Abstract
Objective
To describe self-reported menopausal symptom priorities and their association with demographics and other symptoms among participants in an intervention trial for vasomotor symptoms (VMS).
Methods
Cross-sectional study embedded in the MsFLASH 02 trial, a three by two factorial design of yoga vs. exercise vs. usual activity and omega-3-fatty acid vs. placebo. At baseline, women (n=354) completed hot flash diaries, a card sort task to prioritize symptoms they would most like to alleviate, and standardized questionnaires.
Results
The most common symptom priorities were: VMS (n=322), sleep (n=191), concentration (n=140), and fatigue (n=116). In multivariate models, (1) women who chose VMS as their top priority symptom (n=210) reported significantly greater VMS severity (p=0.004) and never smoking (p=0.012) and (2) women who chose sleep as their top priority symptom (n=100), were more educated (p≤0.001) and had worse sleep quality (p<0.001). ROC curves identified sleep scale scores that were highly predictive of ranking sleep as a top priority symptom.
Conclusions
Among women entering an intervention trial for VMS and with relatively low prevalence of depression and anxiety, VMS was the priority symptom for treatment. A card sort may be a valid tool for quickly assessing symptom priorities in clinical practice and research.
Keywords: Menopause, Symptoms, Vasomotor Symptoms, Sleep Disturbances, Fatigue, Cognitive Functioning
INTRODUCTION
Most women experience multiple, concurrent menopausal symptoms at midlife.1–5 Although vasomotor symptoms (VMS) have been the focus of most intervention studies,6, 7 they do not occur in isolation. VMS frequently co-occur with disturbed sleep and mood, fatigue, trouble remembering or concentrating, and/or sexual difficulties.1–3, 8–10 These co-occurring symptoms are typically not assessed or are considered secondary outcomes, with few trials specifically targeting more than one symptom.6, 7, 11, 12 Research shows that symptoms act synergistically and that the impact of co-occurring symptoms on an individual is multiplicative rather than additive.9, 13 Thus, addressing multiple co-occurring symptoms is vital to improving midlife women’s quality of life.
Little is known about how women view the relative importance of their menopausal symptoms.14 During clinic visits, symptoms are often discussed with providers but not necessarily ranked by relative importance which may result in under-treatment of the symptom women would most like to alleviate. Investigating the relative importance of menopausal symptoms to each woman would provide useful information for understanding how to design research trials to alleviate co-occurring symptoms and prioritize therapeutic discussions and treatment recommendations by providers in clinical practice. Furthermore, understanding demographic correlates of symptom priorities would be informative for identifying at risk patients in clinical practice and designing research sampling plans. For example, it is likely that symptom priorities vary by race given that higher rates of VMS15 and disturbed sleep16–18 are reported by African American women compared to white women. In addition, understanding whether symptom priorities are correlated with ratings and scores on other symptom measures, such as diaries or standardized scales, is important for both research and practice. Symptoms that exceed cutoff scores (i.e. those that are more severe) are likely to be more salient, but when several symptoms exceed cutoff scores, women may differ on how they prioritize each symptom’s relative importance.
The study objective was to describe menopausal symptom priorities among women participating in a behavioral intervention trial for VMS treatment and their association with demographic characteristics and other symptom measures. We anticipated that distinct patterns of symptom priorities would emerge and that these would be associated with demographic correlates and cutoff scores.
METHODS
Design
This cross-sectional study was embedded within the second trial conducted by the United States research network, MsFLASH (Menopause Strategies Finding Lasting Answers to Symptoms and Health). Details of the MsFLASH protocol and research study are published elsewhere.19, 20 Briefly, the trial was a three by two factorial design comparing yoga and exercise vs. usual activity and omega-3 supplements vs. placebo for the treatment of VMS (primary outcome) and menopausal symptoms of disturbed sleep and mood, pain, sexual function, and quality of life (secondary outcomes). All data reported here are from the baseline data collection. Procedures were approved by the institutional review boards at each clinical site (Indianapolis, Oakland, and Seattle) and the Data Coordinating Center in Seattle. All research participants provided written, informed consent and authorization to use protected health information.
Sample
Participants were recruited between February 2011 and January 2012. Mass mailings were sent to women aged 40–62 years using purchased lists and health-plan enrollment files. Women were screened via telephone for eligibility and then via a two-week VMS diary and questionnaire. Potentially eligible women completed a third week of diaries. Eligible women were 40–62 years old; in the menopausal transition or early postmenopause or had had a hysterectomy with FSH >20 mIU/mL and estradiol ≤50 pg/mL; and in generally good health. The VMS eligibility criteria were: ≥14 hot flashes/night sweats per week recorded on daily VMS diaries for 3 weeks; VMS rated as bothersome or severe on 4 or more occasions per week; and the VMS frequency in week 3 did not decrease > 50% from the average weekly levels in weeks 1 and 2. Exclusion criteria included: BMI > 37; use of hormonal contraceptives or hormones in the past month; use of prescription or over-the-counter treatments for VMS in the past month; unstable medical conditions; current user of one of the study interventions or a related activity (i.e., yoga, tai chi, qi gong, meditation, regular exercise, omega-3 fatty acid supplements, frequent consumption of fish); contraindications to exercise (e.g., physical limitations), yoga, or omega-3 (e.g., allergy to soy or fish); or a major depressive episode in the past 3 months.
Procedures
Study screening was done via telephone followed by two weeks of at-home daily VMS diaries. Women whose diaries indicated they met VMS inclusion criteria completed two baseline clinic visits scheduled one week apart before being randomized to the interventions. All measures used for this study were collected during the trial’s baseline data collection period.
Measures
Symptom priorities were assessed using a card sort methodology. Women were given a set of 12 symptom cards: hot flashes or night sweats; disturbed sleep; feeling tired or worn out (fatigue); trouble remembering or concentrating (concentration); loss of interest in sex; vaginal dryness or pain with sexual intercourse; uncontrollable loss of urine; mood swings; feeling irritable; aches and pains; headaches; or heart palpitations. Research staff instructed women to select three cards representing the top three symptoms they would “most like to get rid of or be free of.” Once the three cards were selected from the deck, research staff then asked women to rank order the cards from one to three, with one representing the top symptom, two the second symptom, and three the third symptom they would most like to alleviate. Responses were recorded onto a paper form and data entered by study staff.
VMS frequency, severity and bother were recorded twice daily for three weeks; two weeks prior to the first baseline visit and during the one week between the two baseline visits. Women were instructed to use the diaries in the morning to write down the number, severity rating, and bother rating of their nighttime VMS and similarly use the diaries at bedtime to record daytime VMS. Severity was rated as mild, moderate, or severe. Bother was rated as not at all, a little, moderate, or a lot. Ratings were used to calculate daily mean frequency, severity, and bother.
Hot flash interference was assessed with the 10-item Hot Flash Related Daily Interference Scale (HFRDIS).21 Participants rated the degree to which hot flashes interfered with each item during the previous week using a 0 (do not interfere) to 10 (completely interfere) scale. This uni-dimensional scale is best represented by an overall mean score (sum of items/10) with higher scores representing higher levels of daily interference.22
Sleep was assessed using two scales; the 18-item Pittsburgh Sleep Quality Index (PSQI)23, 24 and the 7-item Insomnia Severity Index (ISI).25, 26 Both were used since the PSQI focuses broadly on overall sleep quality and the ISI is more specific to insomnia symptoms. PSQI global scores above 5 indicate poor sleep quality and above 8 indicate very poor sleep quality and daytime fatigue.27 ISI insomnia severity is interpreted using total scores as none (0–7), subthreshold (8–14), moderate (15–21), and severe (22–28).25, 26
Data Analysis
Analyses were conducted using SAS Version 9.3. Sample demographics were analyzed using descriptive statistics (n=355). Symptoms were ranked by number of top priority symptoms and the total number of first, second, and third priority symptoms. Frequencies and a Venn diagram were used to evaluate how symptoms were prioritized and how the overlap among symptoms was distributed within the sample. Participants were categorized by top priority symptoms. VMS and disturbed sleep were the most highly prioritized symptoms (VMS 210 first + 78 second place votes; disturbed sleep 48 first + 100 second place votes).
The sample was divided based on symptom priorities. We performed several analyses between (1) women who did and did not pick VMS as their top priority symptom and (2) women who did and did not pick disturbed sleep as their top priority symptom. Univariate and then multivariate comparisons and receiver operating characteristic (ROC) curve analyses were performed as outlined below.
Demographics, hot flash diary, and hot flash interference scores were compared using chi-square and t-tests between women who rated VMS as their first priority symptom to alleviate (n=210) and women who did not (n=144). We fit a logistic regression model estimating the probability of picking VMS symptoms as the first priority to alleviate as a function of VMS frequency, severity, and interference, adjusted for those baseline characteristics with a p value less than 0.2 from the univariate tests. VMS bother was not included in the model because of its high correlation with VMS severity.
Similarly, we compared participant characteristics, PSQI, and ISI scores using chi-square and t-tests between women who rated disturbed sleep as their first priority symptom to alleviate (n=48) and those who did not (n=296). We fit two logistic regression models – one as a function of PSQI scores and one as a function of ISI scores – both controlling for baseline characteristics with p values less than 0.2 from univariate tests.
We plotted ROC curves and calculated the area under the ROC curves (AUC) from three unadjusted logistic regression models – one estimating the probability of choosing VMS as the priority symptom as a function of HFRDIS, and two models estimating the probability of choosing disturbed sleep as the priority symptom as a function of PSQI and ISI, respectively. If AUC ≥ 0.7, indicating that the measurement had at least a fair level of efficacy in predicting participant choice, then we applied the ROC curve to select an optimal cut point in the scale for predicting a participant’s choice, treating sensitivity and specificity as equally important.
RESULTS
The sample included 354 women with card sort data. Most of the women were in their 50’s (82%), white (64%) or African-American (26%), college graduates (62%) and married or partnered (66%). The majority had never smoked (65%) and were non-drinkers (38%) or reported drinking 1–7 alcoholic beverages per week (44%). BMI varied: 34% had a BMI < 25, 41% had a BMI from 25–30, and 25% had a BMI > 30. Most (82%) were postmenopausal, and reported fair/good (37%) or very good (45%) health.
Table 1 shows the total number of first-, second-, and third-ranked symptom priorities. The top 4 symptoms women most wanted to alleviate were hot flashes (n=322), disturbed sleep (n=191), trouble remembering or concentrating (n=140), and fatigue (feeling tired/worn out) (n=116). VMS was the most highly prioritized symptom yet only 59% (n=210) of our study sample rated VMS as the top symptom they would most like to eliminate whereas 41% (n=144) picked another symptom. Disturbed sleep was the second most highly prioritized: 48 women picked disturbed sleep as the symptom they would most like to alleviate versus 296 who picked another symptom.
Table 1.
Priorities for Menopausal Symptoms Women Would Most Like to Alleviate
| Priority symptom | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1st | % | 2nd | % | 3rd | % | Overall | % | |
| Hot Flashes/night sweats | 210 | 59.2 | 75 | 21.1 | 37 | 10.4 | 322 | 90.7 |
| Disturbed sleep | 48 | 13.5 | 100 | 28.2 | 43 | 12.1 | 191 | 53.8 |
| Remembering/concentrating | 27 | 7.6 | 40 | 11.3 | 73 | 20.6 | 140 | 39.4 |
| Fatigue | 27 | 7.6 | 40 | 11.3 | 49 | 13.8 | 116 | 32.7 |
| Loss of interest in sex | 14 | 3.9 | 25 | 7.0 | 29 | 8.2 | 68 | 19.2 |
| Vaginal dryness or pain with sexual intercourse | 9 | 2.5 | 17 | 4.8 | 39 | 11.0 | 65 | 18.3 |
| Uncontrollable loss of urine | 5 | 1.4 | 14 | 3.9 | 10 | 2.8 | 29 | 8.2 |
| Mood swings | 4 | 1.1 | 14 | 3.9 | 15 | 4.2 | 33 | 9.3 |
| Feeling irritable | 4 | 1.1 | 12 | 3.4 | 22 | 6.2 | 38 | 10.7 |
| Aches and pains | 3 | 0.9 | 8 | 2.3 | 19 | 5.4 | 30 | 8.5 |
| Headaches | 2 | 0.6 | 5 | 1.4 | 4 | 1.1 | 11 | 3.1 |
| Heart palpitations | 1 | 0.3 | 4 | 1.1 | 8 | 2.3 | 13 | 3.7 |
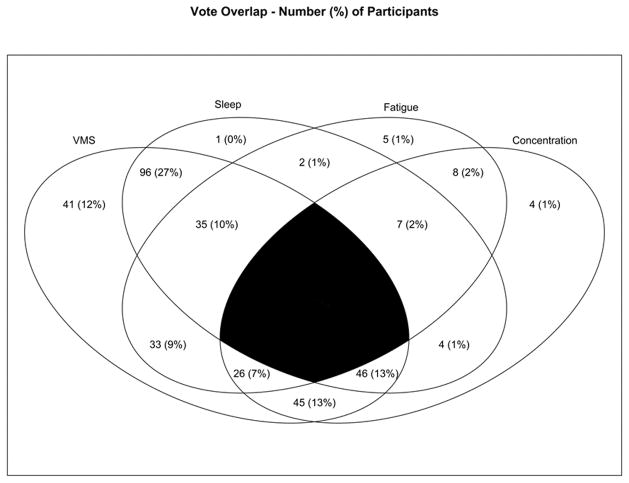
Figure 1 shows co-occurrence among the top four symptoms. All but 2 participants selected at least 1 of the top 4 symptoms, thus the sample size for this figure is n=353. The most common co-occurrence was to select both VMS and disturbed sleep (n=96, 27% of the total sample) as two of the three priority symptoms to alleviate. In contrast, only 1 person picked disturbed sleep but not one of the other most highly prioritized symptoms.
Figure 1. Co-occurrence of Menopausal Symptoms Prioritized as Those that Women Would Most Like to Alleviate.
Venn diagram depicting overlap among symptom priorities. Numbers are n (%). VMS are vasomotor symptoms. Sleep indicates disturbed sleep. Fatigue refers to feeling tired or worn out. Concentration refers to trouble remembering or concentrating. The four symptoms appeared most often as one of the top three symptoms women would most want to alleviate. There was 1 (0.28%) participant who did not pick any of the top four symptoms, thus this figure reflects n=353. The black center reflects the area of overlap not assessed since women rated their top 3 and not 4 symptoms.
VMS as the Top Symptom Priority
Table 2 shows unadjusted differences between women who selected VMS and those that did not. Compared to women who picked symptoms other than VMS as their top symptom to alleviate (n=145), women who picked VMS (n=210) were significantly more likely to be African-American (p=0.02), had significantly greater VMS frequency (p=0.019), severity (p=.005), bother (p<0.001), and tended to report greater VMS interference (p=0.052) (Table 2).
Table 2.
Demographic Differences between Women Based on First Priority Symptom Chosen to Alleviate
| Chose VMS | Chose Other | Chose Disturbed Sleep | Chose Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p | N | % | N | % | p | |
| Age group | 0.390 | 0.482 | ||||||||
| 42–49 years | 8 | 3.8 | 11 | 7.6 | 3 | 6.3 | 16 | 5.2 | ||
| 50–54 years | 101 | 48.1 | 61 | 42.4 | 19 | 39.6 | 143 | 46.7 | ||
| 55–59 years | 76 | 36.2 | 54 | 37.5 | 22 | 45.8 | 108 | 35.3 | ||
| 60–62 years | 25 | 11.9 | 18 | 12.5 | 4 | 8.3 | 39 | 12.7 | ||
| Ethnicity | 0.019 | 0.654 | ||||||||
| White | 130 | 61.9 | 97 | 67.4 | 33 | 68.8 | 194 | 63.4 | ||
| African American | 65 | 31.0 | 28 | 19.4 | 10 | 20.8 | 83 | 27.1 | ||
| Other / Unknown | 15 | 7.1 | 19 | 13.2 | 5 | 10.4 | 29 | 9.5 | ||
| Education | 0.273 | 0.044 | ||||||||
| <= High school diploma or GED | 16 | 7.6 | 5 | 3.5 | 0 | 0.0 | 21 | 6.9 | ||
| Some post high school | 62 | 29.5 | 50 | 34.7 | 10 | 20.8 | 102 | 33.3 | ||
| College graduate | 131 | 62.4 | 89 | 61.8 | 38 | 79.2 | 182 | 59.5 | ||
| Marital status | 0.544 | 0.093 | ||||||||
| Never married | 18 | 8.6 | 16 | 11.1 | 9 | 18.8 | 25 | 8.2 | ||
| Divorced or separated | 40 | 19.0 | 36 | 25 | 10 | 20.8 | 66 | 21.6 | ||
| Widowed | 4 | 1.9 | 3 | 2.1 | 1 | 2.1 | 6 | 2 | ||
| Married/Partnered | 147 | 70.0 | 88 | 61.1 | 27 | 56.3 | 208 | 68 | ||
| Smoking | 0.097 | 0.079 | ||||||||
| Never | 147 | 70.0 | 84 | 58.3 | 30 | 62.5 | 201 | 65.7 | ||
| Past | 43 | 20.5 | 46 | 31.9 | 16 | 33.3 | 73 | 23.9 | ||
| Current | 19 | 9.0 | 13 | 9 | 1 | 2.1 | 31 | 10.1 | ||
| Alcoholic drinks/week | 0.489 | 0.106 | ||||||||
| 0 | 81 | 38.6 | 55 | 38.2 | 16 | 33.3 | 120 | 39.2 | ||
| 1 to <7 | 95 | 45.2 | 61 | 42.4 | 18 | 37.5 | 138 | 45.1 | ||
| 7+ | 32 | 15.2 | 28 | 19.4 | 14 | 29.2 | 46 | 15 | ||
| Body Mass Index | 0.990 | 0.535 | ||||||||
| < 25 | 73 | 34.8 | 49 | 34 | 14 | 29.2 | 108 | 35.3 | ||
| 25 to <30 | 85 | 40.5 | 59 | 41 | 23 | 47.9 | 121 | 39.5 | ||
| >= 30 | 52 | 24.8 | 36 | 25 | 11 | 22.9 | 77 | 25.2 | ||
| Menopausal Status | 0.443 | 0.486 | ||||||||
| Postmenopausal | 167 | 79.5 | 122 | 84.7 | 42 | 87.5 | 247 | 80.7 | ||
| Late transition | 38 | 18.1 | 20 | 13.9 | 5 | 10.4 | 53 | 17.3 | ||
| Early transition | 5 | 2.4 | 2 | 1.4 | 1 | 2.1 | 6 | 2 | ||
| Health Rating | 0.314 | 0.600 | ||||||||
| Excellent | 39 | 18.6 | 19 | 13.2 | 8 | 16.7 | 50 | 16.3 | ||
| Very good | 97 | 46.2 | 64 | 44.4 | 18 | 37.5 | 143 | 46.7 | ||
| Good / fair | 73 | 61 | 42.4 | 22 | 112 | 36.6 | ||||
| ISI | <0.001 | |||||||||
| <= 15 | 165 | 78.6 | 95 | 66.0 | 0.008 | 21 | 43.8 | 239 | 78.1 | |
| >15 (insomnia) | 45 | 21.4 | 49 | 34.0 | 27 | 56.3 | 67 | 21.9 | ||
| PSQI | <0.001 | |||||||||
| < 8 | 106 | 50.5 | 57 | 39.6 | 0.021 | 11 | 22.9 | 152 | 49.7 | |
| >= 8 (poor sleep) | 95 | 45.2 | 85 | 59.0 | 37 | 77.1 | 143 | 46.7 | ||
| M | SD | M | SD | p | M | SD | M | SD | p | |
| VMS diary frequency | 4.7 | 2.5 | 4.0 | 2.5 | 0.005 | 3.8 | 1.9 | 4.5 | 2.6 | 0.079 |
| VMS diary severity | 1.1 | 0.4 | 0.9 | 0.4 | <0.001 | 0.8 | 0.4 | 1.0 | 0.4 | 0.005 |
| VMS diary bother | 2.0 | 0.5 | 1.8 | 0.5 | <0.001 | 1.8 | 0.5 | 2.0 | 0.5 | 0.004 |
| HFRDIS | 34.3 | 21.9 | 29.7 | 21.1 | 0.052 | 30.8 | 18.3 | 32.7 | 22.2 | 0.594 |
GED = general equivalency diploma; VMS = vasomotor symptoms; HFRDIS = Hot Flash Related Daily Interference Scale; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index
Table 3 shows results of an adjusted logistic regression model identifying factors associated with selecting VMS as the top priority symptom to alleviate. Those who picked VMS reported greater VMS severity (OR 2.55, p=0.004) and were more likely to have never smoked (OR 1.90, p=0.012) after adjusting for the other variables. The AUC based on HFRDIS score was 0.56 (n=328, 95% CI 0.50, 0.63), indicating a level of predictive accuracy that was no better than chance.
Table 3.
Multivariate Logistic Regression Identifying Factors Associated with Women who Chose Hot Flashes as Priority Symptom to Alleviate
| Odds Ratio (95% CI) | P-value | |
|---|---|---|
| HFRDIS | 1.00 (0.99, 1.02) | 0.410 |
| VMS Frequency | 1.07 (0.96, 1.19) | 0.204 |
| VMS Severity | 2.55 (1.35, 4.82) | 0.004 |
| Ethnicity | 0.136 | |
| White | (Reference) | |
| African American | 1.56 (0.88, 2.77) | |
| Other / Unknown | 0.68 (0.31, 1.48) | |
| Smoking | 0.012 | |
| Never | 1.90 (0.82, 4.38) | |
| Past | 0.87 (0.35, 2.14) | |
| Current | (Reference) |
HFRDIS = Hot Flash Related Daily Interference Scale; VMS = vasomotor symptoms
Disturbed Sleep as Top Symptom Priority
Table 2 shows unadjusted differences between women who selected disturbed sleep as their top priority symptom to alleviate and those that did not. Compared to women who picked symptoms other than sleep as their top symptom to alleviate (n=306), women who picked disturbed sleep as their top symptom (n=48) were significantly more likely to have Insomnia Severity Index scores > 15 (p<0.001) and Pittsburgh Sleep Quality Index scores > 8 (p<0.001). They also tended to have more education (p=0.044).
Table 4 shows the results of adjusted logistic regression models predicting disturbed sleep as a top priority symptom. Both ISI (OR 1.22, P<0.001) and PSQI (OR 1.26, P<0.001) remained significant after adjusting for education, marital status, and smoking and drinking habits. Education also remained significant in both models and had similar effect sizes in each (OR=1.54 for PSQI and 1.50 for ISI).
Table 4.
Multivariate Logistic Regression Models Identifying Factors Associated with Women who Chose Disturbed Sleep as a Priority Symptom to Alleviate
| PSQI Model | ISI Model | |||
|---|---|---|---|---|
|
| ||||
| Factor | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p |
| Education | 1.54 (1.19, 1.97) | < .001 | 1.50 (1.16, 1.93) | 0.002 |
|
| ||||
| Marital Status | 0.167 | 0.079 | ||
| Never married | 3.05 (1.15, 8.13) | 3.70 (1.16, 1.94) | ||
| Divorced or separated | 1.40 (0.59, 3.33) | 1.83 (0.75, 4.50) | ||
| Widowed | 1.56 (0.16, 15.22) | 1.59 (0.15, 17.28) | ||
| Married/Partnered | (Reference) | (Reference) | ||
| Smoking | 0.341 | 0.356 | ||
| Never | (Reference) | (Reference) | ||
| Past | 1.36 (0.62, 2.95) | 1.35 (0.62, 2.93) | ||
| Current | 0.30 (0.04, 2.40) | 0.30 (0.04, 2.43) | ||
| Alcoholic drinks/week | 0.273 | 0.232 | ||
| None | (Reference) | (Reference) | ||
| 1 to < 7 | 1.06 (0.48, 2.35) | 1.13 (0.49, 2.59) | ||
| 7+ | 2.02 (0.80, 5.13) | 2.20 (0.84, 5.75) | ||
| PSQI | 1.26 (1.13, 1.40) | <.001 | ||
| ISI | 1.22 (1.13, 1.31) | <.001 | ||
PSQI = Pittsburgh Sleep Quality Index; ISI = Insomnia Severity Index; CI = confidence interval
The AUC was 0.76 for ISI (N=350, 95% CI 0.70, 0.83) and 0.71 for PSQI (N=344, 95% CI 0.64, 0.78), indicating that both provided a fair level of accuracy for predicting whether or not a participant would pick disturbed sleep as most bothersome.
If sensitivity and specificity were treated as equally important, then a PSQI score of 9 was optimal to indicate a participant picked disturbed sleep as a top priority symptom to alleviate (sensitivity 66.7, specificity 63.9). This cutoff had a Youden’s index of 0.31 and would correctly classify 32 out of 48 women who picked sleep as most bothersome, and 189 out of 296 who picked other symptoms as most bothersome. Similarly, an ISI score of 11 was optimal (sensitivity 89.6, specificity 44.7). This cutoff had the highest Youden’s index, 0.34 and would correctly classify 43 of the 48 women who picked sleep as most bothersome, but would only correctly classify 135 of the 302 women who picked other symptoms as most bothersome.
DISCUSSION
There were three principal findings from this study. First, VMS and disturbed sleep, followed by feeling tired (fatigue) and trouble remembering or concentrating, were overall the most highly prioritized symptoms that women presenting for a VMS treatment trial wanted to alleviate. Only slightly more than half of women enrolled in a trial for VMS treatment chose VMS as the top symptom they would most like to eliminate. Second, race/ethnicity and VMS severity but not HFRDIS scores were associated with women’s ranking of VMS as their top priority symptom to alleviate. Third, educational level and ISI and PSQI scores were associated with ranking disturbed sleep as a top priority symptom to alleviate.
Strengths and weaknesses of this study include the following. This was a large and relatively diverse sample from a multi-site study. Data were carefully annotated using daily diaries, the card sort and questionnaires that were administered during a clinic visit. VMS are the most common symptom associated with the menopausal transition associated with decreased health related quality of life and we focused on those with 2 or more bothersome VMS per day. The sample was limited to women who participated in a treatment trial for VMS and therefore results may not be generalizable to midlife women who are already receiving VMS treatment or who choose not to seek VMS treatment. However, it is important to note that even though these women were participating in a VMS treatment study, for 41% of them, VMS was not their priority symptom. In addition, women using selective serotonin reuptake inhibitors for VMS were excluded which may have disproportionately eliminated women with mood problems, an important menopausal symptom documented in other studies.2 This may at least partially account for the low ranking of mood and irritability in the card sort. The great majority of mid-life women experience frequent and bothersome VMS during the menopausal transition, and understanding the priorities for symptom relief among this large population group has a high degree of clinical relevance.
The emergence of disturbed sleep as a top priority symptom among this sample of women seeking treatment for VMS was not surprising. Numerous large population-based studies have documented the co-occurrence of VMS and disturbed sleep.2, 5, 28 In addition, our study participants from this analysis were similar to participants in our other trials, nearly one-third of whom had moderate to severe insomnia on the ISI and 40% of whom had poor subjective sleep quality on the PSQI.29
The emergence of low energy and problems with memory/concentration difficulties as top symptom priorities serves as an important reminder for assessing these symptoms in clinical practice and research. These symptoms were not assessed in our network trials except with these card sort data. Changes in concentration and memory were part of the symptom cluster documented in women participating in the Seattle Midlife Women’s Health Study.2 Similarly, verbal memory performance was related to the objectively recorded hot flash frequency in a study of 29 midlife women with moderate to severe hot flashes.30 Fatigue and memory/ concentration problems may be part of a cascade of symptoms resulting from disturbed sleep.
Pain did not appear as a top priority symptom despite other studies showing it to be commonly experienced during menopause. In the Seattle Midlife Women’s Health study, mood and pain also emerged as important symptoms, 2 which differs from our results showing these were, respectively, the 8th and 10th most prioritized symptoms. Similarly, in the Penn Ovarian Aging study, aches and joint pain were among a handful of symptoms most commonly reported in the late transitional stage.10
When evaluating VMS as a top symptom to alleviate, VMS severity but not frequency or interference emerged as significant correlates. VMS frequency and severity were moderately correlated (r=0.36) and when the model was run without severity (not shown), frequency was significant (OR=1.13, p=0.02). However, severity with an odds ratio of 2.66 seems more salient. HFRDIS findings may reflect the importance of VMS severity in a woman’s life and/or limitations of the HFRDIS. In particular, the HFRDIS includes items related to VMS interference with sleep, concentration and sexuality,31 which may have resulted in it being a less than ideal measure for differentiating women who selected VMS as the top symptom to alleviate and women who picked other symptoms such as disturbed sleep, concentration problems, or sexual concerns.
Importantly, associations between symptom priorities and diary VMS severity or standardized sleep measures indicate the card sort may be a valid and efficient method for quickly assessing symptom priorities in clinical practice or research. Because VMS are often co-occurring with other menopausal symptoms, not all of which can be addressed at once or may not be fully controlled, the card sort could help clinicians identify women’s priorities for which symptoms to target first so treatment can be tailored accordingly. The card sort could be used as the first step of an assessment algorithm to guide more detailed symptom assessments tailored only to the subset of symptoms women would most like to alleviate. This could result in greater efficiency and speed of symptom assessments in clinical practice and research, while retaining a focus on those symptoms women are most concerned about managing. Ultimately, the card sort assessment method with a tailored treatment approach could improve symptom management by reducing the burden and impact of these symptoms.
Future research directions include repeating the card sort study in a more broadly selected sample of midlife women with bothersome menopause symptoms that may or may not include VMS to determine the replicability of our findings. Understanding the prioritization of symptoms in women not presenting for VMS trials might result in different findings. We also recommend studying the implementation of the card sort in clinical practice to assess feasibility in busy practice settings and potential impact on the clinical care of midlife women. In theory, a computerized version of the card sort could be directly linked to the electronic medical record to facilitate tracking and provide efficiency in clinical practice.
Acknowledgments
This study was funded by the National Institutes of Health as a cooperative agreement issued by the National Institute on Aging (NIA), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Research on Women’s Health (ORWH), and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA. At Indiana University, the project was funded in part with support from the Indiana Clinical and Translational Sciences Institute, grant UL1RR02571 from the NIH, National Center for Research Resources, Clinical and Translational Sciences Award.
Footnotes
Disclosures: Mr. Hohensee and Drs. Carpenter, Woods, Otte, Guthrie, Newton, Sternfeld, Lau, and LaCroix have no disclosures. Dr. Joffe has grant support from Teva and Merck and is an advisor/consultant for Noven, Merck, and Mitsubishi Tanabe. Dr. Cohen has research support from AstraZeneca, Bristol-Myers Squibb/Otsuka, Cephalon, Inc., Ortho-McNeil Janssen, Sunovion Pharmaceuticals, Inc., and Takeda/Lundbeck. He is an advisor/consultant for JDS Therapeutics LLC. Dr. Reed has grant funding from Bayer Pharmaceuticals.
Contributor Information
Janet S. Carpenter, Email: carpentj@iu.edu, Science of Nursing Care, School of Nursing, Indiana University, Indianapolis, IN.
Nancy Fugate Woods, Email: nfwoods@uw.edu, Biobehavioral Nursing, University of Washington, Seattle, WA.
Julie L. Otte, Email: jlelam@iu.edu, Science of Nursing Care, School of Nursing, Indiana University, Indianapolis, IN.
Katherine A. Guthrie, Email: kguthrie@fhcrc.org, Fred Hutchinson Cancer Research Center, Seattle, WA.
Chancellor Hohensee, Email: chohense@whi.org, Fred Hutchinson Cancer Research Center, Seattle, WA.
Katherine M. Newton, Email: newton.k@ghc.org, Group Health Research Institute, Seattle, WA.
Hadine Joffe, Email: hjoffe@partners.org, Harvard Medical School, Department of Psychiatry, Brigham and Women’s Hospital and Dana Farber Cancer Institute, Boston, MA.
Lee Cohen, Email: lcohen2@mgh.harvard.edu, Center for Women’s Mental Health; Perinatal and Reproductive Psychiatry Clinical Research Program, Massachusetts General Hospital, Boston, MA.
Barbara Sternfeld, Email: Barbara.sternfeld@kp.org, Division of Research, Kaiser Permanent Medical program of Northern California, Oakland, CA.
R. Jane Lau, Email: rjlau@iupui.edu, Department of Obstetrics and Gynecology, School of Medicine, Indiana University, Indianapolis, IN.
Susan D. Reed, Email: reeds@uw.edu, Departments of Obstetrics/Gynecology and Epidemiology, University of Washington School of Medicine, Seattle, WA.
Andrea Z. LaCroix, Email: alacroix@ucsd.edu, Department of Preventive Medicine, University of California San Diego, San Diego, CA.
References
- 1.Cray LA, Woods NF, Mitchell ES. Identifying symptom clusters during the menopausal transition: observations from the Seattle Midlife Women’s Health Study. Climacteric. 2013;16(5):539–49. doi: 10.3109/13697137.2012.746657. [DOI] [PubMed] [Google Scholar]
- 2.Cray LA, Woods NF, Herting JR, Mitchell ES. Symptom clusters during the late reproductive stage through the early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2012;19(8):864–9. doi: 10.1097/gme.0b013e31824790a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2011;14(2):252–61. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: A systematic review. Climacteric. 2007;10(3):197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 5.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 6.Fisher WI, Johnson AK, Elkins GR, Otte JL, Burns DS, Yu M, Carpenter JS. Risk factors, pathophysiology, and treatment of hot flashes in cancer. CA: a cancer journal for clinicians. 2013;63(3):167–92. doi: 10.3322/caac.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rada G, Capurro D, Pantoja T, Corbalan J, Moreno G, Letelier LM, Vera C. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923. doi: 10.1002/14651858.CD004923.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Zaslavsky O, Cochrane BB, Herting JR, Thompson HJ, Woods NF, Lacroix A. Application of person-centered analytic methodology in longitudinal research: exemplars from the Women’s Health Initiative Clinical Trial data. Res Nurs Health. 2014;37(1):53–64. doi: 10.1002/nur.21575. [DOI] [PubMed] [Google Scholar]
- 9.Cray L, Woods NF, Mitchell ES. Symptom clusters during the late menopausal transition stage: observations from the Seattle Midlife Women’s Health Study. Menopause. 2010;17(5):972–7. doi: 10.1097/gme.0b013e3181dd1f95. [DOI] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, Sheng L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2):230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 11.Woods NF, Mitchell ES, Schnall JG, Cray L, Ismail R, Taylor-Swanson L, Thomas A. Effects of mind-body therapies on symptom clusters during the menopausal transition. Climacteric. 2014;17(1):10–22. doi: 10.3109/13697137.2013.828198. [DOI] [PubMed] [Google Scholar]
- 12.Ismail R, Taylor-Swanson L, Thomas A, Schnall JG, Cray L, Mitchell ES, Woods NF. Effects of herbal preparations on symptom clusters during the menopausal transition. Climacteric. 2014;18(1):1–18. doi: 10.3109/13697137.2014.900746. [DOI] [PubMed] [Google Scholar]
- 13.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. The American journal of medicine. 2005;118(Suppl 12B):14–24. doi: 10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152(5):463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 16.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep medicine. 2011;12(3):209–14. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behavioral sleep medicine. 2010;8(4):246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 18.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behavioral sleep medicine. 2006;4(1):29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- 19.Sternfeld B, Lacroix A, Caan BJ, Dunn AL, Newton KM, Reed SD, Guthrie KA, Booth-Laforce C, Sherman KJ, Cohen L, Freeman MP, Carpenter JS, Hunt JR, Roberts M, Ensrud KE. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: The MsFLASH experience. Contemporary clinical trials. 2013;35(1):25–34. doi: 10.1016/j.cct.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton KM, Carpenter JS, Guthrie KA, Anderson GL, Caan B, Cohen LS, Ensrud KE, Freeman EW, Joffe H, Sternfeld B, Reed SD, Sherman S, Sammel MD, Kroenke K, Larson JC, Lacroix AZ. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22(6):979–89. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter JS, Rand KL. Modeling the hot flash experience in breast cancer survivors. Menopause. 2008;15(3):469–75. doi: 10.1097/gme.0b013e3181591db7. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14(4):331–8. [PubMed] [Google Scholar]
- 25.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 26.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 28.Ensrud KE, Stone KL, Blackwell TL, Sawaya GF, Tagliaferri M, Diem SJ, Grady D. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16(2):286–92. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Joffe H, Guthrie KA, Larson JC, Reed SD, Newton KM, Sternfeld B, Lacroix AZ, Landis CA, Woods NF, Freeman EW. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19(8):848–55. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15(5):848–56. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. Journal of pain and symptom management. 2001;22(6):979–89. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]