Abstract
Inflammatory activation of microglia and β amyloid (Aβ) deposition are considered to work both independently and synergistically to contribute to the increased risk of Alzheimer’s disease (AD). Recent studies indicate that long-term use of phenolic compounds provides protection against AD, primarily due to their anti-inflammatory actions. We previously suggested that phenolic compound curcumin ameliorated phagocytosis possibly through its anti-inflammatory effects rather than direct regulation of phagocytic function in electromagnetic field-exposed N9 microglial cells (N9 cells). Here, we explored the prostaglandin-E2 (PGE2)-related signaling pathway that involved in curcumin-mediated phagocytosis in fibrillar β-amyloid peptide (1–42) (fAβ42)-stimulated N9 cells. Treatment with fAβ42 increased phagocytosis of fluorescent-labeled latex beads in N9 cells. This increase was attenuated in a dose-dependent manner by endogenous and exogenous PGE2, as well as a selective EP2 or protein kinase A (PKA) agonist, but not by an EP4 agonist. We also found that an antagonist of EP2, but not EP4, abolished the reduction effect of PGE2 on fAβ42-induced microglial phagocytosis. Additionally, the increased expression of endogenous PGE2, EP2, and cyclic adenosine monophosphate (AMP), and activation of vasodilator-stimulated phosphoprotein, cyclic AMP responsive element-binding protein, and PKA were depressed by curcumin administration. This reduction led to the amelioration of the phagocytic abilities of PGE2-stimulated N9 cells. Taken together, these data suggested that curcumin restored the attenuating effect of PGE2 on fAβ42-induced microglial phagocytosis via a signaling mechanism involving EP2 and PKA. Moreover, due to its immune modulatory effects, curcumin may be a promising pharmacological candidate for neurodegenerative diseases.
Introduction
Alzheimer’s disease (AD) is the foremost form of dementia, and is increasing as the population ages. It is defined by two cardinal pathologic features: senile plaques and neurofibrillary degeneration [1]. Inexplicably, most cases of AD are associated with decreased clearance and degradation of amyloid beta (Aβ) [2] and increased secretion of inflammatory mediators, both associated with the phenotypic activation of microglial cells. It is widely accepted that beneficial strategies against AD may be attributabled to the promotion of phagocytosis and inhibition of the pro-inflammatory response in microglia.
Chronic inflammation has long been hypothesized to be a driving force in promoting the development of AD, leadings to increasing studies for exploring whether inflammatory products have a direct or indirect effect on Aβ clearance. In addition, it is important to investigate the pro-inflammatory mediators that regulate the clearance of Aβ by microglia. Prior studies have shown that pro-inflammatory cytokines act selectively to regulate microglial phagocytosis and Aβ load [3, 4]. Additional evidences have revealed that inducible isoform cyclooxygenase 2 (COX-2)-derived prostaglandin (PG) E2 mediates potentiation of the inflammatory response and amyloid plaque formation [5, 6]. Moreover, it has been proposed that microglial PGE2 receptor subtype 2 (EP2) signaling contributes to Aβ plaque burden in AD transgenic mice [7, 8], and that EP2 signaling suppresses microglial phagocytosis of Aβ42 in primary microglia cultures [9, 10]. These studies suggest that pharmacologic compounds targeting microglial EP2 would be an effective therapeutic option for AD.
Several studies demonstrate that natural compounds limiting neuroinflammation and promoting Aβ clearance may be more efficacious at ameliorating microglia-associated neurodegenerative diseases. Among these immuno-modulators, curcumin is the active compound in turmeric (Curcuma longa), an herbal medicine that has been widely used for many centuries in India and China [11, 12]. Recent experimental data further support the therapeutic potential of curcumin in the pathophysiology of AD. Curcumin proved to be immunomodulatory, inhibiting neurotoxicity and increasing the index of phagocytosis [13, 14]. In a previous work, we demonstrated that curcumin may ameliorate defective microglial phagocytosis via its anti-inflammatory effects, and not by direct regulation of phagocytic function in electromagnetic field-exposed N9 cells [15]. Although several cytokines have been shown to modulate phagocytic ability of microglia in vitro [3, 16], the exact pro-inflammatory molecule underlying the salutary effect of curcumin on microglial phagocytosis in AD is unidentified.
Recent evidence suggests that curcumin inhibits the production of microglia-derived PGE2 in response to inflammatory stimulation [17]. Given the fact that PGE2 is highly released in the AD brain [5, 6] and has a depressed effect on microglial phagocytosis [7, 8], we hypothesized that curcumin regulates microglial phagocytosis via PGE2 and its related signaling pathway. Herein, we first tested whether both exogenous and endogenous PGE2 are involved in immunomodulatory phagocytosis in fAβ42-stimulated N9 microglial cells (N9 cells). We then evaluated the ability of curcumin to ameliorate phagocytic abilities of PGE2 and fAβ42-stimulated N9 cells. Our results demonstrated that curcumin positively regulates microglial phagocytotic activity through inhibition of PGE2-EP2 signaling in Aβ42-stimulated N9 cells. The results may provide critical information supporting the therapeutic use of curcumin in neurologic disorders associated with activated microglia.
Materials and Methods
Cell culture and treatment
The immortalized murine microglial cell line N9 was a gift from Dr. Yun Bai (Department of Genetics, Third Military Medical University, China), and was original established by immortalization of day 13 embryonic brain cultures with the 3RV retrovirus carrying an activated v-myc oncogene as previously described [18, 19]. Briefly, cells were grown in Iscove's modified Dulbecco's medium (IMDM; HyClone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). Cells were seeded in 25 cm2 T-flasks (5×106 cells/flask), 6-well plates (5×105 cells/well), 24-well plates (1.5×105 cells/well) or 96-well plates (1×104 cells/well) at 37°C in a humidified 5% CO2 atmosphere. N9 cells were passaged every three days with 1:4 split ratio and used at passages 3–10.
After 24 h incubation, cell culture medium was replaced with serum-free IMDM supplemented with the compounds of interest, and incubated for 15 or 30 min at 37°C. Synthetic β-amyloid peptide (1–42) (Aβ42; GL Biochem, Shanghai, China) was incubated at 37°C for 7 days in medium to promote fibril formation. Pharmacologic agents used in different experiments included a solvent control (tissue culture grade dimethylsulfoxide (DMSO), 30 min; Sigma-Aldrich); curcumin (30 min, 5, 10 and 20 μM; Sigma-Aldrich); antagonists of PG receptors EP1-4 (GW848687X (5 μM), AH6809, L-798106 (Sigma-Aldrich), and GW627368X (each 15 min,10 μM; Cayman Chemicals, Ann Arbor, MI, USA, unless otherwise indicated)); inhibitor of PKA (H89 (30 min, 20 μM; Sigma-Aldrich)); agonists of EP1-4 (17-phenyl trinor Prostaglandin E2 (PTPE2), butaprost, sulprostone, and L-902,688 (5 μM) (each 15 min, 10 μM; Cayman Chemicals, unless otherwise indicated)); activator of PKA (Adenosine 3ʹ,5ʹ-cyclic Monophosphate, N6-Benzoyl-, Sodium Salt (6-Bnz-cAMP) (30 min, 500 μM; Calbiochem EMD Millipore Corporation, Billerica, MA, USA)). Doses of pharmacologic agents were chosen based on prior specificity studies, and were shown to not alter the growth characteristics of tested cell lines. Then, cells were treated with or without fibrillar Aβ peptide (1–42) (fAβ42, 1 and 5 μM; GL Biochem) and/or prostaglandin-E2 (PGE2, 1, 5 and 10 μM; Cayman Chemicals) for 1, 3, or 6 h before they were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads, or to staining and protein extraction. To investigate the effect of endogenous expression of PGE2 on microglial phagocytosis, cells were treated with Escherichia coli LPS (0.1, 0.5 and 1 μg/ml; Sigma-Aldrich).
Cell viability
Cell Counting Kit-8 assay (CCK-8; Dojindo, Shanghai, China) was used to indirectly assess cell viability dependent of several proliferation-related elements including dehydrogenase, NAD(H), NADP(H), and mitochondrial activity. Briefly, cells were seeded at a density of 3 × 103 cells/well for 24 h or 5 × 103 cells/well for 3 and 6 h in the well of 96-well plates in IMDM medium at 37°C and then cultured using various concentrations of fAβ42 and LPS. At the end of the culture period, 10 μl of CCK-8 solution was added to each well of the culture plate. After a 2-h incubation at 37°C, absorbance at 450 nm was measured with a plate reader (BioTek Epoch, Winooski, VT, USA). A control was performed in parallel to monitor the influence of IMDM medium on the assays. Cell viability was expressed as a percentage of the control cell culture value using the following formula: Cell viability = (absorption of sample—absorption of background)/ (absorption of control—absorption of background) × 100%.
Phagocytosis assay
Phagocytosis was determined with latex beads (1 μm particular carboxylate-modified polystyrene, excitation/emission 470/540, Sigma-Aldrich), as previously described with slight modifications [10]. In brief, latex beads were diluted to 0.00125% with medium, and applied to N9 cells after sham or pharmacologic treatment in culture medium at 37°C for 1 h. After incubation, a washing step with cold serum-free medium was performed to interrupt any interaction between phagocytosing microglia and uningested beads. The phagocytic ability of N9 cells was evaluated using the fluorescence intensity of the engulfed beads on a flow cytometer and fluorescence microscopy. In the fluorescence-activated cell sorting (FACS) analysis, cells were collected and washed three times with ice-cold phosphate-buffered saline (PBS), and resuspended in 250 μl ice-cold PBS. The cell suspension was applied to an Accuri™ C6 flow cytometer (BD Biosciences, San Jose, CA). FACS analysis of 10,000 events (cells) was collected for each sample. In the microscopy assay, the number of ingested beads per cell and the number of phagocytosed cells were counted to determine the mean number of ingested beads per cell, in a blinded fashion with a minimum of 500 cells per slide. Five replicates were used for each experimental condition. The positive control was N9 cells treated with beads alone. Images of phagocytosing cells were captured using a LSM 780 confocal laser scanning microscope (Carl Zeiss GmbH, Jena, Germany).
Immunofluorescent staining
The cells were seeded on glass cover slips in the well of 24- well plates, and then washed three times with ice-cold PBS. Cells were then fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100 in 1% BSA-containing PBS for 15 min at room temperature. After being fixed and permeabilized, the cells were blocked with goat serum (Zhongshan Goldenbridge Biotechnology (ZsBio), Beijing, China) for 20 min at room temperature and washed three times in PBS. To visualize the morphologic features of cells, cell cultures were incubated with rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1, 1:200; Wako Pure Chemical Industries, Osaka, Japan) and a second antibody chicken anti-rabbit IgG-CF™ 633 (1:500; Sigma-Aldrich) for 1 h at 37°C in the dark. Cell cultures were then washed and mounted with aqueous-based anti-fade mounting medium. Fluorescence was detected with a LSM 780 confocal laser scanning microscope (Carl Zeiss GmbH, Jena, Germany).
Enzyme immunoassay (EIA) of PGE2 and cAMP
Cells were seeded in 6-well plates. After pharmacologic treatment of N9 cells in culture medium, cell culture supernatants were collected and PGE2 levels quantified by EIA according to the manufacturer's instructions (Cayman Chemicals). For cAMP experiments, culture supernatants were aspirated and the cells were washed three times with ice-cold PBS, and re-suspended in 100 μl ice-cold PBS. 10 μl of the cell collections were quantified using a cell counter (TC20, Bio-Rad, Hercules, CA, USA). Immediately thereafter, 10 μl of 1N HCl was added to the remainder of the 90 μl collections, incubated for 10 min at room temperature, and centrifuged at 600 x g for 10 min at 2–8°C to remove cellular debris. The sample was neutralized with 10 μl of 1N NaOH. Subsequently, each sample was diluted two-fold with Calibrator Diluent RD5-55 and intracellular cAMP levels were determined immediately to minimize degradation, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
Immunoblot analysis
Cells were washed with ice-cold PBS and scraped in RIPA lysis buffer containing protease and phosphatase inhibitors (Roche, Penzberg, Germany). Whole cell extracts (80 μg/lane) were separated by 10% SDS-polyacrylamide gel and then transferred onto PVDF membranes (Bio-Rad). The membranes were blocked in PBS with 5% non-fat milk for 1 h and then incubated with primary antibodies recognizing EP2 (1:200; Cayman Chemicals), EP4 (1:100; Cayman Chemicals), phospho-CREB (Ser133, 1:1000; Cell Signaling Technology, Danvers, MA, USA), and phospho-VASP (Ser157, 1:500; Cell Signaling Technology). Membranes were washed four times for 5 min each in tris-buffered saline Tween-20 (TBST) and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (ZsBio) for 1 h at room temperature. After the incubation, the membranes were reacted with enhanced chemiluminescence reagent (Bio-Rad) and the signal was detected using a ChemiDoc MP gel imaging system (Bio-Rad). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000; Cell Signaling Technology) was used as internal controls. Protein bands were semi-quantified by densitometric analysis using ImageJ 1.49.
Statistical Analysis
Statistical analysis was performed using SPSS software. Each experiment was repeated a minimum of three times and the data expressed as means ± SEM. The normality of data was verified by Kolmogorov-Smirnov before further analysis. Statistical differences between the groups were assessed by one or two-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was established at P < 0.05, unless otherwise indicated.
Results
Effect of LPS, PGE2, and fAβ42 on the viability of N9 cells
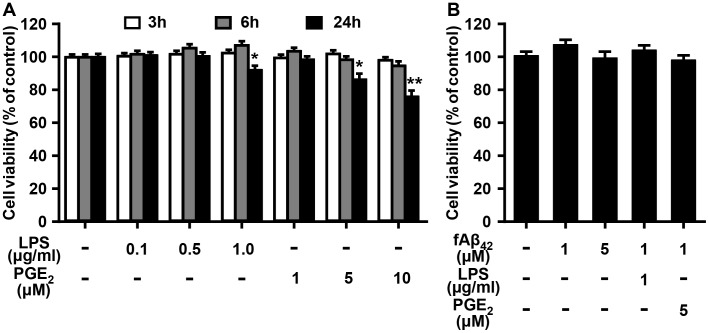
Cell viability of N9 cells was indirectly assessed in the dose-response and time-course stimulation of the LPS and PGE2 by proliferation-based CCK-8 assay. LPS and PGE2 were shown to be cytotoxic to N9 cells with a concentration of 1 μg/ml and 5 μM, respectively (Fig 1A). No significant changes in cell viability were determined at the 3 and 6 h points of LPS and PGE2 treatment. There were significant changes in cell survival when N9 cells were exposed to 1 μg/ml LPS and 5 or 10 μM PGE2 for 24 h. We then measured the effect of fAβ42 on N9 cell viability. Not surprisingly, fAβ42 in the concentration of 1–5 μM did not affect the viability of N9 cells for 6 h treatment (Fig 1B), which is in line with previous reports demonstrating that fAβ42 at the concentration of 1–5 μM will maintain the viability and enhanced microglial phagocytic function of microglial cells in vitro [10, 20]. Moreover, combined treatment of fAβ42 with LPS or PGE2 for 6 h also had no effect on cell viability of N9 cells (Fig 1B). These findings suggest that cell viability was not affected within 6 h treatment of LPS, PGE2, and fAβ42 treatment at the given concentration in microglial cells.
Fig 1. Effect of LPS, PGE2, and/or fAβ42 on cell viability in cultured N9 cells.
N9 cells were stimulated with LPS (0.1, 0.5, and 1 μg/ml) and PGE2 (1, 5, and 10 μM) in the presence or absence of fAβ42 (1 and 5 μM) for 3, 6, and 24 h. At the end of the incubation period, the CCK-8 assay was performed. (A) Dose response and time course effect of LPS and PGE2 on N9 cell viability. Statistical significance was determined by two-way ANOVA followed by Tukey’s test. (B) Dosage response and its combined effect with LPS or PGE2 for 6 h on N9 cell viability. Statistical significance was determined by One-way ANOVA followed by Tukey’s test. For (A) and (B) in each experimental condition, 5–8 replicate wells in a 96-well plate were tested. The results are expressed as % of the untreated control, and are presented as means ± standard error of the mean (SEM) of three independent experiments. *P <0.05, **P <0.01 vs. the untreated control group. con, control; LPS, lipopolysaccharide; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42).
The reduction effect of PGE2 on fAβ42-induced phagocytosis in N9 cells
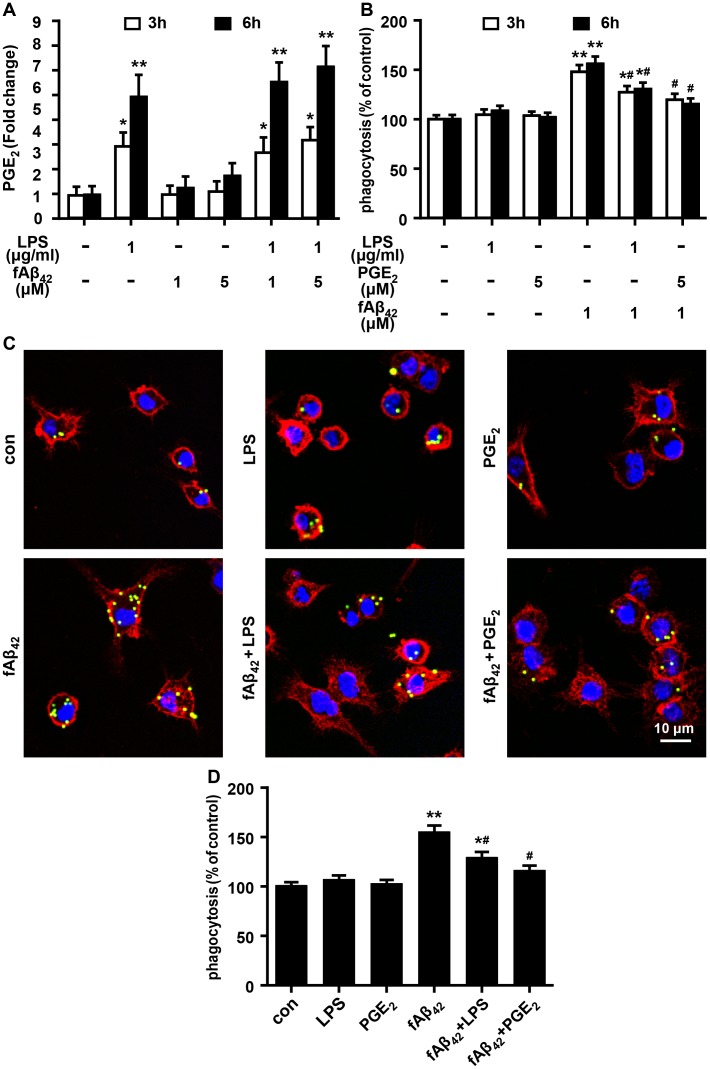
Given that Aβ42 fibrils are much less cytotoxic than oligomeric Aβ1–42 (oAβ42) in the induction of inflammation [20], we measured levels of PGE2 production in LPS and/or fAβ42-stimulated N9 cells, and found that fAβ42 treatment did not resulted in increase in PGE2 level, while LPS with or without fAβ42 treatment produced significantly higher levels of PGE2 (Fig 2A). These findings indicate the potential of LPS to mimic external stimuli to produce endogenous PGE2. To minimize the production of endogenous PGE2 induced by fAβ42, fAβ42 at a concentration of 1 μM was used in the following experiments of microglial phagocytosis, by using fluorescent-labeled latex beads to mimic the phagocytic model. As shown in Fig 2B, treatment with 1 μM fAβ42 for 3 and 6 h significantly increased phagocytosis of latex beads in N9 cells by FACS analysis. In addition, fAβ42-induced phagocytosis was dramatically reduced by exogenous PGE2 or LPS. Considering the rapid actions of PGE2 [21], LPS and PGE2 at the time point of 3 h served as the proper time point in the following experiment. Immunolocalization and confocal microscopy provided further evidence for a phagocytic response, showing a strong increase in phagocytic ability of fAβ42-stimulated N9 cells at 3 h (Fig 2C and 2D). Similarly, fAβ42-induced phagocytosis was reduced by exogenous PGE2 (5 μM) and LPS (1 μg/ml) at 3 h in N9 cells (Fig 2C and 2D). Moreover, cell cultures treated with PGE2, LPS or DMSO (S1 Fig) alone showed no significant changes in microglial phagocytosis in N9 cells cultured without fAβ42 treatment. Our observations confirm that PGE2 can act as a potential risk factor for impaired microglial phagocytosis.
Fig 2. Effect of LPS and PGE2 on fAβ42-induced phagocytosis in N9 cells.
N9 cells were stimulated with LPS (1 μg/ml) and PGE2 (5 μM) in the presence or absence of fAβ42 (1 and 5 μM) for 3 and 6 h. Then, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%) for the phagocytosis assay on a flow cytometer and fluorescence microscopy. (A) Enzyme immunoassay of PGE2 in N9 cells following the stimulation with LPS, fAβ42 (1 and 5 μM), individually and in combination. Experiments were performed with three replicates for each experimental condition. Data are presented relative to control and are presented as means ± SEM of five independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. (B) Average fluorescence intensity of latex beads ingested per group estimated using a flow cytometer. FACS analysis for each group were normalized compared as % of the untreated control. (C) Microscopy images of beads phagocytosis in N9 cells supplied with LPS and PGE2 in the presence or absence of fAβ42 (1 μM) for 3 h. Scale bar: 10 μm. (D) Quantification of phagocytosis. Phagocytic ratios were calculated from (C) as indicated in Methods. The results are normalized to the mean number of beads each cell engulfed (1 and 3 mean beads/cell were normalized as 100% and 150%, respectively). For (B) and (D), data are presented as means ± SEM of three independent experiments. Statistical significance was determined by two-way ANOVA followed by Tukey’s test. For (A), (B), and (D),*P < 0.05, **P <0.01 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; LPS, lipopolysaccharide; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42).
EP2 and EP4 Receptors is associated with phagocytic depression
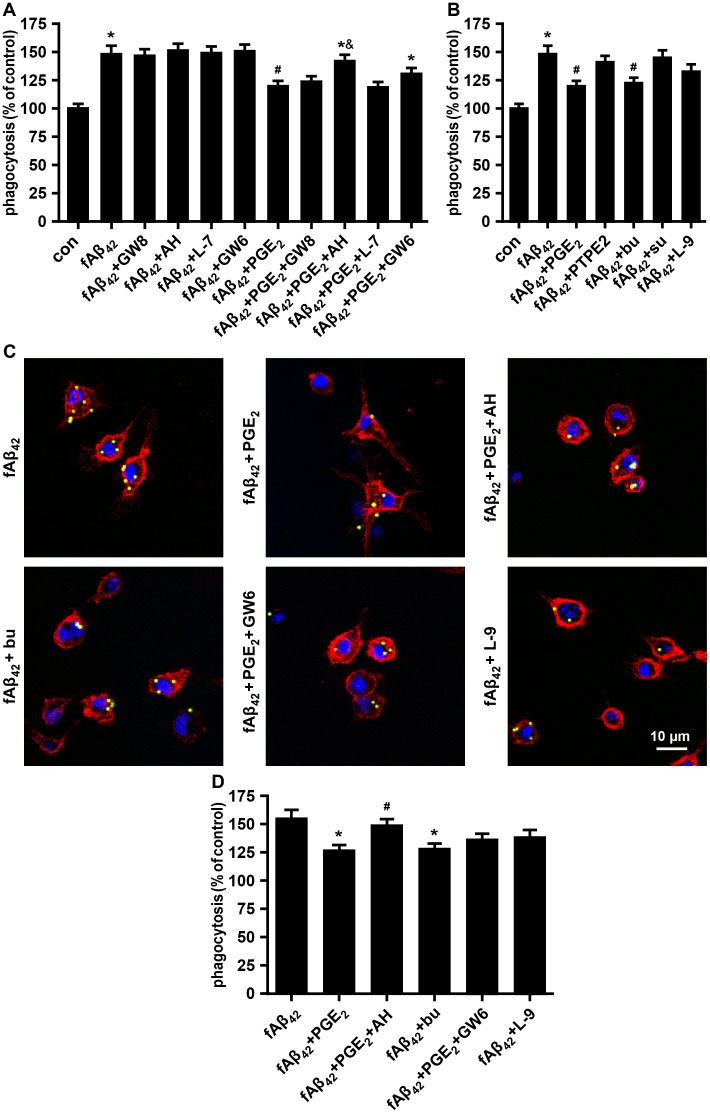
PGE2-related changes in functional microglial activation may be blocked by inhibiting EP receptors. Accordingly, we performed a phagocytosis assay after blocking these receptors with selective antagonists. The FACS analysis indicated that the reduction effect of 5 μM PGE2 on fAβ42-induced phagocytosis was dramatically prevented by 10 μM AH6809 (EP2 antagonist), and was slightly prevented by 10 μM GW627368X (EP4 antagonist) (Fig 3A). In contrast, 5 μM GW848687X (EP1 antagonist) and 10 μM L-798106 (EP3 antagonist) showed no reversal effect of the reduced phagocytosis by the action of PGE2 in fAβ42-stimulated N9 cells. To further confirm the subtypes of the E-prostanoid receptor involved, cells were pretreated with EP1-4 agonists. It was found that 10 μM butaprost (EP2 agonist) exposure decreased the capacity of fAβ42-stimulated N9 cells to ingest latex beads, while 5 μM L-902,688 (EP4 agonist) was effective but not quite as potent (Fig 3B). No alteration of phagocytosis in N9 cells was observed with pretreatment of 10 μM 17-phenyl trinor prostaglandin E2 (PTPE2, EP1 agonist) and sulprostone (EP3 agonist). Preliminary tests were performed at different concentrations of these agents to establish the optimal dose (S2 and S3 Figs). We also found a similar pattern of phagocytosis function in N9 cells with pretreatment of the antagonist and agonist for EP2 and EP4, respectively, in the microscopy assay. PGE2-induced impaired microglial phagocytosis in fAβ42-stimulated N9 cells was prevented by AH6809, but not by GW627368X (Fig 3B, 3C and 3D). Fig 3C and 3D also shows a more robust decrease phagocytosis of beads in N9 cells with the pretreatment of 10 μM butaprost than 5 μM L-902,688. Taken together, these results demonstrated that the reduction effect of PGE2 on fAβ42-induced phagocytosis appeared to be mainly mediated by the EP2 receptor rather than the EP4 receptor.
Fig 3. Effect of E-prostanoid receptors on fAβ42-induced phagocytosis with or without PGE2 in N9 cells.
N9 cells were pretreated with antagonists (A) or agonists (B) of PG receptors EP1-4. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. (A) N9 cells were pretreated with antagonists of PG receptors EP1-4 (GW848687X (5 μM), AH6809 (10 μM), L-798106 (10 μM), and GW627368X (10 μM) (each 15 min)). (B) N9 cells were pretreated with agonists of EP1-4 (10 μM PTPE2, 10 μM butaprost, 10 μM sulprostone, and 5 μM L-902,688; 15 min each). (C) Microscopy images of beads phagocytosis in N9 cells supplied with antagonists or agonists of EP2 and EP4 for 15 min prior to fAβ42 (1 μM) in the presence or absence of PGE2 (5 μM). Scale bar: 10 μm. (D) Quantification of phagocytosis. Phagocytic ratios were calculated from (C) as indicated in Methods. For (A) and (B), the results are expressed as % of the untreated control. For (D), the results are normalized to the mean number of beads each cell engulfed (1 and 3 mean beads/cell were normalized as 100% and 150%, respectively). Data are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. For (A) and (B), *P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group; &P < 0.05 vs the fAβ42 plused PGE2-stimulated group. For (D), *P < 0.05 vs the fAβ42-stimulated group; #P < 0.05 vs the fAβ42 plused PGE2-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); GW8, GW848687X; AH, AH6809; L-7, L-798106; GW6, GW627368X; PTPE2, 17-phenyl trinor Prostaglandin E2; bu, butaprost; su, sulprostone; L-9, L-902,688.
Curcumin ameliorates EP2-dependent phagocytic depression
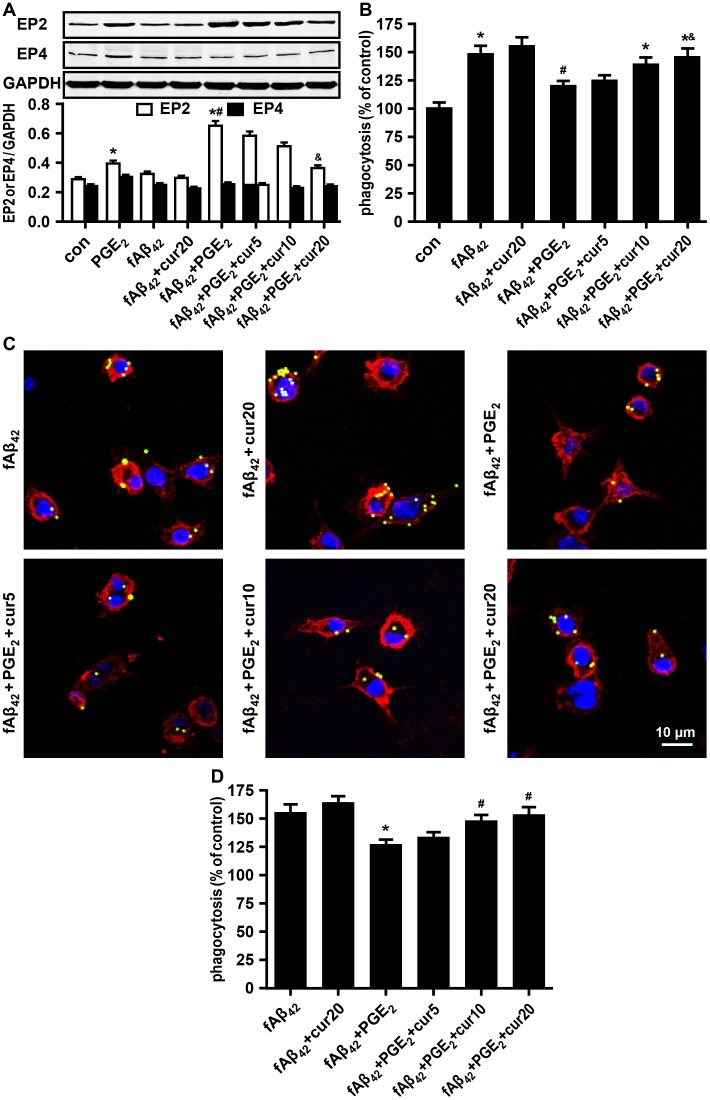
The data above demonstrate that PGE2 inhibits phagocytosis in partial enhanced EP receptors in fAβ42-stimulated N9 cells, while EP2-dependent signaling activation is sufficient to suppress phagocytosis. We found that PGE2 significant increase the expression of EP2. Corresponding to no change of levels of PGE2 production in fAβ42 and/or curcumin-treated N9 cells (Fig 2A and S4 Fig), expression of EP2 and EP4 was not changed in fAβ42-stimulated N9 cells at 3 h (Fig 4A). A decreasing trend of fold change was found for EP2 expression by curcumin preconditioning in PGE2 and fAβ42 co-treated N9 cells (Fig 4A). Immunoblotting showed no change in EP4 expression in each group. We also investigated the effect of curcumin on improving microglial phagocytic function. Clearly, curcumin pretreatment concomitantly improves microglial phagocytosis in a dose-dependent manner against the reduction effect of PGE2 on fAβ42-induced phagocytosis in N9 cells (Fig 4B). Confocal microscopy provided further evidence for the amelioration of phagocytosis, showing a dose-dependent restoration of phagocytic ability by curcumin in PGE2 and fAβ42 co-treated N9 cells (Fig 4C and 4D). However, curcumin alone did not enhance microglial phagocytosis induced by fAβ42 (Fig 4B, 4C and 4D), or decrease EP2 expression in the control group (Fig 4A). These results suggest that EP2 signaling might be involved in the curcumin-mediated improvement of inhibition induced by PGE2 on fAβ42-stimulated microglial phagocytosis.
Fig 4. Effect of curcumin on fAβ42-induced phagocytosis with or without PGE2 in N9 cells.
N9 cells were pretreated with curcumin (5, 10, and 20 μM) for 30 min prior to fAβ42 (1 μM) treatment in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Then, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). (A) Western blot quantification of EP2 and EP4. Curcumin significantly depressed the expression of EP2 in a dose-dependent manner 3 h after treatment with fAβ42 (1 μM) in the presence or absence of PGE2 (5 μM). (B) Curcumin concomitantly improves microglial phagocytosis in a dose-dependent manner 3 h after fAβ42 (1 μM) treatment in the presence or absence of PGE2 (5 μM). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. (C) Beads phagocytosis of N9 cells was restored by curcumin pre-conditioning for the phagocytosis assay by fluorescence microscopy. Scale bar: 10 μm. (D) Quantification of phagocytosis. Phagocytic ratios were calculated from (C) as indicated in Methods. For (B), the results are expressed as % of the untreated control. For (D), the results are normalized to the mean number of beads each cell engulfed (1 and 3 mean beads/cell were normalized as 100% and 150%, respectively). All data are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. For (A) and (B), *P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. &P < 0.05 vs the fAβ42 plused PGE2-stimulated group. For (D), *P < 0.05 vs the fAβ42-stimulated group; #P < 0.05 vs the fAβ42 plused PGE2-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); cur, curcumin.
Curcumin ameliorates phagocytosis by inhibition of cAMP increase and PKA activation
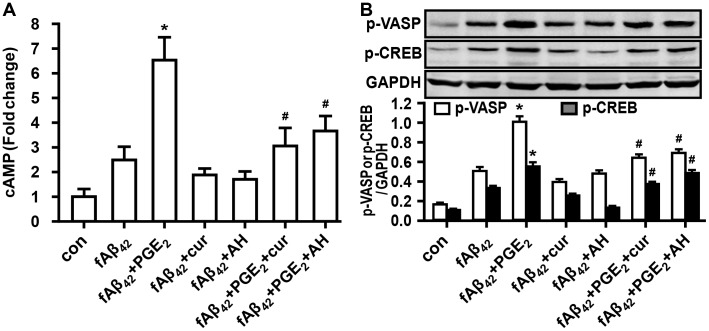
It is well known that both EP2 and EP4 receptors stimulate an increase in cAMP in various immune cells. Our results demonstrated that PGE2 caused a significant enhancement of increased levels of intracellular cAMP, which was blocked by pretreatment of 10 μM AH 6809 and 20 μM curcumin in fAβ42-stimulated N9 cells (Fig 5A). Meanwhile, cells pretreated with AH 6809 or curcumin alone showed a low level of cAMP in fAβ42-stimulated cell groups (Fig 5A), showing similar none affected pattern of fAβ42-induced phagocytosis with the pretreatment of AH 6809 or curcumin alone in the aforementioned description (Figs 3A, 4B and 4C). Given the above results, we focused our studies on the binding protein of cAMP, i.e., the PKA effector. Because the phosphorylation of vasodilator-stimulated phosphoprotein (VASP) is a typical surrogate of PKA activity [22, 23], we assessed the phosphorylation of VASP in the restoration of PGE2 impaired phagocytiosis of N9 cells incubated in curcumin. We also investigated the downstream phosphorylation of the transcription factor CREB for PKA. The immunoblot analysis revealed that the VASP phosphorylation and downstream CREB signaling were increased in fAβ42-stimulated N9 cells with PGE2 preconditioning. The addition of curcumin modified the expression of p-VASP and p-CREB in the inhibitory effect induced by PGE2 on fAβ42-stimulated N9 cells (Fig 5B).
Fig 5. Involvement of cAMP-PKA activity in the restoration of PGE2 impaired phagocytiosis of N9 cells incubated in curcumin.
N9 cells were pretreated with curcumin (20 μM) for 30 min or AH6809 (10 μM) for 15 min prior to fAβ42 (1 μM) treatment in the presence or absence of exogenous PGE2 (5 μM) for 3 h. (A) Cells were lysed and increase of intracellular cAMP was measured by enzyme immunoassay. Experiments were performed with three replicates for each experimental condition. Data are presented relative to control and are presented as means ± SEM of five independent experiments. (B) Cells were lysed and immunoblot analysis was performed for p-VASP, a marker of PKA activation, and p-CREB, which is a selectively activated downstream signaling factor of PKA. Data are presented as means ± SEM of three independent experiments. For (A) and (B), statistical significance was determined by one-way ANOVA followed by Tukey’s test. *P < 0.05 vs the fAβ42-stimulated group; #P < 0.05 vs the fAβ42 plused PGE2-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); cur, curcumin; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; p-VASP, vasodilator-stimulated phosphoprotein; CREB, cyclic AMP responsive element-binding protein.
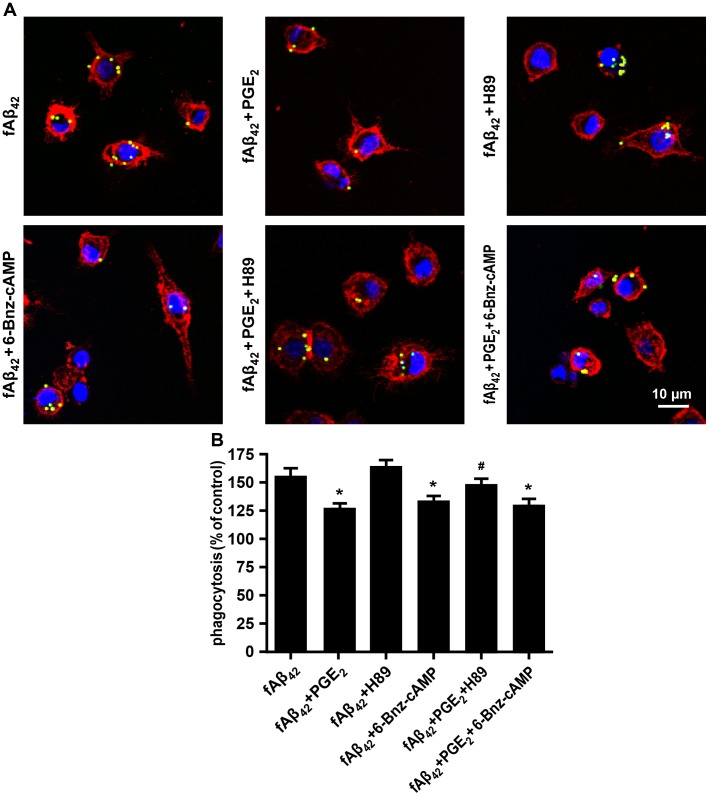
To further test whether PKA activation mediated the actions of cAMP on the inhibitory effect of PGE2 in fAβ42-stimulated N9 cells, various concentrations of H89 (PKA inhibitor) and adenosine 3ʹ,5ʹ-cyclic monophosphate, N6-Benzoyl-, sodium salt (6-Bnz-cAMP) (PKA activator) were used. Pharmacologic studies showed that 20 μM H89 abolished the inhibitory effect of PGE2 on fAβ42-stimulated microglial phagocytosis, while 500 μM 6-Bnz-cAMP significant reduced fAβ42-induced phagocytosis of latex beads in N9 cells. (Fig 6A, 6B and S5 Fig), suggesting the partial involvement of PKA activity in PGE2-induced impaired phagocytosis via EP2. In contrast, H89 alone did not affect microglial phagocytosis induced by fAβ42. Taken together, these results indicate that curcumin may restore the inhibitory effect of PGE2 on Aβ42-induced microglial phagocytosis via a signaling mechanism involving EP2 and PKA.
Fig 6. PGE2-mediated inhibition of phagocytos in fAβ42-stimulated N9 cells is PKA dependent.
N9 cells were pretreated with PKA inhibitor H89 (20 μM) or PKA activator 6-Bnz-cAMP (500 μM) for 30 min. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). (A) Microscopy images of bead phagocytosis in N9 cells were captured using a confocal laser scanning microscope. Scale bar: 10 μm. (B) Quantification of phagocytosis. Phagocytic ratios were calculated from (A) as indicated in Methods. The results are normalized to the mean number of beads each cell engulfed (1 and 3 mean beads/cell were normalized as 100% and 150%, respectively). Data are presented as means ± SEM of three independent experiments. Statistical significance was determined by two-way ANOVA followed by Tukey’s test. *P < 0.05 vs the fAβ42-stimulated group; #P < 0.05 vs the fAβ42 plused PGE2-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); cur, curcumin; PKA, protein kinase A; 6-Bnz-cAMP, Adenosine 3ʹ,5ʹ-cyclic Monophosphate, N6-Benzoyl-, Sodium Salt.
Discussion
In the present study, we observed the immunoregulation of microglial phagocytic ability by the inflammatory mediator PGE2 in fAβ42-stimulated N9 cells. We found a significant decrease of fAβ42-activated microglial phagocytosis of fluorescent-labeled latex beads by exogenous PGE2 or LPS-induced endogenous PGE2. Our data is in agreement with the classic story that impaired phagocytosis and increased expression of PGE2 occurs in the brains of AD patients, suggesting a failure in the phagocytic clearance ability in a PGE2-related chronic inflammatory environment. Hence, the pro-inflammatory PGE2 appears to have a nonredundant immunosuppressive effect of microglial phagocytosis in fAβ42-stimulated N9 cells. Among EP receptors, EP2 was shown to have the highest inhibition in microglial phagocytosis, exhibiting functional activity in response to PGE2. Furthermore, incubation of cells with curcumin, a potent immunomodulating therapeutic natural compound in AD, prevents PGE2 from suppressing fAβ42-elicited phagocytosis, through the inhibition of EP2 activity or its downstream cAMP-PKA-CREB signaling.
It appears that early in the AD process, microglia play a neuroprotective role by promoting Aβ phagocytosis, degradation, and clearance [24]. However, with disease progression, microglia transform to a pro-inflammatory phenotype, lose their ability to clear Aβ, and produce pro-inflammatory cytokines which in turn reduce Aβ uptake and degradation [25]. These distinct effects may be explained by molecular phenotypes and effector functions, depending on the induced signaling pathway of microglial response. Recent studies increasing evidence for the microglial pro-inflammatory responses in the development of AD. It has been reported that the inflammatory and oxidative milieu (LPS or tert-butyl hydroperoxide) significantly downregulates fAβ42-induced scavenger receptors in microglial cells [20]. Pro-inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) have been shown to inhibit fAβ-stimulated phagocytosis of fluorescent microspheres in BV-2 microglial cells [3]. However, the exact cytokines act selectively to regulate microglial phagocytosis in AD brain are not fully understood yet. The prominent pro-inflammatory responses in the AD brain are the rapid induction of COX-2 and prostaglandins [5, 26]. In particular, PGE2 is of most interest in the development of AD, as it is initially significantly elevated in patients with very early stage or probable AD [27]. Recently, Johansson et al. indicated that microglial chemotaxis and Aβ clearance were restored by a knockout microglia-specific PGE2 receptor [28]. In the present study, sequential treatment was designed to mimic and investigate the roles of mature Aβ-fibrils and PGE2-related inflammation in the AD brain. We validated the fact that treatment with 1 μM fAβ42 for 3 and 6 h significantly increased phagocytosis of latex beads in N9 cells, while increased phagocytosis was dramatically reduced by exogenous PGE2 or endogenous PGE2 induced by LPS. Combined with previous epidemiologic evidence demonstrating that long-term nonsteroidal anti-inflammatory drugs have beneficial effects on reducing AD risk [29, 30], we speculate that pharmacologic agents targeting the anti-inflammatory and pro-phagocytic activities of microglia may be helpful for preventing the inhibitory effect of PGE2 on Aβ42-induced phagocytosis, which is thus beneficial for suppressing AD development.
Prior work has suggested that PGE2, a pivotal immunosignaling agent in models of AD, can exert both detrimental and beneficial effects depending on the function of the EP receptors. EP1 receptor has been reported to aggravate neurotoxicity in AD and cerebral ischemia [31]. EP2 signaling is associated with pro-inflammatory gene upregulation, and the inhibition of beneficial chemokine production and Aβ clearance underlying aging and/or Aβ42 accumulation [28]. EP3 might be a pro-inflammatory signal to generate increased Aβ peptides [32]. In contrast, the EP4 receptor has been shown to suppress toxic inflammatory responses to Aβ42 and potentiate phagocytosis of Aβ42 [33]. Although EP receptors were abundantly expressed in glial cells and could play some role in neuronal interaction, it is important to note the unique function of microglial cells linked to their toxic inflammatory responses and potentiation of phagocytic effects. In this study, the agonists and antagonists of EP1-4 were used to confirm which subtype of the E-prostaniod receptor involved in the regulation of microglial phagocytosis. Our results demonstrated that the reduction effect of PGE2 on fAβ42-induced phagocytosis appeared to be mainly mediated by the EP2 and EP4 receptor rather than the EP1 and EP3 receptor. Moreover, an increase in cAMP levels were observed in N9 cells incubated with the selective EP2 or EP4 agonists butaprost or L-902,688, respectively, indicating the activation of cAMP by the activity of EP2 and EP4 receptors. These results suggest the participation of EP2 and/or EP4 receptors in fAβ42-stimulated N9 cells with exogenously supplied PGE2. In an effort to reveal the essential role of microglial EP receptors in the action of PGE2, we used EP antagonists and agonists to explore whether EP2 or EP4 mediate the reduction effect of PGE2 action in N9 cells. We found that PGE2-induced impairement of microglial phagocytosis by fAβ42-stimulated N9 cells was prevented by the EP2 antagonist AH6809, but not by the EP4 antagonist GW627368X. Moreover, the EIA assay showed significant abolishment of cAMP levels by the EP2 specific antagonist AH6809, suggesting a potent role of EP2 in mediating PGE2 action in N9 cells. Otherwise, a more robust decrease phagocytosis of beads was observed in N9 cells with the pretreatment of EP2 agonist butaprost than the EP4 agonist L-902,688. The immunoblot assay identified a significant increase in EP2 levels in cells treated with both fAβ42 and PGE2, as compared to those left untreated. However, EP4 expression was unaffected in all groups. Indeed, several lines of evidence point to microglial EP2 activity as a potentially inhibitor of microglial clearance of Aβ and plaques using receptor-knockout animals [7, 9, 34]. Thus, pharmacologic intervention targeting microglial EP2 signaling may provide critical aid to improve Aβ-related microglial phagocytosis in AD brain.
Epidemiologic studies have suggested that diets rich in phenolic compounds may have preventive effects on the development of dementia or AD. Previous animal studies of curcumin, a phenolic yellow curry pigment, has shown its neuroprotective effects in neurodegenerative conditions such as AD [35, 36]. In vivo studies also showed that curcumin could prevent Aβ-infusion induced spatial memory deficits, as well as reduced Aβ deposits in Sprague-Dawley rats [37]. Furthermore, transgenic mouse model studies demonstrated that curcumin seems to reduce amyloid deposits in a manner that promotes disaggregation of existing Aβ deposits and prevents aggregation of those newly formed [38–40]. In our previous study, curcumin was proven to ameliorate the defective phagocytic ability of microglia in a pro-inflammatory condition [15]. In support of these, curcumin has been found to prevent the LPS-mediated induction of cyclooxygenase-2 against pro-inflammatory response [41, 42]. Although curcumin is widely accepted as a potent modulator of microglial gene expression involving various adhesion molecules, chemokine, and pro-inflammatory cytokines (including PGE2), less gene that associated with the direct regulation of phagocytosis was detected [43]. However, whether a direct association exists between the restoration of microglial phagocytosis and the inhibition of PGE2-induced EP receptor subtype activity by curcumin pretreatment remains poorly understood. Here, we examined the phagocytic ability of PGE2 and fAβ42-stimulated N9 cells with curcumin pre-conditioning. Considered the potential role of PGE2-EP2 signaling in AD [7, 9, 34] and the immunomodulator role of curcumin in microglial pro-inflammatory and phagocytic responses [14–15, 36–37, 39], we tested whether curcumin regulate the PGE2-mediated depression of microglial phagocytosis through PGE2-EP2 signaling in mimicked neuropathologic feature of AD with a hallmark of amount production of fAβ42 and PGE2 [5–8, 27]. Our results revealed that curcumin alone did not change the phagocytic ability in the fAβ42-stimulated N9 cells without the co-treatment of inflammatory mediator PGE2. When PGE2 plus fAβ42-mediated stimulation of microglial phagocytosis, curcumin restored the attenuated phagocytic effect by PGE2. These results indicate that curcumin itself cannot improve microglial phagocytosis in our experiment condition, it just work when the microglial phagocytosis impaired with a PGE2-related pro-inflammatory response. In support of this, fAβ42 is accepted as a weak agonist of inflammatory responses [20], no change of PGE2 was observed in the fAβ42-stimulated N9 cells. Our results also showed that curcumin effectively reverses the inhibitory effect of PGE2 on fAβ42-induced phagocytosis, with a parallel increase in EP2 expression. Moreover, our results showed no detectable change of EP4 expression for each condition, including the curcumin-treated group. Thus, these results suggest that PGE2-EP2 signaling pathway in microglia might be a pharmacologic target of curcumin for the treatment of AD.
Although the activation of the EP2 receptor by PGE2 appears to be correlated to amyloid burden in AD [5–8, 27], the signaling pathways modulated by curcumin that contribute to its efficacy in the promotion of phagocytosis against AD remain unknown. A recent study suggested that upregulation of DOCK2, an upstream regulator of EP2 receptor, contributes to Aβ plaque burden [8]. However, the downstream targets of microglial EP remain elusive. Theoretically, a pivotal role of EP receptor-stimulated, cAMP-dependent effects of PGE2 can be explained by the activation of PKA and CREB in a variety of mammalian cells [44]. Moreover, microglial EP2-PKA signaling was found to be critical to establish the pharmacologic function of an active nature component triptolide in the LPS-induced microglial inflammatory responses [45]. In the present study, we delineated the strong suppression of the activation of cAMP, PKA, and CREB by curcumin against the impaired phagocytosis induced by PGE2 in fAβ42-stimulated N9 cells. Furthermore, our data also showed that pharmacological inhibitor of PKA, H89, restored the phagocytic functions following PGE2 action in fAβ42-stimulated N9 cells. Our results combined with previous studies, suggest, in partial, the involvement of EP2-PKA effects on the role of curcumin in the restoration of impaired phagocytosis in fAβ42-stimulated N9 cells with PGE2.
Although our findings indicated that curcumin ameliorated defective microglial phagocytosis via the inhibition of the PGE2-EP2-PKA pathway in N9 cells, it is important to note some limitations of the current models we tested. The immortalized murine microglial cell line N9, elicited a profound pro-phagocytic effect in response to acute stimulation with fibrillar Aβ42. Notably, in vivo, chronic neuroinflammation and the building blocks of a dynamic and complex network of intermediates such as oligomers, fibrils, and plaques are involved in the pathogenic cascade of AD. Herein, just the fibril form of Aβ was occupied to mimic the late stage of AD. This discrepancy is mainly due to the differences between acute in vitro responses of curcumin to exogenous PGE2 and Aβ42 treatment in an immortalized microglial population, and a chronic in vivo response in the whole cerebral cortex and hippocampus to long-term complex interaction between endogenous PGE2 and Aβ42 for several years. Moreover, various inflammatory mediators exist in the AD brain and, besides PGE2, most are likely involved in the regulation of the capacity of microglial phagocytosis either directly or indirectly [3]. In this study we only focused on the pharmacological intervention of PGE2 by curcumin in N9 cells following acute fibrillar Aβ42 stimulation. Hence, despite the pharmacologic outcome of curcumin in our study, the basis for the effects of curcumin over various time course and the involvement of different cytokines in the AD brain remains to be solved. Further experiments are warranted to explore the detailed preventive mechanisms of curcumin underlying neurologic disorders.
In summary, we demonstrated that curcumin restored the inhibitory effect of PGE2 on phagocytosis of Aβ42-stimulated N9 microglial cells. Moreover, curcumin ameliorated phagocytic abilities via inhibition of the PGE2-EP2 system with partial involvement in a cAMP-PKA-dependent manner. Further studies targeting the precise downstream signaling of the EP2 receptor hold promise to reveal the therapeutic mechanisms of curcumin for AD and other neurodegenerative diseases.
Supporting Information
N9 cells were stimulated with DMSO (0.1 and 0.2%) for 3 and 6 h. Then, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%) for the phagocytosis assay on a flow cytometer. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. con, control; DMSO, dimethylsulfoxide.
(TIF)
N9 cells were pretreated with dosage of antagonists of PG receptors EP1-4. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); GW8, GW848687X; AH, AH6809; L-7, L-798106; GW6, GW627368X.
(TIF)
N9 cells were pretreated with dosage of agonists of PG receptors EP1-4. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); PTPE2, 17-phenyl trinor Prostaglandin E2; bu, butaprost; su, sulprostone; L-9, L-902,688.
(TIF)
N9 cells were pretreated with or without curcumin (10 μM) for 30 min prior to fAβ42 (1 μM) treatment for 3 h. Enzyme immunoassay of PGE2 was performed as described in Methods. Experiments were performed with three replicates for each experimental condition. Data are presented relative to control and are presented as means ± SEM of five independent experiments. Statistical significance was determined by two-way ANOVA followed by Tukey’s test. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); Cur, curcumin.
(TIF)
N9 cells were pretreated with dosage of PKA inhibitor H89 or PKA activator 6-Bnz-cAMP for 30 min. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); 6-Bnz-cAMP, Adenosine 3ʹ,5ʹ-cyclic Monophosphate, N6-Benzoyl-, Sodium Salt.
(TIF)
Acknowledgments
The authors are grateful to Dr. Yun Bai for the N9 cell line. The authors would like to thank Haiying Ran and Yang Liu for their skillful technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the National Natural Science Foundation of China (81172647). The funder's website is http://www.nsfc.gov.cn/. The corresponding author XY received the funding for this work. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yankner BA, Mesulam MM. Seminars in medicine of the Beth Israel Hospital, Boston. beta-Amyloid and the pathogenesis of Alzheimer's disease. N Engl J Med. 1991; 325: 1849–1857. 10.1056/NEJM199112263252605 [DOI] [PubMed] [Google Scholar]
- 2.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010; 330: 1774 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005; 25: 8240–8249. 10.1523/JNEUROSCI.1808-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock AA, Ilkjaer L, Clausen BH, Villadsen B, Dissing-Olesen L, Bendixen AT, et al. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav Immun. 2015; 48: 86–101. 10.1016/j.bbi.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Xiang Z, Ho L, Yemul S, Zhao Z, Qing W, Pompl P, et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 2002; 10: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Guan PP, Wang T, Yu X, Guo JJ, Wang ZY. Aggravation of Alzheimer's disease due to the COX-2-mediated reciprocal regulation of IL-1beta and Abeta between glial and neuron cells. Aging Cell. 2014; 13: 605–615. 10.1111/acel.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J Neurosci. 2005; 25: 10180–10187. 10.1523/JNEUROSCI.3591-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimino PJ, Yang Y, Li X, Hemingway JF, Cherne MK, Khademi SB, et al. Ablation of the microglial protein DOCK2 reduces amyloid burden in a mouse model of Alzheimer's disease. Exp Mol Pathol. 2013; 94: 366–371. 10.1016/j.yexmp.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005; 166: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagano T, Kimura SH, Takemura M. Prostaglandin E2 reduces amyloid beta-induced phagocytosis in cultured rat microglia. Brain Res. 2010; 1323: 11–17. 10.1016/j.brainres.2010.01.086 [DOI] [PubMed] [Google Scholar]
- 11.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991; 57: 1–7. 10.1055/s-2006-960004 [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006; 78: 2081–2087. 10.1016/j.lfs.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 13.Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and antioxidant prevention of Alzheimer's disease: lessons from in vitro and animal models. Ann N Y Acad Sci. 2004; 1035: 68–84. 10.1196/annals.1332.005 [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Fiala M, Cashman J, Sayre J, Espinosa A, Mahanian M, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2006; 10: 1–7. [DOI] [PubMed] [Google Scholar]
- 15.He GL, Liu Y, Li M, Chen CH, Gao P, Yu ZP, et al. The amelioration of phagocytic ability in microglial cells by curcumin through the inhibition of EMF-induced pro-inflammatory responses. J Neuroinflammation. 2014; 11: 49 10.1186/1742-2094-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith ME, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998; 54: 68–78. [DOI] [PubMed] [Google Scholar]
- 17.Jin CY, Lee JD, Park C, Choi YH, Kim GY. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin. 2007; 28(10):1645–51. 10.1111/j.1745-7254.2007.00651.x [DOI] [PubMed] [Google Scholar]
- 18.Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, et al. Monokine production by microglial cell clones. Eur J Immunol. 1989; 19: 1443–1448. 10.1002/eji.1830190815 [DOI] [PubMed] [Google Scholar]
- 19.Corradin SB, Mauel J, Donini SD, Quattrocchi E, Ricciardi-Castagnoli P. Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia. 1993; 7: 255–262. 10.1002/glia.440070309 [DOI] [PubMed] [Google Scholar]
- 20.Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, et al. Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: implications for Alzheimer's disease. Mol Neurodegener. 2011; 6: 45 10.1186/1750-1326-6-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima F, Kapoor M, Kawai S, Yang L, Aronoff DM, Crofford LJ. Prostaglandin E2 activates Rap1 via EP2/EP4 receptors and cAMP-signaling in rheumatoid synovial fibroblasts: involvement of Epac1 and PKA. Prostaglandins Other Lipid Mediat. 2009; 89: 26–33. 10.1016/j.prostaglandins.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002; 16: 583–585. [DOI] [PubMed] [Google Scholar]
- 23.Benz PM, Feller SM, Sickmann A, Walter U, Renne T. Prostaglandin-induced VASP phosphorylation controls alpha II-spectrin breakdown in apoptotic cells. Int Immunopharmacol. 2008; 8: 319–324. 10.1016/j.intimp.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 24.El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer's disease: therapeutic implications. Trends Pharmacol Sci. 2008; 29: 626–632. 10.1016/j.tips.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 25.Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008; 28: 11650–11661. 10.1523/JNEUROSCI.3024-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000; 21: 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Combrinck M, Williams J, De Berardinis MA, Warden D, Puopolo M, Smith AD, et al. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006; 77: 85–88. 10.1136/jnnp.2005.063131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, et al. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer's disease models. J Clin Invest. 2015; 125: 350–364. 10.1172/JCI77487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996; 47: 425–432. [DOI] [PubMed] [Google Scholar]
- 30.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997; 48: 626–632. [DOI] [PubMed] [Google Scholar]
- 31.Zhen G, Kim YT, Li RC, Yocum J, Kapoor N, Langer J, et al. PGE2 EP1 receptor exacerbated neurotoxicity in a mouse model of cerebral ischemia and Alzheimer's disease. Neurobiol Aging. 2012; 33: 2215–2219. 10.1016/j.neurobiolaging.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Wang Q, Johansson JU, Liang X, Woodling NS, Priyam P, et al. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol. 2012; 72: 788–798. 10.1002/ana.23677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodling NS, Wang Q, Priyam PG, Larkin P, Shi J, Johansson JU, et al. Suppression of Alzheimer-associated inflammation by microglial prostaglandin-E2 EP4 receptor signaling. J Neurosci. 2014; 34: 5882–5894. 10.1523/JNEUROSCI.0410-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keene CD, Chang RC, Lopez-Yglesias AH, Shalloway BR, Sokal I, Li X, et al. Suppressed accumulation of cerebral amyloid {beta} peptides in aged transgenic Alzheimer's disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol. 2010; 177: 346–354. 10.2353/ajpath.2010.090840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001; 21: 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008; 326: 196–208. 10.1124/jpet.108.137455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001; 22: 993–1005. [DOI] [PubMed] [Google Scholar]
- 38.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005; 280: 5892–5901. 10.1074/jbc.M404751200 [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007; 102: 1095–1104. 10.1111/j.1471-4159.2007.04613.x [DOI] [PubMed] [Google Scholar]
- 40.Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M. Natural Phenolic Compounds as Therapeutic and Preventive Agents for Cerebral Amyloidosis. Adv Exp Med Biol. 2015; 863: 79–94. 10.1007/978-3-319-18365-7_4 [DOI] [PubMed] [Google Scholar]
- 41.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003; 171:6072–6079. [DOI] [PubMed] [Google Scholar]
- 42.Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, et al. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappaB bindings in BV2 microglial cells. J Pharmacol Sci. 2004; 94:325–328. 10.1254/jphs.94.325 [DOI] [PubMed] [Google Scholar]
- 43.Karlstetter M, Lippe E, Walczak Y, Moehle C, Aslanidis A, Mirza M, et al. Curcumin is a potent modulator of microglial gene expression and migration. J Neuroinflammation. 2011; 8:125 10.1186/1742-2094-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003; 74: 143–153 [DOI] [PubMed] [Google Scholar]
- 45.Zhang T, Gong X, Hu G, Wang X. EP2-PKA signaling is suppressed by triptolide in lipopolysaccharide-induced microglia activation. J Neuroinflammation. 2015; 12: 50 10.1186/s12974-015-0275-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N9 cells were stimulated with DMSO (0.1 and 0.2%) for 3 and 6 h. Then, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%) for the phagocytosis assay on a flow cytometer. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. con, control; DMSO, dimethylsulfoxide.
(TIF)
N9 cells were pretreated with dosage of antagonists of PG receptors EP1-4. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); GW8, GW848687X; AH, AH6809; L-7, L-798106; GW6, GW627368X.
(TIF)
N9 cells were pretreated with dosage of agonists of PG receptors EP1-4. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). Average fluorescence intensity of latex beads ingested and normalized phagocytosis analysis were estimated for each group using FACS analysis. The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); PTPE2, 17-phenyl trinor Prostaglandin E2; bu, butaprost; su, sulprostone; L-9, L-902,688.
(TIF)
N9 cells were pretreated with or without curcumin (10 μM) for 30 min prior to fAβ42 (1 μM) treatment for 3 h. Enzyme immunoassay of PGE2 was performed as described in Methods. Experiments were performed with three replicates for each experimental condition. Data are presented relative to control and are presented as means ± SEM of five independent experiments. Statistical significance was determined by two-way ANOVA followed by Tukey’s test. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); Cur, curcumin.
(TIF)
N9 cells were pretreated with dosage of PKA inhibitor H89 or PKA activator 6-Bnz-cAMP for 30 min. Then, cells were stimulated with fAβ42 (1 μM) in the presence or absence of exogenous PGE2 (5 μM) for 3 h. Subsequently, cells were subjected to a 1 h process of phagocytosis of fluorescent-labeled latex beads (0.00125%). The results are expressed as % of the untreated control, and are presented as means ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.*P < 0.05 vs the untreated control group; #P < 0.05 vs the fAβ42-stimulated group. con, control; PGE2, prostaglandin E2; fAβ42, fibrillar Aβ peptide (1–42); 6-Bnz-cAMP, Adenosine 3ʹ,5ʹ-cyclic Monophosphate, N6-Benzoyl-, Sodium Salt.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.