Abstract
Lymphomas arise from clonal expansions of B, T, or NK cells at different stages of differentiation. Because they occur in the immunocyte-rich lymphoid tissues, they are easily accessible to antibodies and cell-based immunotherapy. Expressing chimeric antigen receptors (CARs) on T cells is a means of combining the antigen-binding site of a monoclonal antibody with the activating machinery of a T cell, enabling antigen recognition independent of major histocompatibility complex restriction, while retaining the desirable antitumor properties of a T cell. Here, we discuss the basic design of CARs and their potential advantages and disadvantages over other immune therapies for lymphomas. We review current clinical trials in the field and consider strategies to improve the in vivo function and safety of immune cells expressing CARs. The ultimate driver of CAR development and implementation for lymphoma will be the demonstration of their ability to safely and cost-effectively cure these malignancies.
Keywords: immunotherapy, adoptive T cell therapy, CD19, CD20, CD30, kappa light chain
HUMAN LYMPHOMAS
Human lymphomas have historically been separated into non-Hodgkin and Hodgkin lymphoma (NHL and HL). NHL includes a broad group of lymphoid malignancies that arise from clonal expansions of B, T, or natural killer (NK) cells at various stages of differentiation. B cell lymphomas are usually derived from germinal center or postgerminal center B cells, whereas T and NK cell lymphomas may arise at any stage of normal T or NK cell lymphopoiesis. Although malignant cells acquire genetic abnormalities, they also retain many of the phenotypic characteristics of their normal counterparts. Target antigens for immunotherapy are, therefore, generally expressed on both lymphoma cells and their nonmalignant counterparts (1).
In contrast, the malignant Reed-Sternberg (RS) cells of HL have an unusual expression of hematopoietic markers that has no normal counterpart. Although the cell from which HL originates was long debated, microdissection studies recently showed that RS cells possess clonal heavy- and light-chain immunoglobulin (Ig) gene rearrangements, and thus HL is likely derived from crippled germinal center cells (2). HL also differs from NHL in that the malignant RS cells are relatively rare, and the more prominent, nonmalignant, infiltrating cells in the microenvironment play an important role in HL biology (2).
SUITABILITY OF NON-HODGKIN AND HODGKIN LYMPHOMA FOR IMMUNOTHERAPY
Despite their biological differences, both HL and NHL have proven to be good targets for immunotherapy. Both lymphomas occur in the immune-rich lymphoid tissues and are therefore easily accessible to antibodies and cell-based immunotherapy. Moreover, T cells targeted to tumor-associated antigens expressed by B cell lymphomas are likely to receive the costimulation they require if they are to pass through immune checkpoints, as B cells are excellent antigen-presenting cells. In addition, lymphomas express both lineage-restricted (e.g., CD19) and unique (e.g., Ig idiotype) tumor antigens that can be targeted by antibodies and/or effector cells (3). Finally, 35–45% of all human lymphomas are associated with persistent infection with Epstein-Barr virus (EBV), so that antigens associated with viral latency can be detected in many patients with HL or with many NHL subtypes, including Burkitt lymphoma, NK/T cell lymphomas, and diffuse large B cell lymphoma (DLBCL). These viral antigens can be successfully targeted with EBV-specific T cells (4, 5).
Although the majority of target antigens on human lymphomas include lineage-restricted antigens that are also present on normal B cells and some T cells, eradication of normal as well as malignant lymphocytes may be considered an acceptable toxicity. Hence, antibodies to CD20 are included in most B cell lymphoma treatment regimens, and the expression of CD30 both on the RS cells of HL and on a subpopulation of activated T and NK cells has not prevented the use of CD30 antibody to treat HL (6).
HOW CAN CAR-T CELLS BUILD UPON LYMPHOMA IMMUNOTHERAPY?
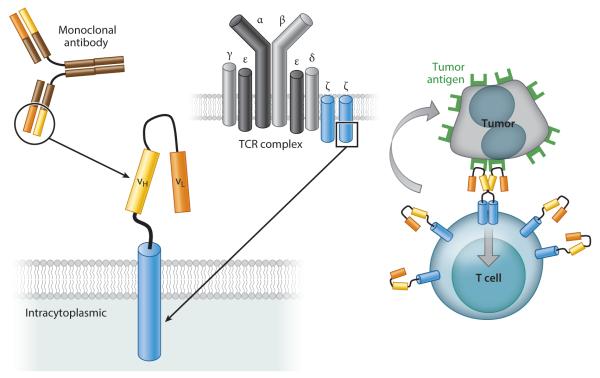
Chimeric antigen receptors (CARs) were first developed in the mid-1980s (7). In 1993, the Eshhar group (8) modified the concept to use (a) an extracellular domain (ectodomain) from a single-chain variable fragment (scFv), composed of the antigen-binding regions of both heavy and light chains of a monoclonal antibody (mAb); (b) a transmembrane domain; and (c) an intracellular domain (endodomain) with a cell-signaling component derived from the T cell receptor (TCR) ζ chain (Figures 1 and 2). Most subsequent CARs have followed this same structural pattern, with incorporation of accessory or costimulatory signaling components (Figure 2). In contrast to conventional T cells, which rely on their native TCRs for tumor antigen recognition, CAR-T cells recognize unprocessed antigen and therefore kill tumor cells independently of their expression of major histocompatibility complex (MHC) antigens.
Figure 1.
The basic structure of first-generation chimeric antigen receptors (CARs). The most common CARs combine the extracellular antigen-recognition site of a monoclonal antibody and the intracellular domains of a T cell receptor (TCR) complex molecule. Clustering of CARs induced by antigen binding on the surface of tumor cells initiates signal transduction that leads to T cell activation and killing of tumor cells.
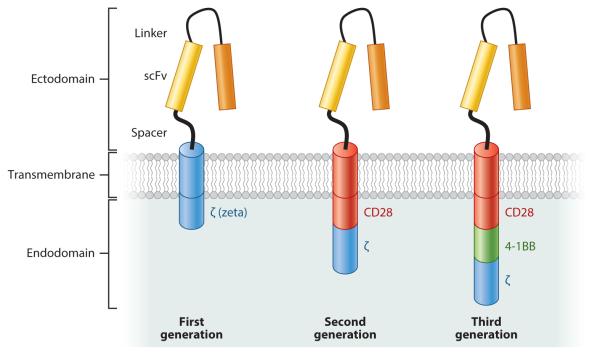
Figure 2.
Three generations of chimeric antigen receptors (CARs). First-generation CARs include an extracellular domain (ectodomain), usually derived from a single-chain variable fragment (scFv), composed of the antigen-binding regions of both heavy and light chains of a monoclonal antibody; a transmembrane domain; and an intracellular domain (endodomain) with a cell-signaling component derived from the T cell receptor, usually the ζ chain. Most subsequent CARs have followed this same structural pattern, with incorporation of one (second-generation CARs) or more (third-generation CARs) accessory or costimulatory signaling components, such as CD28, CD137 (4-1BB), and CD134 (OX40). These additional costimulatory endodomains improve T cell activation and proliferation, and thus may promote killing of target tumor cells.
Design of CAR Ectodomains
To date, all CARs in use for lymphoma have been based on scFvs (Table 1), whose effectiveness depends in part on the affinity of the CAR itself and on the properties of the antigenic epitope recognized. For instance, CARs containing high-affinity scFvs for ROR1 confer greater effector function to T cells than those containing low-affinity scFvs (9). However, the location of the recognized epitope on the antigen also affects CAR function (10, 11). For example, T cells expressing a CAR with an scFv that recognizes a membrane-proximal epitope on CD22 have greater antileukemic activity than CAR-T cells recognizing a distal epitope (10).
Table 1.
Chimeric antigen receptor (CAR) targets in development or in use for lymphomas
| Antigen | Malignancy lineage | CAR ectodomain | Clinical trial |
|---|---|---|---|
| BCMA | B cell | scFv | Unavailablea |
| CD19 | B cell | scFv | See Table 2 |
| CD20 | B cell | scFv | See Table 2 |
| CD22 | B cell | scFv | Ongoing (90) |
| CD30 | B cell and T cell | scFv | See Table 2 |
| CD70 | B cell and T cell | CD70 ligand | Unavailable |
| ROR1 | B cell | scFv | Ongoing (91) |
| κ | B cell | scFv | See Table 2 |
Clinical trial available only for multiple myeloma.
Other components of the ectodomain may also influence CAR effectiveness, such as the presence of flexible linker sequences in the scFv and the type of elements connecting the ecto- to the endodomain (hinge and transmembrane regions). The hinge and transmembrane regions can affect CAR-T cell function profoundly by modifying the length and flexibility of the resulting CAR, its cell surface density, its tendency to self-aggregate and produce T cell exhaustion by tonic signaling, and its potential binding to molecules other than the intended target antigen. For example, a CD19-specific CAR with a CD3-ζ transmembrane domain is less stable over time on the cell surface of T cells than a CD19-specific CAR with the same scFv but a CD28 transmembrane domain (12) (G. Dotti, B. Savoldo, unpublished data). Also, for instance, CD19-CARs with a hinge derived from the IgG4 CH2–CH3 region are functional in vitro but may have impaired antitumor activity in vivo due to interaction between the Fc domain within the hinge and Fc receptor-bearing myeloid cells (13).
Design of CAR Endodomains
Upon antigen recognition, CAR endodomains transmit activation and costimulatory signals to T cells. T cell activation relies on the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic CD3-ζ domain of the TCR complex (14). In addition to this stimulus from TCR engagement (signal 1), T cells require costimulation (signal 2) for sustained growth and function, as TCR stimulation without costimulation induces T cell anergy. The endodomain of early CARs contained only the CD3-ζ signaling component (Figure 1). To improve CAR-T cell function and persistence, costimulatory endodomains (such as CD28, OX40, or 4-1BB) were incorporated into so-called second- (and even third-) generation CARs (Figure 2) to ensure the transgenic T cells are fully activated after engaging their specific target (15–19).
The superiority of second- over first-generation CAR-transduced T cells was decisively demonstrated in a study comparing the two constructs head to head (12). This phase I trial treated subjects with refractory or relapsed B cell lymphomas, mostly DLBCL, who were simultaneously infused with two autologous T cell products. Both products were retrovirally transduced with a CD19-CAR, but one CAR encoded both the CD28 and ζ endodomains whereas the other included only the ζ endodomain. This strategy allowed direct measurement of the consequences of adding a CD28 costimulatory endodomain to CAR-redirected T cells in the same subject and established that T cells bearing a second-generation CAR that contains the CD28 endodomain have enhanced in vivo proliferation and survival compared to T cells expressing a first-generation CAR lacking CD28. The contribution of costimulatory domains is discussed in detail in the section on CD19-CAR trials. All the subsequent studies described in the “Lymphoma Antigens Targeted in Current Clinical Trials” section used second-generation CARs, except where indicated.
Advantages and Disadvantages of Using CARs
Even though mAbs such as CD20 have been highly successful in the treatment of lymphomas, T cells expressing CARs directed at the same or related antigens additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion, and long-term persistence. Moreover, because CAR-T cells have MHC-unrestricted activity, they can circumvent some of the major mechanisms by which tumors avoid MHC-restricted T cell recognition, such as the downregulation of human leukocyte antigen (HLA) class I molecules and defective antigen processing (20–23). Finally, CARs can target nonprotein antigens, which allows them to recognize lymphoma tumor antigens derived from carbohydrates or glycolipids, which are not detectable by conventional TCRs.
T CELL EXPRESSION OF CARS
Although it is possible to transiently express CARs in T cells by transfecting them with naked DNA plasmids or mRNA (for instance, to more safely test them for toxicity, since any adverse effects should reverse once the transgene is eliminated), in most instances the goal is to obtain sustained expression to ensure durability of response (24). Replication-defective retroviruses (gammaretroviruses or lentiviruses) can integrate a reverse-transcribed sequence into the host cell DNA through the action of a viral integrase, thus becoming part of that cell’s genome (25). Because retroviral production under good manufacturing practices is time consuming and expensive, some groups have adopted nonviral methods for permanent transduction, specifically transposon-based systems, including Sleeping Beauty (26) and PiggyBac (27), which lead to stable integration of the transgene, although these systems may be less effective overall.
Ideally, CAR molecules should be grafted onto a subset of T cells that can traffic to tumor sites, receive appropriate costimulation, and expand and persist in vivo. Lymphoma studies have focused on αβ-TCR+ T cells, and investigators are now trying to use phenotypic profiling to identify the optimal subset within this population (28, 29). T cells with a memory-associated phenotype may be optimal for in vivo persistence, and investigators have used positively selected cells expressing a central memory–associated marker (CD62L) to express CD19-directed CARs in a clinical study at the Fred Hutchinson Cancer Research Center. More recently, a T cell subset has been identified with even greater proliferative potential and longer survival in vivo (28). These T stem cell memory populations can differentiate into memory and effector populations, but their ultimate value in human CAR studies has not yet been established.
An alternative to T cell selection based on surface phenotype is to physiologically select T cells that have an established capacity to act as effector T cells, enter the memory pool, and re-expand on re-exposure to antigens in vivo. Virus-specific T cells (VSTs) have these abilities (5, 30). VSTs also express chemokine receptors that should allow them to traffic to sites of lymphoma (31). Moreover, the ability of CAR-VSTs to interact through their native TCR with viral antigens on professional antigen-presenting cells may provide a range of costimulatory signals that enhance their persistence after adoptive transfer and that increase their activity against tumor targets, which is mediated through their transgenic CAR (32).
In addition to CAR gene expression in αβ T cells, T cells with γδ-TCR may also be amenable to transduction with a CAR and provide additional functionality (33). Similarly, other lymphocyte populations may offer specific advantages and can also be transduced using the same methods. For instance, invariant-chain TCR T cells (or NK-T cells) may have preferential tumor trafficking properties and inherent activity against tumor-associated macrophages, while NK cells may have additional intrinsic antitumor lytic potential (34). None of these alternative cell sources has been used yet for CAR therapy of lymphoma.
CHOICE OF LYMPHOMA ANTIGEN FOR CAR TARGETING
Unlike the native TCR, the majority of ScFv-based CARs only recognize intact target antigens expressed directly on the cell surface, rather than peptide fragments from processed proteins that are presented in association with MHC molecules. This limited recognition hinders CAR-T cells’ ability to detect most truly tumor-specific antigenic epitopes (since these are usually derived from internal mutant oncogenes and translocations). Unfortunately, normal B or T cells also express the majority of lymphoma target antigens suitable for CAR recognition.
CAR-T cells can, for example, be used to target a highly and consistently expressed lineage-specific antigen (e.g., CD19, CD20, CD22) resulting in elimination of malignant B cells. However, because these antigens are also expressed by their normal counterparts, B cell ablation is a frequent side effect, albeit relatively benign because replacement therapy using intravenous Ig is feasible. In general, however, it might be preferable to target more restricted lineage-associated antigens, such as BCMA (B cell maturation antigen), which is predominantly expressed by plasma cells and subsets of mature B cells (35). As another example, in many B cell malignancies it is possible to target either the κ or the λ light chain associated with all cells of the (clonal) malignancy, and this is desirable to spare normal (polyclonal) B cells expressing the reciprocal light chain and maintain immune function (18).
The argument in favor of targeting an entire lineage is less acceptable for T cell lymphomas because T cell function is less amenable to replacement therapies than that of B cells. Nonetheless, as discussed below, a more selectively expressed T lineage marker, such as CD30, may be acceptable, particularly if expression is high on the tumor cells.
Targeting single antigens carries the inherent risk of immune escape (36–38), which can be reduced by targeting multiple antigens. Expressing multiple CARs in T cells also has the potential to increase safety by generating T cells that recognize a unique antigen pattern that is only present on tumor cells or their associated stroma (39).
LYMPHOMA ANTIGENS TARGETED IN CURRENT CLINICAL TRIALS CD19
With the important exceptions of hematopoietic stem cells and plasma cells, CD19 is expressed during all stages of B cell differentiation and is maintained on the vast majority of cells that have undergone neoplastic transformation (40), such as in B cell NHL and chronic lymphocytic leukemia (CLL). Most of the initial CAR-T cell trials in B cell malignancies, including NHL and acute lymphoblastic leukemia (ALL), targeted this antigen. Results in NHL, albeit impressive, have not been as striking as in ALL, for which complete remission rates of ~90% have been described (41, 42).
CAR ζ-chain signaling is insufficient for CAR T cell persistence
Early experience treating B cell malignancies with CD19-CAR T cells demonstrated the feasibility of the approach but also its lack of objective antitumor effects. All of these trials used first-generation CARs with a single signaling domain (derived from the ζ chain of the TCR complex) (43). In one of these studies, two patients with refractory follicular lymphoma received T cells expressing a CD19-CAR after undergoing treatment with lymphodepleting doses of fludarabine. The T cells had undergone polyclonal activation with a CD3 antibody (OKT3), plasmid electroporation, and hygromycin selection (for which the plasmid also encoded a resistance gene). After CAR-T cell infusion, patients received low-dose subcutaneous interleukin (IL)-2 injections. Transferred T cells were detectable by polymerase chain reaction (PCR) for fewer than seven days. As expected given the cells’ limited persistence, neither clinical responses nor overt toxicities were observed. Of note, cellular antitransgene immune rejection responses were documented in both patients, although whether this activity was directed at the CAR or the hygromycin resistance gene is unknown (43).
Results from such trials using first-generation CAR-T cells demonstrated that a single stimulatory domain was insufficient to fully activate the chimeric T cells and confirmed that host lymphopenia also facilitates expansion of adoptively transferred T cells. Lymphopenia creates “space” for the oncoming adoptively transferred cells and enhances their homeostatic expansion while also depleting the endogenous regulatory T cells, which normally secrete inhibitory cytokines [e.g., transforming growth factor β (TGFβ) and IL-10] that limit effector T cell expansion (44). Additionally, T cell growth homeostatic cytokines, such as IL-7 and IL-15, which ordinarily exist in limiting amounts, may become readily available owing to less competition and increased production by lymphopoietic stromal cells (45).
An early report from a trial using a second-generation, CD28-containing CD19-CAR described one patient with advanced follicular lymphoma, who was treated with a preparative chemotherapy regimen followed by autologous T cells retrovirally modified to express the CAR. The patient’s tumor underwent partial regression, and B cells were absent from circulation for at least 39 weeks after T cell infusion, despite recovery of other blood series. The CD19-CAR transgene was detected in the peripheral blood up to 27 weeks after infusion (46). Nonetheless, in vivo expansion of these second-generation CAR T cells was still modest, and clinical responses were limited. In another trial of six patients with DLBCL, only two had transient stable disease and four had disease progression. The inadequate activity suggested that alternative costimulatory domains (or a different CAR design) might be necessary for more potent activation of chimeric T cells.
Later-acting costimulatory domains may be more efficacious than CD28
Although costimulatory signals from CD28 seemed to improve expansion and persistence, a trial using a second-generation CAR incorporating 4-1BB (CD137) as an alternative costimulatory domain (47) reported the most dramatic expansion and clinical activity in indolent B cell malignancies. CD28 costimulation is usually provided physiologically by professional antigen-presenting cells and represents an “early” costimulatory signal, but “late” costimulatory molecules, including members of the tumor necrosis factor receptor (TNFR) family such as OX40 (also known as CD134) and 4-1BB (CD137), also play crucial roles. After binding to their specific ligands, these molecules recruit TNFR-associated-factor (TRAF) adapter proteins, which represent an entirely distinct activation pathway from CD28 costimulation and may be associated with more potent activation of T cells (48), at least in certain disease settings.
The first three patients reported from this second-generation, 4-1BB-containing CD19-CAR trial had large-burden, relapsed B cell CLL. They were infused with autologous CAR-T cells after receiving lymphodepleting chemotherapy (47, 49). In contrast to other trials, a lentivirus was used to transfect T cells. These CAR-T cells had a >1,000-fold expansion in vivo, trafficked to bone marrow, and continued to express functional CARs at high levels for at least six months. Despite large tumor burdens, results were impressive: two long-term complete remissions and one prolonged partial remission were seen in the three CLL patients treated. Each infused CAR-T cell was calculated to have eradicated at least 1,000 CLL cells on average. Significant adverse effects were noted, however, including an acute systemic inflammatory response syndrome (fever with hypotension, respiratory distress, or tumor lysis syndrome) as well as late on-target, off-tumor toxicities, namely B cell aplasia associated with decreased numbers of plasma cells and hypogammaglobulinemia (see the section on toxicities below).
Nonetheless, the issue of whether late costimulatory domains are always better than early ones is far from being settled. CD28-containing CD19-CARs have continued to show encouraging activity in other trials, reports from which have reinforced the need for lymphodepletion prior to CAR-T cell infusion, at least in the autologous setting. For example, the outcomes of seven additional patients were described in an update (50) to the single-patient report (46) mentioned in the previous section and, more recently, the same group published results in 13 other NHL patients (51). Nine patients achieved complete remission, which lasted up to 23 months. Adverse events included long-term depletion of normal B cells and prominent elevations in serum levels of inflammatory cytokines, which appeared to correlate with the severity of acute toxicities (fever and hypotension). In addition, central nervous system toxicity, of unclear etiology, was observed in some patients.
A similar second-generation, CD28-containing CAR was used in another trial in which eight CLL patients (and one ALL patient) were treated (52). All patients tolerated the CAR-T cell infusions well, but one patient had rapid clinical deterioration and died less than 48 h after CAR-T cell infusion (see section on toxicities below). Some of the other patients developed fever with or without hypotension a few days after T cell infusion. One of the patients with CLL had a partial response, and none developed B cell aplasia. Persistence of infused CAR-T cells was inversely proportional to the tumor burden but enhanced by prior cyclophosphamide administration, further favoring the use of lymphodepleting chemotherapy before CAR-T cell infusion.
All the CD19-CAR trials described above used cell products generated from autologous T cells. Two additional protocols, summarized in Table 2, employed allogeneic cells (53, 54).
Table 2.
Published results from clinical trials of CAR-T cells targeting non-Hodgkin and Hodgkin lymphoma
| Antigen targeted |
Diseases | N | Construct | CAR gene transfer |
T cell origin |
Auxiliary therapy | SAEs | Outcomes |
|---|---|---|---|---|---|---|---|---|
| CD19 (43) | FL | 2 | scFv + CH2CH3 + CD4TM + CD3ζ (first generation) |
Electroporation (hygromycin selection) |
Autologous | FLU (post T cell infusion) and IL-2 |
None | 2 NR |
| CD19 (46) | FL | 1 | scFv + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | Lymphodepletion (CTX/FLU) and IL-2 |
None | 1 PR |
| CD19 (12) | DLBCL, transformed FL |
6 | scFv + CH2CH3 ± CD28 + CD3ζ (first and second generation) |
Retroviral | Autologous | None | None | 2 SD, 4 NR |
| CD19 (47, 49) | CLL/SLL | 3 | scFv + CD8TM + 4-1BB + CD3ζ (second generation) |
Lentiviral | Autologous | Lymphodepletion (BEN or CTX/PTS) |
TLS, CRS, BC aplasia |
2 CR, 1 PR |
| CD19 (52) | CLL/SLLa | 8 | scFv + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | None or lymphodepletion (CTX) |
Fever, death | 1 PR, 2 SD, 4 NR, 1 death |
| CD19 (50) | FL, CLL/SLL, SMZL |
8 | scFv + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | Lymphodepletion (CTX/FLU) and IL-2 |
Mild CRS, BC aplasia |
1 CR, 5 PR, 1 SD, 1 NE |
| CD19 (51) | DLBCL, PMBCL, CLL/SLL, SMZL |
15 | scFv + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | Lymphodepletion (CTX/FLU) |
TLS, CRS, CNS toxicity, death |
8 CR, 4 PR, 1 SD, 1 death, 1 NE |
| CD19 (92) | CLL/SLL, transformed CLL/SLLa |
4 | scFv + CH2CH3 + CD28 + CD3ζ (second generation) |
Retroviral | Allogeneic | Allo-HSCT preparative regimen; none immediately before T cell infusion |
None | 1 PR, 1 SD, 2 NR |
| CD19 (93) | CLL/SLL, DLBCL, MCL |
10 | scFv + CD28 + CD3ζ (second generation) |
Retroviral | Allogeneic | Allo-HSCT preparative regimen, DLI; none immediately before T cell infusion |
TLS, CRS, fever | 1 CR, 1 PR, 6 SD, 2 NR |
| CD20 (43) | DLBCL | 2 | scFv + CH2CH3 + CD4TM + CD3ζ (first generation) |
Electroporation (G418 selection) |
Autologous | Post-ASCT | None | 2 cCR |
| CD20 (55) | FL, MCL | 7 | scFv + CH2CH3 + CD4TM + CD3ζ (first generation) |
Electroporation (hygromycin selection) |
Autologous | None or IL-2 | None | 2 cCR, 1 PR, 4 SD |
| CD30 (56) | HL, ALCL | 9 | scFv + CH2CH3 + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | None or post-ASCT |
None | 1 CR, 4 SD, 3 NR, 1 NE |
| κ(57) | DLBCL, transformed FL, CLL/SLL, LPL, MCLa |
7 | scFv + CH2CH3 + CD28 + CD3ζ (second generation) |
Retroviral | Autologous | None or low dose CTX |
None | 2 CR, 1 PR, 4 NR |
Trial included patients with acute lymphoblastic leukemia or multiple myeloma but these are excluded from this table.
Abbreviations: ALCL: anaplastic large cell lymphoma, FL: follicular lymphoma, DLBCL: diffuse large B cell lymphoma, CLL/SLL: chronic lymphocytic leukemia/small lymphocytic lymphoma, SMZL: splenic marginal zone lymphoma, PMBCL: primary mediastinal B cell lymphoma, LPL: lymphoplasmacytic lymphoma, scFv: single-chain variable fragment: patient, TM: transmembrane segment, CTX: cyclophosphamide, FLU: fludarabine, BEN: bendamustine, PTS: pentostatin, Allo-HSCT: allogeneic hematopoietic stem cell transplantation, DLI: donor lymphocyte infusion, TLS: tumor lysis syndrome, CRS: cytokine release syndrome, BC: B cell, CNS: central nervous system, NR: no response, SD: stable disease, PR: partial response, cCR: continued complete response (i.e., patient had no evidence of disease before and after infusion), CR: complete response, NE: not evaluable.
CD20
A first-generation CAR targeting CD20 has been used in a few studies. In one of these, seven patients with follicular or mantle cell lymphomas received CD20-specific CAR-modified T cell infusions, with minimal toxicities. T cells were subjected to polyclonal activation, plasmid electroporation, and neomycin selection. The modified T cells persisted in vivo up to nine weeks in patients, who also received low-dose subcutaneous IL-2 injections. Two patients had continued complete responses, one achieved a partial response, and four had stable disease (55). In another study, two patients with recurrent DLBCL were treated with cloned CD8+ T cells expressing another first-generation CD20-CAR (and neomycin resistance) after autologous hematopoietic stem cell transplantation. Neither clinical responses nor overt toxicities were observed. In that trial, the transferred T cells were detectable by PCR for fewer than seven days (43).
CD30
Almost all HLs and some NHLs express the CD30 antigen at both diagnosis and relapse, and mAbs targeting CD30 produce objective antitumor responses. The effects of mAbs, however, appear to be limited in duration, encouraging the substitution of CD30-CARs on longer-lived T cells. A phase I dose escalation study of activated autologous CAR-CD30-T cells treated nine patients with relapsed/refractory EBV1/N CD30+ HL or NHL (seven with HL and two with CD30+ anaplastic large cell lymphoma). Eight of these patients had either relapsed or progressed after treatment with the CD30 mAb brentuximab. CAR-T cell infusions produced no attributable adverse events; in particular, the frequency of T cell precursors targeting cytomegalovirus, EBV, adenovirus, and influenza virus remained unchanged by treatment (assuaging an important concern, since CD30 is upregulated on some activated T cells). The molecular signal from the CAR-T cells peaked at one week following infusion, but decreased by four weeks. Of eight evaluable patients, six weeks after treatment, four patients had stable disease, one had a complete response, and one had a partial response, while three had disease progression (56; C. Ramos, G. Dotti, B. Savoldo, unpublished data).
κ Light Chain
As mentioned above, by taking advantage of the clonal restriction of mature B cell malignancies, which express either a κ or λ light Ig chain, it may be feasible to target B cell malignancies more selectively. A CAR specific for the κ light chain, for example, should selectively target κ+ lymphoma cells and spare the normal B cells expressing the nontargeted λ light chain, thus minimizing damage to humoral immunity. To assess the value of this approach, nine NHL/CLL patients were treated with a κ-directed CAR (57). Infusions were well tolerated without side effects. A CAR-κ-specific Q-PCR assay showed that molecular signals peaked 1–2 weeks after infusion and remained detectable for at least six weeks and up to nine months. Of the seven patients with relapsed NHL, two entered complete remission (after two and three infusions), one had a partial response, and four progressed; and both patients with CLL progressed before or shortly after the six-week evaluation. These data indicate that infusion of CAR-κ-T cells is safe and can be effective in patients with κ+ lymphoma (57). We are currently preparing studies with modified light-chain CARs and with increased conditioning.
OVERALL SAFETY OF CAR-T CELLS FOR LYMPHOMA
Although the results of several of the above studies confirm the promise of CAR-T cell therapy for lymphoma, they also reveal two concerns. The first is that significant, even fatal, treatment-related toxicity may occur, and the second is that the effectiveness of the approach for lymphoma appears lower than for acute B cell leukemia. In order for the therapy to succeed, both the safety and efficacy of CAR-T cells will need to be improved.
Toxicities
The most striking acute safety concern is an example of an on-target toxicity, namely systemic inflammatory response syndrome (SIRS) or cytokine release syndrome (CRS). This toxicity is attributable to rapid and extensive activation of infused CAR-T cells upon antigen engagement, with general perturbation of the immune system, and the associated release of high levels of proinflammatory cytokines, such as TNFα and IL-6 (49, 58). To reduce the likelihood or severity of CRS, investigators are modifying T cell dose escalation and have introduced the prompt use of antibodies blocking the effects of TNFα and IL-6.
Longer-term on-target toxicities are attributable to the consequences of destruction of the normal tissues expressing the targeted antigen, such as B cell aplasia and ultimately hypogammaglobulinemia (41, 47, 49, 55, 58–60). On-target toxicity may be reduced by targeting antigens that are more restricted in their expression, such as the κ light chain of Ig as described above (57), or by targeting multiple antigens when their combination occurs only in tumor cells. Alternatively, patients may receive T cells with only transient expression of the CAR, for example after electroporation of mRNA encoding the receptor (61–63). Unlike T cells transduced with a genome-integrating vector, in which each daughter cell contains the same transgene, translated to the same level, mRNA-transduced T cells express the transgene for a finite period of time (depending on the stability of the mRNA and the translated protein); moreover, levels of expression diminish as the cells divide, and the transcripts become progressively diluted. However, since CAR-T cells may expand 1,000–10,000-fold over 7–10 days, this dilutional effect may take place too quickly for the therapy to be effective.
There are also concerns about toxicity from the gene delivery system, in particular the insertional mutagenesis induced by gamma-retroviral vectors (47, 49, 58, 64) that has led to T cell leukemias following retroviral vector–mediated gene transfer to CD34+ hematopoietic progenitor cells (65). Alternatively, there may be transduction of circulating tumor cells during preparation of the CAR-T cells, and it is conceivable that such inadvertent “marking experiments” would introduce a new driver mutation (66). Insertional mutagenesis leading to uncontrolled proliferation of T lymphocytes (including CAR-T cells) has not yet occurred, perhaps because integration is occurring into more differentiated cells with fewer developmental pathways open to disruption by integration events. Although oncogenicity from retroviruses is currently only a hypothetical concern for CAR-T cells, there is considerable interest in developing vector systems that retain significant genomic integration capacity but are based on DNA plasmids such as the transposon/transposase systems, which may be less likely to integrate in critical sites in the genome (67, 68).
Safety Switches
The specific measures outlined above may all be beneficial, but the inherent potential of T cells to persist and expand means that the associated toxicities may show corresponding persistence and worsen with time. Thus, there is a strong incentive to use engineered T cells that also express a suicide or safety switch along with the CAR. These cells retain their long-term capacity for engraftment, expansion, and expression, but can be eliminated quickly by the activation of the suicide gene in the event of toxicity. Investigators have developed a safety switch based on the caspase-9 molecule that rapidly induces apoptosis in the cell upon exposure to an otherwise bioinert small molecule (chemical inducer of dimerization) (69, 70). Other studies use transfer of the herpesvirus thymidine kinase enzyme that phosphorylates a prodrug such as ganciclovir to an inhibitory nucleoside, or transduce the T cells with a surface-expressed protein that can be targeted in vivo by a lytic mAb, but these may act more slowly or less completely than the caspase-based system.
INCREASING EFFICACY OF CARS
Malignant cells and their supporting stroma have developed a multiplicity of means to evade or subvert the immune system. Many tumors, including most lymphomas, secrete immunosuppressive cytokines, attract immunosuppressive cells, inhibit dendritic cell maturation, express molecules on the cell surface that suppress immune cells, and create a metabolic environment that is immunosuppressive (Figure 3). Although T cell costimulation mediated by CD28 or 4-1BB endodomains in CAR molecules may mitigate some of the above inhibitory effects, other causes of T cell anergy are more difficult to overcome (71).
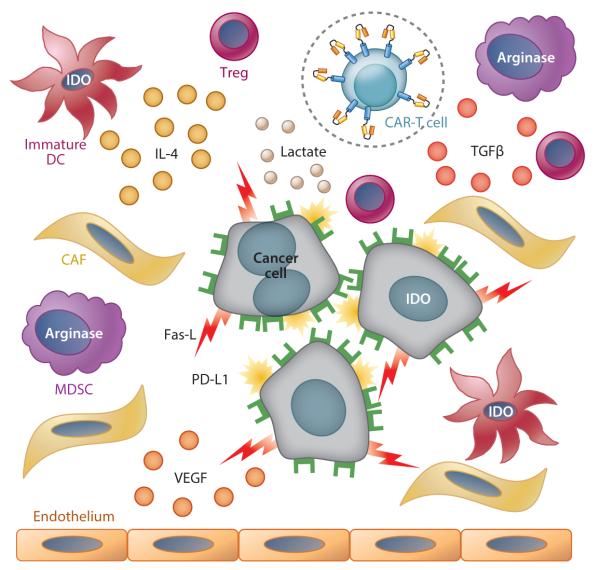
Figure 3.
Tumor strategies for immune evasion. Malignant cells and their supporting stroma secrete immunosuppressive cytokines, such as transforming growth factor β (TGFβ); attract immunosuppressive cells, such T regulatory cells (Tregs) and myeloid-derived suppressive cells (MDSCs); inhibit dendritic cell (DC) maturation; express immunosuppressive molecules on their surface, including Fas ligand and PD-L1 (programmed death 1 ligand); and create a metabolic environment that is immunosuppressive, including high lactate levels, low tryptophan levels, and high kynurenine levels [through the activity of indoleamine 2,3-dioxygenase (IDO) in tumor cells and immature DCs], as well as low arginine levels (through the activity of arginase in MDSCs). Malignant cells and stroma also secrete vascular endothelial growth factor (VEGF), which promotes tumor vascularization and growth via recruitment of endothelial cells. Possible countermeasure strategies include increasing the level of CAR-T cell activation or decreasing physiological downregulation (e.g., by autocrine production of IL-15 or inclusion of additional costimulatory domains); engineering the CAR-T cells to be resistant to tumor immune evasion strategies (such as expressing a dominant negative receptor for TGFβ in CAR-T cells); and targeting the cellular components of tumor stroma [cancer-associated fibroblasts (CAFs) and endothelial cells] using an additional CAR.
Three broad approaches have been adopted as countermeasures to overcome tumor immunosuppression: (a) increasing the level of CAR-T cell activation or decreasing physiological downregulation by checkpoint molecules; (b) engineering the CAR-T cells to be resistant to the inhibitory cytokines used by the tumor; and (c) targeting the cellular components of tumor stroma. Any one countermeasure may affect more than one mechanism of tumor immunosuppression (Figure 3).
Overcoming Checkpoint Inhibition
Inhibiting downregulatory signals with mAbs enables CAR-T cells to overcome the “false” checkpoint signals presented by many lymphomas. Antibodies that block the cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), the programmed death 1 (PD-1) receptor, or the PD-1 ligand (PD-L1) in the tumor microenvironment have produced encouraging clinical results as single agents, for example in HL (72). Several investigators are now combining these agents with CAR-T cells in patients with lymphoma.
As an alternative or complementary solution, it is possible to supply immunostimulatory signals that reduce the inhibitory effects of the checkpoint signals on the CAR-T cells. For example, IL-15 is mainly produced by monocytes, macrophages, and dendritic cells. IL-15 promotes the proliferation of T lymphocytes and also prevents apoptosis and exhaustion (73, 74), reverses anergy (73), stimulates long-lasting antigen-experienced memory cells (75), and overcomes Treg-mediated inhibition (76–79). IL-15 can be used either as a growth factor for the ex vivo expansion of CAR-T cells, where it may “imprint” long-lasting resistance to Tregs (80, 81), or as a recombinant protein in vivo to support T cell expansion after adoptive transfer (80), thereby enhancing the antitumor activity of adoptively transferred T cells. CAR-T cells can be genetically modified to produce their own IL-15 and achieve the hoped-for benefits at the tumor site while avoiding the toxicity associated with systemic administration of the cytokine (76, 78). Local production of cytokines such as IL-7 and IL-12 may be equally beneficial.
Overcoming Inhibitory Cytokines
CAR-T cells can be engineered to be resistant to inhibitory cytokines, such as IL-4 and TGFβ, which are widely used by tumors as an immune evasion strategy. For example, TGFβ promotes tumor growth and limits effector T cell function through SMAD-mediated pathways, resulting in decreased expression of cytolytic gene products such as perforin, decreased cell proliferation, and increased apoptosis (82, 83). These detrimental effects can be negated by modifying T cells to express a dominant-negative TGFβ receptor type II (TGFβ-DNR), which lacks most of the cytoplasmic component including the kinase domain (84, 85). DNR expression interferes with TGFβ signaling, thereby blocking TGFβ-induced SMAD2 phosphorylation so that T cell effector function is sustained even in the presence of TGFβ. This approach has shown benefit in patients with relapsed/resistant EBV-associated HL/NHL who were treated with TGFβ-resistant T cells specific for EBV antigens. Clinical benefit was observed, including complete responses (85), even in patients who had failed treatment with EBV-specific T cells expressing only wild-type TGFβ receptor type II. Other lymphoma studies in which the TGFβ-DNR is expressed in CAR-T cells are now being planned.
Targeting the Cellular Components of Tumor Stroma
Lymphomas have a stromal compartment that supports tumor growth directly through paracrine secretion of growth factors and provision of nutrients, and that also contributes to tumor-induced immunosuppression (86–89). This compartment may be a suitable target for CAR-T cell therapy, but as yet no clinical trials have been reported.
HOW WILL CAR-T CELL THERAPY DEVELOP?
CAR-T cells will require a new approach to drug development and distribution. The conventional pharmaceutical model was created for items that have low manufacturing costs, are sold at high prices, and ameliorate rather than eradicate diseases. Unlike most pharmaceuticals, CAR-T cells have the potential to be “one and done” therapy, meaning a single treatment could prove curative. CAR-T cells are also expensive to manufacture. Moreover, we will likely need to combine CAR therapy with other expensive targeted therapies such as checkpoint antibodies for optimal results, making it difficult to know how to price and pay for these agents.
The complexity of CAR therapies also implies that their development will require multiple small-scale iterations of clinical trials followed by modifications and further clinical trials—akin to the beta testing and version upgrades of the software industry rather than the pattern for conventional therapeutics, in which interruption of progression through phases I–III tends to be a terminal event for the agent rather than an opportunity for an upgrade. The ultimate driver of the development and implementation of CAR-T cells in lymphoma will be demonstrating their increasing success in safely and cost-effectively curing disease.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Leukemia and Lymphoma Society Specialized Center of Research (grant 7018) and the National Institutes of Health National Cancer Institute (grant 3P50CA126752).
Footnotes
DISCLOSURE STATEMENT
M.K.B. is a cofounder of Viracyte, serves on the scientific advisory boards of Blue Bird Bio, Conkwest, PLC, TESSA Therapeutics, and Harvard Medical School, and serves on data safety monitoring boards for Boston Children’s Hospital and Great Ormond Street Hospital. H.E.H. is a cofounder of Viracyte, has a licensing agreement with Cell Medica, and serves on an advisory board for Chimerix; her center has a collaborative research agreement with Celgene.
LITERATURE CITED
- 1.Weinstock DM, Dalla-Favera R, Gascoyne RD, et al. A roadmap for discovery and translation in lymphoma. Blood. 2015;125:2175–77. doi: 10.1182/blood-2015-01-623777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J. Clin. Invest. 2012;122:3439–47. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Neelapu SS, Gause BL, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J. Clin. Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–62. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 7.Becker ML, Near R, Mudgett-Hunter M, et al. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989;58:911–21. doi: 10.1016/0092-8674(89)90943-4. [DOI] [PubMed] [Google Scholar]
- 8.Stancovski I, Schindler DG, Waks T, et al. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J. Immunol. 1993;151:6577–82. [PubMed] [Google Scholar]
- 9.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hombach AA, Schildgen V, Heuser C, et al. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J. Immunol. 2007;178:4650–57. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 12.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Investig. 2011;121:1822–26. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 2015;3:125–35. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 15.Krause A, Guo HF, Latouche JB, et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 1998;188:619–26. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–97. [PubMed] [Google Scholar]
- 17.Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005;12:933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–97. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsen MK, Restifo NP, Cohen PA, et al. Defective major histocompatibility complex class I expression in a sarcomatoid renal cell carcinoma cell line. J. Immunother. Emphasis Tumor Immunol. 1995;17:222–28. doi: 10.1097/00002371-199505000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou Y, Basha G, Seipp RP, et al. Combining the antigen processing components TAP and Tapasin elicits enhanced tumor-free survival. Clin. Cancer Res. 2008;14:1494–501. doi: 10.1158/1078-0432.CCR-07-1066. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67:1887–92. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- 23.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 2009;361:478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–78. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 25.Varmus H. Retroviruses. Science. 1988;240:1427–35. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- 26.Geurts AM, Yang Y, Clark KJ, et al. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol. Ther. 2003;8:108–17. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 27.Wilson MH, Coates CJ, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 2007;15:139–45. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer. 2012;12:671–84. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 31.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 32.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rischer M, Pscherer S, Duwe S, et al. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 2004;126:583–92. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 34.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013;19:2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottschalk S, Ng CY, Perez M, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–43. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 37.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. PNAS. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 39.Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 2013;21:2087–101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma. 1995;18:385–97. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 41.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin. Immunol. 2007;19:318–30. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kochenderfer J, Dudley M, Feldman S, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–49. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brentjens R, Rivière I, Park J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos C, Ballard B, Liu E, et al. Chimeric T cells for therapy of CD30+ Hodgkin and non-Hodgkin lymphomas (HL & NHL) ASCGT Annu. Meet. Abstr. Mol. Ther. 2015;23 Abstr. C-9. [Google Scholar]
- 57.Ramos CA, Savoldo B, Liu E, et al. Clinical responses in patients infused with T lymphocytes redirected to target κ-light immunoglobulin chain. ASH Annu. Meet. Abstr. Blood. 2013;122:506. [Google Scholar]
- 58.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrett DM, Zhao Y, Liu X, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum. Gene Ther. 2011;22:1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almasbak H, Rian E, Hoel HJ, et al. Transiently redirected T cells for adoptive transfer. Cytotherapy. 2011;13:629–40. doi: 10.3109/14653249.2010.542461. [DOI] [PubMed] [Google Scholar]
- 64.Bear AS, Morgan RA, Cornetta K, et al. Replication-competent retroviruses in gene-modified T cells used in clinical trials: Is it time to revise the testing requirements? Mol. Ther. 2012;20:246–49. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavazzana-Calvo M, Fischer A, Hacein-Bey-Abina S, Aiuti A. Gene therapy for primary immunodeficiencies: part 1. Curr. Opin. Immunol. 2012;24:580–84. doi: 10.1016/j.coi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Brenner MK, Rill DR, Moen RC, et al. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993;341:85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- 67.Hackett PB, Largaespada DA, Switzer KC, Cooper LJ. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl. Res. 2013;161:265–83. doi: 10.1016/j.trsl.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazawa Y, Huye LE, Salsman VS, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol. Ther. 2011;19:2133–43. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou X, Di Stasi A, Tey SK, et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood. 2014;123:3895–905. doi: 10.1182/blood-2014-01-551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–16. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–19. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 2001;7:114–18. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 74.Mueller K, Schweier O, Pircher H. Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur. J. Immunol. 2008;38:2874–85. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- 75.Ochoa MC, Mazzolini G, Hervas-Stubbs S, et al. Interleukin-15 in gene therapy of cancer. Curr. Gene Ther. 2013;13:15–30. doi: 10.2174/156652313804806561. [DOI] [PubMed] [Google Scholar]
- 76.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu C, Hughes MS, Zheng Z, et al. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J. Immunol. 2005;175:7226–34. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perna SK, De Angelis B, Pagliara D, et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin. Cancer Res. 2013;19:106–17. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 81.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. PNAS. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akhurst RJ. TGF beta signaling in health and disease. Nat. Genet. 2004;36:790–92. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 83.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–87. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 85.Bollard CM, Dotti G, Gottschalk S, et al. Administration of tumor-specific cytotoxic T lymphocytes engineered to resist TGF-β to patients with EBV-associated lymphomas. ASH Annu. Meet. Abstr. Blood. 2010;116:560. [Google Scholar]
- 86.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res. 2011;1:482–97. [PMC free article] [PubMed] [Google Scholar]
- 87.Kakarla S, Song XT, Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy. 2012;4:1129–38. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marx J. Cancer biology. All in the stroma: cancer’s Cosa Nostra. Science. 2008;320:38–41. doi: 10.1126/science.320.5872.38. [DOI] [PubMed] [Google Scholar]
- 89.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 90.National Cancer Institute . ClinicalTrials.gov. Natl. Libr. Med.; Bethesda, MD: 2014. Anti-CD22 chimeric receptor T cells in pediatric and young adults with recurrent or refractory CD22-expressing B cell malignancies. http://clinicaltrials.gov/show/NCT02315612. [Google Scholar]
- 91.MD Anderson Cancer Center. CLL Global Research Foundation Alliance . ClinicalTrials.gov. Natl. Libr. Med.; Bethesda, MD: 2014. Autologous ROR1R-CAR-T cells for chronic lymphocytic leukemia (CLL) http://clinicaltrials.gov/show/NCT02194374. [Google Scholar]
- 92.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected-virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase I study. Blood. 2013;122:2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kochenderfer J, Dudley M, Carpenter R, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]