Abstract
Introduction: Oncolytic viruses are experimental cancer therapies being translated to the clinic. They are unique in their ability to amplify within the body, therefore requiring careful monitoring of viral replication and biodistribution. Traditional monitoring strategies fail to recapitulate the dynamic nature of oncolytic virotherapy. Consequently, clinically relevant, noninvasive, high resolution strategies are needed to effectively track virotherapy in real time.
Areas covered: The expression of the sodium iodide symporter (NIS) reporter gene is tightly coupled to viral genome replication and mediates radioisotope concentration, allowing noninvasive molecular nuclear imaging of active viral infection with high resolution. This provides insight into replication kinetics, biodistribution, the impact of vector design, administration, and dosing on therapeutic outcomes, and highlights the heterogeneity of spatial distribution and temporal evolution of infection. NIS-mediated imaging in clinical trials confirms the feasibility of this technology to noninvasively and longitudinally observe oncolytic virus infection, replication, and distribution.
Expert opinion: NIS-mediated imaging provides detailed functional and molecular information on the evolution of oncolytic virus infection in living animals. The use of NIS reporter gene imaging has rapidly advanced to provide unparalleled insight into the spatial and temporal context of oncolytic infection which will be integral to optimization of oncolytic treatment strategies.
Keywords: molecular imaging, NIS, oncolytic virus, reporter gene
Article Highlights.
The expression of the sodium iodide symporter (NIS) reporter transgene and subsequent radiotracer concentration can be coupled with viral replication and the oncolytic life cycle.
NIS-mediated imaging allows noninvasive tracking of the spatiotemporal context of viral distribution and replication.
Advances in nuclear molecular imaging systems allow for very detailed and high-resolution imaging and quantitative analysis of intratumoral infection.
NIS-mediated imaging provides quantitative means for noninvasively analyzing oncolytic virus delivery, replication and biodistribution, and provides insight into the effects of genomic design, administration route, viral dose, tumor target and barriers to therapeutic success.
Oncolytic viruses encoding the NIS transgene are successfully being used to monitor oncolytic virus infection in the clinic.
NIS-mediated imaging can be used to identify barriers to oncolytic success and guide oncolytic virotherapy optimizations.
This box summarizes key points contained in the article.
1. Introduction
With the continued need for new and improved cancer treatments, oncolytic virotherapies (OV) are emerging as efficacious and feasible experimental therapeutics. Oncolytic virotherapies are biological therapies that employ viruses with engineered or evolved preferential tumor tropism to induce tumor cell death and cross-prime antitumor immunological responses to effectively clear tumors.[1] There are a number of viruses currently undergoing clinical translation, including adenovirus (Ad), herpes simplex virus (HSV), measles virus (MV), vesicular stomatitis virus (VSV) and vaccinia virus (VV).[2] As an experimental therapy, and especially relevant with replication competent viruses in which the mechanism of action includes amplification at sites of tumor growth in contrast to conventional drug therapies, there is a heightened need for continual pharmacologic monitoring to track pharmacokinetics, safety, efficacy and toxicity associated with OV treatment.[3] With OV, it is necessary to understand evolution of infection with changing biodistribution and kinetics of active and spreading centers of infection. Comprehensive pharmacokinetic monitoring of replication-competent virotherapy therefore requires the ability to observe where virus particles go, how many target cells get infected, if the viral genome is expressed and to what extent, how long infection and replication occur, and if viral infection spreads or progeny are released.[3]
Methods used to monitor pharmacokinetics, safety and toxicity of OV in the preclinical and clinical settings include biopsy and histopathological and molecular analysis to observe infection, immune infiltrates and tissue damage. The collection of blood and tissue samples allows for monitoring of viral shedding, infectious viral recovery and viral genome copies to address safety and replication. Clinical observation is used to identify and follow adverse events. Clinically, biopsy is the gold standard for monitoring the therapeutic effect of viral oncolysis; however, biopsy typically samples a small portion of the entire tumor or target organ with a high probability of missing vector persistence or yielding only partial information about infection at a single time point.[4,5] For instance, in organs such as the prostate, a single needle biopsy captures less than 1% of the total tissue being surveyed. Therefore, these monitoring methods can be invasive, are prone to sampling error, and are limited in their ability to comprehensively relay the location, magnitude, persistence and dynamic nature of viral infection.[6,7]
Improved pharmacologic monitoring will not only alleviate problems with current monitoring methods but also has the potential to be used to address current barriers to therapeutic success of OV. There is variability in preclinical and clinical therapeutic response to OV.[2,8] Understanding the cause for this variability is paramount to optimizing current treatment strategies and will require the ability to correlate therapeutic outcomes with in vivo distribution and replication. Gene therapy approaches, and arguably OV approaches, have fallen short of clinical expectations largely due to limited ability to efficiently transduce tumors. This has been called the fundamental barrier to effective cancer gene therapy.[9,10] Similarly, limited success with oncolytic viruses has been tied to the inability to adequately deliver and initiate sufficient amounts of infection within tumor targets.[8] Noninvasive monitoring techniques will assist in the understanding of delivery and transduction-related barriers necessary for improving therapeutic success of cancer gene therapy and virotherapy approaches.
A noninvasive strategy that allows for serial monitoring of infection can be informative of virotherapy pharmacokinetics and pharmacodynamics and can help to elucidate barriers to successful treatment. These needs can be met by engineering the virus to express reporter genes to facilitate noninvasive molecular imaging at sites of infection. Molecular imaging, defined as the ability to visualize and quantitatively measure the function of biological and cellular processes noninvasively, provides critical information on transduction efficiency, duration and distribution of gene expression with high sensitivity even in deep tissue using localization and detection of nuclear radionuclide tracers.[11,12] Reporter transgenes used for deep-tissue nuclear molecular imaging of OV include enzymes like herpes virus thymidine kinase, receptors such as the dopamine-2 receptor (D2R) and somatostatin receptor 2 (hSSRT2), and transporters such as the human norepinephrine transporter (hNET) and the sodium iodide symporter (NIS).[13] The use of NIS offers potential advantages above other reporter gene systems including the ability to reflect cell viability as its concentrative function is lost with cell apoptosis while enzymes and receptors may still retain detectable function.[14] In contrast to receptor-based reporters like hSSTR2 with stoichiometric-binding relationships, transporters like NIS provide signal amplification through transport-mediated concentrative intracellular accumulation of substrate for improved sensitivity of detection.[15] Additionally, unlike all other radiotracer-based reporter gene systems, NIS is able to concentrate carrier-free radiotracers for convenient use. Therefore, we focus on the utility of in vivo NIS-mediated nuclear molecular imaging to increase our current understanding of OV and discuss how NIS-mediated imaging can be used to improve oncolytic treatment strategies.
1.1. NIS as a reporter gene
The sodium iodide symporter is a 643 amino acid transmembrane glycoprotein that allows iodide uptake and concentration for organification in the thyroid and is also expressed in extrathyroidal tissue including the salivary gland, gastric mucosa and mammary gland.[16–18] Due to its ability to concentrate iodide, NIS has been used for more than 70 years in the detection and treatment of thyroid disorders, demonstrating clinical versatility and practicality of NIS-mediated iodide uptake.[19] The cloning of the NIS gene made it possible to concentrate iodide in other tissue types that do not normally express NIS. The ectopic expression of NIS has been shown to allow radioiodide accumulation at or above levels of thyroid cells without interfering with basic cellular biochemistry.[20–22] This widens the scope of NIS-mediated radiotherapy and imaging beyond the thyroid. The genomic size of the NIS cDNA, 1929 nucleotides, allows insertion into many different oncolytic virus vectors to be used for reporter gene functions. Functional NIS facilitates the concentration of iodide as well as gamma-emitting radioisotopes of iodide (123I–, 124I–, 125I–, 131I–), tetrafluoroborate ([F18]BF4 –) or technetium in the form of anionic pertechnetate (99m TcO4 −), hereafter collectively referred to as radiotracers, that are both readily available and clinically approved for nuclear imaging applications. Imaging modalities such as gamma cameras, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) detect areas of radiotracer concentration that can be visualized and quantified. When used in parallel with X-ray computed tomography (CT), the detailed anatomical context of NIS-expressing infected cells can be seen (Figure 1). Table 1 shows the available radiotracers for NIS-mediated imaging along with their clinically relevant half-lives and energy emissions with each instrument, demonstrating the preclinical and clinical versatility of this tool.
Figure 1.
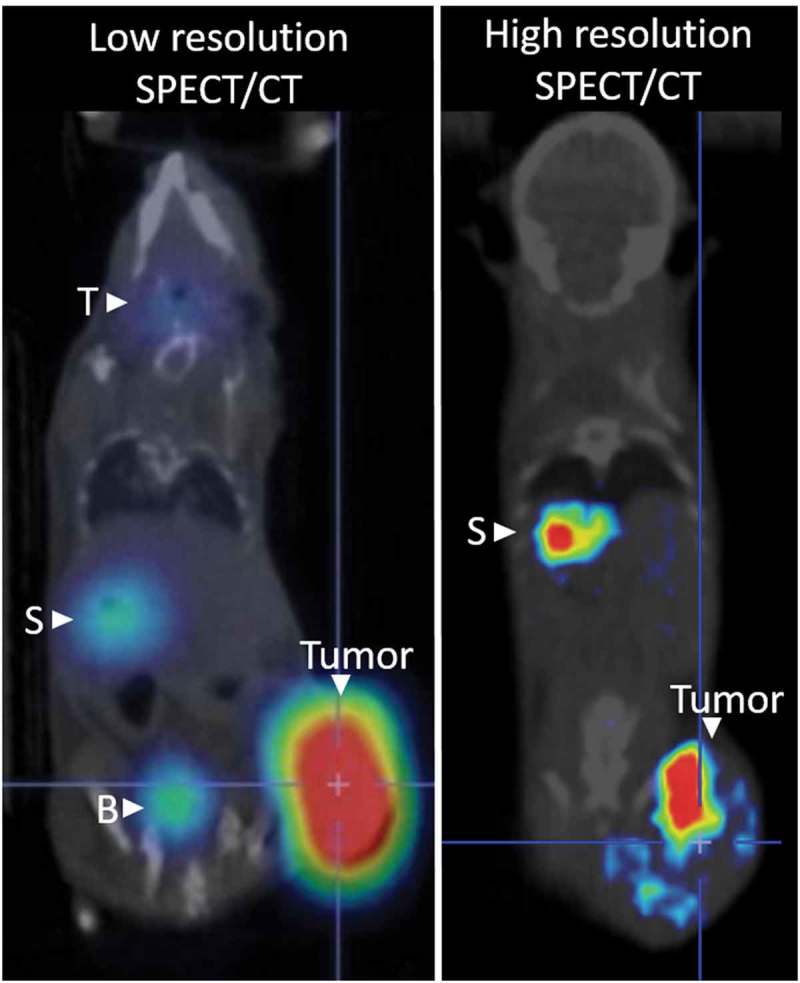
Representative whole body nuclear imaging of mice infected with oncolytic virus expressing NIS following NIS-mediated uptake of radiotracer. The thyroid (T), stomach (S) and bladder (B) can be detected due to endogenous NIS expression and radiotracer excretion. Low-resolution SPECT/CT imaging clearly identifies intratumoral radiotracer uptake. High-resolution SPECT/CT imaging allows for spatial resolution of intratumoral infected centers.
Table 1.
Available radiotracers and corresponding half-lives, decay mode and major γ emission energy for NIS-mediated imaging with SPECT of PET instrumentation.
| Imaging modality | Radiotracer | Half-life | Decay mode | Major γ emission energy (MeV) |
|---|---|---|---|---|
| SPECT | 123I | 13.2 h | EC | 0.159 |
| 125I | 59.4 d | EC | 0.027 | |
| 131I | 8.0 d | β− | 0.364 | |
| 99mTc | 6.0 h | IT | 0.140 | |
| 188Re | 17.0 h | β− | 0.155 | |
| PET | 124I | 4.2 d | β+ | 0.511 |
| 18F | 109.8 m | β+ | 0.511 |
Major γ emission energy is the mean energy with the greatest percentage of emission.
Created with data taken from [81].
d: day; h: hour; m: minute; β−: beta decay; β+: positron decay; EC: electron capture; IT: isomeric transition; SPECT: single-photon emission computed tomography; PET: positron emission tomography.
Transient and stable NIS expression capable of concentrating significant iodide uptake in vitro and in vivo allowing for imaging with planar, SPECT and PET techniques has been demonstrated in multiple tumor types, confirming the ability to use NIS as a functional reporter gene to monitor intratumoral infection (Figure 1).[20–29] Additionally, NIS-mediated iodide uptake can be used to enhance tumor cell death and bystander killing induced by oncolytic viruses by concentrating beta-emitting radioisotopes for what has been termed radiovirotherapy. Beta-emitting isotopes such as 131I not only lead to DNA damage but also enhance viral uptake, viral gene expression and viral replication.[30] The use of NIS-expressing viruses to combine oncolytic and radiation-induced cell damage has been well studied and shown to enhance oncolytic efficacy preclinically.[14,22,23,26,28,31–45] The strengths and weaknesses of this approach are further reviewed by Touchefeu et al.[30] Although the radiovirotherapy application of NIS as a therapeutic gene is outside the scope of this review in which we focus on the role of NIS as a reporter gene, it is worth mentioning as the lessons learned from NIS-mediated molecular imaging of OV will allow for optimization of radiovirotherapy applications as well. Here we discuss how the utility of NIS imaging renders it a highly relevant reporter gene to monitor the fate of oncolytic infection.
2. Detection of viral replication and biodistribution
2.1. Replication
As viral gene expression is highly regulated, it is possible to monitor pharmacokinetics of infection based on the expression of transgenes carried by the replicating virus.[13] Therefore, monitoring of intratumoral infection using NIS is possible because NIS transgene expression is tightly coupled with viral genome translation and replication. Radiotracer uptake can then be noninvasively monitored to reflect NIS expression, serving as a surrogate for viral genome amplification and gene expression. This correlation has been repeatedly confirmed through autoradiography and immunohistochemistry (IHC) analysis showing coincidence of NIS expression, viral infection, and ex vivo radiotracer concentration, correlation of viral protein expression and in vitro radiotracer uptake activity levels, and quantification of NIS gene and viral genome copy numbers.[46–52]
Changes in radiotracer concentration detected with serial noninvasive imaging will allow for real-time tracking of infection progression in vivo. Figure 2 shows how functional NIS expression will correlate with the longevity of viral gene expression, allowing tracking of radiotracer uptake throughout the life cycle of viral infection within a tumor. Prior to maximum radiotracer uptake during initial infection, the NIS gene needs to be translated and the protein translocated and inserted into the cell membrane to form a functional transporter allowing for minimal radiotracer uptake. Active replication corresponds with strong uptake intensity that may increase with spread of infection or plateau with persistence of infection. After maximum radiotracer uptake during the late prelytic phase of infection, NIS transporter function could be impaired, and following cell oncolysis, radiotracer concentration would be lost. Therefore, serial imaging will allow observation of the kinetics of oncolytic infection and the status of viral replication. In example, IHC analysis of tumors explanted following serial monitoring of NIS-mediated radiotracer uptake in animals bearing pancreatic tumors infected with oncolytic vaccinia virus encoding NIS showed that successful radiotracer uptake was located in regions where adequate blood flow, viable tissue and viral proteins were present. A loss of uptake signal intensity correlated with tumor death and necrosis.[53] Similarly, while NIS was detectable with IHC analysis in tumors harvested d1, d2 and d3 following infection using measles virus encoding NIS, radiotracer uptake was only detected with SPECT/CT d1 and d2. This corresponds with IHC showing NIS localized at the cell membrane of intact cells only on d1 and d2. By d3, IHC showed a diffuse signal for NIS protein within areas of cell death that correlated with the inability to detect radiotracer uptake with SPECT/CT, as only healthy cells are capable of concentrating the radiotracer.[40]
Figure 2.
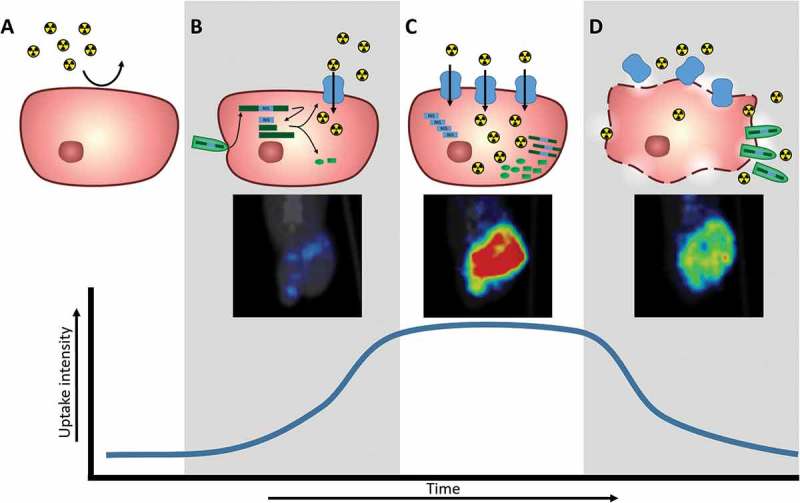
Schematic of NIS expression at the cellular level and corresponding NIS-mediated radiotracer uptake detected and quantified with nuclear imaging techniques. A. Tumor cell does not naturally express NIS and therefore does not concentrate radiotracers. B. Upon viral infection, the viral genome (green) will be replicated and viral genes, along with transgenes (blue), will be transcribed and translated into functional proteins. Expression of the NIS transgene (blue) leads to production of NIS protein that allows radiotracer uptake, corresponding with nuclear imaging detection. C. As viral replication continues, there is increased expression of viral genes and transgenes, leading to increased NIS protein for increased radiotracer uptake detected by nuclear imaging. D. As viral proteins continue to be produced, viral particles are assembled and exit the cell through budding or lysis. Cytopathic effects on cell cause loss of radiotracer uptake capabilities, corresponding to decrease in nuclear imaging detection.
Beyond dynamic monitoring of gene and viral persistence, serial monitoring of NIS-mediated uptake can distinguish between replicating and non-replicating vectors. In prostate cancer using replicating and non-replicating Ad expressing NIS, both vectors mediated similar uptake d1, but by d2 increased uptake was seen in animals treated with replication-competent vectors that continued to increase with time while uptake detected in animals treated with the non-replicating virus did not increase and was eventually undetectable.[46] Persistence of replication can be monitored by sustained uptake activity. Persistence of replication out to 14 days was detected and did not result in accumulation of loss-of-function mutations within the NIS transgene in MV encoding NIS.[51] With a conditionally replicating adenovirus in a prostate tumor model, strong and sustained NIS-mediated radiotracer uptake confirmed the ability to detect intratumoral viral persistence 1 month after virus administration.[44] Table 2 presents a detailed list of preclinical and clinical studies performed using nuclear imaging techniques to detect viral infection noninvasively as well as follow the kinetics of viral replication when noted. The use of NIS-mediated imaging has shown that the kinetics of in vivo viral replication is variable and dependent on virus, tumor, dose and administration route (Table 2).
Table 2.
Table of oncolytic viruses expressing the NIS transgene to mediate radiotracer uptake for in vivo nuclear imaging monitoring of infection, separated by oncolytic virus and tumor type.**
| Virus |
Recombinant |
Tumor type |
Route |
In vivo imaging modality |
In vivo replication tracking |
Ref. |
|
|---|---|---|---|---|---|---|---|
| Radiotracer |
Instrument |
||||||
| Preclinical | |||||||
| ADENOVIRUS | |||||||
| Ad-CMV-hNIS9, Ad-CMV-hNIS (rep-) | glioma | IT | 99mTcO4− | gamma camera scintigraphy | [49] | ||
| Ad-CMV-rNIS (AdNIS)(rep-) | mammary, cervical | IT | 123I | gamma camera scintigraphy | [62] | ||
| Ad-CMV-hNIS (rep-) | prostate | IT [28], intra- prostatic [57] | 123I | ] | [28,57] | ||
| pancreatic | IT, IV | 124I | PET | [25] | |||
| hepatocellular carcinoma | Intra-portal | 123I, 131I | gamma camera scintigraphy | [68] | |||
| Ad5-yCD/mutTK(SR39)rep-hNIS (rep+) | prostate | IT | 99mTcO4− | gamma camera scintigraphy | peak d5, undetectable by d7 | [46] | |
| Ad-MUC1-hNIS (rep-) | breast | IT | 123I | gamma camera scintigraphy | [33] | ||
| pancreatic | IT, IV | 123I | gamma camera scintigraphy | [34] | |||
| ovarian | IT | 123I | gamma camera scintigraphy | [35] | |||
| Ad-MUC1-NIS (3ʹ minimal MUC1 promoter) (rep-) | pancreatic cancer | IT | 123I | gamma camera scintigraphy | [31] | ||
| AdIP1-NIS (rep+) | colon | IT | 99mTcO4− | SPECT/CT | positive d1, peak d2, undetectable by d5 | [48] | |
| AdAM6-NIS (CR) | colon | IT | 99mTcO4− | SPECT/CT | positive d1, peak d3, undetectable by d5 | [48] | |
| Ad5-CEA-NIS (rep-) | medullary thyroid | IT | 123I | gamma camera scintigraphy | [43] | ||
| AdSERE (rep-) | breast | IT | 99mTcO4− | SPECT/CT | [14] | ||
| AdIP2 (CR) | colorectal | IT, IV | 99mTcO4− | SPECT/CT | positive d1, peak d2, undetectable by d3 | [40] | |
| AdPLEN (rep-) | hepatocellular carcinoma | IT | 131I | gamma camera scintigraphy | [58] | ||
| Ad5AMUCH_RSV-NIS (CR) | breast | IT | 123I | gamma camera scintigraphy | [59] | ||
| Ad5/3-∆24-hNIS (CR) | prostate | IT | 123I | gamma camera scintigraphy | [38] | ||
| Ad Ad5PB_RSV-NIS (CR) | prostate | IT | 99mTcO4− | SPECT/CT | positive d1, persistence at 1mo | [44] | |
| AdAM7 (rep+) | peritoneal (ovarian) | IP | 99mTcO4− | SPECT/CT | positive d1, peak d2, undetectable by d8 | [82] | |
| Ad5-AFP-NIS (rep-) |
hepatocellular carcinoma | IT | 123I, 188Re | gamma camera scintigraphy | [83] | ||
| Ad-SUR-NIS (rep-) |
prostate | IT | 99mTcO4− | gamma camera scintigraphy | [84] | ||
| Ad5/3-hTERT-hNIS (CR) | prostate | IT | 123I | gamma camera scintigraphy, SPECT/CT | [70] | ||
| Ad5/3PB-ADP-hNIS, Ad5/3PB-hNIS, Ad5PB-RSV-hNIS (CR) | prostate | IT | 99mTcO4− | SPECT/CT | virus dependent; positive d1, persistence at d14 | [85] | |
| Ad5/3-ADP-hNIS, Ad5/3-hNIS, Ad5-ADP-hNIS, Ad5-hNIS (rep+) | lung adenocarcinoma | IT | 99mTcO4− | SPECT/CT | virus dependent; positive d1, peak or plateau d4 | [86] | |
| Ad5-E1/AFP-E3/NIS (CR) | hepatocellular carcinoma | IT | 123I | gamma camera scintigraphy | peak d3 | [87] | |
| HERPES SIMPLEX VIRUS | |||||||
| HSV-NIS | prostate | IT, IV | 99mTcO4− | SPECT/CT | administration route dependent; positive d3, peak d8, undetectable by d15 | [88] | |
| MEASLES VIRUS | |||||||
| MV-NIS | myeloma | IV | 123I [24,47,89] 124I [24] | ] | [24,47,89] | ||
| ovarian | IT, IV | 99mTcO4− | gamma camera scintigraphy | [26] | |||
| hepatocellular carcinoma | IV | 123I | gamma camera scintigraphy | peak between d7 and d10 | [45] | ||
| pancreatic | IT, IV | 123I [7,41,51,64], 99mTcO4− [90] | gamma camera scintigraphy (planar SPECT) [7,41,64], SPECT/CT [7,41,51,64,90] | peak d2, undetectable by d8 [7] variable among mice, peak between d2 and d6, undetectable by d9 [41] |
[7,41,51,64,90] | ||
| prostate | IT, IV | 123I | gamma camera scintigraphy | administration route dependent; peak d4 (IT), d14 (IV), persistence at d36 (IV) | [39] | ||
| medullo-blastoma | IT | 131I | bioluminescent (Cherenkov) imaging | [91] | |||
| glioblastoma | IT | 123I, 99mTcO4− | gamma camera scintigraphy | peak d3, persistence at d20 | [92] | ||
| squamous cell carcinoma | IT | 125I | SPECT/CT | peak d3 | [93] | ||
| anaplastic thyroid | IT | 99mTcO4- | SPECT/CT | peak d3, persistence at d22 | [94] | ||
| osteosarcoma | IT, IV |
99mTcO4−, Na [18F]BF4 |
SPECT/CT, PET/CT | [95] | |||
| malignant peripheral nerve sheath | IT | 125I | SPECT/CT | [96] | |||
| MV-IFNβ-NIS | mesothelioma | IT | 125I | SPECT/CT | [56] | ||
| VESICULAR STOMATITIS VIRUS | |||||||
| VSV-∆51-NIS | myeloma | IT, IV | 123I | gamma camera scintigraphy | [37] | ||
| VSV-IFNβ-NIS | myeloma | IV | 99mTcO4− | SPECT/CT | ] | [50, 63] | |
| VACCINIA VIRUS | |||||||
| GLV-1h153 | pancreatic | IT,IV | 124I [15,53,69], 99mTcO4− [53,69] | PET [15,53,69], PET/CT [53,69], gamma camera scintigraphy [53,69] | ] | [15, 53, 69] | |
| anaplastic thyroid | IT | 99mTcO4- | gamma camera scintigraphy | [97] | |||
| malignant pleural mesothelioma | IP | 131I | SPECT/CT | [79] | |||
| breast | IT, resection wound | 124I | PET | [36,66] | |||
| gastric | IT | 124I, 99mTcO4- | PET, gamma camera scintigraphy | [98] | |||
| colorectal peritoneal carcinomatosis | IP | 131I | SPECT/CT | [99] | |||
| VV-NIS-C and VV-NIS-W | endometrial | IV | 125I | SPECT/CT | tumor model and virus strain dependent; persistence at d21 | [72] | |
| CLINICAL | |||||||
| ADENOVIRUS | |||||||
| Ad5-yCD/utTK(SR39) rep-hNIS | prostate | intra-prostatic | 99mTcO4− | SPECT/CT | patient dependent; peak d1–d2, undetectable by d4–d8 | [76] | |
| Ad5/3-∆24-hNIS | metastatic cervical carcinoma | IT | 123I, 99mTcO4− | SPECT/CT | no detection | [74] | |
| Measles Virus | |||||||
| MV-NIS | myeloma | IV | 123I | SPECT/CT | patient and plasmacytoma dependent; undetectable by d28 | [55] | |
| ovarian cancer | IP | 123I | SPECT/CT | patient dependent | [77] | ||
Virus structure, tumor target, administration route and nuclear imaging radiotracer and instrument used are noted. The use of NIS-mediated imaging to track kinetics of infection is noted when applicable.
Ad: adenovirus; CR: conditionally replicative; d: day; HSV: herpes simplex virus; IP: intraperitoneal; IT: intratumoral; IV: intravenous; MV: measles virus; rep−: replication deficient; rep+: replication competent; VSV: vesicular stomatitis virus; VV: vaccinia virus; CMV: cytomegalovirus.
2.2. Biodistribution
NIS-mediated imaging can be used to detect areas of radiotracer concentration throughout the body. Radiotracer is naturally concentrated in the thyroid, stomach, lactating mammary glands and salivary glands due to endogenous expression, and can also be detected in the kidney and bladder due to their roles in excretion. Beyond this, NIS-mediated uptake is localized to sites of viral infection so that NIS-mediated imaging is a useful tool to look at distribution of viral infection throughout the whole body. NIS-mediated imaging can demonstrate tumor specificity of infection and lack of off-target infection or viral dissemination to sites with close anatomical proximity. Specificity of intratumoral infection has been demonstrated using NIS-mediated imaging for many of the replicating oncolytic viruses in Table 2, including the ability to detect specificity of systemically administered virus infection within tumors. The rapid advances in three-dimensional nuclear imaging instrumentation and co-registration with CT allow increased spatial resolution and the ability to separate organs that naturally concentrate radiotracer and organs of interest, such as the liver or pancreas in close proximity to the stomach, and the prostate in close proximity to the bladder.[7, 34, 46] For example, NIS-mediated imaging was able to distinguish infection within the canine prostate despite close proximity to the bladder following direct intraprostatic injection of replication-competent Ad encoding NIS.[46] The use of contrast agents can further increase the ability to differentiate organs by blocking endogenous uptake prior to administration of the radiotracer.[54]
The use of systemically administered viruses also widens the applicability of NIS-mediated imaging beyond tracking infection in single tumors to imaging treatment of widespread disease.[55] Systemically administered tumor-selective viruses encoding the NIS-transgene can also be used for exploratory localization of tumors and metastatic lesions. A related application of this technology has been used to detect positive surgical margins after tumor resection.[56] Imaging of biodistribution can be used to detect tumors susceptible to OV and to inform vector design for targeted therapies. For example, vectors designed for targeting specific cancers such as mucin 1 (MUC1)-, carcinoembryonic antigen (CEA)- and alpha-fetoprotein (AFP)-expressing, estrogen positive, and androgen positive tumor cells have benefited from the use of NIS imaging to verify targeted infection via noninvasive imaging of radiotracer uptake.[14,31,33–35,43,44,57–59] The use of targeted vectors in combination with NIS-mediated imaging may be a useful tool for diagnostic tumor genotyping or predicting patient response.
2.3. Sensitivity and quantification
Our ability to use nuclear imaging to comprehensively monitor oncolytic infection is dependent on the sensitivity and resolution of the available imaging technologies to resolve radiotracer uptake at the cellular level. SPECT imaging uses gamma cameras to detect emitted photons, but the signal is degraded due to multiple factors including intrinsic abilities of the detector, geometric limitations imposed by collimation, and scatter of photons within tissue and the collimator producing volumetric images with clinical resolution of ~7.5–10 mm.[42,60] To meet demands for superior 3D images, manufacturers of small-animal imaging instruments such as the most current U-SPECT II system (MILabs) are making machines with increased resolution such that spatial resolution less than 0.25 mm can be achieved.[61] The use of PET imaging, which detects coincident photons resulting from positron-electron annihilation reactions without the need for collimation, allows for increased clinical resolution of 2–5 mm.[60] Current small-animal PET detectors are reported to have resolution approaching 1 mm. Fortunately, NIS reporter gene imaging can either be used with SPECT or PET radiotracers, giving investigators numerous options depending on availability of instrumentation, necessary resolution or sensitivity (Table 3). Figure 1 shows the benefits of increasing resolution achieved by advancing imaging technologies to detect intratumoral infection from a whole body scan, showing the lowest resolution from early gamma scintigraphy detection, enhanced detection with SPECT/CT or PET/CT systems, and finally highest resolution that allows for intratumoral infectious centers to be resolved with the newest small animal SPECT/CT technologies.
Table 3.
Resolution and sensitivity of imaging instruments used to detect oncolytic virus replication via NIS mediated radiotracer uptake.
| Instrument | Minimum resolution (mm) | Sensitivity at minimum resolution (%) | Reference |
|---|---|---|---|
| Clinical | |||
| SPECT | ~10 | 0.03 | [60,100] |
| PET | ~5 | 3 | [60,100] |
| Preclinical (1st generation) | |||
| SPECT | ~1.2 | 0.3 | [100] |
| PET | ~1.5 | 4 | [100] |
| Preclinical (2nd generation) | |||
| SPECT | |||
| U-SPECT II | 0.25 | 0.39 | [61] |
| X-SPECT | 0.75 | 0.06 | [101] |
| nanoSPECT | 0.6 | 0.07 | [101] |
| PET | |||
| inveon | 1.15 | 11.1 | [102,103] |
| Focus 120 | 1.5 | 7 | [104] |
Spatial resolution (overall spatial resolution for the entire camera system) refers to the ability to detect discrete sources of radioactivity and is measured as the smallest diameter of radioactive sources (capillaries) separated by the same distance that remain distinguishable.[61] Sensitivity is defined as the percentage of emitted events that are able to be detected and recorded at the given resolution.[42].
With NIS expression being highly coupled to viral replication, radiotracer uptake activity detected by nuclear imaging can be used for quantitative comparisons of viral replication and persistence and used to estimate the number of infected cells within a tumor. Quantitative analysis is performed on nuclear images using image analysis software. The specific accumulation within the tumor is determined by defining regions or volumes of interest (ROI, VOI, respectively) to quantify the contained intensity of signal, measured as radioactivity. Absolute quantification, the measure of activity per unit of mass or volume, allows for comparison of uptake intensity across animals. This measurement can be adjusted to compensate for differences in background levels of radiotracer between images, and can be used to calculate fraction or number of NIS-expressing cells within a volume. For more accurate comparisons across multiple animals in which injected dose of radiotracer can vary, the percentage of injected dose per gram of tissue (%ID/g) is used which takes the absolute uptake relative to the whole body uptake by measuring the activities of the administration syringe before and after radiotracer injection.
2.4. Intratumoral distribution
NIS-mediated imaging is limited in its ability to resolve small populations of NIS-positive cells. The ability to differentiate intratumoral infection centers or identify metastatic tumor lesions, residual disease or resection margins will depend on the ability to detect small cell populations. Technical and biological limitations for NIS-mediated imaging exist like the instrument sensitivity and resolution, specific and nonspecific uptake, tumor-to-normal tissue uptake ratio such that solitary lesions are only detectable when their size and NIS-mediated uptake sufficiently meet specific instrument requirements.[49] With improvements in resolution, it is now possible to observe the spatial context and distribution of individual infectious centers within a single tumor (Figure 1). Until recently, NIS-mediated uptake could be detected with planar gamma camera scintigraphy, SPECT or PET but did not allow for spatial resolution of infection within the tumor and depended on invasive tumor harvest and IHC analysis to look at infection with high resolution. Previously, while IHC analysis showed that NIS expression was not uniformly distributed throughout the tumor with only 30% of cells transduced and areas lacking transduction, NIS-mediated imaging showed positive radiotracer concentration at the tumor site.[62] Although NIS-mediated imaging could identify whole body biodistribution and replication kinetics, it was not informative on the intratumoral heterogeneity. Importantly, more recent preclinical work using X-SPECT and U-SPECT instrumentation, imaging can now differentiate intratumoral infectious centers.[51,63] Further, high-resolution NIS-mediated imaging shows spatial heterogeneity of viral distribution both locally within a single tumor and across animals of the same treatment group.[63]
The measurement of uptake intensity using noninvasive nuclear imaging techniques has been verified to be a true reflection of the whole tumor activity by ex vivo analysis of explanted tumors after imaging.[51,52,64,65] Table 3 lists the sensitivity and resolution of selected imaging modalities that have been used for detection of oncolytic viruses, highlighting the improvements in spatial resolution and sensitivity with new instruments. With the improvements in nuclear imaging, intratumoral biodistribution can now be quantitatively evaluated, and the causes of infection heterogeneity and its implications for tumor response can be further explored. However, to increase clinical utility, quantitative indices are needed to relate the meaning of uptake detection with clinically relevant therapeutic responses. This includes understanding how positive uptake detection relates to the volume of tissue being imaged. For instance, investigators established sensitivity of X-SPECT to detect positive uptake in NIS-expressing tumor lesions as small as 3 mm.[27] With advances in imaging technology, it has since been reported that the minimum number of NIS-expressing cells detectable in a cluster is ~100 million with microPET (Focus 120 microPET, Concorde Microsystems Inc.).[66] With experiment-specific imaging settings and taking into account the specific tumor cell/stroma ratio, it was estimated that the X-SPECT could resolve a zone as small as 2 × 105 infected cells from background using MV expressing NIS in BxPC-3 tumors.[51,67] Further, Penheiter et al. worked to determine the minimum percentage of NIS-expressing cells necessary for detection via nuclear imaging.[51] Using an older SPECT/CT system (X-SPECT, Gamma Medica), they identified that the minimum percentage of NIS-expressing cells within a tumor to allow detection of radiotracer activity above background was 2.7%, confirmed by both NIS RNA copy yield and IHC quantification. Further, they showed that individual infected centers within a tumor were able to be spatially resolved if their centers were more than 3.9 mm apart and each center comprised at least four times the amount of infected cells required for whole tumor detection.[51] These useful metrics can then be used to predict percentage of infected cells in future treated animals. However, because of the variability between tumor models and oncolytic viruses, it will be necessary to perform such validations to approximate the sensitivity of the NIS reporter with each virus–tumor combination. With updated instrumentation with increased resolution, such as the U-SPECT II, it is anticipated that the minimum amount of infected cells and the distance between infected centers will continue to decrease.
3. NIS-mediated imaging and treatment strategy
The use of NIS-mediated imaging to provide detailed noninvasive information regarding viral replication and biodistribution within a single animal can be used to compare and gain insight into specific treatment parameters such as dose, viral structure, tumor target, administration technique, as well as look at changes that occur over time to determine effects of efflux, replication and persistence of virus. It is important to note that NIS-mediated image analysis and interpretation will be dependent on experimental conditions and will require careful consideration of the different factors that will affect radiotracer uptake in order to best inform oncolytic optimizations. Therefore, lessons regarding how NIS-mediated imaging and quantitative treatment comparisons should be approached and relevant therapeutic observations will be discussed.
3.1. Tracer efflux
Timing of imaging is an essential consideration, especially in the absence of a retention mechanism as is the case with iodide concentrated by ectopic NIS expression. Radiotracer efflux rates will affect detection and quantification of viral infection. Recirculation and reuptake of radiotracers may take place in the tumor allowing for dynamic equilibrium.[68] In vivo monitoring showed that although absolute value of radiotracer uptake decreased over the 8 hours following radiotracer administration, the ratio of tumor-specific uptake relative to background increased with time meaning background radiotracer levels decreased more rapidly than tumor, indicating retention.[69] While slow efflux is a property of an individual cell dependent on the presence of efflux proteins, efficient reuptake is a property of the environment and most likely dictated by the geometry of surrounding cells and extracellular environment; close proximity to other cells expressing NIS and higher concentrations of effluent or pooled iodide maximizes reuptake and retention of the isotope within the tumor.[23] In support of this idea, the kinetics of radiotracer accumulation was observed using short-term serial imaging 24 h after infection with an Ad expressing NIS. A plateau in uptake intensity was reached after 2 h but variation in uptake intensity was seen between tumors in the same animal likely due to differences in tumor size. Smaller tumors with fewer blood vessels to feed iodine uptake and less cells to allow for NIS expression for reuptake and retention had less radiotracer uptake.[70] Higher doses resulting in a greater density of infection may also allow for better imaging by increasing the opportunity for reuptake thereby increasing the biological half-life of radiotracers. Dose-dependent accumulation of radiotracer resulted in dose-dependent biological half-lives, with administration of 5 × 108 or 1 × 109 PFU Ad expressing NIS resulted in 7.5% ID/g compared to 12% ID/g and biological half-lives of 6.1 h compared to 23.6 h, respectively in tumors.[43] Due to the considerable temporal variation in NIS-mediated radioisotope uptake and retention, short-term serial imaging will be necessary to determine optimal imaging protocols prior to the use of serial imaging to gain insight to the evolution of infection over time or to inform clinical practice.
3.2. Dose and administration route
NIS-mediated radiotracer uptake occurs in a viral dose-dependent manner, shown in both in vitro uptake assays and in vivo monitoring of mice administered escalating doses of NIS-expressing viruses.[15,31,40,41,43,45,48,49,59,71] Prior to induction of cytotoxicity, radiotracer uptake increases with viral dose, with some viruses showing a maximum level of uptake that plateaus above a certain multiplicity of infection in in vitro assays, suggesting a maximal level of NIS expression per cell can be achieved.[31] This may correlate with maximal infection burden, explaining why tumors in animals treated with low viral doses initially experience a slower increase in intratumoral radiotracer uptake relative to animals treated at higher doses. However, the intensity of radiotracer uptake eventually peaks followed by a decrease, as if the low-dose tumor has reached the same level of infection burden achieved by higher doses earlier on.[63] Although, the relationship between dose and uptake may not be linear, as seen with an Ad expressing NIS in which a 10-fold increase in viral dose (108 to 109 vp) resulted in only a 2-fold increase in radiotracer uptake in vivo.[46] These observations are important both for understanding dosing and in interpreting the meaning of increased uptake intensity as it correlates to higher viral replication in the tumor.
Both IT and IV administered virus pharmacokinetics have been monitored by NIS-mediated imaging, and differences in viral replication dependent on administration route have been detected, as noted in Table 2. Direct IT injection results in increased radiotracer uptake signal early on, most likely due to the higher density of infected cells within a given location of the tumor. Administration route also caused differences in time of peak uptake intensity as well as persistence of replication of MV expressing NIS in a prostate tumor model.[39] The use of NIS-mediated imaging can be informative of the differences in viral replication and persistence that occur as a result of both dose and administration route that can influence clinical protocol.
3.3. Differences in kinetics
Observing replication kinetics can be useful in predicting response as increasing radiotracer activity correlated with increased therapeutic tumor response.[41,53] Although a loss of radiotracer uptake can be attributed to viral clearance, it has been repeatedly shown that a rise and subsequent loss in radiotracer activity also corresponds with tumor response. With systemic MV expressing NIS to target prostate cancer, a regression in tumor size correlated with a peak and then loss of viral infection, while stable disease had underlying persistence of viral infection and NIS-mediated imaging.[39] Similarly, when VV expressing NIS was used to treat mice bearing endometrial tumors, radiotracer uptake increased as infection spread in enlarging tumors and decreased with decreasing tumor size as the tumor responded to therapy.[72] Therefore, in similar scenarios, an increase in uptake intensity early after infection may suggest an increased dose of virus delivered to the tumor, and a subsequent loss in uptake intensity can be predictive of tumor response.
NIS imaging can also be used to monitor differences in OV replication and efficacy as a result of vector design and genomic architecture. The location of NIS insertion within the virus may affect the resulting kinetics of NIS expression and ultimately efficacy of the oncolytic. It is necessary to understand how the insertion of the transgene at different sites within the genome will affect the relative expression levels, especially for quantitative NIS-mediated imaging.[73] For instance, in one study the positioning of NIS cDNA within the Ad E1 locus compared to the E3 locus led to far lower hNIS expression.[48] Genetic engineering and transcriptional targeting can also affect the kinetics of virus spread. Insertion of the NIS gene in the wild type compared to targeted background of Ad led to differences in time of peak uptake intensity.[48] While promoter specificity can be used to enhance targeting, it may also effect NIS expression. Ad expressing NIS under control of either the MUC1 or CMV promoter showed similar uptake capabilities in vitro, but in vivo imaging showed clear transduction with the CMV promoter and less intense uptake with MUC1-promoter driving NIS expression in the targeted MUC1 positive ovarian xenografts.[35] NIS-mediated imaging can also show differences as a result of additional therapeutic transgenes on oncolytic efficacy. When the IFNβ gene was inserted into the MV genome carrying NIS compared to the same recombinant MV genome carrying green fluorescent protein and NIS, in vitro assays showed similar growth kinetics.[56] The in vivo study showed similar radiotracer uptake up to day 7, but by d14 decreased radiotracer uptake was detected in the MV-IFN-NIS-treated mice relative to the IFNβ-negative counterpart, likely due to the immune response to the virus leading to enhanced antitumor and antiviral effects.[56] Therefore, the effects of immune activation and resultant viral clearance can be noninvasively monitored. It is also important to note that NIS-mediated uptake requires viable cell populations, and so this technology may not be useful for tracking rapid infection in which cells may die prior to functional NIS expression or sufficient radiotracer accumulation. In such a case, constructs optimized for rapid oncolysis and featuring replication-coupled transgene expression might not be optimal for detection.[74]
3.4. Heterogeneity comparisons
An important observation made possible by NIS-mediated imaging is the heterogeneity of spatiotemporal context of intratumoral infection within and across animals of the same treatment group. For instance, MV expressing NIS was used to treat pancreatic tumor-bearing mice and a large range of intratumoral iodide uptake was observed in the animals. Additionally, serial imaging showed considerable temporal variability in peak tumor iodide localization. The dramatic heterogeneity of intratumoral infection distribution was confirmed with IHC analysis.[41] Similarly, when mice bearing multiple myeloma tumor models treated with systemic VSV encoding NIS were imaged with high resolution, differences in spatial distribution pattern and radiotracer uptake kinetics could be seen for separate infected foci within the same tumor.[63] The non-uniformity of intratumoral distribution may be a barrier to translation. The use of NIS-mediated imaging could allow insight into targeting methods to address the causes of tumor heterogeneity such as variation in promoter activity, inability of viral delivery/replication, regions including stromal, necrotic, hyperbaric, hypoxic that are likely to hamper the penetration of the virus to the entire tumor, incomplete transduction due to polarized expression of receptors.[8,70] These delivery-related causes of heterogeneous infection distribution are also likely to impact delivery of radiotracers throughout the entire tumor. Additionally, vascular shutdown as a result of viral infection, as seen with VSV and VV in subcutaneous colon cancer tumor models, could hinder delivery of radiotracers and affect imaging results.[75] To our knowledge, work has yet to be done to explore the impact of vascular shutdown on radiotracer delivery and NIS-mediated imaging results. It will be necessary to consider how intratumoral infection and the causes of infection distribution heterogeneity may influence radiotracer delivery and interpretation of imaging results. Further, there is considerable variability in the radiotracer background signal among different regions of a tumor likely due to the heterogeneity of extracellular fluid content, with lower background associated with densely packed cells and higher backgrounds with lower cell densities or blood pooling in necrotic regions.[51] Consideration of background signal variability is possible using quantitative software and will be important for accurate depictions of infection burden, especially with zones of moderate infection correlated with only moderate radiotracer uptake.
4. Clinical lessons
Oncolytic viruses expressing NIS are now being used in clinical trials to identify feasibility and limitations of this technology in humans and can provide important insight into pharmacokinetics of these experimental therapeutics. Nuclear imaging has been used to clinically track intratumoral localization and infection with both Ad and MV expressing NIS (Table 2).[55,74,76,77] Clinical use and lessons from the use of NIS-mediated monitoring of OV thus far are described here.
In a phase I trial, Barton et al. administered Ad expressing NIS using intraprostatic administration to treat prostate cancer.[76] At the lower dose, 1 × 1011 vp divided into multiple injections, no radiotracer uptake was detected in the prostate when imaging was performed 3 days post administration. This could be because the total dose given at any single site of injection, 3.3 × 1010 vp, was near the threshold of dose per deposit necessary for detection identified in preclinical canine studies, 1-3 × 1010 vp.[46] This could also be due to missing the peak of viral replication prior to the first imaging time point. Positive intratumoral radiotracer uptake was detected in 7 of 9 patients treated at the higher dose of 1 × 1012 vp divided into two deposits of 5 × 1011 vp. This confirmed the ability to detect Ad gene expression in the prostate with close proximity to the bladder. In these patients, the peak of NIS expression was detected 1–2 days after administration and detectable up to 7 days post administration, although the kinetics of radiotracer uptake differed between patients. Even though radiotracer uptake was undetectable at later imaging time points, Ad DNA persisted in the blood of one patient for at least 145 days. This further suggests a threshold dose necessary for clinical detection using imaging techniques. This also indicates that Ad could persist in the prostate for a prolonged period of time although high levels of gene expression are short lived. It was possible to see distinct but overlapping regions of uptake corresponding with where the divided doses had been injected in one patient. These both confirm the ability to resolve regions of infection and demonstrate the sensitivity of the imaging to detect infection when at least 5 × 1011 vp are deposited. Lastly, there was no extraprostatic radiotracer accumulation showing lack of Ad dissemination, although infection at extraprostatic sites could go undetected if the amount of infected cells within a focal region was not above the critical threshold necessary for detection. For quantitative analysis, the gene expression volume was measured for each patient relative to the mean pixel intensity of that volume. While the volume of gene expression was relatively consistent, the mean pixel intensity per unit volume, a measure of gene expression, showed some variation. Knowledge of gene expression and gene expression volume can be used for comparison with future clinical outcomes. This could aid in establishing a minimum threshold dose necessary for clinical outcomes, planning future dosing strategies to determine number of injections necessary to cover a known volume, or to target gene expression to areas of greater tumor burden.[76]
In a compassionate use case, Rajecki et al. used an Ad expressing NIS in metastatic cervical carcinoma tumors.[74] This patient was treated with a total of 3 × 1011 vp divided for intratumoral administration to tumors in the pelvis and a liver metastasis. Radiotracer uptake within the tumors was not detected following administration. An increase in viral DNA in patient serum indicated replication, suggesting the administered dose may have been active but did not exceed a minimum threshold dose necessary for detection by nuclear imaging. Alternatively, differences in vector design may contribute to the lack of detection, in which the virus used here expressed NIS under the control of the E3 promoter, while the virus used by Barton et al. expressed NIS under control of the ubiquitous CMV promoter that allows more rapid and robust NIS expression. It is possible that the rapid lytic cycle of the Ad used here could limit the amount of NIS expressed before cell lysis. This use of nuclear imaging can teach valuable lessons about the expression of transgenes dependent on virus design and shows that viruses optimized for rapid and potent oncolytic activity may not be suited for tracking with NIS-mediated imaging.[74]
In a phase I trial of MV expressing NIS for treatment of refractory multiple myeloma, Russell et al. clearly tracked intratumoral infection following systemic administration using NIS-mediated imaging.[55] Imaging results and clinical responses were presented from two patients, each treated intravenously with a single dose of 1 × 1011 TCID50 MV expressing NIS. In the first patient with a frontal plasmacytoma, radiotracer uptake was localized to the lesion and detectable above background on d8, with increase in uptake intensity d15, indicating propagation of infection. In the second patient with numerous plasmacytomas in the lower extremities, radiotracer uptake was detected in several plasmacytomas on d8, with decreased intensity d15, and undetectable uptake by d28. The intensity of uptake varied between tumors of similar size, indicating the heterogeneity of virus replication even within the same patient. Even so, positive NIS-mediated imaging in both patients correlated with clinical response as measured by M protein reduction and resolution of bone marrow plasmacytosis. Here NIS-mediated imaging was able to demonstrate the clinical feasibility of systemic OV by showing the ability to specifically target multiple sites of tumor growth after systemic administration within a patient.[55] The use of NIS-mediated imaging was also able to reveal differences in radiotracer uptake intensity and kinetics, confirming the clinical relevance of the heterogeneity of intratumoral infection detected preclinically. This demonstrates the continued need to monitor infection noninvasively and calls for the need for better clinical imaging modalities to better understand the heterogeneous nature of viral biodistribution.
NIS-mediated imaging was also used by Galanis et al. to clinically monitor MV expressing NIS infection in resistant ovarian cancer patients following intrapleural administration monthly for up to 6 cycles.[77] Radiotracer uptake mediated by NIS expression was detected in 3 out of 13 patients treated at the highest dose level, 1 × 109 TCID50. The first patient had positive radiotracer uptake on d8 and d15 of the first treatment cycle which became undetectable prior to cycle 2, and was then detectable again on d8, d15 and d21 of the second cycle. This confirms the ability of NIS to detect changes in viral replication, especially in the case of retreatment or potentially in detecting significant secondary viremia. A second patient had radiotracer uptake above background only on d8 of cycle 1, and the third patient had positive uptake on d15 of the first cycle.[77] This study demonstrated the temporal heterogeneity of NIS-mediated radiotracer uptake detection that could signify differences in intratumoral virus replication kinetics or initial intratumoral deposition of virus particles.
These clinical applications demonstrate the utility of NIS-mediated imaging of radiotracer uptake to monitor oncolytic virus infection and propagation. Clinical use of NIS-mediated imaging revealed that there is a threshold level of infection necessary for detection using nuclear imaging, and this threshold may differ with virus. Further, differences in vector design may affect the utility of the NIS reporter gene to track infection. The variability in NIS-mediated detection of infection indicates that there are still barriers to achieving reliable tumor transduction that need to be addressed moving forward. There are seven ongoing clinical trials with oncolytic viruses expressing NIS (NCT00408590, NCT02192775, NCT02364713, NCT02068794, NCT01503177, NCT00450814, NCT01846091). It is hoped that the use of NIS-mediated imaging in these trials will continue to inform clinicians of noninvasive pharmacokinetics and allow for correlations with treatment response.
5. Conclusions
Although there are many oncolytic viruses entering clinical trials, there have been only a small number of complete tumor responses documented.[8,55] Therefore, there is a continued need to optimize current therapies. Molecular nuclear imaging of OV using NIS reporter gene expression to mediate radiotracer uptake is an important and informative technology to facilitate improvements in current treatment protocols. The ability to monitor oncolytic virus infection, replication and biodistribution using NIS-mediated imaging has been confirmed. Changes in radiotracer uptake intensity within a single animal can be serially detected and reflect the oncolytic cycle from infection, replication and expression of viral and transgenes, and cytopathic effects. Whole body nuclear imaging confirms tumor-specific infection and can be used to detect off-target replication or to diagnostically locate tumor deposits. With improvements in nuclear imaging technology, individual centers of infection within a single tumor are now able to be spatially differentiated and followed over time.
The use of NIS-mediated imaging can be used to inform optimizations in treatment protocol. First, the dynamic nature of OV requires serial monitoring from early time points in order to relay comprehensive information regarding in vivo infection and to establish specific imaging protocols for future use. Quantitative image analysis of NIS-mediated radiotracer uptake identified differences in uptake as a result of treatment dose, administration route and virus design. Such quantitative analysis can be used to inform and improve mathematical models developed to predict therapeutic efficacy. For instance, the mathematical model by Bailey et al. identified replication kinetic, delivery and distribution to be key factors in predicting therapeutic outcome, all of which can now be noninvasively monitored.[78] The use of NIS-mediated imaging also identified the variability of intratumoral infection and replication kinetics both within a single tumor and between multiple tumors. This variability is also detected clinically, highlighting continued need for therapeutic optimization. With NIS-mediated imaging, barriers to oncolytic efficacy can be both detected and studied by providing a means to noninvasively and quantitatively compare improvements in viral transduction or replication. Further, the observed heterogeneity in both replication kinetics and biodistribution indicates the need for patient-specific pharmacokinetic monitoring. The use of molecular imaging can go beyond mere detection of intratumoral infection and readily inform treatment optimizations through noninvasively monitoring of viral replication and biodistribution in real time.
6. Expert opinion
As the use of OV continues to increase, the use of NIS-mediated noninvasive imaging will allow for more rapid advances in OV optimization. Together, knowledge of biodistribution and kinetics of infection will allow for better understanding of OV and help to inform clinical practice and dose determination. Using NIS as a marker for noninvasive monitoring of infection gives clinicians the ability to correlate tumor response and toxicity with the actions of OV therapy, including information on viral kinetics during treatment, changes in tumor size, biodistribution and monitor potential viral toxicity.[79]
Monitoring of virus-driven NIS expression is a safe, noninvasive way to address questions pertaining to the oncolytic activity in cancer patients, facilitate dose optimization and development of individualized treatments—quantification of uptake by NIS can provide important dosimetric information for optimal dosing.[39] Predictive mathematical models, such as those using VSV to target multiple myeloma and adenovirus to target prostate cancer, can be generated or improved upon using information from molecular imaging to facilitate dose optimization for complete tumor coverage with gene expression.[71,78] For oncolytic viruses with limited clinical efficacy, combination treatments may be beneficial and molecular imaging of viral spread in tumors could provide unique information to rationalize combination treatments. For example, the NIS transgene also confers the potential for radiovirotherapy to improve tumor response relative to oncolytic virus alone in preclinical studies.[26,31,32,34,37–40,43,59] The noninvasive monitoring of viral replication kinetics can be informative for planning delivery of targeted radiation via NIS expression. The utility of NIS as a reporter gene to gain insight into viral replication and distribution is essential to radiovirotherapy success as radiotracer efflux and the heterogeneity of distribution and replication can impact therapeutic response.
Imaging could allow for identification of location of infection before continuing with 131I treatment and be used to identify optimal timing for administration. NIS-mediated imaging could also be used to predict response to treatment and the need for additional dosing or combination with other anti-cancer therapies. Knowledge of delivery, especially intratumoral distribution, could be used to facilitate personalized approaches by pinpointing untreated tumor areas for reinjection to improve tumor coverage.[51] The heterogeneity detected both in replication kinetics and biodistribution of infection warrants the continued development of enhanced imaging modalities to further understand mechanisms underlying the heterogeneity of infection and ultimately tumor response. Noninvasive monitoring can be used as a tool for quantitative analysis when targeting causes of heterogeneity, such as using different injection and infusion techniques or altering the tumor microenvironment.[8,51,80]This could lead to a better understanding of the causes and cures for heterogeneity of viral infection and replication that are current barriers to success.
It is our opinion that noninvasive, serial NIS-mediated imaging of OV is a clinically valuable tool to predict treatment outcome and response, identify treatment gaps, and better understand and therefore target barriers to OV efficacy. This will require continued advancement of imaging technologies to improve clinical ability to resolve increasingly small deposits of NIS-expressing cells and development of quantitative indexes specific to each virus, tumor and instrument combination to correlate uptake activity at the voxel level with the underlying molecular meaning in terms of number of infected cells. The use of NIS reporter gene imaging is rapidly advancing and can now provide unparalleled insight into the spatial and temporal context of oncolytic infection both preclinically and clinically, which will be integral to optimization of oncolytic treatment strategies.
Declaration of interest
This work was supported by Al and Mary Agnes McQuinn, and Gary and Anita Klesch. SJR and Mayo Clinic have an interest in the NIS reporter gene technology. The technology has been licensed to Imanis Life Sciences. SJR and Mayo Clinic own shares in Imanis Life Sciences.
Funding Statement
This work was funded in great part by National Science Foundation Grant No. 0732233, “Learning Progressions for Scientific Inquiry: A Model Implementation in the Context of Energy.”
Bibliography
- Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
**Detailed review of oncolytic virotherapy
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
**Review detailing the status of oncolytic virotherapy clinical trials.
- Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28(7):326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill S, Warren R, Venook A. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8(4):308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- Swisher SG, Roth JA, Nemunaitis J. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91(9):763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- Richard-Fiardo P, Franken PR, Harrington KJ. The use of molecular imaging of gene expression by radiotracers in gene therapy. Expert Opin Biol Ther. 2011;11(10):1273–1285. doi: 10.1517/14712598.2011.588596. [DOI] [PubMed] [Google Scholar]
- Carlson SK, Classic KL, Hadac EM. Quantitative molecular imaging of viral therapy for pancreatic cancer using an engineered measles virus expressing the sodium-iodide symporter reporter gene. AJR Am J Roentgenol. 2009;192(1):279–287. doi: 10.2214/AJR.08.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Russell SJ. Heterogeneous delivery is a barrier to the translational advancement of oncolytic virotherapy for treating solid tumors. Virus Adapt Treat. 2014;6:11–31. [Google Scholar]
*Review highlights issues related to intratumoral delivery as a barrier to oncolytic success.
- Schmidt-Wolf G, Schmidt-Wolf IG. Human cancer and gene therapy. Ann Hematol. 1994;69(6):273–279. doi: 10.1007/BF01696555. [DOI] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchmiy AA, Efimov GA, Nedospasov SA. Methods for in vivo molecular imaging. Biochemistry (Mosc) 2012;77(12):1339–1353. doi: 10.1134/S0006297912120012. [DOI] [PubMed] [Google Scholar]
- Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65(7):500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad D, Fong Y. Molecular imaging of oncolytic viral therapy. Mol Ther Oncolytics. 2015;1:14007. doi: 10.1038/mto.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Comprehensive review of available oncolytic virus reporter genes for molecular imaging purposes.
- Montiel-Equihua CA, Martin-Duque P, De La Vieja A. Targeting sodium/iodide symporter gene expression for estrogen-regulated imaging and therapy in breast cancer. Cancer Gene Ther. 2008;15(7):465–473. doi: 10.1038/cgt.2008.6. [DOI] [PubMed] [Google Scholar]
- Haddad D, Chen NG, Zhang Q. Insertion of the human sodium iodide symporter to facilitate deep tissue imaging does not alter oncolytic or replication capability of a novel vaccinia virus. J Transl Med. 2011;9:36. doi: 10.1186/1479-5876-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
*First use of NIS-mediated imaging with a VV expressing NIS, and the first of many studies with oncolytic virus GLV-1h153.
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
*First characterization and cloning of the rNIS gene.
- Smanik PA, Liu Q, Furminger TL. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun. 1996;226(2):339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
*First cloning of the hNIS gene
- Spitzweg C, Joba W, Eisenmenger W. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab. 1998;83(5):1746–1751. doi: 10.1210/jcem.83.5.4839. [DOI] [PubMed] [Google Scholar]
*Characterization of NIS gene expression in extrathyroidal tissues including salivary gland, mammary gland and gastric mucosa.
- Riesco-Eizaguirre G, Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol. 2006;155(4):495–512. doi: 10.1530/eje.1.02257. [DOI] [PubMed] [Google Scholar]
- Dadachova E, Carrasco N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin Nucl Med. 2004;34(1):23–31. doi: 10.1053/j.semnuclmed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Haberkorn U, Altmann A, Eisenhut M. Functional genomics and proteomics: the role of nuclear medicine. Eur J Nucl Med Mol Imaging. 2002;29(1):115–132. doi: 10.1007/s00259-001-0682-4. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Harrington KJ, Pinke LA. Clinical review 132: the sodium iodide symporter and its potential role in cancer therapy. J Clin Endocrinol Metab. 2001;86(7):3327–3335. doi: 10.1210/jcem.86.7.7641. [DOI] [PubMed] [Google Scholar]
- Dingli D, Bergert ER, Bajzer Z. Dynamic iodide trapping by tumor cells expressing the thyroidal sodium iodide symporter. Biochem Biophys Res Commun. 2004;325(1):157–166. doi: 10.1016/j.bbrc.2004.09.219. [DOI] [PubMed] [Google Scholar]
- Dingli D, Kemp BJ, O’Connor MK. Combined I-124 positron emission tomography/computed tomography imaging of NIS gene expression in animal models of stably transfected and intravenously transfected tumor. Mol Imaging Biol. 2006;8(1):16–23. doi: 10.1007/s11307-005-0025-0. [DOI] [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye EO, Glaser M. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13(14):1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Pham L, O’Connor MK. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12(6):1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- Marsee DK, Shen DH, MacDonald LR. Imaging of metastatic pulmonary tumors following NIS gene transfer using single photon emission computed tomography. Cancer Gene Ther. 2004;11(2):121–127. doi: 10.1038/sj.cgt.7700661. [DOI] [PubMed] [Google Scholar]
*Showed high-resolution capabilities of NIS-mediated uptake and identified 3 mm as the minimal size for identification of tumor deposits with imaging.
- Spitzweg C, Dietz AB, O’Connor MK. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001;8(20):1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, O’Connor MK, Bergert ER. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60(22):6526–6530. [PubMed] [Google Scholar]
- Touchefeu Y, Franken P, Harrington KJ. Radiovirotherapy: principles and prospects in oncology. Curr Pharm Des. 2012;18(22):3313–3320. doi: 10.2174/1381612811209023313. [DOI] [PubMed] [Google Scholar]
- Chen RF, Li ZH, Pan QH. In vivo radioiodide imaging and treatment of pancreatic cancer xenografts after MUC1 promoter-driven expression of the human sodium-iodide symporter. Pancreatology. 2007;7(5 – 6):505–513. doi: 10.1159/000108968. [DOI] [PubMed] [Google Scholar]
- Dingli D, Russell SJ, Morris JC., 3rd. In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90(6):1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O’Connor MK. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005;11(4):1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O’Connor MK. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006;17(6):661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O’Connor MK. Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Ther. 2006;13(1):60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- Gholami S, Chen CH, Lou E. Vaccinia virus GLV-1h153 in combination with 131I shows increased efficiency in treating triple-negative breast cancer. Faseb J. 2014;28(2):676–682. doi: 10.1096/fj.13-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Carlson SK, Classic KL. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood. 2007;110(7):2342–2350. doi: 10.1182/blood-2007-01-065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Intratumoral spread of VSV expressing NIS could be noninvasively and serially imaged, identifying variability in radiotracer uptake due to administration route.
- Hakkarainen T, Rajecki M, Sarparanta M. Targeted radiotherapy for prostate cancer with an oncolytic adenovirus coding for human sodium iodide symporter. Clin Cancer Res. 2009;15(17):5396–5403. doi: 10.1158/1078-0432.CCR-08-2571. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Iankov ID, Allen C. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol Ther. 2009;17(12):2041–2048. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerlinck I, Merron A, Baril P. Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res. 2009;15(21):6595–6601. doi: 10.1158/1078-0432.CCR-09-0262. [DOI] [PubMed] [Google Scholar]
- Penheiter AR, Wegman TR, Classic KL. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am J Roentgenol. 2010;195(2):341–349. doi: 10.2214/AJR.09.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Identified a relationship between NIS-mediated radiotracer uptake and peak tumor volume reduction
- Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29(3):193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Baker CH, Bergert ER. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum Gene Ther. 2007;18(10):916–924. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
*NIS-mediated imaging revealed dose-dependent accumulation and biological half-life of radiotracer.
- Trujillo MA, Oneal MJ, McDonough S. A probasin promoter, conditionally replicating adenovirus that expresses the sodium iodide symporter (NIS) for radiovirotherapy of prostate cancer. Gene Ther. 2010;17(11):1325–1332. doi: 10.1038/gt.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechacz B, Splinter PL, Greiner S. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44(6):1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- Barton KN, Tyson D, Stricker H. GENIS: gene expression of sodium iodide symporter for noninvasive imaging of gene therapy vectors and quantification of gene expression in vivo. Mol Ther. 2003;8(3):508–518. doi: 10.1016/s1525-0016(03)00153-9. [DOI] [PubMed] [Google Scholar]
**First time showing the ability to differentiate NIS-mediated uptake in the prostate from nearby bladder in canines
- Dingli D, Peng KW, Harvey ME. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
**First time showing the ability to use gamma camera serial imaging as a surrogate for MV-NIS replication after intravenous injection.
- Merron A, Peerlinck I, Martin-Duque P. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14(24):1731–1738. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
*Demonstrated the kinetics of Ad replication could be monitored with molecular imaging, attributing differences in kinetics to viral design
- Cho JY, Xing S, Liu X. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000;7(9):740–749. doi: 10.1038/sj.gt.3301170. [DOI] [PubMed] [Google Scholar]
- Naik S, Nace R, Federspiel MJ. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26(8):1870–1878. doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter AR, Griesmann GE, Federspiel MJ. Pinhole micro-SPECT/CT for noninvasive monitoring and quantitation of oncolytic virus dispersion and percent infection in solid tumors. Gene Ther. 2012;19(3):279–287. doi: 10.1038/gt.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
**Identified detection threshold for quantitative imaging analysis and developed quantititavie indexes of correlation between NIS-mediated radiotracer uptake, percent of infected cells, and distance between infected regions necessary for reliable resolution.
- Groot-Wassink T, Aboagye EO, Wang Y. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther. 2004;9(3):436–442. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
*Showed that PET images obtained from NIS-mediated radiotracer uptake could provide quantitative information on gene expression.
- Haddad D, Zanzonico PB, Carlin S. A vaccinia virus encoding the human sodium iodide symporter facilitates long-term image monitoring of virotherapy and targeted radiotherapy of pancreatic cancer. J Nucl Med. 2012;53(12):1933–1942. doi: 10.2967/jnumed.112.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksanpaisan L, Pham L, McIvor S. Oral contrast enhances the resolution of in-life NIS reporter gene imaging. Cancer Gene Ther. 2013;20(11):638–641. doi: 10.1038/cgt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Federspiel MJ, Peng KW. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89(7):926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
**First reported use of NIS-reporter gene to noninvasively track oncolytic infection in patients after systemic administration.
- Li H, Peng KW, Dingli D. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17(8):550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer RM, Schatz SM, Bergert ER. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodide imaging and therapy of locally recurrent prostate cancer. Mol Ther. 2005;12(5):835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Huang R, Kuang AR. AFP promoter enhancer increased specific expression of the human sodium iodide symporter (hNIS) for targeted radioiodine therapy of hepatocellular carcinoma. Cancer Invest. 2009;27(6):673–681. doi: 10.1080/07357900802620885. [DOI] [PubMed] [Google Scholar]
- Trujillo MA, Oneal MJ, Davydova J. Construction of an MUC-1 promoter driven, conditionally replicating adenovirus that expresses the sodium iodide symporter for gene therapy of breast cancer. Breast Cancer Res. 2009;11(4):R53. doi: 10.1186/bcr2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Differential targeting with selective promoters led to differences in infection detectable by nuclear imaging
- Histed SN, Lindenberg ML, Mena E. Review of functional/anatomical imaging in oncology. Nucl Med Commun. 2012;33(4):349–361. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko O, Van Der Have F, Villena JL. Quarter-millimeter-resolution molecular mouse imaging with U-SPECT+ Mol Imaging. 2014;13(0):1–8. doi: 10.2310/7290.2014.00053. [DOI] [PubMed] [Google Scholar]
*Imaging resolution as small as a quarter of an mm was achieved using the U-SPECT II system.
- Boland A, Ricard M, Opolon P. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60(13):3484–3492. [PubMed] [Google Scholar]
- Miller A, Suksanpaisan L, Naik S. Reporter gene imaging identifies intratumoral infection voids as a critical barrier to systemic oncolytic virus efficacy. Mol Ther Oncolytics. 2014;1 doi: 10.1038/mto.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
**High resolution SPECT/CT imaging of NIS-mediated uptake reveals heterogeneity of spatial distribution and temporal evolution of intratumoral infected centers.
- Carlson SK, Classic KL, Hadac EM. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006;8(6):324–332. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
*Quantitative use of NIS-mediated radiotracer showing strong relationship between ROI analysis and ex vivo tumor activity.
- Cheng D, Wang Y, Liu X. Comparison of 18F PET and 99mTc SPECT imaging in phantoms and in tumored mice. Bioconjug Chem. 2010;21(8):1565–1570. doi: 10.1021/bc1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami S, Chen CH, Belin LJ. Vaccinia virus GLV-1h153 is a novel agent for detection and effective local control of positive surgical margins for breast cancer. Breast Cancer Res. 2013;15(2):R26. doi: 10.1186/bcr3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Used NIS-mediated radiotracer uptake to identify positive surgical margins after tumor resection, identifying diagnostic potential of the NIS transgene.
- Penheiter AR, Russell SJ, Carlson SK. The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr Gene Ther. 2012;12(1):33–47. doi: 10.2174/156652312799789235. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Comprehensive review of NIS reporter gene functions.
- Faivre J, Clerc J, Gerolami R. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Cancer Res. 2004;64(21):8045–8051. doi: 10.1158/0008-5472.CAN-04-0893. [DOI] [PubMed] [Google Scholar]
- Haddad D, Chen CH, Carlin S. Imaging characteristics, tissue distribution, and spread of a novel oncolytic vaccinia virus carrying the human sodium iodide symporter. PLoS One. 2012;7(8):e41647. doi: 10.1371/journal.pone.0041647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki M, Sarparanta M, Hakkarainen T. SPECT/CT imaging of hNIS-expression after intravenous delivery of an oncolytic adenovirus and 131I. PLoS One. 2012;7(3):e32871. doi: 10.1371/journal.pone.0032871. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Discrepancy between tumor size and radioiodide accumulation indicating incomplete penetration of the tumor and highlighting importance of active replication at time of imaging.
- Barton KN, Freytag SO, Nurushev T. A model for optimizing adenoviral delivery in human cancer gene therapy trials. Hum Gene Ther. 2007;18(6):562–572. doi: 10.1089/hum.2007.004. [DOI] [PubMed] [Google Scholar]
- Liu YP, Wang J, Avanzato VA. Oncolytic vaccinia virotherapy for endometrial cancer. Gynecol Oncol. 2014;132(3):722–729. doi: 10.1016/j.ygyno.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham L, Nakamura T, Gabriela Rosales A. Concordant activity of transgene expression cassettes inserted into E1, E3 and E4 cloning sites in the adenovirus genome. J Gene Med. 2009;11(3):197–206. doi: 10.1002/jgm.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki M, Kangasmaki A, Laasonen L. Sodium iodide symporter SPECT imaging of a patient treated with oncolytic adenovirus Ad5/3-Delta24-hNIS. Mol Ther. 2011;19(4):629–631. doi: 10.1038/mt.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Clinical NIS-mediated radiotracer uptake was unable to detect replication of oncolytic Ad expressing NIS in a compassionate-use case, demonstrating the presence of imaging detection thresholds and impacts of vector design.
- Breitbach CJ, Paterson JM, Lemay CG. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15(9):1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Barton KN, Stricker H, Brown SL. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16(10):1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
**First reported use of NIS-reporter gene to noninvasively track oncolytic infection in human patients.
- Galanis E, Atherton PJ, Maurer MJ. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75:22–30. doi: 10.1158/0008-5472.CAN-14-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Clinical use of NIS-reporter gene to track infection noninvasively in ovarian cancer patients and confirmed the ability to detect changes in viral replication and identified temporal heterogeneity in replication between patients.
- Bailey K, Kirk A, Naik S. Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS One. 2013;8(9):e73759. doi: 10.1371/journal.pone.0073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin LJ, Ady JW, Lewis C. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery. 2013;154(3):486–495. doi: 10.1016/j.surg.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D, Cascino MD, Josic K. Mathematical modeling of cancer radiovirotherapy. Math Biosci. 2006;199(1):55–78. doi: 10.1016/j.mbs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Mettler FA, Guiberteau MJ. Essentials of nuclear medicine imaging. 6th. Philadelphia (PA): Saunders/Elsevier; 2012. [Google Scholar]
- Merron A, Baril P, Martin-Duque P. Assessment of the Na/I symporter as a reporter gene to visualize oncolytic adenovirus propagation in peritoneal tumours. Eur J Nucl Med Mol Imaging. 2010;37(7):1377–1385. doi: 10.1007/s00259-009-1379-3. [DOI] [PubMed] [Google Scholar]
- Klutz K, Willhauck MJ, Wunderlich N. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22(11):1403–1412. doi: 10.1089/hum.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Zhao Z, Ma X. Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter. Cancer Gene Ther. 2011;18(2):144–152. doi: 10.1038/cgt.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneal MJ, Trujillo MA, Davydova J. Characterization of infectivity-enhanced conditionally replicating adenovectors for prostate cancer radiovirotherapy. Hum Gene Ther. 2012;23(9):951–959. doi: 10.1089/hum.2012.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneal MJ, Trujillo MA, Davydova J. Effect of increased viral replication and infectivity enhancement on radioiodide uptake and oncolytic activity of adenovirus vectors expressing the sodium iodide symporter. Cancer Gene Ther. 2013;20(3):195–200. doi: 10.1038/cgt.2013.4. [DOI] [PubMed] [Google Scholar]
- Grünwald GK, Klutz K, Willhauck MJ. Sodium iodide symporter (NIS)-mediated radiovirotherapy of hepatocellular cancer using a conditionally replicating adenovirus. Gene Ther. 2013;20(6):625–633. doi: 10.1038/gt.2012.79. [DOI] [PubMed] [Google Scholar]
- Li H, Nakashima H, Decklever TD. HSV-NIS, an oncolytic herpes simplex virus type 1 encoding human sodium iodide symporter for preclinical prostate cancer radiovirotherapy. Cancer Gene Ther. 2013;20(8):478–485. doi: 10.1038/cgt.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RM, Greiner SM, Harvey ME. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82(6):700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter AR, Dingli D, Bender CE. Monitoring the initial delivery of an oncolytic measles virus encoding the human sodium iodide symporter to solid tumors using contrast-enhanced computed tomography. J Gene Med. 2012;14(9–10):590–597. doi: 10.1002/jgm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzen B, Pierson CR, Russell SJ. Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer. 2012;12:508. doi: 10.1186/1471-2407-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opyrchal M, Allen C, Iankov I. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS) Hum Gene Ther. 2012;23(4):419–427. doi: 10.1089/hum.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Peng KW, Russell SJ. Oncolytic measles virus encoding thyroidal sodium iodide symporter for squamous cell cancer of the head and neck radiovirotherapy. Hum Gene Ther. 2012;23(3):295–301. doi: 10.1089/hum.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi HV, Madde P, McDonough SJ. Preclinical efficacy of the oncolytic measles virus expressing the sodium iodide symporter in iodine non-avid anaplastic thyroid cancer: a novel therapeutic agent allowing noninvasive imaging and radioiodine therapy. Cancer Gene Ther. 2012;19(9):659–665. doi: 10.1038/cgt.2012.47. [DOI] [PubMed] [Google Scholar]
- Domingo-Musibay E, Allen C, Kurokawa C. Measles Edmonston vaccine strain derivatives have potent oncolytic activity against osteosarcoma. Cancer Gene Ther. 2014;21(11):483–490. doi: 10.1038/cgt.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyle DR, Escobar DZ, Peng KW. Oncolytic measles virus as a novel therapy for malignant peripheral nerve sheath tumors. Gene. 2015;565:140–145. doi: 10.1016/j.gene.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Gholami S, Haddad D, Chen CH. Novel therapy for anaplastic thyroid carcinoma cells using an oncolytic vaccinia virus carrying the human sodium iodide symporter. Surgery. 2011;150(6):1040–1047. doi: 10.1016/j.surg.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Jun KH, Gholami S, Song TJ. A novel oncolytic viral therapy and imaging technique for gastric cancer using a genetically engineered vaccinia virus carrying the human sodium iodide symporter. J Exp Clin Cancer Res. 2014;33:2. doi: 10.1186/1756-9966-33-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveno C, Mojica K, Ady JW. Gene therapy using therapeutic and diagnostic recombinant oncolytic vaccinia virus GLV-1h153 for management of colorectal peritoneal carcinomatosis. Surgery. 2015;157(2):331–337. doi: 10.1016/j.surg.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen FP, Vanderheyden JL. The future of SPECT in a time of PET. Nucl Med Biol. 2007;34(7):733–735. doi: 10.1016/j.nucmedbio.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Deleye S, Van HR, Verhaeghe J. Performance evaluation of small-animal multipinhole muSPECT scanners for mouse imaging. Eur J Nucl Med Mol Imaging. 2013;40(5):744–758. doi: 10.1007/s00259-012-2326-2. [DOI] [PubMed] [Google Scholar]
**Detailed experimental comparison of current microSPECT/CT imaging system.
- Constantinescu CC, Mukherjee J. Performance evaluation of an Inveon PET preclinical scanner. Phys Med Biol. 2009;54(9):2885–2899. doi: 10.1088/0031-9155/54/9/020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EP, Disselhorst JA, Brom M. Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med. 2009;50(1):139–147. doi: 10.2967/jnumed.108.055152. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee JS, Im KC. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007;48(9):1527–1535. doi: 10.2967/jnumed.107.040550. [DOI] [PubMed] [Google Scholar]


