (Hays et al., 2005; Richardson, 2008; Barton et al., 2013). Changes to plankton communities will vary, but in many locations these communities may become less productive because higher temperatures favour longer, less productive planktonic food chains (McKinnon et al., 2007; Morán et al., 2010). Greater thermal stratification of the water column will reduce nutrient enrichment of the surface layers that are the most important for planktonic productivity (Poloczanska et al., 2007; Brander, 2010; Doney et al., 2012). Steinacher et al. (2010) reported results from four climate-change models predicting a 2–20% reduction in global marine primary production by 2100, with declines in mid to low latitudes due to reduced nutrient input into the eutrophic zone. Future changes in plankton communities will be superimposed on a resource that is inherently variable on a broad range of spatial and temporal scales (Sorokin and Sorokin, 2009). Owing to the likely effects of increasing ocean temperature on the productivity of plankton communities, and their inherent variability, it is important to understand the consequences of these changes for planktivorous organisms.
The effects of food supply on larval fishes are well known, with both faster growth and shorter PLDs being observed with increased food supply (McCormick and Molony, 1992; Green and McCormick, 1999; Meekan et al., 2003; Sponaugle et al., 2009). However, with regard to climate change and the potential change in plankton productivity, it is the interaction between food supply and temperature that may hold the most relevance. While the growth rate of fishes with an unlimited food supply generally increases with increasing temperature, the effects of temperature may be detrimental in food-poor environments. Higher metabolic rates with increasing temperature may lead to faster growth, but only if the availability of food is sufficient to fuel the higher metabolic demands (Munday et al., 2008a). Food is rarely unlimited in the natural environment, and fish on a fixed ration may not grow as fast with increasing temperature, owing to increasing energetic demands.
In this study, we experimentally investigated the independent and interacting effects of elevated water temperature and varying food availability on the larval development and performance of the coral reef damselfish, Amphiprion percula. We reared larvae at three temperatures and with three food supplies in a full orthogonal design to examine effects on larval duration, larval growth, body condition, survivorship, and metabolic rate. We tested the following hypotheses: (i) elevated temperatures would decrease PLD and increase average daily growth, length at metamorphosis, body condition at metamorphosis, and survivorship to metamorphosis; (ii) decreased food supply would increase PLD and decrease daily growth, length at metamorphosis, body condition at metamorphosis, and survivorship to metamorphosis; and (iii) increased temperature would increase metabolic rate, leading to a lower performance at the same level of food availability.
Materials and methods
Study species and brood stock maintenance
Five breeding pairs of the coral reef damselfish (A. percula) were captured from reefs in the Cairns region of northern Great Barrier Reef and transported to the Marine and Aquaculture Research Facilities Unit at James Cook University, Townsville, Queensland, Australia. Pairs were maintained at 29 ± 0.5°C in 60 l outdoor aquaria and fed 0.075 g of Aquaculture Nutrition 12/20 pellets (Proaqua Pty Ltd, Coorparoo, Queensland, Australia) twice per day. Pairs were provided with half a terracotta pot for shelter and to serve as a structure for egg deposition. The pots were inspected each morning for the presence of eggs.
Larval rearing conditions
On the afternoon when hatching was predicted (6–8 days after eggs were laid), pots containing the eggs were transferred to another 60 l aquarium (29.2°C) inside an experimental laboratory, where hatching occurred within a few hours after darkness. Larvae were reared in the 60 l aquarium for two days in a semi-closed system; kept isolated during the day to facilitate feeding, then slowly flushed with filtered seawater each morning prior to light. Each morning after flushing, green Nannochloropsis spp. paste (Reed Mariculture, Campbell, CA, USA) was added to the water until the bottom of the aquarium could not be seen, equating to ∼4 million cells ml−1 (Moorhead and Zeng, 2011). This was done to dissipate light and maintain the nutritional value of the rotifers (Brachionus sp.) that were fed to the larvae at a density of 10 rotifers ml−1 each morning for the first two days. The number of larvae surviving until the third day ranged from ∼50 to 400 among clutches of eggs.
Experimental design
Larval A. percula were subjected to three feeding regimens and three temperatures in a full orthogonal design (i.e. nine treatments). Temperatures were chosen to represent present-day summer averages in the Cairns region of the Great Barrier Reef where the brood stock were collected (29.2°C) and relevant end-of-century climate change projections for this location; i.e. +1.5°C (30.7°C) and +3°C (32.2°C). Food availability was manipulated by increasing the time lag between feeds, with larvae being provided with constant food daily, every second day, or every third day.
On the third morning post-hatch (before feeding), larvae from each clutch that were visually in good condition (i.e. displaying normal swimming behaviour and balance) were gently collected in a glass beaker and arbitrarily distributed among nine to 18 (one or two vessels per treatment, depending on the number of larvae in the clutch) 3 l culture vessels made of 150 mm polyvinyl chloride pipe as described by Moorhead and Zeng (2011). Five to 10 larvae were stocked in each replicate culture vessel at each treatment level. Three culture vessels were placed in each of three to six temperature-controlled water baths (one or two for each temperature treatment). The entire protocol was repeated seven times using progeny from four adult pairs until each treatment level had a starting sample size of >100 individuals in 11 replicate culture vessels per treatment (Table 1). The temperature of the water baths and the location of the vessels within the water baths were randomly modified for each run of the experiment to negate any influence of individual vessels or location within the laboratory on results.
Table 1:
Number of culture vessels (replicate tanks), number of larval Amphiprion percula stocked in each treatment level at the start of the experiment, number of vessels containing live larvae at the end of the experiment, and number of surviving larvae among vessels at the conclusion of the experiment
| Temperature (°C) | Food availability | Number of vessels | Number of larvae stocked at start | Number of vessels with surviving larvae | Number of surviving larvae among vessels |
|---|---|---|---|---|---|
| 29.2 | L | 11 | 104 | 7 | 20 |
| 29.2 | M | 11 | 105 | 6 | 23 |
| 29.2 | H | 11 | 105 | 8 | 51 |
| 30.7 | L | 11 | 105 | 8 | 21 |
| 30.7 | M | 11 | 105 | 7 | 37 |
| 30.7 | H | 11 | 105 | 9 | 46 |
| 32.2 | L | 11 | 105 | 8 | 28 |
| 32.2 | M | 11 | 105 | 8 | 38 |
| 32.2 | H | 11 | 105 | 8 | 36 |
Abbreviations: H, fed every day; L, fed every third day; and M, fed every second day.
All larval fish were fed immediately after transfer to the experimental set-up. In addition to the rotifers, their diet was enriched with newly hatched Artemia sp. nauplii (INVE technologies, Amphur Pakkred, Thailand) fed at a rate of 1 ml−1. The vessels were ‘greened’ with Nannochloropsis spp. as described in the previous subsection. Temperatures were increased slowly (by 0.5–1°C every 8 h, dependent on treatment) over 24 h to reach treatment levels. Feeding manipulations began the day after transfer. Water exchange of vessels was carried out each morning using filtered seawater before the lights came on to flush out uneaten prey and faeces. The photoperiod was maintained at 14 h light–10 h dark during the trials.
Larvae were carefully checked for metamorphosis by torchlight each morning before they had an opportunity to feed. Larvae were considered metamorphosed when their post-orbital stripe became fully pigmented, which always occurred between eight and 19 days post hatch. This pigmentation coincides with a shift in habitat use and a change to benthic colouration (I. M. McLeod, personal observation), which is typical of most damselfishes (McCormick et al., 2002) and has been used as a diagnostic tool for metamorphosis and settlement for a congeneric species, Amphiprion melanopus (Green and McCormick, 1999).
Standard length and body condition
Metamorphosed larvae were removed from culture vessels and killed using an overdose of clove oil. Larvae were then immediately transferred to a 4% phosphate-buffered formaldehyde solution, and the following measurements were taken within 48 h. Larvae were removed from the preservative, blotted dry, weighed (to the nearest 0.1 mg), and photographed in a lateral position on a 0.5 mm plastic grid. Standard length (SL) to the nearest 0.01 mm was estimated for each fish from the digital photograph using image analysis software (ImageJ version 1.45 s; National Institutes of Health, USA). Body condition (hereafter, condition factor) was calculated as the blotted weight at metamorphosis after controlling for standard length at metamorphosis using analysis of covariance (ANCOVA).
Pelagic larval duration and average daily growth
Individual PLD was calculated as the number of days between hatching and metamorphosis. Larval growth was assumed to be linear during the larval stage, as it is for the congener A. melanopus (Green and Fisher, 2004). Individual growth rates were estimated according to the formula: Rg = (Lm − Lh)/Tm, where Rg is the rate of growth in millimetres per day, Lm is the standard length (in millimetres) at metamorphosis, Lh is the standard length at hatching (=3.79 mm), and Tm is the time (in days) from hatching to metamorphosis. Standard length at hatching was determined by sampling 10 newly hatched A. percula each from three clutches. These larvae were measured using the same methodology as for the metamorphosed juveniles. The mean standard length at hatching among clutches was used for the hatch length in the above calculations.
Survivorship
Survivorship was calculated as the number of larvae placed into each vessel, minus the number of larvae that did not survive to metamorphosis.
Larval respirometry
Intermittent-flow respirometry was used to determine routine O2 consumption rates (M·O2Routine), a standard measure of metabolic rate (Willmer et al., 2005). Oxygen consumption rates were measured at 8 days post-hatch for larvae raised at the lowest (29.2°C) and highest (32.2°C) temperatures and the lowest (food available every third day) and highest (food available daily) food availability treatments. A total of 43 larvae were tested (seven to 16 per treatment). Larvae were starved for 24–26 h before trials began.
Larvae were placed individually into 4.8 ml glass vials that served as respirometry chambers. Chambers were submerged in an aquarium maintained at the same temperature at which the larvae were reared. To reduce light levels and external disturbance, a lid was placed over the opaque aquarium during the habituation period and left in place during measurements of M·O2Routine. Water was continuously recirculated within each respirometer using a closed circuit connected to a peristaltic pump, which ensured homogeneous O2 tension throughout the apparatus. The total volume of the respirometer chamber and tubing circuit was 1 ± 1 ml. A submersible pump connected to a timer was used to flush the respirometer chambers with aerated water intermittently at 5 ml min−1, and excess water flushed from each respirometer overflowed from a small standpipe that extended immediately above the water surface in the aquarium.
Preliminary observations indicated that larvae took 15 min in the respirometer to exhibit normal behaviour, an observation that was supported by M·O2Routine measurements plateauing after this time (I. M. McLeod, unpublished data). To avoid the early period of stress, M·O2Routine measurements commenced ∼15 min after the larvae entered the respirometer and continued for a total of four 15 min measurements separated by 5 min flush cycles.
The temperature-compensated O2 concentration of the water within each chamber was continuously recorded (1 Hz) using oxygen-sensitive REDFLASH dye on contactless spots (2 mm) adhered to the inside of each chamber and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via fibre-optic cables. To reduce background bacterial O2 consumption, seawater used for the respirometers was UV sterilized, and the system was cleaned with 70% ethanol each day, or more often if background respiration exceeded 10%. In addition, background respiration was measured before and after each trial and used to correct fish M·O2Routine measurements assuming a linear change in background respiration (i.e. subtracted from the values calculated for the whole animal). At the end of the respirometry trials, larvae were killed with an overdose of clove oil, blotted dry with a paper towel, and weighed with scales accurate to 0.1 mg.
Data analysis
Two-factor ANOVA followed by Tukey's post hoc comparisons of means were used to test the effects of temperature and food availability on PLD, average daily growth, standard length at metamorphosis, individual metabolic rate, and weight-adjusted metabolic rate. The assumptions of normality and homogeneous variances for each performance variable were tested using Levene's test and graphically analysed using residual and Q–Q plots. A natural log transformation was required for the PLD data and a square root transformation was required for individual metabolic rate data to conform to the assumption of homogeneity of variance.
Condition factor at metamorphosis was compared among treatments using a two-factor ANCOVA. Temperature and food availability were the independent variables, weight at metamorphosis was the dependent variable, and standard length at metamorphosis was the covariate. The adjusted weight at metamorphosis from this analysis, which controlled for standard length at metamorphosis, was used as our measure of body condition (condition factor). There was a linear relationship between weight and standard length for each treatment, as assessed by visual assessment of a scatterplot. Standardized residuals for the treatments and for the overall model were normally distributed, as assessed by Shapiro–Wilks test. There was homoscedasticity and homogeneity of variances, as assessed by visual inspection of a scatterplot, and Levene's test of homogeneity of variance, respectively. Post hoc analysis for the ANCOVA was performed with a Bonferroni adjustment.
Individual fish within vessels were pooled across vessels for the analysis. A nested ANOVA design was not appropriate, because some vessels had only one fish surviving to metamorphosis. Using the mean value for each vessel produced near-identical results in performance variables (revealed through ANOVA and ANCOVA). The exception was average daily growth, where there was no significant interaction between the temperature and food availability treatments using the mean vessel values, but there was a significant interaction when individuals were pooled among vessels. This was because of low statistical power due to the limited number of vessels with surviving larvae per treatment (six to nine among trials; Table 1). Partial eta-squared (η2) was calculated as part of the ANOVA and ANCOVA analyses, to provide a standardized measure of the relative effects of treatments on performance variables.
There were significant differences in performance variables among clutches, but consistent trends in effects of temperature and food availability (revealed through careful analysis of stem-and-leaf plots and histograms). Given that clutch effects were not the focus of the present study, individuals were pooled across clutches for the analysis. Logistic regression was used to ascertain the effects of temperature and food availability on the likelihood that individual larvae survived to metamorphosis. Individual larvae were also pooled across vessels for this analysis.
Larval respirometry data for a total of 43 larvae were analysed using LabChart version 6.1.3, (AD Instruments, Colorado Springs, CO, USA). The M·O2Routine (in milligrams per kilogram per hour) was calculated from the average of the final three slopes of O2 concentration vs. time, minus the background O2 consumption. The Q10 temperature coefficient was calculated using the following formula: Q10 = (R2/R1)10/(T2 −T1), where T1 and T2 are the temperatures over which the change was recorded, R1 is the value of the measured variable at T1, and R2 is the value of the variable at T2. All statistical analyses were conducted using the statistical package SPSS Statistics version 20 (IBM™ SPSS™ Inc. 2011).
Results
Pelagic larval duration and growth
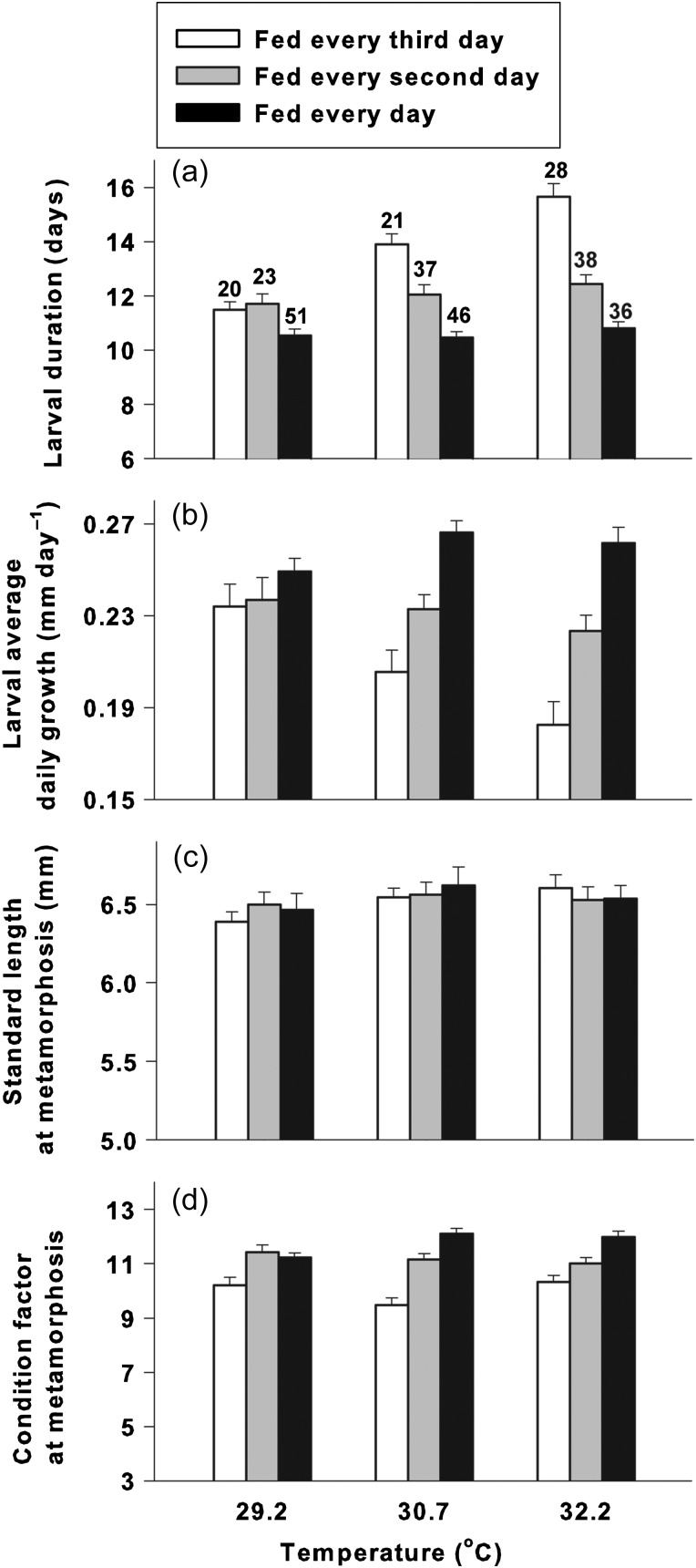
The average PLD of A. percula ranged from 10.5 ± 0.2 days (mean ± SEM) in the 30.7°C and high food availability treatment to 15.6 ± 0.5 days in the 32.2°C and low food availability treatment (i.e. a 50% increase; Fig. 1a). There was a significant interaction (F4,291 = 6.4, P < 0.001, partial η2 = 0.081) between temperature and food availability on PLD (Table 2). Access to food had a stronger effect (partial η2 = 0.286) than temperature (partial η2 = 0.093). The significant interaction was due to a much greater effect of food availability on PLD at elevated temperatures compared with the baseline temperature (29.2°C). Post hoc tests showed that at 29.2°C the individual PLDs were significantly longer in the medium (fed every second day) than in the high (fed every day) food availability treatment. At 30.7°C, PLDs were significantly longer for the medium and low food availability treatments compared with the high food availability treatment, and PLDs were significantly longer for the low compared with the medium food availability treatment.
Figure 1:
Mean pelagic larval duration (a), larval average daily growth (b), standard length at metamorphosis (c), and body condition at metamorphosis (expressed as weight in milligrams adjusted for standard length; d) for Amphiprion percula raised at 29.2, 30.7, and 32.2°C on a low (open bars), medium (shaded bars) or high level of food availability (filled bars). Error bars denote ± SEM. The numbers above bars indicate numbers of larvae included in the analysisQ10.
Table 2:
ANOVA and ANCOVA table for pelagic larval duration, larval average daily growth, standard length at metamorphosis, condition at metamorphosis (ANCOVA), and routine metabolism (M·O2Routine; individual and weight adjusted) for larval A. percula reared in nine combinations of water temperature and food availability
| Source | d.f. | Mean square | F | P value | Effect size (partial η2) |
|---|---|---|---|---|---|
| Pelagic larval duration | |||||
| Temperature | 2 | 0.366 | 15 | <0.0001* | 0.093 |
| Food availability | 2 | 1.42 | 58.3 | <0.0001* | 0.286 |
| Temperature × food availability | 4 | 0.156 | 6.39 | <0.0001* | 0.081 |
| Error | 291 | 0.024 | |||
| Average daily growth | |||||
| Temperature | 2 | 0.0000748 | 4.198 | 0.016* | 0.028 |
| Food availability | 2 | 0.001 | 35.132 | <0.0001* | 0.194 |
| Temperature × food availability | 4 | 0.0000821 | 4.609 | 0.001* | 0.06 |
| Error | 291 | 0.0000178 | |||
| Standard length at metamorphosis | |||||
| Temperature | 2 | 0.381 | 1.782 | 0.17 | 0.012 |
| Food availability | 2 | 0.018 | 0.084 | 0.919 | 0.001 |
| Temperature × food availability | 4 | 0.092 | 0.431 | 0.786 | 0.006 |
| Error | 291 | 0.214 | |||
| Condition factor at metamorphosis (ANCOVA) | |||||
| Length (covariate) | 1 | 0.001 | 458.26 | <0.0001 | 0.612 |
| Temperature | 2 | 1.15 | 0.687 | 0.504 | 0.005 |
| Food availability | 2 | 69.8 | 41.944 | <0.0001* | 0.224 |
| Temperature × food availability | 4 | 7.346 | 4.41 | 0.002* | 0.057 |
| Error | 290 | 1.67 | |||
| Individual M·O2Routine | |||||
| Temperature | 1 | 0 | 19.95 | <0.0001* | 0.216 |
| Food availability | 1 | 0 | 10.7 | 0.002* | 0.338 |
| Temperature × food availability | 1 | 0.0000255 | 2.56 | 0.117 | 0.062 |
| Error | 39 | 0.00001 | |||
| Individual M·O2Routine (weight adjusted) | |||||
| Temperature | 1 | 306 | 6.48 | 0.015* | 0.142 |
| Food availability | 1 | 1.82 | 0.039 | 0.845 | 0.001 |
| Temperature × food availability | 1 | 1.94 | 0.041 | 0.84 | 0.001 |
| Error | 39 | 47.2 | Error | 39 | 47.2 |
There was also a significant interaction (F4,291 = 4.6, P = 0.01, partial η2 = 0.060) between temperature and food availability on the average growth rate between hatching and metamorphosis (Table 2 and Fig. 1b). Again, the food availability had a stronger effect (partial η2 = 0.194) than temperature (partial η2 = 0.0280). The interaction was due to a much greater effect of food availability on average daily growth at elevated temperatures compared with the baseline temperature (29.2°C). Specifically, temperature had no effect on growth for the high or medium food availability treatments, but for the low food availability treatment, growth was significantly lower at 32.2 than at 29.2°C. Growth ranged from 0.27 ± 0.0052 mm day−1 in the 30.7°C and high food supply treatment to 0.018 ± 0.01 mm day−1 in the 32.2°C and low food availability treatment (i.e. a 31% decrease; Fig. 1b).
Standard length at metamorphosis
Amphiprion percula metamorphosed at a standard length of 6.5 ± 0.5 mm across all treatments (Fig. 1c). There was no significant effect of temperature (F2,291 = 1.8, P = 0.17, partial η2 = 0.012) or access to food (F2,291 = 0.09, P = 0.92, partial η2 = 0.001) on SL at metamorphosis, and no interaction between temperature or food availability on SL at metamorphosis (F4,291 = 0.43, P = 0.79, partial η2 = 0.006; Table 2).
Body condition (condition factor) at metamorphosis
After adjusting for standard length at metamorphosis (through an ANCOVA), there was a significant interaction (F4,290 = 4.41, P = 0.02, partial η2 = 0.057) between temperature and food availability on the average blotted weight at metamorphosis (condition factor; Table 2 and Fig. 1d). The significant interaction was due to a greater effect of food availability for the 30.7°C treatments than at 29.2°C. Mean condition factor ranged from 11.2 at 30.7°C and with high food availability to 9.47 with the low food availability at that temperature. Food availability (partial η2 = 0.224) had a stronger effect than temperature (partial η2 = 0.005). Condition factor was always lower in the low food availability treatments than in the high or medium food availability treatments.
Survival to metamorphosis
Among treatments, 31.8% of larvae survived to metamorphosis, ranging from 19.0% in the 29.2°C, low food availability treatment to 49.0% in the 29.2°C, high food availability treatment (Table 1). The logistic regression model (x2(2) = 16, P < 0.001) explained 49.7% of the variance in survival to metamorphosis and correctly classified 62.7% of cases. Food availability but not temperature was a statistically significant predictor of survival to metamorphosis. Larvae in the high food availability treatments (42.4% survival) were 1.4 times more likely to survive than those in the medium food availability treatments (31.1%), and 1.9 times more likely to survive to metamorphosis than those in the low food availability treatments (21.9%).
Larval respirometry
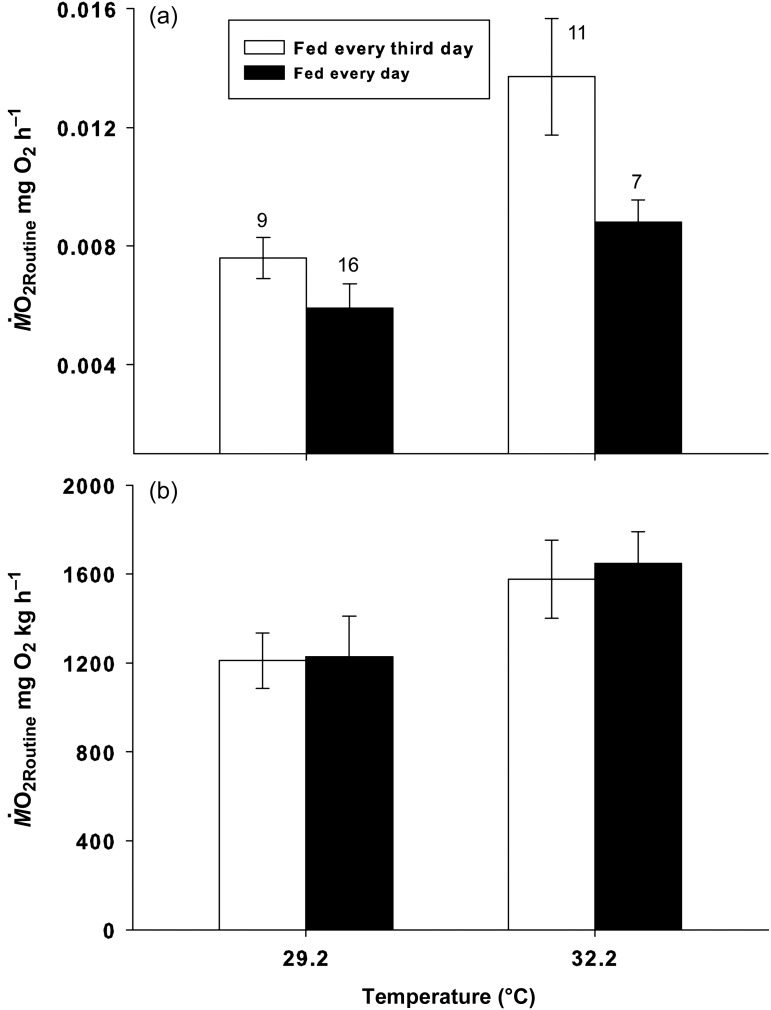
Both temperature (F1,39 = 20, P < 0.0001, partial η2 = 0.338) and food availability (F1,39 = 10.7, P = 0.002, partial η2 = 0.216) significantly affected individual larval O2 consumption. However, there was no interaction between temperature and food availability (F1,39 = 2.56, P = 0.117, partial η2 = 0.062). Mean O2 consumption ranged from 0.0059 ± 0.00082 mg O2 h−1 for larvae at 29.2°C and fed every third day to 0.013 ± 0.0059 mg O2 h−1 for larvae at 32.2°C and fed every day (i.e. a 120% increase; Fig. 2a). However, fish in the high food availability treatments were heavier on day 8 when they were tested. When M·O2Routine was corrected for the individual weights of the larval fish, temperature (F1,39 = 6.5, P = 0.015, partial η2 = 0.142; Fig. 2b) but not food supply (partial η2 = 0.001) significantly affected M·O2Routine, with no significant interaction. Mean M·O2Routine was 122 ± 101 mg O2 kg−1 h−1 at 29.2°C and 162 ± 107 mg O2 kg−1 h−1 at 32.2°C (i.e. a 33% increase). The Q10 coefficient for M·O2Routine calculated over this 3°C increase in temperature was 2.59.
Figure 2:
Mean routine individual oxygen consumption rates (M·O2Routine; expressed as milligrams of O2 per hour; a) and weight-adjusted individual oxygen consumption rates (M·O2Routine; expressed as milligrams of O2 per kilogram per hour; b) for 8-day-old A. percula raised at 29.2 and 32.2°C on a low (open bars) or high level of food availability (filled bars). Error bars denote ± SEM. The numbers above bars indicate numbers of larvae included in the analysis.
Discussion
Our results suggest that climate-induced increases in ocean temperature and variation in planktonic food supply may impact the development and metabolism of larval reef fishes. Overall, larval A. percula grew more slowly and took longer to metamorphose and settle at higher temperatures and with reduced access to food, with a highly significant interaction between these factors. However, neither temperature nor access to food affected the length at metamorphosis. Fish from the lower access to food treatments had a lower condition factor and decreased survivorship. Temperature affected routine oxygen consumption rates (M·O2Routine), which were significantly higher at 32.2°C than at 29.2°C. The differences in individual M·O2Routine between the high and low food availability treatments were explained by the lower weight of the larvae fed every third day. When the metabolic rate was corrected for individual weight, there was no influence of food availability on M·O2Routine.
The direct effects of temperature included an increase in PLD, a lower growth rate, and an increase in metabolic rates at higher temperatures. Previous research investigating the effects of temperature on larval coral reef fish growth and PLD showed that higher temperatures lead to increased growth and shorter PLDs (e.g. McCormick and Moloney, 1995; Green and Fisher, 2004; Sponaugle et al., 2006; Takahashi et al., 2012). Furthermore, in a recent review, Munday et al. (2009) postulated that the limited evidence available suggests that a 3°C increase in sea surface temperatures would reduce the PLD of larval reef fishes by 12–25%. However, this estimate was made by extrapolating results from experiments and field studies looking at temperatures within their natural range, because there were few data available regarding the effects of elevated temperatures on these traits. Importantly, our results show that developmental rates and PLDs may not continue a linear relationship with temperature beyond the range of temperatures typically experienced by the population. Consequently, extrapolations based on present-day variation may be inappropriate for projecting the consequences of future temperature increases on the early life history traits of tropical fishes.
Food availability affected survivorship to metamorphosis, with reduced survivorship in the lower food availability treatments. Amphiprion percula are capable of feeding immediately upon hatching, and feeding treatments were not commenced until the fourth day post-hatch. Hjort (1914) first emphasized the importance of food limitation at the time of first feeding for fish larvae. If food was reduced over the first three days, overall survivorship may have been reduced further, suggesting that our findings, although substantial and significant, may still be conservative. In nature, mortality is extreme in the larval phase, and so small changes in mortality rates could have important ramifications for recruitment to adult populations (Jones, 1991; Leis, 1991).
Access to food had a major impact on growth rates and PLD, with longer PLDs and slower average growth rates in the lower food availability treatments. Food availability also had a profound effect on condition factor at metamorphosis, with lower food availability treatments resulting in lower weight for a given body length. These results are consistent with previous experiments that tested the effects of reduced food for larval coral reef fish (e.g. McCormick and Molony, 1992; Green and McCormick, 1999). Lowered condition factor and growth associated with suboptimal feeding in the larval environment is likely to affect the likelihood of survival well after the fish have metamorphosed and settled (Hoey and McCormick, 2004; Gagliano et al., 2007; McCormick and Gagliano, 2009). Poor feeding history has a lasting effect on metabolism, and this may lead to behavioural trade-offs associated with the balance between feeding and predator vigilance (Metcalfe and Monaghan, 2001). Fishes that have had poor growth histories sometimes compensate by over-performing in growth once feeding conditions improve, and this over-performance in growth can be at the cost of behaviours that directly affect survival (Pechenik, 1990; Gagliano and McCormick, 2007).
The interacting effects of elevated temperature and reduced food availability resulted in much longer PLDs and slower growth rates, suggesting that the harmful effects of ocean warming are likely to be most severe if accompanied by a declining planktonic food supply. The increase in PLD would be likely to lead to important ecological consequences, because the larval life stage has the highest risk of mortality through predation (Houde, 1987; Bailey and Houde, 1989). A longer PLD increases the length of time that larval fish are exposed to the high-risk pelagic environment, thus indirectly reducing the probability of survival. This contrasts with predictions of increased larval survival by some analyses that have primarily synthesized PLD data for many species within their present-day temperature range (e.g. O'Connor et al., 2007). Predictions of increased larval survival may be inaccurate, especially for tropical species, if they do not account for the shape of the thermal reaction norm in PLD above the range of natural variation and incorporate the possible effects of reduced food supply (Steinacher et al., 2010). For those larvae that survive longer PLDs, this might have some implications for the spatial scale of larval dispersal, and thus, the scale of connectivity for some populations, which could have flow-on effects for population dynamics and sustainability (Munday et al., 2009).
It is difficult to estimate the relative importance of temperature and food supply for larval performance in the wild using the results of this study, because very little is known about the temporal variability in food supply and larval feeding in the wild. Owing to this lack of information and logistical constraints, the food treatments used in the present study were chosen to represent high, medium, and low access to food for comparative purposes, and may not be indicative of natural food supply. Nevertheless, they enabled us to examine experimentally the relationship between metabolism and food availability, and how performance variables are affected. In our experiments, the difference in effect sizes show that food supply was more important than temperature for PLD, larval growth, and condition factor. These results contrast with those of Meekan et al. (2003), who showed that over two consecutive summers, water temperature explained 30% and zooplankton abundance only 3.5–4.1% of the variance in growth for a tropical damsel fish, Pomacentrus coelestis.
In our study, metabolic rate increased with increased temperature, a result consistent with findings from previous studies (reviewed by Houde, 1989; Rombough, 1997; Peck et al., 2012). In a recent review, Peck et al. (2012) found that a 10°C increase in temperature was accompanied by a 1.2- to 4.3-fold increase in larval M·O2Routine (Q10 = 1.2–4.3) across 15 families of marine fish. In the present study, the Q10 was ∼2.6, similar to the average Q10 (2.31) found by Peck et al. (2012). Elevated M·O2Routine and therefore energy use at higher temperatures may leave less energy available for growth, resulting in the longer time to metamorphosis, especially when food supplies are low. In our laboratory experiment, the tanks were static, with no flow during the day, and with high concentrations of nutritious prey, so overall energy use is likely to be lower than in nature. Consequently, the effects of reduced access to food may be more severe in the natural environment than the results of this experiment indicate, because larvae in nature must swim against ocean currents and are likely to consume prey of lesser nutritional value.
Larvae settled at a remarkably consistent length across treatments, indicating that reaching a precise size was important for metamorphosis in A. percula. Fish in the lower access to food treatments also had a lower condition factor, indicating that it is taking longer for the fish in the lower food availability treatments to become competent to settle, rather than delaying metamorphosis as some other coral reef will do when denied access to the preferred settlement structure (McCormick, 1999). Across marine fish species, there is more variation in age than size at metamorphosis (Chambers and Leggett, 1987). However, our results contrast with results for a congener, A. melanopus, in which length at settlement was significantly longer at lower temperatures (Green and Fisher, 2004) and shorter in lower food availability treatments (Green and McCormick, 1999). A larger size at settlement may offer some survival advantages (Sogard, 1997; Perez and Munch, 2010). Perhaps the disadvantage of settling at a smaller size outweighs the potential reduction in survivorship that would result from an extended PLD.
Conclusions
This study highlights the potential interacting effects that higher ocean temperatures and reductions in food supply will have on larval coral reef fishes. The M·O2Routine was higher at higher temperatures, indicating a potential mechanism for the differences in growth and development in relationship to food supply. Indeed, the greater effect of temperature in the lowest food availability conditions could be because there is insufficient food to meet the increased metabolic costs. Overall, our results indicate that despite previous predictions of a positive influence of ocean warming on larval development and survival, variable food supply alters how temperature affects growth and development. These observations demonstrate that increased temperature and reduced food supply will affect the life history and demography of larval fish, possibly affecting recruitment processes and population dynamics. Further work is required to determine whether these results are general for a range of other fish species. The potential for coral reef fish populations to adapt to climate-change-associated elevated temperatures and variable access to food during the larval phase is unknown, but is a critical area of future research (Munday et al., 2009, 2012). Future studies could use quantitative genetic breeding designs (Lynch and Walsh, 1998) to test the heritability of individual variation in the response of larval fish to elevated temperature and reduced food supply. Such experiments are logistically challenging, but ultimately essential for predicting the potential for populations to adapt to rapid climate change. This work is urgent, given the potential susceptibility of fish larvae to almost every predicted change facing the oceanic environment.
Acknowledgements
The authors would like to thank Colin Wen, Matthew Mitchell, Jennifer Donelson, Jonathan Moorhead, and the staff from the Marine & Aquaculture Research Facilities Unit at James Cook University for their advice and assistance with the experiments. This research was conducted under James Cook University ethics approval A1684. This work was supported by James Cook University Graduate Research Fund grants and Great Barrier Reef Marine Protection Agency Science for Management Awards to I. M. McLeod and A. S. Wenger, AIMS@JCU funding to I. M. McLeod and T. D. Clark, and Australian Research Council Linkage Grant (LP 100200561) funding awarded to G. P. Jones and colleagues.
References
- 1.Bailey KM, Houde ED. (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25: 1–83. [Google Scholar]
- 2.Barton AD, Pershing AJ, Litchman E, Record NR, Edwards KF, Finkel ZV, Kiørboe T, Ward BA. (2013) The biogeography of marine plankton traits. Ecol Lett 16: 522–534. [DOI] [PubMed] [Google Scholar]
- 3.Brander K. (2010) Impacts of climate change on fisheries. J Mar Syst 79: 389–402. [Google Scholar]
- 4.Chambers RC, Leggett WC. (1987) Size and age at metamorphosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Can J Fish Aquat Sci 44: 1936–1947. [Google Scholar]
- 5.Dillon ME, Wang G, Huey RB. (2010) Global metabolic impacts of recent climate warming. Nature 467: 704–706. [DOI] [PubMed] [Google Scholar]
- 6.Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM. (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401: 233–243. [Google Scholar]
- 7.Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, et al. (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4: 11–37. [DOI] [PubMed] [Google Scholar]
- 8.Gagliano M, McCormick MI. (2007) Compensating in the wild: is flexible growth the key to early juvenile survival? Oikos 116: 111–120. [Google Scholar]
- 9.Gagliano M, McCormick MI, Meekan MG. (2007) Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc R Soc B Biol Sci 274: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganachaud AS, Gupta AS, Orr JC, Wijffles SE, Ridgway KR, Hemer MA, Maes C, Steinberg CR, Tribollet AD, Qiu B, et al. (2011) Observed and expected changes to the tropical pacific ocean. In Bell JD, Hobday AJ, eds, Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change. Secretariat of the Pacific Community, Noumea, pp 101–187. [Google Scholar]
- 11.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. (2001) Effects of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- 12.Glynn PW. (1991) Coral reef bleaching in the 1980s and possible connections with global warming. Trends Ecol Evol 6: 175–179. [DOI] [PubMed] [Google Scholar]
- 13.Green BS, McCormick MI. (1999) Influence of larval feeding history on the body condition of Amphiprion melanopus. J Fish Biol 55: 1273–1289. [Google Scholar]
- 14.Green BS, Fisher R. (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299: 115–132. [Google Scholar]
- 15.Hays GC, Richardson AJ, Robinson C. (2005) Climate change and marine plankton. Trends Ecol Evol 20: 337–344. [DOI] [PubMed] [Google Scholar]
- 16.Hjort J. (1914) Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapp P-V. Cons Int Explor Mer 20: 1–228. [Google Scholar]
- 17.Hoegh-Guldberg O. (1999) Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshw Res 50: 839–866. [Google Scholar]
- 18.Hoey AS, McCormick MI. (2004) Selective predation for low body condition at the larval-juvenile transition of a coral reef fish. Oecologia 139: 23–29. [DOI] [PubMed] [Google Scholar]
- 19.Houde ED. (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2: 17–29. [Google Scholar]
- 20.Houde ED. (1989) Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. Fish Bull 87: 471–495. [Google Scholar]
- 21.Johansen JL, Jones GP. (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Change Biol 17: 2971–2979. [Google Scholar]
- 22.Jones GP. (1991) Postrecruitment processes in the ecology of coral reef fish populations: a multifactorial perspective. In Sale PF, ed., The Ecology of Fishes on Coral Reefs. Academic Press, San Diego, pp 183–230. [Google Scholar]
- 23.Leis JM. (1991) The pelagic stage of reef fishes: the larval biology of coral reef fishes. In Sale PF, ed., The Ecology of Fishes on Coral Reefs. Academic Press, San Diego, ppp 183–230. [Google Scholar]
- 24.Lough J. (2007) Climate and climate change on the Great Barrier Reef. In Johnson JE, Marshall PA, eds, Climate Change and the Great Barrier Reef. Great Barrier Reef Marine Park Authority and Australian Greenhouse Office, Australia, pp 14–50. [Google Scholar]
- 25.Lynch M, Walsh B. (1998) Genetics and Analysis of Quantitative Traits. Sinauer Associates, Inc, Massachusetts. [Google Scholar]
- 26.McCormick MI, Molony BW. (1992) Effects of feeding history on the growth characteristics of a reef fish at settlement. Mar Biol 114: 165–173. [Google Scholar]
- 27.McCormick MI, Moloney BW. (1995) Influence of water temperature during the larval stage on size, age and body condition of a tropical reef fish at settlement. Mar Ecol Prog Ser 118: 59–68. [Google Scholar]
- 28.McCormick MI. (1999) Delayed metamorphosis of a tropical reef fish (Acanthurus triostegus): a field experiment. Mar Ecol Prog Ser 176: 25–38. [Google Scholar]
- 29.McCormick MI, Makey L, Dufour V. (2002) Comparative study of metamorphosis in tropical reef fishes. Mar Biol 141: 841–853. [Google Scholar]
- 30.McCormick MI, Gagliano M. (2009) Carry-over effects – the importance of a good start. Proceedings of the 11th International Coral Reef Symposium, pp 305–310. [Google Scholar]
- 31.McKinnon AD, Richardson AJ, Burford MA, Furnas MJ. (2007) Vulnerability of Great Barrier Reef plankton to climate change. In Johnson JE, Marshall PA, eds, Climate Change and the Great Barrier Reef: A Vulnerability Assessment. Great Barrier Reef Marine Park Authority, Townsville, pp 121–152. [Google Scholar]
- 32.Meehl GA, Stocker TF, Collins WD, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, et al. (2007) Global climate projections. In Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, eds, Climate Change 2007: The Physical Science Basis. Cambridge University Press, Cambridge and New York, pp 747–845. [Google Scholar]
- 33.Meekan MG, Carleton JH, McKinnon AD, Flynn K, Furnas M. (2003) What determines the growth of tropical reef fish larvae in the plankton: food or temperature? Mar Ecol Prog Ser 256: 193–204. [Google Scholar]
- 34.Metcalfe NB, Monaghan P. (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16: 254–260. [DOI] [PubMed] [Google Scholar]
- 35.Moorhead JA, Zeng C. (2011) Breeding of the forktail blenny Meiacanthus atrodorsalis: broodstock management and larval rearing. Aquaculture 318: 248–252. [Google Scholar]
- 36.Morán XAG, López-Urrutia T, Calvo-Díaz A, Li WKW. (2010) Increasing importance of small phytoplankton in a warmer ocean. Glob Change Biol 16: 1137–1144. [Google Scholar]
- 37.Munday PL, Jones GP, Pratchett MS, Williams AJ. (2008a) Climate change and the future for coral reef fishes. Fish and Fisheries 9: 261–285. [Google Scholar]
- 38.Munday PL, Kingsford MJ, O'Callaghan M, Donelson JM. (2008b) Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 27: 927–931. [Google Scholar]
- 39.Munday PL, Leis JM, Lough JM, Paris CB, Kingsford M, Berumen ML, Lambrechts J. (2009) Climate change and coral reef connectivity. Coral Reefs 28: 379–393. [Google Scholar]
- 40.Munday PL, McCormick MI, Nilsson GE. (2012) Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future?. J Exp Biol 215: 3865–3873. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson GE, Crawley N, Lunde IG, Munday PL. (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol 15: 1405–1412. [Google Scholar]
- 42.O'Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM. (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA 104: 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pankhurst NW, Munday PL. (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62: 1015–1026. [Google Scholar]
- 44.Pechenik JA. (1990) Delayed metamorphosis by larvae of benthic marine invertebrates: does it occur? Is there a price to pay? Ophelia 32: 63–94. [Google Scholar]
- 45.Peck MA, Huebert KB, Llopiz JK. (2012) Intrinsic and extrinsic factors driving match-mismatch dynamics during the early life history of marine fishes. Adv Ecol Res 47: 177–302. [Google Scholar]
- 46.Perez KO, Munch SB. (2010) Extreme selection on size in the early lives of fish. Evolution 64: 2450–2457. [DOI] [PubMed] [Google Scholar]
- 47.Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, Kunz TJ, Matear R, Milton DA, Okey TA, Richardson AJ. (2007) Climate change and Australian marine life. Oceanogr Mar Biol Annu Rev 45: 409–480. [Google Scholar]
- 48.Pörtner HO, Farrell AP. (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- 49.Pratchett MS, Munday PL, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR. (2008) Effects of climate-induced coral bleaching on coral-reef fishes-ecological and economic consequences. Oceanogr Mar Biol 46: 251–296. [Google Scholar]
- 50.Richardson AJ. (2008) In hot water: zooplankton and climate change. ICES J Mar Sci 65: 279–295. [Google Scholar]
- 51.Rombough PJ. (1997) The effects of temperature on embryonic and larval development. In Wood CM, McDonald DG, eds, Global Warming: Implications for Freshwater and Marine Fish. Cambridge University Press, Cambridge, UK, pp 177–223. [Google Scholar]
- 52.Sogard SM. (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60: 1129–1157. [Google Scholar]
- 53.Sorokin YI, Sorokin PY. (2009) Analysis of plankton in the southern Great Barrier Reef: abundance and roles in throphodynamics. J Mar Biol Assoc UK 89: 235–241. [Google Scholar]
- 54.Sponaugle S, Grorud-Colvert K, Pinkard D. (2006) Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys. Mar Ecol Prog Ser 308: 1–15. [Google Scholar]
- 55.Sponaugle S, Llopiz JK, Havel LN, Rankin TL. (2009) Spatial variation in larval growth and gut fullness in a coral reef fish. Mar Ecol Prog Ser 383: 239–249. [Google Scholar]
- 56.Steinacher M, Joos F, Frölicher TL, Bopp L, Cadule P, Cocco V, Doney SC, Gehlen M, Lindsay K, Moore JK, et al. (2010) Projected 21st century decrease in marine productivity: a multi-model analysis. Biogeosciences 7: 979–1005. [Google Scholar]
- 57.Stillman JH. (2003) Acclimation capacity underlies susceptibility to climate change. Science 301: 65. [DOI] [PubMed] [Google Scholar]
- 58.Sunday JM, Bates AE, Dulvy NK. (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc R Soc B Biol Sci 278: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi M, McCormick MI, Munday PL, Jones GP. (2012) Influence of seasonal and latitudinal temperature variation on early life-history traits of a coral reef fish. Mar Freshw Res 63: 856–864. [Google Scholar]
- 60.Tewksbury JJ, Huey RB, Deutsch CA. (2008) Ecology: putting the heat on tropical animals. Science 320: 1296–1297. [DOI] [PubMed] [Google Scholar]
- 61.Willmer P, Stone G, Johnston I. (2005) Environmental Physiology of Animals, Ed 2 Wiley, Oxford. [Google Scholar]
- 62.Wilson DT, Meekan MG. (2002) Growth-related advantages for survival to the point of replenishment in the coral reef fish Stegastes partitus (Pomacentridae). Mar Ecol Prog Ser 231: 247–260. [Google Scholar]
- 63.Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC. (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Change Biol 12: 2220–2234. [Google Scholar]
- 64.Wood CM, McDonald DG. (1997) Global Warming: Implications for Freshwater and Marine Fish. Cambridge University Press, Cambridge, UK. [Google Scholar]