Abstract
Background
Bartonella bacilliformis is the etiological agent of Carrion’s disease, a neglected tropical poverty-linked illness. This infection is endemic of Andean regions and it is estimated that approximately 1.7 million of South Americans are at risk. This bacterium is a fastidious slow growing microorganism, which is difficult and cumbersome to isolate from clinical sources, thereby hindering the availability of phylogenetic relationship of clinical samples. The aim of this study was to perform Multi Locus Sequence Typing of B. bacilliformis directly in blood from patients diagnosed with Oroya fever during an outbreak in Northern Peru.
Methodology/Principal Findings
DNA extracted among blood samples from patients diagnosed with Oroya’s fever were analyzed with MLST, with the amplification of 7 genetic loci (ftsZ, flaA, ribC, rnpB, rpoB, bvrR and groEL) and a phylogenetic analysis of the different Sequence Types (ST) was performed. A total of 4 different ST were identified. The most frequently found was ST1 present in 66% of samples. Additionally, two samples presented a new allelic profile, belonging to new STs (ST 9 and ST 10), which were closely related to ST1.
Conclusions/Significance
The present data demonstrate that B. bacilliformis MLST studies may be possible directly from blood samples, being a promising approach for epidemiological studies. During the outbreak the STs of B. bacilliformis were found to be heterogeneous, albeit closely related, probably reflecting the evolution from a common ancestor colonizing the area. Additional studies including new samples and areas are needed, in order to obtain better knowledge of phylogenetic scenario B. bacilliformis.
Author Summary
The bacteria Bartonella bacilliformis is the etiological agent of Carrion’s disease, which is a neglected poverty-related disease, related to Mountain Andean valleys of Peru, Colombia and Ecuador. This disease, in absence of treatment presents a high mortality during the acute phase, called Oroya’s Fever. The second phase is characterized by the development of dermal eruptions, known as “Verruga peruana” (Peruvian wart). This bacterium is a fastidious slow growing microorganism, being difficult and cumbersome to isolate from clinical sources. Then, the available data about phylogenetic relationship in clinical samples are really scarce, but suggesting high variability. The aim of the study was to perform direct blood analysis of B. bacilliformis Multi Locus Sequence Typing (MLST), a genotyping tool, in patients with Oroya fever during an outbreak. The present study demonstrates that the direct blood PCR, followed by nucleotide sequencing and MLST is a technique useful in the phylogenic characterization of this fastidious microorganism endemic from Andean regions. In this study, we demonstrate that the outbreak of Oroya’s fever was caused by closely related Sequence Typing (ST) microorganisms and, additionally, new STs have been described.
Introduction
Carrion’s disease is neglected tropical neglected poverty-linked illness caused by Bartonella bacilliformis. This infection is endemic in low-income areas of Peru, specifically related to Andean regions from Peru, Ecuador and Colombia, covering roughly 145,000Km2 only in Peru, and it is estimated that approximately 1.7 million of South Americans are at risk [1–3]. This illness has two phases: the first, named Oroya’s Fever, mainly affects young children (>60% of cases) and is characterized by fever, acute bacteremia at about 60 days and severe hemolytic anemia [2,4]. Complications are common in this phase, and secondary infections are also frequent due to transient immunosuppression [5]. In the absence of adequate treatment, high levels of mortality (44% to 88%) have been reported [2,4]. The second phase is called “Verruga Peruana” (Peruvian Wart), in which the bacterium induces the proliferation of endothelial cells, resulting in a series of cutaneous lesions [6]. A variety of verrugal lesions are presented in the chronic phase: miliary, nodular and mular [1]. Asymptomatic carriers have also been described in the population from endemic areas (0.5–45%) [7].
B. bacilliformis is a fastidious slow growing microorganism, which is difficult and cumbersome to culture and isolate from clinical sources [2]. Thus, the data available about the phylogenetic relationship of clinical samples of B. bacilliformis are scarce and non-uniform. Indeed, to the best of our knowledge no studies on clonal relations based on Pulsed Field Gel Electrophoresis (PFGE) have been performed, and molecular approaches have been based on PCR methodologies, including Repetitive Extragenic Palindromic PCR (REP-PCR), Enterobacterial Repetitive Intergenic Consensus (ERIC-PCR), Amplified Fragment Length Polymorphism (AFLP), Infrequent Restriction Endonuclease Site PCR (IRS-PCR), analysis of the 16S-23S ribosomal DNA intergenic spacer regions or analysis of the sequence of specific genetic loci such as gltA, ialB and flaA [8–10]. This latter methodology resembles a Multi-locus sequence typing (MLST) technology. MLST approaches are based on housekeeping gene sequencing, being robust, standardized methodology useful to develop epidemiological and evolutionary studies [11]. In fact, MLST schedules have been developed to analyze the phylogenetic relationships of Bartonella henselae [12], and adapted to other Bartonella species, including Bartonella quintana [13] and Bartonella bovis [14]. Furthermore, the use of MLST has been useful in the identification of Bartonella ancashi, new specie of Bartonella genus, closely related to B. bacilliformis [15]. Regarding B. bacilliformis, a MLST schedule has recently been developed based on the sequence of 7 housekeeping genes (bvrR, ribC, ftsZ, groEL, flaA, rnP and rpoB) [16], with 8 different ST being detected in 43 isolates. However, it should be considered that due to the relative isolation of the Andean valleys, the population structure of B. bacilliformis might differ between different endemic areas.
The aim of the study was to perform direct blood MLST of B. bacilliformis from patients diagnosed with Oroya Fever during an outbreak in Northern Peru.
Materials and Methods
Samples
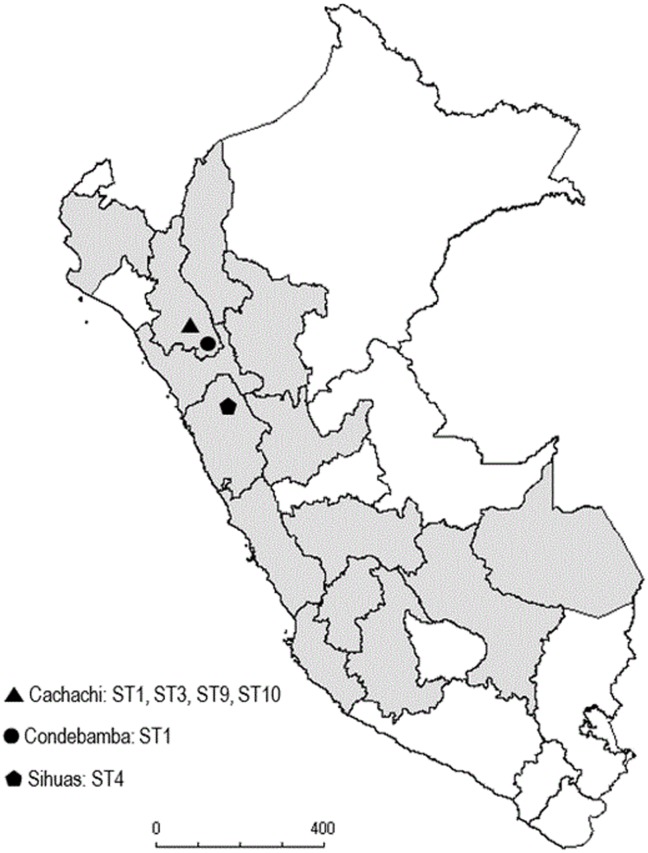
Seven blood samples from Cachachi (Department of Cajamarca in Northern Peru) were collected during March and April 2009 from patients clinically diagnosed with Oroya Fever. Additionally, another two blood samples were collected from Oroya’s Fever patients living in the Condebamba (Cajamarca Department, 50 Km from Cachachi) and Ancash Department in November and October 2011, respectively. Finally, two collection strains isolated in 1941 (CIP 57.19; NCTC12135) and 1949 (CIP 57.18; NCTC12134) from the Pasteur Institute Collection and previously described as belonging to Sequence Type 3 [16] were used as controls (Fig 1). The clinical data and disease presentation of some patients were obtained.
Fig 1. Map of the geographical distribution of Carrion’s disease in Peru with the distribution of the SequenceTypes location.
Ethical statement
All adult participants provided written informed consent. The study were submitted, revised and approved by the Ethics and Research Committees of the Universidad Peruana de Ciencias Aplicadas in Peru and Hospital Clinic of Barcelona in Spain.
Detection of Bartonella bacilliformis
The presence of B. bacilliformis in all the blood samples was confirmed by PCR amplification of 438 bp of the 16S rRNA gene of B. bacillifomis (5’CCTTCA GTTMGGCTGGATC-3’ and 5’-GCCYCCTTGCGGTTAGCACA-3’) as previously described [17]. In all cases the identity of the amplified fragments was confirmed after being visualized in 1.5% agarose gel stained with Sybr Safe and gel recovered using Wizard SV gel and PCR clean up system, (Promega, Madison, WI, USA) following manufacturer's instructions and were sequenced by Macrogen (Seoul, Korea).
DNA extraction
The DNA was extracted from 200 μl of blood sample and directly from the control bacterial strains using a commercial extraction kit (High Pure Kit Preparation template, Roche Applied Science, Mannheim, Germany). Blood and bacterial DNA obtained after extraction were eluted in 100 μl of nuclease free water and then processed or stored at -20°C until use.
MLST genes amplification
Internal fragments of the 7 genetic loci (ftsZ, flaA, ribC, rnpB, rpoB, bvrR and groEL) included in the B. bacilliformis MLST schedule were amplified as previously described [16]. Reaction mixtures were exposed to denaturation at 96°C for 5 min followed by 50 cycles of 96°C for 40 sec, 55°C for 40 sec and 72°C for 50 sec, with a final extension step of 72°C for 10 minutes. Amplified fragments were visualized in 1.5% agarose gel stained with Sybr Safe and subsequently gel recovered using Wizard SV gel and PCR clean up system, (Promega, Madison, WI, USA) following manufacturer’s instructions and sequenced by Macrogen (Seoul, Korea).
Phylogenetic analysis
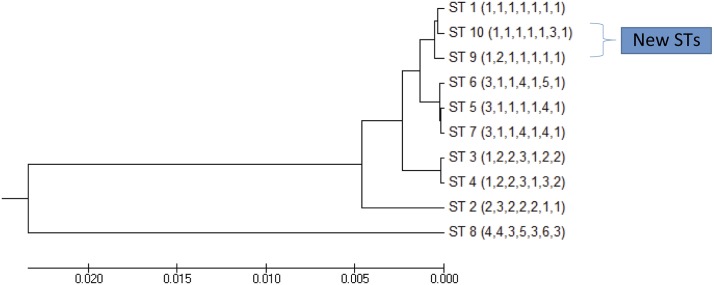
Phylogenetic relationship analyses were conducted using MEGA version 5 [18]. The phylogenetic tree was constructed by UPGMA (Unweighted Pair Group Method with Arithmetic Mean Analysis). The phylogenetic tree was inferred from 500 bootstrap replicates. The sequences of all the alleles described previously were obtained from Genbank (accession numbers JF326267 to JF326294) and were ordered according to the corresponding Sequence Type (ST) in order to develop the phylogenetic tree.
Results
The mean age of patients studied was 25.9 years (SD = 13.77, IC95% = 19.5–32.3), 44.4% being female. Among the 5 patients from whom clinical data were recovered, all (100%) presented fever (>38°C) and malaise, 4 (80%) reported chills, myalgia and pallor, 3 (60%) headache, 2 (40%) reported jaundice and arthralgia and only one patient (20%) presented vomiting (Table 1). In 3 cases the treatment was recorded, in all cases being ciprofloxacin alone (2 cases) or with ceftriaxone (1 case) during 14 days.
Table 1. Clinical and epidemiological characteristics of Oroya Fever infected patients collected during the outbreak in Northern of Peru.
| Sample ID | Carrion phase | Age | Gender | Fever | Malaise | Chills | Myalgia | Arthralgia | Headache | Vomiting | Pallor | Jaundice |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC 1 | Acute | 15 years | Male | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| EC 2 | Acute | 25 years | Female | + | + | + | + | + | + | + | + | - |
| EC 8 | Acute | 16 years | Female | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| EC 14 | Acute | 33 years | Male | + | + | + | + | - | + | - | + | - |
| EC 35 | Acute | 47 years | Male | + | + | + | + | + | + | - | - | - |
| EC 44 | Acute | 32 years | Male | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| EC 48 | Acute | 35 years | Female | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| EC 125 | Acute | 29 days | Male | + | + | - | - | - | - | - | + | + |
| EC 129 | Acute | 30 years | Female | + | + | + | + | - | - | - | + | + |
ID: Identification; ND.- Non determined
Among the 9 blood samples analyzed, a total of 4 different B. bacilliformis STs were identified. The most frequently found was ST1, present in 6 out of 9 (66%) samples, all from the Cajamarca Department (5 out of 7 belonging to the Cachachi outbreak, and that of Condebamba), while the sample from the Ancash Department belonged to ST4 (Fig 1). Additionally, two samples from the Cachachi outbreak presents a new allelic profile, belonging to new STs, which were classified as ST9 (1,2,1,1,1,1,1) and ST10 (1,1,1,1,1,3,1) respectively. The 2 collection strains were classified as ST3 (Table 2).
Table 2. Multi-locus sequence typing (MLST) information of Oroya Fever samples.
| MLST allelic profile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Locality, District, Department | Date | ftsZ | flaA | ribC | rnpB | rpoB | bvrR | groEL | ST |
| 1 | Carrizal, Cachachi, Cajamarca | march-09 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | ST10 |
| 2 | Chuquibamba, Cachichi, Cajamarca | march-09 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ST9 |
| 8 | Shirac, Cachachi, Cajamarca | march-09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| 14 | Shirac, Cachachi, Cajamarca | march-09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| 35 | Pampa Mirador, Cachachi, Cajamarca | march-09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| 44 | Moncada, Cachachi, Cajamarca | march-09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| 48 | Picachos, Cachachi, Cajamarca | may-09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| 125 | Pisgullo, Sihuas, Ancash | oct-11 | 1 | 2 | 2 | 3 | 1 | 3 | 3 | ST4 |
| 129 | Huañinba, Condebamba, Cajamarca | nov-11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ST1 |
| c57.17 | Collection strain | - | 1 | 2 | 2 | 3 | 1 | 2 | 3 | ST3 |
| c57.18 | Collection strain | - | 1 | 2 | 2 | 3 | 1 | 2 | 3 | ST3 |
ID: Identification; ST: Sequence Typing
On determination of phylogenetic relationships between the ST9 and ST10 and the previously described ST, they were found to be closely related to ST1, differing in only 1 of the 7 alleles (Fig 2).
Fig 2. Phylogenetic tree of all the Sequence Types of B. bacilliformis as constructed an UPGMA cluster analysis with Bootstrap method.
Discussion
Studies on the clonality and phylogeny of B. bacilliformis are scarce. This may be due to the slow growth of this bacterium and a series of specific requirements which directly affect the culture. The present study demonstrates MLST studies of B. bacilliformis may be performed directly from blood samples thereby avoiding the difficult step of culturing this microorganism. However, a series of limitations that may limit the usefulness of this technique should be taken into account. Among these, definitive results may not be obtained in the hypothetical case of polyclonal infections when the infecting isolates belong to different STs. Along this line, although to the best of our knowledge coinfection by two different B. bacilliformis clones has not been described to date, coinfections by different Bartonella variants has been reported in cotton rats [19]. In the present report, new MLST were not related to artefactual overlapping of sequences belonging to different B. bacilliformis causing a concomitant infection, because no double peaks were observed in any DNA sequence. Another limitation is that direct blood PCR approaches in the study of asymptomatic B. bacilliformis carriers do not have enough power due to the low bacterial burden [20].
The present study demonstrates the heterogeneity of the B. bacilliformis population. Highly clonality has also been found in other species of Bartonella, such as B. quintana, a re-emerging pathogen causing trench fever [13]. Thus, 3 different ST (ST1, ST9 and ST10) were recovered amongst the samples analyzed from the Cachachi outbreak. However, it is of note that these 3 STs were closely related to each another, and thus may reflect the evolution from a common ancestor colonizing the area.
To date only one study has determined the MLST of B. bacilliformis isolates [16]. In this study ST1 was found to be widely distributed in central and northern areas of Peru, accounting for 46% of the samples analyzed, including samples from the 1960's. In addition, ST1 has been detected in the neighboring San Martin Department, and thus, its presence in the Cajamarca Department is not surprising. ST2, ST3, ST4 and ST8 have been described in the center of the country, similar the present sample belonging to ST4. Meanwhile, ST5 has been observed in southern isolates and ST6 and ST7 in the north of the country.
Some techniques such as PFGE or REP-PCR are useful for the description of clones and specific outbreak characterization, as for example virulence, being of special interest to study recent genetic events. On the other hand, techniques such as MLST classification describe ancient genetic differentiations that may underlie more in depth differences [11–13]. For example, specific STs could possess increased virulence or may have a greater facility to develop either acute or chronic infection, or to remain undetected in asymptomatic carriers. Unfortunately, the scarce data on STs of B. bacilliformis make it difficult to delineate these aspects.
The clinical data of only a few patients were recorded; however the symptoms reported are in accordance with those more extensively described, such as the presence of fever, pallor, malaise and headache in acute cases, [17]. Some of these symptoms are a consequence of hematological complications, such as pallor, while others like vomiting or jaundice are related to gastrointestinal problems [21]. Although this disease mainly affects children under 14 years of age (more than 60% of cases) [22], in our study the youngest patient of the Cachachi outbreak was 15 years old. This may be related to the outbreak nature of the samples. Fortunately, all patients who receipt treatment respond well to the treatment. The currently recommended treatment for the acute phase of Oroya’s Fever includes the use of ciprofloxacin as first line therapy in adults and children >14 years, while chloramphenicol, cotrimoxazole, amoxicillin plus clavulanic acid and ceftriaxone are used as a second line or children and pregnant women [23]. Fortunately, up to now, the levels of antibiotic resistance reported among B. bacilliformis have shown that this microorganism is highly susceptible to the antibiotics tested [24].
In summary, this is the first report of MLST of B. bacilliformis performed in direct blood samples, with two new ST variants being described. Present data highlight the need to extend the studies to new samples and geographical areas, in order to provide a better picture of the situation, which will allow specific STs of B. bacilliformis to be associated with clinical symptoms, and the severity or phase of the disease.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported by the Spanish Network for the Research in Infectious Diseases [REIPI RD12/0015],by Generalitat de Catalunya, Departament d’Universitats, Recerca i Societat de la Informació [2014 SGR 26] and by by a grant of the Instituto de Salud Carlos III - Spain (PI11/00983) which included FEDER funds (JR). MJP has a postdoctoral fellowship from CONCYTEC/FONDECYT (grant number: CG05-2013-FONDECYT). JR has a fellowship from the program I3, of the ISCIII [grant number CES11/012]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ihler GM. Bartonella bacilliformis: dangerous pathogen slowly emerging from deep background. FEMS Microbiol Lett 1996; 144:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez Clemente N, Ugarte-Gil CA, Maguiña C, Pachas P, Blazes D, Bailey R et al. Bartonella bacilliformis: a systematic review of the literature to guide the research agenda for elimination. PLoS Negl Trop Dis. 2012; 6:e1819 10.1371/journal.pntd.0001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pachas PE. Enfermedad de Carrión (Bartonellosis) en el Peru. Lima: Ministerio de Salud, OGE, INS; 2001. [Google Scholar]
- 4.Maguiña C, Guerra H, Ventosilla P. Bartonellosis. Clin Dermatol. 2009; 27:271–280. 10.1016/j.clindermatol.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Ticona E, Huaroto L, Garcia Y, Vargas L, Madariaga MG The pathophysiology of the acute phase of human bartonellosis resembles AIDS. Med Hypotheses 2010; 74:45–49. 10.1016/j.mehy.2009.06.054 [DOI] [PubMed] [Google Scholar]
- 6.Maguiña C, Gotuzzo E Bartonellosis—new and old. Infect Dis Clin North Am.2000; 14:1–22. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlin J, Laughlin LW, Romero S, Solorzano N, Gordon S, Andre RG et al. Epidemiology of endemic Bartonella bacilliformis: A prospective cohort study in a Peruvian mountain valley community. J Infect Dis 2002; 186: 983–990. [DOI] [PubMed] [Google Scholar]
- 8.Birtles RJ, Fry NK, Ventosilla P, Cáceres AG, Sánchez E, Vizcarra H et al. Identification of Bartonella bacilliformis genotypes and their relevance to epidemiological investigations of human bartonellosis. J Clin Microbiol. 2002; 40: 3606–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambuch TM, Handley SA, Ellis B, Chamberlin J, Romero S, Regnery R. Population genetic analysis of Bartonella bacilliformis isolates from areas of Peru where Carrion's disease is endemic and epidemic. J Clin Microbiol. 2004; 42:3675–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padilla RC, Ventura EG Genotipificación de aislamientos de Bartonella bacilliformis por amplificación de elementos repetitivos mediante el uso de REP-PCR y ERIC-PCR. Rev Peru Med Exp Clin 2003; 20: 128–131. [Google Scholar]
- 11.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998; 95: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iredell J, Blanckenberg D, Arvand M, Grauling S, Feil EJ, Birtles RJ. Characterization of the natural population of Bartonella henselae by multilocus sequence typing. J Clin Microbiol. 2003; 41: 5071–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvand M, Raoult D, Feil EJ. Multi-locus sequence typing of a geographically and temporally diverse sample of the highly clonal human pathogen Bartonella quintana. PLoS One 2010; 5: e9765 10.1371/journal.pone.0009765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai Y, Malania L, Alvarez Castillo D, Moran D, Boonmar S, Chanlun A et al. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS One 2013; 21;8:e80894 10.1371/journal.pone.0080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Maguiña C et al. Molecular typing of "Candidatus Bartonella ancashi," a new human pathogen causing verruga peruana. J Clin Microbiol 2013; 51:3865–3868 10.1128/JCM.01226-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaloner GL, Ventosilla Palmira, Birtles RJ. Multi-Locus sequence analysis reveals profound genetic diversity among isolates of the human pathogen Bartonella bacilliformis. PLoS Negl Trop Dis. 2011; 5: e1248 10.1371/journal.pntd.0001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.del Valle Mendoza J, Silva Caso W, Tinco Valdez C, Pons MJ, del Valle LJ, Casabona Oré V et al. (2014) Diagnosis of Carrion's disease by direct blood PCR in thin blood smear negative samples. PLoS One 20;9:e92283 10.1371/journal.pone.0092283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS, Kosoy M Analysis of multi-strain Bartonella pathogens in natural host population—do they behave as species or minor genetic variants? Epidemics 2010; 2: 165–172. 10.1016/j.epidem.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Gomes C, Silva W, Tinco C, Martínez-Puchol, Pons MJ et al. Evaluation of three PCR schemes for detection of Bartonella bacilliformis in blood samples: sensitivity, specificity and applicability. Int J Infect Dis 2014; 21: 367. [Google Scholar]
- 21.Maguiña C, Gotuzzo E, Carcelén A, Salinas C, Cok J, Recavarren S et al. Compromiso gastrointestinal en bartonelosis o enfermedad de Carrión. Rev Gastroent Perú 1997; 17:31–43. [PubMed] [Google Scholar]
- 22.Pachas P. Epidemiología de la bartonelosis en la Región Chavín Libro de resúmenes de trabajos libres. IX Congreso Nacional de Medicina Interna; Lima: 1996. [Google Scholar]
- 23.Tarazona A, Maguiña C, López de Guimaraes D, Montoya M, Pachas PT erapia antibiótica para el manejo de la bartonelosis o enfermedad de Carrión en el Perú. Rev Peru Med Exp Salud Publ. 2006; 23:188–200. [Google Scholar]
- 24.Silva-Caso W, Pons MJ, Ruiz J, del Valle-Mendoza J. Antibiotic resistance in Bartonella bacilliformis clinical isolates from endemic area of Peru. J Global Antimicrob Resist 2015; 3: 222–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.