Abstract
The treatment of articular cartilage injury and disease has become an increasingly relevant part of orthopaedic care. Articular cartilage transplantation, in the form of osteochondral allografting, is one of the most established techniques for restoration of articular cartilage. Our research efforts over the last two decades have supported the transformation of this procedure from experimental “niche” status to a cornerstone of orthopaedic practice. In this Kappa Delta paper, we describe our translational and clinical science contributions to this transformation: (1) to enhance the ability of tissue banks to process and deliver viable tissue to surgeons and patients, (2) to improve the biological understanding of in vivo cartilage and bone remodeling following osteochondral allograft (OCA) transplantation in an animal model system, (3) to define effective surgical techniques and pitfalls, and (4) to identify and clarify clinical indications and outcomes. The combination of coordinated basic and clinical studies is part of our continuing comprehensive academic OCA transplant program. Taken together, the results have led to the current standards for OCA processing and storage prior to implantation and also novel observations and mechanisms of the biological and clinical behavior of OCA transplants in vivo. Thus, OCA transplantation is now a successful and increasingly available treatment for patients with disabling osteoarticular cartilage pathology.
Keywords: osteochondral allografts, cartilage repair
Introduction
Osteochondral allografts (OCA) repair large articular cartilage defects by restoring mature, hyaline cartilage in a biologically, structurally, and functionally appropriate manner.1, 2 Such OCA were historically implanted fresh, within 7 days of donor death, and are useful for the treatment of a wide spectrum of articular pathology, particularly conditions that involved both the osseous and chondral component.1, 2 While the use of osteochondral transplants in biologic joint reconstructions was reported in the early twentieth century,3 the use of such fresh OCA did not regain clinical interest and utilization until the end of the century. Even then, fresh OCA transplantation was relegated to a few specialized university centers with dedicated institutional tissue banks, limiting the number of procedures that could be performed.
Here, we summarize our body of work on the development of OCA storage protocols to increase the supply of suitable donor tissue, the establishment of an appropriate animal model to systematically analyze cartilage repair and remodeling in vivo, the identification of appropriate indications to refine patient selection, and the improvement of surgical techniques. Together, the findings allow achievement of increasingly consistent and reproducible outcomes in cartilage repair scenarios. This manuscript is a synopsis of our basic science, translational studies, and clinical experience spanning over two decades and encompassing over 800 OCA procedures. As a result, OCA transplantation has emerged with an important role in the clinical algorithm of cartilage restoration nationwide.
Sustaining Long-Term Chondrocyte Viability During Allograft Storage
The fundamental paradigm of fresh OCA transplantation is that viable chondrocytes survive storage and subsequent transplantation while maintaining their metabolic activity and sustaining their surrounding matrix to provide an intact structural and functional unit to replace diseased articular tissue. Grafts retrieved after fresh osteochondral allografting have contained viable chondrocytes up to 29 years after implantation.4 Chondrocyte viability in OCA is generally considered to be critical to graft survivorship and clinical outcome.5 In order to make fresh OCA accessible to the community surgeon, prolonged graft storage would be advantageous to allow time for logistical considerations and for the completion of microbiologic and serologic testing prior to release, as required by the FDA since 2002.6 This section describes our efforts to develop OCA storage protocols that aim to preserve long-term chondrocyte viability prior to implantation.
For these in vitro studies, osteochondral samples were harvested, stored, and analyzed for chondrocyte viability, metabolic activity, biochemical content, and biomechanical properties. OCAs were isolated from adult human or goat knees (cores or hemi-joints) and stored at various temperatures, durations, and solutions (see Table 1).
Table 1.
Development of In Vitro OCA Storage Protocols to Preserve Long-Term Chondrocyte Viability: Key Studies
| Tissue Type | Temp [°C] | Duration [days] | Storage Solution | Ref |
|---|---|---|---|---|
| Adult Human | 4 | 28 | TCM | 8 |
| Adult Human | 4 | 28 | TCM Lactated Ringers |
7 |
| Adult Human | 4 | 28 | TCM TCM + 10% serum |
9 |
| Adult Goat | 4 | 28 | TCM TCM + TNF-inhibitor (10 μg/mL etanercept) |
11 |
| Adult Goat (cores & joint scale) |
4 37 |
28 28 |
TCM + 10% serum TCM + 0%, 2%, or 10% serum |
12, 13 |
In general, 4°C storage of OCA supports biologically viable cartilage for relatively short durations (2–4 weeks), but the tissue is locked in a quiescent state (Figure 1). Through the in vitro storage studies, we determined (1) that OCA stored traditionally in lactated Ringer’s solution should be implanted within 7 days of donor death (as appropriate for the empirical university model);7 (2) the cartilage matrix is preserved during storage in tissue culture medium (TCM) at 4°C for 28 days, but the chondrocytes necessary to maintain the matrix after transplantation decreases over time;8 (3) chondrocyte viability was higher after storage at 4°C for 28 days when additional nutrients (i.e., serum) were added to the media and the superficial zone was a target for decreased viability after 14 days;9 (4) chondrocyte death during storage is likely mediated by apoptosis10 and modifications to the apoptotic response by adding a TNF inhibitor to TCM can improve chondrocyte viability during 4°C storage for up to 28 days11 (5) 37 °C storage of OCA supports long-term (≥4 weeks) chondrocyte viability, especially at the articular surface,12 but cartilage of joint-scale OCAs may need additional anabolic stimuli or catabolic inhibitors to maintain matrix (e.g., GAG) content;13 and (6) the results of in vitro storage data in human and goat tissue was consistent with that of clinical practice: 4°C stored grafts have lower chondrocyte viability at the time of OCA transplantation versus fresh grafts.14
Figure 1. Summary of Results for In Vitro Storage of OCA.
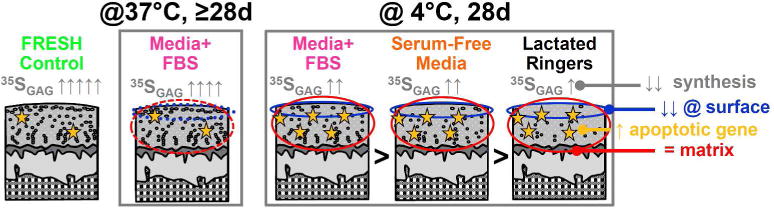
Chondrocyte viability is higher when OCA are stored at 4°C in TCM with greater nutritional content. TCM with fetal bovine serum (FBS) preserves viability better than serum free media and Lactated Ringers, but there is still substantial cell death versus fresh controls and OCA stored at 37°C, especially at the vulnerable superficial zone. At 4°C, while cells are vulnerable to death and relatively quiescent, cartilage matrix content and structure are maintained, but matrix metabolism is decreased and expression of proapoptotic genes are increased. 37 °C storage of OCA supports long-term chondrocyte viability, especially at the articular surface, but cartilage matrix content can be modulated.
From these efforts, the standard method of tissue banks that commercialize OCA is to use TCM with added supplementation of fetal bovine serum to preserve chondrocyte viability during screening and processing. Such allograft storage in the U.S. has led to an increased supply of fresh OCA, and allowed osteochondral allografting to be used outside of specialized university centers.
Biological and Structural Aspects of In Vivo Cartilage and Bone Remodeling
Animal models allow for a systematic analysis of the efficacy and mechanisms of cartilage repair and remodeling. Data obtained from human allograft retrievals is skewed toward poor outcomes, since analysis is performed on “failed” cases, where revision or conversion to total joint arthroplasty was required.15–17 In addition, clinical evaluations are not sensitive to early stage degeneration. We designed studies with intra-disciplinary and multi-scale analyses of cartilage repair in an animal model to elucidate tissue changes indicative of early stage degeneration, and to provide insight into the time-course of cartilage remodeling and/or deterioration.
Adult Boer goats were operated in one knee with Institutional Animal Care and Use Committee approval.18, 19 Each operated knee received two site-matched OCA, one implanted in to the medial femoral condyle (MFC) and one into the lateral trochlea sites (LT). Grafts of different storage treatments (frozen, fresh, or stored at 4°C for 14 or 28 days) were implanted alternatingly into the MFC and LT sites. Contralateral knees served as non-operated controls. At 6 or 12 months, both knees were analyzed, assessing macroscopic and histologic structure, and cartilage (cellular and matrix composition, biomechanical function) and bone (micro-computed tomography (μCT) bone morphometric and histology) properties.
In summary, in vivo OCA repair efficacy appears to be consistent with an overall inverse relationship between cartilage stiffness and structural measures of cartilage degeneration demonstrating that 4°C stored grafts (with reduced cellularity at the cartilage surface) are somewhat susceptible to tissue degeneration and communication between cartilage and bone may affect biological and structural remodeling during osteochondral repair (Figure 2). Through these in vivo animal studies, we determined (1) at 6 months, frozen OCA already exhibited clear progression toward failure, with loss of chondrocytes, reduced proteoglycan content and cartilage stiffness, and associated surface and/or bone collapse, while fresh OCA preserved depth-dependent tissue properties similar to non-operated cartilage, and thus maintained their capacity for biological homeostasis;19 (2) proteoglycan-4 (PRG4)-secreting function of OCA is maintained based on its state at implantation;20 (3) frozen OCA show similar changes at 12 months that were evident after 6 months whereas 4°C stored OCA exhibit reduced performance and variable long-term outcomes which were associated with reduced cellularity at the articular surface versus consistently good repair by fresh OCA;18 (4) the presence of bone cysts (void areas diameter greater than 1mm by μCT) were widespread and bone structure was altered by OCA relative to contralateral non-operated control knees;21 and (5) OCA repair patterns all exhibited basal cysts (located near the base of the implant), and occurred (a) in isolation, (b) with subchondral cysts (located near the bone-cartilage interface) and subchondral bone (ScB) channels, (c) with ScB channels, or (d) with subchondral cysts, ScB channels, and ScB erosion suggesting that bone cysts occurring after OCA may result from aberrant mechanobiology.21
Figure 2. Summary of Results for In Vivo OCA Repair.
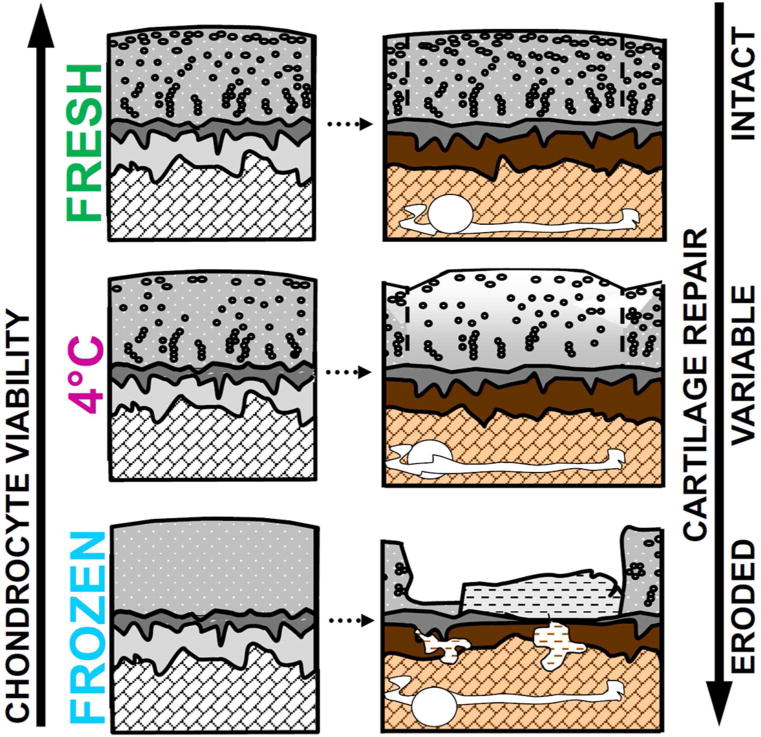
Implantation of fresh OCA resulted in consistently good (and intact) cartilage repair outcomes with high cartilage cellularity, whereas 4°C stored OCA had variable results, and the cartilage had decreased cellularity and was somewhat susceptible to degeneration. Frozen OCA exhibited clear progression toward failure (erosion), with loss of chondrocytes, reduced proteoglycan content and cartilage stiffness, and associated surface and/or bone collapse.
Current MRI grading scales used for cartilage repair or osteoarthritis are not targeted to evaluate OCA repair that have both chondral and osseous components. Thus, we were motivated by the need for non-invasive characterization of OCA and the ability to refine MRI technology to predict clinical outcomes. We introduced a comprehensive MRI scoring system for OCA and validated such scoring system with histopathologic, μCT, and biomechanical reference standards in an adult goat model of in vivo OCA repair with variable treatment options, ranging from outstanding to poor repair.22 Nine primary features for each graft (including, cartilage signal, fill, lateral integration, surface congruity, calcified cartilage integrity, subchondral bone plate congruity, subchondral bone signal, osseous integration, and presence of cystic changes) and four ancillary features of the joint (including, opposing cartilage, meniscal tears, synovitis, and fat-pad scarring) were defined for an accurate description of in vivo repair, which may help standardize reporting of MR findings after OCA repair.
Since the repair outcomes from 4°C stored OCA were inconsistent compared to fresh OCA, we identified a novel use of PRG4 secretion as a biomarker of OCA health and performance, biophysical and biological mechanisms that may be key in osseous repair, and clinically translatable MRI imaging analyses designed specifically to evaluate OCA repair. The results of these studies allow us to better predict OCA outcomes and devise strategies to provide more suitable tissue for transplantation, which in turn will help improve long-term repair efficacy in the clinic. Future studies investigating bone metabolism and its relationship to cyst structure during cyst development may further elucidate potential biological mechanisms of cyst formation within the setting of OCA repair.
Refining Surgical Techniques to Achieve Reproducible Results
The commercialization of OCA tissue allowed surgeons outside of specialized centers to access such tissue, and as a result, the application of OCA increased by an order of magnitude. However, the overwhelming acceptance and success of OCA transplantation would not have been possible without our efforts to provide surgeons with our best practices. Over the years, we have published numerous articles describing technical, logistical, and surgical details needed to further the widespread application of OCA in cartilage repair scenarios.5, 23–33 The operative procedure to implant OCA demands precision to achieve reproducible results and minimize early graft failure related to surgical technique.
The results from the following studies forced us to reconsider earlier surgical protocols, which called for a tight interference fit and often necessitated strong mallet impaction and larger portions of bone to anchor the graft to the host, and to consider alternative strategies to increase the supply of suitable graft tissue. Examining the in vitro biomechanical features of impact loading on chondrocyte death indicated that impact insertion of OCA generates damaging loading impulses sufficient to cause chondrocyte death; such chondrocyte death was particularly in the superficial zone in a pattern that was similar to that which occurs with prolonged storage and occurred with a mechanism of cell death that was consistent with apoptosis mediated by the activation of caspases.34 Similarly examining in vivo graft insertion parameters during animal studies indicated that OCA inserted with ~100 taps resulted in poor outcomes, which were associated with decreased in cellularity and matrix content (Figure 3).18 Thus, a simple technical modification of minimizing excessive impact loading to preserve chondrocyte viability during insertion may be critically important to in vivo repair efficacy.
Figure 3. Relationship of Insertion Parameters and Cartilage Structure in In Vivo Animal Model.
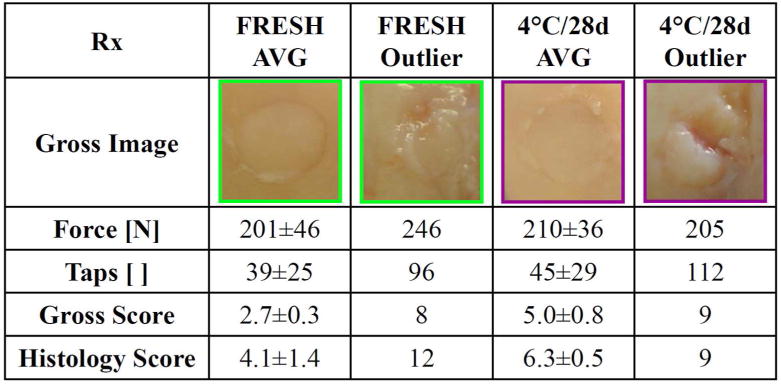
Excessive taps during surgical insertion resulted in poor outcomes after 12 months in vivo from Pallante et al.18
Precise fitting of the allograft and appropriate restoration of the articular anatomy is essential to long-term success.5, 23–33 The surgical technique for osteochondral allografting depends on the joint and surface to be grafted. Here, we highlight a few of the important considerations. In general, for femoral condyle lesions, a small arthrotomy is made to expose the cartilage defect and the lesion site is prepared. Then, a cylindrical plug or shell (free-hand) graft is harvested from the donor to match the defect. The donor OCA is carefully inserted (typically using short gentle impaction or manually pressed) until the cartilage is flush with the surrounding host cartilage. Trochlea OCA are more technically challenging, as the anatomy is complex and care must be taken to restore joint tophography. For the patella, complete resurfacing can be performed in a manner similar to arthroplasty, whereas smaller lesions can be treated with dowel-type grafts (similar to femoral condyle lesions). Tibial plateau OCA are inserted under fluoroscopic guidance in a surgical technique that is similar to unicompartmental arthroplasty. The graft is typically thicker (minimum 10mm) and fixed with interfragmentary screw fixation, and care must be taken to protect the cruciate ligament and meniscal attachments. Common to all OCA procedures are relative contraindications, which include uncorrected ligamentous instability of the joint, or axial malalignment of the limb. These biomechanical parameters should be optimized prior to OCA implantation, via ligament reconstruction or corrective osteotomy. When an osteotomy is planned on the joint surface opposite the OCA (i.e. MFC OCA with tibial osteotomy), the procedures are performed concomitantly. Conversely, if OCA and osteotomy involve the same bone (commonly lateral femoral condyle OCA with distal femoral osteotomy), then the procedures are staged to allow revascularization of the graft bed, with the osteotomy performed 6 months prior to OCA.
The critical step in the OCA procedure is to define the shape, size, and exact location of the defect so that an exact orthotopic graft can be harvested from the donor. Donor and recipient are matched solely on the basis of size within 3mm, determined using a mediolateral dimension of the tibia measured 0.5cm below the joint surface. In addition, the amount of allograft bone is typically limited to a few millimeters (3–4mm), which is sufficient to provide stable fixation, but minimize the volume of transplanted bone. If bone involvement is extensive (>10mm), autograft bone can be used to fill the defect. If the graft is large or is not circumferentially contained, additional fixation (absorbable pins) may be required.
Defining the Clinical Algorithm to Treat Chondral & Osteochondral Lesions with OCA
The establishment of a comprehensive clinical database allows us to prospectively evaluate outcomes of all OCA procedures since 1997, thereby defining appropriate indications, techniques, and optimum patient care (Table 2). Overall, a total of 527 knees in 467 patients (279 male and 188 female with mean age 34, range 14–68) underwent OCA for the following diagnoses with institutional review board approval: traumatic cartilage injury (35%), osteochondritis dissecans (30%), degenerative cartilage lesions (12%), osteonecrosis (8%), early osteoarthritis (6%), revision allografting (6%), and osteochondral fracture (3%).35 Some patients (88%) had undergone previous surgeries (average 2.5, range 1–13), which is unsurprising for a tertiary referral center. All patients were pre- and post-operatively evaluated using an 18-point modified D’Aubigne and Postel scale, which measures function, range of motion, and absence of pain, allocating 1–6 points each, for a maximum of 18 points. Overall, clinical scores improved from 12.1 preoperatively to 16.2 postoperatively (p<0.001). The majority of patients reported less pain (93%), were satisfied with their outcomes (96%), and would undergo OCA again under similar circumstances (90%). Sixty-five grafts failed and additional surgery (revision-21, TKA-40) was required, with a mean time to re-operation of 38 months. Thus, OCA is useful for a wide spectrum of knee joint pathology and results in significant improvement in pain and function, with high satisfaction in the majority of patients.
Table 2.
Patient Characteristics and OCA Details
| Variable | Mean±SD or % |
|---|---|
| Age (yrs) | 34±11.8 |
| Male | 53.1% |
| Diagnosis | |
| Osteochondritis dissecans | 26.8% |
| Degenerative chondral lesion | 22.6% |
| Traumatic chondral injury | 14.7% |
| Osteoarthritis | 12.4% |
| Osteonecrosis | 9.3% |
| Fracture | 7.1% |
| Failed osteochondral allograft | 7.1% |
| Previous surgery on affected joint | 90.7% |
| Number of previous surgeries | 2.6 (1.8) |
| Graft location | |
| Femoral condyle (medial) | 35.3% |
| Femoral condyle (lateral) | 18.4% |
| Tibial plateau (medial) | 1.1% |
| Tibial plateau (lateral) | 2.5% |
| Patella | 7.6% |
| Trochlea | 5.4% |
| Combo (2 locations) | 26.3% |
| Combo (3 locations) | 3.4% |
| Number of grafts | 1.5±0.7 |
| Total graft area (cm2) | 10.1±7.0 |
Overall, long-term clinical follow-up studies have generally shown success rates between 70–90% for a variety of indications (Table 3) Survivorship results at 12 years are summarized in Figure 4). OCA proved to be a successful, effective, and durable treatment option for osteochondritis dissecans (OCD),36 osteonecrosis,37 chondral and osteochondral lesions of the femoral condyles38 and patellofemoral joint,39 in young adolescent (≤18 years),40 and in OCA revisions.41 In the study investigating femoral condyle lesions, it should be noted that the average lesion size (8cm2, range 1–27cm2) was rather large, at the upper limit of what is deemed reliably addressable by either microfracture, osteochondral autologous transfer, or autologous chondrocyte implantation, and that all patients had failed an average of two such alternate procedures at the time of initial presentation; OCA was not the primary method of treatment, but instead used as a salvage situation. For such situations, small fragment fresh OCA had survivorship rates of 82% at 10y and 74% at 15y, which were consistent with other published studies.38 We also demonstrated that OCA is an acceptable primary treatment method (patients had no previous surgeries) for chondral and osteochondral defects, which are generally large in size (≥10 cm2) and have subchondral bone involvement, with survivorship results of ~90% at 5 years and ~75% at 10 years.42
Table 3.
Summary of Outcomes for Clinical OCA Studies
| Diagnosis | Location | Knees/Patients [N/N] |
Age in years [mean (range)] |
Follow-Up in years [mean (range)] |
Outcome | Ref |
|---|---|---|---|---|---|---|
| OCD | Femoral condyle | 66/64 | 29 (15–54) | 7.7 (2–22) | 72% good to excellent 12.9* to 16.1** | |
| Osteonecrosis | Femoral condyle | 28/22 | 24 (26–44) | 5.6 (2–20) | 89% survived 11.3* to 15.8** | |
| Focal traumatic or degenerative | Femoral condyle | 129/122 | 33 (15–68) | 13.5 (2–28) | 79% good to excellent 12.1* to 16.0** | |
| Various | Patellofemoral | 20/18 | 42 (19–64) | 7.8 | 75% successful 11.7* to 16.3** | |
| Various – adolescent | Various | 43/39 | 16 (11–18) | 8.4 (2–27) | 88% good to excellent | |
| Various – no previous surgery | Various | 55/61 | 33 (15–67) | 7.6 (2–23) | 86% extremely or satisfied | |
| OCA Revision | Various | 33/33 | 37 (17–65) | 10.6 (2–27) | 63% good to excellent 14.8** |
Merle D’Aubigne & Postel scale
pre-operatively and
post-operatively.
Figure 4. OCA Survivorship at 12 years by Indication.
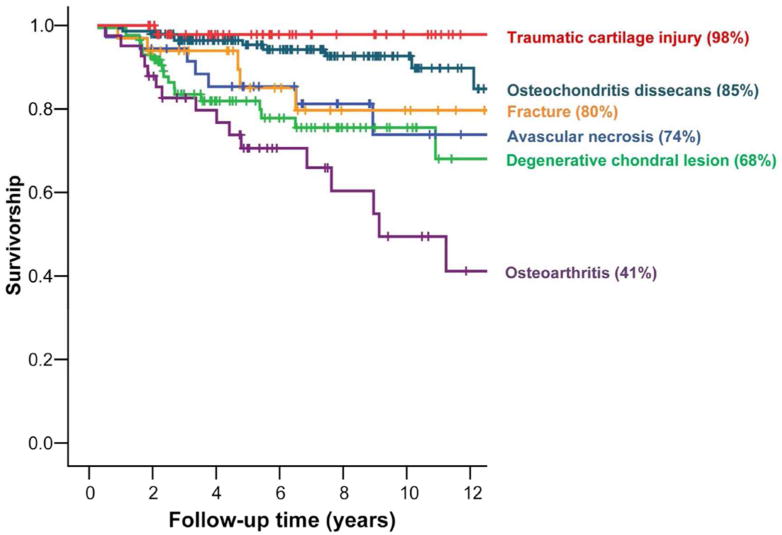
OCA survivorship varied between ~70–90% for most cartilage indications, with the lowest survivorship (~40%) in patients with osteoarthritis.
Preliminary data suggest that fresh OCA are immunogenic in humans. The development of humoral and cellular immune responses to OCA is associated with prolonged inflammatory reactions, cartilage degeneration, and delayed graft incorporations in animal models,43–45 but the biologic effect of immune responses in humans is largely unknown. In a study comparing the magnetic resonance (MR) findings after OCA with antibody responses, the MR appearance of the graft-host interface correlated to antibody response of OCA and humoral immune response was associated with more inflammation and less complete incorporation after OCA placement.46 Additionally, unlike other forms of allogeneic transplantation, fresh OCA are not HLA or ABO blood group matched prior to implanting. The immunologic ramifications of this procedure remain an important consideration and might allow its use to further improve the treatment and prevent graft failures secondary to host rejection. In a study examining the relationship between development of anti-HLA antibodies after OCA with graft size and clinical outcome, positive HLA-antibody screens were found in 70% of patients with large (19/27), 54% of patients with medium (20/37), and 6% of patients with small (1/16) OCA post-operative, but graft survivorship was similar in positive anti-HLA antibody (64%) and negative anti-HLA antibody (79%) patients (p=0.15), as were Knee Society function scores (88.3 vs. 84.6, p=0.5).47
Summary of Clinical Relevance
Over the last decade, the transition of OCA transplantation from a “niche” procedure performed in specialized centers to widespread adoption in the orthopaedic community has been the result of both translational science and clinical studies designed to safely advance this complex surgical technology into common clinical practice. This goal required development of fresh OCA storage protocols that allows tissue banks to provide grafts that maintain adequate tissue characteristics during the storage period. The series of OCA storage studies, reviewed here, have provided the fundamental knowledge governing current storage protocols and have led to much greater access to OCA tissue for surgeons and their patients.
The establishment of a reliable animal model is not only useful to study efficacy in a realistic in vivo model, but also to assess cartilage and bone remodeling following OCA. In particular, thee evaluation was an integrative, inter-disciplinary, and multi-scale approach. The endpoints measured encompassed both structural and biological parameters of cartilage and bone, using a variety of traditional (biomechanical, histological, and biochemical) and modern (micro-computed tomography, confocal microscopy) technology. Since data from human OCA retrievals is skewed toward poor outcomes, using systematic animal models is critical to elucidate tissue changes of early stage degeneration, and to provide insight into the time-course of cartilage remodeling and/or deterioration. Analyzing cartilage and bone biology, structure, and function in an integrative manner identified properties of the osteochondral tissue that are critical to repair efficacy and potential mechanisms of graft success/failure.
The unique and complex surgical techniques employed with the implantation of OCA have also been a barrier to their use. The development of reproducible surgical technique and identifying potential pitfalls such as excessive impact loading of the allograft during insertion has been essential in guiding surgeons adopting this technique in their practice and in obtaining satisfactory clinical outcomes. In addition, the retrieval and subsequent study of failed allografts has provided great insight into the behavior of OCA in vivo. Retrieved OCA have shown presence of living chondrocytes, variable replacement of graft bone, and a lack of histologic evidence of immune mediated graft rejection. These finding clearly support the fundamental premises upon which the OCA procedure is based. Newer data on the humoral reaction to OCA provide further insight into the immunologic response to these transplants, which merits further investigation.
Finally, the clinical outcome studies presented in this manuscript are essential in understanding and defining the appropriate indications and expected outcomes of osteochondral allograft procedures. Our studies have shown OCA to be uniquely useful in the treatment of osteochondritis dissecans and osteonecrosis as well as in situations where isolated chondral lesions are either too large for or have failed other cartilage repair procedures. The use of OCA in more advanced disease states such as osteoarthritis of the knee and ankle, though demonstrating more modest clinical results, have provided an alternative to prosthetic arthroplasty in the younger population, as well as insight into one potential pathway to the achievement of a “biological arthroplasty”.
Conclusions
Our research encompassing translational science including large animal studies, and clinical science, has helped to define the field of modern OCA transplantation. The studies described above have provided the basis for rapid translational application, and been vital to establishing osteochondral allografting as an increasingly important treatment for articular cartilage injury and disease. Our translational science studies have allowed determination of not only the biologic behavior of OCA, but also storage conditions in which chondrocyte viability can be preserved for up to 2–4 weeks prior to surgical implantation. Collectively, these findings have transformed tissue bank practices for graft storage nationwide. Likewise, translational research, such as our graft impaction study and analysis of OCA retrievals, has helped refine surgical technique for OCA transplantation. The establishment of an animal model to systematically study in vivo cartilage and bone remodeling following OCA has allowed us to identify properties of the osteochondral tissue that are critical to repair efficacy and potential mechanisms of graft success/failure. In addition, our clinical outcome program has been instrumental in defining appropriate indications and expectations for osteochondral allografting. The future promise and utility of OCA relies on continued scientific endeavor to realize its potential in biologic cartilage restoration and bioarthroplasty.
Acknowledgments
The authors would like to thank Julie McCauley, MPHc, Pamela A. Pudilo, BSN, and Albert C. Chen, Ph.D. for contributions to the work in this manuscript. The principal author has received benefits for personal or professional use from research funding received from Allosource and Musculoskeletal Transplant Foundation. These studies were supported in the following entities: NIH (AR049380 & AR055367), UCSD Academic Senate, Allosource Inc., Musculoskeletal Transplant Foundation, intramural support from Scripps Clinic, and an award to UCSD under HHMI Professors Program (for RLS).
Footnotes
All authors have made substantial contributions to the research design and interpretation of the data presented in addition to critical revisions and approval of the final manuscript.
References
- 1.Alford JW, Cole BJ. Cartilage restoration, part 1: Basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–42. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Lexer E. Joint transplantation. Clin Orthop Relat Res. 1985;197:4–10. [PubMed] [Google Scholar]
- 4.Jamali AA, Hatcher SL, You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years. A case report. J Bone Joint Surg Am. 2007;89:166–9. doi: 10.2106/JBJS.F.00618. [DOI] [PubMed] [Google Scholar]
- 5.Görtz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–84. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Guidance for industry: Screening and testing of human tissue intended for transplantation. Ed by Services, Usdohah, Food and Drug Administration Center for Biologics Evaluation and Research, 2002
- 7.Ball ST, Amiel D, Williams SK, et al. The effects of storage media on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–52. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 8.Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111–20. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Pennock AT, Wagner F, Robertson CM, et al. Prolonged storage of osteochondral allografts: Does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19:265–72. doi: 10.1055/s-0030-1248117. [DOI] [PubMed] [Google Scholar]
- 10.Robertson CM, Allen RT, Pennock AT, et al. Upregulation of apoptotic and matrix-related gene expression during fresh osteochondral allograft storage. Clin Orthop Relat Res. 2006;442:260–6. doi: 10.1097/01.blo.0000187058.42820.39. [DOI] [PubMed] [Google Scholar]
- 11.Linn MS, Chase DC, Healey RM, et al. Etanercept enhances preservation of osteochondral allograft viability. Am J Sports Med. 2011;39:1494–9. doi: 10.1177/0363546511398645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallante AL, Bae WC, Chen AC, et al. Chondrocyte viability is higher after prolonged storage at 37 degrees c than at 4 degrees c for osteochondral grafts. Am J Sports Med. 2009;37(Suppl 1):24S–32S. doi: 10.1177/0363546509351496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty WJ, Pallante AL, Rone RJ, et al. The proteoglycan metabolism of articular cartilage in joint-scale culture. Tissue Eng Part A. 2010;16:1717–27. doi: 10.1089/ten.tea.2009.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen RT, Robertson CM, Pennock AT, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33:1479–84. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 15.Kandel RA, Gross AE, Ganel A, et al. Histopathology of failed osteoarticular shell allografts. Clin Orthop Relat Res. 1985:103–10. [PubMed] [Google Scholar]
- 16.Oakeshott RD, Farine I, Pritzker KPH, et al. A clinical and histologic analysis of failed fresh osteochondral allografts. Clin Orthop Relat Res. 1988;233:283–94. [PubMed] [Google Scholar]
- 17.Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022–32. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 18.Pallante AL, Chen AC, Ball ST, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40:1814–23. doi: 10.1177/0363546512449321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallante AL, Gortz S, Chen AC, et al. Treatment of articular cartilage defects in the goat with frozen versus fresh osteochondral allografts: Effects on cartilage stiffness, zonal composition, and structure at six months. J Bone Joint Surg. 2012;94:1984–95. doi: 10.2106/JBJS.K.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallante-Kichura AL, Chen AC, Temple-Wong MM, et al. In vivo efficacy of fresh vs. Frozen osteochondral allografts in the goat at 6 months is associated with prg4 secretion. J Orthop Res. 2013;6:880–6. doi: 10.1002/jor.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallante-Kichura AL, Cory E, Bugbee WD, et al. Bone cysts after osteochondral allograft repair of cartilage defects in goats suggest abnormal interaction between subchondral bone and overlying synovial joint tissues. Bone. 2013;57:259–68. doi: 10.1016/j.bone.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang EY, Pallante-Kichura AL, Bae WC, et al. Development of a comphrensive osteochondral allograft mri scoring system (ocamriss) with histopathologic, micro-computed tomography, and biomechanical validation. Cartilage. 2014;5:16–27. doi: 10.1177/1947603513514436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugbee WD. Fresh osteochondral grafting. Operative Tech in Sports Med. 2000;8:158–62. [Google Scholar]
- 24.Bugbee WD. Fresh osteochondral allografts. Semin Arthroplasty. 2000;11:1–7. [Google Scholar]
- 25.Bugbee WD. Alternative to arthroplasty of the knee: Biological resurfacing. Curr Op Orthop. 2001;12:1–7. [Google Scholar]
- 26.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15:191–5. [PubMed] [Google Scholar]
- 27.Bugbee WD. Fresh osteochondral grafts for the knee. Tech Knee Surg. 2004;3:68–76. [Google Scholar]
- 28.Bugbee WD, Cavallo M, S G. Osteochondral allograft transplantation in the knee. J Knee Surg. 2012;25:109–16. doi: 10.1055/s-0032-1313743. [DOI] [PubMed] [Google Scholar]
- 29.Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67–75. doi: 10.1016/s0278-5919(05)70130-7. [DOI] [PubMed] [Google Scholar]
- 30.Bugbee WD, Tirico LE. Osteochondral allograft reconstruction: Improvements in surgical techniques and allograft proccesing. Oper Tech Orthop. 2014;24:19–26. [Google Scholar]
- 31.Gomoll AH, Filardo G, Almqvist KF, et al. Surgical treatment for early osteoarthritis. Part ii: Allografts and concurrent procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20:468–86. doi: 10.1007/s00167-011-1714-7. [DOI] [PubMed] [Google Scholar]
- 32.Görtz S, Bugbee WD. Fresh osteochondral allografts: Graft processing and clinical applications. J Knee Surg. 2006;19:231–40. doi: 10.1055/s-0030-1248112. [DOI] [PubMed] [Google Scholar]
- 33.Sherman SL, Garrity J, Bauer KL, et al. Fresh osteochondral allograft transplantation for the knee: Current concepts review. J Am Acad Orthop Surg. 2014;22:121–33. doi: 10.5435/JAAOS-22-02-121. [DOI] [PubMed] [Google Scholar]
- 34.Borazjani BH, Chen AC, Bae WC, et al. The effect of impact on chondrocyte viability during the insertion of human osteochondral grafts. J Bone Joint Surg. 2006;88:1934–43. doi: 10.2106/JBJS.E.00992. [DOI] [PubMed] [Google Scholar]
- 35.Bugbee WD, De Young A, Gortz S. Twenty-five year experience with osteochondral allografting in the knee. Trans Am Acad Ortho Surg. 2011;78:197. [Google Scholar]
- 36.Emmerson BC, Gortz S, Jamali AA, et al. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–14. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 37.Gortz S, De Young A, Bugbee WD. Fresh osteochondral allografting for steriod-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269–78. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy YD, Gortz S, Pulido PA, et al. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231–7. doi: 10.1007/s11999-012-2556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamali AA, Emmerson BC, Chung C, et al. Fresh osteochondral allografts: Results in the patellofemoral joint. Clin Orthop Relat Res. 2005:176–85. [PubMed] [Google Scholar]
- 40.Murphy RT, Pennock AT, Bugbee WD. Osteochondral allografting in the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42:635–40. doi: 10.1177/0363546513516747. [DOI] [PubMed] [Google Scholar]
- 41.Horton MT, Pulido PA, McCauley JC, et al. Revision osteochondral allograft transplantation: Do they work? Am J Sports Med. 2013;41:2507–11. doi: 10.1177/0363546513500628. [DOI] [PubMed] [Google Scholar]
- 42.Briggs DT, Sadr KN, Pulido PA, et al. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015 doi: 10.1177/1947603515595072. submitted 5 May 2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987;69-A:573–82. [PubMed] [Google Scholar]
- 44.Stevenson S, Dannucci GA, Sharkey NA, et al. The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1989;71-A:1297–307. [PubMed] [Google Scholar]
- 45.Stevenson S, Li XQ, Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1991;73-A:1143–56. [PubMed] [Google Scholar]
- 46.Sirlin CB, Brossmann J, Boutin RD, et al. Shell osteochondral allografts of the knee: Comparison of mr imaging findings and immunologic responses. Radiology. 2001;219:35–43. doi: 10.1148/radiology.219.1.r01ap0435. [DOI] [PubMed] [Google Scholar]
- 47.Hunt HE, Sadr K, DeYoung AJ, et al. The role of immunologic response in fresh osteochondral allografting of the knee. Am J Sports Med. 2014;42:886–91. doi: 10.1177/0363546513518733. [DOI] [PubMed] [Google Scholar]


