Introduction
Membrane fusion is defined as the consolidation of two membrane bilayers and a subsequent mixing of the two previously separated aqueous compartments. Membrane fusion processes are mediated and regulated by a growing family of soluble and integral membrane proteins termed fusion proteins.1,2 Fusion proteins share structural and functional properties and aid in the thermodynamically unfavorable fusion event by promoting hydrophobic interactions that favor fusion.1–3
Within retinal photoreceptor rod cells membrane fusion is a component step of at least three essential cellular processes. Fusion is necessary for the delivery of proteins and lipids in vesicles from the rod inner segment (site of synthesis) to the rod outer segment (ROS). Two additional fusion processes preserve the unique architecture of the outer segment by maintaining the outer segment at a constant length. The coordinated processes of disk morphogenesis and compensatory disk shedding4 require the fusion of two opposing membranes: the fusion of two outgrowing inside-out disk rims for morphogenesis,5 and disk–plasma membrane fusion for disk packet formation.6 Fusion during disk packet formation is documented in microscopy studies in which an analysis of dye penetration into distinct regions of the ROS found that large molecules do not enter the narrow bands of the dye-stained region of the ROS, suggesting a fusion of the plasma membrane with the disk membranes.7,8 This fusion is mediated by a fusion protein unique to photoreceptors: peripherin/rds. In this chapter we describe the protocols used in photoreceptor cell-free fusion assays and the characterization of peripherin/rds as a rod cell-specific fusion protein.6,9–13
General Principles Involved in Design of Fusion Assays
Membrane fusion must satisfy two criteria: (1) the merger of two membrane bilayers and (2) the subsequent mixing of the aqueous contents.14 To characterize the molecular mechanism of fusion, it is necessary to evaluate the two phenomena separately by distinct albeit complementary techniques. In biological systems, the measurement of aqueous contents mixing is often difficult or impossible to achieve and thus is often inferred from the detection of lipid mixing. Unfortunately, the formation of a fusion pore independent of aqueous contents mixing (i.e., hemifusion), can be misinterpreted as fusion.15 The careful design and rigorous interpretation of control experiments avoid such misinterpretations. Aqueous contents mixing in some biological systems is now detectable with the development of molecular genetics techniques that rely on the cytoplasmic activation of reporter genes on fusion of two distinct cell populations.16 Fusion processes are measured in vitro by a variety of techniques described elsewhere.17,18
The most sensitive and reliable measurements of the kinetics of a fusion process require biophysical techniques,19 the most well characterized of which is fluorescence spectroscopy. Fluorescence-based biological fusion assays are broadly divided into two categories: probe dilution20 and resonance energy transfer,21 both of which measure the mixing of the lipids within the membranes. Both of these techniques have been utilized in the design of photoreceptor specific cell-free fusion assays.
Rod Outer Segment Membrane Fusion
In practice, lipid mixing is measured by the incorporation of a fluorescent probe into the membrane bilayer and a change in fluorescence emission, accessed either through resonance energy transfer pairs, or via a release of self-quenching, on fusion of the labeled membrane with a suitable target membrane. In photoreceptors, the fusion assay used most often is based on a relief of octadecylrhodamine B chloride (R18) self-quenching as two membrane bilayers mix. When the R18-labeled membrane fuses with unlabeled target membrane, the lipid-like probe is effectively diluted by its subsequent lateral diffusion within the target membrane. Probe dilution is detected as a progressive linear increase in fluorescence intensity, which is proportional to the extent of fusion.21 This technique allows kinetic and quantitative measurements of fusion between labeled membranes and both artificial and biological membranes.19–21 The typical protocol for R18 labeling of bovine ROS plasma membrane and ensuing fusion follows.
Preparation and Labeling of Rod Outer Segment Plasma Membrane
Materials
Purified ROS plasma membrane vesicles
Octadecylrhodamine B chloride (R18; Molecular Probes, Eugene, OR)
Sephadex G-75 (Pharmacia, Piscataway, NJ)
Column buffer: 100 mM NaCl, 10 mM glycine, 0.1 mM EDTA, pH 7.5
Calcium chelating buffer: 5 mM HEPES, 1 mM EDTA, pH 7.4
ROS disks and plasma membranes are isolated from either fresh or frozen bovine retinas.22 The plasma membrane vesicles are purified free from ROS disk membranes by binding to ricin120–agarose (Sigma, St. Louis, MO) and separated by continuous sucrose density gradient centrifugation.22 The ROS plasma membrane bound to ricin–agarose is recovered as a pellet in the gradient. The plasma membrane is eluted from the ricin–agarose in a Pasteur pipette column with 1 M galactose in 0.1 M sodium borate, pH 8.0. The resulting plasma membrane vesicles are washed free of galactose (spin at 50,000 rpm for 40 min at 10°) and resuspended in calcium chelating buffer.23 The phospholipid content of the membrane is determined24,25 and the final concentration adjusted to 2.0 mM phosphate. ROS plasma membrane vesicles must be labeled and used within a 24-hr period; otherwise vesicles become leaky and give spurious results. Both the preparation and labeling of the ROS plasma membrane are performed under dim red light.
R18 Labeling of Rod Outer Segment Plasma Membrane
The freshly isolated ROS plasma membrane vesicles are labeled with R18.6 A stock solution of R18 (10 mg/ml) is prepared in chloroform–methanol (1:1, v/v) and stored at −20°. An aliquot of this solution is removed, dried under a stream of nitrogen, and reconstituted in a minimal volume of ethanol. R18 is incorporated into the ROS plasma membrane at self-quenching concentrations, equivalent to approximately 5 mol% relative to the phospholipid content of the ROS plasma membrane. Typically, a 2-ml suspension of ROS plasma membrane (rhodopsin concentration, 1 mg/ml) is added to 10 μl of 10 nmol of R18, in ethanol, under vigorous vortexing. Since the final incubation volume is 2 ml, the final ethanol concentration is less than 1% (v/v). The mixture is rocked gently at room temperature for 30–60 min in the dark.6
The unincorporated probe is removed by chromatography on a Sephadex G-75 column (total volume of the column, 3–4 ml). The unincorporated R18 adsorbs to the top of the column, and the R18-labeled plasma membrane is recovered in the void volume and identified by absorbance at 214 nm. Free probe can also be removed with bovine serum albumin,11 followed by sucrose density gradient centrifugation. In addition to being more time consuming, the bovine serum albumin (BSA) procedure may also remove membrane lipids and alter bilayer properties. For the remainder of this chapter R18-labeled plasma membrane is abbreviated as R18–PM. To quantitate the incorporation of R18 into the plasma membrane, the labeled vesicles are extracted with chloroform–methanol (2:l).26 The fluorescence intensity of an aliquot of the extract is measured and compared with the intensity of known amounts of R18 in chloroform, which increase linearly up to concentrations of 1 nmol/ml.27
Preparation of Target Membranes
The accurate measurement of membrane fusion processes by relief of self-quenching requires that the concentration of target membrane be at least 30- to 100-fold greater than that of the R18-labeled species.28 In our experiments a surface area ratio between R18–PM and target membranes of 1:50 or 1:100 yields satisfactory results, although the ratio of labeled membrane to unlabeled membrane should be optimized for each system.28 R18–PM fuse with a variety of target membranes including ROS disk membranes, disk rim-specific vesicles, and disk lipid recombinant vesicles containing peripherin/rds. The preparation of these target vesicles is described below.
Disk Membranes
Disk membranes are routinely isolated during the preparation of plasma membrane from a continuous sucrose density gradient at 30% sucrose.22 In our experience, 50 frozen bovine retinas routinely yield 20–25 mg of disk membrane rhodopsin. The disk membranes are washed free of sucrose and resuspended in calcium chelating buffer23 to a rhodopsin concentration of 2.5 mg/ml for phosphate determination and diluted with chelating buffer to a final phosphate concentration of 1 mM for fusion assays.
Disk Rim-Specific Vesicles
Rim-specific vesicles have a protein content that closely mimicks the protein content of the disk rim region, i.e., enriched in peripherin/rds,29,30 rom-1,31 and the rim–ABC protein,32 with negligible levels of rhodopsin. These vesicles are prepared from disk membranes isolated as described in the preceding section. The disk membranes are solubilized with octylglucoside (OG) and the solubilized mixture subjected to concanavalin A affinity chromatography.33,34 The unbound fraction from the concanavalin A column9 contains total disk lipids and the peripherin/rds and rom-1, which do not bind to the column. This unbound fraction is collected and concentrated to a final volume of 5–8 ml, using an Amicon (Danvers, MA) concentrator (YM10 filter). Disk rim-specific vesicles form spontaneously after the removal of OG by dialysis for 24–48 hr against 1 M NaCl, 10 mM HEPES, pH 7.4, with two changes of buffer. The rim-specific vesicles are not used for fusion unless the residual OG concentration (determined as described35) is less than 0.05 mol% relative to phospholipid. If the OG concentration is higher, the vesicles are dialyzed for an additional 24 hr in the presence of SM-2 BioBeads (Bio-Rad, Hercules, CA). After dialysis, the vesicles are subject to five freeze–thaw cycles [liquid nitrogen (freeze)/room temperature (thaw)]. The volume of vesicles is adjusted with calcium chelating buffer to a final phospholipid concentration of 1 mM.
Peripherin/rds Recombinants
Peripherin/rds is purified from bovine retinas by a combination of concanavalin A affinity chromatography and chromatofocusing techniques.9 Nonphosphorylated peripherin/rds is isolated from the chromatofocusing column at its pI of 4.7, and phosphorylated peripherin/rds at a pI of 4.21. Peripherin/rds and phosphoperipherin/rds recombinants are prepared by detergent dialysis.36 In the preparation of peripherin/rds recombinants, the purified protein is recombined with vesicles prepared from extracted disk membrane lipids.12 Since retinal has been shown to induce lipid-mediated fusion in photoreceptors, we reduce the retinal Schiff-base linkage with NaCNBH3,37 thereby eliminating any retinal-induced effects on fusion. To prepare disk lipid small unilamellar vesicles (SUVs) for recombination, freshly prepared 2 M NaCNBH3 in 1 M acetic acid is added to freshly isolated disk membranes in a 2:1 (v/v) ratio in the dark and allowed to sit at room temperature for 45 min. The treated disk membranes are washed and resuspended in 10 mM HEPES, pH 7.4, and extracted with 2:1 chloroform–methanol.26 The extracted lipids are dried under a stream of nitrogen, lyophilized overnight, and resuspended in 10 mM HEPES, pH 7.4. SUVs are prepared from the liposomes.38 If necessary, the amount of residual retinal(ol) can be quantitated by high-performance liquid chromatography (HPLC).39
The disk lipid SUVs are recombined with purified peripherin/rds, isolated by chromatofocusing. The peripherin/rds-containing fractions are combined and the pH adjusted to 7.0 with 0.1 M imidazole, pH 8.0. The sample is concentrated to one-tenth its original volume with a Centricon 30 filter device (30,000 MW cutoff filter). The ultrafiltration device is centrifuged at 7500g at 4°, time variable, using a fixed-angle rotor. The concentrated purified peripherin/rds in 20–30 mM OG is added to disk lipid SUVs with vigorous vortexing and the mixture placed in an ice bath for 1 hr. The resulting solution should be cloudy, indicating that the OG concentration in the recombinant is below the critical micelle concentration of the detergent. Routinely, a ratio of 1 mol of peripherin/rds to 100–300 mol of phospholipid is used. The recombinant is then dialyzed against 10 mM HEPES, 0.5 M NaCl, pH 7.4, for 24–48 hr, with three changes of buffer, with the final buffer change containing SM-2 BioBeads (Bio-Rad). Under these experimental conditions, less than 0.05% OG remains associated with the recombinants. The recombinant-containing peripherin/rds in the disk lipid vesicles is purified on a continuous 10–40% (w/w) sucrose density gradient, spun at 25,000 rpm for 8 hr at 4°. The recombinant is routinely isolated as a single band at approximately 20–30% sucrose, depending on the final phospholipid/protein ratio. Peripherin/rds in the recombinant is oriented asymmetrically, such that all of the peripherin/rds molecules are inserted with their extra-diskal side out. This orientation is confirmed by immunofluorescence with anti-peripherin/rds monoclonal antibody 2B6 to the C terminus (generously provided by R. Molday29) or by trypsinolysis.9,10 The peripherin/rds recombinants are subject to five freeze–thaw cycles: rapid freezing in liquid nitrogen followed by thawing at room temperature to form large unilamellar vesicles called peripherin/rds LUVs. Disk lipid vesicles containing no peripherin/rds or rhodopsin recombinants, which do not to fuse with R18– PM, are used as controls.9,12,36
R18 Fusion Assay
Fusion between R18–PM and target membranes is detected as an increase in fluorescence intensity at 586 nm on dilution of R18 throughout the target membrane. In designing these assays it is essential that the amount of lipid in the R18–PM be 50-fold lower than the amount of phospholipid in the target membranes. Therefore, we routinely adjust the phospholipid concentration of the target membrane to 1 mM. The assays are performed in a total volume of either 3 or 1 ml. The calcium concentration of the target membrane is adjusted by the addition of CaCl2 to the cuvette prior to or simultaneously with the initiation of fusion. Fluorescence and light scattering are monitored with λex 560 nm and λem 586 nm on a Perkin-Elmer (Norwalk, CT) LS50 B spectrofluorimeter or equivalent.
The target membranes are equilibrated to the appropriate temperature while stirring in a thermostatted cuvette turret. Fusion is initiated by the addition of 50 μl of R18–PM to this suspension of target membrane. The change in R18 fluorescence is monitored continuously and increases linearly as the probe dilutes into the target membrane (see Fig. 1). The increase in observed fluorescence intensity is proportional to the membrane fusion.6,21 The fluorescence intensity of the target membrane without addition of plasma membrane is taken as baseline fluorescence and is equal to 0% fusion. Fluorescence at 100%, equal to 100% fusion, is determined by the addition of 100 μl of 10% Triton X-100 to the membrane mixture. Triton X-100 does not interfere with rhodamine fluorescence and the only correction factor required is that due to dilution. The initial rate of fusion (IRF) is determined as the percent increase in fluorescence intensity as a function of time21 as shown in Eq. (1).
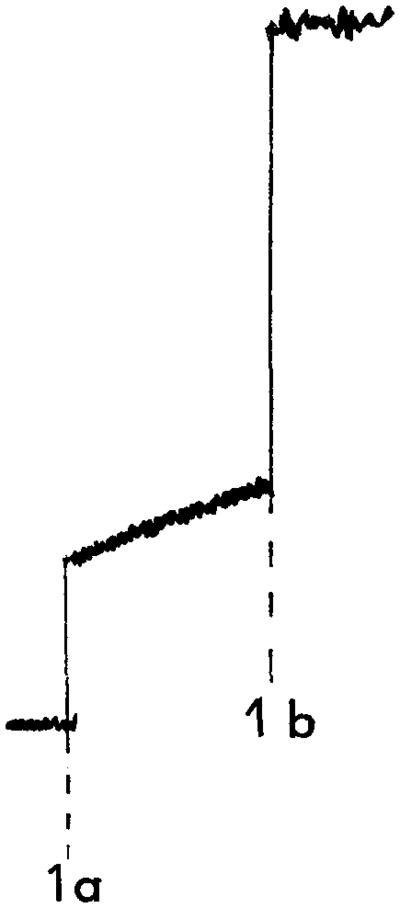
Fig. 1.
Membrane fusion between R18–PM and ROS disk membranes. A representative tracing of the change in fluorescence observed with the R18 lipid mixing assay, at 37°, is shown. (a) Fusion is initiated with the simultaneous addition of R18–PM and calcium ([Ca2+]free = 45 mM) to the disk membranes, (b) The addition of Triton X-100. [Reprinted with permission from K. Boesze-Battaglia, A. D. Albert, and P. L. Yeagle, Biochemistry 31, 3733 (1992); American Chemical Society.]
| (1) |
where slope R18 is equal to the linear change in R18 fluorescence over time, F0 is the fluorescence intensity at baseline (i.e., in the absence of labeled R18–PM), and Ff is the fluorescence intensity on the addition of Triton X-100. If a lag time is observed the slope R18 is determined only after the lag phase.
Analysis of Fluorescence Data
The parameters obtained from the fluorescence data used to characterize the fusion process are as follows: (1) the initial rate of fusion, determined from the slope of the fluorescence increase (Figs. 1 and 2), (2) the extent of fusion, determined on an overnight incubation between R18–PM and target membranes, and (3), in some cases, the lag time prior to the initiation of fusion (Fig. 2).
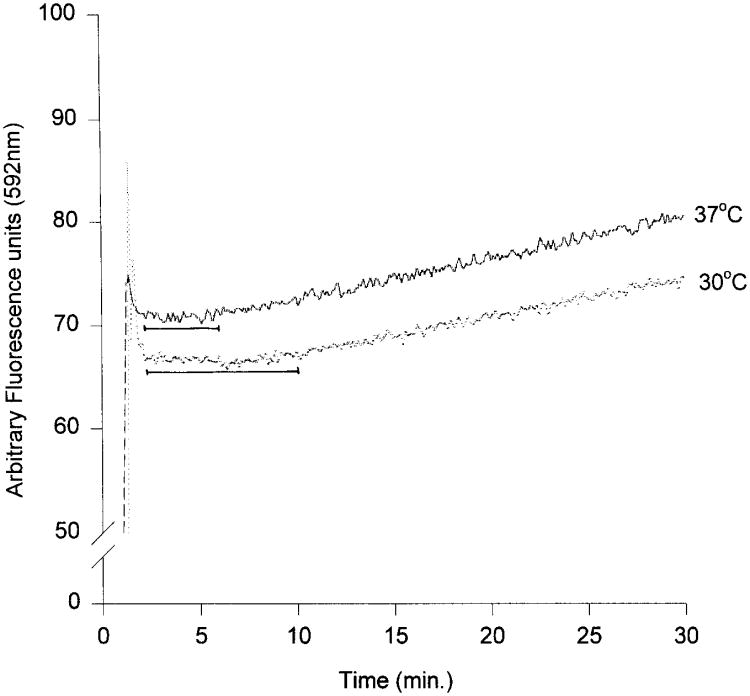
Fig. 2.
Membrane fusion between R18–PM and peripherin/rds recombinants. A representative tracing of the increase in fluorescence intensity of R18 at 592 nm over time (minutes) on the addition of R18–PM (at 15 sec) to peripherin/rds recombinants is shown at 30 and 37°. The horizontal solid lines indicate the lag times. The initial rate of fusion is calculated from the slope of the change in fluorescence intensity after the lag time indicated. [Reprinted with permission from K. Boesze-Battaglia, O. P. Lamba, A. Napoli, S. Sinha, and Y. Guo, Biochemistry 37, 9477 (1998); American Chemical Society.]
Characteristics of Fusion between R18-Labeled Plasma Membrane and Target Membranes
Fusion between R18–PM and the target membranes share common characteristics: fusion increases linearly over time, requires nanomolar levels of calcium, and is completely inhibited by the addition of 100 μl of 1 M EGTA. Fusion is temperature dependent, with the highest initial rates at 37° and almost no detectable fusion above 45° or below 30°.
Disk Membranes
A representative tracing of the increase in fluorescence intensity observed upon the fusion of R18–PM with isolated disk membranes6 is shown in Fig. 1. Fusion is initiated by the simultaneous addition (Fig. 1, 1a) of R18–PM and calcium to a suspension of disk membranes at 37°. Maximal fusion is determined by the addition of Triton X-100 (Fig. 1, 1b). The slope of the fluorescence increase is used to calculate the initial rate of fusion. The fusion between disk membranes and R18–PM can be inhibited by modifications of endogenous peripherin/rds, such as trypsinolysis of the disk membranes,9,10 the addition of anti-peripherin monoclonal antibody 2B6,9 or the addition of low concentrations of the peptide PP-5,10 corresponding to amino acids 311 to 325 of bovine peripherin/rds.
Disk Rim-Specific Vesicles or Peripherin/rds Recombinants
When R18–PM fuse with either rim-specific vesicles or peripherin/rds recombinants, a qualitatively different fluorescence pattern is observed as shown in Fig 2. When fusion of R18–PM with peripherin/rds recombinants is measured at 37° a lag phase lasting 120 sec occurs, during which time little or no fusion is observed.10 This lag time increases to almost 300 sec when the temperature is lowered to 30°. Similar results are obtained with rim-specific vesicles, with the exception that the lag phase is shorter in duration; for example, at 37°, the lag phase is 60–100 sec. A comparison of these parameters is given in Table I.
Table I. Comparison of R18-Labeled Plasma Membrane Fusion with Various Target Membranesa.
| Target membrane | Addition | Lag time (sec) | IRF (%) = ΔF/time |
|---|---|---|---|
| Disks | 10 μM Ca2+ | <20 | 2.9 ± 0.04 |
| Rim-specific vesicles | 10 μM Ca2+ | 20–50 | 0.26 ± 0.02 |
| Peripherin/rds LUVs | 10 μM Ca2+ | 120 | 0.30 ± 0.03 |
Fusion of R18–PMs with disk membranes,12 isolated rim-specific vesicles, and peripherin/rds recombinant LUVs23 at 37°. The initial rate of fusion is given as the percent change in fluorescence intensity per unit time (minutes with disk membranes and seconds with rim-specific vesicles and peripherin/rds LUVs).
To ensure that the observed lag phase results from fusion-related events, both the temperature dependence and the effect of proteolysis or phospholipase treatment on fusion should be analyzed. A lag in the onset of fusion should increase when the temperature is lowered. If the fusion is protein mediated, the lag phase would also change in duration on proteolysis of the proposed fusion protein. Conversely, if the fusion is lipid mediated then any alteration in lipid properties after phospholipase treatment would affect a true lag phase.
Critical Notes Regarding R18 Dequenching Assay
The use of endogenous probes for the measurement of membrane fusion requires that a number of critical factors be considered and controls designed to account for specious results. Under some experimental conditions, a spontaneous transfer of R18 between membranes can occur in the absence of membrane merging and fusion.40 Such transfer has not been seen to any appreciable extent with R18–PM, when these membranes are incubated with phosphatidylcholine LUVs.6 In our experience, spontaneous transfer of R18 from plasma membrane vesicles to phosphatidylcholine SUVs or LUVs is indicative of permeable vesicles due to prolonged storage (longer than 24 hr).
Fusion should also be confirmed by alternative techniques, i.e., sucrose density gradient centrifugation (allows the separation of fused from unfused species11,12) or microscopy.17,18 Alternatively, the addition of high concentrations of unlabeled plasma membrane should inhibit R18 dequenching, indicating that the increase in fluorescence was due to the merger of two membranes. Conversely, binding of R18-labeled membranes to the target membrane should result in no dequenching or in a nonlinear change in R18 fluorescence. Investigators are strongly encouraged, when possible, to compare different fusion assays in the same system and to characterize known fusion properties of the membranes (i.e., specific inhibitors, temperature dependence, cation dependence, membrane specificity).
Characterization of Molecular Basis of Peripherin/rds-Mediated Fusion
Peripherin/rds is the first rod cell-specific fusion protein to be identified. Fusion is mediated through at least one region of peripherin/rds, from residues 311 to 325. A peptide analog to this region, called PP-5, promotes membrane adhesion and membrane destabilization,9,10,13 two prerequisite steps for fusion.41
An initial strategy in the identification of a candidate fusion protein and subsequent characterization of a fusogenic region within such a protein relies on proteolysis studies. Trypsinolysis of peripherin/rds led to the identification of a fusion-promoting region.9,10 The target membranes are incubated with trypsin (final concentration, 0.2 μg/ml) for 30 min in the dark at 37°. The reaction is stopped by the addition of trypsin inhibitor (final concentration, 0.4 mg/ml), and the samples are washed in 10 mM HEPES, pH 7.2, and centrifuged at 60,000 rpm for 40 min. The pellet is resuspended in 10 mM HEPES, pH 7.2, for the fusion assay; the supernatant containing the tryptic fragments is reserved on ice. Trypsinolysis of disk membranes and peripherin/rds recombinants under these conditions results in the cleavage of a 12.5-kDa band immunoreactive with anti-peripherin/rds antibody 2B6, which was found to correspond to the C terminus of peripherin/rds. Cleavage of this peptide fragment inhibited fusion of disks and peripherin/rds recombinants with R18–PM.10 Fusion activity is restored with the subsequent addition of the tryptic fragments at a ratio equal to that in the native membrane.10 The size of the tryptic fragments is determined by gradient gel electrophoresis42 and/or HPLC.43 Such experiments provide evidence that the tryptic fragments contain a fusion-promoting region of peripherin/rds that retained biological activity, as determined on the basis of the restoration of fusion activity, and led to the synthesis of overlapping C-terminal peptides (Table II).
Table II. Aligned Amino Acid Sequence of Synthetic C-Terminal Peripherin/rds Ppetidesa.
| Peptide | Amino acid sequence | |||
|---|---|---|---|---|
| PP-3 | V-E-A-E-G-E-D-A-G-Q-A-P-A-A-G | |||
| PP-4 | S-V-K-K-L-G-K-G-N-Q-V-E-A | |||
| PP-5 | V-P-E-T-W-K-A-F-L-E-S-V-K-K-L | |||
| PP-6 | L-K-S-V-P-E-T-W-K-A-F-L | |||
| PP-7 | W-K-A-F-L | |||
| Residue No. | H2N-308-------311--------315--------------321-----------------------------331-----------------------------------------------345-COOH | |||
Left to right.
Peptide-Induced Aqueous Contents Mixing as Measure of Fusion
The fusion-promoting activity of tryptic fragments and synthetic peptides is evaluated directly in an assay measuring the mixing of the aqueous contents of model membranes. This approach allows the investigator to determine if a peptide satisfies both criteria for fusion, i.e., membrane lipid and aqueous contents mixing. A fluorescence-based contents mixing assay using 8-aminonaphthalene-1,3,6-trisulfonic acid and p-xylene bispyridinium bromide (ANTS and DPX, respectively) has been chosen since these membranes are not biologically active. This assay is based on the principle that the fluorescence of one probe, ANTS, is quenched by a second probe, DPX; such probes are called quenching pairs.44 The two fluorophores are encapsulated separately into two different populations of vesicles. On fusion and contents mixing a collisional quenching of the ANTS occurs and is detected as a decrease in fluorescence.44,45
Materials
ANTS: 8-Aminonaphthalene-l,3,6-trisulfonic acid, disodium salt (Molecular Probes); store in dark
DPX: p-Xylene bispyridium bromide (Molecular Probes); store in dark
PS: Phosphatidylserine (bovine brain; Avanti Polar Lipids, Alabaster, AL)
N-Methyl-DOPE: N-Monomethyldioleoylphosphatidylethanolamine (Avanti Polar Lipids)
ANTS buffer: 10 mM Glycine, 45 mM NaCl, 25 mM ANTS, pH 9.5
DPX buffer: 10 mM Glycine, 90 mM DPX, pH 9.5
Preparation of ANTS- or DPX-Containing Vesicles
Large unilamellar vesicles (LUVs) encapsulating either ANTS or DPX are prepared from 95 mol% N-methyl-DOPE and 5 mol% PS (bovine brain).45,46 The dried lipids are resuspended in buffer containing either ANTS or DPX, subjected to five freeze–thaw cycles in liquid nitrogen, and extruded through a polycarbonate filter (Poretics, Pleasanton, CA; 467 mm, pore size 0.1 μm), using a Nuclepore (Pleasanton, CA) 47-mm in-line filter holder.45 Encapsulated fluorescent probe is separated from unincorporated probe on a Sephadex G-50 column eluted with 100 mM NaCl, 10 mM glycine, and 0.1 mM EDTA, pH 9.5. The vesicles containing the encapsulated probe elute with the void volume. Both the ANTS-containing and the DPX-containing vesicles are resuspended in 10 mM glycine, pH 9.5, to a final 1 mM phosphate concentration. The pH of the solutions must be accurate since N-monomethyl-DOPE will form nonbilayer structures below pH 8.0, resulting in vesicle aggregation.
ANTS-DPX Contents Mixing Assay
Fluorescence intensity is monitored at λex 380 nm and λem 510 nm. In a 3-ml fusion reaction mixture, the thermostatted cuvette contains 30 μl of ANTS-containing vesicles, 270 μ1 of DPX-containing vesicles, and 2.7 ml of column buffer (100 mM NaCl, 10 mM glycine, and 0.1 mM EDTA, pH 9.5). Fusion is initiated by lowering the pH of the fusion mixture from 9.5 to 4.5 (Fig. 3a) by the addition of 2 M sodium acetate–acetic acid (25–50 μl). Fusion is measured as a decrease in fluorescence intensity as the aqueous contents are mixed and an ANTS–DPX complex is formed. Baseline fluorescence is the intensity obtained with the shutters closed. The 100% fluorescence level is the initial fluorescence intensity before lowering the pH. The IRF between ANTS- and DPX-containing vesicles is calculated as shown in Eq. (2)47:
Fig. 3.
Membrane fusion detected by aqueous contents mixing of ANTS- and DPX-containing vesicles. Shown is a representative tracing of the decrease in fluorescence at 510 nm over time (seconds) as the pH of the fusion mixture (ANTS vesicles and DPX vesicles) is lowered with 2 M sodium acetate (a) at 37°.
| (2) |
where slopeANTS/DPX is equal to the linear change in ANTS fluorescence over time, F0 is the fluorescence intensity prior to the initiation of fusion with acetic acid and Ff is the fluorescence intensity with the shutters closed.
Effect of Peripherin/rds Peptides on ANTS–DPX Fusion
To determine if the various synthetic peptides to the C terminus of peripherin/rds are fusogenic, the ANTS–DPX fusion mixture is preincubated with the peptide at the desired temperature prior to the initiation of fusion. Routinely, the effect of a single peptide concentration on fusion is studied within a temperature range of 28 to 42°. The peptide concentrations used are in the millimolar to nanomolar range. Such studies, while necessary to describe a mechanistic pathway, should be performed to complement the fusion assays described above.
Pitfalls
ANTS–DPX Fusion
Transient perturbations, analogous to hemifusion, can be distinguished from fusion by ANTS–DPX leakage assays.48 The ANTS–DPX assay cannot be used to measure aqueous contents mixing of SUVs because ANTS binds excessively to such vesicles.49
Synthetic Peptides
To confirm the specificity of a peptide-induced fusion, a scrambled peptide (same amino acids in a different sequence), a fusogenic peptide from a different system, e.g., a viral fusion peptide, or a nonspecific peptide at high concentration should be tested. The fusogenic peptide should produce an effect at physiologically relevant peptide concentrations. Peptide-induced fusion in a model membrane system should affect fusion in the biological cell-free assay system. In photoreceptors, PP-5 inhibits fusion between disk and R18–PM when added in a 1:1 ratio with peripherin/rds.10 The secondary structure of the peptide should be evaluated in the fusion mixture and an effort made to denature this structure and show that the denatured species is unable to induce fusion.
Peripherin/rds Mutagenesis
Fusion-promoting region(s) of peripherin/rds can also be identified by mutagenesis. Peripherin/rds mutagenesis studies require a viable cell expression system in which the fusion competence of the cells or of the proteins harvested from the cells can be determined. The protocol for the determination of this fusion competency in Madin–Darby canine kidney (MDCK) cells is given below. A similar strategy can potentially be employed to characterize other cell expression systems.
Establishment of Fusion Competency of MDCK Cells Expressing Human Peripherin/rds
MDCK strain II cells stably transfected with human peripherin/rds with an Xpress N-terminal tag50 have been generously provided by R. Kim. The peripherin/rds is localized to the basolateral MDCK plasma membrane, and oriented with the N-terminal epitope tag and the C terminus on the extracellular surface.50 MDCK cells expressing human peripherin/rds are grown to confluence in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine calf serum, and isolated by scraping. The cells cannot be harvested with trypsin, since trypsinolysis hydrolyzes the fusogenic domain of peripherin/rds. The cells are resuspended in 145 mM NaCl, 5 mM KC1, 10 mM HEPES, pH 7.4. Fusion between whole MDCK cells and R18–PM is measured as described above. On the addition of ROS R18–PM to peripherin/rds-expressing MDCK cells at 37° an increase in R18 fluorescence was observed. The initial rate of fusion under these conditions is 0.35 ± 0.040 ΔF(fluorescence)/sec. Fusion between R18–PM and peripherin/rds-expressing cells is inhibited by the addition of 1.0 mM EGTA and by trypsinolysis of the MDCK cell plasma membranes. Fusion is not detected with R18–PM and MDCK cells containing no peripherin/rds. The R18 fluorescence profile seen in these assays is qualitatively similar to that shown in Fig. 2, with a lag time of 60–90 sec.
Resonance Energy Transfer-Based Lipid Mixing Assay
The R18 dequenching assay described above requires large quantities of MDCK cells and may not be usable if fewer cells are available. An alternative technique requiring less material and relying on resonance energy transfer (RET) may be used. Detection of lipid mixing by RET requires that two membrane preparations be labeled with a fluorescent probe at non-self-quenching concentrations. In the RET assay, pairs of fluorophores are used in which one fluorophore, designated the energy donor, has an emission band that overlaps with the excitation band of the second fluorophore, called the energy acceptor.51,52 When these two fluorophores are in close proximity, as would occur in membrane bilayers on fusion and lipid mixing, there is a transfer of the excited state energy from a donor to an acceptor.53 Thus the acceptor fluorophore fluoresces as though it has been excited directly. A large number of lipid probes satisfies the criteria for efficient energy transfer, and they are called energy transfer couples.18 RET between two fluorophores has a number of advantages51–53 when compared with the R18 lipid mixing assay for use in the MDCK cell system: (1) The RET method is extremely sensitive, requiring only small quantities of target membrane since the ratio of the two membranes undergoing fusion is equal; and (2) RET assays are adapted to be performed in 96-well plates, using a fluorescence plate reader in a total fusion reaction volume of 200 μl.54
Two approaches have been described in using RET techniques. In the first, a single membrane species is labeled with two fluorophores55; in the second, the fluorophores are incorporated into two separate membrane species and fusion is detected as an increase in fluorescence intensity of the acceptor fluorophore.54,56 In the MDCK cell system we have employed the latter approach, using R18–PM and F18-labeled MDCK cell plasma membranes, with a fluorescence plate reader at room temperature.54,56
Labeling of MDCK Cells and Rod Outer Segment Plasma Membrane with Fluorescent Membrane Probes
Purified ROS plasma membrane vesicles are labeled with R18 exactly as described above, with the exception that the concentration of R18 incorporated is equal to 0.8 to 1.0% relative to the membrane phospholipid. Unincorporated R18 is removed by chromatography on a Sephadex G-75 column (total volume of the column, 1–2 ml).
The stably transfected MDCK cells expressing human peripherin/rds are grown to confluence and isolated by scraping. The cells are resuspended in 145 mM NaCl, 5 mM KC1, 10 mM HEPES, pH 7.4, and labeled with 5-(N-octadecanoyl)aminofluorescein (F18), following the same protocol as described above for R18-labeled plasma membrane. The F18 is added at 0.80–1.0 mol% relative to phospholipid in the MDCK cells. Routinely, phospholipid phosphate24,25 is determined for each experiment and correlated to cell number. The F18 is removed by chromatography on a Sephadex G-75 column (total volume of the column, 1–2 ml).
R18–F18 Fusion Assay
Fusion between R18-labeled plasma membrane and F18-labeled MDCK cells is measured on a Perkin-Elmer LS 50B spectrofluorimeter equipped with a fluorescence plate reader (model L225 0137). Fusion is initiated by the addition of R18–PM to F18-labeled MDCK cells present in the 96-well plates. The change in fluorescence intensity is measured continuously for 2–10 min with λex 470 nm (F18 excitation) and at λem 524 nm (emission wavelength F18) and λem 592 nm (emission wavelength R18). The fluorescence intensity obtained with double-labeled plasma membrane is taken as maximal fusion. The extent of fusion (R) is calculated as shown in Eq. (3)54:
| (3) |
where I524 and I592 are the fluorescence intensities at 524 and 592 nm, respectively. The subscripts c and t represent the initial time point and any time thereafter, respectively. On fusion, fluorescence intensity increases at 592 nm and decreases at 524 nm, with the λex of 470 nm. To confirm that resonance energy transfer is occurring the emission scan of R18 from 500 to 680 nm is recorded. This assay may be performed on a larger scale in a thermostatted cuvette or on coverslips.56
Advantages of MDCK Cell Line
Peripherin/rds expressed in COS-1 cells is localized to the intracellular membranes and is abnormally glycosylated.57 For studying fusion processes the MDCK cell line has some important advantages over COS-1 cells: (1) In MDCK cells, peripherin/rds is also expressed on the plasma membrane surface, thus allowing whole cells to be used in fusion assays and easier manipulation of the membranes; (2) MDCK cells are stably transfected with human peripherin/rds, allowing a direct correlation between the fusion-promoting activity of peripherin/rds and human peripherin/rds disease-linked mutations; and (3) the human peripherin/rds expressed in MDCK cells is glycosylated and forms dimers as occurs in native photoreceptors.50
Pitfalls of Expression Systems
COS-1 and MDCK cells allow production of large quantities of peripherin/rds and peripherin/rds mutants for recombination and subsequent fusion studies. In addition to the commonly encountered shortcomings of cell expression systems, localization of peripherin/rds to the intracellular membranes of COS-1 cells may result in artifactual characterization of peripherin/rds-mediated fusion processes owing to the presence of endogenous proteins involved in other fusion processes. For example, in COS-1 cells peripherin/rds is localized to the intracellular membranes, which also contain a family of intracellular fusion proteins58,59 that may inhibit or enhance peripherin/rds-mediated fusion. In lieu of another expression system, such shortcomings can be overcome by characterizing in detail the specificity of the fusion pathway. Although the overexpression of the fusion protein may be essential for the isolation of large quantities of protein, membrane fusion processes dependent on this protein may actually be inhibited by excessive amounts of expressed protein. This possibility becomes more likely if a specific oligomeric form of the protein is required for fusion competency.
Pitfalls of Resonance Energy Transfer Assays
To identify and exclude extensive membrane aggregation as a contributor to energy transfer, EGTA or EDTA, known to decrease peripherin/rds-promoted vesicle aggregation, would selectively decrease RET efficiency due to aggregation. In pure liposome preparations, changes in the behavior of the membrane lipids may alter the quantum efficiency of various fluorophores, with changes in fluorescence observed due to factors other than fusion.60
Analysis of Individual Steps Contributing to Membrane Fusion
Once a region of a candidate fusion protein has been shown to promote fusion, the ability of this region to mediate the individual steps in fusion can be evaluated, taking into consideration the four steps of membrane fusion41: (1) aggregation of the fusing membranes, (2) close approach of the lipid bilayers, (3) destabilization of the membrane bilayer at the point of fusion (two bilayers that are closely opposed may not necessarily fuse), and (4) mixing of the components of the lipid bilayer and of the aqueous contents, to form a new structure. The protocols below describe how two mechanistic criteria necessary for fusion, membrane aggregation and destabilization,61 were met for PP-5.
Membrane Aggregation–Adhesion
The Ca2+-dependent aggregation of SUVs or LUVs can be monitored continuously as a change in absorbance at 380 nm in a Perkin-Elmer 2.0 UV/Vis spectrometer or equivalent equipped with a thermostatted cuvette holder. As the vesicles aggregate and become larger an increase in scattered light is detected as a change in absorbance, recorded every 0.1 min for 10 min.23 The SUVs are composed of phosphatidylcholine– phosphatidylserine–cholesterol (PC:PS:Choi, 4:4:1; final phosphate concentration, 1.5 mM).10,13 Aggregation is initiated with the addition of CaCl2 (final concentration, 16 μM Ca2+). Reversible aggregation is distinguished from fusion by the addition of 1 M EDTA to a final concentration of 33 mM and the absorbance is monitored for an additional 3 min. To determine the effect of individual peptides on aggregation, the vesicles are preincubated with peptide for 10 min at the indicated temperatures prior to the addition of calcium. The kinetic parameters of membrane adhesion can be directly calculated from the fluorescence data obtained in the R18 lipid mixing experiments, assuming a mass action kinetic model.62
Membrane Bilayer Destabilization
Membrane bilayer destabilization can be inferred fluorimetrically with the fluorescent dye 3,3′-diethylthiodicarbocyanine iodide (diS-C2-5; Molecular Probes) as a collapse in a valinomycin-induced diffusion potential in model membranes.61,63 SUVs composed of PC:PS: Choi in a 4:4:1 molar ratio are prepared by probe sonication in the presence of K+-containing buffer (50 mM K2S04, 10 mM HEPES–SO4, pH 6.8).38 An aliquot of the SUVs (phospholipid concentration, 36 nmol) is added to 10 ml of isotonic buffer (50 mM Na2S04,10 mM HEPES-SO4, pH 6.8) containing 10 μl of the fluorescent probe diS-C2-5 (stock, 1 mM) and incubated at 37° until a stable baseline fluorescence is established. The addition of valinomycin to a final concentration of 10−7 M selectively permeabilizes the vesicles to K+, creating a negative diffusion potential inside the vesicles and resulting in the quenching of the fluorescence of the dye. Peptides from a 1-mg/ml stock in either distilled H20 or K+-free buffer are added in 25-, 75-, or 100-μl aliquots. Fluorescence is recorded at λex 620 nm and λem 670 nm for 90 min after the addition of the various peptides. An increase in fluorescence intensity on the addition of peptides is indicative of a dissipation of the diffusion potential due to peptide-induced destabilization. To quantitate the total fluorescence recovered, fluorescence is monitored before the addition of valinomycin, after the addition of valinomycin, and after the addition of the desired peptide. The percent fluorescence recovery is calculated61 as shown in Eq. (4):
| (4) |
It is the fluorescence after the addition of the peptide, at time t, I0 is fluorescence after the addition of valinomycin, and If is the fluorescence intensity prior to the addition of valinomycin. Mellitin (final concentration, 9.0 μg/ml) is added to confirm that the increase in fluorescence observed is due to a collapse in the diffusion potential. Mellitin should dissipate the valinomycin-induced diffusion potential even after the addition of those peptides that have no destabilizing effect.
Membrane destabilization is inferred from the fluorescence experiments described above. Changes in bilayer structure can be measured directly using 31 P NMR64–66 and freeze–fracture electron microscopy.67
Conclusions
This chapter has summarized the technical advantages of fluorescence-based fusion assays as applied to retinal rod cells and shown how these assays led to the identification of peripherin/rds as a photoreceptor-specific fusion protein. It is important to note an added benefit of this approach, namely that the data obtained can be readily analyzed in the framework of a mass action kinetic model for fusion.68,69 Such a model depicts the overall fusion reaction as consisting of two distinct steps: a second-order aggregation reaction and the actual fusion event, which is first order. Spontaneous disk–plasma membrane fusion in the ROS11 has been shown to conform to such a model. Therefore, by using a variety of experimental conditions the rate constants of the aggregation step and fusion can be calculated, thereby providing greater understanding of the molecular mechanism of fusion in the ROS.
Acknowledgments
This work was supported by National Institute of Health Grant EY10420. The author thanks Dr. Robert Molday for generously providing anti-peripherin/rds 2B6 antibody. The author is grateful to Dr. R. Schimmel for continuous support, thoughtful discussions, and critical reading of the manuscript.
References
- 1.Düzgüneş N. In: Subcellular Biochemistry. Roodyn D, editor. Plenum Press; London: 1985. p. 195. [Google Scholar]
- 2.Pecheur E, Sainte Marie J, Bienvenue A, Hoekstra D. J Membr Biol. 1999;167:1. doi: 10.1007/s002329900466. [DOI] [PubMed] [Google Scholar]
- 3.White JM. Science. 1992;258:917. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 4.Young RW. Invest Ophthalmol Vis Sci. 1976;15:700. [PubMed] [Google Scholar]
- 5.Steinberg RH, Fisher SK, Anderson DH. J Comp Neurol. 1980;190:501. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- 6.Boesze-Battaglia K, Albert AD, Yeagle PL. Biochemistry. 1992;31:3733. doi: 10.1021/bi00130a002. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto B, Besharse JC. Invest Ophthalmol Vis Sci. 1985;26:628. [PubMed] [Google Scholar]
- 8.Besharse JC, Hageman G. Invest Ophthalmol Vis Sci. 1990;31:57. [PubMed] [Google Scholar]
- 9.Boesze-Battaglia K, Kong F, Lamba OP, Stefano FP, Williams DS. Biochemistry. 1997;22:6835. doi: 10.1021/bi9627370. [DOI] [PubMed] [Google Scholar]
- 10.Boesze-Battaglia K, Lamba OP, Napoli A, Sinha S, Guo Y. Biochemistry. 1998;37:9477. doi: 10.1021/bi980173p. [DOI] [PubMed] [Google Scholar]
- 11.Boesze-Battaglia K. Invest Ophthalmol Vis Sci. 1997;38:487. [PubMed] [Google Scholar]
- 12.Boesze-Battaglia K, Yeagle PL. Invest Ophthalmol Vis Sci. 1992;33:484. [PubMed] [Google Scholar]
- 13.Boesze-Battaglia K, Stefano FP, Fenner M, Napoli AA., Jr Biochim Biophys Acta. 2000 doi: 10.1016/s0005-2736(99)00226-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberman EA, Nenashev VA. Biofizika. 1968;13:193. [PubMed] [Google Scholar]
- 15.Monck JR, Fernandez JM. Curr Opin Cell Biol. 1996;8:524. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 16.Nussbaum O, Broder CC, Berger EA. J Virol. 1994;68:5411. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Düzgüneş N. Methods Enzymol. 1993;220 doi: 10.1016/0076-6879(93)20069-f. [DOI] [PubMed] [Google Scholar]
- 18.Düzgüneş N. Methods Enzymol. 1993;221 doi: 10.1016/0076-6879(93)21009-w. [DOI] [PubMed] [Google Scholar]
- 19.Düzgüneş N, Wilschut J, Papahadjopoulos D. In: Physical Methods in Biological Membranes and Their Model Systems. Conti F, Blumberg WE, DeGier J, Pocchiari F, editors. Plenum; New York: 1985. p. 193. [Google Scholar]
- 20.Keller PM, Person S, Snipes W. J Cell Sci. 1977;28:167. doi: 10.1242/jcs.28.1.167. [DOI] [PubMed] [Google Scholar]
- 21.Hoekstra D, Boer TD, Klappe K, Wilschut J. Biochemistry. 1984;23:5675. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- 22.Boesze-Battaglia K, Albert AD. Exp Eye Res. 1989;49:699. doi: 10.1016/s0014-4835(89)80064-8. [DOI] [PubMed] [Google Scholar]
- 23.Robertson S, Potter J. J Pharmacol. 1984;5:63. [Google Scholar]
- 24.Bartlett GR. J Biol Chem. 1959;234:466. [PubMed] [Google Scholar]
- 25.Litman BJ. Biochemistry. 1973;13:2545. doi: 10.1021/bi00737a028. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane-Stanley MGA. J Biol Chem. 1957;226:497. [PubMed] [Google Scholar]
- 27.Hoekstra D, Klappe K. J Virol. 1986;58:87. doi: 10.1128/jvi.58.1.87-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clague MJ, Schoch C, Zech L, Blumenthal R. Biochemistry. 1990;29:1303. doi: 10.1021/bi00457a028. [DOI] [PubMed] [Google Scholar]
- 29.Connell G, Molday RS. Biochemistry. 1990;29:4691. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- 30.Arikawa K, Molday L, Molday RS, Williams DS. J Cell Biol. 1992;116:659. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortiz OL, Molday RS. Invest Ophthalmol Vis Sci. 1996;37:352. [PubMed] [Google Scholar]
- 32.Illing M, Molday L, Molday RS. J Biol Chem. 1997;272:10303. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 33.Adams AJ, Tanaka M, Shichi H. Exp Eye Res. 1978;27:595. doi: 10.1016/0014-4835(78)90144-6. [DOI] [PubMed] [Google Scholar]
- 34.Litman BJ. Methods Enzymol. 1982;81:150. doi: 10.1016/s0076-6879(82)81025-2. [DOI] [PubMed] [Google Scholar]
- 35.Jermyn MA. Anal Biochem. 1975;68:332. doi: 10.1016/0003-2697(75)90713-7. [DOI] [PubMed] [Google Scholar]
- 36.Jackson M, Litman BJ. Biochemistry. 1982;21:5601. doi: 10.1021/bi00265a033. [DOI] [PubMed] [Google Scholar]
- 37.Fager RS, Sejnowski P, Abrahamson EW. Biochem Biophys Res Commun. 1972;47:1244. doi: 10.1016/0006-291x(72)90968-0. [DOI] [PubMed] [Google Scholar]
- 38.Barenholtz Y, Gibbs D, Litman BJ, Thompson T, Carlson FD. Biochemistry. 1977;16:2806. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 39.Boesze-Battaglia K, Fliesler SJ, Jun L, Young JE, Yeagle PL. Biochim Biophys Acta. 1992;1111:256. doi: 10.1016/0005-2736(92)90318-g. [DOI] [PubMed] [Google Scholar]
- 40.Loyter A, Citovsky V, Blumenthal R. Methods Biochem Anal. 1988;33:128. doi: 10.1002/9780470110546.ch4. [DOI] [PubMed] [Google Scholar]
- 41.Bentz J, Ellens H. Colloids Surf. 1988;30:65. [Google Scholar]
- 42.Schagger H, von Jagow G. Anal Biochem. 1987;166:368. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 43.McDowell JH, Nawrocki JP, Hargrave PA. Biochemistry. 1993;32:4968. doi: 10.1021/bi00069a036. [DOI] [PubMed] [Google Scholar]
- 44.Ellens H, Bentz J, Szoka FC. Biochemistry. 1984;23:1532. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- 45.Szoka F, Olson F, Heath T, Vail W, Mayhew E, Papahadjopoulos D. Biochim Biophys Acta. 1980;601:559. doi: 10.1016/0005-2736(80)90558-1. [DOI] [PubMed] [Google Scholar]
- 46.Ellens H, Bentz J, Szoka FC. Biochemistry. 1985;24:3099. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- 47.Kelsey D, Flanagan T, Young JE, Yeagle PL. Virology. 1991;182:690. doi: 10.1016/0042-6822(91)90610-n. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Kimura S, Imanishi Y. Biochim Biphys Acta. 1996;1283:37. doi: 10.1016/0005-2736(96)00071-5. [DOI] [PubMed] [Google Scholar]
- 49.Düizgüneş N, Bentz J. In: Spectroscopic Membrane Probes. Loew LM, editor. Vol. 1. CRC Press; Boca Raton, Florida: 1988. p. 117. [Google Scholar]
- 50.Kim RY. Invest Ophthalmol Vis Sci. 1998;39:S677. [PubMed] [Google Scholar]
- 51.Stryer L. Annu Rev Biochem. 1978;47:819. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 52.Forster TZ. Naturforsch A. 1949;4A:321. [Google Scholar]
- 53.Hoekstra D. Biochemistry. 1982;21:1055. doi: 10.1021/bi00534a036. [DOI] [PubMed] [Google Scholar]
- 54.Partearroyo MA, Cabezon E, Nieva JL, Alonso A, Foni RM. Biochim Biophys Acta. 1994;1189:175. doi: 10.1016/0005-2736(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 55.Driessen AJM, Hoekstra D, Scherphof G, Kalicharan RD, Wilschut J. J Biol Chem. 1985;260:10880. [PubMed] [Google Scholar]
- 56.Litwin V, Nagashima K, Ryder AM, Chun-Huey C, Carver JM, Olson WC, Alizon M, Hasel K, Maddon PJ, Alloway GP. J Virol. 996;70:6437. doi: 10.1128/jvi.70.9.6437-6441.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg AFX, Moritz L, Molday RS. Biochemistry. 1995;34:14213. doi: 10.1021/bi00043a028. [DOI] [PubMed] [Google Scholar]
- 58.Liniai M. J Neurochem. 1997;69:1781. doi: 10.1046/j.1471-4159.1997.69051781.x. [DOI] [PubMed] [Google Scholar]
- 59.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. Nature (London) 1993;362:318. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 60.Hoekstra D, Düzgüneş N. Methods Enzymol. 1993;220:15. doi: 10.1016/0076-6879(93)20070-j. [DOI] [PubMed] [Google Scholar]
- 61.Kliger Y, Aharon A, Rapaport D, Jones P, Blumenthal R, Shai Y. J Biol Chem. 1997;272:13496. doi: 10.1074/jbc.272.21.13496. [DOI] [PubMed] [Google Scholar]
- 62.Nir S, Klappe K, Hoekstra D. Biochemistry. 1986;25:8261. doi: 10.1021/bi00373a020. [DOI] [PubMed] [Google Scholar]
- 63.Loew LM, Rosenberg I, Bridge M, Gitler C. Biochemistry. 1983;22:837. doi: 10.1021/bi00273a020. [DOI] [PubMed] [Google Scholar]
- 64.Albert AD, Sen A, Yeagle PL. Biochim Biophys Acta. 1984;771:28. doi: 10.1016/0005-2736(84)90106-8. [DOI] [PubMed] [Google Scholar]
- 65.Mollevanger L, DeGrip WJ. FEBS Lett. 1984;169:256. doi: 10.1016/0014-5793(84)80329-4. [DOI] [PubMed] [Google Scholar]
- 66.DeGrip WJ, Drenthe EHS, Van Echteld CJA, De Kruijf B, Verkleij AJ. Biochim Biophys Acta. 1979;558:330. doi: 10.1016/0005-2736(79)90269-4. [DOI] [PubMed] [Google Scholar]
- 67.Hui SW, Stewart TP, Boni LT, Yeagle PL. Science. 1981;212:921. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- 68.Nir S, Stegmann T, Wilschut J. Biochemistry. 1986;25:257. doi: 10.1021/bi00349a036. [DOI] [PubMed] [Google Scholar]
- 69.Bentz J, Nir S, Covell DG. Biophys J. 1988;54:449. doi: 10.1016/S0006-3495(88)82978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]