Endothelial cells are highly heterogeneous1 and participate in angiogenesis, an integrated set of responses in which new blood vessels are formed from existing ones. During angiogenesis, endothelial cells exhibit increased migration and proliferation2 and contribute to the reorganization of the extracellular matrix. Angiogenesis occurs during embryonic development and is also initiated during wound healing and pathological conditions such as ischemia. While most often the revascularization of ischemic tissues is highly beneficial, under certain conditions, it can be harmful. For example, pathological angiogenesis in the retina during diabetic retinopathy can result in blindness3. A number of molecules influence angiogenesis, including vascular endothelial growth factor (VEGF)4, Notch5 and Wnt6. In this edition of Atherosclerosis, Thrombosis, and Vascular Biology Mao et al. identify another key player in this process: the LDL receptor related protein 1 (LRP1) by demonstrating a significant role for LRP1 in orchestrating angiogenesis during retinal neovascularization.
LRP1, is a highly efficient endocytic as well as a signal transducing receptor that binds multiple ligands7-10, and modulates signaling pathways by regulating the extracellular levels of growth factors and binding adaptor molecules to its intracellular domain (ICD). LRP1 plays an important role in the development11, 12 and maintenance of the vasculature13, 14. Deletion of the Lrp1 gene in mice results in early embryonic lethality11 due to extensive hemorrhaging occurring around E13.512. The underlying vascular defect results from a failure to recruit and maintain vascular smooth muscle cells and pericytes of vessels resulting in extreme dilation of the aorta with a thin and disorganized smooth muscle cell layer and discontinuity of the vascular endothelium. Interestingly, the phenotype observed for Lrp1-/- embryos resembles that of mice genetically deficient in sphingosine-1-phosphate (SIP) receptor S1P1. Like the Lrp1-/- embryos, the S1pr1-/- embryos also exhibit embryonic hemorrhage due to failure to recruit vascular smooth muscle cells and pericytes15. This suggests that LRP1 might regulate the S1P signaling pathway12. LRP1 is also required for appropriate vascular development in zebrafish, where loss of Lrp1a results in a disrupted vascular phenotype associated with excess bone morphogenic protein signaling16.
LRP1 is ubiquitously expressed in numerous cell types, including brain endothelium, neurons, smooth muscle cells, astrocytes, macrophages, fibroblasts and hepatocytes17. While LRP1 is expressed in high levels in most cells, protein levels in the endothelium are low. Its expression is regulated by a variety of pathological conditions such as hypoxia18-20 and Alzheimer's disease21 or by changes in physiological conditions such as aging22. The relatively low level of LRP1 expression in endothelium is tightly regulated by physiological conditions reflecting its important role in this tissue. The first role identified for endothelial LRP1 occurs at the blood brain barrier, where LRP1 functions to avert accumulation of amyloid-β (Aβ) in brain which is the key event in Alzheimer's disease pathogenesis22. In addition to its endocytic and clearance role of Aβ in the brain, in endothelial cells, LRP1 ligands may also undergo transcytosis23, 24. While some studies contradict a role for LRP1 in mediating the efflux of Aβ across the blood-brain barrier, 25, 26 an elegant study employing a brain endothelial-specific LRP1 knockout mouse model convincingly demonstrated the importance of this function for endothelial LRP1 in transporting Aβ across blood-brain barrier27.
A second function for LRP1 expressed in the endothelium is revealed in the current study. Using a mouse model of oxygen-induced retinopathy, Mao et al. found that mice in which LRP1 is selectively deleted in endothelial cells display significantly more neovascularization response in the retina under hypoxic stress. To address the potential mechanisms involved, Mao et al. discovered that LRP1 directly interacts with poly(ADP-ribose) polymerase-1 (PARP-1) and co-localizes with this molecule in the nucleus. PARP-1 is a ubiquitous nuclear DNA base repair enzyme28 that is activated in response to DNA damage in eukaryotes29. In the nucleus, activated PARP-1 catalyzes the transfer of ADP-ribose from nicotinamide adenine dinucleotide (NAD+) onto nuclear acceptor proteins including histones and PARP-1 itself30. This process initiates chromatin relaxation and subsequent recruitment of DNA repair proteins. These conformational changes in chromatin lead to diverse biological processes including chromatin remodeling, transcriptional regulation, DNA repair, cell proliferation and apoptosis31.
Since mature forms of LRP1 are located on the plasma membrane and within endosomal compartments, and since PARP-1 is primarily found within the nucleus, the question arises as to how these two molecules could co-localize. The answer may lie in the fact that LRP1 undergoes Regulated Intramembrane Proteolysis (RIP). RIP is a process in which sequential proteolysis of a transmembrane protein ultimately leads to release of its ICD. May et al.32 demonstrated that the LRP1 intracellular domain (ICD) is released by presenilin-1 following shedding of the ectodomain in an event that is enhanced by activation of protein kinase C, but not influenced by LRP1 ligands (Fig 1a). RIP of LRP1 has at least two major consequences that have been well defined. First, release of the LRP1-ICD suppresses inflammation by reducing the transcription of lipopolysaccharide (LPS)-inducible genes33. This occurs when the LRP1-ICD translocates to the nucleus and associates with interferon regulatory factor 3 (IRF-3) promoting its nuclear export (Fig 1b). This pathway is clearly a feedback inhibitory pathway, since PS1-mediated cleavage of LRP1 in macrophages is increased by treatment with LPS. The net result of this pathway is attenuation of TNF-α and IL-6 secretion in response to LPS.
Figure 1. Regulated Intramembrane Proteolysis (RIP) of LRP1 regulates inflammation and endothelial cell proliferation.
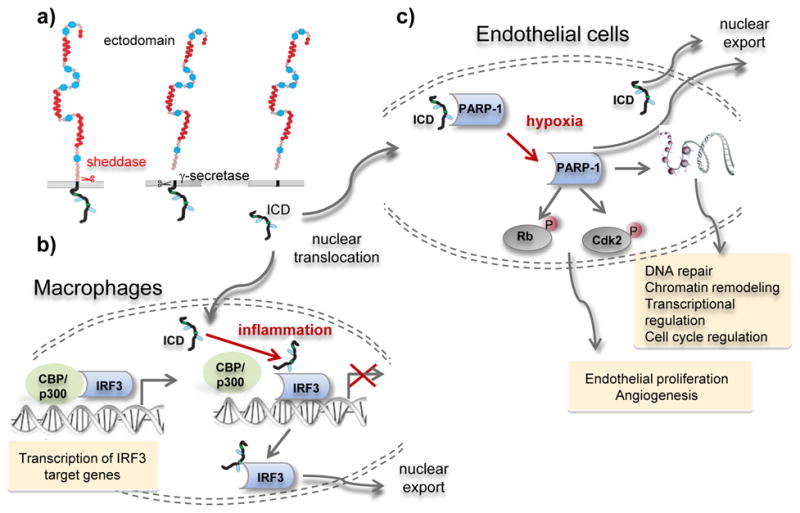
a) RIP is a sequential process that first involves shedding of the LRP1 ectodomain, likely via a ADAM-like protease. This is followed by presenillin-1 mediated cleavge of shed LRP1 releasing the LRP1-ICD which can diffuse to the nucleus. b) In macrophages, the LRP1-ICD attenuates LPS-mediated inflammation by associating with IRF3 resulting in nuclear export of this transcription factor. c) In endothelial cells, the LRP1-ICD associates with PARP-1, attenuating its function. Under conditions of hypoxia, the interaction between LRP1-ICD and PARP-1 is weakened, and PARP-1 is freed to mediate the activation of Cdk2 and inhibition of Rb leading to angiogenesis.
The current paper identifies a second consequence of RIP in endothelial cells in which LRP1 regulates hypoxia-mediated angiogenesis (Fig 1e). The precise mechanism of how this occurs still needs to be established, but the results suggest that LRP1 regulates endothelial cell proliferation by preventing cell cycle progression. During the premitotic stage of the cell cycle, multiple checkpoints ensure the integrity of the genome34 and several crucial proteins play critical roles in these check points. These include cyclin-dependent kinases (Cdks) including Cdk2, which are Ser/Thr kinases that play a central role in the eukaryotic cell division cycle35 by mediating the phosphorylation of retinoblastoma (Rb) to facilitate the G1/S transition in cell cycle36. Mao et al. provided evidence that LRP1 knockdown in human retinal microvascular endothelial cells significantly increases Cdk2 activity as detected by increased phosphorylation at Thr160. Similarly, LRP1 knockdown increased Rb phosphorylation at Ser807/811 leading to Rb inactivation resulting in release of sequestered E2F transcription factors. The studies also revealed that inhibition of PARP-1 rescues these effects resulting from LRP1 knockdown. The conclusion from this work is that LRP1 expression attenuates the phosphorylation of Cdk2 and Rb in normoxia conditions by associating with PARP-1. In hypoxic conditions, despite the fact that LRP1 mRNA expression and protein levels increase18-20, the interaction between LRP1 and PARP-1 is diminished and PARP-1 is freed from the LRP1-ICD leading to its increased activation. This in turn increases Cdk2 and Rb phosphorylation driving cell proliferation (Fig 1c).
While the potential of LRP1 to regulate PARP-1 is an exciting new discovery, this finding raises several important questions that need to be further examined. First, since the binding site on the LRP1-ICD for PARP-1 has been localized to the last 33 amino acids within the ICD, a deletion mutant of LRP1 would be instrumental in teasing out the molecular mechanisms of LRP1/PARP-1 interactions in endothelial cell biology. Second, the role of LRP1/PARP-1 interactions in other pathological and physiological situations needs to be investigated. For example, does this interaction play any role in cancer biology? Additionally, through detailed studies of PARP-1 inhibitors additional roles of PARP-1 are emerging, including regulation of inflammatory mediators37, regulation transcription factor activity38 and regulation of sex hormone signaling39 and cell division40. What is the potential role of LRP1 in regulating PARP-1 activation in these senerios? Further, PARP-1 has been reported to undergo activation during long-term memory41, and it is interesting to consider the possibility that LRP1 may influence this pathway as well. The answers to these questions will prove to be exciting areas for further investigation.
Acknowledgments
This work was supported by grants HL072929 and HL114379 (DKS) from the National Institutes of Health. SCM was supported by a Scientist Development Grant from the American Heart Association.
References
- 1.Kim JD, Lee HW, Jin SW. Diversity is in my veins: role of bone morphogenetic protein signaling during venous morphogenesis in zebrafish illustrates the heterogeneity within endothelial cells. Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):1838–45. doi: 10.1161/ATVBAHA.114.303219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011 May 19;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005 Dec 15;438(7070):960–6. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 4.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995 Jul;15(7 Pt 1):4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009 Feb;16(2):196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010 Jun 15;18(6):938–49. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001 Sep;108(6):779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL Receptor-Related Protein 1: Unique Tissue-Specific Functions Revealed by Selective Gene Knockout Studies. Physiol Rev. 2008 Jul;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005 Oct;62(19-20):2325–38. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher P, Herz J. Signaling through LRP1: Protection from atherosclerosis and beyond. Biochem Pharmacol. 2011 Jan 1;81(1):1–5. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herz J, Clouthier DE, Hammer RE. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1993;73:428. doi: 10.1016/0092-8674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima C, Haffner P, Goerke SM, Zurhove K, Adelmann G, Frotscher M, Herz J, Bock HH, May P. The lipoprotein receptor LRP1 modulates sphingosine-1-phosphate signaling and is essential for vascular development. Development. 2014 Dec;141(23):4513–25. doi: 10.1242/dev.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher P, Gotthardt M, Li WP, Anderson RGW, Herz J. LRP: Role in Vascular Wall Integrity and Protection from Atherosclerosis. Science. 2003 Apr 11;300(5617):329. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 14.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. LRP1 Protects the Vasculature by Regulating Levels of Connective Tissue Growth Factor and HtrA1. Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2137–46. doi: 10.1161/ATVBAHA.113.301893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000 Oct;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ, III, Jin SW, Patterson C. LRP1-Dependent Endocytic Mechanism Governs the Signaling Output of the Bmp System in Endothelial Cells and in Angiogenesis. Circ Res. 2012 Aug 17;111(5):564–74. doi: 10.1161/CIRCRESAHA.112.274597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moestrup SK, Gliemann J, Pallesen G. Distribution of the α2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–82. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 18.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000 Feb 15;60(4):883–7. [PubMed] [Google Scholar]
- 19.Montel V, Gaultier A, Lester RD, Campana WM, Gonias SL. The low-density lipoprotein receptor-related protein regulates cancer cell survival and metastasis development. Cancer Res. 2007 Oct 15;67(20):9817–24. doi: 10.1158/0008-5472.CAN-07-0683. [DOI] [PubMed] [Google Scholar]
- 20.Castellano J, Aledo R, Sendra J, Costales P, Juan-Babot O, Badimon L, Llorente-Cortes V. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1alpha in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1411–20. doi: 10.1161/ATVBAHA.111.225490. [DOI] [PubMed] [Google Scholar]
- 21.Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000 Nov 1;106(9):1159–66. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000 Dec;106(12):1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan W, Kastin AJ, Zankel TC, van KP, Terasaki T, Bu G. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J Cell Sci. 2004 Oct 1;117(Pt 21):5071–8. doi: 10.1242/jcs.01381. [DOI] [PubMed] [Google Scholar]
- 24.Benchenane K, Berezowski V, Fernandez-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005 May;36(5):1065–70. doi: 10.1161/01.STR.0000163050.39122.4f. [DOI] [PubMed] [Google Scholar]
- 25.Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008 Apr;30(1):94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito S, Ueno T, Ohtsuki S, Terasaki T. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: involvement of an LRP-1-independent pathway. J Neurochem. 2010 Jun;113(5):1356–63. doi: 10.1111/j.1471-4159.2010.06708.x. [DOI] [PubMed] [Google Scholar]
- 27.Storck SE, Meister S, Nahrath J, et al. Endothelial LRP1 transports amyloid-beta1-42 across the blood-brain barrier. J Clin Invest. 2015 Nov 30; doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003 Jun;31(6):446–54. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 29.Grube K, Burkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11759–63. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999 Sep 1;342(Pt 2):249–68. [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber V, Dantzer F, Ame JC, de MG. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006 Jul;7(7):517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 32.May P, Reddy YK, Herz J. Proteolytic processing of LRP mediates regulated release of its intracellular domain. J Biol Chem. 2002 Mar 20;277(21):18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 33.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1(47):ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996 Dec 6;274(5293):1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 35.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–17. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ewen ME. The cell cycle and the retinoblastoma protein family. Cancer Metastasis Rev. 1994 Mar;13(1):45–66. doi: 10.1007/BF00690418. [DOI] [PubMed] [Google Scholar]
- 37.Haddad M, Rhinn H, Bloquel C, Coqueran B, Szabo C, Plotkine M, Scherman D, Margaill I. Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol. 2006 Sep;149(1):23–30. doi: 10.1038/sj.bjp.0706837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002 Sep;59(9):1534–53. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright RH, Castellano G, Bonet J, Le DF, Font-Mateu J, Ballare C, Nacht AS, Soronellas D, Oliva B, Beato M. CDK2-dependent activation of PARP-1 is required for hormonal gene regulation in breast cancer cells. Genes Dev. 2012 Sep 1;26(17):1972–83. doi: 10.1101/gad.193193.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang P, Jacobson MK, Mitchison TJ. Poly(ADP-ribose) is required for spindle assembly and structure. Nature. 2004 Dec 2;432(7017):645–9. doi: 10.1038/nature03061. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg S, Visochek L, Giladi E, Gozes I, Cohen-Armon M. PolyADP-ribosylation is required for long-term memory formation in mammals. J Neurochem. 2009 Oct;111(1):72–9. doi: 10.1111/j.1471-4159.2009.06296.x. [DOI] [PubMed] [Google Scholar]


