The i-STAT system, a portable clinical analyzer, is increasingly being used to assess blood parameters in fish. This study validated the i-STAT system on rainbow trout blood under a broad range of conditions. Results indicate that the i-STAT is not an appropriate tool for assessing most blood parameters in rainbow trout.
Keywords: Blood oxygen tension, blood pH, haematocrit, portable clinical analyser, rainbow trout
Abstract
Portable clinical analysers, such as the i-STAT system, are increasingly being used for blood analysis in animal ecology and physiology because of their portability and easy operation. Although originally conceived for clinical application and to replace robust but lengthy techniques, researchers have extended the use of the i-STAT system outside of humans and even to poikilothermic fish, with only limited validation. The present study analysed a range of blood parameters [pH, haematocrit (Hct), haemoglobin (Hb), HCO3−, partial pressure of CO2 (PCO2), partial pressure of O2 (PO2), Hb saturation (sO2) and Na+ concentration] in a model teleost fish (rainbow trout, Oncorhynchus mykiss) using the i-STAT system (CG8+ cartridges) and established laboratory techniques. This methodological comparison was performed at two temperatures (10 and 20°C), two haematocrits (low and high) and three PCO2 levels (0.5, 1.0 and 1.5%). Our results indicate that pH was measured accurately with the i-STAT system over a physiological pH range and using the i-STAT temperature correction. Haematocrit was consistently underestimated by the i-STAT, while the measurements of Na+, PCO2, HCO3− and PO2 were variably inaccurate over the range of values typically found in fish. The algorithm that the i-STAT uses to calculate sO2 did not yield meaningful results on rainbow trout blood. Application of conversion factors to correct i-STAT measurements is not recommended, due to significant effects of temperature, Hct and PCO2 on the measurement errors and complex interactions may exist. In conclusion, the i-STAT system can easily generate fast results from rainbow trout whole blood, but many are inaccurate values.
Introduction
The analysis of blood parameters plays a key role in determining an organism's health- and/or physiological status. Thus, blood sampling and analysis are routinely performed in human and veterinary medicine, as well as more broadly in physiological research, including the growing fields of wildlife surveillance and conservation physiology (Cooke et al., 2013). While adequate blood sampling is now easily accomplished using cannulation and arterial puncture or venipuncture techniques, analysis typically requires sophisticated laboratory equipment, trained personnel and a considerable time investment. Some parameters, such as plasma ion and haemoglobin (Hb) concentrations, can be assessed after freezing (a prerequisite for many field studies in biology), but other blood parameters (e.g. acid–base status and blood gases) need to be measured immediately after collection for maximal accuracy. This requirement sets boundaries for the blood parameters that can be assessed using conventional techniques in remote locations and has been a considerable constraint for remote patient care and for field research on the physiology of non-domesticated animal taxa. However, the recent advances in medical analytical instruments have allowed the development of portable devices capable of accurately assessing a variety of relevant blood parameters; one of the most widely used systems today is the i-STAT® (Abbot Point of Care Inc., Princeton, NJ, USA).
The i-STAT is a rugged, light-weight, battery-powered, handheld device that allows simultaneous measurement of many blood parameters in minutes and using merely 100 μl of whole blood. Owing to these undisputed advantages over conventional analytical techniques, the i-STAT system is gaining popularity in biological research, for the analysis of blood on site and on a wide range of species, including mammals (e.g. Stockard et al., 2007), birds (e.g. Paula et al., 2008), reptiles (e.g. Harms et al., 2003) and a large number of fish species (see Stoot et al., 2014). In fact, the measurement of blood gases and acid–base status of many wild animals became possible only after the introduction of portable clinical analysers, such as the i-STAT system; and still today, it is often the only viable method available to researchers working in the field who are interested in measuring these important physiological parameters.
However, it is of note that the i-STAT system was originally conceived for medical application and that the measurement and especially the calculation of many parameters are based on constants and algorithms derived for human blood. It seems unlikely that a system designed for a single species could account for differences in blood characteristics between species and even taxonomic classes. Perhaps of greatest concern should be the effect of temperature (if different from 37°C, as in all ectothermic animals) on the accurate assessment of blood acid–base status and blood gases, because complex interactions between these factors exist upon closed system temperature changes (Malte et al., 2014). In addition, the measurement of haematocrit (Hct) may be subject to bias, because some of the studied species, such as birds, reptiles and fish, have nucleated red blood cells (RBCs) that will differ substantially from human RBCs in cell size and shape, metabolic activity, Hb content and Hb isoforms (Nikinmaa, 1990). The analysis of blood parameters in fishes is particularly challenging, because some species have unique Hb characteristics, such as a strong Bohr/Haldane effect (Bohr et al., 1904; Brauner and Jensen, 1999) or even a Root effect (Root, 1931; Brittain, 2005), which respond to changes in blood gases and acid–base status; these factors cannot be accounted for by algorithms designed for human blood.
Despite these limitations, Stoot et al. (2014) identified 27 studies in the last decade that have used the i-STAT system to measure blood parameters in several elasmobranch and teleost species and in a variety of environmental and physiological conditions (Kojima et al., 2004; Harrenstien et al., 2005; Foss et al., 2006, 2007, 2012; Choi et al., 2007; Eliason et al., 2007; Mandelman and Farrington, 2007a, b; Olsvik et al., 2007; Suski et al., 2007; Brill et al., 2008; Imsland et al., 2008; Petri et al., 2008; Remen et al., 2008; Mandelman and Skomal, 2009; DiMaggio et al., 2010; Gallagher et al., 2010; Meland et al., 2010; Olsvik et al., 2010; Brooks et al., 2011, 2012; Cicia et al., 2012; Frick et al., 2012; Hyatt et al., 2012; Naples et al., 2012; Roth and Rotabakk, 2012). While the authors of some of these studies recognized that certain blood parameters were not accurate, they argued that relative differences were still meaningful and worthwhile assessing (e.g. Mandelman and Farrington, 2007b; Cooke et al., 2008; Gallagher et al., 2010). Moreover, to the best of our knowledge, only three studies on fish have validated i-STAT results with conventional laboratory techniques (Harrenstien et al., 2005; DiMaggio et al., 2010; Gallagher et al., 2010). DiMaggio et al. (2010; validation for Hct, sodium, potassium and chloride) concluded that the i-STAT is not a reliable tool to assess Hct or plasma ions in seminole killifish (Fundulus seminolis). Both Harrenstien et al. [2005; validation for urea nitrogen, glucose, sodium, potassium, chloride, pH, partial pressure of CO2 (PCO2), total CO2 (TCO2), HCO3−, base excess, Hct and Hb] and Gallagher et al. [2010; validation for pH, PCO2, partial pressure of O2 (PO2), Hb saturation (sO2) and lactate] reported significant differences between measurements performed with the i-STAT and those using conventional techniques on two elasmobranch species and two rockfish species, respectively. However, latter authors concluded that in the tested conditions and applying appropriate conversion factors, the i-STAT system was a useful tool for measuring blood parameters. Whether these conversion factors will hold over a broader range of conditions has not been addressed previously; therefore, extrapolation of the determined conversion factors beyond the tested conditions is considered problematic.
As outlined above, the following three factors are most likely to bias i-STAT measurements: (i) temperature; (ii) differences in nucleated vs. non-nucleated RBC, hence Hct; and (iii) blood gas concentrations and acid–base status (that vary with PCO2), especially in teleosts that have a strong Bohr/Root shift (such as rainbow trout, Oncorhynchus mykiss). In our view, addressing these potentially confounding factors is important for a thorough validation of the i-STAT system for its use in the analysis of fish blood.
The aim of the present study was to validate the i-STAT system for the measurement of blood parameters in the model teleost, rainbow trout, over a broader range of conditions. Our choice of parameters was based on previous studies, most of which used the i-STAT system to measure pH, PCO2 and PO2. In addition, we deemed necessary a validation of the important blood parameters Hct and Hb, because of the fundamental differences between fish and mammalian RBCs and between fish Hb and that of other vertebrates. Therefore, blood samples were experimentally adjusted to one of two Hct levels (low, 20%; and high, 30%) and equilibrated in tonometers at two temperatures (10 and 20°C) and to three levels of PCO2 (0.5, 1.0 and 1.5%). We measured whole-blood Hct, Hb concentration, sO2 and pH and plasma PCO2, HCO3− and Na+ using the i-STAT system and compared these values with those measured using conventional techniques. While the i-STAT system offers tremendous benefits for field studies in experimental biology, this potential can be realized only after a thorough technical validation of the obtained measurements. Therefore, the present results provide guidelines for an appropriate implementation of the i-STAT system into future research on fish.
Materials and methods
Animals and housing
Rainbow trout, Oncorhynchus mykiss Walbaum 1792 (300–600 g), were obtained from Spring Valley Trout Farm (Langley, British Columbia, Canada) and were held at the University of British Columbia aquatic facilities in 4000 l tanks supplied with flow-through dechlorinated municipal tap water (Vancouver, BC, Canada), under a natural photoperiod, at 12°C. Animals were fed three times a week to satiation using commercial trout pellets (Skretting, Orient 4-0, Vancouver, British Columbia, Canada), but feeding was suspended 24 h before blood collection. All procedures were strictly according to the guidelines specified by the Canadian Council on Animal Care (UBC protocol no. A07-0080).
Blood collection
In preparation for surgery, fish were anaesthetized for 2 min in 0.5 g l−1 MS222 (tricaine methanosulfonate) buffered with sodium bicarbonate (1 g l−1 NaHCO3−). When unresponsive, animals were placed on a surgery table, and their gills were continuously perfused with aerated water (12°C) containing a maintenance anaesthetic dose (0.2 g l−1 buffered MS222). An indwelling cannula (PE50) was chronically implanted into the dorsal aorta according to the method described by Soivio et al. (1975). After surgery, the gills were perfused with cooled, aerated water until responsiveness was regained, and animals were transferred to individual flow-through Perspex boxes supplied with aerated water at 12°C. The cannula was flushed with heparinized Cortland saline (10 i.u. ml−1, lithium heparin; Sigma H0878), and animals were allowed to recover from surgery for 24 h. Blood samples of up to 5 ml were taken from undisturbed fish and were drawn from a cannula into a pre-heparinized syringe. Blood was pooled from up to six fish and kept on ice until analysis.
Haematocrit was measured in triplicate and then standardized to a predetermined Hct by either removing or adding plasma that had previously been obtained by caudal puncture (immediately frozen at −80°C). A 3 ml aliquot of blood was loaded into each of six Eschweiler tonometers placed in a thermostated water bath and equilibrated with a water-saturated gas mixture. All experimental conditions were chosen to mimic the in vivo status of rainbow trout venous blood, as described by Rummer (2010). Blood samples in tonometers were equilibrated to treatment conditions for 1 h before analysis using custom-mixed gases (O2, CO2 and N2) from a DIGAMIX 275 6KM 422 Woesthoff pump (Bochum, Germany).
Experimental design
The i-STAT system was validated over a broad range of conditions for three factors (Hct, temperature and PCO2). Six replicate samples (n = 6) were run for each combination of factors, and the experimental unit was a single tonometer containing blood pooled from up to six fish. This design resulted in a total of 72 blood samples, which represented blood pooled from 36 donor fish.
Haematocrit was set to either low (∼20%) or high (∼30%); these values are within the normal range for rainbow trout (Gallaugher and Farrell, 1998). Two temperatures were tested, either 10 or 20°C, and these temperatures were used during conventional blood analyses. The PCO2 was tested at three levels by setting the composition of the equilibration gas mixture during tonometry to 0.5 (∼3.8 mmHg), 1.0 (∼7.5 mmHg) or 1.5% (∼11.3 mmHg), which mimicked the in vivo venous PCO2 tension range of rainbow trout (Rummer, 2010). The PO2 was nominally set to 6% (∼45.9 mmHg) to approximate Hb O2 saturations of 50–100%. Nitrogen was used as a balance gas.
Sampling protocol
The six tonometers were sampled sequentially after equilibration intervals of 45 min using heparinized, gas-tight Hamilton syringes of appropriate volume. Each time, three subsamples were taken from a tonometer. A 90 μl subsample was immediately loaded into the i-STAT cartridge; a 20 μl subsample was used for the analysis of total O2 (TO2); and a subsample of 500 μl was removed for the analysis of pH, Hct, Hb concentration, TCO2 and Na+ concentration. First, whole-blood pH was measured at treatment temperature, Hct was measured in triplicate and samples for the measurement of Hb concentration were stored at 4°C (analysis was within 48 h). The remainder of the blood sample was centrifuged (10 000 g for 2 min); 20 μl of plasma were stored at −80°C for the analysis of Na+ concentration, and TCO2 was measured on the remaining plasma.
Analysis
Measurements were performed using the VetScan i-STAT 1 System (SN:704534-C; software version JAMS 135c/CLEW A26; Abaxis, Union City, CA, USA) with the i-STAT CG8+ cartridge test (ABBT-03P77-25). Cartridges were stored in the dark in their original packaging at 4°C. Before use, cartridges were allowed to equilibrate to room temperature overnight. Analysis was at room temperature, using the temperature correction function of the i-STAT system for the two experimental temperatures.
Using an established laboratory technique, Hct was measured by filling microcapillary tubes (10 μl) and centrifuging at 17 000 g for 3 min. Likewise, Hb concentration was measured in duplicate with a Beckman 640 spectrophotometer (Brea, CA, USA) using the cyanomethaemoglobin method and an extinction coefficient of 11 mmol−1 cm−1 at 540 nm. Whole-blood pH measurements were performed using a Radiometer BMS 3 Mk2 and a Radiometer acid–base analyser PHM73 (Copenhagen, Denmark) with a thermostated pH electrode at the respective treatment temperature. Whole-blood TO2 content was measured according to Tucker (1967). Haemoglobin saturation was calculated from TO2 after subtracting physically dissolved O2 according to Boutilier et al. (1984) and dividing by the theoretical maximal carrying capacity of the rinsed RBCs based upon the tetrameric Hb concentration obtained spectrophotometrically according to Tucker (1967). Total CO2 in the blood plasma was measured using a Corning 965 CO2 analyser (Corning, USA), after centrifuging whole blood for 5 min at 5000 g. Plasma HCO3− concentration and PCO2 were calculated from the plasma pH and TCO2 using the Henderson–Hasselbalch equation and the physicochemical parameters reported by Boutilier et al. (1984). Plasma Na+ concentration was measured after a 2000:1 dilution with milliQ de-ionized water (resistivity >18.2 μΩ cm−1) and using a Varian AA240FS atomic absorption spectrophotometer (Palo Alto, CA, USA).
Data analysis
All data were analysed with RStudio v0.98.501 (RStudio Inc., Boston, MA, USA). i-STAT measurements were compared with control measurements by regression analysis using the original data. The measurement error for the i-STAT values relative to control measurements was calculated as follows: δ = (i-STAT-control)/control × 100. The δ data were then compared with control measurements either by regression analysis or by fitting a non-linear model to the data [linear, logarithmic and exponential models were compared using the Akaike information criterion (AIC), and the model with the best fit was used as representative for the data]. Normality of distribution was tested with the Shapiro–Wilk test (P < 0.05), and homogeneity of variances was tested with Levene's test (P < 0.05). The effects of temperature and Hct on δ were tested with the Welch independent samples t-test (P < 0.05, n = 36) and PCO2 with one-way ANOVA (P < 0.05, n = 24), using the absolute values of δ (i.e. by treating all values as positive). In some cases, this transformation led to a significant deviation of the distribution from normality, which could not be remediated by data transformation; in this situation, the effects of temperature and Hct on δ were tested with the Wilcoxon rank sum test (P < 0.05, n = 36) and PCO2 with the Kruskal–Wallis rank sum test (P < 0.05, n = 24). All data are presented as means ± SEM.
Results
In general, we perceived the i-STAT system as very user friendly. All 72 i-STAT CG8+ cartridges used in the present study performed adequately. There was no clotting inside the cartridges or erroneous readings for other reasons. Measurements were performed on a mere 90 μl of whole blood, and all procedures could easily be carried out by a single person within a few minutes. The control measurements on the same blood parameters, in contrast, required two people working simultaneously for about 45 min per sample (excluding the measurements of Hb and Na+ concentrations that were performed on preserved samples at a later point in time).
pH
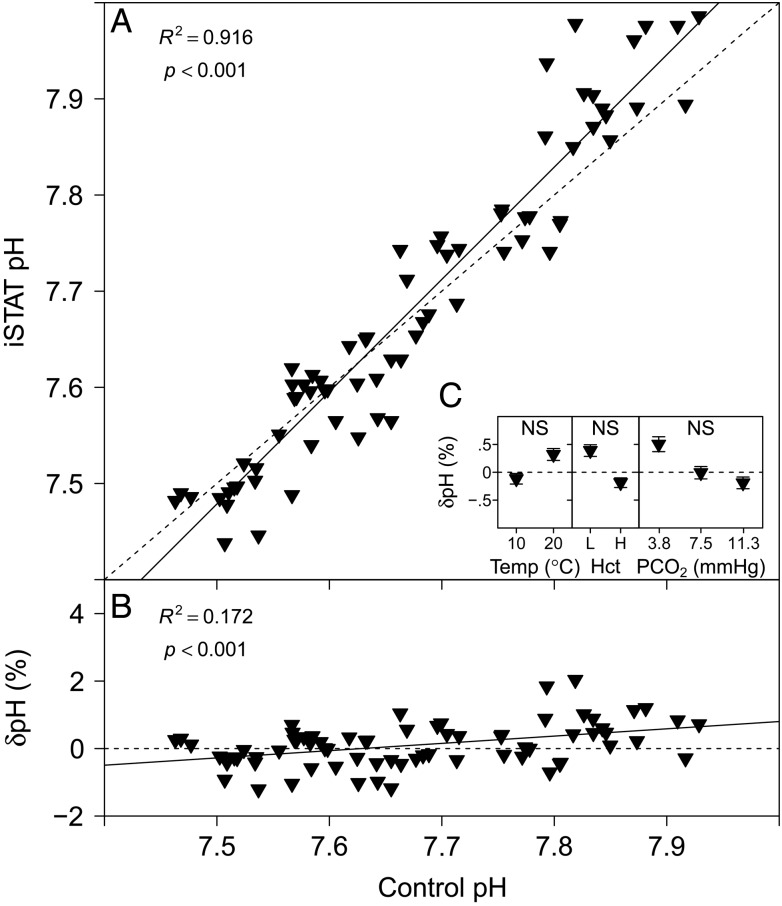
The pH measurements performed with the i-STAT system are compared with measurements made with a thermostated pH electrode in Fig. 1A. A highly significant linear relationship was found between i-STAT and control pH (Table 1). The relative error of pH measurements performed with the i-STAT, δpH (expressed as a percentage), is compared with the control pH in Fig. 1B, and the parameter estimates for the linear relationship are given in Table 1. No significant effects on δpH were detected for temperature (P = 0.871, n = 36), Hct (P = 0.065, n = 36) or PCO2 (P = 0.347, n = 24; Fig. 1C).
Figure 1:
(A) Measurements of pH with the i-STAT system vs. control measurements of pH with a thermostated pH electrode. (B) The relative error of i-STAT pH measurements, δpH (expressed as a %), [calculated as: (i-STAT pH − control pH)/control pH × 100] vs. control pH. Continuous lines represent the fitted linear models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C ), haematocrit (Hct; L, low; H, high) and partial pressure of CO2 (PCO2; in mmHg) on δpH (expressed as a %). Significant effects within treatments are indicated as NS for non-significant. Data are means ± SEM, and statistical analysis was performed on the absolute δpH values.
Table 1:
Parameter estimates (means ± SEM), r2 and P-values for the relationships between i-STAT vs. control measurements and i-STAT measurement errors, δ(x) (expressed as a percentage), vs. control measurements (n = 72)
| Measurement | a | b | c | r2 | P-value |
|---|---|---|---|---|---|
| pH | −1.280 ± 0.322 | 1.168 ± 0.042 | 0.916 | <0.001 | |
| δpH | −16.455 ± 4.171 | 2.157 ± 0.543 | 0.172 | <0.001 | |
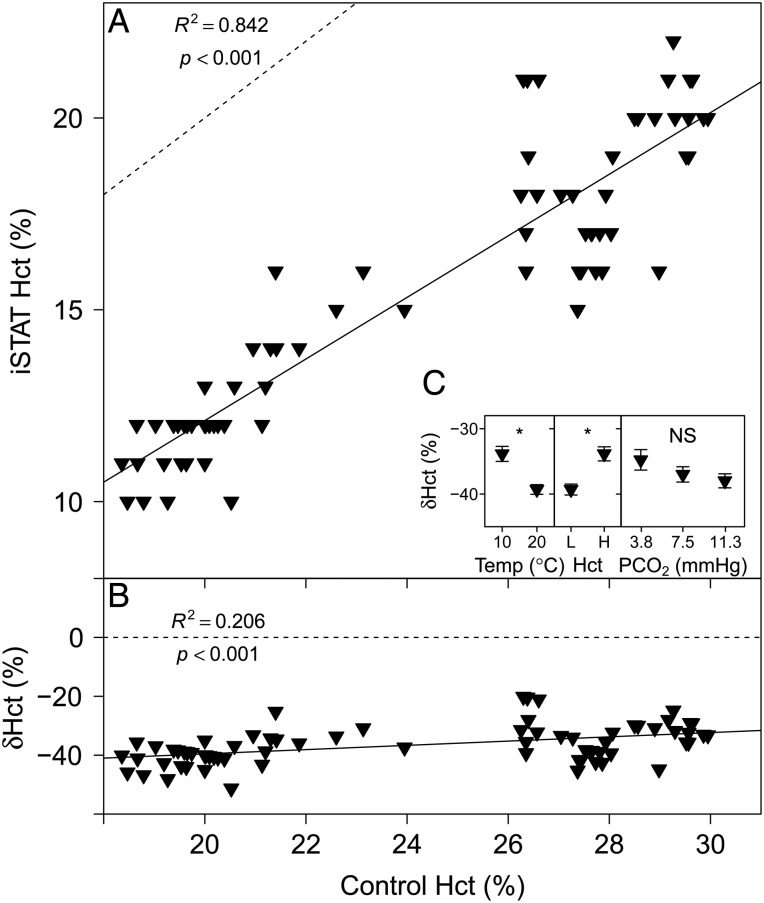
| Hct | −3.945 ± 1.008 | 0.803 ± 0.041 | 0.842 | <0.001 | |
| δHct | −54.006 ± 4.009 | 0.723 ± 0.164 | 0.206 | <0.001 | |
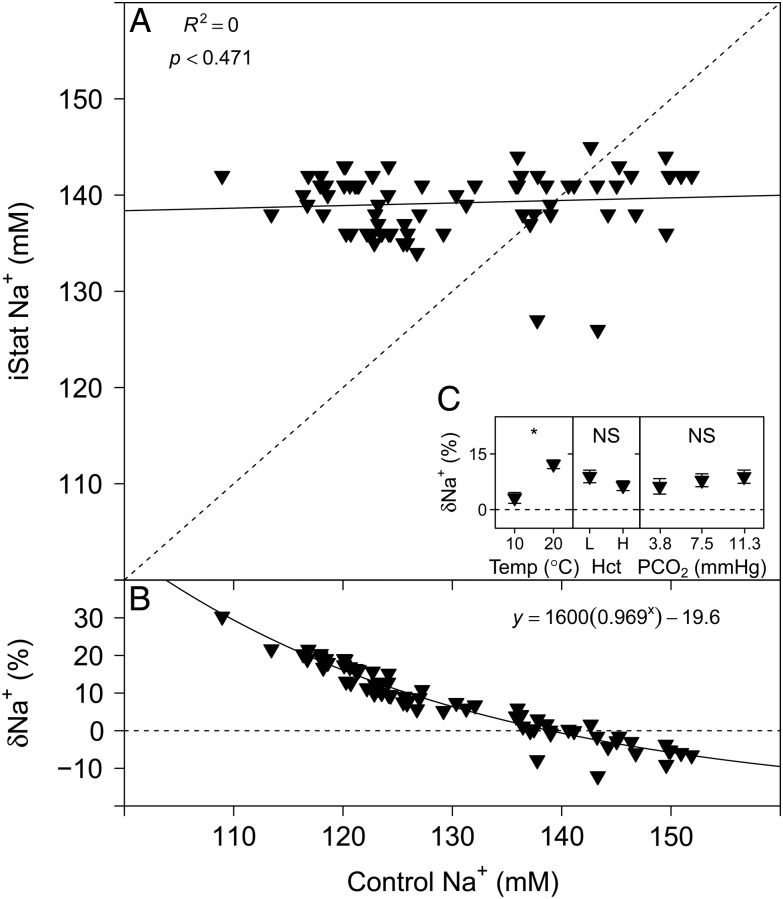
| Na+ | 135.701 ± 4.821 | 0.027 ± 0.037 | −0.007 | 0.471 | |
| δNa+ | 1600 ± 1012 | 0.969 ± 0.006* | −19.562 ± 5.189* | ||
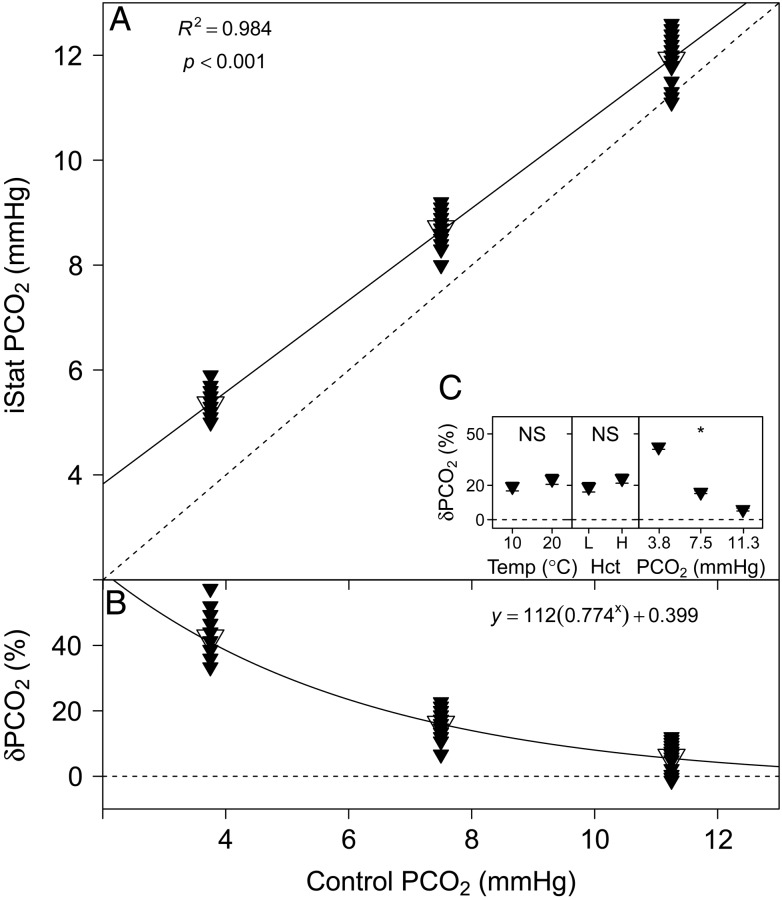
| PCO2 | 2.076 ± 0.106 | 0.876 ± 0.013 | 0.984 | <0.001 | |
| δPCO2 | 111.730 ± 12.543* | 0.774 ± 0.036* | −0.399 ± 3.369 | ||
| HCO3− | 3.150 ± 0.491 | 0.836 ± 0.044 | 0.838 | <0.001 | |
| δHCO3− | 41.961 ± 4.300 | −2.630 ± 0.381 | 0.396 | <0.001 | |
| sO2 | 96.990 ± 0.316 | 0.033 ± 0.004 | 0.425 | <0.001 | |
| δsO2 | 562.073 ± 9.798* | 0.970 ± 0.001* | −26.055 ± 1.311* |
Abbreviations: Hb, haemoglobin; Hct, haematocrit; PCO2, partial pressure of CO2; PO2, partial pressure of O2; and sO2, haemoglobin saturation, δ, measurement error.
Linear relationships according to: i-STAT(x) = a + b × control(x); and δ(x) = a + b × control(x).
Non-linear relationships according to: i-STAT(x) = a × bcontrol(x)−c.
*Parameter estimates in non-linear models marked with ‘*’ are statistically significant (t-test, P < 0.001).
Haematocrit
The Hct measurements performed with the i-STAT system are compared with measurements in microcapillary tubes in Fig. 2A. A highly significant linear relationship was found between i-STAT and control Hct measurements (Table 1). The relative error of Hct measurements performed with the i-STAT, δHct (expressed as a percentage), compared with control Hct was a constant underestimate (Fig. 2B), and the parameter estimates for the linear relationship are given in Table 1. Temperature (P < 0.001) and Hct (P < 0.001) had a significant effect on δHct, but no significant effect of PCO2 on δHct was detected (P = 0.204; Fig. 2C).
Figure 2:
(A) Measurements of Hct with the i-STAT system vs. control measurements of Hct in microcapillary tubes. (B) The relative error of i-STAT Hct measurements, δHct (expressed as a %), [calculated as: (i-STAT Hct − control Hct)/control Hct × 100] vs. control Hct. Continuous lines represent the fitted linear models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C ), Hct (L, low; H, high) and PCO2 (in mmHg) on δHct (expressed as a %). Significant effects within treatments are indicated as NS for non-significant and ‘*’ at the P < 0.05 level. Data are means ± SEM, and statistical analysis was performed on the absolute δHct values.
Sodium
The Na+ measurements performed with the i-STAT are compared with measurements made with a flame photometer in Fig. 3A. No evident relationship was detected between i-STAT Na+ and control Na+ (Table 1). The relative error of Na+ measurements performed with the i-STAT, δNa+ (expressed as a percentage), is compared with control Na+ in Fig. 3B and was best described by an exponential model (linear AIC, 358.24; logarithmic AIC, 350.46; and exponential AIC, 339.83), with the parameter estimates given in Table 1. Temperature (P < 0.001) had a significant effect on δNa+, but no significant effects of Hct (P = 0.054) or PCO2 on δNa+ were detected (P = 0.997; Fig. 3C).
Figure 3:
(A) Measurements of Na+ with the i-STAT system vs. control measurements of Na+ with a flame photometer. (B) The relative error of i-STAT Na+ measurements, δNa+ (expressed as a %), [calculated as: (i-STAT Hct − control Hct)/control Hct × 100] vs. control Hct. Continuous lines represent the fitted models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C ), Hct (L, low; H, high) and PCO2 (in mmHg) on δNa+ (expressed as a %). Significant effects within treatments are indicated as NS for non-significant and ‘*’ at the P < 0.05 level. Data are means ± SEM, and statistical analysis was performed on the absolute δNa+ values.
Partial pressure of CO2
The PCO2 measurements performed with the i-STAT are compared with the set tonometer values in Fig. 4A. A highly significant linear relationship was found between i-STAT and control PCO2 (Table 1). The relative error of PCO2 measurements performed with the i-STAT, δPCO2 (expressed as a percentage), is compared with control PCO2 in Fig. 4B and was best described by an exponential model (linear AIC, 472.95; logarithmic AIC, 445.18; and exponential AIC, 440.02), with the parameter estimates given in Table 1. Partial pressure of CO2 had a significant effect (P < 0.001) on δPCO2, but no significant effects of temperature (P = 0.260) or Hct were detected (P = 0.067; Fig. 4C).
Figure 4:
(A) Measurements of PCO2 with the i-STAT system vs. set PCO2. Mean values are indicated by the larger, open symbols. (B) The relative error of i-STAT PCO2 measurements, δPCO2 (expressed as a % ), [calculated as: (i-STAT PCO2 − control PCO2)/control PCO2 × 100] vs. control PCO2. Continuous lines represent the fitted models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C ), Hct (L, low; H, high) and PCO2 (in mmHg) on δPCO2 (expressed as a %). Significant effects within treatments are indicated as NS for non-significant and ‘*’ at the P < 0.05 level. Data are means ± SEM, and statistical analysis was performed on the absolute δPCO2 values.
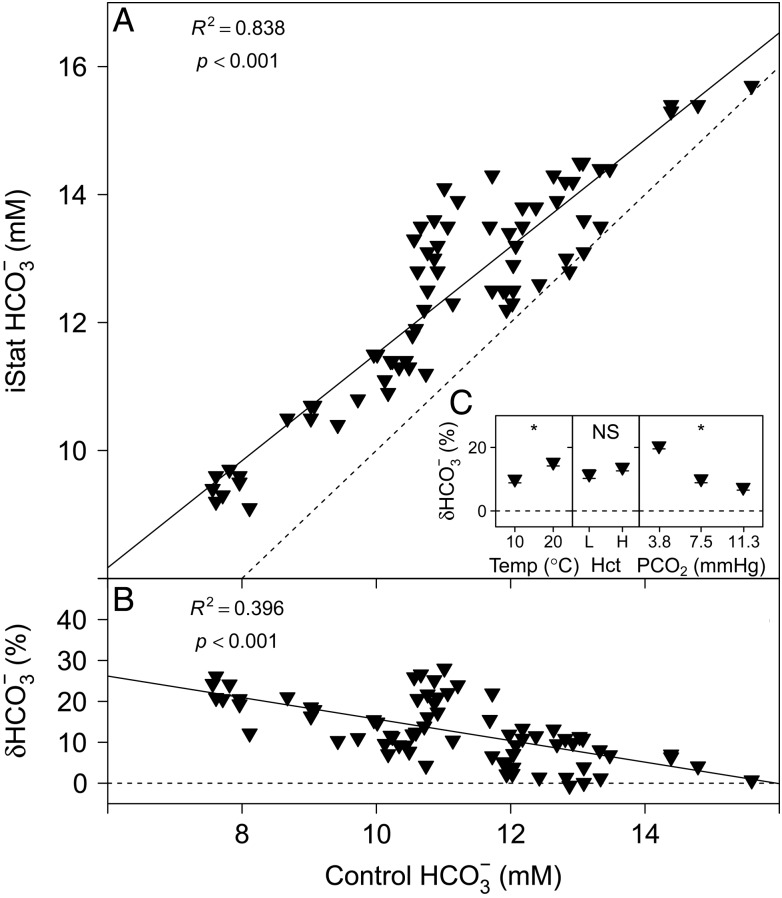
Bicarbonate
The HCO3− measurements with the i-STAT are compared with conventional laboratory techniques in Fig. 5A. A highly significant linear relationship was found between i-STAT and control HCO3− measurements (Table 1). The relative error of HCO3− measurements performed with the i-STAT, δHCO3− (expressed as a percentage), is compared with control HCO3− in Fig. 5B, and the parameter estimates for the linear relationship are given in Table 1. Temperature (P = 0.002) and PCO2 (P < 0.001) had significant effects on δHCO3−, but no significant effect of Hct on δHCO3− was detected (P = 0.232; Fig. 5C).
Figure 5:
(A) Measurements of HCO3− with the i-STAT system vs. control HCO3− calculated from total CO2 and pH. (B) The relative error of i-STAT HCO3− measurements, δHCO3− (%), [calculated as: (i-STAT HCO3− − control HCO3−)/control HCO3− × 100] vs. control HCO3−. Continuous lines represent the fitted linear models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C ), Hct (L, low; H, high) and PCO2 (in mmHg) on δHCO3− (expressed as a %). Significant effects within treatments are indicated as NS for non-significant and ‘*’ at the P < 0.05 level. Data are means ± SEM, and statistical analysis was performed on the absolute δHCO3− values.
Partial pressure of O2
All treatments were measured at a PO2 of 45.9 mmHg (∼6% O2); therefore, the effect of PO2 on i-STAT PO2 measurements was not assessed and the results are not presented in a figure. The overall mean of i-STAT PO2 measurements was 77.9 ± 4.0 mmHg and the mean relative error of measurements, δPO2 (expressed as a percentage), was 69.6 ± 8.6. A significant effect of temperature (P < 0.001) on δPO2 was detected, and mean values were 121.3 ± 11.3 and 17.9 ± 4.4 at 10 and 20°C, respectively. Also PCO2 had a significant effect (P < 0.001) on δPO2, and mean values were 121.6 ± 15.9, 67.1 ± 12.4 and 20.1 ± 7.3% at 3.8, 7.5 and 11.3 mmHg PCO2, respectively. Haematocrit did not have a significant effect on δPO2 (P = 0.258).
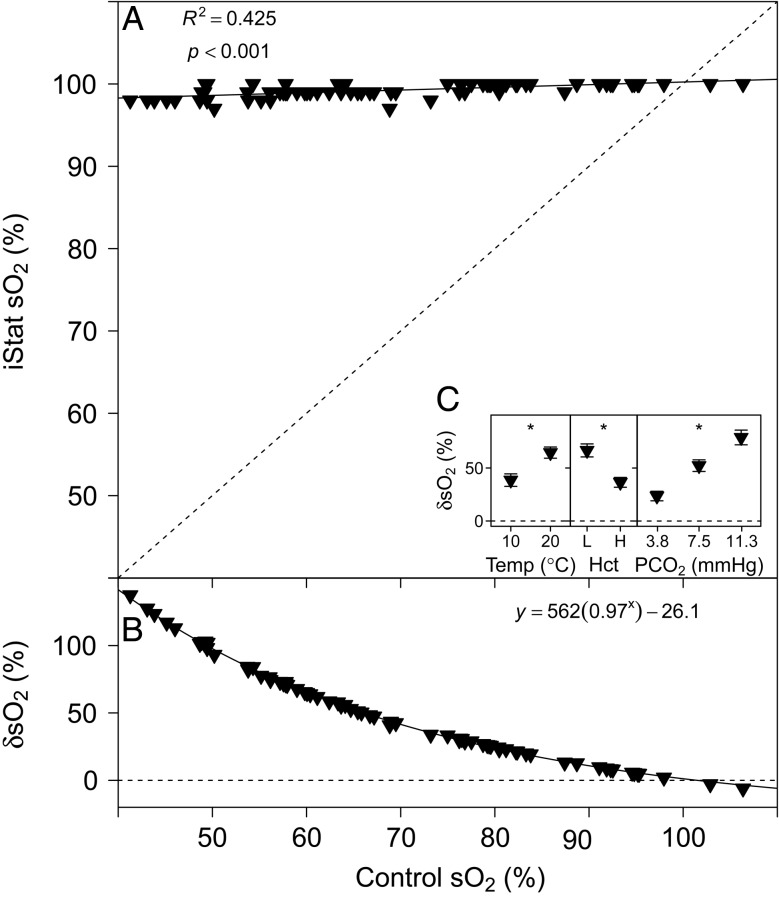
Haemoglobin saturation
The sO2 measurements performed with the i-STAT are compared with the Tucker method in Fig. 6A. A highly significant linear relationship was found between i-STAT and control pH measurements (Table 1). The relative error of sO2 measurements performed with the i-STAT, δsO2 (expressed as a percentage), is compared with control measurements in Fig. 6B and was best described by an exponential model (linear AIC, 515.73; logarithmic AIC, 419.20; and exponential AIC, 236.87), with the parameter estimates given in Table 1. Temperature (P < 0.001), Hct (P < 0.001) and PCO2 had a significant effect on δsO2 (P < 0.001; Fig. 6C).
Figure 6:
(A) Measurements of haemoglobin saturation (sO2) with the i-STAT system vs. control sO2 calculated by the Tucker method. (B) The relative error of i-STAT sO2 measurements, δsO2 (expressed as a %), [calculated as: (i-STAT sO2 − control HCO3−)/control sO2 × 100] vs. control sO2. Continuous lines represent the fitted models and dashed lines represent the lines of identity. (C) Effects of temperature (in °C), Hct (L, low; H, high) and PCO2 (in mmHg) on δsO2 (expressed as a %). Significant effects within treatments are indicated as ‘*’ at the P < 0.05 level. Data are means ± SEM, and statistical analysis was performed on the absolute δsO2 values.
Discussion
The measurement of rainbow trout whole-blood pH with the i-STAT system yielded accurate and precise results over the physiological pH range. The relative measurement error (δpH) over this range was within ∼2% of control measurements performed with a thermostated pH electrode (Fig. 1B). Harrenstien et al. (2005) have previously described i-STAT measurement errors for pH of −5% in black rockfish (Sebastes melanops). Gallagher et al. (2010) used the i-STAT on elasmobranchs and found similar measurement errors for pH of −3.8 and −4.4% for sandbar sharks and smooth dogfish, respectively (calculated values at pH 7.5 based on the described linear relationships between control pH and i-STAT pH). Temperature had no significant effect on i-STAT pH measurements; thus, the integrated temperature correction algorithm seems to account adequately for the effects of a closed system temperature change within the cartridge (Malte et al., 2014). Haematocrit did not have a significant effect on i-STAT pH measurements, which suggests that any differences in RBC size or nucleation state should not affect pH measurements. In addition, no significant effect of PCO2 was detected on i-STAT pH measurements.
In contrast, the fairly basic Hct measurement performed by the i-STAT system was consistently and significantly lower than the values measured with the standard microcapillary tubes (Fig. 2A). Over the range of tested Hct values (20–30%), measurements performed with the i-STAT system were typically 40% lower than control values (Fig. 2B); this is in line with previous studies. DiMaggio et al. (2010) described a similar measurement error for i-STAT Hct of −44.5% in semiole killifish. Harrenstien et al. (2005) found a measurement error of −30% in black rockfish, attributing this bias to differences in RBC shape between humans and fish.
The i-STAT system measures Hct indirectly by means of whole-blood conductometry (Table 2). While plasma itself is relatively conductive to electrical currents, RBCs are not; therefore, a relationship exists between the fraction of volume that is occupied by RBCs (i.e. Hct) and the conductivity of a whole-blood sample. In the i-STAT system, corrections are applied for sample temperature and sample volume (by first measuring the conductivity of a calibrant within the cartridge) and for plasma conductivity (by measuring Na+ and K+ concentrations; i-STAT Technical Bulletin, 2011, 2013a, e). All these factors are integrated into the calculation of Hct using an algorithm designed for human blood. As opposed to human RBCs, fish RBCs are nucleated, and therefore larger and different in shape (elliptical in fishes vs. double concave in humans) for a given Hb concentration (Nikinmaa, 1990). Considering the higher metabolic activity, differences in Hb isoforms and membrane composition, differences in RBC conductivity are possible. Samples measured at 10°C had a significantly lower δHct than samples measured at 20°C; thus, higher temperatures seem to have a negative effect on the accuracy of Hct measurements with the i-STAT on fish whole blood; this may be due to a temperature effect on membrane fluidity and therefore conductive properties of RBCs. In addition, Na+ concentration (which is integrated into Hct calculations) was not reliably measured in fish plasma.
Table 2:
Measured and calculated blood parameters reported by the i-STAT system with CG8+ cartridges
| Parameter | Methoda |
|---|---|
| pH | Direct potentiometry |
| Hct | Whole-blood conductometry |
| Hb | Calculated from Hct |
| Na+ | Ion-selective electrode potentiometry |
| PO2 | Amperometry |
| PCO2 | Direct potentiometry |
| HCO3− | Calculated from pH and PCO2 |
| sO2 | Calculated from PO2, pH and HCO3− |
Abbreviations: Hb, haemoglobin; Hct, haematocrit; PCO2, partial pressure of CO2; PO2, partial pressure of O2; and sO2, haemoglobin saturation.
aRetrieved from the i-STAT Technical Bulletin (2013a, b, c, d, e).
The plasma of fish living in dilute freshwaters typically has ion concentrations that are lower than those found in humans (Lago et al., 2008). Although the i-STAT system corrects conductivity readings for differences in plasma Na+ and K+ concentrations, no other ions are taken into account, and their concentrations may affect plasma conductivity and thus the accuracy of Hct measurements (i-STAT Technical Bulletin, 2011). Furthermore, i-STAT Hct is sensitive to the concentration of other non-conductive elements in the plasma, i.e. proteins and lipids. At Hct levels <40% and total protein concentrations <6.5 g/dl, measurements of conductivity will lead to an underestimation of Hct (i-STAT Technical Bulletin, 2011). Haematocrit levels in the present study were <30%, and total protein levels found in rainbow trout are ∼2.1 g/dl (Milligan and Wood, 1982). A significant effect of Hct on δHct indicates that having more RBCs in the blood sample will increase the accuracy of i-STAT Hct measurements; this may suggest that the confounding effects are due to differences in plasma conductivity between humans and fish, as opposed to differences in RBC conductivity. The strong Bohr/Haldane effect found in trout and the associated RBC swelling upon acidification and H+ buffering by Hb (Jensen, 2004) did not seem to affect i-STAT Hct measurements, because PCO2 did not have a significant effect on δHct.
The present results support previous findings that the i-STAT system underestimates Hct in fish blood; this bias was sensitive to temperature and Hct, and may be sensitive to plasma composition. Therefore, correction of i-STAT Hct measurements on fish blood using conversion factors is not recommended. If the nucleated state of fish RBCs contributed to an inaccurate Hct assessment by the i-STAT, we suspect that similar if not identical problems may be encountered with any blood that has nucleated RBCs. This would bode poorly for the majority of vertebrate animals, because over half of the species are fishes, ∼40% comprise the amphibians, reptiles and birds, while <10% are mammals that have non-nucleated erythrocytes.
Plasma Na+ concentration was assessed over the range of 110–150 mm. All fish were housed in the same conditions, and blood samples were pooled from several fish; therefore, initially, all samples had similar plasma Na+ concentrations. However, to obtain different Hct levels, samples were diluted with plasma obtained by caudal puncture. During such stress, rainbow trout RBCs will actively take up Na+ by β-adrenergically activated sodium-proton-exchangers (β-NHE) activity, thereby lowering plasma Na+ levels (Borgese et al., 1987; Nikinmaa, 1990). Given that the plasma for dilution was frozen and stored for several days, no direct effect of catecholamines on blood samples was expected (Caldwell et al., 2006); the relatively large range in plasma Na+ concentrations can be attributed to our procedures rather than the variability in the fish that were sampled. Yet, the range of plasma Na+ concentrations is physiologically representative for freshwater fishes housed in dilute water (Vancouver tap water, [Na+] 60 mm; Fu et al., 2010). Over this range, no significant relationship was detected between i-STAT Na+ and control Na+ measurements with a flame photometer (Fig. 3A). The observed measurement error, δNa+, decreased from about +20% at 110 mm to −10% at 150 mm Na+, following an exponential relationship (Fig. 3B). Surprisingly, temperature had a significant effect on δNa+, and a non-significant trend (P = 0.054) suggests that Hct may affect i-STAT Na+ measurements outside the Hct range studied here. The i-STAT system measures Na+ by ion-selective potentiometry (Table 2); a calibrant solution is measured as reference, and the results are calculated based on the Nernst equation (i-STAT Technical Bulletin, 2013e). This method may be sensitive to the concentration of other ions of similar charge or size. Most fish are ammoneotelic. Thus, the relatively high concentrations of NH4+ in the plasma (0.1–0.8 mm NH4+; Wright and Wood, 1985; McDonald and Milligan, 1992) are a candidate to cause interference with i-STAT Na+ measurements; this possibility, however, requires further testing. Previous studies have found the i-STAT to underestimate Na+ concentration by −26% in semiole killifish (at 180 mm Na+; DiMaggio et al., 2010) and by −8% in marine black rockfish (at 170 mm Na+; Harrenstien et al., 2005). The latter findings are similar to the present results and, according to the exponential model fitted to the data, δNa+ at 170 mm would be −12% (Fig. 3B). Our results suggest that i-STAT measurements of Na+ in fish whole blood are most accurate around 140 mm, but at lower or higher concentrations the results become unreliable; the cause for this bias remains unclear. Therefore, and due to the significant effect of temperature on δNa+, we do not recommend using conversion factors to correct i-STAT Na+ measurements.
For the tested range of PCO2 values (3.8–11.3 mmHg), measurements performed with the i-STAT system were generally higher than set control values (Fig. 4A). At 3.8 mmHg, the i-STAT overestimated PCO2 by 40%. Gallagher et al. (2010) have described the i-STAT to overestimate PCO2 by more than 350% in sandbar sharks and smooth dogfish (at 3.5 mmHg). The results of Harrenstien et al. (2005) had an error closer to the present study, with the i-STAT overestimating PCO2 by 67% in black rockfish (at 10 mmHg). The significant effect of PCO2 on the measurement error, δPCO2, suggests that the accuracy of i-STAT PCO2 measurements is highly dependent on PCO2 itself (Fig. 4C), and accuracy improves at higher PCO2 tensions. In fact, the exponential relationship between δPCO2 and control PCO2 suggests that above 19 mmHg PCO2, δPCO2 is <1% (Fig. 4B). Therefore, it seems that the i-STAT system can measure PCO2 accurately at higher PCO2 levels, such as expected for humans; however, measurements in the PCO2 range of most fishes (arterial PCO2 in rainbow trout, ∼2.6 mmHg; Lessard et al., 1995; Brauner and Randall, 1998) will be associated with a high and potentially variable measurement error. The i-STAT measures PCO2 by direct potentiometry, and the final result is calculated based on the Nernst equation, after measuring a calibrant with known CO2 content as a reference (Table 2). Although the reportable range for PCO2 in the i-STAT is given as 5–130 mmHg, it seems that the low values typically present in fish cannot be measured accurately.
Bicarbonate values assessed with the i-STAT system on whole blood of rainbow trout were significantly higher than control values (Fig. 5A). The δHCO3− for the i-STAT system was ∼20% at 8 mm HCO3− and decreased towards higher HCO3− concentrations (Fig. 5B). This is lower than the −47% (at 13 mm HCO3−) reported by Harrenstien et al. (2005). The i-STAT system calculates HCO3− from measured pH and PCO2 values (Table 2). Therefore, it is not surprising that the PCO2 level had a significant effect on δHCO3−, which corresponds with the effect of PCO2 on δPCO2 reported above (Fig. 4C). Both pH and PCO2 measurements in the i-STAT system are temperature corrected, and no significant effects of temperature were detected on these measurements. However, a significant effect of temperature on δHCO3− indicates that the algorithm used by the i-STAT to calculate HCO3− concentration is not representative for the effect of temperature on HCO3− concentration in fish plasma (i-STAT Technical Bulletin, 2013b).
Partial pressure of O2 in this study was assessed with a single level, to keep the number of factor combinations manageable. Over all treatments, mean PO2 measured with the i-STAT was 77.9 ± 4.0 mmHg (at a set PO2 of 45.9 mmHg), and the mean measurement error, δPO2, was 69.6 ± 8.6%. This measurement error for i-STAT PO2 is similar to the previously described 51.8% for sandbar sharks and 77.4% for smooth dogfish (Gallagher et al., 2010). The i-STAT measures PO2 amperiometrically (Table 2), with a platinum cathode behind a gas-permeable membrane. This method is temperature sensitive, and the i-STAT technical documentation warns not to cool blood samples before measurement, because this may lead to an overestimation of PO2 (i-STAT Technical Bulletin, 2013d). The present results describe a significant effect of temperature on δPO2, and PO2 measurements with the i-STAT were more accurate at 20°C (mean δPO2 = 17.9 ± 4.4%) than at 10°C (mean δPO2 = 121.3 ± 11.3%). Given that the upper lethal temperature of rainbow trout is 23–25°C (Black, 1953), accurate PO2 measurements within the physiological temperature range of this species do not seem possible with the i-STAT system. Low PCO2 levels also decreased the accuracy of i-STAT PO2 measurements, and a significant effect of PCO2 on δPO2 was detected. Whether PCO2 affects i-STAT PO2 measurement directly or via changes in pH is unclear from the present data. At typical arterial PCO2 levels in trout (∼2.6 mmHg; Lessard et al., 1995), the i-STAT system does not yield satisfactory accuracy for the measurements of PO2 (δPO2 > 67.1 ± 12.4%).
Results for the calculated parameter sO2 yielded a highly significant linear relationship between i-STAT sO2 and control sO2 obtained with the Tucker method (Fig. 6A). The control values obtained in the present study are considered representative for rainbow trout whole blood, as substantiated by previous studies that have used the same tonometry method under similar conditions of PO2, PCO2 and temperature (Rummer, 2010; Rummer and Brauner, 2011). However, for the entire range of control sO2 values (40–100%) the i-STAT system reported sO2 values of 97–100%, i.e. full Hb saturation. The algorithm that the i-STAT system uses to calculate sO2 (Table 2) is based on a human oxygen equilibrium curve and assumes a constant Hct and ‘normal’ Hb affinity for O2, i.e. it does not account for inter-specific differences in Hb–O2 affinity, Hb isoform multiplicity, the effect of organic phosphates on Hb–O2 affinity or the presence of dysfunctional Hb species (carboxy-, met- and sulf-Hb; i-STAT Technical Bulletin, 2013d). While many of these factors may be relatively predictable and constant in human blood, in fishes this is not the case (Nikinmaa, 1990; Jensen et al., 1998), and inter-specific variability, differences in environmental conditions and differences in the physiological state of the animal may significantly bias sO2 measurements performed with the i-STAT. In addition, some teleost fish species, such as trout, exhibit a strong Bohr/Root effect (Bohr et al., 1904; Root, 1931), which is clearly not accounted for in the algorithm for the calculation of sO2 used by the i-STAT system. In trout, a higher PCO2 tension will decrease sO2 due to the combined effects of acidification (hence increase in H+ concentration) and due to the increase in CO2 concentration. Both H+ and CO2 can bind to Root-effect Hb and will lead to a change in conformation that stabilizes the deoxygenated state and hence leads to lower sO2 values at the same PO2 (Brittain, 2005). Human Hb has a moderate Bohr effect and no Root effect; therefore, changes in pH or PCO2 do not affect sO2 to the same degree as in fish. In the i-STAT, this is reflected in the significantly higher δsO2 at higher PCO2 tensions, despite a more accurate measurement of PO2 at higher PCO2 tensions. The measurement of i-STAT sO2 was significantly more accurate at higher Hct levels and at lower temperatures. Given that the calculation of sO2 is a function of PO2, it was expected that factors that would lead to an inaccurate determination of PO2 would equally be reflected in an inaccurate calculation of sO2. This was not the case, and the significant temperature and PCO2 effects were opposite to those effects observed for PO2. Although unexpected, this emphasizes the complex interactions that must exist between factors and provides a warning against using simple linear relationships for correction of i-STAT results. Since the temperature correction algorithm of the i-STAT system is based on human blood characteristics, using this function may increase the measurement error beyond that caused by a closed system temperature change (i.e. heating the sample to 37°C). However, Gallagher et al. (2010) manually temperature corrected raw values generated by the i-STAT using previously derived equations (Mandelman and Skomal, 2009) and, likewise, concluded that the i-STAT was unable to determine sO2 reliably in the studied elasmobranch species, suggesting that the problem goes beyond mere differences in temperature [i-STAT raw values for pH, PCO2 and PO2 were reliably corrected to 25°C using the equations derived by Mandelman and Skomal (2009)]. The characteristics of Hb in humans and fish are fundamentally different (Jensen et al., 1998); therefore, the i-STAT (and algorithms based on human constants) cannot simply yield representative results for sO2 in fish.
Conclusion
Measurements of pH were accurate under the tested conditions, and the i-STAT is considered an appropriate tool for assessing the acid–base status of blood in rainbow trout. Haematocrit was consistently lower than control values, but significant effects of temperature, Hct and plasma composition on the accuracy of i-STAT Hct measurements would make correction of results complex. The accuracy of i-STAT measurements of plasma Na+ concentration, PCO2, HCO3− and PO2 was dependent on the measured range and associated with a high measurement error at those values typically expected for rainbow trout. In agreement with previous work, measurements of sO2 with the i-STAT system were not meaningful on rainbow trout whole blood, presumably due to large differences between the characteristics of Hb in humans and fish. Given the large number of studies that routinely use portable clinical analysers (including the i-STAT system) to measure blood parameters on a variety of species, the present results emphasize the need for a thorough validation of these devices within the specific study conditions. Our results indicate that, for the accurate measurement of most blood parameters in fishes (save for pH), there is no alternative to the time-consuming but well-validated laboratory methodology.
Acknowledgements
Thanks are due to Bill Milsom for providing the i-STAT system and to Sabine Lague for instructions on proper use. This study was supported by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grants to C.J.B. and A.P.F. A.P.F. holds a Canada Research Chair.
References
- Black EC. (1953) Upper lethal temperatures of some British Columbia freshwater fishes. J Fish Board Can 10: 196–210. [Google Scholar]
- Bohr C, Hasselbalch K, Krogh A. (1904) About a new biological relation of high importance that the blood carbonic acid tension exercises on its oxygen binding. Skand Arch Physiol 16: 402–412. [Google Scholar]
- Borgese F, Garcia-Romeu F, Motais R. (1987) Control of cell volume and ion transport by β-adrenergic catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol 382: 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier RG, Heming TA, Iwama GK. (1984) Physicochemical parameters for use in fish respiratory physiology. In Hoar WS, Randall DJ, eds, Fish Physiology, Vol. XA. Academic Press, New York, pp 403–426. [Google Scholar]
- Brauner CJ, Jensen BJ. (1999) O2 and CO2 exchange in fish: The nonlinear release of Bohr/Haldane protons with oxygenation. In Val AL, Almeida-Val VMF, eds, Biology of Tropical Fishes. INPA, Manaus, pp 393–400. [Google Scholar]
- Brauner CJ, Randall D. (1998) The linkage between oxygen and carbon dioxide transport. In Perry SF, II, Tufts BL, eds, Fish Physiology, Vol. XVII: Fish Respiration. Academic Press, San Diego, pp 283–320. [Google Scholar]
- Brill R, Bushnell P, Schroff S, Seifert R, Galvin M. (2008) Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J Exp Mar Biol Ecol 354: 132–143. [Google Scholar]
- Brittain T. (2005) Root effect hemoglobins. J Inorg Biochem 99: 120–129. [DOI] [PubMed] [Google Scholar]
- Brooks EJ, Sloman KA, Liss S, Hassan-Hassanein L, Danylchuk AJ, Cooke SJ, Mandelman JW, Skomal GB, Sims DW, Suski CD. (2011) The stress physiology of extended duration tonic immobility in the juvenile lemon shark, Negaprion brevirostris (Poey 1868). J Exp Mar Biol Ecol 409: 351–360. [Google Scholar]
- Brooks EJ, Mandelman JW, Sloman KA, Liss S, Danylchuk AJ, Cooke SJ, Skomal GB, Philipp DP, Sims DW, Suski CD. (2012) The physiological response of the Caribbean reef shark (Carcharhinus perezi) to longline capture. Comp Biochem Physiol A Mol Integr Physiol 162: 94–100. [DOI] [PubMed] [Google Scholar]
- Caldwell S, Rummer JL, Brauner CJ. (2006) Blood sampling techniques and storage duration: effects on the presence and magnitude of the red blood cell β-adrenergic response in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 144: 188–195. [DOI] [PubMed] [Google Scholar]
- Choi K, Lehmann D, Harms C, Law J. (2007) Acute hypoxia–reperfusion triggers immunocompromise in Nile tilapia. J Aquat Anim Health 19: 128–140. [DOI] [PubMed] [Google Scholar]
- Cicia AM, Schlenker LS, Sulikowski JA, Mandelman JW. (2012) Seasonal variations in the physiological stress response to discrete bouts of aerial exposure in the little skate, Leucoraja erinacea. Comp Biochem Physiol A Mol Integr Physiol 162: 130–138. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Suski CD, Danylchuk SE, Danylchuk AJ, Donaldson MR, Pullen C, Bulte G, O'Toole A, Murchie KJ, Koppelman JB, et al. (2008) Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools. J Fish Biol 73: 1351–1375. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio MA, Ohs CL, Petty BD. (2010) Evaluation of a point-of-care blood analyzer for use in determination of select hematological indices in the seminole killifish. N Am J Aquacult 72: 261–268. [Google Scholar]
- Eliason E, Farrell A, Kiessling A, Karlsson A, Djordjevic B. (2007) Validation of the hepatic portal vein cannulation technique using Atlantic salmon (Salmo salar L.). Comp Biochem Physiol A Mol Integr Physiol 146: S187–S187. [Google Scholar]
- Foss A, Kristensen T, Åtland Å, Hustveit H, Hovland H, Øfsti A, Imsland AK. (2006) Effects of water reuse and stocking density on water quality, blood physiology and growth rate of juvenile cod (Gadus morhua). Aquaculture 256: 255–263. [Google Scholar]
- Foss A, Imsland AK, Roth B, Schram E, Stefansson SO. (2007) Interactive effects of oxygen saturation and ammonia on growth and blood physiology in juvenile turbot. Aquaculture 271: 244–251. [Google Scholar]
- Foss A, Grimsbø E, Vikingstad E, Nortvedt R, Slinde E, Roth B. (2012) Live chilling of Atlantic salmon: physiological response to handling and temperature decrease on welfare. Fish Physiol Biochem 38: 565–571. [DOI] [PubMed] [Google Scholar]
- Frick LH, Walker TI, Reina RD. (2012) Immediate and delayed effects of gill-net capture on acid–base balance and intramuscular lactate concentration of gummy sharks, Mustelus antarcticus. Comp Biochem Physiol A Mol Integr Physiol 162: 88–93. [DOI] [PubMed] [Google Scholar]
- Fu C, Wilson JM, Rombough PJ, Brauner CJ. (2010) Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc Biol Sci 277: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AJ, Frick LH, Bushnell PG, Brill RW, Mandelman JW. (2010) Blood gas, oxygen saturation, pH, and lactate values in elasmobranch blood measured with a commercially available portable clinical analyzer and standard laboratory instruments. J Aquat Anim Health 22: 229–234. [DOI] [PubMed] [Google Scholar]
- Gallaugher P, Farrell AP. (1998) Hematocrit and blood oxygen-carrying capacity. In Perry SF, II, Tufts BL, eds, Fish Physiology, Vol. XVII: Fish Respiration: Academic Press, San Diego, pp 185–227. [Google Scholar]
- Harms CA, Mallo KM, Ross PM, Segars A. (2003) Venous blood gases and lactates of wild loggerhead sea turtles (Caretta caretta) following two capture techniques. J Wildlife Dis 39: 366–374. [DOI] [PubMed] [Google Scholar]
- Harrenstien LA, Tornquist SJ, Miller-Morgan TJ, Fodness BG, Clifford KE. (2005) Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. J Am Vet Med Assoc 226: 255–265. [DOI] [PubMed] [Google Scholar]
- Hyatt MW, Anderson PA, O'Donnell PM, Berzins IK. (2012) Assessment of acid–base derangements among bonnethead (Sphyrna tiburo), bull (Carcharhinus leucas), and lemon (Negaprion brevirostris) sharks from gillnet and longline capture and handling methods. Comp Biochem Physiol A Mol Integr Physiol 162: 113–120. [DOI] [PubMed] [Google Scholar]
- Imsland AK, Gunnarsson S, Asgeirsson A, Roth B, Schram E, Foss A. (2008) Commercial-scale validation of temperature-step rearing on growth physiology in turbot, Scophthalmus maximus. J World Aquacult Soc 39: 684–691. [Google Scholar]
- i-STAT Technical Bulletin (2011) Hematocrit Determination in the i-STAT System and comparison to Other Methods. Abbott Point of Care Inc, Abbott Park, IL, USA. [Google Scholar]
- i-STAT Technical Bulletin (2013a) Hematocrit/Hct and Calculated Hemoglobin. Abbott Point of Care Inc., Abbott Park, IL, USA. [Google Scholar]
- i-STAT Technical Bulletin (2013b) PCO2 and Calculated Values for HCO3, Base Excess and Anion Gap. Abbott Point of Care Inc., Abbott Park, IL, USA. [Google Scholar]
- i-STAT Technical Bulletin (2013c) pH. Abbott Point of Care Inc, Abbott Park, IL, USA. [Google Scholar]
- i-STAT Technical Bulletin (2013d) PO2 and Calculated Oxygen Saturated/sO2. Abbott Point of Care Inc, Abbott Park, IL, USA. [Google Scholar]
- i-STAT Technical Bulletin (2013e) Sodium/Na+. Abbott Point of Care Inc, Abbott Park, IL, USA. [Google Scholar]
- Jensen FB. (2004) Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand 182: 215–227. [DOI] [PubMed] [Google Scholar]
- Jensen FB, Fago A, Weber RE. (1998) Hemoglobin structure and function. Fish Physiol 17: 1–40. [Google Scholar]
- Kojima T, Ishii M, Kobayashi M, Shimizu M. (2004) Blood parameters and electrocardiogram in squeezed fish simulating the effect of net damage and recovery. Fish Sci 70: 860–866. [Google Scholar]
- Lago RM, Pencina MJ, Wang TJ, Lanier KJ, D'Agostino RB, Sr, Kannel WB, Vasan RS. (2008) Interindividual variation in serum sodium and longitudinal blood pressure tracking in the Framingham Heart Study. J Hypertens 26: 2121–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J, Val A, Aota S, Randall D. (1995) Why is there no carbonic anhydrase activity available to fish plasma? J Exp Biol 198: 31–38. [DOI] [PubMed] [Google Scholar]
- McDonald D, Milligan CL. (1992) Chemical properties of the blood. In Hoar WS, Randall D, Farrell A, eds, Fish Physiology, Vol. XIIB: The Cardiovascular System. Academic Press, New York. [Google Scholar]
- Malte CL, Jakobsen SL, Wang T. (2014) A critical evaluation of automated blood gas measurements in comparative respiratory physiology. Comp Biochem Physiol A: Mol Integr Physiol, doi:10.1016/j.cbpa.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Mandelman JW, Farrington MA. (2007a) The estimated short-term discard mortality of a trawled elasmobranch, the spiny dogfish (Squalus acanthias). Fish Res 83: 238–245. [Google Scholar]
- Mandelman JW, Farrington MA. (2007b) The physiological status and mortality associated with otter-trawl capture, transport, and captivity of an exploited elasmobranch, Squalus acanthias. ICES J Mar Sci 64: 122–130. [Google Scholar]
- Mandelman JW, Skomal GB. (2009) Differential sensitivity to capture stress assessed by blood acid–base status in five carcharhinid sharks. J Comp Physiol B 179: 267–277. [DOI] [PubMed] [Google Scholar]
- Meland S, Heier LS, Salbu B, Tollefsen KE, Farmen E, Rosseland BO. (2010) Exposure of brown trout (Salmo trutta, L.) to tunnel wash water runoff — chemical characterisation and biological impact. Sci Total Environ 408: 2646–2656. [DOI] [PubMed] [Google Scholar]
- Milligan CL, Wood CM. (1982) Disturbances in haematology, fluid volume distribution and circulatory function associated with low environmental pH in the rainbow trout, Salmo gairdneri. J Exp Biol 99: 397–415. [Google Scholar]
- Naples LM, Mylniczenko ND, Zachariah TT, Wilborn RE, Young FA. (2012) Evaluation of critical care blood analytes assessed with a point-of-care portable blood analyzer in wild and aquarium-housed elasmobranchs and the influence of phlebotomy site on results. J Am Vet Med Assoc 241: 117–125. [DOI] [PubMed] [Google Scholar]
- Nikinmaa M. (1990) Vertebrate Red Blood Cells. Adaptations of Function to Respiratory Requirements. Zoophysiology. Springer, Berlin. [Google Scholar]
- Olsvik PA, Lie KK, Hevrøy EM. (2007) Do anesthetics and sampling strategies affect transcription analysis of fish tissues? BMC Mol Biol 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsvik PA, Kroglund F, Finstad B, Kristensen T. (2010) Effects of the fungicide azoxystrobin on Atlantic salmon (Salmo salar, L.) smolt. Ecotox Environ Safety 73: 1852–1861. [DOI] [PubMed] [Google Scholar]
- Paula VV, Fantoni DT, Otsuki DA, Auler JO., Jr (2008) Blood-gas and electrolyte values for Amazon parrots (Amazona aestiva). Pesquisa Vet Brasil 28: 108–112. [Google Scholar]
- Petri D, Hamre K, Lundebye AK. (2008) Retention of the synthetic antioxidant butylated hydroxyanisole in Atlantic salmon (Salmo salar) fillets. Aquacult Nutr 14: 453–458. [Google Scholar]
- Remen M, Imsland AK, Stefansson SO, Jonassen TM, Foss A. (2008) Interactive effects of ammonia and oxygen on growth and physiological status of juvenile Atlantic cod (Gadus morhua). Aquaculture 274: 292–299. [Google Scholar]
- Root RW. (1931) The respiratory function of the blood of marine fishes. Biol Bull 61: 427–456. [Google Scholar]
- Roth B, Rotabakk BT. (2012) Stress associated with commercial longlining and recreational fishing of saithe (Pollachius virens) and the subsequent effect on blood gases and chemistry. Fish Res 115: 110–114. [Google Scholar]
- Rummer JL. (2010) A novel mechanism for enhancing tissue oxygen delivery in teleost fishes. PhD thesis University of British Columbia, Vancouver. [Google Scholar]
- Rummer JL, Brauner CJ. (2011) Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in rainbow trout, Oncorhynchus mykiss. J Exp Biol 214: 2319–2328. [DOI] [PubMed] [Google Scholar]
- Soivio A, Nynolm K, Westman K. (1975) Technique for repeated sampling of blood of individual resting fish. J Exp Biol 63: 207–217. [DOI] [PubMed] [Google Scholar]
- Stockard T, Levenson D, Berg L, Fransioli J, Baranov E, Ponganis P. (2007) Blood oxygen depletion during rest-associated apneas of northern elephant seals (Mirounga angustirostris). J Exp Biol 210: 2607–2617. [DOI] [PubMed] [Google Scholar]
- Stoot LJ, Cairns NA, Cull F, Taylor JJ, Jeffrey JD, Morin F, Mandelman JW, Clark TD, Cooke SJ. (2014) Use of portable blood physiology point-of-care devices for basic and applied research on vertebrates: a review. Conserv Physiol 2: doi:10.1093/conphys/cou011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suski CD, Cooke SJ, Danylchuk AJ, O'Connor CM, Gravel MA, Redpath T, Hanson KC, Gingerich AJ, Murchie KJ, Danylchuk SE, et al. (2007) Physiological disturbance and recovery dynamics of bonefish (Albula vulpes), a tropical marine fish, in response to variable exercise and exposure to air. Comp Biochem Physiol A Mol Integr Physiol 148: 664–673. [DOI] [PubMed] [Google Scholar]
- Tucker VA. (1967) Method for oxygen content and dissociation curves on microliter blood samples. J Appl Physiol 23: 410–414. [DOI] [PubMed] [Google Scholar]
- Wright PA, Wood CM. (1985) An analysis of branchial ammonia excretion in the fresh water rainbow trout: effects of environmental pH change and sodium uptake blocked. J Exp Biol 114: 320–353. [Google Scholar]