Abstract
Opsoclonus myoclonus ataxia syndrome (OMAS) is an autoimmune disorder characterized by rapid, random, conjugate eye movements (opsoclonus), myoclonus, and ataxia. Given these symptoms, autoantibodies targeting the cerebellum or brainstem could mediate the disease or be markers of autoimmunity. In a subset of patients with OMAS, we identified such autoantibodies, which bind to non-synaptic puncta on the surface of live cultured cerebellar and brainstem neuronal dendrites. These findings implicate autoimmunity to a neuronal surface antigen in the pathophysiology of OMAS. Identification of the targeted antigen(s) could elucidate the mechanisms underlying OMAS and provide a biomarker for diagnosis and response to therapy.
Keywords: Opsoclonus myoclonus ataxia syndrome, paraneoplastic neurological syndrome, autoantibodies, cerebellar neurons, brainstem neurons
1. Introduction
Opsoclonus myoclonus ataxia syndrome (OMAS) is a rare but devastating disorder involving the acute onset of opsoclonus (rapid, random, multidirectional saccadic eye movements without intersaccadic intervals), myoclonus, and ataxia, as well as disordered mood or behavior. OMAS occurs as either a paraneoplastic or post-infectious autoimmune disorder (Digre, 1986; Wong, 2007). In children 50% of cases are paraneoplastic and are associated with neuroblastoma; the remaining pediatric cases believed to be post-infectious or to result from neuroblastoma that has regressed prior to onset of symptoms (Panzer and Dalmau, 2011). OMAS also occurs in adults, where the associated tumors include breast, ovarian, and small cell lung cancers (Luque et al., 1991). Patients with OMAS, regardless of tumor status, are treated with immunosuppressive therapies with variable responses, often with residual long-term neurocognitive deficits (Catsman-Berrevoets et al., 2009; De Grandis et al., 2009).
Despite the initial description of OMAS more than 50 years ago (Kinsbourne, 1962), little is understood about its underlying pathophysiology. For patients with paraneoplastic disease, expression of neuronal antigens within the tumor might trigger an autoimmune response that spreads to the brain. For patients with idiopathic OMAS, exposure to a virus may trigger a similar event. The symptoms of OMAS may point to the autoimmune target(s) in the cerebellum or pons. Ataxia results from dysfunction of the cerebellum, or cerebellar inflow / outflow tracts within the pons, midbrain, and thalamus. Opsoclonus is thought to originate from either the cerebellum (Wong et al., 2001) or dysfunction of omnipause neurons in the pons (Kim et al., 2007; Ramat et al., 2008). As there is a minimal brain inflammation in OMAS (Kilgo and Schwartze, 1984), autoantibodies in OMAS may directly bind to their target antigen, disrupting its function without causing significant inflammatory tissue destruction, analogous to what is seen in encephalidities associated with known neuronal surface antigens (Bien et al., 2012; Dalmau et al., 2007). Various studies have reported autoantibodies in OMAS, including antibodies, now believed to be non-specific, directed agaist neurofilament proteins (Braxton et al., 1989; Noetzel et al., 1987) as well as recent reports describing antibodies to neurotransmitter receptors in a few patients with symptoms of OMAS as part of broader neuroimmune disorder (Höftberger et al., 2013; Petit-Pedrol et al., 2014; Smith et al., 2011). In several larger series, although broad anti-neuronal reactivity is seen, no single autoantibody specific for OMAS has been identified (Antunes et al., 2000; Bataller et al., 2003). Studies using flow cytometry have found serum antibodies recognizing neuroblastoma cells and cerebellar granule cells, but these techniques disrupt neuronal architecture and no specific autoantigen has been identified (Blaes et al., 2005; Korfei et al., 2005).
Previous attempts to identify pathogenic antibodies in OMAS have largely involved attempts to determine binding to antigens in fixed tissue specimens, which may alter surface epitopes, (Lang and Vincent, 1996), or are limited by the study of pure populations of single cell types (Blaes et al., 2005; Korfei et al., 2005). To broaden the scope of screened antigens without introducing fixation artifact, we therefore evaluated OMAS-antibody binding in live, mixed, cell cultures from rat cerebellum and brainstem using the techniques successfully employed by our group to identify autoantibodies in anti-NMDA receptor encephalitis (Dalmau et al., 2007) and other disorders mediated by antibodies to cell surface autoantigens, such as AMPA receptors and GABA receptors (Lai et al., 2009; Lancaster et al., 2010). We identified and characterized antibodies to a neuronal surface antigen in 4 out of 42 subjects with OMAS.
2. Patients and Methods
2.1. Patient material
Cerebrospinal fluid (CSF) and serum was collected in accordance with the University of Pennsylvania Institutional Review Board guidelines, and informed consent was obtained from each subject. After collection, samples were stored at −80°C. Samples were obtained from a repository containing serum and CSF from subjects with possible neuroimmune disease. All OMAS samples present in the repository were screened, after exclusion of those noted to have known paraneoplastic antibodies, such as Ri. 35 serum and 29 CSF samples were available, of which 20 samples were paired serum and CSF. Of subjects with known ages, 25 subjects were adults (average age 41 years, range = 21 – 74 years), and 10 were children (average age of 8 years, range = 1 – 17 years. Samples from subjects with other neurological disease were used as negative controls (26 serum samples, 15 CSF, of which 15 were paired serum and CSF. 18 samples were from adults (average age 46 years, range = 24 – 82 years), and 8 were from children (average age of 10 years, range = 3 – 18 years).
2.2. Methods
2.2.1 Cell cultures
Mixed cerebellar and brainstem cultures were prepared from E18 rat embyros. Cerebellums were cut, digested with trypsin-DNase, and mechanically triturated. Cells were washed with additional DNase, BME, and CMF-PBS. Cells were resuspended in BME supplemented with horse serum, fetal bovine serum, L-glutamine and glucose, and plated at 600 cells/μL on poly-L-lysine coated coverslips in 24 well plates. Cells were cultured at 37 °C in 5% CO2 atmosphere. After 24 hours in culture, the media was replaced with serum free medium supplemented with B27 and N2, which was changed every 4–5 days. Cells cultured in this manner should contain a mixed population of neurons that is not enriched for granule cells (Banker and Goslin, 1998). For some experiments, cultured rat hippocampal or cortical neurons were employed, prepared as described previously (Buchhalter and Dichter, 1991).
2.2.2. Autoantibody screening
Patient samples were diluted in media (CSF 1:5, serum 1:100) and incubated on live cultured neurons for 30 minutes at 37°C. Each experiment included CSF from at least one individual with other neurologic disease as a negative control, and one sample from a patient with either anti-AMPA receptor encephalitis or anti-NMDA receptor encephalitis as a positive control. After incubation with patient samples, cells were fixed with 4% paraformaldehyde, and immunostaining was performed. Briefly, coverslips were washed with PBS, permeabilized in 0.3% Triton X-100 in PBS, and then blocked with bovine serum albumin (BSA). Cells were then incubated overnight in chicken anti-MAP primary antibody (1:5000, Abcam 5392) to label neurons and their dendrites. The following day, coverslips were washed with PBS and placed in secondary antibody (1:1000 Alexa Fluor 488 goat anti-human and 1:1000 Alexa Fluor 568 goat anti-chicken) for one hour at 37°C. After washing, cells were mounted on glass slides in mounting medium with DAPI (Vectashield) to label nuclei, and stored at 4°C until imaging. To further characterize positive samples, the following primary antibodies were used to stain cerebellar or cortical neurons: to label axons: rabbit anti-Tau (1:1000, gift of Virginia Lee), to label glia: mouse anti-GFAP (1:2000, Sigma, G-3893), to label presynaptic structures: mouse anti-synaptophysin (1:250, Sigma, S-5768, to label postsynaptic structures mouse anti-PSD-95 (1:500, UC Davis/NIH NeuroMab Facility, #73-028, AB-10698024) or mouse anti-gephryin (1:250, Synaptic Systems #147-011). The following secondary antibodies were employed: Alexa Fluor 568 or 594 goat anti-mouse or anti-rabbit as appropriate, diluted 1:1000.
2.2.3. Western blotting
Lysates were prepared from cultured cerebellar neurons at 8 DIV. Proteins were electrophoresed and transferred to nitrocellulose membranes. Blots were blocked with milk, incubated overnight with serum diluted 1:1000, washed, incubated with HRP-linked anti-human IgG secondary antibody (Jackson, 1:10,000) and developed with chemiluminescent substrate (SuperSignal, Thermo).
2.2.4. Tissue section immunostaining
Adult mouse brains were preserved in PFA, embedded in OTC, and frozen. Sagittal sections were cut, blocked with goat serum, and then incubated overnight in serum diluted 1:75. The following day, sections were washed, then incubated with Alexa Fluor 488 goat anti-human IgG diluted 1:1000, washed, and mounted on glass slides in mounting medium with DAPI to label nuclei.
2.2.3. Immunostaining in transfected cells
HEK293 cells were cultured and transiently transfected as described (Wu et al., 2007). Briefly, cells were plated and cultured on poly-L-lysine-coated glass coverslips. Calcium phosphate transfection was performed one day later using 2 μg of total DNA per milliliter of medium. The following constructs were transfected: GluA2, GluN1, GlyRα1, LGI, and Caspr. Transfected HEK293 cells were stained as described previously. (Dalmau et al., 2008) Briefly, 16–24 h after transfection cells were fixed in 4% paraformaldehyde in PBS, washed with PBS, permeabilized with Triton X-100, and then blocked with BSA. Cells were then incubated overnight with patient serum (1:100) or CSF (1:5) and commercially available antibodies against the following transfected proteins: GluA2 (rabbit polyclonal 07-598, Millipore), GluN1 (mouse monoclonal 556-308, BD Biosciences), GlyRα1 (mouse monoclonal mAb4a 1:200, Synaptic Systems), LGI (rabbit polyclonal 93228 1:10,000, Abcam), and Caspr (rabbit polyclonal 30868, Abcam). The following day, coverslips were washed with PBS and placed in secondary antibody (1:1000 Alexa Fluor 488 goat anti-human IgG and 1:1000 Alexa 568 goat anti-mouse or anti-rabbit diluted in PBS) for one hour at 37°C. After washing, cells were mounted on glass slides in mounting medium with DAPI (Vectashield), and stored at 4°C until imaging.
3. Results
3.1. Patient characteristics
We screened samples from subjects with OMAS obtained from a repository containing serum and CSF from subjects with possible neuroimmune disease. All OMAS samples present in the repository were screened (35 serum and 29 CSF samples, of which 20 samples were paired serum and CSF). Of subjects with known ages, 25 subjects were adults (average age 41 years, range = 21 – 74 years), and 10 were children (average age of 8 years, range = 1 – 17 years). 23% of adult patients had associated tumors (ovarian teratoma, n=4; lymphoma, n=2; breast cancer, n=1; prostate cancer, n=1; and lung adenocarcinoma, n=1). 30% (n = 3) of pediatric patients had neuroblastoma with no other tumor types present (Table 1A). Samples from subjects with other neurological disease were used as negative controls (26 serum samples, 15 CSF, of which 15 were paired serum and CSF). 18 samples were from adults (average age 46 years, range = 24 – 82 years), and 8 were from children (average age of 10 years, range = 3 – 18 years).
Table 1.
Demographic features of subjects with opsoclonus myoclonus ataxia syndrome (OMAS)
| Female | Male | Age > 18 | Age < 18 | Tumor present | Tumor absent | |
|---|---|---|---|---|---|---|
| Percent of samples (n = 42) | 62.5 | 37. 5 | 54 | 46 | 26 | 74 |
| N positive* | 4 | 0 | 3 | 1 | 1 | 3 |
Positive for antibodies to a cerebellar/brainstem surface antigen
3.2. Immunostaining in cultured neurons
Serum samples from four OMAS subjects contained antibodies that bound to puncta on the surface of cultured live cerebellar/brainstem neurons (Table 1A–B). CSF was available for one of these subjects, but did not contain detectable anti-neuronal surface antibodies by this assay. Three of these subjects had a CSF lymphocytic pleocytosis, and one subject had an associated tumor. Two of these subjects had additional neurological symptoms: dysphagia (n=1) and arm rigidity (n=1). No control samples had detectable anti-neuronal surface antibodies (0 of 26 control serum samples, p<0.05, student’s t-test, one-tailed, two-samples, equal variance).
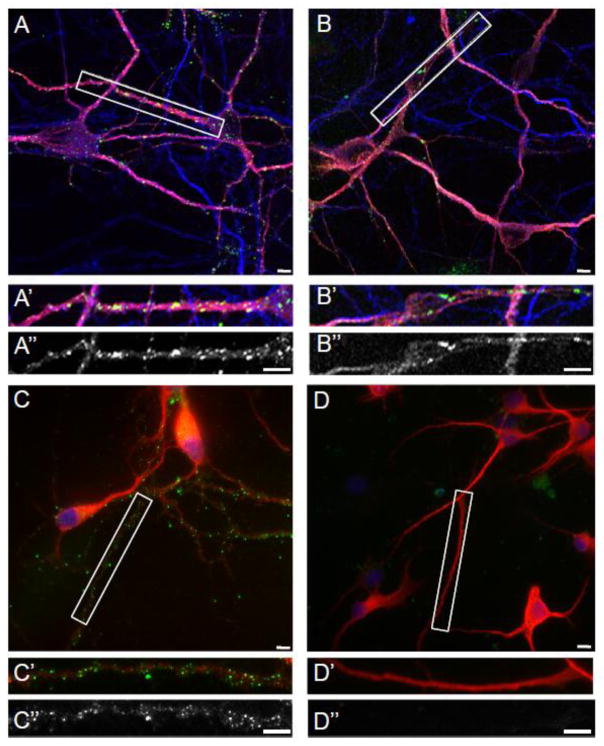
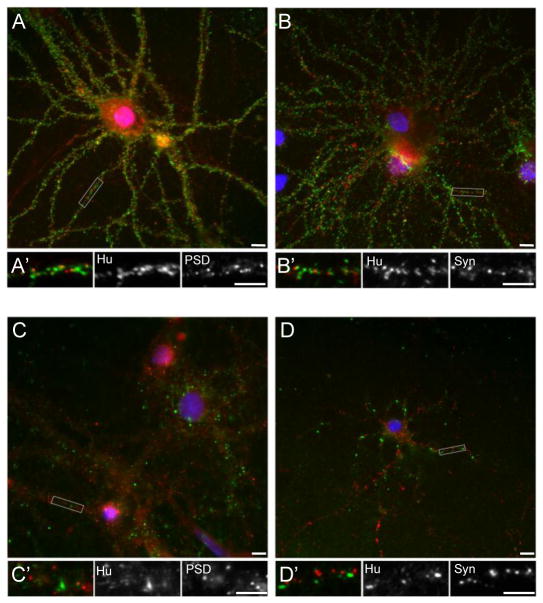
These anti-neuronal surface antibodies bound to puncta on neuronal dendrites but not axons (Fig. 1B). Anti-neuronal surface antibodies were also detected in positive control samples from subjects with anti-AMPAR encephalitis (Fig. 1A) and anti-Tr associated cerebellar degeneration (Fig. 1C), but not in negative control samples (Fig. 1D). The puncta recognized by OMAS antibodies did not colocalize with the presynaptic marker synaptophysin (Fig. 2D), PSD-95, a post-synaptic scaffolding protein at excitatory synapses (Fig. 2C), or gephyrin, a post-synaptic scaffolding protein at inhibitory synapses (data not shown). In contrast, anti-neuronal surface antibodies from serum of a subject with anti-AMPAR encephalitis recognized puncta that colocalized with PSD-95 (Fig. 2B) but not synaptophysin (Fig. 2A), as expected. Taken together, these results indicate that the antigen recognized by OMAS antibodies is dendritic but likely extrasynaptic.
Figure 1.
Reactivity of OMAS patient sera with rat cerebellar/brainstem neuronal cultures. Serum was incubated on live rat cerebellar cultures. Following fixation, fluorescent immunostaining was performed and human IgG labelled with Alexa 488 conjugated secondary antibodies. For A–D, dendrites are labeled with MAP2 (red). For A–B, axons are labeled with Tau (blue) For C–D, nuclei are labelled with DAPI (blue). (A) Positive control using serum from a patient with anti-AMPAR encephalitis (green); (A′) Higher magnification view of boxed area in (A); (A″) Grayscale image of patient-antibody staining of the boxed area. (B) Dendritic puncta recognized by serum from a patient with OMAS (green). (B′) Higher magnification view of boxed area in (B); (B″) Grayscale image of patient-antibody staining of the boxed area. (C) Dendritic puncta recognized by antibodies from a patient with anti-Tr associated cerebellar degeneration (green). (C′) Higher magnification view of boxed area in (C); (C″) Grayscale image of patient-antibody staining of the boxed area. (D). No anti-neuronal surface reactivity is seen using negative control serum. (D′) Higher magnification view of boxed area in (D); (D″) Grayscale image of patient-antibody staining of the boxed area. Scale bars = 10 μm.
Figure 2.
Characterization of the dendritic surface antigen recognized by positive OMAS samples. Serum was incubated on live rat cortical cultures. Following fixation, fluorescent immunostaining was performed using primary antibodies against presynaptic (synaptophysin) or postsynaptic (PSD-95) elements, and human IgG labelled with Alexa 488 conjugated secondary antibodies. (A) Serum from a patient with AMPA-R encephalitis labels puncta (green) that colocalize with PSD-95 (red). (A′) Higher magnification view of boxed area in (A), followed by grayscale images of this boxed area as indicated. (B) Serum from a patient with AMPA-R encephalitis labels puncta (green) that are adjacent to, but do not colocalize with, synaptophysin (red). (B′) Higher magnification view of boxed area in (B), followed by grayscale images of this boxed area as indicated. (C) Positive OMAS serum labels puncta (green) that do not colocalize with PSD-95 (red). (C′) Higher magnification view of boxed area in (C), followed by grayscale images of this boxed area as indicated. (D) Positive OMAS serum labels puncta (green) that do not colocalize with synaptophysin (red). (D′) Higher magnification view of boxed area in (D), followed by grayscale images of this boxed area as indicated. Scale bars = 10 μm.
3.3. Western blot
In order to characterize the antigen further, lysate from cerebellar/brainstem cultures was prepared, and patients’ antibodies were used to perform Western blot analysis. Each positive OMAS sample showed a distinct pattern of recognized proteins within this lysate (Supplemental Fig. 1). However, anti-AMPAR encephalitis serum and negative control sera also recognized numerous proteins in this lysate. Therefore, these banding patterns primarily reflect non-specific binding to multiple intracellular proteins. Of note, there did not appear to be a common protein recognized by all four OMAS samples, indicating that this antigen may not be the same in all four patients or may not be recognized using this technique.
3.4. Immunostaining in tissue sections
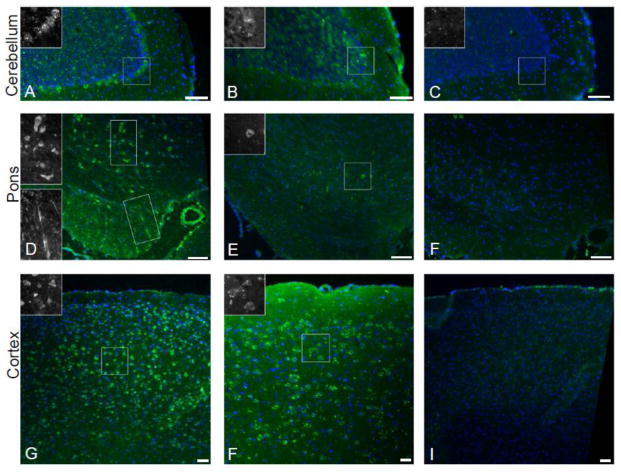
There was sufficient available sample to evaluate staining in rat brain tissue sections for three of the positive OMAS samples. These showed varied antibody binding, including, in some samples, labeling of cerebellar Purkinje cells (Figure 3A) as well as cell bodies and processes in the pons (Figure 3D–E). In parallel experiments, negative control serum did not show staining of brain tissue sections (Figure 3C, F, I). Each OMAS sample showed a distinct staining pattern (Table 3), again indicating that the antigen of interest may be distinct in each patient.
Figure 3.
Characterize of brain tissue section reactivity of positive OMAS samples. Serum was diluted and applied to adult mouse brain sections and human IgG was labelled with Alexa 488 conjugated secondary antibody (green); nuclei were labeled with DAPI (blue). (A) Antibodies from patient 1 recognized Purkinje cells in the cerebellum, inset provides higher magnification grayscale view of boxed region containing Purkinje cell bodies. (B) Antibodies from patient 2 do not have specific cerebellar reactivity. (C) There is no significant staining using negative control serum in cerebellum. (D) Antibodies from patient 1 recognize cell bodies and processes within the pons, insets provide higher magnification grayscale views of the boxed regions. (E) Antibodies from patient 2 recognize some cell bodies in the pons, inset provides higher magnification grayscale view of the boxed region. (F) There is no significant staining using negative control serum in pons. (G) Antibodies from patient 1 recognize cell bodies in the cortex, inset provides higher magnification grayscale view of the boxed region. (H) Antibodies from patient 2 recognize cell bodies in the cortex, inset provides higher magnification grayscale view of the boxed region. (I) There is no significant staining using negative control serum in cortex. Scale bars = 50 μm.
Table 3.
Summary of brain section immunoreactivity in positive samples
| Subject | Cortex | Cerebellum | Pons |
|---|---|---|---|
| 1 | Numerous + cells | + Purkinje cell layer | Numerous + cells Transverse fibers |
| 2 | Scattered + cells | Negative | Rare + cells |
| 3 | Negative | + Purkinje cell layer | Negative |
3.5. Candidate antigen analysis
A candidate antigen approach was also pursued. The four positive OMAS samples did not have reactivity against the following known neuronal surface autoantigens, over-expressed in HEK cells: GluA2, GluN1, GlyRα1, LGI, and Caspr. Positive controls included testing of samples from subjects with anti-AMPAR encephalitis (anti-GluA2), anti-NMDAR encephalitis (anti-GluN1), and encephalitis associated with voltage-gated potassium channel complex antibodies (anti-LGI and anti-Caspr) (data not shown). Taken together, these data suggest that the autoantigen in these OMAS patients may be novel.
4. Discussion
Here, we report that a subset of patients with OMAS produce antibodies that recognize a surface antigen on cultured cerebellar and brainstem neurons. This antigen is located in non-synaptic puncta on neuronal dendrites. Similar techniques have been used to identify the likely pathogenic antigens in several newly described autoimmune synaptic encephalidites, such as anti-NMDAR encephalitis and anti-AMPAR encephalitis (Dalmau et al., 2007; Lai et al., 2009). As our study looked for binding to surface antigens on live neurons, it is distinct from previous attempts to identify pathogenic OMAS antibodies; most of these studies were limited by the use of fixed tissue specimens, Western blot, or flow cytometry of pure cell populations. Directly pathogenic autoantibodies are likely to bind to accessible surface proteins (Ances et al., 2005), and the epitopes on such extracellular antigens may not be detected by staining in tissue section or of lysates on Western blot as they are altered by fixation or denaturation (Lang and Vincent, 1996). Furthermore, these techniques allow for detection of antibodies that bind to intracellular proteins, which are less likely to be disease-relevant and which may be non-specifically recognized by serum antibodies from subjects without neuroinflammatory disease. Therefore, the antibodies detected in our study, targeting neuronal surface antigen(s), are more likely to be directly pathogenic, as compared to prior studies reporting antibodies to proteins such as neurofilament (Noetzel et al., 1987).
Using this approach, only a minority of screened samples recognized surface neuronal antigens. It may be that only a minority of patients with OMAS have antibodies recognizing surface proteins on neurons. However it is also possible that such antibodies to extracellular antigens actually are present in a higher proportion of patients, but not detected by our assay. The target antigen(s) may not be highly expressed at sufficient levels in tissue culture or the antibodies may not be not well-detected by this technique. Furthermore, there may be biases in the OMAS samples included in the repository; in particular, patients with typical OMAS, such as toddlers with our without neuroblastoma, are under-represented, and patients with more unusual presentations, such as atypical associated neurologic symptoms, are likely over-represented. Therefore, it is difficult to state whether our autoantibody detection rate of approximately 10% would be representative of the typical OMAS population.
Despite these limitations, discovery of antibodies against a non-synaptic antigen on neuronal dendrites in a subset of OMAS patients may be an important clue as to the pathogenesis of this disease. Previous work by Blaes et al., (2005) detect antibodies from patients with OMAS that bound to cultured cerebellar granule cells by flow cytometry, but did not further characterize the antigen. As our technique is distinct, it is not clear if we are detecting autoantibodies with the same reactivity. However, our work significantly expands upon these previous findings by establishing that the neuronal surface antigen is likely non-synaptic and present on neuronal dendrites.
Identification of the recognized antigen will provide insight regarding OMAS pathophysiology. Understanding the protein networks and cellular mechanisms disrupted by these autoantibodies may be relevant even in antibody-negative patients, as similar disease pathways may be involved. Future work can validate these antibodies as a marker of disease activity, with the goal of establishing a biomarker to facilitate diagnosis or track response to therapy. In the longer term, understanding the autoantibody-antigen interaction may lead to novel therapeutics approaches to disrupt this interaction, or mitigate its effects. By better understanding the root causes of OMAS we can develop better treatments, resulting in improved patient outcomes.
Supplementary Material
Patient samples have broad reactivity to proteins from cultured cerebellar/brainstem neurons on Western Blot. Proteins from cultured cerebellar neurons were electrophoresed and Western blotting performed by incubation with patient and control serum followed by incubation with HRP-linked anti-human IgG secondary antibody and developed with chemiluminescent substrate. Numerous proteins were detected by antibodies in OMAS patient serum, as well as with negative control serum and serum from a patient with anti-AMPA receptor encephalitis.
Table 2.
Clinical features of subjects with detected anti-neuronal surface antibodies
| Subject | Age (years) | Sex | Tumor status | Additional clinical information |
|---|---|---|---|---|
| 1 | 42 | F | Negative | Also dysphagia. CSF pleocytosis and abnormal FLAIR/T2 signal in the brainstem on MRI. |
| 2 | 70 | F | Negative | History of heavy smoking. |
| 3 | 56 | F | Follicular lymphoma | Also arm rigidity. CSF pleocytosis. |
| 4 | 3 | F | Negative | Febrile prodrome. CSF pleocytosis and abnormal FLAIR/T2 signal in the brainstem on MRI. |
Highlights.
Antibodies to cerebellar/brainstem neurons were found 4/42 subjects with OMAS.
These antibodies bind to non-synaptic surface puncta on neuronal dendrites.
Autoantigen identification may enhance understanding of OMAS pathophysiology.
Acknowledgments
We wish thank Eric Lancaster, MD, PhD (University of Pennsylvania Perlman School of Medicine) for sharing of primary antibodies and the DNA constructs for expression of LGI, Caspr and GlyRα1. This study was supported by National Institute of Health grants T32DA022605, T32NS007413 and K12NS049453, as well as the L. Morton Morley Funds of the Philadelphia Foundation for JAP, and R21NS81439 for DRL, and RO1NS077851 (JD).
Footnotes
Potential Conflicts of Interest
JAP and RA report no conflicts of interest. DRL has a patent for cell-based assay testing for anti-NMDA receptor encephalitis with royalties paid to Eurimmune. JD has a research grant from Euroimmun, and receives royalties for the use of Ma2, NMDAR, GABAbR, and DPPX autoantibody tests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ances BM, Vitaliani R, Taylor Ra, Liebeskind DS, Voloschin A, Houghton DJ, Galetta SL, Dichter M, Alavi A, Rosenfeld MR, Dalmau J. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes N, Khakoo Y, Matthay K, Seeger R, Stram D, Gerstner E, Abrey L, Dalmau J. Antineuronal Antibodies in Patients With Neuroblastoma and Paraneoplastic Opsoclonus-Myoclonus. J Pediatr Hematol Oncol. 2000;22:315–320. doi: 10.1097/00043426-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K, editors. Culturing Nerve Cells. 2. Bradford: 1998. [Google Scholar]
- Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NKV, Dalmau J. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol. 2003;53:347–353. doi: 10.1002/ana.10462. [DOI] [PubMed] [Google Scholar]
- Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, Jellinger Ka, Reuss DE, Ribalta T, Schlegel J, Sutton I, Lassmann H, Bauer J. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–38. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- Blaes F, Fühlhuber V, Korfei M, Tschernatsch M, Behnisch W, Rostasy K, Hero B, Kaps M, Preissner KT. Surface-binding autoantibodies to cerebellar neurons in opsoclonus syndrome. Ann Neurol. 2005;58:313–7. doi: 10.1002/ana.20539. [DOI] [PubMed] [Google Scholar]
- Braxton DB, Williams M, Kamali D, Chin S, Liem R, Latov N. Specificity of human anti-neurofilament autoantibodies. J Neuroimmunol. 1989;21:193–203. doi: 10.1016/0165-5728(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Buchhalter JR, Dichter Ma. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Aarsen FK, Van Hemsbergen MLC, Van Noesel MM, Hakvoort-Cammel FGAJ, Van Den Heuvel-Eibrink MM. Improvement of neurological status and quality of life in children with opsoclonus myoclonus syndrome at long-term follow-up. Pediatr Blood Cancer. 2009;53:1048–1053. doi: 10.1002/pbc.22226. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter Ma, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grandis E, Parodi S, Conte M, Angelini P, Battaglia F, Gandolfo C, Pessagno A, Pistoia V, Mitchell WG, Pike M, Haupt R, Veneselli E. Long-term follow-up of neuroblastoma-associated opsoclonus-myoclonus-ataxia syndrome. Neuropediatrics. 2009;40:103–11. doi: 10.1055/s-0029-1237723. [DOI] [PubMed] [Google Scholar]
- Digre KB. Opsoclonus in adults. Report of three cases and review of the literature. Arch Neurol. 1986;43:1165–1175. doi: 10.1001/archneur.1986.00520110055016. [DOI] [PubMed] [Google Scholar]
- Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, De Felipe A, Saiz A, Dalmau J, Graus F. Encephalitis and GABAB receptor antibodies: Novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgo G, Schwartze G. Opsoclonus. Update on clinical and pathologic associations. J Clin Neuroophthalmol. 1984;4:109–113. [PubMed] [Google Scholar]
- Kim JS, Choi KD, Oh SY, Jeong SH, Oh YM, Kim HJ, Park SH, Ahn JY. Double saccadic pulses and macrosaccadic oscillations from a focal brainstem lesion. J Neurol Sci. 2007;263:118–123. doi: 10.1016/j.jns.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Myoclonic encephalopathy of infants. J Neurol Neurosurg Psychiatry. 1962;25:271–6. doi: 10.1136/jnnp.25.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfei M, Fühlhuber V, Schmidt-Wöll T, Kaps M, Preissner KT, Blaes F. Functional characterisation of autoantibodies from patients with pediatric opsoclonus-myoclonus-syndrome. J Neuroimmunol. 2005;170:150–7. doi: 10.1016/j.jneuroim.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Matà S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B, Vincent A. Autoimmunity to ion-channels and other proteins in paraneoplastic disorders. Curr Opin Immunol. 1996;8:865–871. doi: 10.1016/S0952-7915(96)80017-3. [DOI] [PubMed] [Google Scholar]
- Luque Fa, Furneaux HM, Ferziger R, Rosenblum MK, Wray SH, Schold SC, Glantz MJ, Jaeckle Ka, Biran H, Lesser M. Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol. 1991;29:241–251. doi: 10.1002/ana.410290303. [DOI] [PubMed] [Google Scholar]
- Noetzel MJ, Cawley LP, James VL, Minard BJ, Agrawal HC. Anti-neurofilament protein antibodies in opsoclonus-myoclonus. J Neuroimmunol. 1987;15:137–145. doi: 10.1016/0165-5728(87)90088-9. [DOI] [PubMed] [Google Scholar]
- Panzer J, Dalmau J. Movement disorders in paraneoplastic and autoimmune disease. Curr Opin Neurol. 2011;24:346–53. doi: 10.1097/WCO.0b013e328347b307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalá C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–86. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramat S, Leigh RJ, Zee DS, Shaikh AG, Optican LM. Progress in Brain Research. Elsevier Masson SAS; 2008. Applying saccade models to account for oscillations. [DOI] [PubMed] [Google Scholar]
- Smith JH, Dhamija R, Moseley BD, Sandroni P, Lucchinetti CF, Lennon VA, Kantarci OH. N-methyl-D-aspartate Receptor Autoimmune Encephalitis Presenting With Opsoclonus-Myoclonus. Arch Neurol. 2011;68:1069–72. doi: 10.1001/archneurol.2011.166. [DOI] [PubMed] [Google Scholar]
- Wong A. An update on opsoclonus. Curr Opin Neurol. 2007;20:25–31. doi: 10.1097/WCO.0b013e3280126b51. [DOI] [PubMed] [Google Scholar]
- Wong AMF, Musallam S, Tomlinson RD, Shannon P, Sharpe JA. Opsoclonus in three dimensions: oculographic, neuropathologic and modelling correlates. J Neurol Sci. 2001;189:71–81. doi: 10.1016/s0022-510x(01)00564-0. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter Da, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–87. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient samples have broad reactivity to proteins from cultured cerebellar/brainstem neurons on Western Blot. Proteins from cultured cerebellar neurons were electrophoresed and Western blotting performed by incubation with patient and control serum followed by incubation with HRP-linked anti-human IgG secondary antibody and developed with chemiluminescent substrate. Numerous proteins were detected by antibodies in OMAS patient serum, as well as with negative control serum and serum from a patient with anti-AMPA receptor encephalitis.