ABSTRACT
Depression in HIV/AIDS patients affects adherence and disease progression and often goes unnoticed. DHIVA is a cross-sectional epidemiologic survey, investigating the prevalence of depression in people living with HIV through use of a validated self-administered scale (CES-D-20), as well and the degree of concordance between the physician's perception and patients' reports. A total of 690 HIV-infected patients attending 24 centers across Italy were enrolled. Concordance was calculated by K statistics. Association between depression and subject characteristics were evaluated through univariate and multivariate logistic models (OR and 95%CI). The prevalence of depressive symptoms was 48.8% from patient's questionnaires and 49.5% from physicians' reports, with a low/fair concordance (K = .38, p < .001). CES-D-20 found severe depression in 22.5% of the patients vs 4% identified by physicians. 135/155 (87%) of the severely depressed patients (according to CES-D-20) were considered as non or mildly/moderately depressed by physicians. Risk of severe depression was associated with unemployment (p < .001), previous depression (p < .001), treatment failure (p = .001), and former smoking status (p = .018). Depression is frequent in HIV-infected patients in the HAART era, with significant discrepancy between physician perception and the self-reported CES-D-20 results. Screening should be mandatory in all HIV patients.
KEYWORDS: Depression, HIV, CES-D-20 scale, psychological evaluation
Introduction
With the advent of highly active antiretroviral therapy (HAART), the disease pattern of HIV/AIDS has gradually evolved (Scandlyn, 2000) and the clinical approach to patients has changed, extending beyond pharmacological treatment and posing increasing attention towards the patient's overall well-being and quality of life. In particular, focus is being set on psychological distress and depression which are common to chronic conditions and which have been demonstrated to strongly influence clinical outcomes (Alciati et al., 2001; Hartzell, Janke, & Weintrob, 2008).
Several studies have confirmed a high but variable prevalence of depression among HIV/AIDS patients ranging from 22% to 45% (Benton, 2008; Ciesla & Roberts, 2001; Penzak, Reddy, & Grimsley, 2000). Despite these high-prevalence estimates there is evidence of an appreciable under-diagnosis of depressive symptoms, especially for severe depression (Asch et al., 2003; Rodkjaer et al., 2010; Rabkin, 2008).
As in other chronic settings as in cancer or chronic heart diseases, recognizing depression in HIV/AIDS patients (especially severe depression) and discriminating it from other physiological and emotional states may be difficult for the lack of specific skills to diagnose mental disorders, lack of time in busy hospital settings, and reluctance of the patient to discuss emotional well-being (Krebber et al., 2014; Thombs et al., 2008).
Consequently, appropriate depression individuation would generally require specific evaluation by a psychiatrist or psychologist (Gelenberg, 2009; Rabkin, 2008). However, this type of support is not always available or included in the routine clinical protocols, being left to the single hospitals/centers' initiative which can introduce a significant risk of underestimation (Asch et al., 2003; Israelski et al., 2007).
The aim of this study is to evaluate the extent of missed diagnoses of depression among HIV-infected subjects.
Patients and methods
The DHIVA study is a multicenter cross-sectional study involving 24 clinical HIV Centers across Italy. The primary objective of the study was to compare the degree of concordance between assessments of depression as evaluated by a validated screening tool, the Center of Epidemiologic Studies Depression (CES-D-20) scale (Radloff, 1977) and the perception of the attending physician. Secondary objectives were to assess the prevalence and the severity of depressive symptoms and to describe the correlates of depression.
The study was performed within a fixed 10-day timeframe, enrolling for each site a maximum of 30 consecutive outpatients among those regularly attending the center. Inclusion criteria were age >18, a diagnosis of HIV >6 months, and consent to inclusion in the study; the sole exclusion criterion was the patient's inability to fill out the questionnaire. The attending physicians were asked to evaluate their patients' depression status according to their clinical experience and classify it as absent, mild, moderate, or severe. Each patient enrolled was asked to fill out the self-assessment CES-D-20 questionnaire, that classified the subject into one of four categories: absence of depression, mild, moderate, and severe depression (CES-D-20 score categories: <16, 16–20; 21–25; 26–60, respectively)
Patients were also given a second ad hoc questionnaire (the DHIVA questionnaire, Appendix – Figure A1) investigating subjective parameters and patients' behavioral and social indicators.
The study obtained approval/authorization by ethics committees as requested by local regulations. Prior to the inclusion in the study, all patients provided a signed informed consent form.
Statistical methods
All characteristics were described using frequency distributions for categorical variables and mean, median, standard deviation, interquartile range, and range for quantitative variables.
Agreement between physician's judgment and CES-D-20 results was assessed by means of Cohen's weighted kappa coefficient (Cohen, 1968). The weight matrix allows the specification of the degree of the disagreement. K statistics were calculated using two differently weighted systems (W1 and W2), both based on the “proximity/closeness” of the judgments or the “seriousness” of the disagreement. In the first system, the decrease is constant (1–.66–.33–0), while the second assigns weights so as not to penalize trial contiguous classes (1–.89–.56–0).
Data were analyzed through descriptive statistics and stratified according to the CES-D-20. Association between depression and subject characteristics were evaluated separately through univariate and multivariate logistic models (OR and 95% CI).
To perform univariate and multivariate logistic models, both diagnosis of depression (mild, moderate, and severe) and just severe depression were used as response variables and demographic/clinical conditions as regressors: multivariate logistic regression was performed with variables which were previously found with p < .20 in univariate logistic regression. From the regression model thus obtained the variables that had p-value >.10 were gradually excluded, one at a time. At each single step of the exclusion procedure the variable with the higher p-value was deleted, based on the value of the rank test.
Continuous variables were reported as mean +/− SD; comparisons were performed by a two-tailed independent samples t-test; discrete variables were reported as category counts; the comparisons were performed by a Pearson chi-square test.
Results
The study enrolled 709 patients: of these, 18 were excluded from the analysis due to inclusion criteria violation or incomplete questionnaires, resulting in 690 evaluable patients. The majority of patients were male (72.5%), Caucasian (96.5%), and between 45 and 54 years of age; a large proportion had primary/middle school education (47%), smoked (51%), and was not stably employed (38%) (Table 1). According to results attained by CES-D-20, 48.8% of respondents had some degree of depression: 14.3% of subjects had mild symptoms, 12.0% had moderate symptoms, and 22.5% had severe symptoms. According to the infectious disease physicians, 49.4% of patients suffered from depression: of these, 27% were classified as mild, 18.4% as moderate, and 4% as severe depression (Figure 1).
Table 1. Demographic and clinical data: overall and by gender stratification.
| Factors | Overall | Male (n = 501) | Female (n = 189) | p |
|---|---|---|---|---|
| Age (median, min–max years) | 45 (18–77) | 46 (19–77) | 44 (18–76) | .014 |
| Ethnic group, % (N) | .247 | |||
| White | 96.52 (666) | 96.41 (483) | 96.83 (183) | |
| Hispanic | 1.88 (13) | 2.20 (11) | 1.06 (2) | |
| Black | 1.16 (8) | .80 (4) | 2.12 (4) | |
| Asian | .43 | .60 (3) | .00 (0) | |
| Education, % (N) | ||||
| Primary school | 7.39 (51) | 5.99 (30) | 11.11 (21) | .001 |
| Middle school | 39.57 (273) | 37.72 (189) | 44.44 (84) | |
| High school | 35.80 (247) | 35.93 (180) | 35.45 (67) | |
| University | 14.06 (97) | 17.17 (86) | 5.82 (11) | |
| Unknown | 2.46 (17) | 2.20 (11) | 3.17 (6) | |
| Employment, % (N) | < .001 | |||
| Unemployed | 17.54 (121) | 14.37 (72) | 25.93 (49) | |
| Employed | 62.03 (299) | 69.06 (346) | 43.39 (82) | |
| Self-employed | 18.70 (129) | 16.67 (115) | 7.41 (14) | |
| Occasionally employed | 3.91 (27) | 2.79 (14) | 6.88 (13) | |
| Housewife | 4.64 (32) | 0 (0) | 16.93 (32) | |
| Other | 11.88 (82) | 13.77 (69) | 6.88 (13) | |
| Number of cohabitants (mean +/− SD) | 2.23+/−1.87 | 1.94 +/− 2.32 | 2.32 +/− 1.16 | .001 |
| Smoking | ||||
| Smoker | 50.87 (351) | 49.90 (250) | 53.44 (101) | .791 |
| Non smoker | 30.43 (210) | 30.94 (155) | 29.10 (55) | |
| Ex-smoker | 13.77 (95) | 14.37 (72) | 12.17 (23) | |
| Mode of HIV transmission, % (N) | ||||
| Sexual | 70.43 (486) | 71.46 (358) | 67.72 (128) | .135 |
| Vertical | .87 (6) | .80 (4) | 1.06 (2) | |
| Drug addition | 25.36 (175) | 23.55 (118) | 30.16 (57) | |
| Transfusion/blood products | .72 (5) | 1.00 (5) | .00 (0) | |
| Infection duration (years from HIV diagnosis; mean +/− SD) | 11.7 +/− 7.9 | 10.74 +/− 7.98 | 14.32 +/− 7.12 | < .001 |
| CD4 (cell/cmm; mean +/− SD) | 580 +/−300 | 566 +/− 290 | 607 +/− 328 | .109 |
| Patients with undetectable viral load, % (N) | 72.5 | 372 | 128 | .182 |
| Neoplasias. % (N) | 5.36 (37) | 4.59 (23) | 7.41 (14) | .202 |
| HBV co-infection, % (N) | .582 | |||
| Yes | 8.70 (63) | 9.18 (46) | 7.41 (17) | |
| No | 83.04 (573) | 82.83 (415) | 83.60 (158) | |
| Unknown | 8.26 (57) | 7.98 (40) | 8.99 (17) | |
| HCV co-infection, % (N) | .551 | |||
| Yes | 29.42 (203) | 28.74 (144) | 31.22 (59) | |
| No | 64.64 (446) | 65.47 (328) | 62.43 (118) | |
| Unknown | 5.94 (41) | 5.79 (29) | 6.35 (12) | |
| Cirrhosis, % (N) | 3.04 (21) | 2.79 (14) | 3.70 (7) | .710 |
Figure 1.
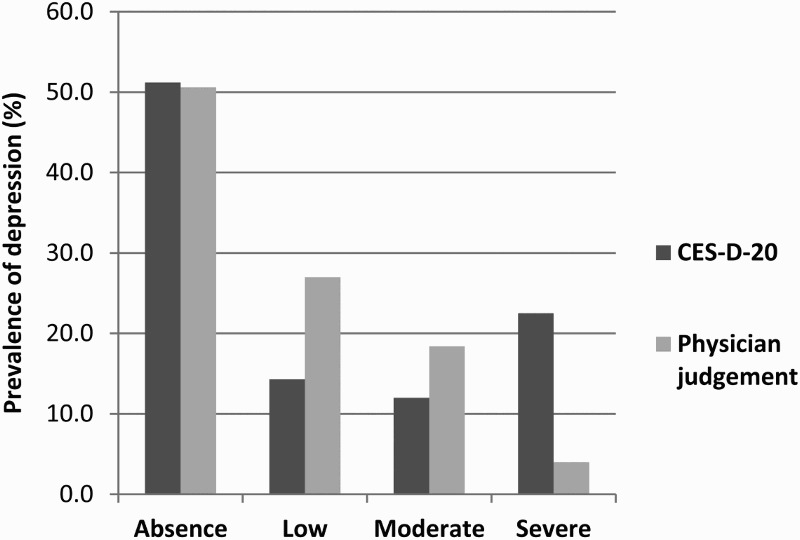
Prevalence of depression. Comparison between assessment by CES-D-20 (dark grey) and by the physician (light grey).
The degree of discrepancy/concordance between CES-D-20 results and clinical evaluation is reported in Table 2. Concordance was full in 296 cases (42.9%), whereas in the remaining 57.1% the two judgments disagreed about the presence or degree of depression.
Table 2. Concordance table between degrees of depression according to CES-D questionnaire and as perceived by the physician: results go from perfect concordance (dark grey boxes) to absolute discordance (white boxes).
| CES-D | |||||
|---|---|---|---|---|---|
| Physician assessment | Absence of depression | Mild depression | Moderate depression | Severe depression | Total |
| Absence of depression | 233a | 52b | 32c | 32d | 349 |
| 33.8a | 7.5b | 4.6c | 4.6d | 50.6% | |
| Mild depression | 95b | 26a | 31b | 34c | 186 |
| 13.8b | 3.8a | 4.5b | 4.9c | 26.9% | |
| Moderate depression | 22c | 19b | 17a | 69b | 127 |
| 3.2c | 2.8b | 2.5a | 10.0b | 18.4% | |
| Severe depression | 3d | 2c | 3b | 20a | 28 |
| 0.44d | 0.3c | 0.4b | 2.9a | 4.1% | |
| Total | 353 | 99 | 83 | 155 | 690 |
| 51.2% | 14.4% | 12.0% | 22.5% | 100.0% | |
a Total concordance
b Mild discordance
c Severe discordance
d Total discordance
Note: Top line: number of patients; bottom line: percentage.
Specifically, in some cases clinicians considered as “not depressed” patients who were depressed according to CES-D-20 criteria (n = 116/690 [16.8%], including 32 [4.6%] who were severely depressed). Conversely, some patients who were classified as not depressed by CES-D-20, were identified as depressed by physicians (n = 120; 17.4%).
Among patients classified as depressed by CES-D-20 (n = 337, Table 3), there was full concordance on the degree of depression in only 18.7% of cases (n = 63/337, dark grey boxes), while the depressive status was overestimated by physicians in 7.1% of cases (24/337) and underestimated in the remaining 74.2% (250/337; light grey boxes). Considering the CES-D-20 categories as a reference, physicians' underestimation was particularly high with regards to moderate (75.9%, n = 63/83) and severe depression (87.1%, n = 135/155).
Table 3. Patients classified as “depressed” (different degrees) by CES-D-20: results go from perfect concordance (dark grey boxes) to discordance (light grey boxes).
| CES-D | ||||
|---|---|---|---|---|
| Physician assessment | Mild depression | Moderate depression | Severe depression | Total |
| Absence of depression | 52b | 32c | 32d | 116 |
| 15.4b | 9.5c | 9.5d | 34.4% | |
| Mild depression | 26a | 31b | 34c | 91 |
| 7.7a | 8.2b | 10.1c | 27.1% | |
| Moderate depression | 19b | 17a | 69b | 105 |
| 5.6b | 5.0a | 20.5b | 31.2% | |
| Severe depression | 2c | 3b | 20a | 25 |
| 0.6c | 0.9b | 5.9a | 7.4% | |
| Total | 99 | 83 | 155 | 337 |
| 29.4% | 24.6% | 45.9% | 100.0% | |
a Total concordance
b Mild discordance
c Severe discordance
d Total discordance
Note: Top line: number of patients; bottom line: percentage.
The comparison of the concordance between the two assessments as measured by K statistics evidenced an overall fair (K = .31 according to W1) or moderate (K = .43 according to W2) concordance between the CES-D-20 and physicians' evaluation.
A high variability of concordance among participating centers (Figure 2) was also observed.
Figure 2.
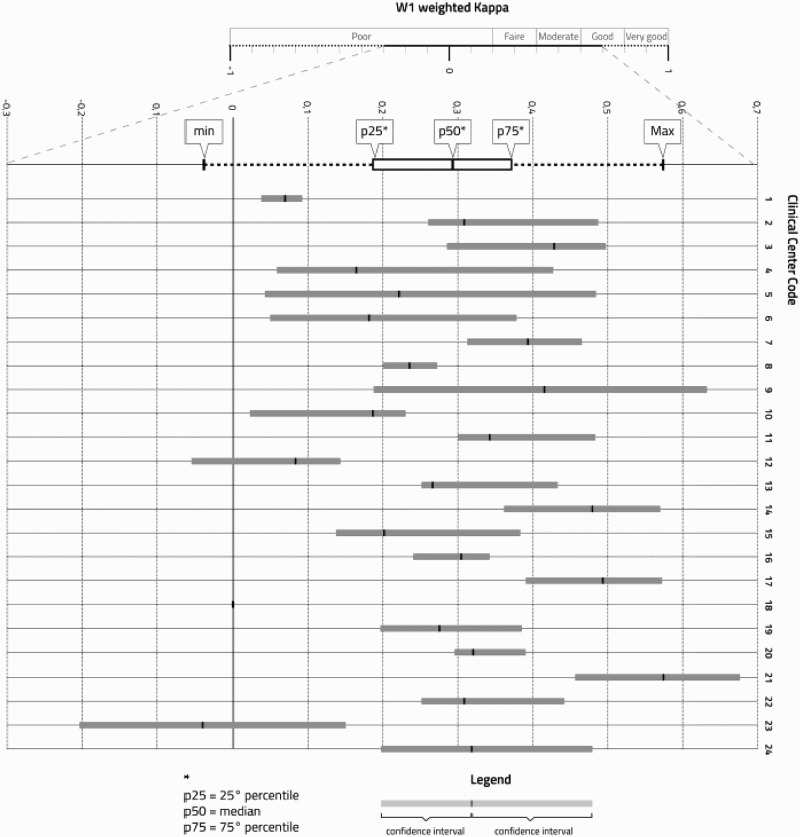
Agreement between physician's assessment and CES-D-20 scores. The weighted K (Cohen's kappa) coefficient measures the agreement between physician's assessment and CES-D-20 scores. Confidence intervals indicate the variability of kappa within each center (among physicians). The weighting system was defined according to the proxmity /closeness of the judgments within contiguous classes (W1, 1-.66-.33-0). Box plots (grey boxes) of weighted Cohen's K coefficients refer to hospitals. The figure shows that K values are scattered over a wide range; increasing distance from the 0 axis indicates a higher degree of concordance.
Factors associated with depression at the univariate level are reported in Appendix (Tables A1 and A2).
Application of the multivariate model on clinical/demographic data showed that the independent factors significantly associated with depression were: un- or under-employment, liver cirrhosis, previous diagnosis of depression, living in smaller households, unknown HBV status, and current use of illicit drugs (Table 4). Multivariate analysis, with data derived from the DHIVA questionnaire, confirmed as independent correlates of depression the unsatisfactory perception of quality of life, sexual dysfunction (other than loss of libido), use of sleeping pills /sedatives, and non-adherence to antiretrovirals in the last week (Table 5).
Table 4. Socio-demographic factors associated with depression: data from multivariate logistic regression from CRF.
| Multivariate logistic model | ||||
|---|---|---|---|---|
| Factors | OR | 95% CI OR | p | |
| Work status vs employed/self-employed | ||||
| Unemployed/occasionally employed | 2.77 | 1.45 | 5.28 | .002 |
| Other status | 1.62 | .91 | 2.91 | .103 |
| HBV infection vs no | ||||
| Yes | .88 | .39 | 2.01 | .765 |
| Unknown | 3.87 | 1.43 | 10.45 | .008 |
| Hepatic cirrhosis: yes vs no | 9.24 | 2.35 | 36.30 | .001 |
| Previous diagnosis of depression: yes vs no | 4.48 | 2.09 | 9.61 | <.001 |
| Household size: >=1 vs 0 | .43 | .19 | .96 | .038 |
| Use of drugs: yes vs no | 6.32 | 1.79 | 22.34 | .004 |
Note: Please refer to Appendix Table A1 for univariate data.
Table 5. Socio-demographic factors associated with depression: data from multivariate logistic regression from DHIVA questionnaire.
| Multivariate logistic model | ||||
|---|---|---|---|---|
| Factors | OR | 95% CI OR | p | |
| Frequency of other sexual disfunctionsavs never | ||||
| Often | 4.72 | 2.58 | 8.64 | <.001 |
| Rarely | 1.60 | .81 | 3.14 | .173 |
| Not answered | 1.97 | .93 | 4.19 | .077 |
| Presence of other family members with depression | ||||
| No | .59 | .34 | 1.03 | .063 |
| Not answered | .83 | .23 | 3.03 | .778 |
| Missing antiretrovirals in the last week | ||||
| No | .26 | .13 | .53 | <.001 |
| Not answered | .33 | .13 | .84 | .020 |
| Frequency of use of sleeping pills/sedatives vs never | ||||
| Often | 4.30 | 1.85 | 10.00 | .001 |
| Rarely | 1.67 | .81 | 3.44 | .162 |
| Not answered | 2.55 | .63 | 10.38 | .189 |
| Quality of life vs satisfactory | ||||
| Acceptable | 6.23 | 3.67 | 10.56 | <.001 |
| Unsatisfactory | 27.05 | 8.69 | 84.22 | <.001 |
| Not answered | 1.11 | .22 | 5.53 | .901 |
Note: Please refer to Appendix Table A2 for univariate data.
aOther than loss of libido.
The number of patients classified by CES-D-20 as having severe depression was 155 (22.46%). The variables found to be associated with severe grade of depression in the univariate model, are listed in the Appendix (Table A3) while the multivariate model is showed in Table 6.
Table 6. Factors significantly associated to severe depression in multivariate logistic model.
| Factors | OR | 95% CI OR | P | |
|---|---|---|---|---|
| Gender (male vs female) | .592 | .341 | 1.027 | .062 |
| Not/occasionally employed vs employed /self-employed | 2.147 | 1.153 | 3.997 | .016 |
| Previous diagnosis of depression: yes vs no | 3.815 | 1.889 | 7.704 | <.001 |
| Treatment failure: >1 vs 0 | 2.618 | 1.348 | 5.086 | .004 |
| Treatment failure: unknown vs 0 | .326 | .108 | .985 | .047 |
| Smoke: ex-smoker vs smoker | .452 | .208 | .981 | .045 |
Note: Please refer to Appendix Table A3 for univariate data.
The parameters associated with concordance/discordance between the physician's judgment and the CES-D-20 assessment were analyzed at a univariate level. The previous diagnosis of depression and previous alcohol abuse are the factors that correlate with concordance between physicians' diagnosis of depression and CES-D-20 depression categories; the chance of being correctly recognized as depressed by the physician was 88% higher in patients with a previous diagnosis of depression (p < .001) and 76% higher in those using alcohol (p = .04). Conversely, depressed patients taking interferon for HCV co-infection had a 10-fold higher risk of not being recognized as depressed by the physician, compared to those not on interferon therapy (Appendix, Table A4).
Finally, the factors significantly involved in attributing depression by physicians to patients who were not depressed per CES-D-20 were: HBV or HCV co-infections (p = .0189 and p = .011), cirrhosis (p = .033), unknown education level (p = .018), employment status (p = .012), previous depression (p = .002), or treatment failure (p = .006) (Appendix, Table A5).
Discussion
In patients living with HIV/AIDS, depression has been reported to be associated with non-adherence to therapy (Horberg et al., 2008; Nel & Kagee, 2011), faster progression of the disease (Kacanek et al., 2010; Pence et al., 2007) and diminished active and problem-focused coping strategy. Depression was considered as a main comorbidity (contributing, incidental, or confounder) for clinical assessment of HIV-associated neurocognitive disorder (HAND, Antinori et al., 2007) and a recent analysis of the CHARTER Research Cohort pointed out that major depressive disorder (MDD) is also linked to viral escape in CSF (Hammond, Crum, & Treisman, 2013).
Despite the burden of depression in HIV/AIDS patients the diagnosis can be frequently missed in this population and it may be difficult to discriminate depressive symptoms from normal fluctuations in mood state. Although questionnaires cannot replace the doctor–patient relationship, self-administered depression scales are generally considered a valid screening tool. The CES-D-20 self-administered depression scale (Radloff, 1977) is widely used to screen for depression status among people with HIV and has proved to be a tool with high sensitivity and somewhat lower specificity (Balsamo & Saggino, 2007). Prevalence of depression in our cohort was high (approximately half of the population, considering all grades of depression and for both the physician and CES-D-20 evaluation).
The two evaluations gave us different prevalence distribution when considering the different categories of depression, thus suggesting that even when a depressive status has been correctly identified it may be difficult to discriminate the depression severity.
Full concordance between CES-D-20 assessment and physicians' judgment on the presence and severity of depression was achieved only in less than one-fifth of cases.
Discrepancy between the physician's perception and the CES-D-20 assessment was found especially in relation to the degree of depression. Considering the CES-D-20 categories as a reference, overestimation occurred in a minority of cases, while underestimation was more common than expected. In particular, 87.1% of subjects with CES-D-20 scores placing them in the severely depressed category were not perceived to be severely depressed by their physicians, with potential consequences for the patients' health and clinical management. The underestimation of depression among patients taking interferon-alpha is especially noteworthy, as its neuropsychiatric side effects are widely described (Mello, Segurado, & Malbergier, 2010).
It is conceivable that a certain degree of depression is considered by physicians so common in patients living with HIV/AIDS, as to be deemed the patient's baseline mood. Some HIV-related symptoms (e.g., fatigue) might further interfere with recognizing depression, and physicians might need specialized training to correctly diagnose depression in this setting.
It is important to note that the main concern of HIV physicians is to maintain an adequate suppression of HIV and an acceptable immunological function in patients, and this can lead them to overlook other clinically relevant aspects such as depression. Furthermore, attending physicians in Infectious Diseases units may not receive any specific training for depression diagnosis and care. We can speculate that the discrepancy of diagnostic accuracy across the participating centers could be explained also by the presence/absence of multidisciplinary teams that include mental health specialists, where it is possible that the multifaceted approach to global patients' health may lead them to monitor also non-strictly virological aspects.
On the other hand, in the presence of HBV and/or HCV co-infection or cirrhotic liver disease, physicians tend to overestimate depression. In these cases, the peculiar Italian epidemiological picture of viral liver diseases (Sagnelli, et al., 2005; Sagnelli et al., 2008) and the high prevalence of drug abuse among HIV patients (Istituto Superiore di Sanità, 2013) may have a confounding effect on clinical judgment. Drug abuse has already been associated with an increased risk of psychological alterations (Psaros et al., 2013) and co-infections with hepatitis B and C viruses represented an additional risk factor for depression (Raison et al., 2006; Weiss & Morgello, 2009). The association between unknown HBV status and depression found in our analysis does not have an obvious explanation: however, the number of patients with unknown HBV status was small (56 subjects), and this association is probably irrelevant. In our study hepatic cirrhosis was strongly associated with depressive symptoms, confirming previous reports (Mells et al., 2013).
Lower educational level and unemployment status were among the most powerful factors associated with depression, with unemployment being a strong predictor for the highest degree of depressive status. Although depression can arise as consequence of patients not being involved in a working activity, depression itself might also be the cause for becoming less efficient in the workplace and eventually lead to losing the job or not maintaining it (Bravo et al., 2010; Raison et al., 2006).
A very strong association has been detected between low perception of quality of life and depressive symptoms thus confirming existing data that suggest that psychological well-being and psychiatric comorbidities are important predictors of quality of life in this population (Briongos-figuero et al., 2011; Degroote et al., 2013; Douaihy & Singh, 2001). Nevertheless we have to take into account that many determinants of quality of life and of depression overlap, therefore the strength of the association may be due to confounders. As shown for other chronic disorders (Grenard et al., 2011) and also HIV infection (Gonzalez et al., 2011), a strong association between depression and medication non-adherence has been found. Since it has been shown that antidepressant treatment improves antiretroviral adherence (Sin & DiMatteo, 2014), clinicians should be aware of these two frequently coexisting conditions and always investigate patients diagnosed with depressive symptomatology for correct pill intake and vice versa.
The number of previous treatment failures and female gender are specifically related to severe depression and did not emerge as correlate of risk for minor degrees of depression. In this respect, our data can be considered in agreement with the previously published literature (Ickovics et al., 2001); it is well established that women generally bear a greater combination of stress factors in relation to family planning, motherhood, lower income, and lack of emotional support compared to men (Mello et al., 2010; Rabkin, 2008) . Severe depression in women is also a predictor of disease progression and higher morbidity: depressed women with HIV/AIDS, however, are at increased risk for non-AIDS related deaths (Cook et al., 2004).
In our study population living alone, sexual dysfunctions, use of sleeping pills were significantly associated with current depression, confirming that people living with HIV may require further clinical, social, and emotional support.
The main limitation of the study is that possible differences among clinicians about specific training and experience on neuropsychiatric aspects have not been collected. Additionally, it has to be taken into account that CES-D-20 is an epidemiological screening tool designed and validated to identify individuals at risk for clinical depression and not to provide a clinical diagnosis. Furthermore, we acknowledge that some symptoms (such as fatigue possibly associated with treatment or disease itself) may cause the CES-D to overestimate the depression thus it is possible that part of discrepancy might have been driven by CES-D-20 false positive or negative.
Finally, the cross-sectional design was suitable to demonstrate association between investigated factors and depressive symptoms, but not causality.
Conclusions
Our study confirms that depression is a very frequent condition among the HIV-positive population also in the HAART era. Factors associated with severe depressive symptomatology were principally socio-demographic characteristics, previous diagnosis of depression, and treatment failure. The most relevant finding of this study, however, was an alarming discrepancy between psychological evaluation obtained by means of a standardized screening tool and by the clinician's assessment. Although clinicians may benefit from more education and training on depression diagnosis, and recognizing the presence of depression correlates may lead to fewer missed diagnoses, the use of a self-reported scale, such as CES-D-20, could represent an immediate and cost-effective screening tool for identifying patients with depressive symptomatology.
Nonetheless HIV has become a chronic infection that extends over several decades. Patients living with HIV/AIDS may often have a low quality of life especially driven by psychological aspects rather than physical symptoms. Greater attention of clinician's towards the quality of life in persons living with HIV/AIDS is mandatory and needs a multidisciplinary approach.
Acknowledgements
The authors wish to thank all patients and all Clinical Centers involved in the DHIVA Study. They also wish to thank Pencil and Papers (Italy) for the editorial assistance in preparing this manuscript.
Appendix
Figure A1.

Dhiva questionnaire
Table A1. Factors associated with the presence of depressive symptoms in univariate logistic models (CRF).
| Univariate logistic model | ||||
|---|---|---|---|---|
| Odds Ratio | [95% Conf. | Interval] | p>t | |
| Educational degree: high school vs middle school/elementary | .57 | .37 | .87 | .010 |
| Education degree: university vs middle school/elementary | .37 | .20 | .70 | .002 |
| Educational degree: unknown vs middle school/elementary | .59 | .19 | .83 | .365 |
| Male vs female | .58 | .38 | .89 | .014 |
| Unemployed/occasionally employed vs employed/self-employed | 3.93 | 2.22 | 6.96 | .000 |
| Other status vs employed/self-employed | 1.56 | .92 | 2.64 | .098 |
| Duration of infection: 12–59 months vs <12 months | 2.56 | .71 | 9.27 | .152 |
| Duration of infection: 60–119 months vs <12 months | 3.07 | .84 | 11.20 | .089 |
| Duration of infection: >120 months vs <12 months | 3.48 | 1.00 | 12.16 | .050 |
| CD4: 200–350 cell/mm3 vs <200 cell/mm3 | 1.02 | .40 | 2.56 | .973 |
| CD4: 351–500 cell/mm3 vs <200 cell/mm3 | .93 | .40 | 2.16 | .862 |
| CD4: >500 cell/mm3 vs <200 cell/mm3 | .79 | .36 | 1.73 | .554 |
| RNA: 50–1000 copies/ml vs <50 copies/ml | 1.15 | .68 | 1.97 | .597 |
| RNA: >1000 copies/ml vs <50 copies/ml | 1.13 | .62 | 2.05 | .692 |
| HBV: yes vs no | 1.38 | .67 | 2.84 | .379 |
| HBV: unknown vs no | 3.09 | 1.29 | 7.41 | .012 |
| HCV: yes vs no | 2.31 | 1.46 | 3.64 | .000 |
| HCV: unknown vs no | 2.71 | 1.20 | 6.12 | .017 |
| Hepatic cirrhosis: yes vs no | 13.96 | 3.75 | 51.89 | .000 |
| Neoplasia: yes vs no | 1.24 | .54 | 2.86 | .605 |
| Previous diagnosis of depression: yes vs no | 3.80 | 1.92 | 7.51 | .000 |
| AIDS events: yes vs no | 1.05 | .67 | 1.65 | .832 |
| Interferon therapy: yes vs no | 1.48 | .37 | 5.85 | .580 |
| Previous use of drugs: yes vs no | 2.50 | 1.60 | 3.91 | .000 |
| Previous use of alcohol: yes vs no | 4.27 | 1.84 | 9.90 | .001 |
| Adherence to ARV therapy | .98 | 0.91 | 1.05 | .499 |
| Mode of transmission: other vs sexual transmission | 1.20 | .52 | 2.76 | .665 |
| Mode of transmission: drug use vs sexual transmission | 2.57 | 1.61 | 4.11 | .000 |
| Family size : >=1 vs 0 | .49 | .24 | 1.00 | .051 |
| Therapy failures: 1 vs 0 | 1.74 | .93 | 3.26 | .084 |
| Therapy failures: >1 vs 0 | 1.61 | 1.00 | 2.58 | .050 |
| Therapy failures: unknown vs 0 | .70 | .32 | 1.52 | .369 |
| Use of drugs: yes vs no | 6.78 | 2.34 | 19.64 | <.001 |
| Use of alcohol: yes vs no | 2.88 | .83 | 10.03 | .097 |
| Smoking: never smoked vs smoker | .55 | .35 | .87 | .010 |
| Smoking: ex-smoker vs smoker | .82 | .47 | 1.43 | .479 |
Table A2. Factors associated with the presence of depressive symptoms in univariate logistic models (DHIVA questionnaire).
| Univariate logistic model | ||||
|---|---|---|---|---|
| Odds Ratio | [95% Conf. | Interval] | p>t | |
| Tiredness: rarely vs never | 1.96 | .98 | 3.93 | .057 |
| Tiredness: often vs never | 8.16 | 3.91 | 17.02 | .000 |
| Tiredness: very often vs never | 9.32 | 3.48 | 24.98 | .000 |
| Tiredness: not answered vs never | 2.95 | 1.07 | 8.20 | .037 |
| Mental confusion: rarely vs never | 3.50 | 2.17 | 5.66 | .000 |
| Mental confusion: often vs never | 7.81 | 3.81 | 15.99 | .000 |
| Mental confusion: very often vs never | 10.20 | 2.67 | 38.99 | .001 |
| Mental confusion: not answered vs never | 2.72 | 1.28 | 5.78 | .009 |
| Sleep disorders: rarely vs never | 2.47 | 1.45 | 4.21 | .001 |
| Sleep disorders: often vs never | 7.26 | 3.93 | 13.42 | .000 |
| Sleep disorders: very often vs never | 21.30 | 7.88 | 57.61 | .000 |
| Sleep disorders: not answered vs never | 4.83 | 2.08 | 11.23 | .000 |
| Anxiety: rarely vs never | 2.51 | 1.46 | 4.30 | .001 |
| Anxiety: often vs never | 13.96 | 7.14 | 27.29 | .000 |
| Anxiety: very often vs never | 144.24 | 29.22 | 712.09 | .000 |
| Anxiety: not answered vs never | 5.82 | 2.73 | 12.42 | .000 |
| Decreased concentration: rarely vs never | 2.85 | 1.77 | 4.58 | .000 |
| Decreased concentration: often vs never | 14.34 | 6.81 | 30.21 | .000 |
| Decreased concentration: very often vs never | 77.82 | 9.68 | 625.77 | .000 |
| Decreased concentration: not answered vs never | 2.55 | 1.09 | 5.98 | .031 |
| Trunk fat: rarely vs never | 1.30 | .75 | 2.24 | .344 |
| Trunk fat: often vs never | 2.57 | 1.40 | 4.75 | .003 |
| Trunk fat: very often vs never | 5.59 | 1.90 | 16.47 | .002 |
| Trunk fat: not answered vs never | 1.90 | 1.01 | 3.56 | .045 |
| Loss of fat in limbs: rarely vs never | 1.19 | .70 | 2.04 | .513 |
| Loss of fat in limbs: often vs never | 2.13 | 1.08 | 4.20 | .030 |
| Loss of fat in limbs: very often vs never | 1.44 | .55 | 3.81 | .458 |
| Loss of fat in limbs: not answered vs never | 1.83 | .96 | 3.48 | .066 |
| Loss of fat in face: rarely vs never | .95 | .55 | 1.66 | .858 |
| Loss of fat in face: often vs never | 2.39 | 1.20 | 4.76 | .014 |
| Loss of fat in face: very often vs never | 2.08 | .75 | 5.77 | .159 |
| Loss of fat in face: not answered vs never | 1.47 | .80 | 2.70 | .219 |
| Loss of libido: rarely vs never | 2.17 | 1.31 | 3.61 | .003 |
| Loss of libido: often vs never | 4.05 | 2.20 | 7.46 | .000 |
| Loss of libido: very often vs never | 7.48 | 2.77 | 20.19 | .000 |
| Loss of libido: not answered vs never | 1.71 | 0.91 | 3.21 | .095 |
| Other sexual disorders: rarely vs never | 1.82 | 1.04 | 3.18 | .035 |
| Other sexual dysfunctions: often vs never | 5.96 | 3.11 | 11.44 | .000 |
| Other sexual dysfunctions: very often vs never | 10.56 | 2.81 | 39.77 | .001 |
| Other sexual dysfunctions: not answered vs never | 2.30 | 1.30 | 4.09 | .004 |
| Financial status: fairly satisfactory vs very satisfactory | 1.84 | .59 | 5.75 | .295 |
| Financial status: acceptable vs very satisfactory | 4.15 | 1.36 | 12.67 | .013 |
| Financial status: unsatisfactory vs very satisfactory | 12.67 | 3.96 | 40.58 | .000 |
| Financial status: not answered vs very satisfactory | 7.90 | 1.75 | 35.79 | .007 |
| Work: fairly satisfactory vs very satisfactory | .92 | .48 | 1.75 | .797 |
| Work: acceptable vs very satisfactory | 2.00 | 1.04 | 3.82 | .037 |
| Work: unsatisfactory vs very satisfactory | 6.38 | 3.05 | 13.35 | .000 |
| Work: not answered vs very satisfactory | 2.04 | .95 | 4.37 | .068 |
| Use of drugs and/or alcohol: rarely vs never | 1.39 | .86 | 2.27 | .178 |
| Use of drugs and/or alcohol: often vs never | 3.77 | 1.69 | 8.40 | .001 |
| Use of drugs and/or alcohol: very often vs never | 2.36 | .87 | 6.41 | .090 |
| Use of drugs and/or alcohol: not answered vs never | 3.00 | 1.16 | 7.75 | .023 |
| Family members suffering from depression: no vs yes | .51 | .32 | .80 | .004 |
| Family members suffering from depression: not answered vs yes | 1.27 | .41 | 3.94 | .676 |
| Seropositivity known to family and/or partner: no vs yes | .84 | .52 | 1.37 | .494 |
| Seropositivity known to family and/or partner: not answered vs yes | 1.62 | .51 | 5.10 | .410 |
| Number of times ARV therapy was taken in past month | .85 | .68 | 1.06 | .161 |
| Forgot to take therapy in past week: no vs yes | .30 | .16 | .56 | .000 |
| Forgot to take therapy in past week: not answered vs no | .33 | .16 | .68 | .003 |
| Therapy discontinuation in past 3 months: no vs yes | .63 | .31 | 1.27 | .197 |
| Therapy discontinuation in past 3 months: not answered vs yes | .56 | .26 | 1.23 | .151 |
| Use of sleeping pills/sedatives: rarely vs never | 1.97 | 1.05 | 3.71 | .036 |
| Use of sleeping pills /sedatives: often vs never | 5.93 | 2.49 | 14.13 | .000 |
| Use of sleeping pills /sedatives: very often vs never | 10.39 | 2.69 | 40.14 | .001 |
| Use of sleeping pills /sedatives: not answered vs never | 1.57 | .89 | 2.79 | .122 |
| Quality of life: fairly satisfactory vs very satisfactory | 15.66 | 4.91 | 49.98 | .000 |
| Quality of life: acceptable vs very satisfactory | 78.10 | 24.22 | 251.87 | .000 |
| Quality of life: unsatisfactory vs very satisfactory | 416.57 | 84.81 | 2046.18 | .000 |
| Quality of life: not answered vs very satisfactory | 31.85 | 9.28 | 109.23 | .000 |
Table A3. Univariate analysis of the factors associated with severe depression.
| Univariate logistic model | ||||
|---|---|---|---|---|
| Odds Ratio | [95% Conf. | Interval] | P>t | |
| Educational degree: high school vs middle school/elementary | .53 | .32 | .88 | 0.01 |
| Educational degree: university vs middle school/elementary | .12 | .04 | .30 | <.001 |
| Male vs female | .42 | .26 | .68 | <.001 |
| Unemployed/occasionally employed vs employed /self-employed | 4.08 | 2.29 | 7.26 | <.001 |
| Duration of infection: 12–59 months vs <12 monthss | 9.60 | 1.14 | 76.78 | .04 |
| Duration of infection: 60–119 months vs <12 months | 11.78 | 1.43 | 96.63 | .02 |
| Duration of infection: >120 months vs <12 months | 15.75 | 2.00 | 124.05 | .01 |
| CD4: 351–500 cell/mm3 vs <200 cell/mm3 | .34 | .13 | .85 | .02 |
| HCV: yes vs no | 2.66 | 1.61 | 4.41 | <.001 |
| Cirrhosis: yes vs no | 4.82 | 1.41 | 16.51 | .01 |
| Previous diagnosis of depression: yes vs no | 4.33 | 2.30 | 8.15 | <.001 |
| Previous use of drugs: yes vs no | 3.20 | 1.96 | 5.22 | <.001 |
| Previous use of alcohol yes vs no | 5.63 | 2.65 | 10.84 | <.001 |
| Mode of transmission: drug use vs sexual | 3.21 | 1.93 | 5.34 | <.001 |
| Family size: >=1 vs 0 | .45 | .22 | .93 | .03 |
| Therapy failure: 1 vs 0 | 2.28 | 1.33 | 3.89 | .00 |
| Use of drugs: yes vs no | 6.46 | 2.57 | 16.24 | <.001 |
| Use of alcohol: yes vs no | 6.27 | 2.00 | 19.65 | .00 |
| Smoking: never smoked vs smoker | .44 | .25 | .76 | .00 |
| Smoking: ex-smoker vs smoker | .44 | .21 | .92 | .03 |
Table A4. Univariate analysis of factors associated with physician's discordance in assessing depression – depressed patients according to CES-D.
| Univariate logistic model | ||||
|---|---|---|---|---|
| Odds Ratio | [95% Conf. | Interval] | p>t | |
| Educational degree: high school vs middle school/elementary | 1.39 | .73 | 2.66 | .311 |
| Educational degree: university vs middle school/elementary | 2.18 | .78 | 6.08 | .134 |
| Educational degree: unknown vs middle school/elementary | 1.49 | .33 | 6.79 | .606 |
| Male vs female | 1.57 | .83 | 2.98 | .163 |
| Not/occasionally employed vs employed/self-employed | .89 | .44 | 1.81 | .746 |
| Other employed condition vs employed/self-employed | .61 | .26 | 1.44 | .258 |
| Duration of infection: 12–59 months vs <12 months | .40 | .05 | 3.06 | .374 |
| Duration of infection: 60–119 months vs <12 months | .31 | .04 | 2.38 | .258 |
| Duration of infection: >120 months vs <12 months | .44 | .06 | 3.19 | .417 |
| CD4: 200–350 cell/mm3 vs <200 cell/mm3 | .58 | .14 | 2.31 | .436 |
| CD4: 351–500 cell/mm3 vs <200 cell/mm3 | .88 | .25 | 3.05 | .838 |
| CD4: >500 cell/mm3 vs <200 cell/mm3 | .79 | .24 | 2.53 | .687 |
| RNA: 50–1000 copies/ml vs <50 copies/ml | 2.02 | .94 | 4.35 | .070 |
| RNA: >1000 copies/ml vs <50 copies/ml | 1.48 | .60 | 3.63 | .388 |
| HBV: yes vs no | .82 | .24 | 2.75 | .742 |
| HBV: unknown vs no | 1.14 | .45 | 2.88 | .776 |
| HCV: yes vs no | .59 | .31 | 1.15 | .124 |
| HCV: unknown vs no | 1.12 | .41 | 3.07 | .822 |
| Cirrhosis: yes vs no | .35 | .08 | 1.59 | .173 |
| Neoplasia: yes vs no | .64 | .19 | 2.26 | .495 |
| Previous diagnosis of depression: yes vs no | .12 | .03 | .41 | .001 |
| AIDS events: yes vs no | 1.02 | .50 | 2.07 | .963 |
| Interpheron therapy: yes vs no | 10.11 | 2.12 | 48.00 | .004 |
| Previous use of drugs: yes vs no | .84 | .45 | 1.58 | .588 |
| Previous use of alcohol: yes vs no | .24 | .06 | .94 | .040 |
| Adherence to ARV therapy | .99 | .88 | 1.11 | .832 |
| Mode of acquisition: other vs sexual | 1.70 | .49 | 5.85 | .398 |
| Mode of acquisition: drug use vs sexual | .80 | .41 | 1.54 | .503 |
| Family size: >=1 vs 0 | 1.33 | .50 | 3.56 | .572 |
| Therapy failure: 1 vs 0 | 1.54 | .62 | 3.81 | .352 |
| Therapy failure: >1 vs 0 | .87 | .42 | 1.80 | .704 |
| Therapy failure: unknown vs 0 | 2.43 | .82 | 8.23 | .152 |
| Use of drugs: yes vs no | 1.06 | .35 | 3.20 | .919 |
| Use of alcohol: yes vs no | 1.51 | .37 | 6.08 | .564 |
| Smoke: never smoker vs smoker | .65 | .32 | 1.31 | .228 |
| Smoke: ex-smoker vs smoker | 1.12 | .48 | 2.60 | .792 |
Table A5. Univariate analysis of factors associated with physician's discordance in assessing depression – Not-depressed patients according to CES-D.
| Modello logistico univariato | ||||
|---|---|---|---|---|
| Odds Ratio | [95% Conf. | Interval] | p>t | |
| Educational degree: high school vs middle school/elementary | 1.55 | .80 | 3.00 | .194 |
| Educational degree: university vs middle school/elementary | 1.28 | .55 | 2.94 | .566 |
| Educational degree: unknown vs middle school/elementary | 7.04 | 1.40 | 35.47 | .018 |
| Male vs female | 1.27 | .64 | 2.50 | .498 |
| Not/occasionally employed vs employed/self-employed | 1.70 | .70 | 4.13 | .241 |
| Other employed condition vs employed/self-employed | 2.65 | 1.24 | 5.68 | .012 |
| Duration of infection: 12–59 months vs <12 months | .30 | .07 | 1.20 | .088 |
| Duration of infection: 60–119 months vs <12 months | .25 | .06 | 1.09 | .065 |
| Duration of infection: >120 months vs <12 months | .40 | .11 | 1.48 | .167 |
| CD4: 200–350 cell/mm3 vs <200 cell/mm3 | .26 | .07 | .97 | .046 |
| CD4: 351–500 cell/mm3 vs <200 cell/mm3 | .38 | .11 | 1.29 | .120 |
| CD4: >500 cell/mm3 vs <200 cell/mm3 | .36 | .11 | 1.11 | .075 |
| RNA: 50–1000 copies/ml vs <50 copies/ml | .53 | .21 | 1.31 | .169 |
| RNA: >1000 copies/ml vs <50 copies/ml | 1.67 | .71 | 3.95 | .239 |
| HBV: yes vs no | 3.31 | 1.23 | 8.93 | .018 |
| HBV: unknown vs no | .27 | .05 | 1.48 | .130 |
| HCV: yes vs no | 2.49 | 1.23 | 5.04 | .011 |
| HCV: unknown vs no | .26 | .05 | 1.37 | .112 |
| Cirrhosis: yes vs no | 13.33 | 1.24 | 143.66 | .033 |
| Neoplasia: yes vs no | .30 | .07 | 1.36 | .119 |
| Previous diagnosis of depression: yes vs no | 7.97 | 2.16 | 29.18 | .002 |
| AIDS events: yes vs no | 1.07 | .54 | 2.12 | .838 |
| Interpheron therapy: yes vs no | 2.09 | .22 | 19.57 | .517 |
| Previous use of drugs: yes vs no | 1.49 | .72 | 3.05 | .280 |
| Previous use of alcohol: yes vs no | 2.26 | .50 | 10.26 | .289 |
| Adherence to ARV therapy | .93 | .84 | 1.03 | .156 |
| Mode of acquisition: other vs sexual | .83 | .24 | 2.87 | .770 |
| Mode of acquisition: drug use vs sexual | 1.65 | .77 | 3.53 | .197 |
| Family size: >=1 vs 0 | .83 | .24 | 2.87 | .768 |
| Therapy failure: 1 vs 0 | 3.59 | 1.45 | 8.87 | .006 |
| Therapy failure: >1 vs 0 | 1.16 | .56 | 2.40 | .693 |
| Therapy failure: unknown vs 0 | 1.89 | .71 | 5.04 | .203 |
| Use of drugs: yes vs no | 4.53 | .62 | 33.23 | .136 |
| Use of alcohol: yes vs no | 5.85 | .72 | 47.35 | .098 |
| Smoke: never smoker vs smoker | .92 | .48 | 1.76 | .807 |
| Smoke: ex-smoker vs smoker | .77 | .34 | 1.77 | .537 |
Disclosures statement
The study was entirely sponsored by AbbVie. The design and study conduct support for this study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication. F.M, G.G, A.M.C and U.d.L.P. are employees of AbbVie and may own AbbVie stocks or options. M.G. is a member of the Panel for the “Italian Guidelines for the Use of Antiretroviral Agents and the Diagnostic-clinical Management of HIV-1 infected Persons”. He has served on the Advisory Boards for Abbvie srl, BMS, Gilead, GSK, Janssen-Cilag, MSD, ViiV. A.A. has received honoraria for consultancy from Abbvie srl, Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen-Clilag, ViiV Healthcare, and has received research founding from Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare. A. A. is a member of the Panel for the “Italian Guidelines for the Use of Antiretroviral Agents and the Diagnostic-clinical Management of HIV-1 infected Persons”. She has served on the Advisory Boards for Abbvie, Merck, Janssen, and Gilead.
DHIVA Study Group
Figoni M., Divisione Malattie Infettive ad Alta Complessità 2, Azienda Ospedaliera D. Cotugno, Napoli;
Andreoni M., Maffongelli G., UOC di Malattie Infettive e Day Hospital Fondazione Policlinico Tor Vergata, Roma;
Mercuri A., Clinica Malattie Infettive Ospedale S. Maria della Misericordia, Perugia;
Carosi G., Motta D., Istituto Malattie Infettive e Tropicali Azienda Ospedaliera Spedali Civili di Brescia;
Cattelan A.M., Sasset L., SOC di Malattie Infettive Ospedale “Santa Maria della Misericordia”, Rovigo;
Cauda R., Ciccarelli N., Istituto di Clinica Malattie Infettive Università Cattolica, Roma;
Chirianni A., Sangiovanni V., III Divisione Malattie Infettive Azienda Ospedaliera D. Cotugno Napoli;
Colangeli V., Magistrelli E., U.O. di Malattie Infettive Azienda Ospedaliera Universitaria Policlinico S. Orsola Malpighi, Bologna;
Di Perri G., Trentini, L., Clinica Universitaria di Malattie Infettive Ospedale Amedeo di Savoia, Torino;
D'Arminio Monforte A., Comi L., Clinica Malattie Infettive Azienda Ospedaliera San Paolo, Milano;
Manzillo E., VIII Malattie Infettive Azienda Ospedaliera D. Cotugno, Napoli;
Lazzarin A., Fondazione San Raffaele del Monte Tabor di Milano;
Leoncini F., Martinelli C.V., SOD Malattie Infettive Azienda Ospedaliera Careggi, Firenze;
Blè C., Ospedale S. Maria Annunziata, Firenze;
Uglietti A., Clinica Malattie Infettive IRCCS Policlinico San Matteo, Pavia;
Montella F., Di Sora F., Dip.to delle Scienze Mediche U.O.C. Medicina 5 ad Indirizzo Immunologico, Roma;
Mughini M.T., Istituto Malattie Infettive ARNAS, A.O. Garibaldi di Nesima, Catania
Clinica Malattie Infettive Azienda Ospedaliera Universitaria di Sassari;
Narciso P., Bellagamba, IV Divisione Malattie Infettive IRCCS, Roma;
Parruti G., Vadini F., U.O Malattie Infettive e Tropicali, Pescara;
Rizzardini G., Capetti A., I°Divisione Malattie Infettive Ospedale L. Sacco di Milano;
Sighinolfi L., Segala D., U.O. Malattie Infettive Azienda Ospedaliero Universitaria di Ferrara
References
- Alciati A., Starace F., Scaramelli B., Campaniello M., Adriani B., Mellado C., Cargnel A. Has there been a decrease in the prevalence of mood disorders in HIV-seropositive individuals since the introduction of combination therapy? European Psychiatry. 2001;(8):491–496. doi: 10.1016/S0924-9338(01)00611-3. [DOI] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., … Wojna V. E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch S. M., Kilbourne A. M., Gifford A. L., Burnam M. A., Turner B., Shapiro M. F., Bozzette S. A. Underdiagnosis of depression in HIV. Journal of General Internal Medicine. 2003;(6):450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo M., Saggino A. Psicoterapia Cognitiva e Comportamentale. 2007. Test per l'assessment della depressione nel contesto italiano: Un'analisi critica [Tests for the assessment of depression in Italian context: A critical review] Retrieved from: http://www.researchgate.net/ [Google Scholar]
- Benton T. D. Depression and HIV/AIDS. Current Psychiatry Reports. 2008;(3):280–285. doi: 10.1007/s11920-008-0045-y. [DOI] [PubMed] [Google Scholar]
- Bravo P., Edwards A., Rollnick S., Elwyn G. Tough decisions faced by people living with HIV: A literature review of psychosocial problems. AIDS Reviews. 2010;(2):76–88. Retrieved from: http://www.aidsreviews.com. [PubMed] [Google Scholar]
- Briongos-figuero L. S., Bachiller-Luque P., Palacios-Martin T., De Luis-Román D., Eiros-Bouza J. M. Depression and health related quality of life among HIV-infected people. European Review for Medical and Pharmacological Sciences. 2011;(8):855–862. Retrieved from: http://www.europeanreview.org. [PubMed] [Google Scholar]
- Ciesla J. A., Roberts J. E. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;(5):725–730. doi: 10.1176/appi.ajp.158.5.725. Retrieved from: http://ajp.psychiatryonline.org/doi/full/10.1176/appi.ajp.158.5.725#_i8. [DOI] [PubMed] [Google Scholar]
- Cohen J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychological Bulletin. 1968;(4):213–220. doi: 10.1037/h0026256. Retrieved from: http://dx.doi.org/10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Grey D., Burke J., Cohen M. H., Gurtman A. C., Richardson J. L., … Hessol N. A. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. American Journal of Public Health. 2004;(7):1133–1140. doi: 10.2105/AJPH.94.7.1133. PMCID: PMC1448411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote S., Vogelaers D. P., Vermeir P., Mariman A., De Rick A., Van Der Gucht B., … Vandijck D. M. Socio-economic, behavioural,(neuro) psychological and clinical determinants of HRQoL in people living with HIV in Belgium: A pilot study. Journal of the International AIDS Society. 2013;(1):18643–189651. doi: 10.7448/IAS.16.1.18643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaihy A., Singh N. Factors affecting quality of life in patients with HIV infection. The AIDS Reader. 2001;(9):450–4. PMID: 11682918 Retrieved from: http://www.europepmc.org. [PubMed] [Google Scholar]
- Estratto notiziario Istituto Superiore di Sanità - Volume 26 – N.7–8 July–August . 2013. ISSN 0394–9303. [Google Scholar]
- Gelenberg A. J. Using assessment tools to screen for, diagnose, and treat major depressive disorder in clinical practice. The Journal of Clinical Psychiatry. 2009:e01–e01. doi: 10.4088/JCP.9058se1c.01gry. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. S., Batchelder A. W., Psaros C., Safren S. A. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2011;(2):721–728. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard J. L., Munjas B. A., Adams J. L., Suttorp M., Maglione M., McGlynn E. A., Gellad W. F. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. Journal of General Internal Medicine. 2011;(10):1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E. R., Crum R. M., Treisman G. J., Mehta S. H., Atkinson J. H., Clifford D. B., … McArthur J. C. 2013. Major depressive disorder in persons with HIV is associated with new-onset of cerebrospinal fluid viral escape. Poster presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2013). Denver, September 10–13, 2013. (Abstract H-1257. ) [Google Scholar]
- Hartzell J. D., Janke I. E., Weintrob A. C. Impact of depression on HIV outcomes in the HAART era. Journal of Antimicrobial Chemotherapy. 2008;(2):246–255. doi: 10.1093/jac/dkn193. [DOI] [PubMed] [Google Scholar]
- Horberg M. A., Silverberg M. J., Hurley L. B., Towner W. J., Klein D. B., Bersoff-Matcha S., … Kovach D. A. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2008;(3):384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- Ickovics J. R., Hamburger M. E., Vlahov D., Schoenbaum E. E., Schuman P., Boland R. J. HIV Epidemiology Research Study Group Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Prentiss D. E., Lubega S., Balmas G., Garcia P., Muhammad M., … Koopman C. Psychiatric co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007;(2):220–225. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]
- Kacanek D., Jacobson D. L., Spiegelman D., Wanke C., Isaac R., Wilson I. B. Incident depression symptoms are associated with poorer HAART adherence: A longitudinal analysis from the nutrition for healthy living (NFHL) study. Journal of Acquired Immune Deficiency Syndromes (1999) 2010;(2):291–300. doi: 10.1097/QAI.0b013e3181b720e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebber A. M. H., Buffart L. M., Kleijn G., Riepma I. C., Bree R., Leemans C. R., … Verdonck-de Leeuw I. M. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology. 2014;(2):121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello V. A., Segurado A. A., Malbergier A. Depression in women living with HIV: Clinical and psychosocial correlates. Archives of Women's Mental Health. 2010;(3):193–199. doi: 10.1007/s00737-009-0094-1. [DOI] [PubMed] [Google Scholar]
- Mells G. F., Pells G., Newton J. L., Bathgate A. J., Burroughs A. K., Heneghan M. A., … Jones D. E. Impact of primary biliary cirrhosis on perceived quality of life: The UK-PBC national study. Hepatology. 2013;(1):273–283. doi: 10.1002/hep.26365. [DOI] [PubMed] [Google Scholar]
- Nel A., Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS Care. 2011;(11):1360–1365. doi: 10.1080/09540121.2011.565025. [DOI] [PubMed] [Google Scholar]
- Pence B. W., Miller W. C., Gaynes B. N., Eron J. J., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2007;(2):159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- Penzak S. R., Reddy Y. S., Grimsley S. R. Depression in patients with HIV infection. American Journal of Health System Pharmacy. 2000;(4):376–389. doi: 10.1093/ajhp/57.4.376. Retrieved from hawaii.edu. [DOI] [PubMed] [Google Scholar]
- Psaros C., O'Cleirigh C., Bullis J. R., Markowitz S. M., Safren S. A. The influence of psychological variables on health-related quality of life among HIV-positive individuals with a history of intravenous drug use. Journal of Psychoactive Drugs. 2013;(4):304–312. doi: 10.1080/02791072.2013.825030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin J. G. HIV and depression: 2008 review and update. Current HIV/AIDS Reports. 2008;(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Radloff L. S. The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raison C. L., Afdhal N. H., Silver J. M., Solomon D. 2006. Neuropsychiatric side effects associated with interferon-alfa plus ribavirin therapy: Recognition and risk factors. UpToDate, Rose, B.D. (Ed.), UpToDate, Waltham, MA. Retrieved from: www.uptodate.com. [Google Scholar]
- Rodkjaer L., Laursen T., Balle N., Sodemann M. Depression in patients with HIV is under-diagnosed: A cross-sectional study in Denmark. HIV Medicine. 2010;(1):46–53. doi: 10.1111/j.1468-1293.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- Sagnelli E., Stroffolini T., Mele A., Almasio P., Coppola N., Ferrigno L., … Filippini P. The importance of HCV on the burden of chronic liver disease in Italy: A multicenter prevalence study of 9,997 cases. Journal of Medical Virology. 2005;(4):522–527. doi: 10.1002/jmv.20313. [DOI] [PubMed] [Google Scholar]
- Sagnelli E., Stroffolini T., Mele A., Imparato M., Almasio P. L. Chronic hepatitis B in Italy: New features of an old disease—approaching the universal prevalence of hepatitis B e antigen—negative cases and the eradication of hepatitis D infection. Clinical Infectious Diseases. 2008;(1):110–113. doi: 10.1086/524074. [DOI] [PubMed] [Google Scholar]
- Scandlyn J. When AIDS became a chronic disease. Western Journal of Medicine. 2000;(2):130–133. doi: 10.1136/ewjm.172.2.130. PMCID: PMC1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin N. L., DiMatteo M. R. Depression treatment enhances adherence to antiretroviral therapy: A meta-analysis. Annals of Behavioral Medicine. 2014;(3):259–269. doi: 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs B. D., de Jonge P., Coyne J. C., Whooley M. A., Frasure-Smith N., Mitchell A. J., … Ziegelstein R. C. Depression screening and patient outcomes in cardiovascular care: A systematic review. JAMA. 2008;(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- Weiss J. J., Morgello S. Psychiatric management of HIV/HCV-coinfected patients beginning treatment for hepatitis C virus infection: Survey of provider practices. General Hospital Psychiatry. 2009;(6):531–537. doi: 10.1016/j.genhosppsych.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


