We discuss the methodological issues associated with measuring stress hormones in wild animals. We discuss five questions that we think should be considered about the use of stress hormone measurements in conservation physiology. We present a meta-analysis showing that human activities consistently increase stress hormone levels across vertebrates.
Keywords: Anthropogenic disturbance, biodiversity, conservation physiology, fitness, meta-analysis, stress
Abstract
Conservation physiology proposes that measures of physiological stress (glucocorticoid levels) can be used to assess the status and future fate of natural populations. Increases in glucocorticoids may reflect a more challenging environment, suggesting that the influence of human activities on free-living animals could be quantified by measuring glucocorticoids. Biomedical studies suggest that chronic increases in glucocorticoids can have detrimental effects on survival and reproduction, which could influence the viability of populations. Here, we discuss the use of measurements of glucocorticoids in conservation physiology. We first provide an overview of the different methods to quantify glucocorticoids and their utility in conservation physiology. We then discuss five questions we think are essential for conservation physiologists to address. We highlight how intrinsic (e.g. sex, reproductive status, age, recent experiences) and ecological factors (e.g. predation, food availability, snowfall) can, by themselves or through their interactions with anthropogenic disturbances, affect the physiological stress response and mask any general patterns about the effects of anthropogenic disturbances on glucocorticoids. Using a meta-analysis, we show that anthropogenic disturbances are consistently associated with increased glucocorticoids regardless of the type of human disturbance. We also show that males may be more sensitive to anthropogenic disturbances than females and that faecal glucocorticoids, but not baseline plasma glucocorticoids, consistently increase in response to anthropogenic disturbances. Finally, we discuss how increases in glucocorticoids in free-living animals can sometimes enhance survival and reproduction. Unfortunately, our literature analysis indicates that this observation has not yet gained traction, and very few studies have shown that increases in glucocorticoid levels resulting from anthropogenic disturbances decrease survival or reproduction. We think that the use of measures of glucocorticoids in conservation physiology has tremendous potential, but there are still a number of methodological concerns, in addition to several crucial questions that should be addressed.
Introduction
The recent loss of global biodiversity is striking. Of the species that have been assessed, the International Union for Conservation of Nature (IUCN) classifies 22% of mammal, 14% of bird, 21% of reptile and 31% of amphibian species as extinct or threatened with extinction (IUCN, 2013). To ameliorate these biodiversity losses, we need a clear understanding of our role in it. How have environmental and anthropogenic disturbances impacted animal survival and reproduction (i.e. fitness)? Ideally, potential changes in fitness could be predicted, but this is made particularly difficult because we generally do not have a benchmark of population (e.g. growth rate, size, distribution) or demographic parameters (e.g. survival, reproduction) against which the predicted changes could be assessed. Conservation physiology proposes to fill this gap by: (i) quantifying the physiological responses of individuals to disturbance; (ii) determining how these responses affect behaviour, survival and reproduction; and (iii) understanding whether individual-level responses scale up to impact the viability of populations (Wikelski and Cooke, 2006; Cooke et al., 2013). Essentially, it will provide a mechanistic link between the individual animals and the demographic outcome.
A major focus in conservation physiology is the causes and consequences of physiological stress (Wingfield et al., 1997; Cockrem, 2005; Walker et al., 2005; Wikelski and Cooke, 2006; Busch and Hayward, 2009). Environmental challenges or anthropogenic disturbances can trigger activation of the vertebrate neuroendocrine axis that often results in the release of stress hormones (glucocorticoids). Glucocorticoids are fundamental to how animals integrate, cope with and respond to both predictable and unpredictable perturbations in their environment, and they are closely tied to individual performance and fitness, and perhaps to population dynamics (Boonstra et al., 1998; Moore and Jessop, 2003; Breuner et al., 2008; Bonier et al., 2009; Crespi et al., 2013). Measures of glucocorticoid levels can therefore provide quantitative information about how environmental changes impact individuals (Tarlow and Blumstein, 2007). They may even act as an ‘early warning system’ to portend future population declines (Wikelski and Cooke, 2006; Drake and Griffen, 2010; Sheriff et al., 2011a; Fefferman and Romero, 2013) and could be used as a ‘trigger point’ in conservation monitoring programmes, where threshold levels of glucocorticoids could be set and predetermined management plans implemented if those thresholds are reached (Lindenmayer et al., 2013).
In this review, we argue that a detailed understanding of physiological stress through measurements of glucocorticoids plays a central role in conservation biology and physiology by aiding our understanding of the consequences of anthropogenic disturbances or global climate change. We caution that there are also many limitations to this approach and a great deal of fundamental research remains to be done in conservation physiology, especially using an experimental approach. We urge more critical and thorough assessments and interpretations of measures of physiological stress, such as glucocorticoid levels obtained from free-living animals.
What is physiological stress in vertebrates?
Stress is an ambiguous word given that ‘stress’ can be used to define both the environmental perturbation (‘stressor’) and the suite of physiological responses to the perturbation (‘stress’) so that the definition becomes somewhat circular (‘stressors cause stress’). Physiological stress is frequently described as the group of adaptive physiological responses (‘general adaptation syndrome’; Seyle, 1936) to an aversive extrinsic stimulus (a ‘stressor’) that helps to restore internal homeostasis (Cannon, 1932) following exposure to the aversive stimulus. However, it is important to remember that the homeostatic set points that the stress response is trying to restore can change seasonally or according to life history stage, and with other intrinsic (e.g. age, body condition, reproductive status) or extrinsic factors (e.g. weather, predation risk). To account for this, McEwen and Wingfield (2003) introduced the concept of allostasis or ‘maintaining constancy through change’ to physiological ecology. Allostasis describes the range of predictive and responsive physiological processes that assist in the maintenance of homeostasis and explicitly recognizes that homeostatic set points are not fixed and that organisms have evolved physiological mechanisms that enable them to maintain some degree of internal constancy in the face of a variable environment (McEwen and Wingfield, 2003).
Here, we define physiological stress as the multidimensional physiological response to predictable and unpredictable environmental stimuli (stressors) that challenge internal stability or homeostasis, though we recognize that these homeostatic set points can fluctuate. This includes physiological stress responses associated with coping with a change in the environment, such as an unpredictable anthropogenic disturbance. It also includes physiological stress responses that occur in anticipation of environmental changes, such as seasonal changes in food availability or temperature, or in preparation for increased energetic requirements, such as during reproduction or migration. Most of the following discussion concerns the most common measure of physiological stress, glucocorticoid levels.
Chronic vs. acute stress in conservation physiology
The vertebrate neuroendocrine response is facilitated largely by the hypothalamic–pituitary–adrenal axis (HPA axis; in birds and mammals) or hypothalamic–pituitary–inter-renal axis (HPI axis; in amphibians, fish and reptiles), which are reviewed elsewhere (Sapolsky et al., 2000; Sapolsky, 2002). Environmental challenges that activate these vertebrate neuroendocrine axes can be either acute or chronic stressors. Acute stressors, such as a severe storm or pursuit by a predator, can elevate glucocorticoid levels in the blood and cause a range of physiological and behavioural changes that facilitate coping with the environmental challenge (Wingfield et al., 1998). Although temporary increases in glucocorticoids during reproduction could theoretically threaten the viability of populations by inhibiting reproduction (e.g. foregoing reproduction entirely, causing abortions or brood abandonment; Young et al., 2006; Ouyang et al., 2012), conservation physiologists are, for the most part, interested in sustained or chronic increases in physiological stress. These chronic increases in glucocorticoid levels are thought to inhibit reproduction and may also reduce survivorship (Sapolsky et al., 2000), though the evidence for this in free-living animals is inconsistent (see sections on Questions 4 and 5 below).
Chronic stress has traditionally been thought to represent a dysregulation of the HPA or HPI axis (Sapolsky et al., 1986) or allostatic (McEwen and Wingfield, 2003) or homeostatic overload (Romero et al., 2009) that is brought on by chronic exposure to unpredictable or uncontrollable environmental challenges. Until recently, most physiological ecologists working on natural populations have accepted this traditional view (Boonstra, 2013). We think that this view is likely to apply to animals exposed to anthropogenic disturbances and, thus, it provides insight into what conservation physiologists should look for. The classic symptoms of chronically stressed individuals are as follows: (i) higher baseline plasma glucocorticoid levels; (ii) increased acute increases in plasma glucocorticoids following an environmental challenge; and (iii) an increased amount of time taken to return plasma glucocorticoid levels back to baseline (Sapolsky et al., 1986, 2000; Sapolsky, 1999; Dickens and Romero, 2013). This dysregulation of the HPA or HPI axis is primarily caused by a loss of negative feedback, in which chronic stress decreases the number of glucocorticoid receptors in key regulatory parts of the brain, such as the hippocampus and hypothalamus (Sapolsky et al., 1984; Romero, 2004; Dickens et al., 2009). Chronically stressed individuals therefore tend to experience a larger cumulative exposure to glucocorticoids, which is thought to induce a series of pathological effects (Sapolsky et al., 1986; Sapolsky, 2005; McEwen and Wingfield, 2010), though again this is mostly restricted to studies in humans or laboratory animals (see sections on Questions 4 and 5 below).
How to measure chronic stress in free-living animals
Conservation physiology is predicated on the assumption that biomarkers of physiological stress can be used to determine reliably whether an environmental challenge (ecological or anthropogenic) induces chronic physiological stress. A crucial endeavour in conservation physiology is therefore to develop standardized methods to quantify chronic stress in free-living animals (Tarlow and Blumstein, 2007; Sheriff et al., 2011b). Glucocorticoid levels are frequently used to quantify physiological or chronic stress (Sapolsky et al., 2000), and there are a number of sources from which glucocorticoids can be measured in free-living animals, namely from blood (plasma or serum), saliva, faeces, urine, hair or feathers (Sheriff et al., 2011b). Each of these different sources carries with it a number of caveats, which have been extensively reviewed elsewhere (Tarlow and Blumstein, 2007; Sheriff et al., 2011b; Breuner et al., 2013). There are also a number of downstream measures (e.g. glucose, free fatty acids, body mass, telomere length, oxidative stress) that can be used as biomarkers of chronic stress. We briefly discuss on these downstream measures very briefly below because they have recently been reviewed elsewhere (Monaghan et al., 2009; Breuner et al., 2013; Dickens and Romero, 2013; Beaulieu and Costantini, 2014). Here, we focus specifically on the potential value of these methods to quantify glucocorticoid levels for conservation physiologists.
Instantaneous measures of stress: plasma, serum or salivary glucocorticoids
Individuals under chronic stress can exhibit elevated baseline blood glucocorticoids, a stronger increase in blood glucocorticoids in response to some environmental challenge or a reduced ability to terminate the stress response (Sapolsky et al., 1986; Sapolsky, 2002; Dickens and Romero, 2013). Baseline glucocorticoids (Bonier et al., 2009), the magnitude of the stress response (Romero and Wikelski, 2001; Wikelski et al., 2002; Blas et al., 2007; Breuner et al., 2008; MacDougall-Shackleton et al., 2009) and the ability to terminate it (Romero and Wikelski, 2010) may also be tied closely to survival in free-living animals.
Baseline glucocorticoid levels can be measured by obtaining blood samples within ∼3 min of initial capture (Romero and Reed, 2005). In fish and perhaps other aquatic species, baseline glucocorticoid levels may also be measured by determining the amount of glucocorticoids or their metabolites that are excreted into holding water (Wysocki et al., 2006; Scott and Ellis, 2007; Scott et al., 2008). However, these measures of glucocorticoids in holding water may actually represent stress-induced glucocorticoid levels because holding water is generally obtained by moving fish from one large aquarium to another (Wong et al., 2008). Salivary glucocorticoid levels obtained from terrestrial vertebrates are also thought to reflect baseline glucocorticoid levels in the blood with a 20 min lag (Kirschbaum and Hellhammer, 1989). The magnitude of the stress response can be assessed by obtaining a series of blood samples at set time points following a standardized restraint stress test (Romero et al., 2008) or an injection of adrenocorticotrophic hormone (Sheriff et al., 2011b). The magnitude of the stress response can also be assessed in fish by measuring the amount of glucocorticoids excreted into holding water after a simulated stressor (Scott and Ellis, 2007; Wong et al., 2008). The ability to terminate the stress response through negative feedback can be assessed by obtaining a blood sample at a set time point following an injection of dexamethasone, which binds to glucocorticoid receptors and should ultimately cause a decrease in the synthesis of glucocorticoids in the adrenal glands (Sapolsky and Altmann, 1991; Boonstra et al., 1998).
In a recent review of all studies purportedly measuring the effects of chronic stress in laboratory and wild animals, Dickens and Romero (2013) found that baseline or stress-induced glucocorticoid levels in blood samples did not change in a consistent manner in response to chronic stress. Baseline or stress-induced plasma glucocorticoid levels sometimes increased, decreased or did not change in response to chronic stress. In contrast, chronic stress consistently decreased the ability to exert negative feedback and terminate the stress response in nearly all studies using dexamethasone injections (n = 19; Dickens and Romero, 2013), though there are some clear exceptions from studies of free-living animals under chronic stress (Boonstra et al., 1998; Sheriff et al., 2011a). The ambiguity in the effects of chronic stress on baseline or stress-induced blood glucocorticoid levels cautions that they may not be useful for conservation physiologists. Some of this ambiguity is likely to be due to the difficulty of obtaining baseline blood samples, at least in mammals (within 3 min of capture; Romero and Reed, 2005), and potentially, habituation to the chronic stress methodology itself that is used in laboratory settings (Cyr and Romero, 2009). It could also be due to differences in measures of total (bound and unbound to corticosteroid-binding globulin) vs. free glucocorticoids (unbound to corticosteroid-binding globulin; Breuner et al., 2013). For example, exposure to physiological stress could decrease corticosteroid-binding globulin, thereby increasing free glucocorticoids (the biologically active portion) while not changing total glucocorticoids (Boonstra et al., 2001).
Unfortunately, measures of the magnitude of the stress response or the ability to terminate the stress response are not practical for most species of conservation concern. They require a series of blood samples to be obtained following restraint and injections (dexamethasone or adrenocorticotrophic hormone) to quantify plasma glucocorticoid levels (Sheriff et al., 2011b). The live-capture, restraint and anaesthesia of mammals to obtain these measures reduces the ability to measure baseline plasma glucocorticoid levels that should be obtained within 3 min of initial capture and can also carry significant costs due to capture-related mortality (Jacques et al., 2009). Measures of salivary glucocorticoid levels in terrestrial vertebrates or measures of glucocorticoids or their metabolites in holding water in aquatic vertebrates have not been used as extensively. Both measures could be useful for conservation physiologists (e.g. Wysocki et al., 2006) given that they may reflect baseline levels (at least in the case of salivary glucocorticoids) but do not require blood drawing. However, obtaining both of these types of samples generally still requires capture and restraint, and so their usefulness in conservation physiology may therefore be limited.
Integrated measures of glucocorticoids: faeces, urine, feathers and hair
Individuals experiencing chronic stress are thought to experience a higher cumulative exposure to glucocorticoids (Sapolsky et al., 1986; Sapolsky, 2005). Integrated measures of glucocorticoid levels circulating in the blood, such as those from faeces, urine, feathers, hair or holding water (for aquatic species only), reflect an integrated average of blood glucocorticoids that individuals have secreted, metabolized and excreted over a species-specific duration (Sheriff et al., 2011b). Integrated measures of glucocorticoids may therefore more closely reflect the cumulative exposure of individuals to glucocorticoids rather than point samples obtained from blood or saliva and may be the only practical measures that conservation physiologists can use to quantify chronic stress.
Faecal glucocorticoid metabolite (FGM) levels have been widely used in conservation physiology and represent one of the least invasive measures of physiological stress because they can be obtained without capture in some species. Faecal glucocorticoid metabolites reflect free (unbound to corticosteroid-binding globulin) plasma glucocorticoid levels (Sheriff et al., 2010) that an individual has experienced over a species-specific amount of time (Palme et al., 2005; Sheriff et al., 2011b). Urinary glucocorticoid levels may be useful in some contexts but are, in general, extremely difficult to collect from free-living species except perhaps arboreal primate species (but see Creel, 2001 for examples).
The use of hair and feathers as integrated measures of glucocorticoids is still in its infancy, and there are a number of methodological concerns associated with these methods that need to be resolved (Sheriff et al., 2011b; Keckeis et al., 2012; Meyer and Novak, 2012). For example, glucocorticoid levels in hair may not be representative of circulating levels of plasma glucocorticoids (Keckeis et al., 2012), and hair sampled from different parts of the body might have different glucocorticoid levels (Ashley et al., 2011; Terwissen et al., 2013).
In support of their usefulness in measuring chronic stress, Dickens and Romero (2013) found that integrated measures of glucocorticoid levels, such as FGM levels, were significantly higher in response to chronic stress. Although one study in captive birds exposed to chronic stress shows that FGM levels decrease instead of increase (Cyr and Romero, 2008), this seems to be an exception. Of all the different methods available, integrated measures of glucocorticoids, especially FGM levels, appear to be the most reliable indicator of chronic stress and are also perhaps the most practical and least invasive (Sheriff et al., 2011b). Of course, with any measure of physiological stress, they need to be compared against baseline data, such as those before the environmental or anthropogenic disturbance, and not necessarily compared with measures of physiological stress from another population (see section on Question 1 below).
Downstream measures of chronic stress
Although our focus is on measures of glucocorticoids, there is considerable unexplained variation in the relationship between measures of glucocorticoids and life-history traits (see section on Question 4 below). This may require a broader analysis of physiological stress, such as by measuring the downstream effects of chronic stress (Breuner et al., 2013). Here, we discuss a limited number of metrics that could be useful for conservation physiologists that are reviewed elsewhere (Monaghan et al., 2009; Breuner et al., 2013; Dickens and Romero, 2013).
Glucose levels are predicted to increase in conditions of chronic stress (Fujiwara et al., 1996), which has been observed in some studies in free-living animals (Boonstra et al., 1998; Ruiz et al., 2002; Clinchy et al., 2004; Sheriff et al., 2011a). Free fatty acids and haematocrit levels in free-living animals have been observed to decrease in environments where individuals were presumed to be under chronic stress (Hellgren et al., 1993; Boonstra et al., 1998; Clinchy et al., 2004; Sheriff et al., 2011a). Immune responses are predicted to decline under chronic stress (Sapolsky et al., 2000; Dhabhar, 2009; Martin, 2009), and this has been observed in a number of studies in free-living animals that have used relatively simple measures of immune function, such as counts of leukocytes and lymphocytes (Baker et al., 1998; Boonstra et al., 1998; Hanssen et al., 2003; Clinchy et al., 2004; Davis, 2005; Lobato et al., 2005; Travers et al., 2010; Sheriff et al., 2011a). Chronic stress is also thought to inhibit reproduction (Rivier and Rivest, 1991; Sapolsky et al., 2000; Kirby et al., 2009), and levels of specific reproductive hormones have been observed to decrease in free-living animals thought to be under chronic stress (testosterone: Sapolsky, 1985; Boonstra et al., 1998; progesterone: Hackländer et al., 2003; and prolactin: Delehanty et al., 1997). Measures of oxidative stress (Monaghan et al., 2009; Beaulieu and Costantini, 2014) or telomere shortening rates (Nussey et al., 2014) potentially caused by increased oxidative damage (von Zglinicki, 2002; Monaghan and Haussmann, 2006; Haussmann et al., 2012) or increased physiological stress (Costantini et al., 2011; Haussmann et al., 2012; Herborn et al., 2014) may be useful downstream measures of chronic stress, but they have yet to be used in a conservation context (but see Beaulieu and Costantini, 2014). A drawback for conservation studies is that most of these downstream metrics of chronic stress necessitate the use of blood sampling, with the exception of some reproductive hormone metabolites that can be measured in faeces (Sheriff et al., 2011b).
Body mass is also predicted to decline under chronic stress, and interestingly, it is a consistent biomarker of chronic stress (Dickens and Romero, 2013). Unfortunately, the measurement of body mass in free-living animals typically requires capture and restraint or ingenious ways of training animals to provide such body mass data (e.g. habituation and training individuals to go on to laboratory balances for a food reward).
As a result of these practical drawbacks of downstream measures of chronic stress and because they simply have not been used as frequently, we narrow our discussion to measures of glucocorticoids or the metabolites of glucocorticoids in plasma/serum, faeces, feathers and hair.
Methodological limitations of measuring chronic stress in wild animals
One of the key considerations in using any method in conservation must be the degree of invasiveness that is possible or desirable (Cooke and O'Connor, 2010). Measures of chronic stress vary in their degree of invasiveness (Sheriff et al., 2011b). However, even with use of the relatively non-invasive methods the application of stress hormones in conservation physiology remains imperfect because of several attributes that we review briefly below.
Species specificity
All methods to measure chronic stress require an appropriate validation for each species (Sheriff et al., 2011b). For example, many studies have validated the use of enzyme immunoassays to measure FGM levels in ground and tree squirrels (Bosson et al., 2009, 2013; Dantzer et al., 2010; Sheriff et al., 2012). Even in these relatively closely related species, an appropriate and thorough validation must occur (Buchanan and Goldsmith, 2004; Touma and Palme, 2005; Sheriff et al., 2011b). For rare or endangered species where it may not be feasible to obtain the samples required for a rigorous validation, it is appropriate to validate methods with captive or zoo animals of the same species.
Daily and seasonal changes
Glucocorticoids fluctuate on a normal circadian rhythm, with peaks in blood occurring immediately prior to daily activity and a continuous slow decline throughout the day (Romero, 2002; Sapolsky, 2002). These changes in glucocorticoid levels can cause changes in all other measures of glucocorticoids or their metabolites (in faeces, urine and saliva), with the possible exception of hair and feathers. Glucocorticoids can also fluctuate seasonally, often with peaks occurring during the breeding season (Harper and Austad, 2001; Sheriff et al., 2012). Thus, the potential for daily and seasonal changes in glucocorticoids needs to be avoided by collecting samples at the same time of day or season, or their effects need to be incorporated into the interpretation of results.
Sex, reproductive status and age
The sex, reproductive status and age of an animal all have the ability to influence adrenal glucocorticoid production (Romero, 2002; see section on Question 1 below). For example, Dantzer et al. (2010) found that reproductive condition significantly affected FGM levels in female North American red squirrels (Tamiasciurus hudsonicus), with pregnant females having the highest glucocorticoid levels and non-breeding females the lowest; however, reproductive condition did not affect FGM levels in males. Thus, it is important to collect samples from individuals of known reproductive status where possible, but also to understand the impact of changes in glucocorticoid levels in order to interpret results appropriately (see further discussion in the section on Question 1 below).
Faecal glucocorticoid metabolite levels
As a result of the non-invasive nature of the technique and the relative ease of collection, FGM measures have become increasingly used with free-ranging animals and in conservation studies. However, several confounding factors that can limit the utility of FGM measures are sometimes overlooked, though they have been highlighted in detail (Buchanan and Goldsmith, 2004; Millspaugh and Washburn, 2004; Palme, 2005; Sheriff et al., 2011b; Goymann, 2012). Dietary changes and metabolic rate of animals can alter the way in which hormones are metabolized or excreted, making comparisons across seasons or between populations eating different foods difficult (Goymann et al., 2006; Dantzer et al., 2011; Goymann, 2012). Faecal glucocorticoid metabolite levels can also be affected by precipitation, temperature and microbial degradation after defaecation (Washburn and Millspaugh, 2002; Stetz et al., 2013). Faecal glucocorticoid metabolites may not be evenly distributed throughout the faecal mass and, thus, the entire mass should be homogenized prior to subsampling (Millspaugh and Washburn, 2003; Sheriff et al., 2011b). Any preservation treatment other than freezing can alter FGM levels (Palme, 2005; Sheriff et al., 2011b) and, because of differing affinities for FGMs, the antibody used in the immunoassay will alter FGM results (Sheriff et al., 2011b). This last point highlights that it is generally not appropriate to compare absolute FGM values from two different studies unless standardized field and laboratory techniques are used.
Essential questions for incorporating measures of physiological stress into conservation physiology
Similar to authors of other reviews, we think that measures of physiological stress, such as glucocorticoids, can be a key tool in conservation physiology. However, we emphasize here that there are five essential questions that should be considered by conservation physiologists if they are to use measures of glucocorticoids to manage populations, to mitigate anthropogenic disturbances or even to inform governmental policy (Cooke and O'Connor, 2010). Our aim within this section is to strengthen the field of conservation physiology by challenging some of the common assumptions in conservation physiology and, more generally, in physiological ecology.
Question 1: how do intrinsic factors modify physiological stress responses to environmental challenges?
A variety of intrinsic factors, such as sex, age, reproductive condition, developmental or adult experiences, or interactions between these factors can influence the magnitude of the physiological stress response to environmental challenges or anthropogenic disturbances (Fig. 1). These intrinsic factors may reduce the ability of conservation physiologists to detect physiological stress responses to an anthropogenic disturbance. For example, in free-living northern spotted owls (Strix occidentalis caurina), there are sex differences in the effect of logging roads on FGM levels. Male owls but not females living in areas closer to logging roads or timber harvest exhibit higher FGM levels (Wasser et al., 1997). Males also exhibit a much more pronounced rise in FGM levels in response to experimental increases in traffic noise compared with females (Hayward et al., 2011). In contrast, in American kestrels (Falco sparverius), only females but not males exhibit higher baseline plasma total corticosterone levels in the presence of anthropogenic disturbances (amount and speed of road traffic, human development: Strasser and Heath, 2013). Thus, not only can there be sex-specific differences in the responsiveness of the HPA or HPI axis to an anthropogenic disturbance, but also the sex that is more responsive may be species specific (see also Ahlering et al., 2013).
Figure 1:
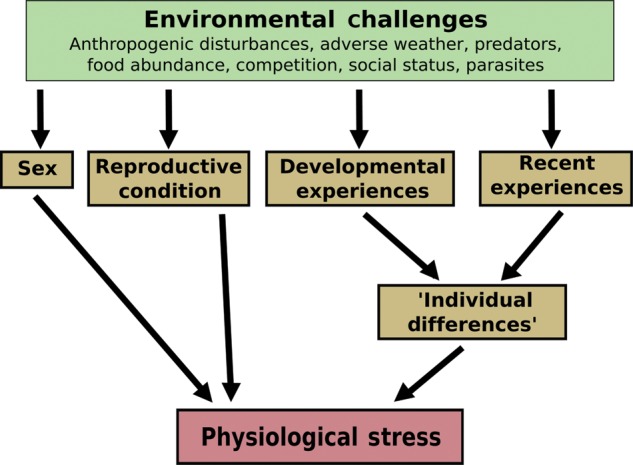
Differences in the intrinsic characteristics of individuals can modify the relationship between environmental challenges (‘stressors’) and measures of physiological stress, such as glucocorticoids. There are many environmental challenges that elicit a physiological stress response, including anthropogenic disturbances and abiotic (weather) or biotic factors (predator or food abundance, competition, social status or parasite load). These anthropogenic and natural environmental challenges can also interact with each other to influence the physiological stress response. The physiological stress response of individuals to these environmental challenges can be modified by the sex of the individual, its reproductive condition (e.g. breeding or non-breeding season, pregnancy, lactation), through ‘individual differences’ that arise due to developmental or recent experiences that either sensitize or desensitize the physiological stress response to environmental challenges.
In seasonal breeders, changes in reproductive state can influence the magnitude of the physiological stress response to an environmental challenge (Handa et al., 1994). It is still quite rare for studies in conservation physiology to examine how differences in reproductive condition affect the physiological stress response to anthropogenic disturbances. In one exception, in maned wolves (Chrysocyon brachyurus) in Brazil, exposure to humans or their activities increased FGM levels in males and non-breeding females but not in reproductively active females (Spercoski et al., 2012). This difference could be because the HPA axis sensitivity of females to environmental challenges tends to be hyporesponsive during pregnancy (Brunton et al., 2008).
A lack of knowledge about the reproductive state of animals from which measures of physiological stress are obtained could reduce the ability of conservation physiologists to make correct inferences about the causes of increased glucocorticoid levels. For example, forest fragmentation has been associated with higher FGM levels in black howler monkeys (Alouatta pigra), yet these differences could have also been caused by differences in reproductive state (Martínez-Mota et al., 2007). Female howler monkeys (n = 2) in the non-fragmented forest were lactating, whereas those in the fragmented forest (n = 2) were not. Given that lactation affects FGM levels (Goymann et al., 2001; Dantzer et al., 2010), this suggests that differences in reproductive status, not fragmentation, may be the cause of these differences in FGM levels in black howler monkeys.
Seasonal changes in reproductive status can also co-occur with changes in anthropogenic disturbances that could lead to false inferences about the causes of physiological stress. For example, exposure to humans through tourism can be associated with increased FGM levels (Barja et al., 2007; Zwijacz-Kozica et al., 2013). However, tourism generally peaks in the spring and summer (Barja et al., 2007; Zwijacz-Kozica et al., 2013), which can coincide with breeding, when FGM levels are elevated (Romero, 2002). As such, tourism pressure and changes in reproductive status co-vary, making it difficult to determine the real cause of changes in glucocorticoid levels without an experimental approach. Future studies employing experimental manipulations of anthropogenic disturbances in both the breeding and non-breeding seasons (Arlettaz et al., 2007; Hayward et al., 2011; Blickley et al., 2012a; Crino et al., 2013) would help to disentangle the multiple causes of increased glucocorticoid levels.
The physiological stress response to environmental challenges can also change with age. In mammalian species, the HPA axis of altricial offspring tends to be hyporesponsive early in life (Wada, 2008), but as individuals grow older, their HPA axis can become hyper-responsive (Sapolsky et al., 1986; Sapolsky and Altmann, 1991). Given that older individuals often exhibit a more prolonged and exaggerated stress response to an environmental challenge, age could magnify the physiological stress response to environmental changes or anthropogenic disturbances. For example, elk (Cervus elaphus) in Yellowstone National Park have higher FGM levels when there is greater snowmobile activity in the area, but only after the effects of age and snow pack depth are controlled for statistically (Creel et al., 2002). Faecal glucocorticoid metabolite levels in elk increase with age (Creel et al., 2002), but it is unknown whether age impacts the physiological stress response to snowmobile activity. Older elk might exhibit a stronger increase in FGM levels in response to snowmobile activity such that the age structure of the population could be an important predictor of the effects of human disturbance on measures of physiological stress in elk or other among-population comparisons.
The presence of inter-individual differences in physiological stress responses may also obscure the ability of conservation physiologists to detect general patterns about the effects of anthropogenic disturbances on measures of glucocorticoids (Romero, 2004; Madliger and Love, 2014). Some individuals may consistently exhibit a greater physiological stress response to an environmental challenge (‘reactive individuals’), whereas others consistently exhibit a more dampened stress response (‘proactive individuals’: Korte et al., 2005; Koolhaas et al., 2010). There are multiple causes of inter-individual variation in HPA axis activity and reactivity, such as differences in developmental or early life experiences (Meaney, 2001; Harris and Seckl, 2011). In adults, distant or recent experiences that elicited a physiological stress response can also sensitize (Romero, 2004) or desensitize (Cyr and Romero, 2009; Lynn et al., 2010) the HPA axis response to the same or a novel environmental challenge in the future (Ellenberg et al., 2006; Walker et al., 2006). There are also a growing number of studies documenting that glucocorticoid levels are repeatable within individuals (e.g. Bosson et al., 2009; Dantzer et al., 2010; Ouyang et al., 2011; Cockrem, 2013; Lendvai et al., 2013). What is perhaps more important than documenting that repeatable individual differences exist, however, is to document whether individuals consistently differ in their physiological stress response to changes in intrinsic characteristics (e.g. age, reproductive condition) and extrinsic characteristics (e.g. food availability, weather patterns, anthropogenic disturbances). For example, plasma glucocorticoid levels increase with age in some humans, but decrease with age in others (Lupien et al., 1995, 1998). This highlights that there is among-individual phenotypic plasticity (Nussey et al., 2007) in the effects of age on glucocorticoid levels. This could mask any general patterns about the overall effects that an environmental challenge or anthropogenic disturbance have on a population (Madliger and Love, 2014). For example, in a population subjected to a new anthropogenic disturbance, reactive individuals might exhibit higher FGM levels, whereas proactive individuals might exhibit lower FGM levels, which could result overall in a non-significant change in FGM levels.
We think that these intrinsic characteristics that influence the magnitude of the physiological stress response to environmental challenges or anthropogenic disturbances (Fig. 1) should be an important consideration for conservation physiologists. If there are differences in the sex ratio, age structure, reproductive condition or proportion of reactive vs. proactive individuals, there may be differences in these measures of glucocorticoids regardless of the presence or absence of the anthropogenic disturbance. Given that anthropogenic disturbances could impose new sources of natural selection on behavioural or endocrine traits (Bonier, 2012; Snell-Rood and Wick, 2013), they could alter these population-level parameters (sex ratio, age structure, etc.) in a non-random fashion. For example, anthropogenic disturbances could select for proactive (or bold) individuals with dampened stress responses to human activities (Atwell et al., 2012). This, in addition to the methodological challenges associated with measuring FGM levels (see ‘Faecal glucocorticoid metabolite levels’ section above), makes among-population comparisons using FGM levels problematic, though of course in some cases they are unavoidable.
Question 2: are anthropogenic disturbances consistently associated with increased glucocorticoid levels?
The foundations of conservation physiology include the implicit assumption that physiological stress responses to an environmental challenge are consistent. Do anthropogenic disturbances consistently and uniformly increase glucocorticoid levels or cause chronic stress? To address this question, we performed a meta-analysis to examine the effect sizes of anthropogenic disturbances on baseline and integrated glucocorticoid levels. We calculated 59 effect sizes of the influence of human disturbance on glucocorticoid levels from 46 studies (Table S1; see supplementary material for detailed methods). We categorized four types of human disturbance: noise or disturbance from machines (n = 20); tourism (n = 13); urban habitat (n = 14); and habitat modification (n = 12). Our analysis included effect sizes from four different classes of vertebrates: Aves (n = 30); Mammalia (n = 20); Amphibia (n = 3); and Reptilia (n = 6). Glucocorticoids were quantified from faecal (n = 27), plasma (n = 28), hair (n = 2), feather (n = 1) and urine (n = 1) samples (Table S1). The plasma samples that we included in the analysis were considered to reflect baseline levels because they were obtained from animals prior to the capture-related increase in glucocorticoid levels (i.e. <3 min: Romero and Reed, 2005). We did not include studies that quantified stress-induced plasma glucocorticoid levels. We considered glucocorticoid and glucocorticoid metabolite levels measured in feathers, faeces, hair and urine to represent integrated glucocorticoid levels (see ‘Integrated measures of glucocorticoids: serum, urine, feathers and hair’ section above).
Across all vertebrate taxa addressed here, species exposed to human disturbance had significantly higher glucocorticoid levels (baseline plasma and integrated glucocorticoid levels together) when we pooled all effect size estimates from these 59 different effect size estimates. Positive effect sizes indicate that levels of glucocorticoids were higher in response to human disturbance. The weighted estimate of the effect size was 0.32 (J corrected Hedges's g; Gurevitch and Hedges, 2001), and the 95% confidence interval (±0.06) indicated that it was significantly greater than zero. An effect size of this magnitude suggests that human disturbance has between a small (0.2) and medium (0.5) effect on glucocorticoid levels (Cohen, 1969). There was one extremely high effect size (10.49) in our analysis, from a study of long-nosed bandicoots (Perameles nasuta), where bandicoots in urban areas had substantially higher faecal glucocorticoid metabolite levels than those in less disturbed areas (Dowle et al., 2013). However, excluding this point from our analysis does not have a large effect on the overall effect size (0.31 ± 0.06), indicating that human disturbance significantly increased glucocorticoid levels regardless of whether this outlier was included or not.
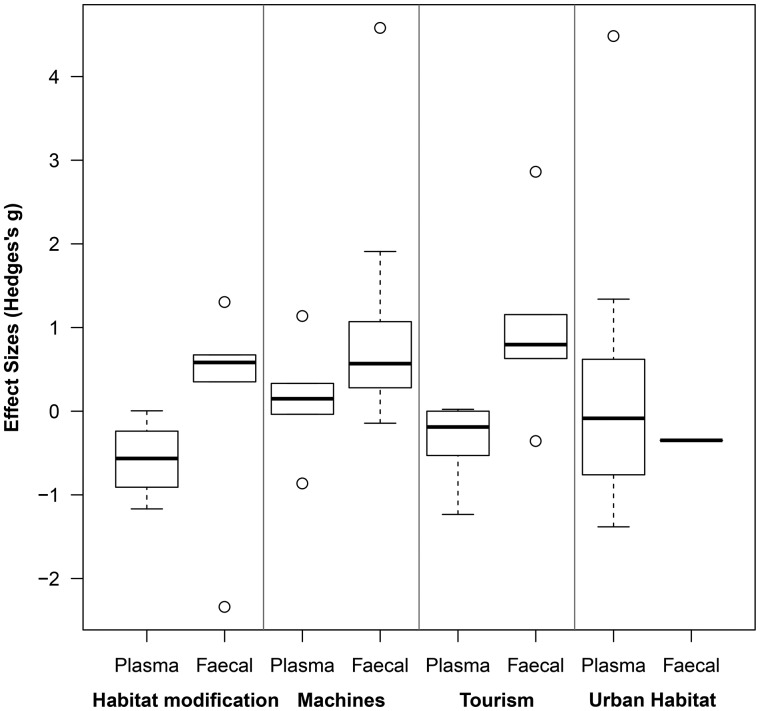
We next examined how the four types of human disturbance, glucocorticoid sample type and taxonomy influenced effect sizes in a single linear mixed-effects model (R package metaphor from Viechtbauer, 2010 in R version 3.0.2: R Development Core Team, 2013). In this analysis, we included only glucocorticoid levels quantified in faecal and baseline plasma samples because we did not have a sufficient amount of estimates with the other sample types (hair, feathers and urine; see above). We pooled the samples from amphibians and reptiles into a group called ‘herptiles’ (n = 9 studies) and compared them with the samples from mammals and birds. We removed the extremely high effect size from the long-nosed bandicoot (10.49: Dowle et al., 2013) from the analysis because its inclusion changed the interpretation of the effect of disturbance type on glucocorticoid levels (disturbance effect with its inclusion z = 2.09, P = 0.04). Excluding the estimate from the long-nosed bandicoot, the type of human disturbance did not have a significant effect on the effect sizes (z = 1.00, P = 0.32; Fig. 2). In this analysis, there was also no significant difference in the effect sizes between herptiles, mammals and birds (z = 0.33, P = 0.74). Interestingly, this analysis did demonstrate that effect sizes were significantly higher when glucocorticoid levels were quantified in faecal samples compared with when they were quantified using baseline plasma samples (z = 2.58, P = 0.01; Fig. 2). Specifically, the weighted estimate of the effect size for FGM levels was 0.51 ± 0.08 (95% confidence interval; medium effect: Cohen, 1969), whereas the effect size for plasma sample estimates was not significantly greater than zero (i.e. the 95% confidence interval overlapped zero = 0.09 ± 0.09). This indicates that anthropogenic disturbances were consistently associated with increased FGM levels, but not with increased baseline plasma glucocorticoid levels.
Figure 2:
The effect of four different types of human disturbance (habitat modification, machines, tourism and urban habitat) and glucocorticoid sample type (plasma or faecal) on the effect size (Hedges's g) associated with human disturbance. Positive effect sizes indicate that glucocorticoid levels were greater in response to human disturbance than in the control conditions. Effect sizes were greater when glucocorticoid levels were calculated using faecal samples compared with blood samples, but there were no differences in effect sizes among the four types of human disturbance (see main text). One extremely high effect size (10.49; Dowle et al., 2013) quantified using faecal samples in the urban habitat category was removed from the figure (see main text). Box (first and third quartiles with median line) and whisker (95% confidence intervals) are shown along with outliers (dots).
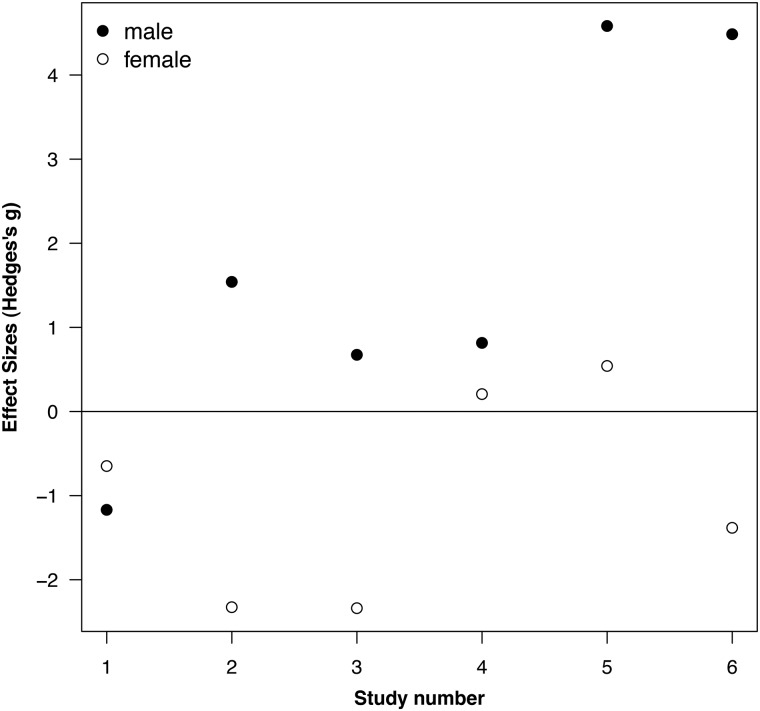
In additional analyses, we found that males and females respond differently to human disturbances. We calculated sex-specific effect sizes from six different studies because the original studies found a significant interaction between human disturbance and sex (see Table S1). In five of the six studies, the effect size for males was greater than the effect size for females (Fig. 3). Interestingly, in three out of five of these studies, males had higher glucocorticoid levels in response to human disturbance, whereas females had lower glucocorticoid levels in response to human disturbance (Fig. 3). When these studies were examined using a linear mixed-effects model, the effect size for males was significantly greater than the effect size for females (z = 2.60, P = 0.009; disturbance and sample type were not included in this model because they were paired). From the remaining studies, we were able to calculate an additional 12 male-specific effect sizes and one additional female-specific effect size. When we included all sex-specific effect sizes in a linear mixed-effects model, once again, the effect sizes for males were greater than the effect sizes for females (z = 2.07, P = 0.04; sample type was non-significant and was removed from the model). These results suggest that if there is a sex difference, males may respond more strongly to human disturbance than females (e.g. Wasser et al., 1997), though other studies found no significant sex differences in the increase in glucocorticoid levels in response to human disturbances (see Table S1).
Figure 3:
A subset of six studies for which we calculated sex-specific effect sizes because there was a significant human disturbance-by-sex interaction. Positive effect sizes indicate that glucocorticoid levels were greater in response to human disturbance than in the control conditions. Based on this subset of studies, males respond more strongly to human disturbance than females (see discussion in main text). See Supporting information Table S1 for the list of these studies.
There are three fundamental messages from our meta-analysis. First, human disturbance is generally associated with an increase in faecal glucocorticoid metabolite levels, but not in baseline glucocorticoids quantified from plasma samples collected within 3 min of initial capture. Faecal glucocorticoid metabolite levels reflect an integrated measure of glucocorticoids and may more closely reflect the cumulative exposure of individuals to glucocorticoids rather than point samples obtained from baseline blood samples. This indicates that baseline plasma glucocorticoid levels do not consistently increase in response to anthropogenic disturbances. Our results again support our suggestion above that FGM levels may be the most useful measure of glucocorticoids in conservation physiology (see ‘Integrated measures of glucocorticoids: serum, urine, feathers and hair’ section above). Second, all types of human disturbance (habitat modification, machines, tourism and urban habitat; Fig. 2) seem to have a similar effect on glucocorticoid levels. These results are encouraging for conservation physiologists because they suggest that we can assume that the relationship between anthropogenic disturbances and this measure of physiological stress is consistent across species and taxa. Unfortunately, they also suggest that any type of human disturbance causes a substantial increase in glucocorticoid levels. Third, glucocorticoid levels of males may increase to a greater extent in response to human disturbance than glucocorticoid levels of females. As we suggested above (Fig. 1), investigating the effects of anthropogenic disturbances on both sexes is important. The results from our meta-analysis indicate that females may not show increased measures of physiological stress compared with males (but see Strasser and Heath, 2013).
Question 3: how do intrinsic and ecological factors interact with anthropogenic factors to influence measures of glucocorticoids?
Interactions between the intrinsic and extrinsic factors that we discussed above may also be important because they could sensitize or desensitize the physiological stress response to anthropogenic disturbances (Romero, 2004). For example, FGM levels in elk are influenced by both snowpack depth (ecological factor) and snowmobile activity (anthropogenic factor: Creel et al., 2002). In years of high snowfall, there is greater snowmobile activity and elk have higher FGM levels. However, this is likely not only because of increased snowmobile activity, but also because elk are more vulnerable to predation by wolves (White et al., 2012) and, thus, their HPA axis is sensitized by this higher predation risk.
Anthropogenic disturbances can also desensitize the HPA axis, causing an inappropriate physiological stress response to real ecological challenges. For example, although exposure to chemical pollutants can increase physiological stress (Wikelski et al., 2001, 2002), some of these pollutants (methylsulfonyl polychlorinated biphenyls) can also antagonize the glucocorticoid receptor (Johansson et al., 1998). If pollutants compete with endogenous glucocorticoids to bind to the glucocorticoid receptor, they could desensitize the HPA axis to ecological factors by inhibiting or limiting the normal physiological stress response (Hontela et al., 1992; Boonstra, 2004; Pottinger et al., 2013). Conservation physiologists could wrongly interpret this situation to suggest that the low measures of glucocorticoids in the animals (due to their now compromised HPA axis) were indicative of healthy populations (see also Verboven et al., 2010 for an example where pollutants may sensitize the HPA axis).
The implementation of a conservation action plan can also influence measures of glucocorticoids in unpredictable ways due to interactions between the anthropogenic intervention and ecological causes of physiological stress. For example, elk in North America are provided with supplemental food during the winter (Putman and Staines, 2004). However, elk that were aggregated in the winter in areas with supplemental food had FGM levels that were >31% higher than those in areas without supplemental food and with lower local densities (Forristal et al., 2012), though these differences in FGM levels could also be attributed to diet (Dantzer et al., 2011; Goymann, 2012). If management decisions were based simply on measures of glucocorticoids, the resultant action would be to stop supplemental feeding and reduce population density, which is rarely the goal of conservation physiology.
Question 4: physiological stress has adverse consequences in humans and laboratory animals, but what about free-living animals?
Short-term increases in glucocorticoids are thought to promote adaptive physiological and behavioural changes, such as those associated with escaping from a predator or coping with a sudden change in weather. Chronic or long-term increases in glucocorticoid levels, however, are widely touted as having detrimental effects, though the evidence comes largely from biomedical studies in humans or laboratory animals (Romero, 2004; Korte et al., 2005; Boonstra, 2013). For example, chronic stress can cause neuronal atrophy in key parts of the brain (Sapolsky, 1996), suppress the immune system (Dhabhar and McEwen, 1997; Korte et al., 2005) and inhibit reproduction (Rivier and Rivest, 1991; Kirby et al., 2009). In contrast, chronic elevations in glucocorticoid levels in free-living animals or those exposed to real ecological challenges can trigger adaptive phenotypic plasticity that enables animals to cope better with that environment (e.g. Dantzer et al., 2013; Middlemis Maher et al., 2013).
Insights from biomedical research about the negative consequences of chronic stress have spurred many ecological studies focused on testing the assumption that fitness is negatively associated with baseline or stress-induced glucocorticoid levels (reviewed by Moore and Jessop, 2003; Breuner et al., 2008; Bonier et al., 2009; Crespi et al., 2013). Specifically, the ‘cort-fitness hypothesis’ predicts that as the number of challenges individuals experience increases, baseline glucocorticoid levels increase and fitness decreases (Bonier et al., 2009). Instead of finding an inverse relationship between baseline plasma glucocorticoid levels and fitness, as would be expected from biomedical studies, Bonier et al. (2009) found inconsistent relationships between plasma glucocorticoids and fitness. Of the different studies in vertebrates examined (n = 53), 51% found a negative relationship between baseline glucocorticoid levels and fitness, while 30% found a positive relationship.
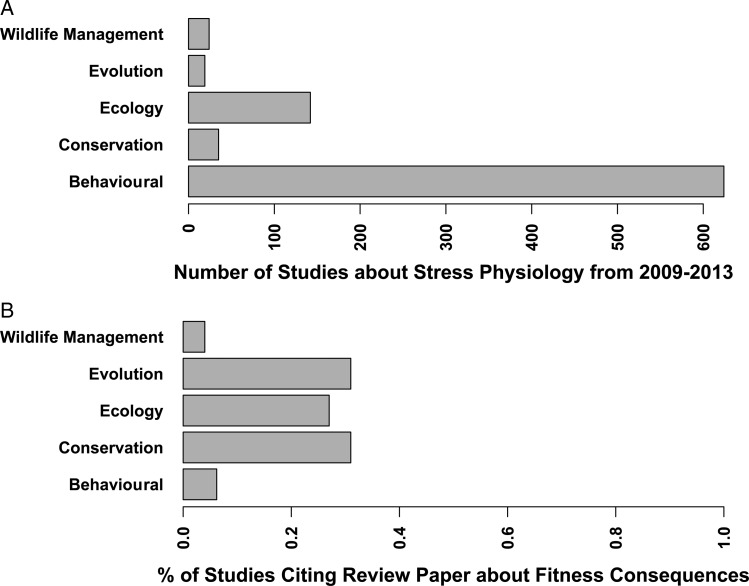
The results from Bonier et al. (2009) highlight the fact that the relationships between measures of glucocorticoids and fitness in free-living animals, and even in laboratory animals, are inconsistent. We assessed the impact of these reviews (Breuner et al., 2008; Bonier et al., 2009; Crespi et al., 2013) showing that the relationship between measures of physiological stress and fitness in free-living animals is inconsistent by locating all the studies about glucocorticoids (Fig. 4) that were published from 2009 to 2013 (after Bonier et al., 2009 was published) in major journals in the fields of behavioural, conservation, ecological, evolutionary and wildlife management research. There are a growing number of studies about stress physiology published in these areas (Fig. 4A), yet many of the recent studies presumably do not acknowledge the inconsistency of the relationship between glucocorticoids and fitness. From 2009 to 2013, only ∼30% of all the publications about glucocorticoids (Fig. 4B) published in the major journals in conservation, ecology and evolution cite one of the major review papers highlighting these inconsistencies (Breuner et al., 2008; Bonier et al., 2009; Crespi et al., 2013). Only 4% of all of the publications about glucocorticoids published from 2009 to 2013 in the major wildlife management journals cite even one of these review papers.
Figure 4:
(A) The number of studies regarding aspects of physiological stress (using keywords ‘glucocorticoids’, ‘cortisol’ or ‘corticosterone’) in behavioural, conservation, ecological, evolutionary and wildlife management research published from 2009 to 2013. (B) The percentage of these studies that cited recent reviews (Breuner et al., 2008; Bonier et al., 2009; Crespi et al., 2013) about the relationships between baseline or stress-induced glucocorticoid levels and survival or reproduction in free-living animals in these different research fields. Search and retrieval of articles was performed through Web of Science on 27 December 2013 (complete list available from corresponding author upon request).
Although the general assumption derived from biomedical research that increased physiological stress or glucocorticoids causes a reduction in survival or reproduction in free-living animals seems dubious, our basic analysis here shows that this criticism has clearly not taken a hold in physiological ecology, conservation physiology and, especially, not in wildlife management. We acknowledge that this is a coarse analysis and that some of these studies are not specifically focused on measuring the relationship between glucocorticoids and fitness (e.g. those in behavioural journals). However, our emphasis is that most studies in the realm of conservation and wildlife management are likely to discuss how glucocorticoids are closely tied to fitness at some point in the publication, yet they do not cite one of these review papers that indicate how the relationship between fitness and glucocorticoids is not uniformly negative. Unfortunately, many of these studies may be subscribing to the biomedical interpretation of the negative effects of physiological stress and not assessing their data in light of studies of free-living animals.
Question 5: do anthropogenic disturbances decrease survival and reproduction?
The results from our meta-analysis indicate that anthropogenic disturbances are consistently associated with increased FGM levels across taxa (Fig. 3). However, the fitness consequences of these anthropogenic disturbances or chronic stress caused by the anthropogenic disturbances are rarely investigated. There are a few studies in birds that show that individuals in areas with greater human disturbance (tourism, proximity to road, noise) have higher baseline (Strasser and Heath, 2013) or stress-induced plasma total glucocorticoid (Müllner et al., 2004; Ellenberg et al., 2007) or FGM levels (Hayward et al., 2011) and also exhibit lower survival (Müllner et al., 2004) or breeding success (Ellenberg et al., 2007; Hayward et al., 2011; Strasser and Heath, 2013). Unfortunately, there are a number of alternative explanations for these patterns. In order to ascertain whether reduced breeding success is caused by increased baseline plasma glucocorticoid levels, future studies need to increase glucocorticoid levels experimentally and observe the effects on breeding success. For example, Blickley et al. (2012a) experimentally increased FGM levels in lekking male greater sage-grouse (Centrocercus urophasianus) using audio playbacks of noises from natural gas drilling and roads, yet the fitness consequences of these experimental increases in glucocorticoids are mostly unknown (but see Blickley et al., 2012b).
Observational studies can also be confounded by the possibility that poor-quality individuals might be forced to breed in suboptimal habitats, such as those near human disturbances. Human disturbance in observational studies is an experimental treatment that it is not applied randomly. Poor-quality individuals might intrinsically show higher baseline plasma glucocorticoid levels and exhibit lower breeding success, but it does not necessarily have to be caused by their proximity to human disturbances. Without an experimental approach, such as randomly applying human disturbances to nests or individuals (e.g. Blickley et al., 2012a, 2012b) distributed within a study area or cross-fostering offspring from a disturbed area to a less disturbed area, it is difficult to determine the direction of causality. A recent study in mountain white-crowned sparrows (Zonotrochia leucophrys oriantha) experimentally manipulated the amount of traffic noise to which nestlings were exposed. In contrast to what might be predicted from observational studies, Crino et al. (2013) found that nestlings exposed to experimentally increased levels of traffic noise were in better condition (ratio of body mass to tarsal length) and had lower stress-induced plasma total corticosterone levels. Increased exposure to traffic noise also did not affect nestling baseline plasma total corticosterone levels, one measure of immune function, or survival to fledgling.
Future studies employing similar experimental manipulations of anthropogenic disturbances (Arlettaz et al., 2007; Hayward et al., 2011; Blickley et al., 2012a; Crino et al., 2013) should help to illuminate the genuine causes and consequences of increases in glucocorticoid levels due to anthropogenic disturbances. In particular, future studies in the context of conservation physiology should experimentally manipulate glucocorticoid levels in individuals in both disturbed and undisturbed areas using audio playbacks (Blickley et al., 2012a, Dantzer et al., 2013), glucocorticoid provisioning or implants (Silverin, 1986; Dantzer et al., 2013), or by manipulating the activity of glucocorticoid receptors (Landys et al., 2004) or corticotrophin-releasing factor receptors (Xiao et al., 2006). Other experimental manipulations, such as provisioning of supplemental food to some individuals in disturbed habitats but not to others, cross-fostering offspring from disturbed to undisturbed habitats, or altering the intensity and location of tourist pressure will help to determine whether the human disturbance is causing the increases in glucocorticoids and declines in fitness or if these patterns are simply the outcome of poor-quality individuals breeding in areas of high disturbance. We recognize that such manipulations are obviously challenging, but they could be performed in surrogate species to help to understand whether a heightened physiological stress response in areas disturbed by human activity does indeed cause a reduction in survival or breeding success.
Finally, increased glucocorticoid levels in disturbed habitats may reflect coping strategies to deal with those anthropogenic disturbances and may not necessarily cause a reduction in survival and/or reproduction. The allostasis framework (McEwen and Wingfield, 2003) would predict that the stress responses of individuals to environmental challenges are generally adapted to both their life-history strategy and their ecology. For example, bird species that live in areas in which the time to reproduce is short (highly seasonal or cold environments) or stochastic (e.g. arid regions where rainfall is highly variable) exhibit lower acute stress responses (Hau et al., 2010). The dampening of the stress response in these challenging environments might allow individuals to continue to breed despite any environmental challenges they experience (Wingfield et al., 1998; Wingfield and Sapolsky, 2003; Hau et al., 2010). Although the strength of phenotypic selection caused by anthropogenic agents might be increased in comparison to other ecological causes of selection (Hendry et al., 2008; Darimont et al., 2009), adaptation to urban or human-disturbed environments should not be any different. It seems reasonable to conclude that selection will favour endocrine phenotypes that enable individuals to continue to reproduce and survive despite human disturbances (Bonier, 2012). For example, selection in human-disturbed areas might favour individuals with dampened stress responses (Partecke et al., 2006; Atwell et al., 2012). Selection favouring a reduction in the acute stress response could also cause relatively rapid shifts in the HPA or HPI axis of populations in disturbed vs. less disturbed areas (e.g. 12 generations: Atwell et al., 2012). Alternatively, this could mean that individuals in disturbed or urban habitats have increased baseline or stress-induced glucocorticoid levels in comparison to those in less disturbed areas. This increase in cumulative exposure to glucocorticoids does not necessarily need to inhibit reproduction (Wingfield and Sapolsky, 2003) and might not influence the viability of individuals or populations. We think that future studies that link together concepts from evolutionary ecology alongside methods in conservation physiology will be particularly beneficial to addressing how animals adapt to human-disturbed environments through correlated changes in behaviour, physiology and life-history traits.
Conclusions
Now I believe that the scattered state of ecology, which is like an active worm that has been chopped into little bits, each admirably brisk, but leading a rather exclusive and lonely existence, and not combining to get anywhere in particular, this disintegrating tendency, in what should be a coherent and united organism, is very largely due to a lack of proper working hypotheses. Charles Elton (1930), Page 68 in Animal Ecology and Evolution
Almost 100 years ago, the animal ecologist Charles Elton (1930) warned about the lack of coherency in ecology due to a lack of ‘proper working hypotheses’, which is ominous given that there was only one professional publication about animal ecology at that time (Journal of Animal Ecology). Importantly, conservation physiology has several working hypotheses, for instance, that measures of glucocorticoids can be used: (i) to document the effects of anthropogenic disturbances on animals; and (ii) to expect or predict future declines in a population or species (Wikelski and Cooke, 2006). However, the underpinnings of these hypotheses are based upon some major assumptions arising from biomedical studies, which seem to be either untested or dubious in free-living animals.
Our review suggests that conservation physiology has not yet gathered all the ‘little bits’ from ecology, evolution and physiology into a single and coherent discipline. This is, of course, not unique; it plagues well-established scientific disciplines and is to be expected from any new scientific discipline. Our review highlights that the physiological stress response to an environmental challenge or anthropogenic disturbance can be modified by a number of intrinsic characteristics that could mask any general patterns. Future studies in conservation physiology should incorporate the effects of sex, reproductive condition and age into their analyses and also be wary of comparisons among populations whose members may differ in these intrinsic characteristics. Our meta-analysis indicates that the presence of anthropogenic disturbances, regardless of their type, consistently and significantly increases glucocorticoid levels in mammals, birds, amphibians and reptiles. However, our further analyses highlight that FGM levels, but not baseline plasma glucocorticoid levels, were higher in response to anthropogenic disturbances and that the effects of anthropogenic disturbances on glucocorticoid levels might be sex specific (Fig. 3). Future studies in conservation physiology must also assess how ecological (food, predators, density) and anthropogenic factors interact with one another to cause or ameliorate physiological stress. Measures of glucocorticoids are used in conservation physiology because they are thought to reflect health or fitness and, therefore, can be used to predict future population declines. This assumption is based upon biomedical studies, and the relationship between fitness and stress physiology is inconsistent in free-living animals and, in some cases, mediates adaptive behavioural or life-history responses to those environmental changes. We think that experimental studies testing the effects of anthropogenic disturbances on measures of physiological stress and their effects on fitness will be particularly valuable in conservation physiology. Finally, there are numerous methodological issues associated with the appropriate measurement of glucocorticoids that need to be considered carefully for all biomarkers of physiological stress but especially for FGM levels.
In this review, we have taken a critical view of the use of measures of glucocorticoids in conservation physiology and wildlife management. We agree with Cooke and O'Connor (2010) that ‘It is also necessary to validate more tools in the “conservation physiology toolbox”, and ensure a thorough understanding of the physiological biomarkers applied to conservation efforts.’ This will undoubtedly require greater coherency in conservation physiology through testing fundamental assumptions and the integration of studies in the other ‘little bits’, including physiology, ecological physiology and evolutionary ecology. We hope that this review exerts stress on the field of conservation physiology to make it a stronger and more robust scientific discipline so that it can be used to slow losses of biodiversity.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Acknowledgements
We thank S. J. Cooke for inviting us to write this review and two anonymous reviewers for helpful comments on a previous version of this manuscript. We thank J. Blickley, R. Rolland, M. Bourbonnais (Foothills Research Institute Grizzly Bear Project), E. Røskaft, M. Dietz and S. French for sharing data for the meta-analysis. We are grateful to R. Palme for informing us about two studies we had initially missed in our meta-analysis. B.D., Q.E.F., M.J.S. and R.B. wrote the paper, Q.E.F. and B.D. performed the meta-analysis, and B.D. performed the literature analysis.
References
- 1.Ahlering MA, Maldonado JE, Eggert LS, Fleischer RC, Western D, Brown JL. (2013) Conservation outside protected areas and the effect of human-dominated landscapes on stress hormones in savannah elephants. Conserv Biol 27: 569–575. [DOI] [PubMed] [Google Scholar]
- 2.Arlettaz R, Patthey P, Baltic M, Leu T, Schaub M, Palme R, Jenni-Eiermann S. (2007) Spreading free-riding snow sports represent a novel serious threat for wildlife. Proc R Soc B Biol Sci 274: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley NT, Barboza PS, Macbeth BJ, Janz DM, Cattet MRL, Booth RK, Wasser SK. (2011) Glucocorticosteroid concentrations in feces and hair of captive caribou and reindeer following adrenocorticotropic hormone challenge. Gen Comp Endocrinol 172: 382–391. [DOI] [PubMed] [Google Scholar]
- 4.Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED. (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23: 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker ML, Gemmell E, Gemmell RT. (1998) Physiological changes in brushtail possums, Trichosurus vulpecula, transferred from the wild to captivity. J Exp Zool 280: 203–212. [DOI] [PubMed] [Google Scholar]
- 6.Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A, Camacho L, Illera JC. (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem 104: 136–142. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu M, Costantini D. (2014) Biomarkers of oxidative status: missing tools in conservation physiology. Conserv Physiol 2: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. (2007) Stress response during development predicts fitness in a wild, long lived vertebrate. Proc Natl Acad Sci USA 104: 8880–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blickley JL, Word KR, Krakauer AH, Phillips JL, Sells SN, Taff CC, Wingfield JC, Patricelli GL. (2012a) Experimental chronic noise is related to elevated fecal corticosteroid metabolites in lekking male Greater Sage-Grouse (Centrocercus urophasianus). PLoS ONE 7: pe50462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blickley JL, Blackwood D, Patricelli GL. (2012b) Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv Biol 26: 461–471. [DOI] [PubMed] [Google Scholar]
- 11.Bonier F. (2012) Hormones in the city: endocrine ecology of urban birds. Horm Behav 61: 763–772. [DOI] [PubMed] [Google Scholar]
- 12.Bonier F, Moore IT, Martin PR, Robertson RJ. (2009) The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163: 208–213. [DOI] [PubMed] [Google Scholar]
- 13.Boonstra R. (2004) Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr Comp Biol 44: 95–108. [DOI] [PubMed] [Google Scholar]
- 14.Boonstra R. (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–23. [Google Scholar]
- 15.Boonstra R, Hik D, Singleton GR, Tinnikov A. (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 79: 371–394. [Google Scholar]
- 16.Boonstra R, McColl CJ, Karels TJ. (2001) Reproduction at all costs: the adaptive stress response of male arctic ground squirrels. Ecology 82: 1930–1946. [Google Scholar]
- 17.Bosson CO, Palme R, Boonstra R. (2009) Assessment of the stress response in Columbian ground squirrels: laboratory and field validation of an enzyme immunoassay for fecal cortisol metabolites. Phys Biochem Zool 82: 291–301. [DOI] [PubMed] [Google Scholar]
- 18.Bosson CO, Palme R, Boonstra R. (2013) Assessing the impact of live-capture, confinement, and translocation on stress and fate in eastern gray squirrels. J Mammal 94: 1401–1411. [Google Scholar]
- 19.Breuner CW, Patterson SH, Hahn TP. (2008) In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol 157: 288–295. [DOI] [PubMed] [Google Scholar]
- 20.Breuner CW, Delehanty B, Boonstra R. (2013) Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct Ecol 27: 24–36. [Google Scholar]
- 21.Brunton PJ, Russell JA, Douglas AJ. (2008) Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol 20: 764–776. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan KL, Goldsmith AR. (2004) Noninvasive endocrine data for behavioural studies: the importance of validation. Anim Behav 67: 183–185. [Google Scholar]
- 23.Busch DS, Hayward LS. (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. [Google Scholar]
- 24.Cannon WB. (1932) The Wisdom of the Body. W. W. Norton & Company, New York. [Google Scholar]
- 25.Clinchy M, Zanette L, Boonstra R, Wingfield JC, Smith JNM. (2004) Balancing food and predator pressure induces chronic stress in songbirds. Proc R Soc B Biol Sci 271: 2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockrem JF. (2005) Conservation and behavioral neuroendocrinology. Horm Behav 48: 492–501. [DOI] [PubMed] [Google Scholar]
- 27.Cockrem JF. (2013) Individual variation in glucocorticoid stress responses in animals. Gen Comp Endocrinol 181: 45–58. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. (1969) Statistical Power Analysis for the Behavioral Sciences. Academic Press, New York. [Google Scholar]
- 29.Cooke SJ, O'Connor CM. (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. [Google Scholar]
- 30.Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costantini D, Marasco V, Møller AP. (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181: 447–456. [DOI] [PubMed] [Google Scholar]
- 32.Creel S. (2001) Social dominance and stress hormones. Trends Ecol Evol 16: 491–497. [Google Scholar]
- 33.Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO. (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16: 809–814. [Google Scholar]
- 34.Crespi EJ, Williams TD, Jessop TS, Delehanty B. (2013) Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct Ecol 27: 93–106. [Google Scholar]
- 35.Crino OL, Johnson EE, Blickley JL, Patricelli GL, Breuner CW. (2013) Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J Exp Biol 216: 2055–2062. [DOI] [PubMed] [Google Scholar]
- 36.Cyr NE, Romero LM. (2008) Fecal glucocorticoid metabolites of experimentally stressed captive and free-living starlings: implications for conservation research. Gen Comp Endocrinol 158: 20–28. [DOI] [PubMed] [Google Scholar]
- 37.Cyr NE, Romero LM. (2009) Identifying hormonal habituation in field studies of stress. Gen Comp Endocrinol 161: 295–303. [DOI] [PubMed] [Google Scholar]
- 38.Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S, Humphries MM, Boonstra R. (2010) Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167: 279–286. [DOI] [PubMed] [Google Scholar]
- 39.Dantzer B, McAdam AG, Palme R, Boutin S, Boonstra R. (2011) How does diet affect fecal steroid hormone metabolite concentrations? An experimental examination in red squirrels. Gen Comp Endocrinol 174: 124–131. [DOI] [PubMed] [Google Scholar]
- 40.Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. (2013) Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340: 1215–1217. [DOI] [PubMed] [Google Scholar]
- 41.Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. (2009) Human predators outpace other agents of trait change in the wild. Proc Natl Acad Sci USA 106: 952–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis AK. (2005) Effects of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76: 334–338. [Google Scholar]
- 43.Delehanty DJ, Oring LW, Fivizzani AJ, Halawani MEE. (1997) Circulating prolactin of incubating male Wilson's phalaropes corresponds to clutch size and environmental stress. Condor 99: 397–405. [Google Scholar]
- 44.Dhabhar FS. (2009) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16: 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhabhar FS, McEwen BS. (1997) Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11: 286–306. [DOI] [PubMed] [Google Scholar]
- 46.Dickens MJ, Romero LM. (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191: 177–189. [DOI] [PubMed] [Google Scholar]
- 47.Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL. (2009) Chronic stress alters glucocorticoid receptor and mineralcorticoid receptor mRNA expression in the European Starling (Sturnus vulgaris). J Neuroendocrinol 21: 832–840. [DOI] [PubMed] [Google Scholar]
- 48.Dowle M, Webster KN, Deane E. (2013) Faecal glucocorticoid metabolite concentrations in the free-ranging bandicoots (Perameles nasuta and Isoodon obesulus) of northern Sydney. Aust Mammal 35: 1–7. [Google Scholar]
- 49.Drake JM, Griffen BD. (2010) Early warning signals of extinction in deteriorating environments. Nature 467: 456–459. [DOI] [PubMed] [Google Scholar]
- 50.Ellenberg U, Mattern T, Seddon PJ, Jorquera GL. (2006) Physiological and reproductive consequences of human disturbance in Humboldt penguins: the need for species-specific visitor management. Biol Conserv 133: 95–106. [Google Scholar]
- 51.Ellenberg U, Setiawan AN, Cree A, Houston DM, Seddon PJ. (2007) Elevated hormonal stress response and reduced reproductive output in Yellow-eyed penguins exposed to unregulated tourism. Gen Comp Endocrinol 152: 54–63. [DOI] [PubMed] [Google Scholar]
- 52.Elton CS. (1930) Animal Ecology and Evolution. Clarendon Press, Oxford. [Google Scholar]
- 53.Fefferman NH, Romero LM. (2013) Can physiological stress alter population persistence? A model with conservation implications. Conserv Physiol 1: doi:10.1093/conphys/cot012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forristal VE, Creel S, Taper ML, Scurlock BM, Cross PC. (2012) Effects of supplemental feeding and aggregation on fecal glucocorticoid metabolite concentrations in elk. J Wildl Manage 76: 694–702. [Google Scholar]
- 55.Fujiwara T, Cherrington AD, Neal DN, McGuinness OP. (1996) Role of cortisol in the metabolic response to stress hormone infusion in the conscious dog. Metabolism 45: 571–578. [DOI] [PubMed] [Google Scholar]
- 56.Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- 57.Goymann W, East ML, Wachter B, Höner OP, Möstl E, Van't Hof TJ, Hofer H. (2001) Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc R Soc B Biol Sci 268: 2453–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goymann W, Trappschuh M, Jensen W, Schwabl I. (2006) Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm Behav 49: 644–653. [DOI] [PubMed] [Google Scholar]
- 59.Gurevitch J, Hedges LV. (2001) Meta-analysis: combining the results of independent experiments. In Scheiner SM, Gurevitch J, eds, Design and Analysis of Ecological Experiments, Ed 2 Oxford University Press, New York, pp 347–369. [Google Scholar]
- 60.Hackländer K, Möstl E, Arnold W. (2003) Reproductive suppression in female Alpine marmots, Marmota marmota. Anim Behav 65: 1133–1140. [Google Scholar]
- 61.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28: 464–476. [DOI] [PubMed] [Google Scholar]
- 62.Hanssen SA, Folstad I, Erikstad KE. (2003) Reduced immunocompetence and cost of reproduction in common eiders. Oecologia 136: 457–464. [DOI] [PubMed] [Google Scholar]
- 63.Harper JM, Austad SN. (2001) Effect of capture and season on fecal glucocorticoid levels in deer mice (Peromyscus maniculatus) and red-backed voles (Clethrionomys gapperi). Gen Comp Endocrinol 123: 337–344. [DOI] [PubMed] [Google Scholar]
- 64.Harris A, Seckl J. (2011) Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59: 279–289. [DOI] [PubMed] [Google Scholar]
- 65.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. (2010) Corticosterone, testosterone and life-history strategies of birds. Proc R Soc B Biol Sci 277: 3203–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. (2012) Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B Biol Sci 279: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayward LS, Bowles AE, Ha JC, Wasser SK. (2011) Impacts of acute and long-term vehicle exposure on physiology and reproductive success of the northern spotted owl. Ecosphere 2: 1–20. [Google Scholar]
- 68.Hellgren EC, Rogers LL, Seal US. (1993) Serum chemistry and hematology of black bears: physiological indices of habitat quality or seasonal patterns. J Mammal 74: 304–315. [Google Scholar]
- 69.Hendry AP, Farrugia TJ, Kinnison M. (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17: 20–29. [DOI] [PubMed] [Google Scholar]
- 70.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. (2014) Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc R Soc B Biol Sci 281: 20133151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hontela A, Rasmussen JB, Audet C, Chevalier G. (1992) Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol 22: 278–283. [DOI] [PubMed] [Google Scholar]
- 72.IUCN (2013) The IUCN Red List of Threatened Species. Version 2013.2, http://www.iucnredlist.org [Google Scholar]
- 73.Jacques CN, Jenks JA, Deperno CS, Sievers JD, Grovenburg TW, Brinkman TJ, Swanson CC, Stillings BA. (2009) Evaluating ungulate mortality associated with helicopter net-gun captures in the northern great plains. J Wildl Manage 73: 1282–1291. [Google Scholar]
- 74.Johansson M, Nilsson S, Lund B-O. (1998) Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environ Health Perspect 106: 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keckeis K, Lepschy M, Schöpper H, Moser L, Troxler J, Palme R. (2012) Hair cortisol: a parameter of chronic stress? Insights from a radiometabolism study in guinea pigs. J Comp Physiol B 182: 985–996. [DOI] [PubMed] [Google Scholar]
- 76.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. (2009) Stress increases putative gonadotropin inhibitory hormone and decreases lutenizing hormone in male rats. Proc Natl Acad Sci USA 106: 11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirschbaum C, Hellhammer DH. (1989) Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22: 150–169. [DOI] [PubMed] [Google Scholar]
- 78.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. (2010) Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31: 307–321. [DOI] [PubMed] [Google Scholar]
- 79.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. (2005) The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 29: 3–38. [DOI] [PubMed] [Google Scholar]
- 80.Landys MM, Piersma T, Ramenofsky M, Wingfield JC. (2004) Role of the low-affinity glucocorticoid receptor in the regulation of behavior and energy metabolism in the migratory red knot Calidris canutus islandica. Physiol Biochem Zool 77: 658–668. [DOI] [PubMed] [Google Scholar]
- 81.Lendvai AZ, Bókony V, Angelier F, Chastel O, Sol D. (2013) Do smart birds stress less? An interspecific relationship between brain size and corticosterone levels. Proc R Soc B Biol Sci 280: 20131734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindenmayer DB, Piggott MP, Wintle BA. (2013) Counting the books while the library burns: why conservation monitoring programs need a plan for action. Front Ecol Environ 11: 549–555. [Google Scholar]
- 83.Lobato E, Moreno J, Merino S, Sanz JJ, Arriero E. (2005) Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca). Ecoscience 12: 27–34. [Google Scholar]
- 84.Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NPV. (1995) Longitudinal study of basal cortisol levels in healthy elderly subjects: evidence for subgroups. Neurobiol Aging 17: 95–105. [DOI] [PubMed] [Google Scholar]
- 85.Lupien SJ, De Leon M, De Santi S, Convit A, Tarshish C, Thakur M, McEwen BS, Hauger RL, Meaney MJ. (1998) Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1: 69–73. [DOI] [PubMed] [Google Scholar]
- 86.Lynn SE, Prince LE, Phillips MM. (2010) A single exposure to an acute stressor has lasting consequences for the hypothalamo–pituitary–adrenal response to stress in free-living birds . Gen Comp Endocrinol 165: 337–344. [DOI] [PubMed] [Google Scholar]
- 87.MacDougall-Shackleton SA, Dindia L, Newman AEM, Potvin DA, Stewart KA, MacDougall-Shackleton EA. (2009) Stress, song and survival in sparrows. Biol Lett 5: 746–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McEwen BS, Wingfield JC. (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- 89.McEwen BS, Wingfield JC. (2010) What's in a name? Integrating homeostasis, allostasis and stress. Horm Behav 57: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madliger CL, Love OP. (2014) The need for a predictive, context-dependent approach to the application of stress hormones in conservation. Conserv Biol 28: 283–287. [DOI] [PubMed] [Google Scholar]
- 91.Martin LM. (2009) Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol 163: 70–76. [DOI] [PubMed] [Google Scholar]
- 92.Martínez-Mota R, Valdespino C, Sánchez-Ramos MA, Serio-Silva JC. (2007) Effects of forest fragmentation on the physiological stress response of black howler monkeys. Anim Conserv 10: 374–379. [Google Scholar]
- 93.Middlemis Maher J, Werner EE, Denver RJ. (2013) Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc R Soc B 280: 20123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meaney MJ. (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161–1192. [DOI] [PubMed] [Google Scholar]
- 95.Meyer JS, Novak MA. (2012) Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 153: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Millspaugh JJ, Washburn BE. (2003) Within-sample variation of fecal glucocorticoid measurements. Gen Comp Endocrinol 132: 21–26. [DOI] [PubMed] [Google Scholar]
- 97.Millspaugh JJ, Washburn BE. (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–199. [DOI] [PubMed] [Google Scholar]
- 98.Monaghan P, Haussmann MF. (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21: 47–53. [DOI] [PubMed] [Google Scholar]
- 99.Monaghan P, Metcalfe NB, Torres R. (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12: 75–92. [DOI] [PubMed] [Google Scholar]
- 100.Moore IT, Jessop TS. (2003) Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav 43: 39–47. [DOI] [PubMed] [Google Scholar]
- 101.Müllner A, Linsenmair KE, Wikelski M. (2004) Exposure to ecotourism reduces survival and affects stress response in hoatzin chicks (Opisthocomus hoazin). Biol Conserv 118: 549–558. [Google Scholar]
- 102.Nussey DH, Wilson AJ, Brommer JE. (2007) The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol 20: 831–844. [DOI] [PubMed] [Google Scholar]
- 103.Nussey DH, Baird D, Barrett E, Boner W, Fairlie J, Gemmell N, Hartmann N, Horn T, Haussmann M, Olsson M, et al. (2014) Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol Evol 5: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ouyang JQ, Hau M, Bonier F. (2011) Within seasons and among years: when are corticosterone levels repeatable? Horm Behav 60: 559–564. [DOI] [PubMed] [Google Scholar]
- 105.Ouyang JQ, Quetting M, Hau M. (2012) Corticosterone and brood abandonment in a passerine bird. Anim Behav 84: 261–268. [Google Scholar]
- 106.Palme R. (2005) Measuring fecal steroids: guidelines for practical application. Ann N Y Acad Sci 1046: 75–80. [DOI] [PubMed] [Google Scholar]
- 107.Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E. (2005) Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion and noninvasive measurement in fecal samples. Ann N Y Acad Sci 1046: 162–171. [DOI] [PubMed] [Google Scholar]
- 108.Partecke J, Schwabl I, Gwinner E. (2006) Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87: 1945–1952. [DOI] [PubMed] [Google Scholar]
- 109.Pottinger TG, Henrys PA, Williams RJ, Mathhiessen P. (2013) The stress response of three-spined sticklebacks is modified in proportion to effluent exposure downstream of wastewater treatment works. Aquat Toxicol 126: 382–392. [DOI] [PubMed] [Google Scholar]
- 110.Putman RJ, Staines BW. (2004) Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: justifications, feeding practice and effectiveness. Mammal Rev 34: 285–306. [Google Scholar]
- 111.R Development Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 112.Rivier C, Rivest S. (1991) Effects of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 45: 523–532. [DOI] [PubMed] [Google Scholar]
- 113.Romero LM. (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128: 1–24. [DOI] [PubMed] [Google Scholar]
- 114.Romero LM. (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255. [DOI] [PubMed] [Google Scholar]
- 115.Romero LM, Reed JM. (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A 140: 73–79. [DOI] [PubMed] [Google Scholar]
- 116.Romero LM, Wikelski M. (2001) Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc Natl Acad Sci USA 98: 7366–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romero LM, Wikelski M. (2010) Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc R Soc B Biol Sci 277: 3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Romero LM, Meister CJ, Cyr NE, Kenagy GJ, Wingfield JC. (2008) Seasonal glucocorticoid responses to capture in wild free-living mammals. Am J Physiol Regul Integr Comp Physiol 294: R614–R622. [DOI] [PubMed] [Google Scholar]
- 119.Romero LM, Dickens MJ, Cyr NE. (2009) The reactive scope model — A new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–389. [DOI] [PubMed] [Google Scholar]
- 120.Ruiz G, Rosenmann M, Novoa FF, Sabat P. (2002) Hematological parameters and stress index in Rufous-collared sparrows dwelling in urban environments. Condor 104: 162–166. [Google Scholar]
- 121.Sapolsky RM. (1985) Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids? Endocrinology 116: 2273–2278. [DOI] [PubMed] [Google Scholar]
- 122.Sapolsky RM. (1996) Why stress is bad for your brain. Science 273: 749–750. [DOI] [PubMed] [Google Scholar]
- 123.Sapolsky RM. (1999) Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 34: 721–732. [DOI] [PubMed] [Google Scholar]
- 124.Sapolsky RM. (2002) Endocrinology of the stress response. In Becker JB, Breedlove SM, Crews D, McCarthy MM, eds, Behavioral Endocrinology, Ed 2 MIT Press, Cambridge, MA, pp 409–450. [Google Scholar]
- 125.Sapolsky RM. (2005) The influence of social hierarchy on primate health. Science 308: 648–652. [DOI] [PubMed] [Google Scholar]
- 126.Sapolsky RM, Altmann J. (1991) Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol Psychiatry 30: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 127.Sapolsky RM, Krey LC, McEwem BS. (1984) Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA 81: 6174–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sapolsky RM, Krey LC, McEwen BS. (1986) The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 7: 284–301. [DOI] [PubMed] [Google Scholar]
- 129.Sapolsky RM, Romero LM, Munck AU. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- 130.Scott AP, Ellis T. (2007) Measurement of fish steroids in water—a review. Gen Comp Endocrinol 153: 392–400. [DOI] [PubMed] [Google Scholar]
- 131.Scott AP, Hirschenhauser K, Bender N, Oliveira R, Earley RL, Sebire M, Ellis T, Pavlidis M, Hubbard PC, Huertas M, et al. (2008) Non-invasive measurement of steroids in fish-holding water: important considerations when applying the procedure to behaviour studies. Behaviour 145: 1307–1328. [Google Scholar]
- 132.Seyle HA. (1936) A syndrome produced by diverse nocuous agents. Nature 138: 32–33. [DOI] [PubMed] [Google Scholar]
- 133.Sheriff MJ, Krebs CJ, Boonstra R. (2010) Assessing stress in animal populations: do fecal and plasma glucocorticoids tell the same story? Gen Comp Endocrinol 166: 614–619. [DOI] [PubMed] [Google Scholar]
- 134.Sheriff MJ, Krebs CJ, Boonstra R. (2011a) From process to pattern: how fluctuating predation risk impacts the stress axis of snowshoe hares during the 10-year cycle. Oecologia 166: 593–605. [DOI] [PubMed] [Google Scholar]
- 135.Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. (2011b) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166: 869–887. [DOI] [PubMed] [Google Scholar]
- 136.Sheriff MJ, Wheeler H, Donker SA, Krebs CJ, Palme R, Hik DS, Boonstra R. (2012) Mountain-top and valley-bottom experiences: the stress axis as an integrator of environmental variability in arctic ground squirrel populations. J Zool 287: 65–75. [Google Scholar]
- 137.Silverin B. (1986) Corticosterone-binding proteins and behaviour effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen Comp Endocrinol 64: 67–74. [DOI] [PubMed] [Google Scholar]
- 138.Snell-Rood EC, Wick N. (2013) Anthropogenic environments exert variable selection on cranial capacity in mammals. Proc R Soc B Biol Sci 280: 20131384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spercoski KM, Morais RN, Morato RG, de Paula RC, Azevedo FC, May-Júnior JA, Santos JP, Reghelin AL, Wildt DE, Songsasen N. (2012) Adrenal activity in maned wolves is higher on farmlands and park boundaries than within protected areas. Gen Comp Endocrinol 179: 232–240. [DOI] [PubMed] [Google Scholar]
- 140.Stetz J, Hunt K, Kendall KC, Wasser SC. (2013) Effects of exposure, diet, and thermoregulation on fecal glucocorticoid measures in wild bears. PLoS ONE 8: pe55967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strasser EH, Heath JA. (2013) Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J Appl Ecol 50: 912–919. [Google Scholar]
- 142.Tarlow EM, Blumstein DT. (2007) Evaluating methods to quantify anthropogenic stressors on wild animals. Appl Anim Behav Sci 102: 429–451. [Google Scholar]
- 143.Terwissen CV, Mastromonaco GF, Murray DL. (2013) Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen Comp Endocrinol 194: 162–167. [DOI] [PubMed] [Google Scholar]
- 144.Touma C, Palme R. (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046: 54–74. [DOI] [PubMed] [Google Scholar]
- 145.Travers M, Clinchy M, Zanette L, Boonstra R, Williams TD. (2010) Indirect predator effects on clutch size and the cost of egg production. Ecol Lett 13: 980–988. [DOI] [PubMed] [Google Scholar]
- 146.Verboven N, Verreault J, Letcher RJ, Gabrielsen GW, Evans NP. (2010) Adrenocortical function of Arctic-breeding glaucous gulls in relation to persistent organic pollutants. Gen Comp Endocrinol 166: 25–32. [DOI] [PubMed] [Google Scholar]
- 147.Viechtbauer W. (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36: 1–48. [Google Scholar]
- 148.von Zglinicki T. (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344. [DOI] [PubMed] [Google Scholar]
- 149.Wada H. (2008) Glucocorticoids: mediators of vertebrate ontogenetic transitions. Gen Comp Endocrinol 156: 441–453. [DOI] [PubMed] [Google Scholar]
- 150.Walker BG, Boersma PD, Wingfield JC. (2005) Field endocrinology and conservation biology. Integr Comp Biol 45: 12–18. [DOI] [PubMed] [Google Scholar]
- 151.Walker BG, Boersma PD, Wingfield JC. (2006) Habituation of adult Magellanic penguins to human visitation as expressed through behavior and corticosterone secretion. Conserv Biol 20: 146–154. [DOI] [PubMed] [Google Scholar]
- 152.Washburn BE, Millspaugh JJ. (2002) Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer feces. Gen Comp Endocrinol 127: 217–222. [DOI] [PubMed] [Google Scholar]
- 153.Wasser SK, Bevis K, King G, Hanson E. (1997) Noninvasive physiological measures of disturbance in the Northern Spotted Owl. Conserv Biol 11: 1019–1022. [Google Scholar]
- 154.White PJ, Proffitt KM, Lemke TO. (2012) Changes in elk distribution and group sizes after wolf restoration. Am Midl Nat 167: 174–187. [Google Scholar]
- 155.Wikelski M, Cooke SJ. (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 156.Wikelski M, Romero LM, Snell HL. (2001) Marine iguanas oiled in the Galapagos. Science 292: 437–438. [DOI] [PubMed] [Google Scholar]
- 157.Wikelski M, Wong V, Chevalier B, Rattenborg N, Snell HL. (2002) Marine iguanas die from trace oil pollution. Nature 417: 607–608. [DOI] [PubMed] [Google Scholar]
- 158.Wingfield JC, Sapolsky RM. (2003) Reproduction and resistance to stress: when, where, and how. J Neuroendocrinol 15: 711–724. [DOI] [PubMed] [Google Scholar]
- 159.Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J. (1997) Environmental stress, field endocrinology, and conservation biology. In Clemmons JR, Bucholz R, eds, Behavioral Approaches to Conservation in the Wild. Cambridge University Press, Cambridge, pp 95–131. [Google Scholar]
- 160.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. (1998) Ecological bases of hormone behavior interactions: the emergency life-history stage. Am Zool 3: 191–206. [Google Scholar]
- 161.Wong SC, Dykstra M, Campbell JM, Earley RL. (2008) Measuring water-borne cortisol in convict cichlids (Amatitlania nigrofasciata): is the procedure a stressor? Behaviour 145: 1283–1305. [Google Scholar]
- 162.Wysocki LE, Dittami JP, Ladich F. (2006) Ship noise and cortisol secretion in European freshwater fishes. Biol Conserv 128: 501–508. [Google Scholar]
- 163.Xiao E, Xia-Zhang L, Vulliemoz N, Rivier J, Ferin M. (2006) Astressin B, a corticotropin-releasing hormone receptor antagonist, accelerates the return to normal luteal function after an inflammatory-like stress challenge in the rhesus monkey. Endocrinology 148: 841–848. [DOI] [PubMed] [Google Scholar]
- 164.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. (2006) Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc Natl Acad Sci USA 103: 12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zwijacz-Kozica T, Selva N, Barja I, Silván G, Martínez-Fernández L, Illera JC, Jodłowski M. (2013) Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol 58: 215–222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.