The oceans are absorbing excess atmospheric CO2, and this is causing ocean acidification. Surprisingly, one coral reef damselfish exhibits enhanced aerobic performance after living at projected future ocean CO2 levels for 17 days. Identifying both the winners and losers under climate change scenarios is vital to conserving marine biodiversity.
Keywords: aerobic scope, climate change, coral reef fish, ocean acidification
Abstract
The uptake of anthropogenic CO2 by the ocean has been suggested to impact marine ecosystems by decreasing the respiratory capacity of fish and other water breathers. We investigated the aerobic metabolic scope of the spiny damselfish, Acanthochromis polyacanthus, from the Great Barrier Reef, Australia when exposed for 17 days to CO2 conditions predicted for the end of the century (946 μatm CO2). Surprisingly, resting O2 consumption rates were significantly lower and maximal O2 consumption rates significantly higher in high-CO2-exposed fish compared with control fish (451 μatm CO2). Consequently, high-CO2-exposed fish exhibited an unexpected increase in absolute (38%) and factorial aerobic scopes (47%). Haematological and muscle water changes associated with exercise were not affected by CO2 treatment. Thus, contrary to predictions, our results suggest that elevated CO2 may enhance aerobic scope of some fish species. Long-term experiments are now required to assess the response to elevated CO2 further, because developmental and transgenerational effects can be dramatic in fish. Ultimately, understanding the variability among species regarding the effects of CO2 on aerobic scope will be critical in predicting the impacts of ocean acidification on marine communities and ecosystems.
Introduction
Atmospheric CO2 levels are rising, leading to a corresponding increase in CO2 and a decrease in pH at the ocean surface, a process known as ocean acidification (Doney, 2010). Future CO2 levels are expected to impact marine ecosystems widely, because the scope for aerobic performance in fish and other water breathers is predicted to decrease at higher CO2 levels (Pörtner and Farrell, 2008). Reductions in aerobic scope (the difference between resting and maximal oxygen consumption rates) result in less energy being available for life-history processes, such as growth and reproduction (Donelson et al., 2010; Pörtner and Peck, 2010). Thus, understanding how elevated CO2 influences aerobic scope is important for predicting the ecological impacts of ocean acidification on marine ecosystems (Ishimatsu et al., 2008; Pörtner and Farrell, 2008; Munday et al., 2012).
Consistent with theoretical predictions (Pörtner and Farrell, 2008), reduced aerobic scope at near-future CO2 levels (∼1000 μatm) has been demonstrated in two coral reef cardinalfishes (Ostorhinchus doederleini and Ostorhinchus cyanosoma; Munday et al., 2009). The negative effects of elevated CO2 on cardinalfishes may be attributed to the fish living in tropical waters near the upper end of their thermal range, as they are particularly temperature-sensitive species (Gardiner et al., 2010). However, in other tropical fishes, near-future CO2 levels appear to have a beneficial or hormetic effect on aerobic performance and life-history traits (Miller et al., 2013; Couturier et al., 2013). Recent studies have even demonstrated a mechanistic basis for enhanced oxygen delivery to tissues in the presence of low levels of hypercapnia that is unique to teleost fishes (Rummer and Brauner, 2011; Rummer et al., 2013), but it is unknown how widespread the phenomenon may be. Moreover, at even higher CO2 levels the benefits may disappear, as indicated by research on Atlantic cod (Gadus morhua), in which no changes in oxygen consumption rates were observed at CO2 levels several times higher than end-of-century predictions (Melzner et al., 2009). Likewise, Couturier et al. reported that juvenile damselfish (Pomacentrus amobinensis) no longer exhibited a higher maximal aerobic capacity at CO2 levels only moderately higher than end-of-century predictions (at ∼1400–2400 μatm; Couturier et al., 2013). In fact, most of the earlier studies on fish exposed to CO2 levels 5–50 times greater than end-of-century predictions demonstrated no effect on performance (McKenzie et al., 2003; Deigweiher et al., 2008; Ishimatsu et al., 2008; Baker et al., 2009; reviewed by Brauner and Baker, 2009). Given that the physiological responses observed in fish at near-future CO2 levels (<1000 μatm) may be different from responses at the extreme CO2 levels often used in earlier studies (5000 to >50 000 μatm), it is important to test the effects of climate change-relevant CO2 levels on fish and other marine species to determine if they have generally negative effects, as predicted by theory, or potentially positive effects in some species, as suggested by recent experimental studies.
Habitat may also play an important role in determining the sensitivity of a species to increased CO2. Fluctuating CO2 levels are common in coastal marine ecosystems (Hofmann et al., 2011), and coral reef ecosystems may already experience diurnal fluctuations in CO2 that reach or even exceed the average projected levels for the year 2100 (Melzner et al., 2012; Shaw et al., 2013). Therefore, some species may already show homeostatic adaptations to frequent CO2 fluctuations (McKenzie et al., 2003; Ishimatsu et al., 2008; Baker et al., 2009; Brauner and Baker, 2009) that explain their ability to maintain performance at projected future CO2 levels. Given that increased uptake of CO2 by the ocean will affect both the average CO2 level and the magnitude of extreme CO2 levels, it is important to consider both physiological sensitivity and the habitat occupied in determining which species will exhibit positive and which species will exhibit negative responses to rising CO2 levels in the ocean.
We tested the effect of surface ocean CO2 levels projected for 2100 under Representative Concentrations Pathway 8.5 (RCP 8.5 = 936 μatm; Meinshausen et al., 2011) on resting 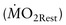 and maximal O2 consumption rates
and maximal O2 consumption rates 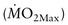 to calculate aerobic scope for the spiny damselfish, Acanthochromis polyacanthus, a model species for studying climate change impacts on reef fishes (Nilsson et al., 2007, 2009; Donelson et al., 2012). In addition to our primary aim, we also measured key haematological and tissue variables to examine the physiological status of CO2-exposed fish immediately following exercise to provide insight into the physiological mechanisms that may underlie the effects of CO2 on aerobic performance.
to calculate aerobic scope for the spiny damselfish, Acanthochromis polyacanthus, a model species for studying climate change impacts on reef fishes (Nilsson et al., 2007, 2009; Donelson et al., 2012). In addition to our primary aim, we also measured key haematological and tissue variables to examine the physiological status of CO2-exposed fish immediately following exercise to provide insight into the physiological mechanisms that may underlie the effects of CO2 on aerobic performance.
Materials and methods
Experimental animals
Acanthochromis polyacanthus (standard length, 63.5 ± 1.0 mm; wet mass, 11.41 ± 0.78 g; means ± SD) were collected from the Lizard Island lagoon (14°40′08′′S; 145°27′34′′E), Great Barrier Reef, Australia and maintained in the laboratory in a flow-through seawater system at ambient summer temperatures (27.3–30.6°C) for ∼14 days prior to CO2 treatment. Fish were then randomly removed from holding aquaria and evenly distributed among four 35 l aquaria supplied with seawater at present-day control CO2 levels (451 μatm) and four with high-CO2 water (946 μatm; Table 1). Fish were kept in CO2 treatments for 17 days and fed to satiation twice daily (NRD pellets; INVE Aquaculture, Salt Lake City, UT, USA), but food was withheld for 24 h prior to sampling or respirometry. All collection, care, and experimental protocols complied with James Cook University Animal Ethics Committee regulations (permit: #A1722).
Table 1:
Mean seawater data (±SEM) and range for each treatment (values to nearest integer, one or two decimal places)
| Temperature (°C) |
pHNBS |
||||||
|---|---|---|---|---|---|---|---|
| Treatment | Mean | Range | Salinity (p.p.t.) | Mean | Range | Total alkalinity (μmol kg seawater−1) | Partial pressure of CO2 (μatm) |
| Control | 29.2 (±0.1) | 27.3–30.6 | 34.5 | 8.14 (±0.01) | 8.11–8.17 | 2272 (±13) | 451 (±16) |
| High CO2 | 29.3 (±0.1) | 27.5–30.3 | 34.5 | 7.87 (±0.01) | 7.84–7.89 | 2258 (±5) | 946 (±29) |
Carbon dioxide treatment
Aquaria were supplied with seawater at present-day control CO2 levels (451 μatm) or high-CO2-equilibrated seawater (946 μatm). High-CO2 seawater was achieved by CO2 dosing a 60 l header tank to a set pHNBS (National Bureau of Standards) to match the surface ocean CO2 level projected for 2100 under RCP 8.5 (Meinshausen et al., 2011). A pH controller (Aqua Medic GmbH, Bissendorf, Germany) delivered a steady stream of CO2 into a powerhead in the bottom of the header tank if the pH rose above the set point. The pH was monitored regularly to ensure that it remained within ±0.05 of desired levels. Individual aquaria received CO2-equilibrated seawater from the 60 l header tank at ∼500 ml min−1. Control aquaria received seawater from a 60 l header tank diffused with ambient air. The temperature in each aquarium was measured twice daily. Seawater total alkalinity and pHNBS for CO2 calculations were measured from replicate water samples of control and high-CO2 water taken at the start and end of the experiment. Total alkalinity was estimated by Gran titration using certified reference materials (Dr A. G. Dickson, Scripps Institution of Oceanography). Average seawater partial pressure of CO2 (pCO2) was calculated using these parameters in CO2SYS (Pierrot et al., 2006) using constants from Dickson and Millero (1987) (Table 1).
Resting and maximal oxygen consumption
Intermittent-flow respirometry has been found to provide a reliable estimate of standard or resting metabolic rates (Roche et al., 2013) and was therefore used to determine resting O2 consumption rates for eight control and eight high-CO2-exposed fish in CO2 conditions. Fish were placed individually into 1615 ml, darkened respirometry chambers submerged in a temperature-controlled aquarium (29°C) and allowed 90 min to habituate to the chamber. Submersible pumps supplied a constant water flow (900 l h−1) from the aquaria through the chambers. In preliminary experiments, we determined that 90 min was ample time to ensure that O2 consumption rates had reached the lowest possible values, after which O2 consumption rates did not vary significantly. Thus, at 90 min, the water flow to each chamber was stopped for 15 min every 30 min over a period of 90 min (Supplementary material, Fig. S1). The time for which the water flow was interrupted was short enough to ensure that O2 did not fall below 80% saturation. The temperature-compensated O2 concentration (in milligrams per litre) of the water within each chamber was continuously recorded (1 s−1) using oxygen-sensitive REDFLASH dye on contactless spots (2 mm) adhered to the inside of each chamber and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via fibre-optic cables. Data were analysed using LabChart version 6.1.3 (ADInstruments, Colorado Springs, CO, USA). The value of 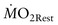 (in milligrams per kilogram per hour) was calculated from the average of the three slopes of O2 concentration (Supplementary material, Fig. S1), minus the background O2 consumption, which was measured daily before and at the end of each trial (assumed linear) and did not exceed 5% of the
(in milligrams per kilogram per hour) was calculated from the average of the three slopes of O2 concentration (Supplementary material, Fig. S1), minus the background O2 consumption, which was measured daily before and at the end of each trial (assumed linear) and did not exceed 5% of the 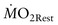 of the fish.
of the fish.
Following the measurement of 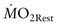 , fish were held in individual mesh baskets for 1 h and fed ad libitum to boost O2 consumption further. The maximal O2 consumption rate was then determined in a circular swim respirometer (Nilsson et al., 2007). To determine maximal oxygen consumption rates, fish were placed individually into a 1612 ml sealed vertical cylinder submerged in a temperature-controlled aquarium (29°C). A water current within the cylinder was created using a magnetic stirring bar and plate (below the cylinder), and the water speed was increased to the maximal speed at which the fish could sustain a steady position (see Nilsson et al., 2007 for a diagram and a detailed description of the set-up). Criteria for obtaining the maximal sustained swimming speed at which
, fish were held in individual mesh baskets for 1 h and fed ad libitum to boost O2 consumption further. The maximal O2 consumption rate was then determined in a circular swim respirometer (Nilsson et al., 2007). To determine maximal oxygen consumption rates, fish were placed individually into a 1612 ml sealed vertical cylinder submerged in a temperature-controlled aquarium (29°C). A water current within the cylinder was created using a magnetic stirring bar and plate (below the cylinder), and the water speed was increased to the maximal speed at which the fish could sustain a steady position (see Nilsson et al., 2007 for a diagram and a detailed description of the set-up). Criteria for obtaining the maximal sustained swimming speed at which 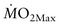 could be determined were that the fish had to be swimming against the current using pectoral fins only while maintaining the same position in the cylinder. Increasing the speed of the water current would result in the fish losing position. The decrease in O2 concentration in the cylinder was monitored with an oxygen probe (WTW OXI 340i, Weilheim, Germany) for up to 7 min, during which time the rate of O2 decline was stable. Data were analysed offline, and
could be determined were that the fish had to be swimming against the current using pectoral fins only while maintaining the same position in the cylinder. Increasing the speed of the water current would result in the fish losing position. The decrease in O2 concentration in the cylinder was monitored with an oxygen probe (WTW OXI 340i, Weilheim, Germany) for up to 7 min, during which time the rate of O2 decline was stable. Data were analysed offline, and 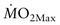 was calculated as described above for
was calculated as described above for 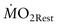 . Absolute
. Absolute 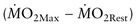 and factorial aerobic scopes
and factorial aerobic scopes 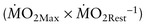 were calculated for each fish.
were calculated for each fish.
Haematological and tissue analyses
Immediately following the measurment of 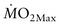 , fish were euthanized by cranial concussion. The caudal fin was severed, blood was collected to analyse haemoglobin, glucose, and lactate concentrations, and epaxial muscle was dissected to calculate the percentage of water in the muscle. Additionally, eight control and eight high-CO2-exposed fish not subjected to respirometry were sampled to determine resting physiological status. Haemoglobin (Hb) concentration in blood was determined using 10 μl of whole blood and the HemoCue® Hb 201 System, Australia Pty Ltd, Tumbi Umbi, NSW, Australia and reported as grams per 100 ml and millimolar haemoglobin tetramer (Hb4) using calibration curves previously verified on this species and according to Clark et al. (2008). Whole blood glucose and lactate concentrations (millimolar) were determined from two 15 μl samples using the Accutrend® Plus (Roche Diagnostics Australia Pty Ltd Dee Why, NSW, Australia). Fulton's body condition factor (K = (W × 100) × L−3, where W is wet mass in grams and L is standard length in millimetres) was calculated to assess the length-to-weight ratio. The volume of plasma was insufficient for other analyses
, fish were euthanized by cranial concussion. The caudal fin was severed, blood was collected to analyse haemoglobin, glucose, and lactate concentrations, and epaxial muscle was dissected to calculate the percentage of water in the muscle. Additionally, eight control and eight high-CO2-exposed fish not subjected to respirometry were sampled to determine resting physiological status. Haemoglobin (Hb) concentration in blood was determined using 10 μl of whole blood and the HemoCue® Hb 201 System, Australia Pty Ltd, Tumbi Umbi, NSW, Australia and reported as grams per 100 ml and millimolar haemoglobin tetramer (Hb4) using calibration curves previously verified on this species and according to Clark et al. (2008). Whole blood glucose and lactate concentrations (millimolar) were determined from two 15 μl samples using the Accutrend® Plus (Roche Diagnostics Australia Pty Ltd Dee Why, NSW, Australia). Fulton's body condition factor (K = (W × 100) × L−3, where W is wet mass in grams and L is standard length in millimetres) was calculated to assess the length-to-weight ratio. The volume of plasma was insufficient for other analyses 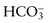 (e.g. Cl−,, total CO2, and catecholamines).
(e.g. Cl−,, total CO2, and catecholamines).
Statistical analyses
Student's paired t-tests were used to compare 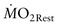 and
and 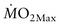 between control and high-CO2-exposed fish. Two-way ANOVAs and Holm–Sidak post hoc tests were used to compare haematological and tissue parameters between control and high-CO2-exposed fish at rest and post-exercise. Statistical analyses were conducted using SigmaPlot (Systat Software, Inc., Chicago, IL, USA).
between control and high-CO2-exposed fish. Two-way ANOVAs and Holm–Sidak post hoc tests were used to compare haematological and tissue parameters between control and high-CO2-exposed fish at rest and post-exercise. Statistical analyses were conducted using SigmaPlot (Systat Software, Inc., Chicago, IL, USA).
Results
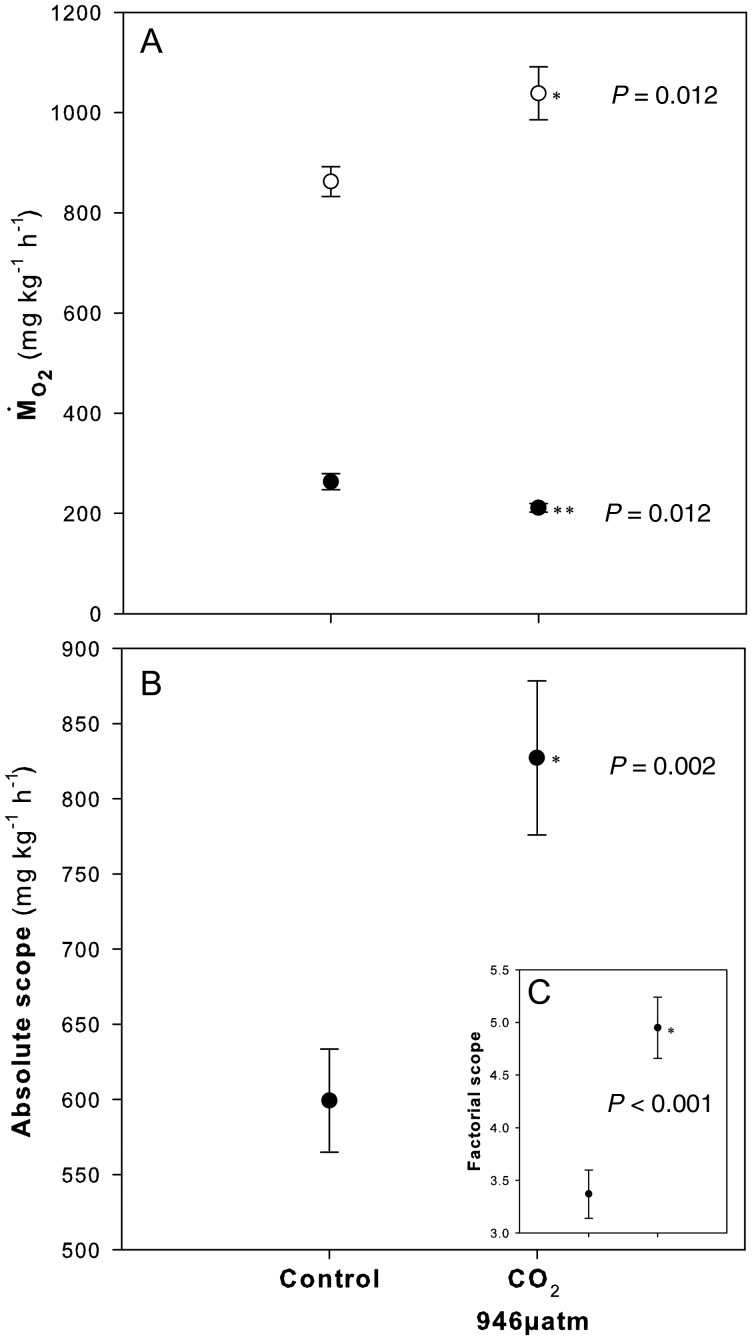
The 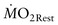 of high-CO2-exposed fish was ∼20% lower than that of control fish (t(14) = 2.866, P = 0.012; Fig. 1A), whereas the
of high-CO2-exposed fish was ∼20% lower than that of control fish (t(14) = 2.866, P = 0.012; Fig. 1A), whereas the 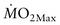 of high-CO2-exposed fish was ∼20% higher than that of control counterparts (t(14) = −2.898, P = 0.012; Fig. 1A). Consequently, high-CO2-exposed fish exhibited a 38 and 47% higher absolute and factorial aerobic scope (t(14) = −3.70, P = 0.002; Fig. 1B; and t(14) = −4.29, P < 0.001; Fig. 1C).
of high-CO2-exposed fish was ∼20% higher than that of control counterparts (t(14) = −2.898, P = 0.012; Fig. 1A). Consequently, high-CO2-exposed fish exhibited a 38 and 47% higher absolute and factorial aerobic scope (t(14) = −3.70, P = 0.002; Fig. 1B; and t(14) = −4.29, P < 0.001; Fig. 1C).
Figure 1:
The effect of 17 days of exposure to high-CO2 on resting and maximal O2 consumption rates and absolute and factorial aerobic scope in spiny damselfish. (A) Resting (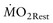 ; filled circles) and maximal oxygen consumption rates (
; filled circles) and maximal oxygen consumption rates (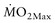 ; open circles). (B) Absolute aerobic scope
; open circles). (B) Absolute aerobic scope 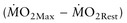 . (C) Factorial aerobic scope
. (C) Factorial aerobic scope 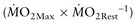 . Values are means ± SEM. Asterisks demarcate significant differences from control values (Student's paired t-test).
. Values are means ± SEM. Asterisks demarcate significant differences from control values (Student's paired t-test).
Exposure to high-CO2 conditions for 17 days had no effect on the physiological parameters examined in resting conditions (Table 2 and Supplementary material, Table S1). Likewise, [Hb], [lactate], [glucose], and muscle water did not differ between control and CO2 treatment groups immediately following measurement of 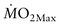 . However, [lactate] and percentage muscle water increased following measurement of
. However, [lactate] and percentage muscle water increased following measurement of 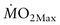 , independent of CO2 treatment (P = 0.045 and P < 0.001, respectively). Body condition did not change in control or high-CO2 conditions (Table 2 and Supplementary material, Table S1). No significant interactions were detected between CO2 treatment and exercise (Supplementary material, Table S1).
, independent of CO2 treatment (P = 0.045 and P < 0.001, respectively). Body condition did not change in control or high-CO2 conditions (Table 2 and Supplementary material, Table S1). No significant interactions were detected between CO2 treatment and exercise (Supplementary material, Table S1).
Table 2:
The effect of high CO2 and maximal swimming on body metrics, blood, and tissue variables of spiny damselfish
| Mass (g) | Standard length (mm) | Condition factor (K) | [Hb] (g 100 ml−1) | [Hb] (mM) | [Lactate] (mM) | [Glucose] (mM) | Muscle water (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Rest | Mean | 10.60 | 62.43 | 0.00424 | 6.50 | 1.03 | 1.33 | 2.87 | 74.25 |
| SEM | 0.94 | 1.57 | 0.00008 | 0.30 | 0.05 | 0.30 | 0.40 | 0.41 | ||
| n | 13 | 13 | 13 | 10 | 10 | 9 | 9 | 13 | ||
| Post-swimming | Mean | 11.68 | 64.74 | 0.00445 | 7.20 | 1.14 | 3.27* | 3.73 | 75.89* | |
| SEM | 0.71 | 1.26 | 0.00011 | 0.31 | 0.05 | 1.20 | 0.49 | 0.28 | ||
| n | 8 | 7 | 7 | 5 | 7 | 3 | 6 | 7 | ||
| High CO2 | Rest | Mean | 13.20 | 65.35 | 0.00427 | 6.80 | 1.08 | 1.58 | 1.69 | 73.46 |
| SEM | 0.37 | 2.69 | 0.00019 | 0.12 | 0.02 | 0.17 | 0.36 | 0.38 | ||
| n | 10 | 10 | 10 | 7 | 5 | 9 | 9 | 10 | ||
| Post-swimming | Mean | 10.17 | 61.59 | 0.00425 | 6.60 | 0.92 | 2.87* | 2.67 | 75.00* | |
| SEM | 1.02 | 2.17 | 0.00015 | 0.44 | 0.14 | 0.67 | 0.42 | 0.82 | ||
| n | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 8 | ||
| Significance | n.s. | n.s. | n.s. | n.s. | n.s. | P = 0.045 | n.s. | P < 0.001 |
Abbreviations: [Hb], haemoglobin concentration; n.s., non-significant. Asterisks demarcate significant differences between rest and post-swimming values within a given parameter; there were no effects of CO2 treatment or interaction between CO2 treatment and exercise (two-way ANOVA).
Discussion
When exposed to CO2 levels relevant to end-of-century projections RCP 8.5 (Meinshausen et al., 2011) for 17 days, spiny damselfish demonstrated an enhanced aerobic scope compared with control fish, contradicting predictions that elevated CO2 will reduce aerobic performance (Ishimatsu et al., 2008; Pörtner and Farrell, 2008). The response differs from the 47% decrease in aerobic scope observed in coral reef cardinalfishes exposed to similar CO2 levels (Munday et al., 2009), as well as the unchanged 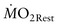 and
and 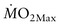 for other teleosts exposed to much higher CO2 levels (McKenzie et al., 2003; Deigweiher et al., 2008; Ishimatsu et al., 2008; Melzner et al., 2009). However, the response found in the present study is similar to the response of another damselfish, juvenile Pomacentrus amboinensis, which exhibited a 28–39% increase in
for other teleosts exposed to much higher CO2 levels (McKenzie et al., 2003; Deigweiher et al., 2008; Ishimatsu et al., 2008; Melzner et al., 2009). However, the response found in the present study is similar to the response of another damselfish, juvenile Pomacentrus amboinensis, which exhibited a 28–39% increase in 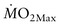 at similar CO2 levels (Couturier et al., 2013). Parameters related to blood oxygen-carrying capacity, energy metabolism, and tissue hydration revealed the expected differences between fish at resting and post-swimming, but were not influenced by CO2 treatment. We discuss potential mechanisms for this unexpected enhancement of performance in conditions of elevated CO2.
at similar CO2 levels (Couturier et al., 2013). Parameters related to blood oxygen-carrying capacity, energy metabolism, and tissue hydration revealed the expected differences between fish at resting and post-swimming, but were not influenced by CO2 treatment. We discuss potential mechanisms for this unexpected enhancement of performance in conditions of elevated CO2.
Resting oxygen consumption
Exposure to very high levels of CO2 (5000 to >50 000 μatm; 5–50 times higher than in present study) and the associated decrease in seawater pH induce hyperventilation and several physiological modifications at the fish gill, including increases in ion and acid–base regulation (Evans et al., 2005; Brauner and Baker, 2009). Within the first 96 h of exposure to high CO2, fish elevate plasma [HCO3−] via equimolar decreases in [Cl−] to counter increased [H+] (Brauner and Baker, 2009; Esbaugh et al., 2012). Ion exchange occurs largely at the gill, and when the main ion transporters operate at higher rates, rearrangements to energy budgets could result (Deigweiher et al., 2010). Increased gill energy requirements (Deigweiher et al., 2010) suggest that 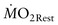 could increase during high-CO2 exposure, yet many studies have shown no change in
could increase during high-CO2 exposure, yet many studies have shown no change in 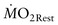 (Ishimatsu et al., 2008; Couturier et al., 2013). Furthermore, in the present study, we observed a decrease in
(Ishimatsu et al., 2008; Couturier et al., 2013). Furthermore, in the present study, we observed a decrease in 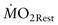 during high-CO2 exposure, suggesting decreased energy demands. A plasma acidosis is a characteristic response to elevated environmental CO2 (Claiborne et al., 2002), but it is important to note that at the climate change-relevant CO2 levels (946 μatm) in this study, environmental pCO2 is still likely to be much lower than the plasma pCO2 of resting fish (Esbaugh et al., 2012). The levels of hypercapnia used here still represent an outward, yet reduced, blood-to-environment CO2 gradient (Esbaugh et al., 2012), but may not be problematic in comparison to higher levels examined in previous studies that would have severely impacted CO2 diffusion.
during high-CO2 exposure, suggesting decreased energy demands. A plasma acidosis is a characteristic response to elevated environmental CO2 (Claiborne et al., 2002), but it is important to note that at the climate change-relevant CO2 levels (946 μatm) in this study, environmental pCO2 is still likely to be much lower than the plasma pCO2 of resting fish (Esbaugh et al., 2012). The levels of hypercapnia used here still represent an outward, yet reduced, blood-to-environment CO2 gradient (Esbaugh et al., 2012), but may not be problematic in comparison to higher levels examined in previous studies that would have severely impacted CO2 diffusion.
Maximal oxygen consumption
Compared with control conditions, high-CO2-exposed spiny damselfish increased 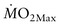 . During maximal aerobic exercise, fish can increase functional respiratory surface areas by increasing gill blood perfusion, pressure, and lamellar recruitment (Wood and Randall, 1973; Evans et al., 2005). While increasing gill surface area may satisfy increased O2 requirements, there may be a cost to osmoregulation (Randall et al., 1972). Marine fish increase drinking rates to compensate for water loss over the gills, but may consequently expend more energy excreting excess ions across the gills. This did not appear to be the case in the present study. Here, exercised fish exhibited an increase in muscle water, which may indicate an increase in drinking; however, the response was uniform between control and CO2 treatment groups. Thus, although the upper limit to aerobic activity may be set by the need to defend ion balance (Gonzalez and McDonald, 1992), this critical threshold may not have been reached at the CO2 levels used in this study. Exercising spiny damselfish may be able to afford increases in the functional respiratory surface area of the gill, thereby boosting O2 uptake, but without significant ion and acid–base disturbances at these low levels of hypercapnia.
. During maximal aerobic exercise, fish can increase functional respiratory surface areas by increasing gill blood perfusion, pressure, and lamellar recruitment (Wood and Randall, 1973; Evans et al., 2005). While increasing gill surface area may satisfy increased O2 requirements, there may be a cost to osmoregulation (Randall et al., 1972). Marine fish increase drinking rates to compensate for water loss over the gills, but may consequently expend more energy excreting excess ions across the gills. This did not appear to be the case in the present study. Here, exercised fish exhibited an increase in muscle water, which may indicate an increase in drinking; however, the response was uniform between control and CO2 treatment groups. Thus, although the upper limit to aerobic activity may be set by the need to defend ion balance (Gonzalez and McDonald, 1992), this critical threshold may not have been reached at the CO2 levels used in this study. Exercising spiny damselfish may be able to afford increases in the functional respiratory surface area of the gill, thereby boosting O2 uptake, but without significant ion and acid–base disturbances at these low levels of hypercapnia.
Whole blood lactate concentrations were also elevated in both control and CO2-treated fish post-exercise, with no effect of CO2. The finding suggests that both groups of fish were reaching roughly the same aerobic/anaerobic threshold and were potentially exerted to a similar extent. Nevertheless, the fish exposed to elevated CO2 for 17 days were able to do this while increasing O2 consumption rates. It may be that exposure to mild hypercapnia, as in this experiment, combined with the stress of exercise resulted in a release of catecholamines into the bloodstream, which has recently been demonstrated in rainbow trout to aid in increasing O2 uptake and potentially delivery (Rummer and Brauner, 2011; Rummer et al., 2013). Clearly, more research is necessary to understand the mechanisms underpinning the increase in aerobic scope during mild hypercapnia observed here in the spiny damselfish.
Significance and perspectives
Contrary to predicted physiological impacts of climate change (Pörtner and Farrell, 2008), aerobic scope of A. polyacanthus was increased upon exposure to predicted end-of-century CO2 levels. The finding adds to a growing number of studies showing that the effect of increased CO2 levels on aerobic performance varies dramatically among fish species, ranging from decreasing aerobic performance (Munday et al., 2009) or no change in aerobic performance (McKenzie et al., 2003; Deigweiher et al., 2008; Ishimatsu et al., 2008; Melzner et al., 2009) to increasing aerobic performance (Couturier et al., 2013). If aerobic scope underpins the performance of fish populations (Pörtner and Peck, 2010; Eliason et al., 2011), ocean acidification could play an important role in altering the relative abundances of species, and thereby ecosystem dynamics and the structure of marine communities, especially in the face of fluctuating CO2 levels in coastal marine ecosystems. In the light of recent findings of strong developmental and transgenerational acclimation effects in fish exposed to elevated CO2 and/or temperature (Miller et al., 2012; Salinas and Munch, 2012; Scott and Johnston, 2012), it is even more important to understand this variable, perhaps species-specific response in aerobic scope that has important implications for the future structure of marine communities.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Acknowledgements
We thank Lizard Island Research Station for excellent research facilities and technical support, as well as Dr A. J. Morash for helpful discussions. This work was supported by the Australian Research Council (P.L.M.); the Australian Research Council Centre of Excellence for Coral Reef Studies (J.L.R., P.L.M.); the University of Oslo and Research Council of Norway (G.E.N., J.A.W.S., C.S.C.); and the United States National Institute of General Medical Sciences of the National Institutes of Health (grant number P20GM103395 to J.A.W.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Baker DW, Matey V, Huynh KT, Wilson JM, Morgan JD, Brauner CJ. (2009) Complete intracellular pH protection during extracellular pH depression is associated with hypercarbia tolerance in white sturgeon, Acipenser transmontanus. Am J Physiol Regul Integr Comp Physiol 296: R1868–R1880. [DOI] [PubMed] [Google Scholar]
- 2.Brauner CJ, Baker DW. (2009) Patterns of acid-base regulation during exposure to hypercarbia in fishes. In ML Glass, SC Wood, eds, Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects. Springer, Berlin, Heidelberg, pp 43–63. [Google Scholar]
- 3.Claiborne JB, Edwards SL, Morrison-Shetlar AI. (2002) Acid–base regulation in fishes: cellular and molecular mechanisms. J Exp Zool 293: 302–319. [DOI] [PubMed] [Google Scholar]
- 4.Clark TD, Eliason EJ, Sandblom E, Hinch SG, Farrell AP. (2008) Calibration of a hand-held haemoglobin analyser for use on fish blood. J Fish Biol 73: 2587–2595. [Google Scholar]
- 5.Couturier CS, Stecyk JAW, Rummer JL, Munday PL, Nilsson GE. (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deigweiher K, Koschnick N, Pörtner H-O, Lucassen M. (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295: R1660–R1670. [DOI] [PubMed] [Google Scholar]
- 7.Deigweiher K, Hirse T, Bock C, Lucassen M, Pörtner H. (2010) Hypercapnia induced shifts in gill energy budgets of Antarctic notothenioids. J Comp Physiol B 180: 347–359. [DOI] [PubMed] [Google Scholar]
- 8.Dickson AG, Millero FJ. (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res A 34: 1733–1743. [Google Scholar]
- 9.Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM. (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401: 233–243. [Google Scholar]
- 10.Donelson JM, Munday PL, McCormick MI, Pitcher CR. (2012) Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Climate Change 2: 30–32. [Google Scholar]
- 11.Doney SC. (2010) The growing human footprint on coastal and open-ocean biogeochemistry. Science 328: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 12.Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP. (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- 13.Esbaugh A, Heuer R, Grosell M. (2012) Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J Comp Physiol B 182: 1–14. [DOI] [PubMed] [Google Scholar]
- 14.Evans DH, Piermarini PM, Choe KP. (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner NM, Munday PL, Nilsson GRE. (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One 5: pe13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez RJ, McDonald DG. (1992) The relationship between oxygen consumption and ion loss in a freshwater fish. J Exp Biol 163: 317–332. [Google Scholar]
- 17.Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, Paytan A, Price NN, Peterson B, Takeshita Y, et al. (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One 6: pe28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishimatsu A, Hayashi M, Kikkawa T. (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- 19.McKenzie DJ, Piccolella M, Valle AZD, Taylor EW, Bolis CL, Steffensen JF. (2003) Tolerance of chronic hypercapnia by the European eel Anguilla anguilla. J Exp Biol 206: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 20.Meinshausen M, Smith S, Calvin K, Daniel J, Kainuma K, Lamarque JF, Matsumoto K, Montzka S, Raper S, Riahi K, et al. (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109: 213–241. [Google Scholar]
- 21.Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H-O, Lucassen M. (2009) Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater. Aquat Toxicol 92: 30–37. [DOI] [PubMed] [Google Scholar]
- 22.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska M, Bange H, Hansen H, Körtzinger A. (2012) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 1–14. [Google Scholar]
- 23.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nature Climate Change 2: 858–861. [Google Scholar]
- 24.Miller GM, Watson S-A, McCormick MI, Munday PL. (2013) Increased CO2 stimulates reproduction in a coral reef fish. Glob Chang Biol doi:10.1111/gcb.12259. [DOI] [PubMed] [Google Scholar]
- 25.Munday PL, Crawley NE, Nilsson GE. (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- 26.Munday PL, McCormick MI, Nilsson GE. (2012) Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J Exp Biol 215: 3865–3873. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson GE, Östlund-Nilsson S, Penfold R, Grutter AS. (2007) From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proc R Soc B Biol Sci 274: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson GE, Crawley N, Lunde IG, Munday PL. (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Chang Biol 15: 1405–1412. [Google Scholar]
- 29.Pierrot D, Lewis E, Wallace DWR. (2006) MS Excel program developed for CO2 system calculations. In: ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center. Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN, USA. [Google Scholar]
- 30.Pörtner HO, Farrell AP. (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- 31.Pörtner HO, Peck MA. (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. [DOI] [PubMed] [Google Scholar]
- 32.Randall DJ, Baumgarten D, Malyusz M. (1972) The relationship between gas and ion transfer across the gills of fishes. Comp Biochem Physiol A Comp Physiol 41: 629–637. [DOI] [PubMed] [Google Scholar]
- 33.Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL. (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. [DOI] [PubMed] [Google Scholar]
- 34.Rummer JL, Brauner CJ. (2011) Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in rainbow trout, Oncorhynchus mykiss. J Exp Biol 214: 2319–2328. [DOI] [PubMed] [Google Scholar]
- 35.Rummer JL, McKenzie DJ, Innocenti A, Supuran CT, Brauner CJ. (2013) Root effect hemoglobin may have evolved to enhance general oxygen delivery. Science 340: 1327–1329. [DOI] [PubMed] [Google Scholar]
- 36.Salinas S, Munch SB. (2012) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15: 159–163. [DOI] [PubMed] [Google Scholar]
- 37.Scott GR, Johnston IA. (2012) Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc Natl Acad Sci USA 109: 14247–14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML. (2013) Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob Chang Biol 19: 1632–1641. [DOI] [PubMed] [Google Scholar]
- 39.Wood CM, Randall DJ. (1973) The influence of swimming activity on water balance in the rainbow trout (Salmo gairdneri). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 82: 257–276. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.