Abstract
The HESI-coordinated RISK21 roadmap and matrix are tools that provide a transparent method to compare exposure and toxicity information and assess whether additional refinement is required to obtain the necessary precision level for a decision regarding safety. A case study of the use of a pyrethroid, “pseudomethrin,” in bed netting to control malaria is presented to demonstrate the application of the roadmap and matrix. The evaluation began with a problem formulation step. The first assessment utilized existing information pertaining to the use and the class of chemistry. At each stage of the step-wise approach, the precision of the toxicity and exposure estimates were refined as necessary by obtaining key data which enabled a decision on safety to be made efficiently and with confidence. The evaluation demonstrated the concept of using existing information within the RISK21 matrix to drive the generation of additional data using a value-of-information approach. The use of the matrix highlighted whether exposure or toxicity required further investigation and emphasized the need to address the default uncertainty factor of 100 at the highest tier of the evaluation. It also showed how new methodology such as the use of in vitro studies and assays could be used to answer the specific questions which arise through the use of the matrix. The matrix also serves as a useful means to communicate progress to stakeholders during an assessment of chemical use.
Keywords: Integrated testing strategy, pyrethroid, risk assessment, tiered approach
Introduction
To address and catalyze further improvements in human health risk assessment, the International Life Sciences Institute (ILSI) Health and Environmental Sciences Institute (HESI) created the Risk Assessment in the 21st Century (RISK21) Project. This multi-sector, international initiative began in 2009 and has involved the active participation of over 120 individuals from 12 countries, 15 government institutions, 20 universities, two non-governmental organizations, and 12 corporations.
This collective effort, RISK21, has resulted in a problem formulation-based conceptual framework called the “roadmap” and a simple exposure–toxicity comparison matrix. The matrix enables exposure and hazard to be evaluated and compared effectively and transparently using all relevant sources of information in a framework designed for efficient and confident decision making. By focusing on data generation related to decision making, animal resource requirements can be greatly reduced and the incorporation of new in vitro and in silico tools into the decision-making process can also be facilitated. The overarching principles of the RISK21 approach and an introduction to the roadmap and visualization matrix are described by Pastoor et al. (2014). The use of the roadmap and matrix has been described in more detail by Embry et al. (2014). The purpose of this paper is to give an example of the application of the roadmap and matrix in a health assessment of a pyrethroid insecticide used on bed nets to reduce transmission of malaria. It is not the purpose of this paper to endorse any of the methods used for assessing exposure or toxicity, but rather to demonstrate how methods can be used within the RISK21 approach. A companion case study (Wolf et al., 2015) examined the use of the framework as a screening tool to prioritize 20 chemicals detected in surface water and groundwater which could potentially appear in drinking water.
Methodology
A case study was created around the use of insecticides in bed nets to prevent mosquito bites and infection with malaria. A theoretical pyrethroid, known as “pseudomethrin,” was presented to the team for evaluation. Its identity was not revealed to the team members, but the team could “commission” studies and receive the results. Pseudomethrin is actually deltamethrin, a registered pyrethroid with a full toxicological database and a range of other in vivo and in vitro data (California EPA 2000). No new experimental data were generated. If data could not be found from an existing source, but were deemed necessary as part of the exercise, then hypothetical values were “created” which were consistent with other pyrethroids in order to allow the exercise to continue. In practice, it was not necessary to create many values; those values that were created are clearly noted in the text of this manuscript as hypothetical values. It should be emphasized that the purpose of the exercise was to assess the utility of the RISK21 approach, not to evaluate or endorse the use of any particular chemical. The idea was to simulate the collection and evaluation of data and information during the assessment of a specific chemical use rather than a general assessment of a chemical. The example was chosen to explore how a chemical from a well-studied class, a so called “nth-in-class” chemical would be addressed. The team was given the charge to use no more than 50 animals to make its decision in an effort to promote innovative thinking.
Stepwise use of the RISK21 roadmap
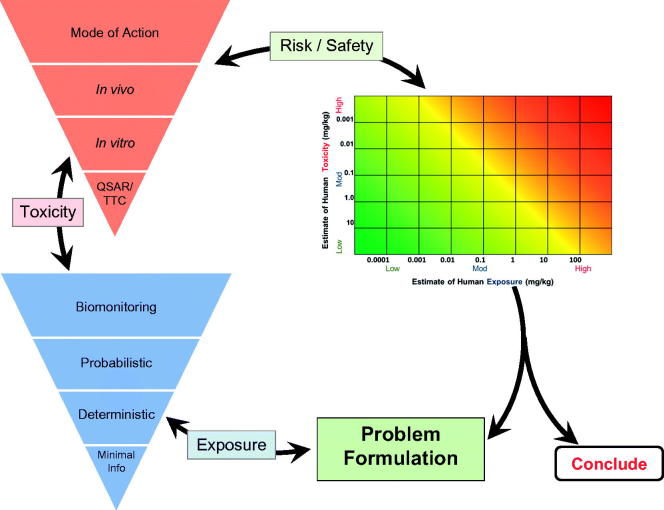
The RISK21 roadmap is summarized in Figure 1. The first step is Problem Formulation – to better understand and explore the context. The team produced the problem formulation statement described in the Section “Problem formulation statement for pseudomethrin”.
Figure 1.
The RISK21 roadmap.
Problem formulation statement for pseudomethrin
The problem is formulated in terms of the scenario under consideration and the context of the scenario, and concludes with a question about safety which is to be addressed in the assessment.
Scenario: The use of bed nets to protect against mosquito bites is widespread in parts of the world where malaria is endemic. The efficacy of the net can be enhanced by impregnating it with an insecticide. Pseudomethrin is a candidate pyrethroid for this use.
Context: Pseudomethrin is manufactured in a modern plant with high standards of containment and industrial hygiene. Risk assessment for manufacture is not considered in this exercise.
Three methods are currently available for the treatment of nets:
“'Do-it-yourself” treatment kits are available for home use. The pesticide is supplied in liquid, powder, or tablet form to be dissolved or dispersed in water. The nets are dipped in the resulting pesticide solution/suspension, and then dried. The pyrethroid is loosely bound to the net and treatment has to be repeated every 3 months.
A central service run by trained personnel is available. Net owners can bring their nets for treatment or re-treatment, thus reducing the risks of exposure of untrained members of the public.
Factory-pretreated nets are supplied for sale, with effectiveness lasting up to 3–4 years as the pyrethroid is more firmly bound to the net. These pre-treated nets reduce potential exposures resulting from the preparation, use, and disposal of insecticide-containing solutions, associated with the need to dip and re-dip nets.
The purpose of the risk assessment was to determine whether pseudomethrin is suitable for the “do-it-yourself” method. This use results in two possible exposure components. The first is dipping the nets in a solution of the insecticide to impregnate the nets. This is considered to be a short-term exposure scenario. The second is sleeping under the nets. This is considered to be a chronic exposure scenario. Pseudomethrin is not being considered for other uses.
The structure and physico-chemical properties of pseudomethrin are known. The pyrethroid is also known to have efficacy in controlling mosquitoes. There are no toxicological data available apart from negative results from a standard battery of in vitro genotoxicity assays which the manufacturer commissions on all compounds that have commercial potential.
Safety question: Would the do-it-yourself method of impregnating mosquito bed netting with pseudomethrin result in unacceptable adverse health effects to people using the nets?
Assemble information
The second step after problem formulation is to assemble the available known information about the scenario. There are various ways of assembling this information but it is helpful to use three broad headings as follows:
Chemical properties
Pseudomethrin is a pyrethroid and there is a wealth of data about this class of chemistry. The structure is known and the major physico-chemical properties are available. After questioning, the team was informed that analysis of structure–activity relationships (SAR) indicated no structural alerts, other than similarity to other pyrethroids, and the physico-chemical properties were within the range of other pyrethroids that had been commercialized. This information can be used in exposure and structure activity models as appropriate.
Use and exposure
This case study demonstrates how the tiered exposure assessment approach can be applied to an “nth-in-class” chemical. The pyrethroids are a class of compounds with well-established uses as insecticides (EPA 2011). As such, existing pyrethroid data can be utilized to estimate substance specific parameters needed for exposure assessment of a hypothetical, novel pyrethroid insecticide developed for application to bed netting (Barlow et al. 2001). In addition, the World Health Organization (WHO) published A Generic Risk Assessment Model for Insecticide Treatment and Subsequent Use of Mosquito Nets which included a comprehensive exposure assessment (WHO 2004), and this was considered to be suitable for use in this case.
Biological activity and adverse effects
The pyrethroids form a class of insecticides developed from natural pyrethrums found in the chrysanthemum family. In addition to similar structural characteristics, their biological activity results from their ability to keep sodium channels in neurons open and thus block nerve conduction (Soderlund et al. 2002). Pyrethroids are active in both insect and mammalian neurons, although there is some selectivity due to absorption and kinetic differences. Their potential to cause adverse effects in laboratory animals has been well studied in regulatory tests, and there are data which indicate that the neuronal effects do occur in humans. Pyrethroids cause two types of adverse neurological effect tremors (T-syndrome) and convulsions (choreathetosis and salivation (CS-syndrome). Both effects are only noted in the presence of the pyrethroid and recovery is rapid and complete and repeated dosing dose not significantly influence their toxic potency. The US Environmental Protection Agency (EPA) has published a pyrethroid cumulative risk assessment addressing 21 of the most commercialized pesticidal compounds (EPA 2011) recognizing a common mode of action. The wealth of toxicological data on the class creates an opportunity to focus data generation on which critical data on pseudomethrin to generate rather than going through a checklist of studies.
First assessment – Tier 0
Tier 0 is defined as an assessment which only uses data which are available and derived from the chemical structure without generating compound-specific data.
Estimation of exposure
Exposure was estimated in four stages.
Assemble the exposure information
The WHO published A Generic Risk Assessment Model for Insecticide Treatment and Subsequent Use of Mosquito Nets in 2004 (WHO 2004) which included a comprehensive exposure assessment for deltamethrin on which this exposure assessment is based. However, the team believed that they were bridging general pyrethroid exposure data from the WHO to pseudomethrin.
Choose and document the logic for estimating exposure
The WHO document defined the worst-case exposure scenario as one in which the bed nets are treated by the user (a “do-it-yourself” dipping treatment). Once every 3 months, families are assumed to prepare the dipping solution from concentrated product and dip the nets, wring them out, and hang them up to dry. Due to the considerable time between exposures, this was considered to be a short-term exposure scenario for adults and children. The other identified exposure scenario results from sleeping under a treated net every night. This could involve an adult, child, or infant and is considered a chronic exposure.
These scenarios represent worst-case exposure estimates. The default exposure parameters identified in the WHO document are used as surrogates for the new pseudomethrin. The WHO document discounted inhalation exposure due to the low vapor pressure of the compound, and only considered the oral exposure route for children sleeping under the bed nets. All three exposure routes (dermal, oral, and inhalation) were considered for this assessment. The reference values for weight, breathing rate, and other key parameters were from the WHO assessment. In addition, the key values for the amount of pseudomethrin in the treating solutions and the resulting concentrations in liquid and on the net were also taken from the WHO assessment.
Produce range or probability distribution for exposure
For dipping the net, the exposure to the hands resulting from dipping the net in the solution is calculated to be 41 mg of pseudomethrin on the skin per event. If a worst-case assumption of 100% dermal absorption is utilized, the amount absorbed would be 0.7 mg/kg/event for a 60 kg adult and 1.0 mg/kg/event for a 40 kg child of 12 which is the age at which children might start to dip nets. The EPA cumulative risk assessment suggests a default value of 5% absorption for pyrethroids, and using this value results in the amount absorbed by an adult as 0.03 mg/kg/event and by a child as 0.05 mg/kg/event. Oral exposure is not anticipated during treatment of the bed nets.
Vapor pressure and the ideal gas law were used toestimate the air concentration resulting from a spill of concentrate as the worst-case exposure scenario indicated that the inhalation exposure route is not significant compared with that of the dermal exposure. As such, the inhalation pathway was not considered as a significant route of exposure. The range of estimates for a single exposure is therefore 0.03–0.7 mg/kg/event for an adult and 0.05–1.0 mg/kg/event for a child. The range is derived from the range of dermal absorption values of 5–100%.
For sleeping under the bed nets, the WHO default is that 30% of the body’s surface area is in contact with the net while sleeping. The WHO document indicates that the recommended insecticide loading on the bed nets is 25 mg/m2; therefore, this value was used to calculate the amount of compound available for transfer from the net to the skin. A worst-case approach would be to assume 100% transfer of product from the net to the skin. The WHO document indicates that 2.5% of the insecticide can be dislodged from the net and transferred to skin, and a range of 2.5–100% was used. Once the dermal exposure is estimated, the assumptions range of 5–100% dermal absorption is then applied. Applying these factors gives the ranges shown in Table 1 for daily exposure.
Table 1.
Doses of pseudomethrin absorbed as calculated for dipping and sleeping under the net in the first assessment.
| Use | Dermal contact (mg/kg/d) | Hand to mouth (mg/kg/d) | Net mouthing (mg/kg/d) | Total or aggregate (mg/kg/d) | |
|---|---|---|---|---|---|
| Net dipping (single exposure) | Adult | 0.03–0.7 | N/A | N/A | 0.03–0.7 |
| Child | 0.05–1.0 | N/A | N/A | 0.05–1.0 | |
| Infant | N/A | N/A | N/A | N/A | |
| Sleeping under net (chronic exposure) | Adult | 0.0002–0.16 | N/A | N/A | 0.0002–0.16 |
| Child | 0.0001–0.08 | 0.000002–0.006 | N/A | 0.0001–0.086 | |
| Infant | 0.0005–0.4 | 0.000007–0.003 | 0.01–0.04 | 0.0106–0.443 |
Oral exposure via hand-to-mouth transfer is considered a relevant exposure pathway for both infants and children. The WHO document assumes that 10% of the amount transferred to the hand is then transferred to the mouth and is available for oral ingestion. The same parameters such as transfer coefficient based on dislodgeable fraction and insecticide loading are then applied. However, worst-case estimates would assume 100% for hand-to-mouth transfer and for net-to-hand transfer. These calculations provide ranges of the estimates of amounts ingested shown in Table 1.
A second oral exposure route is through mouthing of the net by infants. The WHO document estimates the area of bed net mouthed to be 0.005 m2 and assumes that 30% of the pesticide is ingested overnight by the infant. A worst-case estimate would be to assume ingestion of 100% of pseudomethrin from the net area mouthed overnight. These calculations give estimates shown in Table 1.
Once the insecticide is applied to the netting and dried, it is bound to the net and, together with its low vapor pressure, the conclusion can be drawn that it is not expected to volatilize in significant amounts compared with other routes. The formation of inhalable particulates is unlikely given the physical properties of the pyrethroid. It is considered unlikely that it would be absorbed onto airborne particles within the net while sleeping. Inhalation exposure is therefore not included in this assessment.
The overall ranges from all three routes of exposure of the amount of pseudomethrin dermally absorbed and ingested while sleeping under the net are shown in Table 1.
Visualize range or probability distribution for exposure
The assessments of human exposure are expressed in ranges of amount (mg/kg/d) of pseudomethrin absorbed. Therefore, the axis to be used on the RISK21 matrix will be in mg/kg/d absorbed, and the range will be depicted as a separate-shaded area (Embry et al. 2014).
There will be two situations considered: dipping the nets which is a single exposure, and sleeping under the bed net which is a long-term exposure. The range for net dipping is 0.03–1.0 mg/kg/event as a single exposure. The range for sleeping under the net is 0.01–0.4 mg/kg/d as a chronic exposure.
Estimation of toxicity
The next step is to estimate toxicity which is completed in four stages.
Assemble the toxicity information
The pyrethroids form a well-studied class of chemicals. A reference group of 11 pyrethroids was selected because a full suite of toxicological studies and a standardized single dose study was published as part of the assessment for cumulative toxicity (Wolansky et al. 2006). Reviews carried out by the Food and Agriculture Organization of the United Nations (FAO) or EPA for each chemical were inspected and an information table was constructed (Table 2). The table contained the following values where available: rat oral LD50; rat single dose BMD20; rat 28-d, 90 d, and chronic NOAELs; mouse 28-d, 90-d, and chronic NOAELs; dog 90-d and 1-year NOAELs; rat and rabbit developmental toxicology maternal and fetal NOAELs; and rat multigeneration maternal and pup NOAELs. In addition, the lowest NOAEL was selected to correspond to three durations of human exposure: a single dose for a 1-d exposure; 28–90-d for repeat human exposures up to 90 d; 1 year or longer for prolonged human exposure over 90 d.
Table 2.
Key toxicological data for pyrethroids (values in mg/kg bodyweight).
| Chemical | Bifenthrin (FAO 2010) | Cyfluthrin (FAO 2003a) | Cypermethrin (FAO 2006) | Esfenvalerate (FAO 2002a) | Fenpropathrin (FAO 1993) | lambda-cyhalothrin (FAO 2003b) | Permethrin (FAO 1999) | Prallethrin (FAO 2002b) | Resmethrin (FAO 1996) | S-Bioallethrin (FAO 2005) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat | Acute LD50 | 53 | 160 | 90 | 71 | 79 | 225 | 490 | 8000 | 413 | |||
| Oral BMD20 a | 14.3 | 12.6 | 76 | 40.5 | 29 | 8.9 | 156 | 150 | 291 | 135 | |||
| 15–28-d NOAEL | 10 | 20 | 50 | 36 | |||||||||
| 90-d NOAEL | 3.8 | 9.5 | 40 | 7.5 | 17 | 2.5 | 60 | 79 | 20 | ||||
| 2-year – chronic NOAEL | 2.3 | 6.2 | 7.5 | 2 | 5 | 2.3 | 25 | 19 | 3 | 27 | |||
| Mouse | 15–28-d NOAEL | 43 | 60 | 140 | |||||||||
| 90-d NOAEL | 45 | 10 | 374 | ||||||||||
| 2-year – chronic NOAEL | 7.6 | 38.4 | 60 | 4 | 56 | 1.8 | 75 | 6 | 38 | 42 | |||
| Dog | 15–45-d NOAEL | ||||||||||||
| 90-d NOAEL | 2.5 | 6.5 | 12.5 | 7 | 0.5 | 5 | 3 | 80 | 42 | ||||
| 1-year NOAEL | 1.5 | 6.5 | 5.7 | 5 | 3 | 0.5 | 5 | 2.5 | 14 | ||||
| DART | Rat Dev Tox NOAEL Mat | 1 | 3 | 17.5 | 2.5 | 3 | 10 | 225 | 10 | 200 | 20 | ||
| Rat Dev Tox NOAEL Pup | 2 | 30 | 70 | 20 | 10 | 15 | 225 | 300 | 200 | 80 | |||
| Rabbit Dev Tox NOAEL Mat | 2.7 | 20 | 450 | 3 | 6 | 10 | 200 | 30 | 240 | 50 | |||
| Rabbit Dev Tox NOAEL Pup | 8 | 20 | 700 | 20 | 36 | 30 | 1200 | 200 | 240 | 50 | |||
| Rat Repro Parental NOAEL | 3 | 9 | 3.8 | 4.2 | 3 | 2 | 170 | 36 | 4 | 60 | |||
| Rat Repro Pup NOAEL | 5 | 34.7 | 7.5 | 4.2 | 3 | 2 | 170 | 60 | 4 | 180 | |||
| Reference NOAELs | Reference 1-d NOAEL | 14.3 | 12.6 | 76 | 40.5 | 29 | 8.9 | 156 | 150 | 291 | 135 | ||
| Reference 90-d NOAEL | 2.5 | 6.5 | 12.5 | 4.2 | 7 | 0.5 | 5 | 3 | 4 | 20 | |||
| Reference chronic NOAEL | 1.5 | 6.5 | 7.5 | 2 | 3 | 0.5 | 5 | 2.5 | 3 | 14 |
BMD20 values are from EPA (2011).
Choose and document the logic for estimating toxicity
The toxicity data set for the pyrethroids is extensive and covers a range of chemical structures within the series. The data set can be used for a read-across assessment (OECD 2007). It is assumed that pseudomethrin is at least as potent as the most potent pyrethroid which has been studied. In addition, a factor of 10 will be applied to cover the eventuality that pseudomethrin is more potent because the reference range of pyrethroids includes only those which have “survived” the industry selection and development processes which may have rejected more potent compounds. The assumed laboratory animal NOAELs will be subjected to the application of an uncertainty factor of 100 on the assumption that humans are 10 times more sensitive than laboratory animals and that some humans are 10 times more sensitive than typical humans.
The BMD20 dose for a single exposure was used as the reference dose for net dipping which is a single exposure in humans. The lowest NOAEL from the 1-year dog, the 2-year rat, or the developmental and reproductive toxicology studies will be taken as the reference dose for sleeping under a net. All the studies have been conducted via the oral route. As the exposure has been calculated in absorbed dose, no correction has to be made.
Produce range or probability distribution for toxicity
Estimating the BMD20 value for pseudomethrin is the basis for estimating toxicity for net dipping. Based on data variance and sample size, the BMD20 was the most conservative estimate able to predict a significant change from control values (EPA 2011). The pyrethroid with the lowest BMD20 value is lambda-cyhalothrin with a value of 8.9 mg/kg. This value will represent the upper bound of the range. A default uncertainty factor of 100 is applied to this value (10× for interspecies and 10× for intraspecies). To account for the possibility that pseudomethrin might be more potent, an additional factor of 10 is applied to give an assumed BMD20 for pseudomethrin of 0.0089 mg/kg/d.
Infants comprise the sub-population with the highest dose for sleeping under the net; consequently, when looking for the appropriate reference study, developmental and reproductive toxicity studies are inspected first. Bifenthrin has the lowest NOAEL for developmental toxicity of 2 mg/kg/d, and lambda-cyhalothrin has the lowest NOAEL for reproductive toxicity of 2 mg/kg/d. However, the lowest overall NOAEL for chronic exposure is lambda-cyhalothrin with a value of 0.5 mg/kg/d from the 1-year dog study. This value represents the upper bound of the range. To account for the possibility that pseudomethrin might be more potent, a factor of 10 is applied to give an assumed NOAEL for pseudomethrin of 0.05 mg/kg/d. An additional uncertainty factor of 100 (10× for interspecies and 10× for intraspecies) is then applied to this figure to give 0.0005 mg/kg/d.
Visualize range or probability distribution for toxicity
The assessments of the safe level for human toxicity are expressed in ranges of amount (mg/kg/d) of pseudomethrin absorbed. Therefore, the axis to be used on the RISK21 matrix will be in mg/kg/d absorbed and, as the range has been derived by applying uncertainty factors, these will be depicted separately on the matrix (Embry et al. 2014).
There will be two situations considered: dipping the nets which results in a single short-term exposure and sleeping under the bed net which results in a long-term exposure. The human safe dose range for net dipping is 0.0089–8.9 mg/kg/d. The human safe dose range for sleeping under the nets is 0.0005–0.5 mg/kg/d.
Assessment of safety
The next step is the assessment of safety using the RISK21 matrix. This step has three stages.
Choose and document the policy to be used for assessing safety and add to matrix if necessary
The safety assessment will conclude that safety can be assured only when the upper bound of the exposure estimate is less than the lower bound of the human toxicity assessment. The estimate of human toxicity will include uncertainty factors within the range, and, therefore, the RISK21 matrix does not need to be adjusted.
Plot exposure and toxicity ranges or distribution on the RISK21 matrix
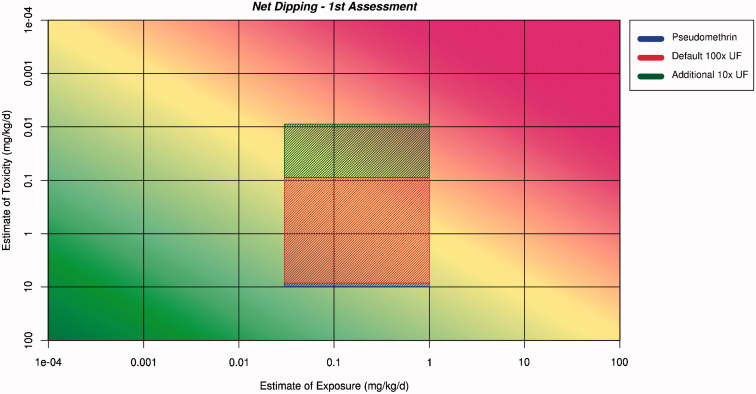
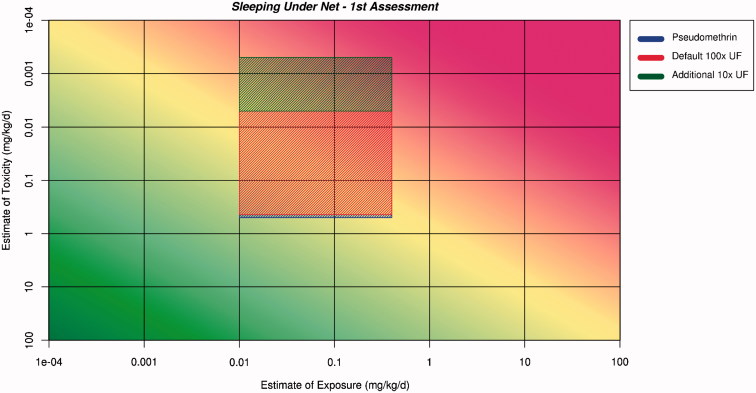
The ranges for the estimates of exposure and toxicity are plotted on the RISK21 matrix. These ranges are then projected across the matrix and an area is formed where they intersect. The shape and position of the intersecting area is used to aid decision making. This has been done for two scenarios: net dipping (Figure 2) and for sleeping under the net for infants, children, and adults (Figure 3).
Figure 2.
Application of the ranges for exposure and toxicity on the RISK21 matrix to form the exposure/toxicity intersect area for net dipping for the first assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
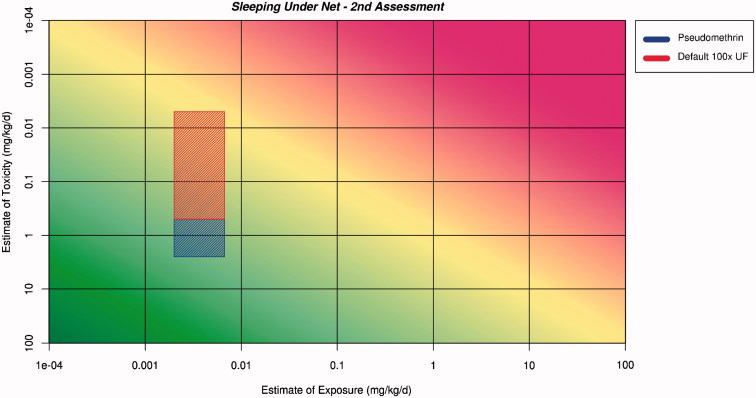
Figure 3.
Application of the ranges for exposure and toxicity on the RISK21 matrix to form the exposure/toxicity intersect area for sleeping under the net for the first assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
Assess safety and decide and document conclusion
The intersect areas for net dipping and sleeping under the net extend into the red area of the matrix. This indicates that there is no margin of exposure, and safety cannot be assured. Both the areas are large in both dimensions, indicating a large degree of uncertainty or lack of precision in both exposure and toxicity estimates. This is a reflection of the conservative assumptions that were used in the derivation of both estimates. Both exposure and toxicity estimates should be subjected to refinement. The conclusion to be documented is as follows: the use of pseudomethrin as an insecticide in mosquito nets cannot be considered safe on the basis of information in Tier 0 which included no data specific to pseudomethrin but which relied upon applying conservative assumptions to the generic pyrethroid data. The resulting large ranges of the estimates for exposure and toxicity suggest that refinement of the estimates for both exposure and toxicity should be undertaken.
Second assessment – Tier 1
The second assessment involves using the RISK21 roadmap for a second time. The results of the first assessment are used to decide on the key pieces of information which will be required to refine the estimates of exposure and toxicity. Tier 1 is defined as an assessment using only in vitro data.
Estimation of exposure
Assemble the exposure information
The information which was used in the first assessment is re-examined to determine where acquisition of new or additional data will make the most impact in increasing the precision of the estimate.
Inspection of the data for net dipping indicates that dermal exposure has both the greatest range of estimate and the greatest impact, and, consequently, should be refined by data generation. Upon examination, it is clear that the width of the range is derived from the values used for dermal absorption. A worst-case value of 100% was used as a maximum, and a value of 5% was used as the minimum based on the value used by the EPA (2011). As dermal absorption is the key factor in determining human exposure for net dipping, this value would have been determined for pseudomethrin. The team requested these data. A value of 2–3% for dermal absorption from human skin for pseudomethrin was determined from in vitro dermal absorption studies; the value for deltamethrin in a published study by Hughes & Edwards (2010) was used for pseudomethrin.
Inspection of the data for sleeping under the net indicates that dermal exposure is again the route with the largest value and range for adults and children, but the oral route also contributes significantly to the exposure estimated for infants. Looking back at the derivation of the estimates for dermal exposure, the percentage absorption figure has a large impact, and therefore the value derived from the in vitro dermal absorption study can be used to increase precision or reduce uncertainty. The other value which contributes to the lack of precision is the estimate for the transfer of pseudomethrin to the skin from the net. The higher bound of the range was defined by the assumption of 100% transfer and the lower end by a default value of 2.5% derived by WHO. It was decided that this important parameter for pseudomethrin would have been determined using transfer estimation studies, and the team requested these data. However, no value was available and a hypothetical value of 0.5–1.5% was created, as described in section Methodology, which was used in the derivation of the estimates of exposure.
The estimation of exposure via the oral route for infants and children assumes that pseudomethrin is transferred to the hand and then to the mouth using the same estimate of transfer as for dermal exposure. The new information derived from the transfer test can be used to reduce uncertainty and to increase precision. There is an additional factor in the case of infants which is net mouthing, i.e., the assumption that 30–100% of pseudomethrin in the area of the net is ingested from mouthing behaviour. This value would have been determined using simulated saliva studies and was requested by the team. No data were available; therefore, a hypothetical value of 1.5–5% ingestion was created to derive the estimate of exposure.
In summary, new information has been derived from the following studies:
In vitro dermal absorption – data from Hughes & Edwards (2010)
Net to skin transfer study – hypothetical data
Net to simulated saliva study – hypothetical data
Choose and document the logic for estimating exposure
The same WHO-based model with specific values will be included rather than default assumptions.
Produce range or probability distribution for exposure
Net dipping: The same calculations are made as previously for net dipping but pseudomethrin-specific values (1–3%) for dermal absorption are used:
Sleeping under the net: The same calculations are made as previously for sleeping under the net but the specific values for dermal absorption (2–3%), the hypothetical values for transfer from skin to mouth (0.5–1.5%), and the hypothetical values for transfer from net to mouth (1.5–5%) are used for the second assessment. The calculated values are shown in Table 3.
Table 3.
Doses of pseudomethrin absorbed calculated for dipping and sleeping under the net in the second assessment.
| Use | Dermal contact (mg/kg/d) | Hand to mouth (mg/kg/d) | Net mouthing (mg/kg/d) | Total or aggregate (mg/kg/d) | |
|---|---|---|---|---|---|
| Net dipping (single exposure) | Adult | 0.028–0.04 | N/A | N/A | 0.028–0.04 |
| Child | 0.04–0.06 | N/A | N/A | 0.04–0.06 | |
| Infant | N/A | N/A | N/A | N/A | |
| Sleeping under net (chronic exposure) | Adult | 1.6 × 10−5–7x10−5 | N/A | N/A | 1.6 × 10−5–7 × 10−5 |
| Child | 8 × 10−6–4 × 10−5 | 3 × 10−6–9 × 10−5 | N/A | 1.1 × 10−5–1.3 × 10−4 | |
| Infant | 4 × 10−5–2 × 10−4 | 1.5 × 10−5–4.5 × 10−4 | 0.002–0.006 | 0.002–0.0067 |
Oral exposure via hand-to-mouth transfer calculations can be adjusted with the new values for net-to-hand transfer. The WHO document assumes that 10% of the amount transferred to the hand is then transferred to the mouth and is available for oral ingestion. The same parameters such as transfer coefficient based on dislodgeable fraction and insecticide loading are then applied. However, worst-case estimates would assume 100% for hand-to-mouth transfer. The hypothetical value range of 0.5–1.5% for net-to-hand transfer will be used. The values calculated for absorption are shown in Table 3.
A second oral exposure route is through net mouthing by infants. The hypothetical value for the simulated saliva test indicates that 5–15% of the pesticide is ingested overnight by the infant. The calculated value for pseudomethrin absorbed is shown in Table 3. Inhalation exposure is not included in this assessment as before (section Produce range or probability distribution for exposure). The overall ranges from all three routes of exposure of the amount of pseudomethrin absorbed via the three routes are shown in Table 3.
Visualize range or probability distribution for exposure
The assessments of human exposure are expressed in ranges as described in section Visualize range or probability distribution for exposure using the refined values. The range for net dipping will be 0.04–0.06 mg/kg/d single short-term exposure. The range for sleeping under the net will be 0.002–0.0067 mg/kg/d chronic exposure.
Estimation of toxicity
Assemble the information for toxicity
The key insecticidal and toxicological property of pyrethroids is to hold open the sodium channel and thus cause neurotoxicity (Wakeling et al. 2012). The team searched for comparative potency data and investigated the utility of an existing in vitro model of this activity which uses microelectroarrays (MEA) in cerebrocortical neuron mouse cultures. Using this model, the relative potency of the 10 reference pyrethroids has been determined (Losa et al. 2009) by comparing the doses required to cause 50% inhibition of activity (IC50). This parameter was requested by the team, and the value was determined for pseudomethrin as 175 nM (value for deltamethrin from Losa et al. 2009). This value lies within the range (25–1685 nM) for the MEA IC50s of the reference range of pyrethroids.
Choose and document the logic for estimating toxicity
The correlation between the MEA IC50, the BMD20, and the chronic reference dose for the pyrethroids was examined. There was a reasonable correlation between the log MEA IC50 and the log BMD20 (r = 0.7), but there was a poor correlation between the log MEA IC50 and log chronic reference NOAEL (r = −0.39). There is also a poor correlation between the BMD20 and the chronic reference NOAEL (r = 0.3), and the log BMD20 and the log chronic reference NOAEL (r = −0.21).
Determining the MEA IC50 provides reassurance that pseudomethrin has a similar potency to the reference pyrethroids. It could be used to predict the BMD20 and thus the reference NOAEL for the single exposure associated with dipping the net. However, the poor correlation of both MEA IC50 and BMD20 with the chronic reference NOAEL limits the use of these values for assessing the chronic exposure associated with sleeping under the nets.
The relationship between the calculated MEA IC50 and the BMD20 ranges between 54.5 and 2.4, with a mean of 19.6. Applying these factors to the MEA IC50 of 175 for pseudomethrin gives a predicted value of 8.9 mg/kg/d (based on the mean) with a range of 3.2–73.7 mg/kg/d. An uncertainty factor of 100 (10× for interspecies and 10× for intraspecies) for extrapolation to humans is applied. It is a matter of judgment as to whether an extra factor is required because this range has been derived from an in vitro assessment. The range covers the variation in the relationship between the in vitro and in vivo values in the reference data set, and includes the most conservative estimate. However, the correlation between the in vitro and in vivo values is not strong, and, therefore, an additional extrapolation factor of three is applied only to the lower bound to give a range of 0.011–74 mg/kg/d for net dipping. The application of additional uncertainty factors for in vitro to in vivo studies in this exercise was not meant to suggest regulatory policy, but solely to highlight where uncertainties may lie. In this manner, areas for data generation that could reduce uncertainties could be more easily identified.
The utility of the MEA IC50 for assessing chronic exposure is difficult to determine. There must be other contributing factors to the chronic reference NOAEL apart from the potency of the pyrethroid on the sodium channel because there is a poor correlation between the MEA IC50 and the chronic reference dose. These factors may include toxicokinetic differences. The poor correlation means that a mathematically based prediction cannot be made, but it could be argued that the range of pyrethroids for which there are data includes the range of contributing factors. In Tier 0 (section “Choose and document the logic for estimating toxicity”), the logic used was to assume that pseudomethrin was as potent as the most potent pyrethroid in the reference range, and a factor of 10 was added to allow for pseudomethrin’s potency. The MEA IC50 for pseudomethrin lies within the reference range, albeit towards the more potent end of the distribution. Therefore, it could be argued that the assumption that pseudomethrin is as potent as the most potent pyrethroid which has an MEA IC50 value which is five times more potent in vitro than pseudomethrin is conservative enough. The chronic reference dose is set on the basis of neurotoxicity for nearly every pyrethroid, except for those where the chronic reference dose is set on liver toxicity. In these cases, the pyrethroids are relatively less potent in vitro and in vivo for neuroactivity, which is not the case for pseudomethrin.
The lowest overall NOAEL for chronic exposure is lambda-cyhalothrin with a value of 0.5 mg/kg/d from the 1-year dog study. This value represents the lower bound of the range where the chronic NOAEL might lie. The upper bound of this range could be set on the basis that the five-fold difference in potency between pseudomethrin and lambda-cyhalothrin might be reflected in the NOAEL. This gives a range of chronic NOAELs of 0.5–2.5 mg/kg/d. An interspecies and intraspecies uncertainty factor of 100 is then applied to this figure to give a range of 0.005–2.5 mg/kg/d.
Produce range or probability distribution for toxicity
The human exposure safe range for net dipping is 0.011–74 mg/kg/d. The human exposure safe range for sleeping under the net is 0.005–2.5 mg/kg/d.
Visualize range or probability distribution for toxicity
The estimates of the safe level for human toxicity are expressed in ranges of amount (mg/kg/d) of pseudomethrin absorbed. Therefore, the axis to be used on the RISK21 matrix will be in mg/kg/d absorbed and the range will be depicted separately (Embry et al. 2014). The portion of the range which represents the 100× interspecies and intraspecies uncertainty factors will be shown as a separate shaded area.
Assessment of safety
Choose and document the policy to be used for assessing safety and add to matrix if necessary
The safety assessment will conclude that safety can be assured when the lower bound of the exposure estimate is less than the lower bound of the human safety assessment. The estimate of human safety will include uncertainty factors within the range, and therefore the RISK21 matrix does not need to be adjusted.
Place visualizations of exposure and toxicity ranges on the RISK21 matrix
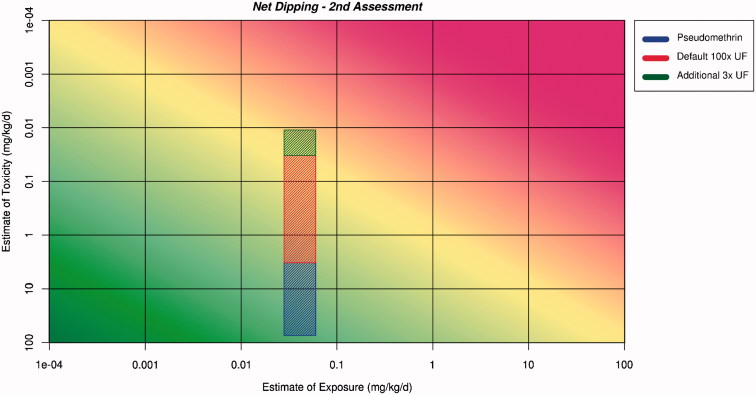
The ranges for the estimates of exposure and toxicity are plotted onto the RISK21 matrix. They are then projected across the matrix, and the shape and position of where they intersect will be used to aid decision-making. This has been completed for the net dipping scenario (Figure 4) and for the sleeping-under-the-net scenario (Figure 5).
Figure 4.
Application of the ranges for exposure and toxicity on the RISK21 matrix to form the exposure/toxicity intersect area for net dipping for the second assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
Figure 5.
Application of the ranges for exposure and toxicity onto the RISK21 matrix to form the exposure/toxicity intersect area for sleeping under the net for the second assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
Assess safety and decide and document conclusion
The second assessment for both net dipping and sleeping under the net has produced intersect areas which indicate that the exposure estimates now have relatively high precision but the toxicity estimates still have relatively low precision. The matrix for net dipping and the matrix for sleeping under the net indicate the large contribution of the 100× uncertainty factor to the lack of precision. The intersect areas in Figures 4 and 5 project into the area of the matrix where safety cannot be assured in both situations. The shape of the intersect areas suggest that attention should be paid to refining the toxicity estimate.
Third assessment – Tier 2
Tier 2 is defined as an assessment involving use and chemical-specific exposure studies or in vivo or ex vivo toxicity studies. The first step in deciding what to do next is to look at the matrix for each use. Net dipping and sleeping under the net both have similar shapes and positioning. They indicate that the estimate for exposure is now much more precise than the estimate for toxicity. They both lie in a position where a decision about safety could be made if the toxicity estimate were more precise. It was decided that the next phase of the assessment should focus on reducing the uncertainty of the toxicity estimate.
Toxicity assessment
Assemble the information for toxicity
The estimation of toxicity at the end of Tier 1 did not include the use of any of the 50 animals allotted. The estimation had relied on the extensive database on chemicals in the pyrethroid class. In Tier 0, the assumption was made that pseudomethrin was 10 times more potent than the most potent pyrethroid in the reference database. In Tier 1, the relative potency of pseudomethrin had been assessed in vitro. The likely range of reference animal NOAELs had to then be estimated, followed by the application of an additional uncertainty factor of 100×. The overall range of toxicity estimations covered over three orders of magnitude for both short-term (0.011–74 mg/kg/d) and longer-term exposure (0.0017–2.5 mg/kg/d), with two orders of magnitude resulting from the standard default uncertainty factor of 100×.
Choose and document the logic for estimating toxicity
The relatively narrow range for predicted animal NOAELs arises because the group of reference compounds has a relatively narrow range of potencies. The range of potencies for all chemicals is up to seven orders of magnitude (Bitsch et al. 2006), but the range for the pyrethroids is 0.5–14 mg/kg/d or 1.5 orders of magnitude. Pseudomethrin’s in vitro potency for the key toxicological effect lies within the same range and, together with similar structure and physical–chemical properties, suggests that its in vivo potency would also lie within this range. Performing conventional regulatory toxicological studies would confirm where in this range pseudomethrin would lie; however, this may not represent a good use of the limited number of animals (50) allowed for this exercise.
Inspection of the database reveals that the chronic reference dose is derived from the 1-year dog study for all of the reference pyrethroids. This indicates that any animal work should be carried out in this species. Furthermore, the ratio between the NOAELs for the 90-d and 1-year studies ranges from one to three. It is possible that ratios of greater than one are the result of differences in dose spacing in the studies. Further inspection of the results of the 90-d studies reveals that the NOAELs are set on neurological effects seen in the first few days of dosing. This suggests that a targeted study in the dog over 3–5 d focusing on clinical signs of neurotoxicity would be the best use of animals. Additionally, it was felt that serial sampling of plasma levels of pseudomethrin could provide additional data useful for modelling without requiring additional animals. This would entail the use of 24 dogs assuming three dogs per sex per dose level and three dose levels plus controls. It should also be noted that the pyrethroids do not cause structural damage to the nervous system at doses that result in clinical signs of neurotoxicity. As such, a terminal sacrifice with histopathological evaluation of tissues is likely not necessary for the proposed study. A 5-d dosing study with pseudomethrin in dogs was “commissioned” by the team. In the study, neurological effects were seen at 10 and 2.5 mg/kg/d but not at 1 mg/kg/d (values derived from clinical observations in 90-d dog study with deltamethrin (California EPA 2000). As such, a NOAEL of 1 mg/kg/d replaced the estimated values used in earlier tiers of the RISK21 matrix evaluation.
Produce range or probability distribution for toxicity
The NOAEL of 1 mg/kg/d from the 5-d study provides a reference NOAEL for both net dipping and sleeping under the net. A default uncertainty factor of 100× for extrapolation to humans is applied to the NOAEL. This provides a range of 0.01–1.0 mg/kg for the toxicity estimate.
Visualize range or probability distribution for toxicity
The range of 0.01–1.0 mg/kg will be shown as a point representing the NOAEL, with a separate shaded area to represent the default 100× uncertainty factor.
Assessment of safety
Choose and document the policy to be used for assessing safety and add to matrix if necessary
The safety assessment will conclude that safety can be assured when the lower bound of the exposure estimate is less than the lower bound of the human safety assessment. The estimate of human safety will include uncertainty factors within the range and, therefore, the RISK21 matrix does not need to be adjusted.
Place visualizations of exposure and toxicity ranges on the RISK21 matrix
The ranges for the estimates of exposure and toxicity are plotted onto the RISK21 matrix. They are then projected across the matrix, and the shape and position of where they intersect will be used to aid decision-making. This has been done for net dipping (Figure 6) and for sleeping under the net (Figure 7).
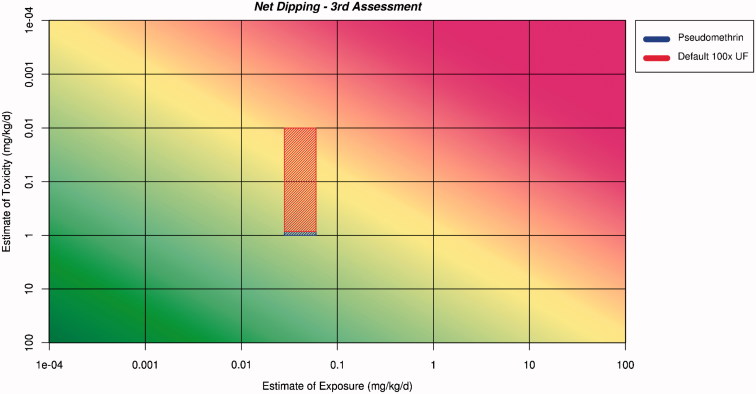
Figure 6.
Application of the ranges for exposure and toxicity on the RISK21 matrix to form the exposure/toxicity intersect area for net dipping for the third assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
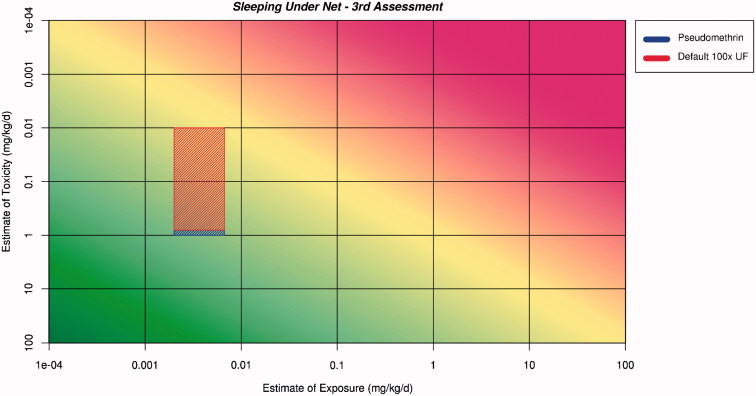
Figure 7.
Application of the ranges for exposure and toxicity onto the RISK21 matrix to form the exposure/toxicity intersect area for sleeping under the net for the third assessment. The area to the left of the yellow shading indicates where exposure is below the human safe level for toxicity.
Assess safety and decide and document conclusion
The intersect area for net dipping protrudes into the area of the matrix where safety cannot be assured. The shape of the intersect area suggests that further refinement of the toxicity estimate would be required. The intersect area for sleeping under the net does not protrude into the area where safety cannot be assured and, therefore, an initial assessment that sleeping under the net would be safe can be made.
Further investigations of toxicity – Tier 3
Tier 3 is defined as an assessment using studies to investigate default extrapolations to determine if they are valid. It may involve mode-of-action studies to determine relevance or kinetic studies to assess comparative internal dose estimation, including time courses.
The assessment concluded that safety could not be assured for net dipping. However, this conclusion was made on the basis of extrapolating the results of an oral study in the dog to dermal exposure in the human. Further lines of investigation would focus on kinetics. The 5-d dog study which was proposed as part of this investigation would provide the base information for Tier 3 investigations. It would be possible to monitor plasma levels of pseudomethrin over time. It is likely that the effects would be shown to be related to plasma concentration rather than area under the curve given the nature of the response.
It should then be possible to develop a physiologically based pharmacokinetic (PBPK) model using values such as hepatic microsomal intrinsic clearance, fraction unbound in plasma, blood:plasma ratio, log P, or Caco-2 permeability, with human values being used for the in vitro values (Leahy 2006). The model could be validated using the data from the 5-d dog study. The primary route of exposure for net dipping is by the dermal route, and the model could then be adapted by using the values from the in vitro dermal absorption study to enable predictions of the plasma concentration curve following dermal exposure. The curve following dermal absorption would likely be flatter and more prolonged, but it would not reach as high a peak plasma level. It would then be possible to model the net dipping exposure and determine whether the threshold for neurological effects would be exceeded. It is a matter of judgment as to how to extrapolate this information to humans. A conventional uncertainty factor of 100 could be applied; however, this would still leave a two-orders-of-magnitude range of values. It could be argued that the PBPK model was based on human values and that a factor of four for interspecies kinetics could be removed (Dorne & Renwick 2005), leaving an uncertainty factor of 25 to be applied to the value.
For net dipping, it would also be necessary to give consideration as to whether pseudomethrin is capable of causing other types of toxicity at relevant doses. The only other finding from the reference pyrethroids was liver toxicity, which appeared in some of the less potent pyrethroids that could be dosed at higher levels. However, this cannot be assumed and should be investigated further. Thomas et al. (2013) have suggested a tiered approach in which chemicals are assessed in a series of in vitro assays for their capability to cause non-specific toxicity and a range of specific toxicities, similar to the range of assays used in ToxCast™. If pseudomethrin was shown not to cause effects in the other assays at concentrations lower than those in the specific in vitro assay for its neurological activity, it could be concluded that the lead effect would be neurotoxicity. This conclusion could not be reached if other effects were indicated at lower concentrations, and it would be necessary to follow up with targeted in vivo studies. It would also be necessary to exclude genotoxicity, but this would have to be done as part of the manufacturer’s initial characterization studies.
Comparison with conventional toxicity assessment
Pseudomethrin is actually deltamethrin. It is interesting to compare the toxicity profile generated by the RISK21 assessment with the profile generated by the conventional regulatory package of studies (Table 4) to better understand how this targeted approach can help achieve the reduced animal use and incorporation of new methodologies into the risk assessment vision of RISK21. A similar profile results from the use of over 2000 animals or the use of 24 animals, although this is only possible because the chemical under consideration belongs to a well-studied class.
Table 4.
Comparison of effects for deltamethrin using full regulatory toxicology package and the studies “commissioned” using the RISK21 roadmap and matrix.
| Full regulatory studies package | RISK21 stepwise assessment | |
|---|---|---|
| Lead effect | Neurotoxicity | Neurotoxicity |
| Other effects | None significant; from full package | None significant; from ToxCast assays |
| Short-term relevant NOAEL | 1 mg/kg from dog study | 1 mg/kg from dog study |
| Long-term relevant NOAEL | 1 mg/kg from dog study | 1 mg/kg from dog study |
| Number of animals used | 2000+ | 24 |
If the chemical had not belonged to a well-studied class, it would have been possible to adopt the data-driven approach proposed by Thomas et al. (2013) to assess the toxicity. Their approach uses a range of assays to determine whether the chemical has cytotoxic activity or specific activity at low concentrations. They also advocate the use of in vitro assays for metabolism and kinetics. Their first stage uses reverse dosimetry to develop an equivalent oral NOAEL in a similar manner to ToxCast™. Thomas et al. (2013) have examined the correlation between the NOAEL derived from this method and the in vivo NOAEL derived from conventional studies. There was a distribution of values, with the median showing that the NOAEL from the in vitro methodology was 1.8 orders of magnitude below the conventional NOAEL, with the upper and lower quartiles between 2.55 and 0.95 orders of magnitude lower than the conventional methodology. This quantifies the uncertainty from this method and means that it can be used within the RISK21 roadmap and matrix. The in vitro NOAEL would be considered to be in the center of the range. The lower end of the range would be considered to be an order of magnitude below this, and the higher end an order of magnitude above it. An additional two orders of magnitude would be applied as the interspecies and intraspecies uncertainty factor. Consequently, if the in vitro methodology predicted an oral NOAEL of 1 mg/kg, the upper limit of the range would be 10 mg/kg and the lower limit would be 0.1 mg/kg. The uncertainty factor would then be applied to the lower limit to give 0.001 mg/kg. The range to be placed on the matrix would be 0.001–10 mg/kg.
Thomas et al. (2013) then advocate the use of targeted in vitro and in vivo studies to follow up on the results for the wider set of assays. This would only be required if the result on the RISK21 matrix showed that it was necessary. If the predicted exposure was low, then the result would be sufficient for a decision to be made with confidence. If this were not the case, then the targeted studies could be used to narrow the range of the predicted toxicity.
Discussion
The purpose of the case history was to explore the RISK21 roadmap and matrix as an aid to risk assessment. The “pseudomethrin” case was chosen as an example of assessing a chemical from a class which was well understood and a use situation which was also well understood. The RISK21 roadmap asks the assessor to use existing knowledge rather than start every assessment as if nothing were known. An artificial constraint of using no more than 50 animals was placed on the exercise to try to stimulate the use of novel methods so that their value could be explored.
The first step in the process was problem formulation, which described the scenarios to be assessed. Once this had been done, a focused search for information took place. This revealed that an exposure model was available and it also revealed a wealth of data on the chemical class. Table 5 summarises the four assessments that were made for the net dipping use, and Table 6 summarises the assessments made for sleeping under the net. The first assessment was made using the physico-chemical properties of pseudomethrin, default values, and conservative assumptions about the toxicity. The first assessment did not allow a safety decision to be made but highlighted the areas to be investigated to provide the most valuable information. Specific values were generated for key factors in determining exposure: dermal absorption, net-to-hand transfer, and net-to-saliva transfer. Applying these factors to the WHO model allowed estimates of exposure to be derived which spanned less than one order of magnitude.
Table 5.
Summary of the assessments made using the RISK21 roadmap and matrix for dipping the net.
| Assessment | Values used | Range | ||
|---|---|---|---|---|
| 1. Tier 0 | Exposure | Default values for dermal absorption in WHO generic model | 0.03–1 mg/kg/d | Safety cannot be assuredRefine both exposure and toxicity |
| Toxicity | Use BMD20 from most potent in series with default 100× with additional factor of 10× | 0.0089–8.9 mg/kg/dAdult and child | ||
| 2. Tier 1 | Exposure | Determine value for dermal absorption in human skin and use in WHO generic model | 0.028–0.06 mg/kg/dAdult and child | Safety cannot be assuredRefine toxicity |
| Toxicity | Determine MEA IC50 as indicator of potency; estimate BMD50 value from MEA IC50. Apply default 100× and additional 3× for in vitro to in vivo | 0.011–74 mg/kg/d | ||
| 3. Tier 2 | Exposure | As assessment 2 | 0.028–0.06 mg/kg/dAdult and child | Safety cannot be assuredRefine toxicity |
| Toxicity | 5-d dog study, neurological NOAEL with 100× extrapolation factor | 0.01–1 mg/kg | ||
| 4. Tier 3 | Exposure | As assessment 2, but derive kinetic profile for absorption to determine Cmax | 0.028–0.06 mg/kg/dAdult and child | Study not done; therefore, conclusion not reached |
| Toxicity | Use kinetic values from 2-d dog study to derive Cmax for neurological effects and compare with exposure Cmax. | Proposed study |
Table 6.
Summary of the assessments made using the RISK21 roadmap and matrix for sleeping under the net.
| Assessment | Values used | Range | Conclusion | |
|---|---|---|---|---|
| 1. Tier 0 | Exposure | Default values for dermal absorption, net-to-skin transfer and net-to-saliva, and use in WHO generic model | 0.01–0.4 mg/kg/dInfant | Safety cannot be assuredRefine both exposure and toxicity |
| Toxicity | Use chronic NOAEL from most potent in series with default 100× with additional factor of 10 | 0.0005–0.5 mg/kg/d | ||
| 2. Tier 1 | Exposure | Determine values for dermal absorption, net-to-skin transfer, and net-to-saliva, and use in WHO generic model | 0.002–0.0067 mg/kg/dInfant | Safety cannot be assuredRefine toxicity |
| Toxicity | Determine MEA IC50 as indicator of potency. Apply default 100× | 0.005–2.5 mg/kg/d | ||
| 3. Tier 2 | Exposure | As assessment 2 | 0.002–0.0067 mg/kg/dInfant | Safety can be assuredEnd assessment |
| Toxicity | Five-day dog study, neurological NOAEL with 100× extrapolation factor, in vitro screens to exclude other toxicity | 0.01–1 mg/kg/d |
The key question which arose from the toxicity assessment was the relative potency of pseudomethrin versus the reference pyrethroids. The MEA IC50 assay allowed a comparison to be made, although the relationship between this in vitro assessment and the in vivo activity was varied. Applying a conservative assumption to the IC50 extrapolation allowed another estimate of the toxicity of pseudomethrin to be made. The visualization of the range for toxicity on the RISK21 matrix highlighted the contribution to the range of the default 100× animal-to-human extrapolation factor. Two factors were highlighted as requiring further investigation: confirmation of the in vivo potency and whether the default 100× factor from conventional studies was appropriate.
The use of the matrix allows the conservative assumptions which were built in to the initial assessments of toxicity to be visualised. In many risk assessments, a point estimate is provided which can give a misleading indication of the precision of the toxicity estimate as it may contain large uncertainty factors which are not acknowledged. The display of a range shows the assessor the level of uncertainty in the estimate of toxicity. If safety can be assured even with a high level of uncertainty over toxicity, then a decision can be made. If not, then the matrix highlights the need to improve the precision of the estimate of toxicity.
The course of action which was proposed focused on the species from which the reference NOAELs is derived, i.e., the dog. It was noted that the lead effect of neuroactivity could be identified and characterized for dose–response during the first 3–4 d of a 90-d or 1-year study. It was then proposed to investigate the comparative kinetics of the oral dose in the dog and the different routes of exposure (dermal from dipping and release from the net via the dermal and oral routes). This course of action would have used 24 dogs in highly targeted studies including kinetics.
In addition to characterizing the neuroactivity, the more general question remained regarding whether pseudomethrin might have the capability to cause other adverse effects. This is an area of much current interest. The methodology proposed by Thomas et al. (2013) addresses this question by postulating two types of toxicity. The first can be called general or non-specific toxicity and is related to cytotoxicity in key organs such as the liver and kidney related to distribution of the chemical or its metabolite. Cytotoxicity assays appear to predict this type of toxicity. The second type of toxicity is specific toxicity and requires the chemical to interact with a biological system in a specific way such as binding to a receptor or inhibiting an enzyme. A second batch of assays identifies this type of activity. The analysis provided by Thomas et al. (2013) shows that this methodology provides an estimated in vivo NOAEL which is an order of magnitude lower than the equivalent in vivo NOAEL, which errs on the side of safety. Putting a chemical through this battery gives guidance as to other areas which may have required further investigation.
The similarity of the profile for toxicity generated by the full package and that generated by the step-wise approach tested here is remarkable. The difference in the resources, time, and animals required to come to similar conclusions is large. However, it must be acknowledged that this case study was designed to examine what could be achieved with a chemical which belonged to a well-characterized class of chemistry. This was selected to test the philosophy inherent in the RISK21 roadmap and matrix that pre-existing knowledge should be used, thus demonstrating the value of this approach where there is knowledge about the chemical class. The challenge remains in applying a new approach to the assessment of chemicals which belong to a class which has not been extensively examined. However, the methodology proposed by Thomas et al. (2013) has suggested ways in which this could be achieved.
The RISK21 roadmap and matrix provided a framework for a stepwise assessment of a chemical with a particular use. It demonstrated the concept of using existing information to drive the generation of additional data using a value-of-information approach. The use of the matrix highlighted whether developing new exposure or toxicity information would have a greater impact on honing the risk assessment and reducing uncertainties. It also showed how new methodologies such as the use of in vitro studies and assays could be used to answer the specific questions which arise through the use of the matrix. The matrix also proved to be a useful way for the team to communicate to others the rationale behind their requests for new studies and the progress of their assessment.
Acknowledgements
The authors gratefully acknowledge the government, academic, and industry scientists of the HESI RISK21 Technical Committee for their contributions to this work. (For a full list of RISK21 participants, see http://www.risk21.org.) The authors also gratefully acknowledge the comments of three anonymous reviewers who were selected by the Editor. These comments were very helpful in revising the manuscript.
Declaration of interest
This manuscript was prepared under the auspices of the International Life Sciences Institute Health and Environmental Sciences Institute (ILSI HESI), a non-profit organization aimed at engaging scientists from academia, government, industry, research institutes, and NGOs to identify and resolve global health and environmental issues. The authors’ affiliations are as shown on the cover page. The authors had sole responsibility for the writing and content of the paper. The views and opinions expressed in the paper are those of the authors, and do not necessarily reflect the views of the authors’ employers or the opinions or policies of the US EPA or NIH. Mention of trade names does not constitute endorsement. None of the authors has recently or is currently involved as an expert witness in litigation or formal government rule making on the subject of this paper. The authors employed by ILSI HESI participated as part of their normal employment. None of the authors received financial support or an honorarium in the preparation of this paper, with the exception of Dr. John Doe whose work was supported by Syngenta. The authors declare that there are no conflicts of interest.
Glossary
Abbreviations
- BMD
benchmark dose
- EPA
United States Environmental Protection Agency
- FAO
Food and Agriculture Organization of the United Nations
- HESI
Health and Environmental Sciences Institute
- ILSI
International Life Sciences Institute
- MEA
microelectroarrays
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Cooperation and Development
- PBPK
physiologically-based pharmacokinetic
- RISK21
Risk Assessment in the 21st Century
- SAR
structure-activity relationship
- WHO
World Health Organization
References
- Barlow S, Sullivan F, Lines J. Risk assessment of the use of deltalmethrin on bednets for the prevention of malaria. Food Chem Toxicol. 2001;39:407–422. doi: 10.1016/s0278-6915(00)00152-6. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Jacobi S, Melber C, Wahnschaffe U, Simetska N, Mangelsdorf I. REPDOSE: a database on repeated dose toxicity studies of commercial chemicals – a multifunctional tool. Regul Toxicol Pharmacol. 2006;46:202–210. doi: 10.1016/j.yrtph.2006.05.013. [DOI] [PubMed] [Google Scholar]
- California EPA. Deltamethrin risk characterization. Sacramento, CA: California Environmental Protection Agency, Health Assessment Section, Medical Toxicology Branch, Department of Pesticide Regulation; 2000. [Google Scholar]
- Dorne JL, Renwick AG. The refinement of uncertainty/safety factors in risk assessment by the incorporation of data on toxicokinetic variability in humans. Toxicol Sci. 2005;86:20–26. doi: 10.1093/toxsci/kfi160. [DOI] [PubMed] [Google Scholar]
- Embry M, Bachman A, Bell D, Boobis A, Cohen S, Dellarco M, Dewhurst I. Risk assessment in the 21st century (RISK21): roadmap and matrix. Crit Rev Toxicol. 2014;44(Suppl 3):6–16. doi: 10.3109/10408444.2014.931924. [DOI] [PubMed] [Google Scholar]
- EPA. Pyrethrins/pyrethroid cumulative risk assessment. Washington, DC: US Environmental Protection Agency, Office of Pesticide Programs; 2011. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 185 for fenpropathin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 1993. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides data sheet 83 for resmethrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 1996. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 331 for permethrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 1999. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 204 for esfenvalerate. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2002a. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 743 for prallethrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2002b. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 385 for cyfluthrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2003a. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 463 for lambda cyhalothrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2003b. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 750 for s-bioallethrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2005. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 118 for cypermethrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2006. [Google Scholar]
- FAO. WHO specifications and evaluations for public health pesticides evaluation report 415 for bifenthrin. Geneva, Switzerland: World Health Organization, Food and Agriculture Organization of the United Nations; 2010. [Google Scholar]
- Hughes MF, Edwards BC. In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicol Appl Pharmacol. 2010;246:29–37. doi: 10.1016/j.taap.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Leahy D. Integrating in vitro ADMET data through generic physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol. 2006;2:619–628. doi: 10.1517/17425255.2.4.619. [DOI] [PubMed] [Google Scholar]
- Losa S, Johnstone A, Shafer T. Relative potencies of type I and type II pyrethroids for inhibition of spontaneous firing in neuronal networks. Abstract # 2126. Toxicologist. 2009 [Google Scholar]
- OECD. Guidance on grouping of chemicals. Environment health and safety publications No 80 ENV/JM/MONO(2007)28. Paris, France: Organisation for Economic Co-operation and Development; 2007. [Google Scholar]
- Pastoor T, Bachman A, Bell D, Cohen S, Dellarco M, Dewhurst I, Doe J. A 21st century roadmap for human health risk assessment. Crit Rev Toxicol. 2014;44(Suppl 3):1–5. doi: 10.3109/10408444.2014.931923. [DOI] [PubMed] [Google Scholar]
- Soderlund D, Clark J, Sheets L, Mullin L, Piccirillo V, Sargent D, Stevens J, Weiner M. Mechanisms of pyrethroid toxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Philbert MA, Auerbach SS, Wetmore BA, Devito MJ, Cote I, Rowlands JC. Incorporating new technologies into toxicity testing and risk assessment: moving from 21st century vision to a data-driven framework. Toxicol Sci. 2013;136:4–18. doi: 10.1093/toxsci/kft178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling EN, Neal AP, Atchison WD. Pyrethroids and their effects on ion channels. In: Soundararajan RP, editor. Pesticides – advances in chemical and botanical pesticides. InTech; 2012. http://www.intechopen.com/books/pesticides-advances-in-chemical-and-botanical-pesticides/pyrethroids-and-their-effects-on-ion-channels [DOI] [Google Scholar]
- WHO. WHO Publication WHO/CDS/WHOPES/GCDPP/2004.6 WHO/PCS/04.1. Geneva, Switzerland: World Health Organization; 2004. A generic risk assessment model for insecticide treatment and subsequent use of mosquito n.ets. [Google Scholar]
- Wolansky M, Gennings C, Crofton K. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol Sci. 2006;89:271–277. doi: 10.1093/toxsci/kfj020. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Bachman A, Barrett G, Bellin C, Goodman JI, Jensen E, Moretto A. Illustrative case using the RISK21 roadmap and matrix: prioritization for evaluation of chemicals found in drinking water. Crit Rev Toxicol; [DOI] [PMC free article] [PubMed] [Google Scholar]