Incidental capture, or bycatch, of elasmobranchs (sharks, skates, and rays) threatens populations worldwide. In this review, elasmobranch sensory biology and ecology are explored to identify potential species- and fishery-specific bycatch reduction techniques for a variety of fishing gear types.
Keywords: Bycatch reduction, elasmobranch fishes, fisheries, sensory
Abstract
Incidental capture, or bycatch, in fisheries represents a substantial threat to the sustainability of elasmobranch populations worldwide. Consequently, researchers are increasingly investigating elasmobranch bycatch reduction methods, including some focused on these species' sensory capabilities, particularly their electrosensory systems. To guide this research, we review current knowledge of elasmobranch sensory biology and feeding ecology with respect to fishing gear interactions and include examples of bycatch reduction methods used for elasmobranchs as well as other taxonomic groups. We discuss potential elasmobranch bycatch reduction strategies for various fishing gear types based on the morphological, physiological, and behavioural characteristics of species within this diverse group. In select examples, we indicate how an understanding of the physiology and sensory biology of vulnerable, bycatch-prone, non-target elasmobranch species can help in the identification of promising options for bycatch reduction. We encourage collaboration among researchers studying bycatch reduction across taxa to provide better understanding of the broad effects of bycatch reduction methods.
Introduction and background
As populations of marine species face increasing declines due to anthropogenic activities, concern over wasteful loss of biomass as incidental catch, or ‘bycatch’, is growing on a global scale (Hall et al., 2000; Lewison et al., 2004; Kelleher, 2005). Levels and composition of bycatch, which can be defined as retained or discarded non-target species as well as unobserved mortality prior to or following landing/release (Gilman, 2011), vary spatially, temporally, and with gear type. With the current level of under-reporting, lack of species identification, unobserved mortality, and incomplete understanding of at-vessel and post-release mortality, the total global bycatch mortality (all species) is difficult to determine, but estimates range from 7.0 to 38.5 million tonnes annually, accounting for up to 40.4% of total catch (Alverson et al., 1994; Kelleher, 2005; Davies et al., 2009). A significant number of marine species affected by high bycatch rates are classified by the International Union for Conservation of Nature (IUCN) as threatened (vulnerable, endangered, or critically endangered).
Research aimed at reducing bycatch of marine mammals, sea birds, and sea turtles has grown since the late 1980s, while prioritization for elasmobranch (shark, skate, and ray) bycatch reduction has lagged behind (Werner et al., 2006). Recent reports of fishery-driven global declines in many elasmobranch populations (e.g. Dulvy et al., 2008; Camhi et al., 2009), high rates of incidental capture in certain fisheries (e.g. Mandelman et al., 2008), high at-vessel mortality rates observed in some species (e.g. Morgan and Burgess, 2007), and K-selected life history traits (Hoenig and Gruber, 1990; Cortés, 1999, 2002; Heppell et al., 1999) have elevated the priority of elasmobranch bycatch reduction within fisheries management. Elasmobranchs differ from other focal species, such as sea birds or dolphins, in that directed elasmobranch fisheries are widespread and sale of incidental elasmobranch catch is profitable in many regions where markets for fins, meat, and other products are accessible (Davies et al., 2009). Nevertheless, incidental capture is detrimental to populations of elasmobranch species, many of which fulfil important ecological roles in their communities (Stevens et al., 2000). Moreover, elasmobranch bycatch and depredation can compromise crew safety and decrease profitability through reduced target catch and gear damage (Gilman et al., 2008).
Elasmobranchs are caught in virtually all types of fishing gear, with rates dependent on the species, size class, gear selectivity, location, and extent of effort, among other factors. Fishing gears can be classified in a variety of ways, including those with nets (e.g. trawls, gill nets, and purse seines), and those with baited hooks. Although trawls and gill nets have variable mesh sizes to improve selectivity for target species, they can be highly indiscriminate for non-target species, capturing any organism that does not fit through the mesh or cannot escape entanglement, and are usually associated with high levels of bycatch (Alverson et al., 1994). While hook fisheries depend on attraction of target predatory species to bait, they can also result in high levels of incidental catch (Gilman et al., 2008). Pelagic longlines, gill nets, and trawls are responsible for the highest annual reported biomass of direct and indirect global elasmobranch catch (Bonfil, 1994; Gilman et al., 2008; Mandelman et al., 2008; Molina and Cooke, 2012).
Over the past 30 years, researchers have identified successful methods to reduce bycatch of various taxa through modifications to fishing practices and gear that often involve sensory-based strategies, including acoustic, visual, and chemical signals. Although typically aimed at one species or group, bycatch mitigation methods can influence bycatch rates of other taxa encountering the same fishing gear. For example, bottom trawls outfitted with excluder or bycatch reduction devices (BRDs), such as turtle excluder devices (TEDs) designed to release sea turtles, can also decrease elasmobranch bycatch (Brewer et al., 1998, 2006; Fennessy and Isaksen, 2007). The opposite can also be true, where modifications that decrease bycatch of one species can increase catch of another. For example, a shift from dolphin-associated purse seine sets towards using fish aggregating devices (FADs) has decreased dolphin bycatch; however, bycatch of sea turtles, sharks, and non-target teleost fish increases with use of FADs (Lewison et al., 2004; Gilman, 2011). For these reasons, it is important to understand the effects of modifications to fishing practices and gear on all target and non-target species.
Several fishing practices and gear modifications have been identified to reduce elasmobranch bycatch in a variety of fisheries, although most direct research on this topic has been applied to hook gear (Molina and Cooke, 2012). For example, the use of circle rather than J hooks, fish instead of squid bait, monofilament in place of steel leaders, and setting at deeper depths for shorter lengths of time can contribute to decreased catch and/or mortality of elasmobranchs on pelagic longlines (Watson et al., 2005; Ward et al., 2009; Gilman, 2011). However, the high level of variability among studies highlights the need for shark-specific controlled experiments to provide more definitive results (Godin et al., 2012). For net fisheries, limits on mesh size, increased tension (e.g. of gill nets), addition of excluder devices (in trawls), and modifications to FAD construction and use (for purse seining) can also reduce elasmobranch bycatch (Brewer et al., 2006; Thorpe and Frierson, 2009; Schaefer and Fuller, 2011). The emerging field of sensory-based deterrents directed at elasmobranchs has centred on the electrosensory system (see O'Connell et al., 2012). While the use of electric and magnetic deterrents to repel elasmobranchs warrants further research and development, it is important to explore additional sensory pathways for bycatch reduction potential.
Bycatch mitigation research has generally aimed either to reduce contact with gear or to facilitate escape once contact occurs (Werner et al., 2006). For elasmobranchs, immediate (at-vessel or capture) and post-release (delayed or discard) mortality rates vary widely both within and between species (Morgan and Burgess, 2007; Skomal and Bernal, 2010). Capture mortality ranges from 15 to 100% by species and gear type, among other factors (e.g. Beerkircher et al., 2002; Moyes et al., 2006; Mandelman and Farrington, 2007; Campana et al., 2009; Thorpe and Frierson, 2009; Morgan and Carlson, 2010). Although estimated short-term post-release survivorship appears high in some species (Moyes et al., 2006; Campana et al., 2009), unaccounted sublethal effects (e.g. cryptic injury, latent pathology, and impaired feeding) can ultimately prove fatal for individuals, with possible population-level consequences (e.g. Borucinska et al., 2002; Frick et al., 2010; Skomal and Mandelman, 2012). For further discussion of this important topic, we refer the reader to Skomal and Bernal (2010). For the purposes of this review, emphasis is placed on reducing interactions of elasmobranchs with gear to decrease both immediate and delayed mortality that may result from contact with fishing gear targeting other species.
Elasmobranchs occupy a wide variety of ecological niches and possess diverse sensory and behavioural adaptations. Gaining a better understanding of this diversity may hold potential for refining or redirecting previously tested methods or developing new, more effective bycatch reduction strategies. Our objectives are as follows: (i) to summarize current knowledge of elasmobranch sensory biology and feeding behaviour related to potential interactions with fishing gear; (ii) to review bycatch reduction research that is directly or indirectly related to elasmobranchs; and (iii) to explore new research directions that may be useful in reducing bycatch in example species associated with some or all of the following factors: low or rapidly declining populations; high incidental capture rates in a given fishery or fisheries; and high or unknown mortality due to capture and handling. Our ultimate goal is to suggest promising new directions for research to reduce unintended interactions between elasmobranchs and fishing gear.
Sensory biology
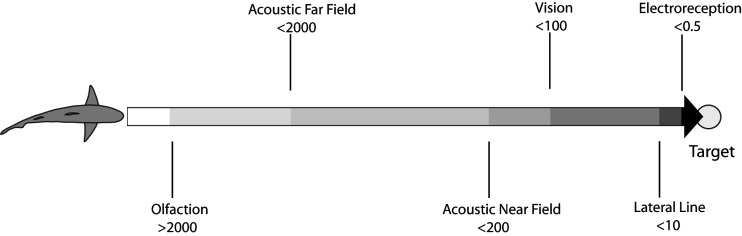
Elasmobranchs use a variety of information from their environment to navigate and locate other organisms (prey, predators, and conspecifics). Chemical, mechanical, visual, and electrical signals can be detected by peripheral sensory systems, interpreted by the central nervous system, and result in physiological and behavioural responses. Use of these signals to locate food may vary by species, distance, prey type and environmental factors. Chemical and acoustic signals propagate long distances from the source, followed by visual, water flow, and finally electrical information at short range (Fig. 1). We review current knowledge of elasmobranch sensory biology to highlight areas where sensory signals may be useful for bycatch reduction. Comparisons with teleosts are included where possible to identify differences that may enhance gear selectivity for target species. We also include examples of new and existing bycatch reduction methods aimed at each sensory system for elasmobranchs as well as other taxa. Our priority is to identify modifications to fishing gear that will reduce bycatch with little or no effect on target catches. For a more thorough introduction to elasmobranch sensory systems, see Gardiner et al. (2012) and Collin (2012).
Figure 1.
Relative transmission/detection distances (in metres) associated with potential sensory signals emitted by a target, such as a prey item. As the elasmobranch approaches the target, additional types of sensory information become available for detection using different sensory modalities. All distances approximate a best-case scenario; however, actual distances are influenced by environmental variables and interspecific variation in sensory systems.
Chemical detection
Elasmobranchs are sensitive to chemical signals via taste, common chemical sense, and olfaction. In seawater, chemical signals as odours can be carried great distances from their source, forming complex plumes, eddies, and intermittent signals (e.g. Webster, 2007). The distribution of odour signals depends on water flow and can be influenced by currents, topography, and the movements of organisms. Olfaction is important, if not essential, for initiation of search behaviour in both elasmobranchs and teleosts; however, additional sensory input is typically needed for location of a potential food source (Atema et al., 1980; Gardiner and Atema, 2007). For example, tracking of odours is accomplished with both the olfactory sytem and the lateral line system (see ‘Mechanical detection: water flow’ below) in tandem (Gardiner and Atema, 2007). Despite the enlarged olfactory organs of elasmobranchs, sensitivity to specific amino acids is similar in threshold (10−9 mol/l) and amino-acid type in both elasmobranchs and teleosts (Meredith and Kajiura, 2010). Differences in behavioural thresholds and sensory organ thresholds are probable, so it is essential that behavioural experiments provide information on sensitivity as well as the nature of the response (attraction/repulsion). Olfaction-related behavioural experiments have been limited by the difficulty of obtaining precise concentration data at the receptor of free-swimming animals (Gardiner et al., 2012).
Sharks display attraction to odours derived from fish and invertebrates (potential prey), particularly those from stressed fish, and repulsion (although variable) to human sweat (Tester, 1963). Some sharks also display an adverse reaction to chemicals derived from a potential predator (Rasmussen and Schmidt, 1992) and toxins such as those produced by the moses sole, Pardachirus marmoratus (Clarke, 1983). Hundreds of chemical compounds have been studied as shark deterrents (e.g. Gilbert, 1968), yet researchers continue efforts to identify one that is effective across a variety of species. Both synthetic surfactants and semiochemicals produced by sharks appear promising (Smith LJ Jr, 1991; Sisneros and Nelson, 2001; E. Stroud, personal communication). Chemical deterrents are an intriguing option to reduce elasmobranch interactions with fishing gear because of their importance in early detection and arousal for prey search behaviours, although control over the chemical dispersion rates remains a challenge. Future studies should be aimed at identifying a chemical that masks attractive odours or elicits a repulsion response in elasmobranchs without altering behaviour of targeted species. Behavioural reactions to conflicting attractive and repulsive odours may be unpredictable, so future research should also include the use of chemical deterrents with artificial bait. Alternatively, attracting elasmobranchs to sites away from fishing gear via ‘remote attracting devices’ is another promising area of current research (Dagorn, 2010). In this scenario, the attractant odour should be specific to elasmobranchs, so that targeted fish are not also attracted. Behavioural and field experiments to test the responses of both target and non-target species are essential in determining effectiveness of candidate deterrents or attractants.
Research on chemical signals for bycatch reduction in other species groups has met varying levels of success. Shark liver oil slicks dripped at the surface during longline setting reduced seabird interactions with fishing gear without influencing fish catch (Pierre and Norden, 2006). However, no noxious chemicals have been identified for successfully reducing sea turtle or marine mammal bycatch (Gearin et al., 1988; Southwood et al., 2008). Experiments with bait type have revealed that using fish instead of squid bait reduces both sea turtle and shark bycatch, without decreasing target catch (e.g. Watson et al., 2005; Gilman, 2011). With sea turtles, the difference in catch is attributed to bait-dependent differences in capture strategy/bait consumption (Gilman, 2011); however, it is not known whether chemical signals emitted by the bait or consumption behaviour contribute to observed decreases in catch rate of elasmobranchs.
Mechanical detection: sound
Mechanical stimuli can be detected by the hearing apparatus, mechanosensory system (discussed separately under ‘Mechanical detection: water flow’ below), and skin (cutaneous receptors) of elasmobranchs. Sounds travel quickly in water and can propagate from metres to kilometres in the ocean, depending on the frequency of the signal. Sound signals consist of pressure and particle displacement components that fall off at different rates with distance from the source, defining the acoustic near and far fields, and may be complicated by interactions with surface or ground interfaces and structures (Myrberg, 2001). Elasmobranchs are sensitive to the particle displacement component of sounds within the range of 20–1000 Hz (Casper and Mann, 2006, 2010), and sharks have been attracted from beyond visible distances by low-frequency, irregularly pulsed sounds, particularly those ranging within 25–50 Hz (Nelson and Gruber, 1963; Banner, 1967; Myrberg et al., 1972, 1976), although laboratory studies have raised questions over sharks' capability of detecting sounds in the acoustic far field (see Casper and Mann, 2006). Abrupt and high-intensity sounds (10× ambient noise) elicited fright or withdrawal responses in sharks within 10 m of the source; however, habituation was observed (Myrberg, 2001). Teleost fishes, such as tunas, targeted by pelagic longlines are also sensitive to low-frequency sounds below 1000 Hz, particularly within 200–500 Hz (Iversen, 1967, 1969; Moein Bartol and Ketten, 2006). Some teleosts are equipped with bony connections to the swim bladder that provide them with a mechanism for detection of the pressure component of sound (See Popper, 2003).
Acoustic cues in the form of high-frequency acoustic pingers can reduce bycatch of some marine mammals and seabirds in gill nets; however, concerns remain over habituation and association of food with pingers, and potential hearing damage associated with higher decibel acoustic harassment devices (e.g. Melvin et al., 1999; Bordino et al., 2002; Barlow and Cameron, 2003). No influences of pingers on catch rate of elasmobranchs have been reported, and they are not expected, given that the frequencies used are beyond the range of known elasmobranch hearing. Predator sounds have also been tested on the behaviour of cetaceans, yielding mixed results (Fish and Vania, 1971; Scordino and Pfeifer, 1993). Seismic air guns were found to keep sea turtles away from a power plant intake, but the intensity of sound required was likely to cause damage to marine organisms at close range (Popper, 2003). Current research seeks to use frequencies that may be more benign. Growing concerns over increasingly high levels of sound in the ocean are a necessary consideration for development of acoustic deterrents (Southwood et al., 2008). The increasing levels of anthropogenic background noise and decreasing absorption of low-frequency sounds with ocean acidification (Hester et al., 2008) are likely to be major factors influencing organisms' abilities to sense and respond to biologically meaningful sounds and raise concern over the introduction of additional sounds into the ocean for bycatch reduction purposes.
Visual detection
The elasmobranch visual system has been comparatively well studied, and its structure and function rival that of higher vertebrates (e.g. Hart et al., 2006; Lisney et al., 2012). Vision is a dominant sensory domain and, in clear waters, can extend tens of metres, providing continuous, near-instantaneous information relative to orientation, movement, habitat, predators, prey, and conspecifics.
The intensity of ambient light in the aquatic environment is dynamic and can vary nine orders of magnitude based on time of day, angle of incidence, scatter, and seasonality (Lythgoe, 1979; McFarland, 1990). The maximal transmission of light occurs at short wavelengths in deep-sea and clear, open-ocean environments (blue), at intermediate wavelengths in coastal waters (green), and at longer wavelengths in estuarine and freshwater (yellow–red; Jerlov, 1968). Positioned within the retina of most elasmobranchs are both rods, which confer sensitivity and resolution in low-light conditions, and cones, which confer sensitivity in bright-light conditions, higher acuity, and potential colour discrimination. The proportions of rods and cones vary by species according to ecological factors, with deeper dwelling species possessing fewer to no cones (e.g. Bozzano, 2004; Hart et al., 2006). The spectral tuning of rod visual pigments correlates with depth; deeper dwelling species have more blue-shifted sensitivities, in contrast to those living nearer to the surface. Cone spectral sensitivity generally matches ambient environmental spectra, yet sharks studied to date appear to be monochromats and may therefore be colourblind (Gruber, 1975; McComb et al., 2010; Hart et al., 2011), although potentially unexplored mechanisms involving comparisons of rod and one cone input may allow sharks to achieve coloir discrimination, as is suggested in pinnipeds (Griebel and Peichl, 2003). In contrast to sharks, some batoids (skates, rays, and guitarfish) possess multiple spectrally distinct cone pigments, suggesting the capacity for colour vision (Hart et al., 2004; Theiss et al., 2007). In terms of behavioural responses, various species of sharks have displayed attraction to certain colours and high-contrast objects (McFadden and Johnson, 1971), although colour discrimination has only recently been conclusively demonstrated in one ray species (Van-Eyk et al., 2011).
The elasmobranch eye possesses distinct morphological and physiological adaptations that maximize visual function in differing habitats. Adaptations include variation in eye size, eye positioning, mobile pupils, elaborate pupillary operculae, and reflective retinal media (Walls, 1942). Elasmobranchs generally possess relatively large visual fields, up to 360 degrees, with varying amounts of binocular vision facilitating depth perception (McComb and Kajiura, 2008; McComb et al., 2009). Species that inhabit the upper 200 m of the open ocean typically have larger eyes and associated areas of the brain, and faster temporal resolution, and are thought to rely more heavily on vision for locating prey (Kajiura et al., 2010; McComb et al., 2010).
Differences between the vision of elasmobranchs (and other bycatch species) and that of target teleosts may prove useful for bycatch mitigation. Several gear modifications to alter the visual appearance of gear have been investigated, particularly in the pelagic environment. Light sticks are frequently used to attract target fish to bait on pelagic longlines, and modifying them is a potential mechanism to reduce sea turtle bycatch (Crognale et al., 2008; Southwood et al., 2008). The cone sensitivities of shark species range from 480 to 561 nm (Hart et al., 2004, 2011; McComb et al., 2010), while striped marlin (Tetrapturus audax) are reported to have multiple visual pigments with peak sensitivity at 436, 488, and 531 nm (Fritches et al., 2003), and adult yellow fin tuna (Thunnus albacares) have peaks at 426 and 483 nm (Loew et al., 2002). The use of chemical lights outside the range of known shark sensitivity and within range of target fishes may help reduce elasmobranch attraction to gear.
Electroluminescent light sticks or light-emitting diodes (LEDs) offer the opportunity to set specific spectral characteristics as well as flicker or flash rates. Temporal resolution, measured as the critical flicker fusion frequency (CFF) or flicker fusion frequency (FFF), varies among species and presents a novel opportunity to explore manipulation of light flicker on longlines in efforts to reduce attraction by non-target species. Flicker and moving stimuli are detected readily by the visual system and, therefore, knowledge of the CFF of target and non-target species would be beneficial for designing bycatch reduction devices. The CFF for a given species varies with temperature and with light/dark adaptation of the eye, thus future experiments should examine the CFF of species in field conditions (e.g. dark or scotopic adaptation and relevant temperatures). The night-time FFF ranges from 30 to 60 Hz in teleost fishes (Horodysky et al., 2008, 2010), including swordfish (Fritches et al., 2005) and tuna (Bullock et al., 1991; Brill et al., 2005), while the scotopic CFF in elasmobranchs ranges from 16 to 25 Hz (McComb et al., 2010). Flicker rates higher than 30 Hz could increase attraction by teleosts, while simultaneously decreasing attraction to species with lower CFF, such as sharks and leatherback sea turtles (Crognale et al., 2008), to which the light would appear steady and dimmer. The determination of CFF at various wavelengths in relevant laboratory conditions for both target and non-target species, coupled with field-testing, would improve our understanding of the applicability of this technology.
Altering the appearance of the bait itself by dying it blue has been found to decrease seabird, though not sea turtle, bycatch in pelagic longline fisheries (e.g. Brothers et al., 1999; Swimmer et al., 2005). The effects of dyed bait on shark catch were not specifically reported in these studies and remain unknown. Lydon and Starr (2005) reported use of additional colours of dye, including green and red, in commercial longline fisheries to increase target catch, though effects of these practices on shark catch are also unknown. Bird-scaring or Tori lines provide an effective visual signal to keep birds from longlines during deployment when they are vulnerable to becoming hooked and drowned by the gear (Alexander et al., 1997). The use of monofilament leaders, which are less visible to teleosts than steel, can increase target species catch while simultaneously decreasing shark catch (Ward et al., 2008), although concurrent gear adjustments are required to avoid increasing seabird bycatch (Gilman et al., 2008). Even though the number of landed sharks decreases, use of monofilament is unlikely to decrease the number of sharks interacting with gear, resulting in more hooks being bitten off and potential delayed mortality (Afonso et al., 2012).
For net-based fisheries, several modifications have been investigated, including increasing the visibility of the net, adding fake predator models, or increasing visibility of escape hatches. Illumination of gill nets can increase visibility of the net, thereby allowing some species, including sea turtles, to avoid entanglement (Wang et al., 2010). Recent field trials suggest that LED lights in or near the ultraviolet range can reduce both sea turtle and elasmobranch bycatch in gill nets while increasing target species catch (J. Wang, personal communication). Visual predator models have also successfully decreased sea turtle bycatch in gill nets, although fish catch, including target species and elasmobranchs, was also reduced (Wang et al., 2010). Difference in colour (white or black) of an excluder grate to reduce the number of spiny or piked dogfish, Squalus acanthias, captured by trawl did not influence shark behaviour, with both colours resulting in a dramatic decrease in spiny dogfish bycatch (Chosid et al., 2012).
Mechanical detection: water flow
The mechanosensory systems of elasmobranchs include the lateral line canals, superficial neuromasts, spiracular organs, and vesicles of Savi. Mechanoreceptor type and distribution vary by species (Maruska, 2001; Jordan, 2008). Components of these systems are sensitive to the velocity or acceleration of water flow around the body or within canals. Signals detected by the lateral line system of elasmobranchs are low frequency (<200 Hz), with velocities and accelerations respectively in the micrometre per second and millimetre per square second range (Maruska and Tricas, 2004). Hydrodynamic signals within this range are important in prey detection for orienting to currents (rheotaxis), in tracking odour plumes (Gardiner and Atema, 2007), and in locating weak water jets similar to those produced by prey (Montgomery and Skipworth, 1997; Jordan et al., 2009). However, compared with teleosts, relatively little is know about the mechanosensory systems of elasmobranchs. In teleosts, water motion caused by currents, other organisms, and the individual itself can provide information used for complex behaviours, including navigation, hydrodynamic imaging, predator and prey localization, and schooling (Coombs and Montgomery, 1999). Water flow signals from a dipole source, such as a prey organism, are relatively short ranging, while currents can provide information essential for navigation and locating distant prey (see ‘Chemical detection’ above). Functional consequences of differences in anatomical structure between elasmobranchs and bony fishes, such as the distribution of neuromasts within lateral line canals and the presence of non-pored canals, as well as morphological variation between species of elasmobranchs, are not well understood.
Bycatch mitigation strategies using hydrodynamic signals have been largely unexplored. We introduce a potential application for bottom trawls, particularly those targeting invertebrates. Elasmobranchs, including both skates and sharks, have been observed to respond only upon contact with trawl gear (Queirolo et al., 2012). Water jets directed downward and forward of the gear could elicit an earlier response, allowing elasmobranchs an opportunity to avoid capture in the trawl.
Electrical detection
The ampullary electrosensory system of elasmobranchs consists of an array of pores at the surface connected to sensory cells by gel-filled canals and is highly sensitive to low-frequency electrical stimuli produced by both non-biological and biological sources. Electric fields within the range of detection by elasmobranch electroreceptors are created by temperature gradients (Brown, 2010), currents moving through the Earth's magnetic field (Kalmijn, 1971), geomagnetic anomalies (Klimley, 1993), underwater electrical cables (Koops, 2000), and bioelectric fields (Kalmijn, 1971). Electric field gradients fall off quickly in seawater, thus weak electric signals, such as those produced by live prey, are detectable only at short range. This distance is influenced by the signal strength and orientation, as well as temperature and salinity, which determine the resistivity of seawater (Kalmijn, 1971). Species with differing evolutionary histories, feeding ecologies, and electrosensory system morphologies have thus far shown similar sensitivity to electric field strengths, with behavioural responses observed below 1 nV/cm from a maximal distance of 30–40 cm (Kajiura and Holland, 2002; Kajiura, 2003; McGowan and Kajiura, 2009; Jordan et al., 2011). While the minimal electric field strength eliciting an orientation response is similar across species, variation in the number and distribution of electrosensory pores, along with recent behavioural work, suggest that different species rely on the electrosensory system to varying degrees to locate and capture prey (Kajiura et al., 2010; Gardiner, 2011). Coastal species and those feeding on benthic prey are most likely to rely heavily on the electrosensory system (Kajiura et al., 2010).
The electrosensory system was lost during bony fish evolution, and is not present in species typically targeted by fishing activities. Thus, the production of electric signals detectable by elasmobranchs but not teleost fishes has been the focus of several recent deterrent-based bycatch reduction studies (e.g. Stoner and Kaimmer, 2008). Magnets, lanthanide metals/alloys, and powered electrical devices have been evaluated as potential deterrents to prevent consumption of baited hooks or to act as a barrier that could prevent capture in nets (e.g. Marcotte and Lowe, 2008; Robbins et al., 2011). All three types of electrical deterrents produce electric signals different from those created by living things and may over-stimulate the sensitive electrosensory system, eliciting an aversion response.
Laboratory and field experiments with lanthanide metals and magnets have reported conflicting results in eliciting avoidance behaviour, decreasing shark catch, and observing habituation (e.g. Kaimmer and Stoner, 2008; Wang et al., 2008; Rigg et al., 2009; Tallack and Mandelman, 2009; O'Connell et al., 2011; Robbins et al., 2011; Hutchinson et al., 2012). Variation in the response to lanthanide metal deterrents has been observed both between and within species (Jordan et al., 2011). Factors such as hunger level and presence of potential competitors may contribute to variation in both laboratory and field settings (Brill et al., 2009; Robbins et al., 2011). In the presence of conspecifics, the dusky smoothhound, Mustelus canis, switched from avoiding food attached to a lanthanide metal (when alone) to preferring it (Jordan et al., 2011). Inter-specific differences have been attributed to ecological variables, with greater deterrent success being found for coastal, benthic-feeding species or age classes (Rigg et al., 2009; Hutchinson et al., 2012). Future studies may achieve greater success when focusing on species that are likely to rely heavily on electroreception to locate prey and are rarely observed to feed in groups.
Deterrents aimed at the electrosensory system present various economic and logistical challenges (see O'Connell et al., 2012). For example, lanthanide metals dissolve in seawater, requiring frequent replacement, which increases the time spent modifying gear and exacerbates cost concerns for materials. Magnets can present additional challenges for handling on board ships and during deployment/recovery of gear. Powered electronic devices have been marketed as shark-attack deterrents, although their effectiveness has rarely been evaluated experimentally (Huveneers et al., 2012). One area of current research is focused on developing battery-powered electrical devices that can be affixed to hook-and-line fishing gear to reduce elasmobranch bycatch (S. Kajiura, personal communication). Whether or not these are more cost effective remains to be seen. High-powered electric fields to exclude sharks from larger areas require considerable amounts of energy and also achieve variable effectiveness (Smith ED, 1991; Marcotte and Lowe, 2008). High-powered electric fields have shown promise in reducing seal depredation on fish caught by gill net (Forrest et al., 2009), indicating that further development of this technology could be useful for reducing gear interactions of multiple non-target taxa.
Sensory modality convergence during feeding events
As an elasmobranch approaches its prey, more sensory cues become detectable (Fig. 1). In the ocean, sound and odour signals entrained in currents can potentially travel long distances from the source and may contain initial information directing marine predators toward food. Depending on water clarity and light levels, vision can aid in prey localization up to several tens of metres away. Electric and hydrodynamic signals in seawater have a short range, and typically elicit orientations by elasmobranchs within a metre of the source.
Multisensory integration is fundamental to the formation of unified sensory perception and is present in a wide range of taxa (e.g. Rowland and Stein, 2008). This topic is beginning to be examined in elasmobranchs, and it is unknown whether new information is added to and integrated with existing information or if switching from reliance on one sensory system to another occurs (Gardiner et al., 2012). When one type of signal becomes obscured, e.g. vision at close range, others, e.g. electroreception, are likely to become increasingly important in directing the final stages of prey capture. Large blind spots in the visual field anterior to sharks are hypothesized to be almost completely overlapped by the sensitivity range of the electrosensory system, resulting in near-seamless sensory function (McComb et al., 2009). Sharks have been observed to use at least two signal types in tandem, e.g. odour and water flow (Gardiner and Atema, 2007), or to have one type override another. For example, blue sharks, Prionace glauca, and dusky smoothhound, Mustelus canis, preferentially bit at a prey-simulating dipole rather than the prey odour source (Kalmijn, 1971).
Recent work indicates that certain sensory modalities direct specific prey-capture behaviours. Bonnethead sharks, Sphyrna tiburo, could locate but not accurately line up to capture food without vision, and could not efficiently strike to ingest food in the absence of electrical stimuli (Gardiner, 2011). The nurse shark, Ginglymostoma cirratum, required olfactory signals for food ingestion to occur even though orientation to the prey was successful with other stimuli (Gardiner, 2011). In the blacktip shark, Carcharhinus limbatus, both visual and olfactory cues were necessary for efficient prey location and capture (Gardiner, 2011). More generally, differences in sensory system morphology and development of the associated regions of the brain suggest a trade-off between vision and electroreception, where oceanic pelagic species may rely more heavily on vision and coastal benthic-feeding species on electroreception (Kajiura et al., 2010). Variation exists, however, and other sensory cues may play an integral role for some species, such as odour in the nurse shark. Use of multiple sensory modalities in locating and capturing prey suggests that the most effective bycatch reduction approach, for at least some species, may involve combinations of strategies simultaneously directed at more than one sensory system.
Signals produced by fishing gear
Whether it involves bait or not, every type of fishing gear emits a variety of signals when deployed underwater. At a minimum, these signals will include hydrodynamic disturbances and sounds created by the gear and/or the vessel deploying gear, and could also include odours, visual signals, and electrical cues. Any gear in contact with the substrate (e.g. weighted gill nets, bottom trawls, and demersal longlines), may generate various sounds, vibrations, and increased turbidity. Bait will contribute additional chemical and visual cues. Any entangled, hooked, or fouling organism may emit a variety of signals, including odours, hydrodynamic disturbances, struggling sounds, visual signals, and bioelectric fields. Here, we outline potential sensory signals emitted by various types of fishing gear that elasmobranchs may encounter and introduce potential bycatch reduction modifications to each gear type as topics for future research (summarized in Table 1).
Table 1.
Potential applications of new and existing bycatch reduction technology by fishing gear and elasmobranch sensory modality
| Sensory modality | Baited hook and line (longline) | Gill net | Trawl | Purse seine |
|---|---|---|---|---|
| Olfaction | Surfactants, semiochemicals | Surfactants, semiochemicals | Remote attraction/bait stations | |
| Bait type | ||||
| Dead sharks | ||||
| Hearing | Not recommended | |||
| Vision | Light sticks: wavelength and flicker | Net illumination | Flashing lights | |
| Bait colour | Net colour | |||
| Leader type/colour | Predator models | |||
| Dead sharks | ||||
| Mechanosensory lateral line/pit organs | Water jets | |||
| Electrosensory | Magnets, lanthanide metals, battery-powered electric devices | Powered electric field ‘barrier’ | Electric pulse generators | |
| Magnetic field ‘barrier’ | ||||
| Other | Pre-net fence (tactile) |
Some of these may potentially be applied to other gear types, and all require additional research and development.
Net fishing gear
Gill nets are typically designed to be difficult to detect visually, although some are outfitted with various colours to attempt to select for certain target species or deter others (e.g. Melvin et al., 1999). Increasingly, gill nets are equipped with high-frequency acoustic pingers, and other modifications, including illumination and stiffer, acoustically reflective materials, continue to be evaluated (Northridge et al., 2003; Werner et al., 2006; Larsen et al., 2007; Trippel et al., 2009; Bordino et al., 2013). Stiffer materials or increased tension can decrease the risk of entanglement of elasmobranchs and other species (e.g. Thorpe et al., 2001). Signals providing early warning of the presence of the net, e.g. visual or electromagnetic cues, may reduce elasmobranch interactions with gear and could override attractive signals produced by captured organisms. Visual enhancements, including lights affixed to nets (Fig. 2A), nets constructed of photoluminescent materials, or predator models, warrant further testing for their influence on elasmobranch and target species catch (see Wang et al., 2010). The effects of different colours of nets on elasmobranchs in artisanal and commercial fisheries are largely unknown. Electrical barriers affixed to the net, either powered or magnetic, could repel elasmobranchs, preventing entanglement. The development of a pre-net ‘fence’, consisting of vertical float lines attached to a weighted line in front of the gill net, may provide visual and tactile cues to keep larger animals from becoming entangled, while smaller target species pass between float lines into the net (M. Kobza, personal communication).
Figure 2.
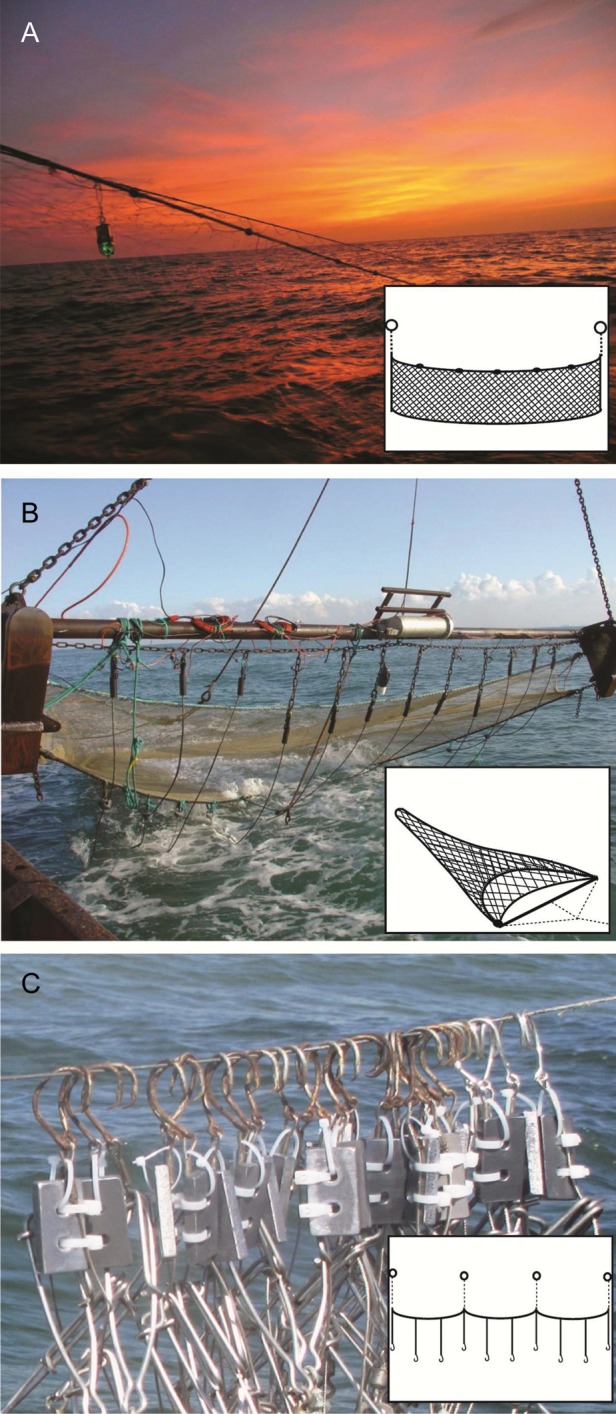
Examples of sensory-based deterrents attached to fishing gear. (A) Illuminated gill net (photograph credit Jesse Senko). (B) Beam trawl fitted with electric pulse generator, electrodes, and raised ground rope (Hovercran shrimp pulse trawl, photograph credit ILVO, Belgium). (C) Longline gear with lanthanide metal secured near hook (photograph credit Kieran Smith). Inset illustrations show examples of each fishing gear type when deployed
Purse seine gear is commonly set over FADs (or organisms such as whale sharks) that can provide hydrodynamic, sound, and odour signals from aggregating fishes and fouling organisms. In one study, odour was suspected to be the major cue for fish to locate FADs, but no difference was found for fish returning to a FAD from downstream or cross-stream starting points, leading the researchers to suggest sound as potentially important (Dempster and Kingsford, 2003). Once the purse seine begins to be deployed around or near the FAD, additional hydrodynamic, chemical, and sound signals are likely to be present. The use of multiple FADs to facilitate species segregation (Schaefer and Fuller, 2011) and/or displaced attractants (e.g. bait stations; Dagorn, 2010) may help to reduce the number of elasmobranchs captured by a purse seine net. Within the net itself, both target and non-target species have been observed to segregate by size class and species, indicating potential for selective release of non-target species and size classes (Muir et al., 2012). The use of sensory cues directing bycatch organisms toward escape routes both before and after setting the net show promise and warrant further research (Dagorn et al., 2012).
Trawl gear dragged along the bottom causes sound, vibration, and hydrodynamic disturbance, and could be detected visibly, depending on light levels and turbidity, which may be increased by the gear itself. Entrained organisms may emit additional chemical and hydrodynamic signals. Trawl gear not in contact with the bottom would produce similar signals without increases in sound, vibration, and turbidity from bottom stirring. For a resting elasmobranch being approached by mobile gear, such as a trawl, signals prior to contact with the gear are likely to be limited to sound and vibrations, followed by visual signals of suspended sediments or the gear itself, because the animal may be upstream of chemical trails. Queirolo et al. (2012) observed sharks and skates to be fairly passive around the mouth of the trawl, possibly relying on camouflage to ‘hide’, staying motionless or swimming no faster than the trawl itself upon contact. Inter-specific differences in shark behaviour around approaching gear have been observed (Lorance and Trenkel, 2006), and herding, which is visually mediated in flatfish (Ryer et al., 2010), can also occur in skates (Winger et al., 2010). Signals including visual (lights), hydrodynamic (water jets), or electric fields could provide an early warning system to benthic elasmobranchs, which may increase reaction time to enable avoidance of approaching gear. Polet et al. (2005) demonstrated that electric pulse generators positioned at the mouth of a trawl (Fig. 2B) successfully caused brown shrimp, the targeted species, to jump upwards, allowing the ground rope to be raised by 10–15 cm with no reduction in shrimp catch. Raising the ground rope allows fishes and other invertebrates to escape under the net and results in less benthic damage (Polet et al., 2005). Although not encountered in the above study, the electric pulse itself may alert elasmobranchs to the approaching gear and facilitate escape either below or away from the trawl.
Baited fishing gear
Bait exudes chemical and visual signals, although the lines themselves can be difficult to detect visually. During deployment of longlines, activity from seabirds may cause additional sounds and disturbances. When baited gear, such as longlines, is deployed, we suspect that elasmobranchs are more likely to approach from downstream, where chemical signals are available. Pelagic sharks rely heavily on vision to locate prey, thus several visual signals warrant further experimentation for use on pelagic longlines, including light stick colour and flicker frequency, bait dying, and leader type or colour. Anecdotal evidence also suggests that the presence of dead sharks on or near fishing gear can decrease subsequent shark catch (S.J.B. Gulak, personal communication). The extent of chemical vs. visual signals contributing to this observed avoidance response is unclear, and controlled experiments are necessary. Several sharks feed on other elasmobranchs, suggesting that chemicals produced by freshly dead individuals may, in some cases, be attractive rather than repulsive, thus further understanding of chemicals produced by freshly dead vs. decaying sharks and the effects of these on nearby elasmobranchs is needed. Research into developing electric/magnetic deterrents for baited gear appears most promising for coastal demersal longlines, where bycatch species are more likely to rely heavily on electroreception (Fig. 2C).
The likelihood of striking at bait depends on a variety of environmental and biological variables, including both feeding motivation and the ability to locate the bait successfully (Stoner, 2004). The likelihood of becoming hooked also depends on feeding behaviour, including how the bait and hook are ingested, which may be influenced by hook type, placement within the bait, and bait type. Detailed knowledge of elasmobranch interactions with bait and fishing gear, however, are lacking.
Feeding behaviour and ecology
While it is not within the scope of this review to present a comprehensive overview of elasmobranch feeding ecology and behaviour, we provide a brief background in the context of potential interactions with fishing gear.
Overview of prey-capture mechanics/strategies
Elasmobranchs exhibit a variety of feeding and prey-capture strategies that vary with habitat and prey type. Strategies include filtering, ambushing, disabling, digging up, and chasing down prey, while capture may include suction and/or ram feeding, with various degrees of jaw protrusion (Motta and Huber, 2012). Prey capture, from mouth opening (expansion) to closing (compression), is typically rapid (100–400 ms), with variation associated with species and prey type (Motta et al., 2002). The feeding apparatus of sharks is relatively simple in comparison to teleosts, and consists of 10 cartilaginous elements that make up and support the jaw (Motta, 2004). Regardless of whether suction or ram feeding occurs, bait and hooks can either enter the buccal cavity or become lodged in the jaw. Sharks are significantly less likely to survive when the hook is swallowed vs. embedded in the jaw (Campana et al., 2009). Both hook size and hook shape can influence the likelihood of swallowing in teleosts and elasmobranchs (Bacheler and Buckel, 2004; Campana et al., 2009), and hook material can affect how long the hook remains in the jaw if the line is cut. Circle hooks were developed to reduce catch rate and swallowing of hooks by sea turtles. While the effects of circle hooks on shark catch rates have been mixed, their use generally reduces at-vessel mortality of both sea turtles and elasmobranchs when compared with J hooks (e.g. Watson et al., 2005; Afonso et al., 2011; Curran and Bigelow, 2011, Godin et al., 2012). Whether the elasmobranch swallows the bait whole or takes multiple bites may be a function of mouth size, bait type, or additional inter-specific differences. The differences in mouth shape, size, and bait ingestion mechanics between elasmobranchs and target species, as a means to reduce bycatch, have not yet been investigated.
Behavioural ecology of elasmobranchs during feeding events
Sharks are generally considered intermittent, asynchronous, opportunistic feeders; however, many species are highly specialized for limited prey types, and most batoids and filter feeders feed more continuously (Motta and Wilga, 2001; Wetherbee and Cortés, 2004; Motta and Huber, 2012). Most elasmobranch species show evidence of being opportunistically selective feeders, choosing preferred prey when it is available (Wetherbee et al., 1990). Many species feed in a solitary manner; however, some form large aggregations and employ co-ordinated efforts to herd prey (See Motta, 2004). Group feeding can provide advantages in terms of locating prey more efficiently, but can also introduce social and competitive factors, which may influence foraging success and prey selectivity. Aggressive feeding aggregations can involve groups of six to hundreds of sharks and may include indiscriminate feeding, accelerated speed, and injury to individuals (e.g. Nelson, 1969). Both the presence of potential competitors and increased hunger level have been suggested as factors that decrease the success of shark deterrents in field and laboratory settings, indicating the need for different strategies for solitary- vs. group-feeding species (Tallack and Mandelman, 2009; Jordan et al., 2011; Robbins et al., 2011).
Behavioural ecology of non-feeding elasmobranch aggregations
Elasmobranch species have been observed to form aggregations for several reasons other than feeding. Schooling or shoaling behaviour can be associated with reproduction or refuging (Klimley and Nelson, 1984). Dominance hierarchies in shoals of sharks appear to be established by size and/or sex, and many species form separate aggregations based on size and/or sex (see Bres, 1993). Many of these aggregations are associated with environmental structures, such as reefs, sea mounts, or FADs, and disperse at regular intervals, such as night-time foraging (Bres, 1993; Filmalter et al., 2011). Species segregation may play a key role in future bycatch reduction strategies for purse seines (Muir et al., 2012). Aggregations of elasmobranchs (and other species) are highly vulnerable to fishing activities, particularly when large nets capable of entrapping entire shoals are used, and high catch numbers can lead to inflated population estimates (Fordham et al., 2006).
Species examples
Sensory system anatomy, feeding ecology, and prey-capture strategies vary widely among elasmobranchs, suggesting the need for more species-specific bycatch mitigation strategies. Here, we discuss in more detail a few species of US and/or international concern, for which regional or global attention to bycatch reduction is warranted. Species were chosen based on one or more of the following factors: (i) conservation concern; (ii) potential for successful bycatch reduction through the use of deterrents and/or gear modifications aimed at reducing interaction with fishing gear; and (iii) high or unknown at-vessel and/or post-release mortality.
Scalloped hammerhead
Populations of hammerhead shark species are estimated to have declined by up to 85% since the 1980s in some regions, such as the northwest Atlantic ocean (Hayes et al., 2009; Jiao et al., 2009). The scalloped hammerhead (Sphyrna lewini) is currently listed by the IUCN as endangered globally and is largely taken as bycatch by pelagic longline and gill-net fishing vessels, although they are also captured by trawls, purse seines, bottom longlines, and inshore artisanal fisheries, particularly at smaller size classes (Baum et al., 2007; Amandè et al., 2010). High at-vessel mortality has been documented for scalloped hammerheads in pelagic longline fisheries (61%; Beerkircher et al., 2002), demersal longlines (91.4%; Morgan and Burgess, 2007), and gill nets (93%; J. Carlson, unpublished data).
As a result of their unique head shape and corresponding sensory arrangements, hammerheads have been the subject of considerable sensory-related research. The expanded cephalofoil has been suggested to provide sensory and manoeuvrability advantages, in addition to its occasional use as a restraint for capturing benthic prey (Strong et al., 1990; Kajiura et al., 2003, 2005; McComb et al., 2009). The location of eyes and nares farther from the mid-line of the body may improve binocular vision and tracking of odour plumes, respectively (Kajiura et al., 2005; McComb et al., 2009). Head shape does not appear to provide hammerhead sharks with enhanced electrosensitivity, although it does increase their lateral search area, which may be particularly useful when scanning for benthic prey (Kajiura and Holland, 2002). Scalloped hammerheads have been observed to disperse from aggregations near a sea mount to feed at night (Klimley, 1988), suggesting that bioluminescence and/or senses other than vision are important for tracking prey.
Based on their sensory systems and ecological roles, we suggest research into multiple sensory modalities to reduce bycatch of scalloped hammerheads and other species that share similar sensory characteristics. For example, we recommend studies investigating visual (e.g. light stick) and chemical cues, as well as electric/magnetic deterrents, which have already demonstrated some success. Scalloped hammerheads avoided a magnetic field created by ferrite magnet blocks (Rigg et al., 2009), and lanthanide metal alloys significantly reduced catch of this species on longlines (Hutchinson et al., 2012). Another species of concern, for which a similar bycatch reduction approach may be successful, is the dusky shark, Carcharhinus obscurus, which also has a high number of electrosensory pores (Kajiura et al., 2010) and has demonstrated avoidance of electrical barriers (Smith ED, 1991). In contrast, the more pelagic blue shark, Prionace glauca, has a low number of electrosensory pores and probably relies more heavily on vision and olfaction to locate prey (Kajiura et al., 2010). Lanthanide metal deterrents have failed to reduce blue shark catch rates, but visual and chemical bycatch reduction strategies may have more success (Hutchinson et al., 2012).
Smalltooth sawfish
Listed by the IUCN as critically endangered, the sawfishes are one of the most threatened families of fishes, having experienced dramatic population declines and near extirpation from areas where they were once common (Adams et al., 2006; Compagno et al., 2006; Charvet-Almeida et al., 2007). For example, the population of smalltooth sawfish, Pristis pectinata, in US waters is essentially restricted to southern Florida (Simpfendorfer et al., 2008). Fishing activities are the greatest threat to this species (Adams et al., 2006). Smalltooth sawfish are taken as bycatch both in bottom longline fisheries and in shrimp trawls, where high mortality is estimated (National Marine Fisheries Service, 2012).
Owing to the high tendency of the saw-like rostrum to become entangled, preventing contact with fishing gear and ensuring safe release if captured are major objectives in sawfish recovery efforts. Sawfish have extensive electrosensory and lateral line systems extending out along the rostrum, which is used both to sense and to manipulate prey (Wueringer et al., 2011a,b, 2012). Observations of sawfish interacting with trawl gear, such as those obtained by video, are crucial for devising effective ways to avoid sawfish capture and minimize mortality. If sawfish remain relatively passive until contact occurs with trawl gear, as observed in other elasmobranchs (Queirolo et al., 2012), an early warning system including water jets and/or electric pulses may facilitate timely reaction and gear avoidance. The combined effects of electric pulses and a raised ground rope could significantly reduce bycatch of many species in addition to sawfish. If capture occurs, catch of narrow sawfish, Anoxypristis cuspidate, in Australia has been shown to decline with use of TEDs in trawl nets (Brewer et al., 2006), although post-release condition is unknown. Although TEDs are also used in Florida waters where smalltooth sawfish occur, the effects of these on sawfish catch and mortality are not known (J. Carlson, personal observation).
Blacknose shark
The blacknose shark, Carcharhinus acronotus, is currently listed by the IUCN as near threatened globally, has experienced a 25% population reduction between the 1950s and 2006 (Siegfried and Brooks, 2007), and is considered over-fished, with over-fishing occurring in the Atlantic ocean (National Marine Fisheries Service, 2011). This species is targeted by some fisheries (Carlson and Bethea, 2007) and is also caught as bycatch in teleost gill net and shrimp trawl fisheries, particularly as juveniles (Shepherd and Myers, 2005; Siegfried and Brooks, 2007).
Although little is known about blacknose shark behaviour, visual characteristics suggest that this species is more active during low-light, crepuscular, or nocturnal periods, and it has a 360 degree dorsal and ventral visual field, with frontal binocular vision (McComb et al., 2009, 2010). Like other coastal pelagic Carcharhinid species, blacknose sharks have a relatively high number of electrosensory pores, suggesting the importance of electroreception in the perception of their environment and location of prey (Kajiura et al., 2010). Capture of blacknose sharks in gill nets is highly dependent on mesh size (Carlson and Cortés, 2003), and modifying this, along with sensory-based approaches, may reduce bycatch in gill-net fisheries. Known visual and electrosensory characteristics suggest that net lighting and electrical deterrents are particularly promising lines of research to pursue for bycatch reduction of blacknose sharks in gill nets. These modifications, as well as pre-net fences, discussed earlier, warrant further study to determine their effectiveness in reducing contact of non-target species with gill nets.
Manta rays
The giant manta, Manta birostris, and reef manta, Manta alfredi, were only recently recognized as separate species (Marshall et al., 2009), and are often confused with devil rays of the genus Mobula. Thus, historical fishery records mix or confuse these species, but both mantas are listed by the IUCN as vulnerable (Marshall et al., 2011). Global populations of mantas are estimated to have decreased by at least 30%, with declines of up to 80% in some regions (Marshall et al., 2011). These species are taken as bycatch in longline (Beerkircher et al., 2002) and, more commonly, in net fisheries, such as purse seines (Coan et al., 2000; Amandè et al., 2010), pelagic trawls (Zeeberg et al., 2006), and gill nets (Trent et al., 1997; White et al., 2006).
Manta rays filter feed on planktonic organisms, either near the surface or at depth, using unfurled cephalic lobes to direct food and water into the mouth. Although few quantitative data on manta sensory anatomy and detection capabilities exist, the electrosensory system appears markedly reduced, while the lateral line is extensive and highly branched (Chu and Wen, 1979). The eyes of mantas are relatively small and oriented for forward, lateral, and downward vision. A captive manta displayed feeding responses to visual and olfactory stimuli (Ari and Correia, 2008), while electromagnetic and olfactory cues are likely to be important in navigation during migrations. Owing to their large size, mantas may be visible to fishers and should be avoided as a first step toward reducing interactions with gear. Manta and mobulid rays were more frequently captured by European purse seiners in the Atlantic ocean in sets on free-swimming tuna rather than in sets on FADs (Amandè et al., 2010). This trend is opposite to that observed for overall bycatch, and for sharks, where 91% were caught in sets with FADs (Amandè et al., 2010).
Priorities for manta ray bycatch reduction include more detailed and quantitative knowledge of their sensory biology, along with observations of mantas interacting with fishing gear. We suggest the use of side-scan sonar, video, or observers to look for any patterns, such as upward or downward swimming, that might be useful in determining avoidance or release strategies for mantas once inside purse seine nets. Observations such as these have contributed to the development of highly effective dolphin bycatch reduction techniques (e.g. Barham et al., 1977; Francis et al., 1992), and are currently being developed for release of sharks from purse seines (Itano et al., 2012). In addition, remote bait stations, such as those currently being explored for sharks (Dagorn, 2010), may assist in diverting mantas away from fishing areas; however, more information on how mantas locate prey and associate with tuna schools is needed.
Conclusions, new directions, and challenges
Over-fishing, including excessive mortality from bycatch, is the largest threat to elasmobranch populations. According to the IUCN, of the 563 elasmobranch species not considered data deficient, 55% are categorized as threatened or near threatened (IUCN Redlist). Concern over depleted elasmobranch populations and waste associated with bycatch has led to a surge in bycatch reduction-related research. Despite attempts to understand and exploit elasmobranch sensory biology for prevention of shark attacks since 1945, no deterrent has, to our knowledge, proved effective for all species and situations tested (Gilbert, 1968; Sisneros and Nelson, 2001; O'Connell et al., 2012). Elasmobranchs are a diverse group of over 1100 species, exhibiting wide ranges in habitat, behaviour, ecology, sensory biology, and neurobiology (Gardiner et al., 2012; White and Last, 2012; Yopak, 2012). For these reasons, we suggest a species- and fishery-specific approach that builds upon current knowledge of sensory biology and behaviour of target and non-target species. While a universally effective deterrent may be unlikely, greater understanding of sensory capabilities and feeding behaviour may shed light on deterrents or combinations of deterrents that will be effective in reducing elasmobranch interactions with particular fisheries. This information, combined with more detailed knowledge of fishing practices (made available through observer programmes, as well as co-operation and collaboration with the fishing industry) can be integrated to develop more effective elasmbranch bycatch reduction strategies.
In order to expand the field of elasmobranch bycatch reduction research, we have identified several opportunities for future research and collaboration. Improving our understanding of how various elasmobranch species sense and interact with fishing gear and how this differs from target species is a key priority. Detailed knowledge of sensory biology and behaviour is lacking for many elasmobranch species, including several that make up large components of bycatch from various fisheries. Combinations of anatomical, behavioural, and field studies are ideal, but not feasible for some species (e.g. rare/protected species and/or those that do not survive in captivity). Identification of specific sensory cues broadcast by a type of fishing gear and evaluation of when, how, and which sensory information is used by a given species should lead toward effective solutions for reducing gear interactions. Several new and existing technologies should undergo further testing for use in elasmobranch bycatch mitigation (Table 1). Selection of which technology is likely to be most effective will depend on the fishery and species involved, particularly for fisheries with high bycatch rates, where one species dominates the bycatch, or when mortality associated with a fishery poses significant risks to threatened populations.
Behavioural observations of elasmobranchs prior to and during capture are needed in order to suggest promising techniques for minimizing vulnerability to fishing gear. Despite a vast body of research on teleosts (e.g. Somerton, 2004; Broadhurst et al., 2007; He, 2009), few studies have directly or indirectly investigated elasmobranch behaviour around fishing gear. Strategies to increase gear selectivity and reduce bycatch of non-target teleost fishes often seek to evaluate and exploit species-specific behavioural tendencies (Breen et al., 2004; Walsh et al., 2004; Videler and He, 2010). For example, in tuna longlining, where shark bycatch occurs, analysis of differences in mouth shape, approach, and ingestion of bait may reveal new hook designs, in addition to J and circle hooks, or novel bait hooking strategies to decrease bycatch. Technology for video or sonar surveillance of trawling and other fishing operations is an important tool for understanding when and how various species, including elasmobranchs, react to approaching gear (e.g. Queirolo et al., 2012). Use of this technology should accompany catch data as new bycatch reduction methods are tested for a more complete understanding of how gear modifications and deterrents influence the behaviour of both target and non-target species.
In addition to adopting an integrative approach, including modelling, laboratory, and field tests to understand consequences of gear alterations on both target and non-target species (Molina and Cooke, 2012), we emphasize the need for greater collaboration among researchers studying bycatch reduction of different taxonomic groups. When testing gear modifications in the field, recording detailed species-specific catch data is vital to the overall goal of bycatch mitigation. Many bycatch reduction studies focusing on other taxa list elasmobranchs among the catch, yet do not include detailed analyses of the effects of gear modifications on elasmobranch catch rate and/or mortality (with notable exceptions, such as Watson et al., 2005). This missing information could provide valuable insights into types of modifications that influence elasmobranch vulnerability to fishing gear. Also, greater collaboration among scientists should streamline efforts to develop technologies that reduce bycatch of all non-target species, rather than decreasing the catch of one species or group while increasing the catch of another.
Bycatch reduction strategies aimed at each sensory system have inherent advantages and challenges based on the nature of the signal and how it can be produced. Many gear modifications and deterrents present logistical, economic, and environmental challenges. For example, chemical deterrents may disperse too rapidly or slowly to be effective, and certain chemicals may pose a pollution risk or could negatively affect marine organisms (see O'Connell et al., 2012). In contrast, chemical deterrents may be fairly cost effective for mass production and be relatively simple to integrate into current fishing practices (e.g. pre-treated baits). Any of the proposed bycatch mitigation methods will be likely to meet with less resistance from the fishing community if their benefits (such as increased or unaffected target catch and/or decreased depredation and gear damage) outweigh their costs in terms of materials and time spent modifying gear.
In addition to development of new techniques to prevent elasmobranch bycatch, increased motivation and incentives for policymakers and the fishing industry to prioritize elasmobranch bycatch reduction are necessary. The demand for shark fins and other elasmobranch products is high and growing in some markets, resulting in disincentives to research, mandate, employ, and enforce the use of methods to minimize elasmobranch bycatch. Several elasmobranch bycatch reduction techniques (e.g. using fish instead of squid bait) have already proved useful, yet have not achieved widespread implementation (Gilman et al., 2008). Economic and political pressures often outweigh scientific advice in fisheries management arenas, contributing to the general state of over-exploitation of global fisheries (e.g. Gilman, 2011). Despite challenges to international co-operation in the management of fisheries and bycatch, broad changes have been relatively successful (e.g. the moratorium on the use of high-seas drift nets and the implementation of TEDs in trawls), lending hope for future progress toward more sustainable fishing. As the human population and demand for seafood continue to rise, development of sustainable fisheries, with minimal bycatch, are increasingly important. Research that integrates the sensory biology and behavioural characteristics of elasmobranch species can help to improve gear selectivity, support more effective fisheries management, and facilitate recovery of threatened species.
Acknowledgements
We thank E. Gilman, C. O'Connell, L. Dagorn, V. Restrepo, E. Stroud, and M. Kobza for insight into on-going research projects and bycatch reduction methods. We also thank the anonymous reviewers whose thoughtful comments helped us to improve the manuscript. Opinions expressed herein are of the authors only and do not imply endorsement by any agency or institution associated with the authors. This work was supported by the Consortium for Wildlife Bycatch Reduction at the New England Aquarium under US Department of Commerce NOAA Award (grant no. NA10NMF4520343).
References
- 1.Adams WF, Fowler SL, Charvet-Almeida P, Faria V, Soto J, Furtado M. (2006) Pristis pectinata. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/18175/0 (last accessed March 2013). [Google Scholar]
- 2.Afonso AS, Hazin FHV, Carvalho F, Pacheco JC, Hazin H, Kerstetter DW, Murie D, Burgess GH. (2011) Fishing gear modifications to reduce elasmobranch mortality in pelagic and bottom longline fisheries off Northeast Brazil. Fish Res 108: 336–343. (doi:10.1016/j.fishres.2011.01.007) [Google Scholar]
- 3.Afonso AS, Santiago R, Hazin H, Hazin FHV. (2012) Shark bycatch and mortality and hook bite-offs in pelagic longlines: interactions between hook types and leader materials. Fish Res 131–133: 9–14. (doi:10.1016/j.fishres.2012.07.001) [Google Scholar]
- 4.Alexander K, Robertson G, Gales R. (1997) The incidental mortality of albatrosses in longline fisheries. In Report on Workshop First International Conference on the Biology and Conservation of Albatrosses, September 1995 Australian Antarctic Division, Hobart, Australia: 44 pp. [Google Scholar]
- 5.Alverson DL, Freeberg MH, Murawaski SA, Pope JG. (1994) A global assessment of fisheries bycatch and discards. FAO Fisheries technical paper no. 339 FAO, Rome, 235 pp. [Google Scholar]
- 6.Amandè MJ, Ariz J, Chassot E, Delgado de Molina A, Gaertner D, Murua H, Pianet R, Ruiz J, Chavance P. (2010) Bycatch of the European purse seine tuna fishery in the Atlantic Ocean for the 2003–2007 period. Aquat Living Resour 23: 353–362. (doi:10.1051/alr/2011003) [Google Scholar]
- 7.Ari C, Correia JP. (2008) Role of sensory cues on food searching behavior of a captive Manta birostris (Chondrichtyes, Mobulidae). Zoo Biol 27: 294–304. (doi:10.1002/zoo.20189) [DOI] [PubMed] [Google Scholar]
- 8.Atema J, Holland K, Ikehara W. (1980) Olfactory responses of yellowfin tuna (Thunnus albacares) to prey odors: chemical search image. J Chem Ecol 6: 457–465. (doi:10.1007/BF01402922) [Google Scholar]
- 9.Bacheler NM, Buckel JA. (2004) Does hook type influence the catch rate, size, and injury of grouper in a North Carolina commercial fishery? Fish Res 69: 303–311. (doi:10.1016/j.fishres.2004.07.001) [Google Scholar]
- 10.Banner A. (1967) Evidence of sensitivity to acoustic displacements in the lemon shark, Negaprion brevirostris (Poey). In Cahn PH, ed., Lateral Line Detectors Indiana University Press, Bloomington, pp. 265–273. [Google Scholar]
- 11.Barham E, Taguchi W, Reilly S. (1977) Porpoise rescue methods in the yellowfin purse seine fishery and the importance of Medina panel mesh size. Mar Fish Rev 39: 1–10. [Google Scholar]
- 12.Barlow J, Cameron GA. (2003) Field experiments show that acoustic pingers reduce marine mammal bycatch in the California drift gill net fishery. Mar Mamm Sci 19: 265–283. (doi:10.1111/j.1748-7692.2003.tb01108.x) [Google Scholar]
- 13.Baum J, Clarke S, Domingo A, Ducrocq M, Lamónaca AF, Gaibor N, Graham R, Jorgensen S, Kotas JE, Medina, et al. (2007) Sphyrna lewini. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/39385/0 (last accessed March 2013). [Google Scholar]
- 14.Beerkircher LR, Cortés E, Shivji M. (2002) Characteristics of shark bycatch observed on pelagic longlines off the southeastern United States, 1992–2000. Mar Fish Rev 64: 40–49. [Google Scholar]
- 15.Bonfil R. (1994) Overview of world elasmobranch fisheries. FAO Fisheries technical paper no. 341 FAO, Rome, 119 pp. [Google Scholar]
- 16.Bordino P, Kraus S, Albareda D, Fazio A, Palmerio A, Mendez M, Botta S. (2002) Reducing incidental mortality of Franciscana dolphin Pontoporia blainvillei with acoustic warning devices attached to fishing nets. Mar Mamm Sci 18: 833–842. (doi:10.1111/j.1748-7692.2002.tb01076.x) [Google Scholar]
- 17.Bordino P, Mackay AI, Werner TB, Northridge SP, Read AJ. (2013) Franciscana bycatch is not reduced by acoustically reflective or physically stiffened gillnets. Endang Species Res 19: in press. [Google Scholar]
- 18.Borucinska J, Kohler N, Natanson L, Skomal G. (2002) Pathology associated with retained fishing hooks in blue sharks, Prionace glauca (L.), with implications for their conservation. J Fish Dis 25: 515–521. (doi:10.1046/j.1365-2761.2002.00396.x) [Google Scholar]
- 19.Bozzano A. (2004) Retinal specialisations in the dogfish Centroscymnus coelolepis from the Mediterranean deep-sea. Sci Mar 68: 185–195. (doi:10.3989/scimar.2004.68s3185) [Google Scholar]
- 20.Breen M, Dyson J, O'Neill FG, Jones E, Haigh M. (2004) Swimming endurance of haddock (Melanogrammus aeglefinus L.) at prolonged and sustained swimming speeds, and its role in their capture by towed fishing gears. ICES J Mar Sci 61: 1071–1079. (doi:10.1016/j.icesjms.2004.06.014) [Google Scholar]
- 21.Bres M. (1993) The behaviour of sharks. Rev Fish Biol Fish 3: 133–159. (doi:10.1007/BF00045229) [Google Scholar]
- 22.Brewer D, Rawlinson N, Eayrs S, Burridge C. (1998) An assessment of bycatch reduction devices in a tropical Australian prawn trawl fishery. Fish Res 36: 195–215. (doi:10.1016/S0165-7836(98)00096-4) [Google Scholar]
- 23.Brewer D, Heales D, Milton D, Dell Q, Fry G, Venables B, Jones P. (2006) The impact of turtle excluder devices and bycatch reduction devices on diverse tropical marine communities in Australia's northern prawn trawl fishery. Fish Res 81: 176–188. (doi:10.1016/j.fishres.2006.07.009) [Google Scholar]
- 24.Brill RW, Bigelow KA, Musyl MK, Fritsches KA, Warrant EJ. (2005) Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Collect Vol Sci Pap ICCAT 57: 142–161. [Google Scholar]
- 25.Brill R, Bushnell P, Smith L, Speaks C, Sundaram R, Stroud E, Wang J. (2009) The repulsive and feeding-deterrent effects of electropositive metals on juvenile sandbar sharks (Carcharhinus plumbeus). Fish Bull 107: 298–307. [Google Scholar]
- 26.Broadhurst MK, Kennelly SJ, Gray C. (2007) Strategies for improving the selectivity of fishing gears. In Kennelly S, ed., Bycatch Reduction in World Fisheries. Springer, Amsterdam, The Netherlands, pp. 1–21. [Google Scholar]
- 27.Brothers NP, Cooper J, Lokkeborg S. (1999) The incidental catch of seabirds in longline fisheries: worldwide review and technical guidelines for mitigation. FAO Fish Circ 937: 1–100. [Google Scholar]
- 28.Brown BR. (2010) Temperature response in electrosensors and thermal voltages in electrolytes. J Biol Phys 36: 121–134. (doi:10.1007/s10867-009-9174-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullock TH, Hoffmann MH, New JG, Nahm FK. (1991) Dynamic properties of visual evoked potentials in the tectum of cartilaginous and bony fishes, with neuroethological implications. J Exp Zool Suppl 5: 142–255. [DOI] [PubMed] [Google Scholar]
- 30.Camhi MD, Valenti SV, Fordham SV, Fowler SL, Gibson C. (2009) The Conservation Status of Pelagic Sharks and Rays: Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop. IUCN Species Survival Commission Shark Specialist Group, Newbury, UK, 78 pp. [Google Scholar]
- 31.Campana SE, Joyce W, Manning MJ. (2009) Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar Ecol Prog Ser 387: 241–253. (doi:10.3354/meps08109) [Google Scholar]
- 32.Carlson JK, Cortés E. (2003) Gillnet selectivity of small coastal sharks off the southeastern United States. Fish Res 60: 405–414. (doi:10.1016/S0165-7836(02)00135-2) [Google Scholar]
- 33.Carlson JK, Bethea DM. (2007) Catch and bycatch in the shark gillnet fishery: 2005–2006. NOAA Technical Memorandum NMFS-SEFSC-522, 26 pp. [Google Scholar]
- 34.Casper BM, Mann DA. (2006) Evoked potential audiograms of the nurse shark (Ginglymostoma cirratum) and the yellow stingray (Urobatis jamaicensis). Environ Biol Fish 76: 101–108. (doi:10.1007/s10641-006-9012-9) [Google Scholar]
- 35.Casper BM, Mann DA. (2010) Field hearing measurements of the Atlantic sharpnose shark Rhizoprionodon terraenovae. J Fish Biol 75: 2768–2776. (doi:10.1111/j.1095-8649.2009.02477.x) [DOI] [PubMed] [Google Scholar]
- 36.Charvet-Almeida P, Faria V, Furtado M, Cook SF, Compagno LJV, Oetinger MI. (2007) Pristis perotteti. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/18176/0 (last accessed March 2013). [Google Scholar]
- 37.Chosid DM, Pol M, Szymanski M, Mirarchi F, Mirarchi A. (2012) Development and observations of a spiny dogfish Squalus acanthias reduction device in a raised footrope silver hake Merluccius bilinearis trawl. Fish Res 114: 66–75. (doi:10.1016/j.fishres.2011.03.007) [Google Scholar]
- 38.Chu YT, Wen WC. (1979) A study of the lateral-line canal system and that of Lorenzini ampullae and tubules of elasmobranchiate fishes of China. In Monograph of Fishes of China, Vol. 2 Science and Technology Press, Shanghai, pp. 117–126. [Google Scholar]
- 39.Clark E. (1983) Shark repellent effect of the Red Sea Moses sole. In Zahurance BJ, ed., Shark Repellents from the Sea: New Perspectives, Westview Press, Boulder, pp. 135–150. [Google Scholar]
- 40.Coan AL, Sakagawa GT, Prescott D, Williams P, Staish K, Yamasaki G. (2000) The 1999 U.S. Central-Western Pacific Tropical Tuna Purse Seine Fishery. Document prepared for the annual meeting of parties to the South Pacific Regional Tuna Treaty 3-10 March 2000. LJ-00-10. [Google Scholar]
- 41.Collin SP. (2012) The neuroecology of cartilaginous fishes: sensory strategies for survival. Brain Behav Evol 80: 80–96. (doi:10.1159/000339870) [DOI] [PubMed] [Google Scholar]
- 42.Compagno LJV, Cook SF, Fowler SL. (2006) Pristis microdon. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/18174/0 (last accessed March 2013). [Google Scholar]
- 43.Coombs S, Montgomery JC. (1999) The enigmatic lateral line system. In Fay RR, Popper AN, eds, Comparative Hearing: Fish and Amphibians. Springer-Verlag, New York, pp. 319–362. [Google Scholar]
- 44.Cortés E. (1999) A stochastic stage-based population model of the sandbar shark in the western North Atlantic. In Musick JA, ed., Life in the Slow Lane. Ecology and Conservation of Long-Lived Marine Animals, Vol 23 American Fisheries Society Symposium, Maryland, USA, pp. 115–136. [Google Scholar]
- 45.Cortés E. (2002) Incorporating uncertainty into demographic modeling: application to shark populations and their conservation. Conserv Biol 16: 1048–1062. (doi:10.1046/j.1523-1739.2002.00423.x) [Google Scholar]
- 46.Crognale MA, Eckert SA, Levenson DH, Harms CA. (2008) Leatherback sea turtle Dermochelys coriacea visual capacities and potential reduction of by-catch by pelagic longline fisheries. Endang Species Res 5: 249–256. (doi:10.3354/esr00112) [Google Scholar]
- 47.Curran D, Bigelow K. (2011) Effects of circle hooks on pelagic catches in the Hawaii-based tuna longline fishery. Fish Res 109: 265–275. (doi:10.1016/j.fishres.2011.02.013) [Google Scholar]
- 48.Dagorn L. (2010) Mitigating bycatch of sharks and finfish by tropical Tuna purse seiners using FADs. ISSF Workshop on Bycatch, Brisbane, Australia, June 26, 2010. [Google Scholar]
- 49.Dagorn L, Filmalter J, Forget F. (2012) Summary of results on the development of methods to reduce the mortality of silky sharks by purse seiners. IOTC-2012-WPEB08-21. [Google Scholar]
- 50.Davies RWD, Cripps SJ, Nickson A, Porter G. (2009) Defining and estimating global marine fisheries bycatch. Mar Policy 33: 661–672. (doi:10.1016/j.marpol.2009.01.003) [Google Scholar]
- 51.Dempster T, Kingsford MJ. (2003) Homing of pelagic fish to fish aggregating devices (FADs): an investigation of the role of sensory cues. Mar Ecol Prog Ser 258: 213–222. (doi:10.3354/meps258213) [Google Scholar]
- 52.Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C, et al. (2008) You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conserv Mar Freshw Ecosyst 18: 459–482. (doi:10.1002/aqc.975) [Google Scholar]
- 53.Fennessy ST, Isaksen B. (2007) Can bycatch reduction devices be implemented successfully on prawn trawlers in the Western Indian Ocean? Afr J Mar Sci 29: 453–463. (doi:10.2989/AJMS.2007.29.3.12.342) [Google Scholar]
- 54.Filmalter JD, Dagorn L, Cowley PD, Taquet M. (2011) First descriptions of the behavior of silky sharks, Carcharhinus falciformis, around drifting fish aggregating devices in the Indian Ocean. Bull Mar Sci 87: 325–337. [Google Scholar]
- 55.Fish JF, Vania JS. (1971) Killer whale, Orcinus orca, sounds repel white whales, Delphinapterus leucas. Fish Bull 69: 531–535. [Google Scholar]
- 56.Fordham S, Fowler SL, Coelho R, Goldman KJ, Francis M. (2006) Squalus acanthias. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/39326/0 (last accessed March 2013). [Google Scholar]
- 57.Forrest KW, Cave JD, Michielsens CGJ, Haulena M, Smith DV. (2009) Evaluation of an electric gradient to deter seal predation on salmon caught in gill-net test fisheries. North Am J Fish Manage 29: 885–894. (doi:10.1577/M08-083.1) [Google Scholar]
- 58.Francis RC, Awbrey FT, Goudey CL, Hall MA, King DM, Medina H, Norris KS, Orbach MK, Payne R, Pikitch E. (1992) Dolphins and the tuna industry. National Research Council. National Academy Press, Washington, DC, 176 pp. [Google Scholar]
- 59.Frick LH, Reina RD, Walker TI. (2010) Stress related changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J Exp Mar Biol Ecol 385: 29–37. (doi:10.1016/j.jembe.2010.01.013) [Google Scholar]
- 60.Fritches KA, Litherland L, Thomas N, Shand J. (2003) Cone visual pigments and retinal mosaics in the striped marlin. J Fish Biol 63: 1347–1351. (doi:10.1046/j.1095-8649.2003.00246.x) [Google Scholar]
- 61.Fritches KA, Brill RW, Warrant EJ. (2005) Warm eyes provide superior vision in swordfishes. Curr Biol 15: 55–58. (doi:10.1016/j.cub.2004.12.064) [DOI] [PubMed] [Google Scholar]
- 62.Gardiner J, Hueter R, Maruska K, Sisneros J, Casper B, Mann D, Dernski L. (2012) Sensory physiology and behavior of elasmobranchs. In Carrier J, Musick J, Heithaus M, eds, Biology of Sharks and Their Relatives, Ed 2 CRC Press, Boca Raton, FL, pp. 349–401. [Google Scholar]
- 63.Gardiner JM, Atema J. (2007) Sharks need the lateral line to locate odor sources: rheotaxis and eddy chemotaxis. J Exp Biol 210: 1925–1934. (doi:10.1242/jeb.000075) [DOI] [PubMed] [Google Scholar]
- 64.Gardiner JM. (2011) Multisensory Integration in Shark Feeding Behaviors. PhD thesis University of South Florida, Tampa, FL. [Google Scholar]
- 65.Gearin PJ, Pfeifer R, Jeffries SJ, DeLong RL, Johnson MA. (1988) Results of the 1986–1987 California sea lion-steelhead trout predation control program at the Hiram M. Chittenden Locks. NWAFC Processed Report, 88–30, 111 pp. [Google Scholar]
- 66.Gilbert PW. (1968) The shark: barbarian and benefactor. Bioscience 18: 946–950. (doi:10.2307/1294435) [Google Scholar]
- 67.Gilman E, Clarke S, Brothers N, Alfaroshigueto J, Mandelman J, Mangel J, Petersen S, Piovano S, Thomson N, Dalzell P. (2008) Shark interactions in pelagic longline fisheries. Mar Policy 32: 1–18. (doi:10.1016/j.marpol.2007.05.001) [Google Scholar]
- 68.Gilman EL. (2011) Bycatch governance and best practice mitigation technology in global tuna fisheries. Mar Policy 35: 590–609. (doi:10.1016/j.marpol.2011.01.021) [Google Scholar]
- 69.Godin AC, Carlson JK, Burgener V. (2012) The effect of circle hooks on shark catchability and at-vessel mortality rates in longline fisheries. Bull Mar Sci 88: 469–483. (doi:10.5343/bms.2011.1054) [Google Scholar]
- 70.Griebel U, Peichl L. (2003) Colour vision in aquatic mammals—facts and open questions. Aquat Mamm 29: 18–30. (doi:10.1578/016754203101024040) [Google Scholar]
- 71.Gruber SH. (1975) Duplex vision in the elasmobranchs: histological, electrophysiological and psychophysical evidence. In Ali MA, ed., Vision in Fishes: New Approaches in Research; Plenum, New York and London, pp. 525–540. [Google Scholar]
- 72.Hall M, Alverson DL, Metuzals KI. (2000) Bycatch: problems and solutions. Mar Pollut Bull 41: 204–219. (doi:10.1016/S0025-326X(00)00111-9) [Google Scholar]
- 73.Hart NS, Lisney TJ, Marshall NJ, Collin SP. (2004) Multiple cone visual pigments and the potential for trichromatic colour vision in two species of elasmobranch. J Exp Biol 207: 4587–4594. (doi:10.1242/jeb.01314) [DOI] [PubMed] [Google Scholar]
- 74.Hart NS, Lisney TJ, Collin SP. (2006) Visual communication in elasmobranchs. In Kapoor BG, Ladich F, Collin SP, Raschi WG, eds, Fish Communication, Vol 2 Science Publishers Inc, Enfield, NH, pp. 337–392. [Google Scholar]
- 75.Hart NS, Theiss SM, Harahush BK, Collin SP. (2011) Microspectrophotometric evidence for cone monochromacy in sharks. Naturwissenschaften 98: 193–201. (doi:10.1007/s00114-010-0758-8) [DOI] [PubMed] [Google Scholar]
- 76.Hayes CG, Jiao Y, Cortés E. (2009) Stock assessment of scalloped hammerhead sharks in the western North Atlantic Ocean and Gulf of Mexico. North Am J Fish Manage 29: 1406–1417. (doi:10.1577/M08-026.1) [Google Scholar]
- 77.He P. (2009) Swimming capacity of marine fishes and its role in capture by fishing gears. In Domenici P, Kapoor BG, eds, Fish Locomotion—an Etho-ecological Approach. Science Publishers, Endfield, NH, pp. 484–412. [Google Scholar]
- 78.Heppell SS, Crowder LB, Menzel TR. (1999) Life table analysis of long-lived marine species, with implications for conservation and management. In Musick JA, ed., Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals. American Fisheries Society, Maryland, USA, pp. 137–148. [Google Scholar]
- 79.Hester KC, Peltzer ET, Kirkwood WJ, Brewer PG. (2008) Unanticipated consequences of ocean acidification: a noisier ocean at lower pH. Geophys Res Lett 35: pL19601. (doi:10.1029/2008GL034913) [Google Scholar]
- 80.Hoenig JM, Gruber SH. (1990) Life-history patterns in the elasmobranchs: implications for fisheries management. In Pratt HL, Jr, Gruber SH, Taniuchi T, eds, Elasmobranchs as Living Resources: Advances in Biology, Ecology, Systematics and the Status of Fisheries. NOAA technical report NMFS 90 US Department of Commerce, Springfield, IL, pp. 1–16. [Google Scholar]
- 81.Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ. (2008) Comparative visual function in five sciaenid fishes inhabiting Chesapeake Bay. J Exp Biol 211: 3601–3612. (doi:10.1242/jeb.023358) [DOI] [PubMed] [Google Scholar]
- 82.Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ. (2010) Comparative visual function in four piscivorous fishes inhabiting Chesapeake Bay. J Exp Biol 213: 1751–1761. (doi:10.1242/jeb.038117) [DOI] [PubMed] [Google Scholar]
- 83.Hutchinson M, Wang JH, Swimmer Y, Holland K, Kohin S, Deward H, Wraith J, Vetter R, Heberer C, Martinez J. (2012) The effects of a lanthanide metal alloy on shark catch rates. Fish Res 131–133: 45–51. (doi:10.1016/j.fishres.2012.07.006) [Google Scholar]
- 84.Huveneers C, Rogers PJ, Semmens J, Beckmann C, Kock AA, Page B, Goldsworthy S. (2012) Effects of the Shark Shield electric deterrent on the behaviour of white sharks (Carcharodon carcharias). Final Report to SafeWork South Australia, SARDI publication no. F2012/000123-1, SARDI Research Report Series No. 632, 61 pp. [Google Scholar]
- 85.Itano D, Muir J, Hutchinson M, Leroy B. (2012) Development and testing of a release panel for sharks and non-target finfish in purse seine gear. WCPFC-SC8-2012/EB-WP-14. [Google Scholar]
- 86.Iversen RTB. (1967) Response of the yellowfin tuna (Thunnus albacares) to underwater sound. In Tavolga WN, ed., Marine Bioacoustics. II. Pergamon Press, Oxford, pp. 105–121. [Google Scholar]
- 87.Iversen RTB. (1969) Auditory thresholds of the scrombrid fish Euthynnus affinis, with comments on the use of sound in tuna fishing. FAO conference on fish behavior in relation to fishing techniques and tactics. FAO Fish Rep 62: 849–859. [Google Scholar]
- 88.Jerlov NG. (1968) Optical Oceanography. Elsevier Oceanography Series 5. Elsevier, Amsterdam, 194 pp. [Google Scholar]
- 89.Jiao Y, Hayes C, Cortés E. (2009) Hierarchical Bayesian approach for population dynamics modelling of fish complexes without species-specific data. ICES J Mar Sci 66: 367–377. (doi:10.1093/icesjms/fsn162) [Google Scholar]
- 90.Jordan LJ, Mandelman JW, Kajiura SM. (2011) Behavioral responses to weak electric fields and a lanthanide metal in two shark species. J Exp Mar Biol Ecol 409: 345–350. (doi:10.1016/j.jembe.2011.09.016) [Google Scholar]
- 91.Jordan LK. (2008) Comparative morphology of stingray lateral line canal and electrosensory systems. J Morphol 269: 1325–1339. (doi:10.1002/jmor.10660) [DOI] [PubMed] [Google Scholar]
- 92.Jordan LK, Kajiura SM, Gordon MS. (2009) Functional consequences of structural differences in stingray sensory systems. Part I: mechanosensory lateral line canals. J Exp Biol 212: 3037–3043. (doi:10.1242/jeb.028712) [DOI] [PubMed] [Google Scholar]
- 93.Kaimmer S, Stoner AW. (2008) Field investigation of rare-earth metal as a deterrent to spiny dogfish in the Pacific halibut fishery. Fish Res 94: 43–47. (doi:10.1016/j.fishres.2008.06.015) [Google Scholar]
- 94.Kajiura S, Cornett A, Yopak K. (2010) Sensory adaptations to the environment: electroreceptors as a case study. In Carrier J, Heithaus M, Musick J, eds, Sharks and Their Relatives: Physiological Adaptations, Behavior, Ecology, Conservation, and Management. CRC Press, London, pp. 393–433. [Google Scholar]
- 95.Kajiura SM. (2003) Electroreception in neonatal bonnethead sharks, Sphyrna tiburo. Mar Biol 143: 603–611. (doi:10.1007/s00227-003-1099-3) [Google Scholar]
- 96.Kajiura SM, Holland KN. (2002) Electroreception in juvenile scalloped hammerhead and sandbar sharks. J Exp Biol 205: 3609–3621. [DOI] [PubMed] [Google Scholar]
- 97.Kajiura SM, Forni JB, Summers AP. (2003) Maneuvering in juvenile carcharhinid and sphyrnid sharks: the role of the hammerhead shark cephalofoil. Zoology 106: 19–28. (doi:10.1078/0944-2006-00086) [DOI] [PubMed] [Google Scholar]
- 98.Kajiura SM, Forni JB, Summers AP. (2005) Olfactory morphology of carcharhinid and sphyrnid sharks: does the cephalofoil confer a sensory advantage? J Morphol 264: 253–263. (doi:10.1002/jmor.10208) [DOI] [PubMed] [Google Scholar]
- 99.Kalmijn AJ. (1971) The electric sense of sharks and rays. J Exp Biol 55: 371–383. [DOI] [PubMed] [Google Scholar]
- 100.Kelleher K. (2005) Discards in the World's Marine Fisheries: An Update. Food and Agriculture Organisation of the United Nations, FAO, Rome, 131 pp. [Google Scholar]
- 101.Klimley AP, Nelson DR. (1984) Diel movement patterns of the scalloped hammerhead shark (Sphyrna lewini) in relation to El Bajo Espiritu Santo: a refuging central-position social system. Behav Ecol Sociobiol 15: 45–54. (doi:10.1007/BF00310214) [Google Scholar]
- 102.Klimley AP. (1988) Diel movements of scalloped hammerhead sharks, Sphyrna lewini Griffith and Smith, to and from a seamount in the Gulf of California. J Fish Biol 33: 751–761. [Google Scholar]
- 103.Klimley AP. (1993) Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar Biol 117: 1–22. [Google Scholar]
- 104.Koops FBJ. (2000) Electric and magnetic fields in consequence of undersea power cables. In Proceedings of International Seminar on Effects of Electromagnetic Fields on the Living Environment, 4–5 October 1999, Ismaning, Germany. ICNIRP, Oberschleissheim, Germany, pp. 189–210. [Google Scholar]
- 105.Larsen F, Eigaard OR, Tougaard J. (2007) Reduction of harbour porpoise (Phocoena phocoena) bycatch by iron-oxide gillnets. Fish Res 85: 270–278. (doi:10.1016/j.fishres.2007.02.011) [Google Scholar]
- 106.Lewison RL, Crowder LB, Read AJ, Freeman SA. (2004) Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol 19: 598–604. (doi:10.1016/j.tree.2004.09.004) [Google Scholar]
- 107.Lisney TJ, Theiss SM, Collin SP, Hart NS. (2012) Vision in elasmobranchs and their relatives: 21st century advances. J Fish Biol 80: 2024–2054. (doi:10.1111/j.1095-8649.2012.03253.x) [DOI] [PubMed] [Google Scholar]
- 108.Loew ER, Fleishman LJ, Foster RG, Provencio I. (2002) Visual pigments and oil droplets in diurnal lizards: a comparative study of Caribbean anoles. J Exp Biol 205: 927–938. [DOI] [PubMed] [Google Scholar]
- 109.Lorance P, Trenkel VM. (2006) Variability in natural behaviour, and observed reactions to an ROV, by mid-slope fish species. J Exp Mar Biol Ecol 332: 106–119. (doi:10.1016/j.jembe.2005.11.007) [Google Scholar]
- 110.Lydon G, Starr P. (2005) Effect of Blue Dyed Bait on Incidental Seabird Mortalities and Fish Catch Rates on a Commercial Longliner Fishing Off East Cape, New Zealand. Unpublished Conservation Services Programme Report Department of Conservation, Wellington, 12 pp. [Google Scholar]
- 111.Lythgoe JN. (1979) The Ecology of Vision. Oxford University Press, Oxford, 256 pp. [Google Scholar]
- 112.McComb DM, Kajiura SM. (2008) Visual fields of four batoid fishes: a comparative study. J Exp Biol 211: 482–490. (doi:10.1242/jeb.014506) [DOI] [PubMed] [Google Scholar]
- 113.McComb DM, Tricas TC, Kajiura SM. (2009) Enhanced visual fields in hammerhead sharks. J Exp Biol 212: 4010–4018. (doi:10.1242/jeb.032615) [DOI] [PubMed] [Google Scholar]
- 114.McComb DM, Frank TM, Hueter RE, Kajiura SM. (2010) Temporal resolution and spectral sensitivity of the visual system of three coastal shark species from different light environments. Physiol Biochem Zool 83: 299–307. [DOI] [PubMed] [Google Scholar]
- 115.McFadden EB, Johnson CS. (1971) Colour and reflectivity of sea survival equipment as related to shark attack. In Abstracts from the Aerospace Medical Association Meeting, Houston, TX. [Google Scholar]
- 116.McFarland WN. (1990) Light in the sea: the optical world of elasmobranchs. J Exp Zool 256suppl.S5: 3–12. (doi:10.1002/jez.1402560503) [Google Scholar]
- 117.McGowan DW, Kajiura SM. (2009) Electroreception in the euryhaline stingray, Dasyatis sabina. J Exp Biol 212: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 118.Mandelman J, Farrington M. (2007) The estimated short-term discard mortality of a trawled elasmobranch, the spiny dogfish (Squalus acanthias). Fish Res 83: 238–245. (doi:10.1016/j.fishres.2006.10.001) [Google Scholar]
- 119.Mandelman J, Cooper PW, Werner TB, Lageaux K. (2008) Shark bycatch and depredation in the U.S. Atlantic pelagic longline fishery. Rev Fish Biol Fish 18: 427–442. (doi:10.1007/s11160-008-9084-z) [Google Scholar]
- 120.Marcotte MM, Lowe CG. (2008) Behavioral responses of two species of sharks to pulsed, direct current electrical fields: testing a potential shark deterrent. Mar Technol Soc J 42: 53–61. (doi:10.4031/002533208786829133) [Google Scholar]
- 121.Marshall A, Bennett MB, Kodja G, Hinojosa-Alvarez S, Galvan-Magana F, Harding M, Stevens G, Kashiwagi T. (2011) Manta birostris. In IUCN 2012. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/198921/0 (last accessed March 2013). [Google Scholar]
- 122.Marshall AD, Compagno LJV, Bennett MB. (2009) Redescription of the genus Manta with resurrection of Manta alfredi (Krefft, 1868) (Chondrichthyes; Myliobatoidei; Mobulidae). Zootaxa 2301: 1–28. [Google Scholar]
- 123.Maruska KP. (2001) Morphology of the mechanosensory lateral line system in elasmobranch fishes: ecological and behavioral considerations. Environ Biol Fish 60: 47–75. (doi:10.1023/A:1007647924559) [Google Scholar]
- 124.Maruska KP, Tricas TC. (2004) Test of the mechanotactile hypothesis: neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray. J Exp Biol 207: 3463–3476. (doi:10.1242/jeb.01140) [DOI] [PubMed] [Google Scholar]
- 125.Melvin E, Parrish JK, Conquest LL. (1999) Novel tools to reduce seabird bycatch in coastal gillnet fisheries. Conserv Biol 13: 1386–1397. (doi:10.1046/j.1523-1739.1999.98426.x) [Google Scholar]
- 126.Meredith TL, Kajiura SM. (2010) Olfactory morphology and physiology of elasmobranchs. J Exp Biol 213: 3449–3456. (doi:10.1242/jeb.045849) [DOI] [PubMed] [Google Scholar]
- 127.Moein Bartol S, Ketten DR. (2006) Turtle and tuna hearing. In Swimmer Y, Brill R, eds, Sea Turtle and Pelagic Fish Sensory Biology: Developing Techniques to Reduce Sea Turtle Bycatch in Longline Fisheries. NOAA (Natl Ocean Atmos Adm) Tech Mem NMFS-PIFSC-7, pp. 98–105. [Google Scholar]
- 128.Molina JM, Cooke SJ. (2012) Trends in shark bycatch research: current status and research needs. Rev Fish Biol Fish 22: 719–737. (doi:10.1007/s11160-012-9269-3) [Google Scholar]
- 129.Montgomery J, Skipworth E. (1997) Detection of weak water jets by the short-tailed stingray Dasyatis brevicaudata (Pisces: Dasyatidae). Copeia 1997: 881–883. (doi:10.2307/1447310) [Google Scholar]
- 130.Morgan A, Burgess GH. (2007) At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf Carib Res Inst 19: 123–129. [Google Scholar]
- 131.Morgan A, Carlson JK. (2010) Capture time, size, and hooking mortality of bottom longline-caught sharks. Fish Res 101: 32–37. (doi:10.1016/j.fishres.2009.09.004) [Google Scholar]
- 132.Motta P, Huber D. (2012) Prey capture behavior and feeding mechanics of elasmobranchs. In Carrier J, Musick J, Heithaus M, eds, Biology of Sharks and Their Relatives, Ed 2 CRC Press, Boca Raton, FL, pp. 153–210. [Google Scholar]
- 133.Motta PJ, Wilga CD. (2001) Advances in the study of feeding mechanisms, mechanics, and behaviors of sharks. Environ Biol Fish 60: 131–156. (doi:10.1023/A:1007649900712) [Google Scholar]
- 134.Motta PJ, Hueter RE, Tricas TC, Summers AP. (2002) Kinematic analysis of suction feeding in the nurse shark, Ginglymostoma cirratum (Orectolobiformes, Ginglyostomatidae). Copeia 2002: 24–38. (doi:10.1643/0045-8511(2002)002[0024:KAOSFI]2.0.CO;2) [Google Scholar]
- 135.Motta PJ. (2004) Prey capture behavior and feeding mechanics of elasmobranchs. In Carrier JC, Musick JA, Heithaus MR, eds, Biology of Sharks and Their Relatives. CRC Press, Boca Raton, FL, 596 pp. [Google Scholar]
- 136.Moyes CD, Fragoso N, Musyl MK, Brill RW. (2006) Predicting postrelease survival in large pelagic fish. Trans Am Fish Soc 135: 1389–1397. (doi:10.1577/T05-224.1) [Google Scholar]
- 137.Muir J, Itano D, Hutchinson M, Leroy B, Holland K. (2012) Behavior of target and non-target species on drifting FADs and when encircled by purse seine gear. WCPFC-SC8-2012/EB-WP-13. [Google Scholar]
- 138.Myrberg AA, Jr, Ha SJ, Walewski S, Banbury JC. (1972) Effectiveness of acoustic signals in attracting epipelagic sharks to an underwater sound source. Bull Mar Sci 22: 92–94. [Google Scholar]
- 139.Myrberg AA, Jr, Gordon CR, Klimley AP. (1976) Attraction of free ranging sharks to low frequency sound, with comments on its biological significance. In Schuijf A, Hawkins AD, eds, Sound Reception in Fishes. Elsevier, New York, pp. 205–228. [Google Scholar]
- 140.Myrberg AA., Jr (2001) The acoustical biology of elasmobranchs. Environ Biol Fish 60: 31–45. (doi:10.1023/A:1007647021634) [Google Scholar]
- 141.National Marine Fisheries Service (NMFS). (2011) SEDAR 21 stock assessment report: HMS Atlantic Blacknose Shark. US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Office of Sustainable Fisheries, Silver Spring, MD, 31 pp. [Google Scholar]
- 142.National Marine Fisheries Service (NMFS). (2012) Reinitiation of Endangered Species Act (ESA) Section 7 Consultation on the Continued Implementation of the Sea Turtle Conservation Regulations, as Proposed to Be Amended, and the Continued Authorization of the Southeast U.S. Shrimp Fisheries in Federal Waters under the Magnuson-Stevens Act. US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Regional Office, St Petersburg, FL, 302 pp. [Google Scholar]
- 143.Nelson DR, Gruber SH. (1963) Sharks: attraction by low-frequency sounds. Science 142: 975–977. (doi:10.1126/science.142.3594.975) [DOI] [PubMed] [Google Scholar]
- 144.Nelson DR. (1969) The silent savages. Oceans 1: 8–22. [Google Scholar]
- 145.Northridge S, Sanderson D, Mackay A, Hammond P. (2003) Analysis and mitigation of cetacean bycatch in UK fisheries. Final Report due to Defra (UK) Project MF0726 Sea Mammal Rescue Unit, University of St. Andrews. Accessed on March 20, 2013. [Google Scholar]
- 146.O'Connell CP, Abel DC, Stroud EM. (2011) Analysis of permanent magnets as elasmobranch bycatch reduction devices in hook-and-line and longline trials. Fish Bull 109: 394–401. [Google Scholar]
- 147.O'Connell C, Stroud EM, He P. (2012) The emerging field of electrosensory and semiochemical shark repellents: mechanisms of detection, overview of past studies, and future directions. Ocean and Coastal Management. http://dx.doi.org/10.1016/j.ocecoaman.2012.11.005 [Google Scholar]
- 148.Pierre JP, Norden WS. (2006) Reducing seabird bycatch in longline fisheries using a natural olfactory deterrent. Biol Conserv 130: 406–415. (doi:10.1016/j.biocon.2006.01.002) [Google Scholar]
- 149.Polet H, Delanghe F, Verschoore R. (2005) On electrical fishing for brown shrimp (Crangon crangon) II. Sea trials. Fish Res 72: 13–27. (doi:10.1016/j.fishres.2004.10.015) [Google Scholar]
- 150.Popper AN. (2003) Effects of anthropogenic sounds on fishes. Fisheries 28: 24–31. (doi:10.1577/1548-8446(2003)28[24:EOASOF]2.0.CO;2) [Google Scholar]
- 151.Queirolo D, Gaete E, Montenegro I, Soriguer MC, Erzini K. (2012) Behaviour of fish by-catch in the mouth of a crustacean trawl. J Fish Biol 80: 2517–2527. (doi:10.1111/j.1095-8649.2012.03305.x) [DOI] [PubMed] [Google Scholar]
- 152.Rasmussen LEL, Schmidt MJ. (1992) Are sharks chemically aware of crocodiles? In Doty RL, Müller-Schwarze D, eds, Chemical Signals in Vertebrates IV. Plenum Press, New York, pp. 335–342. [Google Scholar]
- 153.Rigg DP, Peverell SC, Hearndon M, Seymour JE. (2009) Do elasmobranch reactions to magnetic fields in water show promise for bycatch mitigation? Mar Freshw Res 60: 942–948. (doi:10.1071/MF08180) [Google Scholar]
- 154.Robbins WD, Peddemors VM, Kennelly SJ. (2011) Assessment of permanent magnets and electropositive metals to reduce the line-based capture of Galapagos sharks, Carcharhinus galapagensis. Fish Res 109: 100–106. [Google Scholar]
- 155.Rowland BA, Stein BE. (2008) Temporal profiles in multisensory integration. Front Neurosci 2: 218–224. (doi:10.3389/neuro.01.033.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ryer CH, Rose CS, Iseri PJ. (2010) Flatfish herding behavior in response to trawl sweeps: a comparison of diel responses to conventional sweeps and elevated sweeps. Fish Bull 108: 145–154. [Google Scholar]
- 157.Schaefer KM, Fuller DW. (2011) An overview of the 2011 ISSF/IATTC research cruise for investigating potential solutions for reducing fishing mortality of undesirable sizes of bigeye and yellowfin tunas and sharks in purse-seine sets on drifting FADs. Scientific Committee Seventh Regular Session, August 9–17, 2011, Federated States of Micronesia. WCPFC-SC7-2011/EB-WP-13, Seattle, WA, 5 pp. [Google Scholar]
- 158.Scordino J, Pfeifer B. (1993) Sea lion/steelhead conflict at the Ballard Locks. A history of control efforts to date and a bibliography of technical reports. WDFW Report, 10 pp. [Google Scholar]
- 159.Shepherd TD, Myers RA. (2005) Direct and indirect fishery effects on small coastal elasmobranchs in the northern Gulf of Mexico. Ecol Lett 8: 1095–1104. (doi:10.1111/j.1461-0248.2005.00807.x) [Google Scholar]
- 160.Siegfried KI, Brooks EN. (2007) Assessment of Blacknose, Bonnethead, and Atlantic Sharpnose Sharks with a State- Space, Age-Structured Production Model. Southeast Data, Assessment, and Review Document 13-AW-03. US Department of Commerce, NOAA, NMFS, Southeast Fish Science Center, Panama City, FL. [Google Scholar]
- 161.Simpfendorfer CA, Poulakis GR, O'Donnell PM, Wiley TR. (2008) Growth rates of juvenile smalltooth sawfish, Pristis pectinata, in the western Atlantic. J Fish Biol 72: 711–723. (doi:10.1111/j.1095-8649.2007.01764.x) [Google Scholar]
- 162.Sisneros JA, Nelson DR. (2001) Surfactants as chemical repellants: past, present, and future. Environ Biol Fish 60: 117–129. (doi:10.1023/A:1007612002903) [Google Scholar]
- 163.Skomal GB, Bernal D. (2010) Physiological responses to stress in sharks. In Carrier JC, Musick JA, Heithaus MR, eds, Sharks and Their Relatives II: Biodiversity, Adaptive Physiology and Conservation. CRC Press Taylor Francis Group, Boca Raton, FL, pp. 457–488. [Google Scholar]
- 164.Skomal GB, Mandelman JW. (2012) The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comp Biochem Physiol A Mol Integr Physiol 162: 146–155. (doi:10.1016/j.cbpa.2011.10.002) [DOI] [PubMed] [Google Scholar]
- 165.Smith ED. (1991) Electric shark barrier: initial trials and prospects. Power Eng J 5: 167–176. (doi:10.1049/pe:19910036) [Google Scholar]
- 166.Smith LJ., Jr (1991) The effectiveness of sodium lauryl sulfate as a shark repellent in a laboratory test situation. J Fish Biol 38: 105–113. (doi:10.1111/j.1095-8649.1991.tb03096.x) [Google Scholar]
- 167.Somerton DA. (2004) Do Pacific cod (Gadus macrocephalus) and walleye pollock (Theregra chalcogramma) lack a herding response to the doors, bridles, and mudclouds of survey trawls? ICES J Mar Sci 61: 1186–1189. (doi:10.1016/j.icesjms.2004.06.003) [Google Scholar]
- 168.Southwood A, Fritsches K, Brill R, Swimmer Y. (2008) Sound, chemical, and light detection in sea turtles and pelagic fishes: sensory-based approaches to bycatch reduction in longline fisheries. Endang Species Res 5: 225–238. (doi:10.3354/esr00097) [Google Scholar]
- 169.Stevens J, Bonfil R, Dulvy NK, Walker P. (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57: 476–494. (doi:10.1006/jmsc.2000.0724) [Google Scholar]
- 170.Stoner AW. (2004) Effects of environmental variables on fish feeding ecology: implications for the performance of baited fishing gear and stock assessment. J Fish Biol 65: 1445–1471. (doi:10.1111/j.0022-1112.2004.00593.x) [Google Scholar]
- 171.Stoner AW, Kaimmer SM. (2008) Reducing elasmobranch bycatch: laboratory investigation of rare earth metal and magnetic deterrents with spiny dogfish and Pacific halibut. Fish Res 92: 162–168. (doi:10.1016/j.fishres.2008.01.004) [Google Scholar]
- 172.Strong WR, Jr, Snelson FF, Gruber SH. (1990) Hammerhead shark predation on stingrays: an observation of prey handling by Sphyrna mokarran. Copeia 3: 836–840. (doi:10.2307/1446449) [Google Scholar]
- 173.Swimmer Y, Arauz R, Higgins B, McNaughton L, McCracken M, Ballestero J, Brill R. (2005) Food color and marine turtle feeding behavior: can blue bait reduce turtle bycatch in commercial fisheries? Mar Ecol Prog Ser 295: 273–278. (doi:10.3354/meps295273) [Google Scholar]
- 174.Tallack SML, Mandelman JW. (2009) Do rare-earth metals deter spiny dogfish? A feasibility study on the use of electropositive “mischmetal” to reduce the bycatch of Squalus acanthias by hook gear in the Gulf of Maine. ICES J Mar Sci 66: 315–322. (doi:10.1093/icesjms/fsn215) [Google Scholar]
- 175.Tester AL. (1963) The role of olfaction in shark predation. Pac Sci 17: 145–170. [Google Scholar]
- 176.Theiss SM, Lisney TJ, Collin SP, Hart NS. (2007) Colour vision and visual ecology of the blue-spotted maskray, Dasyatis kuhlii Müller & Henle, 1814. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 193: 67–79. (doi:10.1007/s00359-006-0171-0) [DOI] [PubMed] [Google Scholar]
- 177.Thorpe T, Pabst DA, Beresoff D. (2001) Assessments of modiifed gillnets as means to reduce bycatch in Southeastern North Carolina coastal waters. Report of the North Carolina Marine Fisheries Commission. Fishery Resource Grant Program 00-FEG-09, 43 pp. [Google Scholar]
- 178.Thorpe T, Frierson D. (2009) Bycatch mitigation assessment for sharks caught in coastal anchored gillnets. Fish Res 98: 102–112. (doi:10.1016/j.fishres.2009.04.003) [Google Scholar]
- 179.Trent L, Parshley DE, Carlson JK. (1997) Catch and bycatch in the shark drift gillnet fishery off Georgia and East Florida. Mar Fish Rev 59: 19–28. [Google Scholar]
- 180.Trippel EA, Holy NL, Shepherd TD. (2009) Barium sulphate modified fishing gear as a mitigative measure for cetacean incidental mortalities. J Cetacean Res Manage 10: 235–246. [Google Scholar]
- 181.Van-Eyk SM, Siebeck UE, Champ CM, Marshall J, Hart NS. (2011) Behavioural evidence for colour vision in an elasmobranch. J Exp Biol 214: 4186–4192. (doi:10.1242/jeb.061853) [DOI] [PubMed] [Google Scholar]
- 182.Videler JJ, He P. (2010) Swimming in marine fishes. In He P, ed., Behavior of Marine Fishes: Capture Processes and Conservation Challenges. Wiley-Blackwell, Ames, IA, pp. 5–24. [Google Scholar]
- 183.Walls GL. (1942) The Vertebrate Eye and Its Adaptive Radiation. Cranbrook Institute of Science, Bloomfield Hills, MI, 785 pp. [Google Scholar]
- 184.Walsh SJ, Godø OR, Michalsen K. (2004) Fish behaviour relevant to fish catchability. ICES J Mar Sci 61: 1238–1239. (doi:10.1016/j.icesjms.2004.08.004) [Google Scholar]
- 185.Wang J, McNaughton L, Swimmer Y. (2008) Galapagos and sandbar shark aversion to electropositive metal (Pr-Nd alloy). In Swimmer Y, Wang JH, McNaughton L, eds, Shark Deterrent and Incidental Capture Workshop. April 10–11, US Department of Commerce, NOAA Technical Memorandum NOAA-TM-NMFS-PIFSC-16, Boston, MA, USA, pp. 31–35. [Google Scholar]
- 186.Wang JH, Fisler S, Swimmer Y. (2010) Developing visual deterrents to reduce sea turtle bycatch in gill net fisheries. Mar Ecol Prog Ser 408: 241–250. (doi:10.3354/meps08577) [Google Scholar]
- 187.Ward P, Lawrence E, Darbyshire R, Hindmarsh S. (2008) Largescale experiment shows that nylon leaders reduce shark bycatch and benefit pelagic longline fishers. Fish Res 90: 100–108. (doi:10.1016/j.fishres.2007.09.034) [Google Scholar]
- 188.Ward P, Epe S, Kreutz D, Lawrence E, Robins C, Sands A. (2009) The effects of circle hooks on bycatch and target catches in Australia's pelagic longline fishery. Fish Res 97: 253–262. (doi:10.1016/j.fishres.2009.02.009) [Google Scholar]
- 189.Watson JW, Epperly SP, Shah AK, Foster DG. (2005) Fishing methods to reduce sea turtle mortality associated with pelagic longlines. Can J Fish Aquat Sci 981: 965–981. (doi:10.1139/f05-004) [Google Scholar]
- 190.Webster DR. (2007) Structure of turbulent chemical plumes. In Woodfin RL, ed., Trace Chemical Sensing of Explosives. John Wiley & Sons, New York, pp. 109–129. [Google Scholar]
- 191.Werner T, Kraus S, Read A, Zollett E. (2006) Fishing techniques to reduce the bycatch of threatened marine animals. Mar Technol Soc J 40: 50–68. (doi:10.4031/002533206787353204) [Google Scholar]
- 192.Wetherbee BM, Gruber SH, Cortés E. (1990) Diet, feeding habits, and consumption in sharks, with special reference to the lemon shark, Negaprion brevirostris. In Pratt HL, Jr, Gruber SH, Taniuchi T, eds, Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries, Vol 90 NOAA Technical Report NMFS, pp. 29–48. [Google Scholar]
- 193.Wetherbee BM, Cortés E. (2004) Food consumption and feeding habits. In Carrier JF, Musick JA, Heithaus MR, eds, Biology of Sharks and Their Relatives. CRC Press, Boca Raton, FL, pp. 225–246. [Google Scholar]
- 194.White WT, Giles J, Dharmadi, Potter IC. (2006) Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fish Res 82: 65–73. (doi:10.1016/j.fishres.2006.08.008) [Google Scholar]
- 195.White WT, Last PR. (2012) A review of the taxonomy of chondrichthyan fishes: a modern perspective. J Fish Biol 80: 901–917. (doi:10.1111/j.1095-8649.2011.03192.x) [DOI] [PubMed] [Google Scholar]
- 196.Winger PD, Eayrs S, Glass CW. (2010) Fish behaviour near bottom trawls. In He P, ed., Behaviour of Marine Fishes: Capture Processes and Conservation Challenges, Ed 1 Wiley-Blackwell, Ames, IA, pp. 67–103. [Google Scholar]
- 197.Wueringer BE, Peverell SC, Seymour JE, Squire LJ, Kajiura SM, Collin SP. (2011a) Sensory systems in sawfishes. 1. the ampullae of Lorenzini. Brain Behav Evol 78: 139–149. (doi:10.1159/000329515) [DOI] [PubMed] [Google Scholar]
- 198.Wueringer BE, Peverell SC, Seymour J, Squire L, Jr, Collin SP. (2011b) Sensory systems in sawfishes. 2. The lateral line. Brain Behav Evol 78: 150–161. (doi:10.1159/000329518) [DOI] [PubMed] [Google Scholar]
- 199.Wueringer BE, Squire LJ, Kajiura SM, Hart NS, Collin SP. (2012) The function of the sawfish's saw. Curr Biol 22: R150–R151. (doi:10.1016/j.cub.2012.01.055) [DOI] [PubMed] [Google Scholar]
- 200.Yopak KE. (2012) Neuroecology of cartilaginous fishes: the functional implications of brain scaling. J Fish Biol 80: 1968–2023. (doi:10.1111/j.1095-8649.2012.03254.x) [DOI] [PubMed] [Google Scholar]
- 201.Zeeberg JJ, Corten A, de Graaf E. (2006) By-catch and release of pelagic megafauna in industrial trawler fisheries off Northwest Africa. Fish Res 78: 186–195. (doi:10.1016/j.fishres.2006.01.012) [Google Scholar]