Abstract
Background
Management of osteoarthritis (OA) requires both medical and behavioral strategies, but there is low use of some recommended therapies.
Objectives
This study examined the effectiveness of a combined patient and provider intervention for improving OA outcomes.
Design
Cluster randomized clinical trial with assignment to OA Intervention and Usual Care arms.
Setting
Department of Veterans Affairs Medical Center (VA) in Durham, NC, USA.
Participants
30 providers (clusters) and 300 outpatients with symptomatic hip and / or knee OA
Interventions
The telephone-based patient intervention focused on weight management, physical activity, and cognitive behavioral pain management. The provider intervention involved delivery of patient-specific OA treatment recommendations to primary care providers through the electronic medical record.
Measurements
Primary outcome: Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) total score (range: 0-96) at 12 months. Secondary outcomes: WOMAC function subscale (range: 0-68), WOMAC pain subscale (0-20), physical performance (Short Physical Performance Battery) and depressive symptoms (Patient Health Questionnaire-8). Linear mixed models adjusted for clustering of providers assessed the difference in improvement in outcomes between arms.
Results
At 12-month follow-up, WOMAC scores were 4.1 points lower (indicating improvement) in the OA Intervention arm vs. Usual Care [95% confidence interval (CI) = −7.2, −1.1; p=0.009]. The WOMAC function subscale was 3.3 points lower in the intervention arm [95% CI = −5.7, −1.0; p=0.005]. There was no difference in WOMAC pain subscale scores between arms (p=0.126). Physical performance and depressive symptoms did not differ between the two arms.
Limitations
The study was conducted in one VA medical center.
Conclusions
The combined patient and provider intervention resulted in modest improvement in self-reported physical function in patients with hip and knee OA.
Primary Funding Source
National Institute of Arthritis and Musculoskeletal and Skin Disorders
Clinical Trial Registration Numbers
Background
Osteoarthritis (OA) is one of the most common chronic health conditions and a leading cause of pain and disability (1-5). The prevalence of OA is on the rise, and this trend is expected to continue (6, 7). In addition to the substantial toll at the individual level (8), OA is placing an increasing burden on healthcare systems (9, 10).
Evidence-based guidelines indicate that adequate management of hip and knee OA requires both behavioral and clinical strategies (11-14). Physical activity and weight management are key behavioral strategies for managing hip and knee OA, but the majority of adults with OA are physically inactive and / or overweight (15, 16). Similarly, despite strong evidence supporting several clinical strategies (e.g., joint injections, physical therapy, pain medications), some of these treatments are under-utilized, and studies have shown lrates of adherence to OA quality indicators (17-20). There is a need to improve the OA management at both patient and provider levels. The purpose of our study was to examine a combined patient and provider intervention for managing OA in primary care.
Methods
The institutional review board of the Department of Veterans Affairs Medical Center in Durham, NC (DVAMC) approved this study. Detailed methods have been published previously (21).
Study Design
This was a cluster randomized controlled trial, with primary care providers (PCPs) assigned to OA Intervention or Usual Care control. Randomization was computer-generated, maintained by the study statistician, and stratified based on providers’ volume of female patients (<15% vs. ≥15%). We aimed to enroll ten patient participants, five white and five non-white, from each of 30 PCPs. PCPs assigned to the OA Intervention received the Provider Intervention, and their enrolled patient participants received the Patient Intervention. Patient participants in both arms continued with any usual medical care recommended by their providers. Since all study participants were patients enrolled at the DVAMC, usual care could include any of standard treatments for OA given by a PCP and referral to other providers or specialists. Provider and patient participants in the Usual Care arm received no additional intervention during the study period.
Participants and Recruitment
PCPs in the DVAMC Ambulatory Care Service with patient panels large enough to likely enroll n=10 participants were invited to participate. Patients were eligible if they had hip OA (based on radiographic evidence in the electronic medical record (EMR)) and / or knee OA (based on radiographic evidence in the EMR or meeting American College of Rheumatology clinical criteria (22)), along with patient-reported joint symptoms ( “pain, aching, stiffness or swelling in or around a hip or knee joint with arthritis” that was present or for which patient used pain medications on most days during the last month (23). Participants also had to be overweight (body mass index (BMI) ≥ 25) and not currently meeting Department of Health and Human Services physical activity recommendations (24). Exclusion criteria are summarized in Appendix A. Potential participants were identified from the DVAMC EMR, mailed an introductory letter and called for a screening interview. Eligible patients came to the DVAMC to complete consent and baseline assessments and were subsequently informed of their randomization assignment via telephone. Study team members involved in screening and consent were blinded to randomization. Potential patient participants were blinded to their PCP’s randomization until after baseline assessments.
Interventions
Patient Intervention
This was a twelve-month intervention focusing on physical activity, weight management and cognitive behavioral pain management strategies (21). Telephone calls were scheduled twice per month for the first six months, then monthly for the last six months, and were delivered by one counselor with training in OA and behavior change. Goal-setting and action-planning were major components of the intervention. Motivational interviewing strategies were employed by the counselor throughout the intervention (21, 25, 26). Participants were given written patient educational materials corresponding to intervention topics, an exercise video for patients with OA, and an audio CD of relaxation exercises.
Provider Intervention
This intervention involved delivery of the following patient-specific OA treatment recommendations to PCPs, based on published treatment guidelines (13, 14, 27): Refer to physical therapist, Refer for evaluation for knee brace, Refer to MOVE! (VA’s weight management program that includes physical activity counseling (28)), Perform or refer for intra-articular injection, Recommend topical NSAID or capsaicin, Add gastroprotective agent or remove NSAID, Discuss possibility new / alternate pain medication, Refer to orthopedic evaluation for joint replacement surgery. We developed algorithms (Appendix B) to determine when each treatment option may be reasonable for a PCP to consider for a patient (21). Study team members collected all information needed to complete the algorithm for each patient participant during baseline assessments. Patient-specific recommendations were delivered to PCPs via the EMR as a progress note requiring e-signature. The study team monitored upcoming visits for OA Intervention participants, and recommendations were delivered to PCPs about one week prior to participants’ first routine visit after enrollment. The recommendations remained available to PCPs within the EMR, but no further communications were transmitted to OA intervention providers.
Outcome Measures
Baseline and 12-month follow-up measures were completed in person, except for n=36 participants who could not return to the Durham VAMC at 12-months but completed some measures via telephone. The primary outcome was also assessed via telephone at 6-months. Outcomes assessors were blind to randomization. Participants were reimbursed $25 and $10 for completing in-person and phone assessments, respectively.
Primary Outcome
The primary outcome measure was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), a self-report measure of lower extremity pain (5 items), stiffness (2 items), and function (17 items) in the past two weeks (29-31). All items are rated on a 5-point Likert scale ranging from “none” to “extreme,” (total score range, 0=96, higher scores indicate worse symptoms and function).
Secondary Outcomes
We separately examined the WOMAC pain and function subscales. We also administered the Short Physical Performance Battery (SPPB) (32), a commonly used performance measure in older adults that includes three tests of balance, a timed 8-foot walk, and five chair stands. The total score ranges from 0 (worst performance) to 12 (best performance). . Depressive symptoms were assessed with the Patient Health Questionnaire-8 (PHQ-8) (33), with scores ranging from 0-24.
Process Measures
We collected additional “process measures” related to behaviors or outcomes intermediate to changes in symptoms or function. Self-reported physical activity was measured with the Community Health Activities Model Program for Seniors (CHAMPS) (34, 35), which assesses the frequency and duration of light to vigorous activities during the past four weeks; we report the weekly frequency and duration of all exercise, as well as moderate or higher intensity exercise. The CHAMPS has established test-retest reliability and association with accelerometer-based physical activity among older adults (36). BMI was calculated using measured height and weight; when 12-month follow-up measures were administered via telephone, weight was self-reported.
For all patient participants, we used the DVAMC EMR to examine PCPs’ OA-related referrals, as well as participants’ actual visits for physical therapy, knee braces, joint injections, MOVE!, and orthopedic consultation during their study period. We assessed changes in oral and topical pain medication by participant self-report. For oral pain medications, we defined new use during the study period as: a.) no pain medication use at baseline but some pain medication use at follow-up, OR b.) taking a different pain medication at follow-up than at baseline. For topical creams, we defined new users as those who reported using any type at follow-up but not at baseline. We acknowledge that use of new oral or topical pain medications may not necessarily lead to better pain control. For OA Intervention participants, we also report proportions whose PCP received each treatment recommendation from the study team, and among those, proportions who had referrals and visits for those treatments.
Participant Characteristics
We collected patient participant-reported age, gender, race/ethnicity (white vs. non-white), household financial situation ( “inadequate income” defined as participants who reported that they “just meet basic expenses” or “don’t even have enough to meet basic expenses”), education level, marital status, work status (employed or student vs. other), disabled (positive response to “disabled” in the work status question, regardless of other response(s) selected), self-rated health (excellent, very good or good vs. fair or poor), and duration of OA symptoms. We also report presence of OA in the knee only, hip only or both, based on enrollment data described above. For provider participants, we collected information on clinician category (physician, nurse, nurse practitioner or physician assistant), gender and whether the patient panel was <15% or ≥15% female.
Adverse Events
We inquired at each study encounter about hip and knee injuries or surgeries, per protocol. Information about other adverse events was noted when participants informed the study team during a regular contact, or when an event was discovered during study-related review of medical records.
Sample Size
Our sample size of 300 patient participants was based on detecting a moderate effect size of approximately 0.30 for the difference in mean WOMAC scores between arms, with 80% power and a type-I error rate of 0.05. This translates to a 4.2 point difference at 12-months, which is equivalent to approximately 11% improvement from anticipated mean baseline score; this allowed sufficient power to detect a clinically relevant difference (12-18% based on prior relevant literature) (37-39). We used a two-sample t-test sample size calculation for the between arm difference at 12-months, multiplied by a factor 1-(rho)^2, where rho represents the Pearson correlation between baseline and follow-up outcome measures (0.60) (40). This sample size was then adjusted to reflect provider clustering using an intraclass correlation coefficient (ICC) of 0.02(41) and inflated to compensate for potential attrition (12%). Based on our pilot work we assumed a mean baseline WOMAC score of 38 with a standard deviation of 14.
Data Analyses
Our primary hypothesis was that OA Intervention participants would have significantly greater improvement in WOMAC scores than Usual Care. Analyses involved all randomly assigned participants using all data collected (42). Observations were not deleted due to missing follow-up data (43). The estimation procedure for our analytic technique (linear mixed models) implicitly accommodates missingness when related to prior outcome, or to other baseline covariates in the model (defined as missing at random (MAR)). To assess the primary model’s robustness to missing observations, we multiply imputed missing WOMAC follow-up scores using a Markov chain Monte Carlo (MCMC) algorithm incorporating additional variables beyond those in the linear mixed effects models to strengthen the MAR assumption (Appendix C).
For primary and secondary outcomes, as well as continuous process measures, linear mixed models were fit using the SAS procedure PROC MIXED (44). A random effect to account for clustering of primary care providers and an unstructured covariance structure for the repeated measures over time were used. For CHAMPS outcomes , change from baseline to 12-months was used due to normality assumptions. Predictors in all models included dummy coded follow-up time effect(s) and an indicator variable for the intervention interacting with follow-up time effect(s)(45). This model assumes study arms have equal baseline means, which is appropriate for a randomized control trial and is equivalent in efficiency to an ANCOVA model (46). The final models also included patient participant race and the provider stratification for percentage of female patients . Our primary inference was on the treatment by 12-month follow-up time point parameter, as this was the estimated difference between arms at the end of the study. For CHAMPS 12-month change from baseline scores, model fixed effect terms included baseline CHAMPS score and an indicator variable for study arm. For BMI analysis, in-person weight at 12-month follow-up was the outcome; sensitivity analyses included self-reported weight from participants without an in-person 12-month visit.
In a post-hoc analysis to examine clinically meaningful change in WOMAC scores (based on prior studies of behavioral and rehabilitative interventions) (37, 39, 47), we categorized patients as either improved or not at 12-months, using a 12% improvement in total WOMAC score from baseline (5.8 point reduction) and an 18% improvement from baseline (8.7 point reduction). We used generalized estimating equation (GEE) models (48) with a logit link function and an unstructured correlation structure and used empirical standard errors for inference.
Role of the Funding Source
The funding agency approved the project and monitored progress but had no direct role in the design or conduct of the study, analyses, or decision to submit the manuscript for publication.
Results
Participant Enrollment and Retention
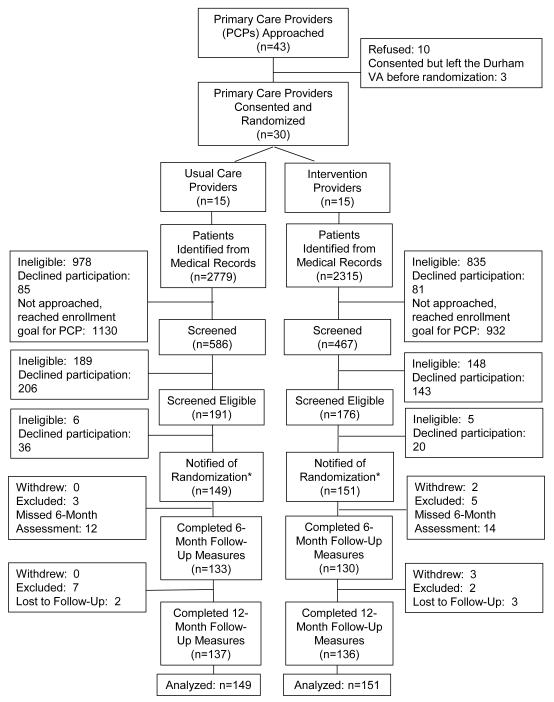
Forty-three PCPs were approached and 30 were enrolled (Figure 1, Table 1). We identified n=5,094 potential patient participants from the EMR. Of 1,503 patients screened by telephone, 356 were eligible and 300 were enrolled and randomized (Table 1). There were 151 patient participants in the OA Intervention and Usual Care arms, respectively. One patient was assigned to a Usual Care provider at the beginning of the recruitment process but switched to an OA Intervention provider prior to being notified of study group assignment. It was determined that the participant should be included in the assigned arm of his provider at the time of notification of randomization assignment (OA Intervention).
Figure 1.
CONSORT Diagram.
* One patient switched from a Usual Care Provider to an Osteoarthritis Intervention Provider before being notified of randomization and was analyzed with the Osteoarthritis Intervention Group.
Table 1.
Provider and Patient Participant Characteristics at Baseline
| Provider Participants | Total Sample (N=30) |
Usual Care (N=15) |
OA Intervention Group (N=15) |
|---|---|---|---|
|
| |||
| Mean Study Patients Per Provider (SD) | 10 (2.1) | 9.9 (1.7) | 10.1 (2.5) |
|
| |||
| < 15% Females in Patient Panel N (%) | 25 (83.3) | 13 (86.7) | 12 (80.0) |
|
| |||
| Men N (%) | 12 (40.0) | 5 (33.3) | 7 (46.7) |
|
| |||
| Provider Type N (%) | |||
| Physician | 19 (63.3) | 9 (60.0) | 10 (66.7) |
| Nurse Practitioner | 3 (10.0) | 0 (0) | 3 (20.0) |
| Physician Assistant | 7 (23.3) | 6 (40.0) | 1 (6.7) |
| Registered Nurse | 1 (3.3) | 0 (0) | 1 (6.7) |
|
| |||
| Patient Participants |
Total Sample
(N=300) |
Usual Care
(N=149) |
OA Intervention Group
(N=151) |
|
| |||
| Mean age (SD), y | 61.1 (9.2) | 61.7 (9.0) | 60.4 (9.4) |
|
| |||
| Men N (%) | 272 (90.7) | 141 (94.6) | 131 (86.8) |
|
| |||
| Non-White Race N (%) | 150 (50.0) | 75 (50.3) | 75 (49.7) |
|
| |||
| Married or Living with Partner N (%) | 199 (66.3) | 106 (71.1) | 93 (61.6) |
|
| |||
| High School Education or Less N (%) | 81 (27.0) | 44 (29.5) | 37 (24.5) |
|
| |||
| Inadequate Income N (%) | 103 (34.3) | 48 (32.2) | 55 (36.4) |
|
| |||
| Employed or Student N (%)* | 127 (42.8) | 60 (40.5) | 67 (45.0) |
|
| |||
| Disabled N (%)* | 98 (33.0) | 53 (35.8) | 45 (30.2) |
|
| |||
| Fair or Poor Health N (%) | 115 (38.3) | 57 (38.3) | 58 (38.4) |
|
| |||
| Mean Body Mass Index (SD), kg/m2 | 33.8 (5.8) | 33.4 (5.7) | 34.3 (6.0) |
|
| |||
| Joint(s) with OA N (%) | |||
| Knee Only | 238 (79.3) | 124 (83.2) | 114 (75.5) |
| Hip Only | 32 (10.7) | 14 (9.4) | 18 (11.9) |
| Knee and hip | 30 (10.0) | 11 (7.4) | 19 (12.6) |
|
| |||
| Mean duration of arthritis symptoms (SD), y* |
14.2 (11.6) | 14.6 (12.1) | 13.8 (11.1) |
|
| |||
| Mean WOMAC score (SD)* | 48.4 (17.5) | 47.8 (17.4) | 48.9 (17.6) |
|
| |||
| Mean SPPB score (SD)* | 8.0 (2.6) | 8.1(2.5) | 8.0 (2.6) |
|
| |||
| Mean PHQ-8 Score (SD)* | 6.8 (5.4) | 6.4 (5.1) | 7.2 (5.6) |
|
| |||
| Median frequency of All Exercise per week; CHAMPS (IQR)* |
14.0 (8.0, 23.0) | 14.0 (8.0, 23.5) | 14.0 (9.0, 22.0) |
|
| |||
| Median duration of All Exercise - hours per week; CHAMPS (IQR)* |
10.0 (4.8, 17.3) | 10.8 (5.0, 17.9)) | 9.8 (4.8, 16.8) |
|
| |||
| Median frequency of Moderate or Greater Intensity Exercise per week; CHAMPS (IQR)* |
5.0 (2.0, 10.0) | 5.0 (2.0, 10.0) | 5.0 (2.0, 9.0) |
|
| |||
| Median duration of Moderate or Greater Intensity Exercise - hours per week; CHAMPS (IQR)* |
3.5 (0.5, 8.3) | 3.5 (0.5, 9.5) | 2.8 (0.5, 7.8) |
OA=Osteoarthritis; SD=Standard Deviation; WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index; SPPB=Short Performance Physical Battery; PHQ-8=Patient Health Questionnaire-8; CHAMPS=Community Health Activities Model Program for Seniors; IQR=Interquartile Range
<= 4 participants missing data for employment, duration of arthritis, WOMAC, PHQ-8; CHAMPS; 16 participants are missing data for the SPPB score (11 in OA intervention and 5 in the control group)
Among enrolled participants, 88% completed 6-month measures, and 91% completed 12-month measures (Figure 1). Seventeen participants were excluded before study completion for the following reasons : hip / knee replacement or other significant hip / knee surgery (n=10), developed serious health condition that would make generalized exercise or diet advice risky (n=3), exclusion criteria present that were not identified prior to consent (n=2), moved out of area and treatment recommendations could not be issued (n=1), and switched from an OA Intervention provider to a Usual Care provider before treatment recommendations were issued (n=1).
Intervention Delivery
The average number of participants enrolled per provider was 10.0 (SD=2.1; range 3-12). OA Intervention participants completed an average of 11.5 phone calls (SD=4.9) out of 18 planned calls, and the average length of calls was 16.6 minutes (SD =12.4). For all but eight patient participants, the study team was able to deliver treatment recommendation(s) to the PCP within the intervention period. Of the eight participants, two were excluded from the study, one withdrew, one was reassigned to a non-study PCP who had not yet seen the patient, two transferred to another VA Medical Center, and two were inadvertently missed by the study team. For some participants (n=26), non-acute visits were scheduled with PCPs not enrolled in the study; in all but one case the covering PCP agreed to receive the recommendations. The mean number of recommendations issued per patient participant was 4.6 (SD = 1.8).
Adverse Events
Four study-related adverse events occurred, but none were associated with the OA intervention.
Primary Outcome
At 12 months, the estimated mean improvement in WOMAC score was −4.0 points (95% Confidence Interval (CI) −6.2, −1.8) in the OA Intervention arm and 0.1 point (95% CI −2.1, 2,3) in the Usual Care arm, with an estimated mean difference of −4.1 points (95% CI −7.2, −1.1; p=0.008) between arms (Table 2). There was improvement in both arms at 6 months compared to baseline, with the mean score for the Usual Care arm rebounding to baseline levels by 12 months. WOMAC scores also increased between 6 and 12 months for the OA Intervention arm but remained below baseline scores. There was a clustering effect for WOMAC scores within provider, with an estimated ICC of 0.02. Similar results were found using the multiply imputed datasets (mean difference = −4.2 (95% CI, −7.3 −1.2; p=0.007). For the post-hoc analysis examining improvement of 12% or 18% of WOMAC score from baseline to 12-months, an estimated 47.2% of participants improved 12% at 12-months in the OA Intervention arm compared to 37.5% in Usual Care (OR 1.3 95% CI (1.0,1.6); p=0.089); 36.1% improved 18% in the OA Intervention arm compared to 28.2% in Usual Care (OR 1.3 95% CI (0.9,1.8); p=0.166).
Table 2.
Estimated Mean Differences between Usual Care and Intervention Groups in Patient Outcomes&
| Outcome^ | Time Point |
Usual Care (N=149) |
OA Intervention (N=151) |
Treatment difference OA-UC (95% CI)* |
P |
|---|---|---|---|---|---|
| WOMAC Total Score | |||||
| Baseline | 48.4 | n/a | n/a | ||
| 6 months | 43.5 | 41.2 | −2.3 (−5.6, 0.9) | 0.162 | |
| 12 months | 48.5 | 44.4 | −4.1 (−7.2, −1.1) | 0.009 | |
|
| |||||
| WOMAC Pain Subscale | |||||
| Baseline | 10.2 | n/a | |||
| 6 months | 8.7 | 8.5 | −0.2 (−0.9, 0.5) | 0.59 | |
| 12 months | 9.9 | 9.4 | −0.5 (−1.2, 0.2) | 0.126 | |
|
| |||||
| WOMAC Function Subscale | |||||
| Baseline | 33.8 | n/a | n/a | ||
| 6 months | 30.7 | 28.7 | −1.9 (−4.4, 0.6) | 0.127 | |
| 12 months | 34.3 | 31.0 | −3.3 (−5.7, −1.0) | 0.005 | |
|
| |||||
| Physical Function (SPPB) | |||||
| Baseline | 8.0 | n/a | n/a | ||
| 12 months | 7.6 | 7.8 | 0.3 (−0.3, 0.9) | 0.38 | |
|
| |||||
| Depressive Symptoms (PHQ-8) | |||||
| Baseline | 6.8 | n/a | n/a | ||
| 12 months | 6.8 | 6.2 | −0.6 (−1.5, 0.3) | 0.160 | |
|
| |||||
| Change from Baseline in Weekly Frequency of all Exercise (CHAMPS) |
12 months | −0.3 | 3.0 | 3.3 (0.8,5.8) | 0.009 |
|
| |||||
| Change from Baseline in Duration (Hours per Week) of All (CHAMPS) |
12 months | 0.2 | 3.9 | 3.6 (1.3,5.9) | 0.003 |
|
| |||||
| Change from Baseline on Weekly Frequency of Moderate or Greater Intensity Exercise (CHAMPS) |
12 months | −1.1 | 0.5 | 1.6 (0.1,3.1) | 0.042 |
|
| |||||
| Change from Baseline in Duration (Hours per Week) in Moderate or Greater Intensity Exercise (CHAMPS) |
12 months | −0.5 | 1.2 | 1.6 (0.3,2.9) | 0.017 |
|
| |||||
| Body Mass Index | |||||
| Baseline | 33.8 | n/a | n/a | ||
| 12 months | 33.6 | 33.5 | −0.1 (−0.5, 0.2) | 0.47 | |
OA=Osteoarthritis; WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index; SPPB=Short Performance Physical Battery; PHQ-8=Patient Health Questionnaire-8; CHAMPS=Community Health Activities Model Program for Seniors
Linear Mixed Model Results; ^37 participants (21 OA Intervention, 16 Usual Care) and 27 participants (15 OA Intervention; 12 Usual Care) have no follow-up data for 6 and 12 months, respectively; additional missing across all time points due to scoring algorithms, refusal to participate, ≤ 6 participants missing WOMAC scores (1 baseline; 2 OA Intervention, 1 Usual Care 6 months; 1 OA Intervention, 1 Usual Care 12 months); 55 missing SPPB score (16 baseline;, 21 OA Intervention, 18 Usual Care 12 months); 19 missing PHQ scores (4 baseline, 5 OA Intervention, 10 Usual Care 5, 12 months); 11 missing frequency and duration of exercise (1 baseline, 4 OA Intervention, 6 Usual Care 12months); 4 missing measured BMI (3 OA Intervention, 1 Usual Care)
Difference of estimated treatments may not match estimated difference in means exactly due to rounding
Secondary Outcomes
We found no difference between arms in the WOMAC pain subscale (p=0.52 at 6 months; p=0.126 at 12 months, Table 2). However, at 12-months, the estimated mean WOMAC physical function score in the OA Intervention arm was 3.3 points (95%CI 1.0, 5.7; p=0.005) lower than Usual Care. For objective physical function and depressive symptoms we found no differences between arms (Table 2). Because some patient participants completed 12-month follow-up assessments by telephone, SPPB scores were missing more often at this time point than the self-report measures (see Table 2 for details).
Process Measures
The OA Intervention arm had greater improvement in both the frequency and duration of all physical activity at 12-months compared to Usual Care: 3.3 more times per week (p=0.009) and 3.6 more hours per week (p=0.003; Table 2). Similar results were found for change in physical activity of moderate or greater intensity, with an increase of 1.6 more times per week and 1.6 more hours per week in the OA Intervention arm compared to Usual Care (p=0.04 and p=0.02, respectively). We found no difference between arms for BMI. Results were similar in sensitivity analyses including patient participants for whom weight was self-reported at 12-months..
OA Treatment Recommendations and Use
Table 3 shows proportions of all patient participants who received specific OA-related referrals and treatments or visits during their study period, by study arm. PCP referrals to several rehabilitative and behavioral therapies were somewhat higher for OA Intervention than Usual Care; these included physical therapy (12% vs. 7%), knee braces (19% vs. 11%), and MOVE! (20% vs. 3%). However, the numbers of patients who actually received these therapies at the Durham VAMC during the study period were low for both study arms. Referrals and receipt of joint injections were similar by arm, as were orthopedic visits. Referrals are not needed for topicals, NSAIDs or alternative pain medication. Similar proportions of patient participants in the OA Intervention and Usual Care arms reported new use of topical creams (NSAIDs or capsaicin) at 12-month follow-up (9% and 7%, respectively). Proportions of patient participants who reported using a new or different pain medication at follow-up were also similar (36% and 35%, respectively).
Table 3.
Provider Referrals and Treatment Receipt
| Usual Care (n=149) |
OA Intervention (n=151) |
|||
|---|---|---|---|---|
|
| ||||
| Treatment / Visit^ | Received Referral from Provider N (%) |
Received Treatment N (%)* |
Received Referral from Provider N (%) |
Received Treatment N (%)* |
|
| ||||
| Physical Therapy† | 10 (6.7) | 2 (20.0) | 18 (11.9) | 1 (5.6) |
| Knee OA | 1 (10.0) | 0 (0.0) | ||
| Hip OA | 1 (10.0) | 1 (5.6) | ||
|
| ||||
| Knee Brace | 17 (11.4) | 3 (17.6) | 29 (19.2) | 5 (17.2) |
|
| ||||
| MOVE! Program | 5 (3.4) | 2 (40.0) | 30 (19.9) | 8 (26.7) |
|
| ||||
| Orthopedic Visit† | 9 (6.0) | 6 (66.7) | 8 (5.3) | 4 (50.0) |
| Knee OA | 5 (55.6) | 3 (37.5) | ||
| Hip OA | 1 (11.1) | 1 (12.5) | ||
|
| ||||
| Joint Injection | 16 (10.7) | 7 (4.7) | 14 (9.3) | 8 (5.3) |
OA=Osteoarthritis; PA=Physical Activity
Missing data: Treatment receipt: Topicals −16 Usual Care, 16 OA Intervention; Pain Medication – 12 Usual Care, 15 OA Intervention,
For physical therapy, knee braces, MOVE! and orthopedic visits, proportions are calculated using participants with referrals (during the study period) as the denominator. For joint injections (which can be done by a primary care provider without a specialty consult), proportions are calculated using all participants in the study arm as the denominator.
Referrals for physical therapy and orthopedic visits did not differentiate between hip and knee OA
Table 4 shows proportions of OA Intervention participants whose PCP received specified treatment recommendations from the study team, and of those, proportions who had PCP referrals and visits for those treatments. The most commonly issued treatment recommendations were for MOVE! (87%) and discussion of new pain medication (83%). PCPs of 16 participants received a recommendation for adding a gastroprotective agent or removing from NSAID; data not shown in table since referral and treatment data were not obtainable from the EMR. Proportions of participants who received referrals from PCPs ranged from 11%-43% of those with a study-issued recommendation. However, numbers of participants who received each treatment were low.
Table 4.
Treatment Recommendations, Provider Referrals and Treatment Receipt in OA Intervention Group
| Treatment / Visit^ | PCP Received Treatment Recommendation from Study Team N (%) |
Received Treatment Recommendation and Referral from PCP N (%)** |
Received Treatment Recommendation, Referral and Treatment N (%)*** |
|---|---|---|---|
|
| |||
| Physical Therapy† | 74 (49.0) | 15 (20.3) | 1 (6.7) |
| Knee OA | 0 (0.0) | ||
| Hip OA | 1 (6.7) | ||
|
| |||
| Knee Brace | 62 (41.1) | 22 (35.5) | 2 (9.1) |
|
| |||
| MOVE! Program ‡ | 131 (86.8) | 30 (22.9) | 8 (26.7) |
| Weight and PA | 110 (72.9) | 25 (22.7) | 7 (28.0) |
| Weight Only | 14 (9.3) | 2 (14.3) | 0 (0.0) |
| PA only | 7 (4.6) | 3 (42.9) | 1 (33.3) |
|
| |||
| Orthopedic Visit† | 18 (11.9) | 2 (11.1) | 0 (0.0) |
| Knee OA | 0 (0.0) | ||
| Hip OA | 0 (0.0) | ||
|
| |||
| Joint Injection | 33 (21.9) | N/A | 9(27.3) |
|
| |||
| Topical NSAID or Capsaicin |
75 (49.7) | N/A | 11 (14.7) |
|
| |||
| Discuss New/ Alternative Pain Medication |
125 (82.8) | N/A | 46 (36.8) |
OA=Osteoarthritis; PA=Physical Activity; PCP = Primary Care Provider; NSAID=Nonsteroidal Anti-inflammatory Drug
Missing data: Treatment recommendations, 8 participants unable to issue reccomendations; Treatment receipt: Topicals −16 Usual Care, 16 OA Intervention; Pain Medication – 12 Usual Care, 15 OA Intervention,
Proportion is based on participants with recommendation as the denominator
For physical therapy, knee braces, MOVE! and orthopedic visits, proportions are calculated using participants with participants that received treatment recommendations and referrals (during the study period) as the denominator. For joint injections (which can be done by a primary care provider without a specialty consult), topical,pain medication variables, proportions are calculated using participants that received treatment recommendations as the denominator.
Treatment recommendations and referrals for physical therapy and orthopedic visits did not differentiate between hip and knee OA;
Participants could receive MOVE! recommendations for physical activity, weight management or both. These recommendations are listed separately, but provider referrals and MOVE! visits did not differentiate.
Discussion
In this study, a patient and provider OA intervention improved the primary outcome (total WOMAC score) at 12-months. WOMAC function subscale scores also improved at 12months. This is important since OA is one of the main contributors to functional limitations in adults (4), leading to loss of abilities to perform daily activities if not mitigated. The WOMAC function score makes up a large portion of the total score (68 out of 96 total possible points), and this likely drove changes in the primary outcome. The intervention also improved physical activity levels. Research indicates only about 10% of patients with OA meet physical activity recommendations (49), and increasing activity levels in these patients is a high public health priority (50). We found no statistically significant or clinically relevant differences between arms for other secondary and process outcomes, including the WOMAC pain subscale, depressive symptoms and BMI (51, 52).
Effects of this OA Intervention on WOMAC total and function subscale scores were modest. In the context of behavioral and rehabilitative interventions for OA, thresholds for clinically meaningful improvement in WOMAC have typically ranged from 12%-18% (37-39). In this study we observed an 8.5% improvement in WOMAC total scores and a 9.8% improvement in WOMAC function scores in the OA Intervention arm, which are below these thresholds. We found that 47% and 36% of OA Intervention patient participants achieved 12% and 18% improvement in WOMAC total scores, respectively. Future analyses will examine whether specific participant characteristics were associated with differential improvement.
For both study arms, WOMAC scores improved at the interim 6-month follow-up assessment, with somewhat greater improvement in the OA Intervention arm. WOMAC scores then increased by 12-months (indicating relapse); scores remained improved compared to baseline for the OA Intervention arm only. In the OA Intervention arm, the decline in response between 6 and 12 months may have been related to the decrease in frequency of intervention calls. There are several possible reasons that effects of this intervention were smaller than those observed in some other studies of behavioral interventions OA (53-57). First, the intervention was less intensive than those examined in most prior studies, involving no in-person visits and relatively brief telephone calls. Although a low-intensity intervention is appealing from a resource and dissemination perspective, more intensive interventions may be necessary to enable patients with OA to achieve and sustain clinically relevant improvements. Second, study participants had greater pain and functional limitations than in samples from most prior studies of this type (53-55). Advanced OA can be more difficult to alleviate with non-surgical options, which may have limited treatment benefits for some of our participants. Third, participants also had a high level of comorbid physical and psychological health conditions (58), which can contribute to outcomes such as pain, function and depressive symptoms (59). Fourth, most similar studies have recruited patients predominantly by self-referral, which may attract primarily highly motivated individuals. By approaching patients proactively and inviting them to participate, our recruitment strategy was designed to identify a sample that was comparable to a typical clinic population in terms of motivation to engage in a lifestyle change intervention. Fifth, specifically related to the SPPB, tests included in this battery may not be highly sensitive to change in this type of patient sample, and recent recommendations indicate that other physical performance tests may be more appropriate in the context of knee and hip OA (60).
This study was novel in its approach to a provider-based intervention for OA, which resulted in greater referrals for some treatments, particularly physical therapy, knee braces and referral to MOVE!. However, participants’ actual use of these therapies did not differ between study arms and was quite low overall. Some participants may have been unwilling or unable to return to the DVAMC for additional treatments, since many of these participants live relatively far from the facility. Some participants may have received these treatments after their observed study period or outside the VA and therefore not captured in our data pull. There is a need for additional work to understand reasons patients may choose not to initiate key behavioral and rehabilitative therapies for OA, particularly when referred by their PCPs. For other clinical treatments addressed in the provider intervention (joint injections, medication use, orthopedic referrals), there were no substantial differences between study arms. PCPs may already recommend these treatments regularly, as suggested by relatively common use of joint injections in both study arms. .
There are several limitations to this study. First, this study design did not allow separate examination of patient and provider interventions. Second, participants received care at a VA medical center, with a high proportion of men. This may limit generalizability of findings. Third, there are limitations to the accuracy of self-reported physical activity data; however, we do not suspect this differed between study arms. Fourth, algorithms for generating treatment recommendations required more extensive data collection than would be feasible in clinical settings. However, simpler sets of criteria could be used to trigger these recommendations in clinical situations.
In conclusion, this patient provider intervention resulted in modest improvements in self-reported physical function and physical activity for Veterans with hip and knee OA. The provider-based intervention seemed particularly useful for increasing referrals for behavioral and rehabilitative programs. However, changes in study outcomes were modest, which may indicate that higher intensity interventions are needed to yield clinically meaningful changes in OA-related outcomes.
Acknowledgements
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs. The authors thank the Ambulatory Care Service of the Durham VA Medical Center for participation in the study, as well as study team members Jennifer Chapman, Karen Juntilla, and Laurie Marbrey. We are also deeply grateful to all of the Veterans who participated in this study.
Financial Support: This project was supported by the Department of Veterans Affairs, Health Services Research and Development Service (IIR 10-126). Dr. Bosworth is funded by a Career Scientist (08-027) award.
Appendix A: Exclusion Criteria
| Other rheumatologic conditions |
| Recent hip or knee surgery or injury |
| On waiting list for arthroplasty |
| Recent hospitalization for cardiovascular or cerebrovascular events |
| Severe neurologic or psychiatric conditions |
| Severe memory loss |
| Terminal illness |
| Nursing home residence |
| Severe hearing or speech impairment |
| Blindness |
| Current participation in another OA intervention or other lifestyle change study |
| Current pregnancy or plans to become pregnant |
| No primary care visits at the Durham VAMC in the past 12 months |
Appendix B: Provider Intervention Recommendations and Algorithms
Refer to physical therapy for evaluation and/or therapeutic exercises
Criteria:
Patient may be interested in being referred for physical therapy for OA if their provider recommends, AND
Patient is not doing lower extremity strengthening exercises ≥2 times per week, AND
Patient indicates being dissatisfied with their ability to perform one more activities on the Satisfaction with Physical Function Scale (walking, lifting / carrying, stair climbing, housework), AND
Patient has not seen a physical therapist for their OA in the past year.
Refer for evaluation for knee brace
Criteria: (for each knee with OA):
Patient is not currently using a knee brace, AND
Patient may be interested in trying a knee brace (or different kind of knee brace) if their provider recommends.
Criteria for Specific Brace Consults (VA-Based Study Only)
Knee Sleeve: Knee pain rating 1-3 (on a 10cm visual analog scale) AND varus/valgus alignment <10°, AND does not indicate knee “buckling”.
Hinged Brace: Knee pain rating >3 (on a 10cm visual analog scale) OR indicates knee “buckling”, AND varus/valgus alignment ≤15°.
Unloader Brace: Knee pain >3 (on a 10 cm visual analog scale) OR indicates knee “buckling,” AND varus/valgus alignment >15°.
Refer to weight management program (MOVE!)
Criteria:
Patient has BMI ≥ 25, AND
Patient may be interested in being referred to a weight management program if their provider recommends.
Refer to physical activity program (MOVE!)
Criteria:
Patient is not doing at least 2 hours and 30 minutes of aerobic activity per week and strengthening exercises ≥2 times per week, AND
Patient may be interested in being referred to a physical activity program if their provider recommends.
Perform or refer for Intra-articular injection
Criteria:
Patient has moderate to severe knee pain (≥6 on a 10cm visual analog scale), AND
Patient has radiographic evidence of OA in that knee, AND
Patient is already taking oral pain medications, AND
Patient has not received a joint injection in the past 6 months, AND
Patient may be interested in having a knee joint injection if their provider recommends.
Recommend or prescribe Topical NSAID or capsaicin
Criteria:
Patient is not currently using topical creams for OA, AND
Patient may be interested in trying a topical cream (or different type of topical cream) if their provider recommends.
Patient reports taking an NSAID (prescription or OTC) but has risk factors for GI bleeding. Consider addition of gastroprotective agent or switch to other pain medication
Criteria:
Patient is currently using an NSAID without gastroprotective agent, AND
Patient has one or more risk factors for GI bleeding: age≥ 75 years, history of peptic ulcer disease or GI bleeding, current glucocorticoid use.
Discuss the possibility of trying a new/alternate pain medication with patient
Criteria:
Patient indicated they may like to talk with their health care provider about the possibility of trying a different pain medication for their arthritis.
Referral to orthopedics for evaluation for joint replacement surgery (if no contraindications to surgery)
Criteria:
Radiographic evidence of OA in that joint, AND
Patient has tried each of the following: pain medications, joint injection, physical therapy, AND
Pain ≥6 (on a 10cm visual analog scale) in that joint, AND
Functional limitation due to OA ≥6 (on a 10 point visual numeric scale), AND
Patient indicated they may be interested in being referred to a specialist for evaluation for potential joint replacement surgery.
Appendix AC. Technical Appendix
Table A1C1.
Estimated means, 95% Confidence Intervals (CIs) for WOMAC Total Score from Linear Mixed assuming unconstrained baseline means
| Outcome^ | Time Point | Usual Care (N=149) |
OA Intervention (N=151) |
Treatment difference (95% CI)* |
p |
|---|---|---|---|---|---|
| WOMAC Total Score | |||||
| Baseline | 47.9 | 48.9 | |||
| 6 months | 43.1 | 41.6 | −2.5 (−5.9, 0.9) | 0.14 | |
| 12 months | 48.1 | 44.8 | −4.4 (−7.5, −1.2) | 0.008 |
Continuous Outcome Code for primary and secondary outcomes
title “Mixed analysis w/ race stratification for WOMACTotal”; ods output estimates=estimates_womactotal; proc mixed data=analyzeall order=data; class physicianid studyid studyper; model womactotal=c_racestrata c_mdstrata month6 month12 month6*Randgrp month12*Randgrp/ solution ddfm=kr outPm=OutPred cl; random physicianid; repeated studyper /subject=studyid type=un rcorr; estimate 'common baseline' intercept 1 /cl; estimate 'UC 6-month' intercept 1 month6 1 /cl; estimate 'TRT 6-month' intercept 1 month6 1 month6*Randgrp 1 /cl; estimate 'UC 12-month' intercept 1 month12 1 /cl; estimate 'TRT 12-month' intercept 1 month12 1 month12*Randgrp 1 /cl; estimate 'UC 12-month-baseline' month12 1 /cl; estimate 'TRT 12-month-baseline' month12 1 month12*Randgrp 1 /cl; run;
CHAMPS Outcome Code
title “Mixed analysis on change for c&outcome.”; proc mixed data=changes order=data plot; class physicianid studyid ; model cmodexerfrq=modexerfrq c_racestrata c_mdstrata Randgrp/ solution ddfm=kr residual cl; random physicianid; estimate 'UC 12-month' intercept 1 modexerfrq &bmean. /cl; estimate 'TRT 12-month' intercept 1 modexerfrq &bmean. Randgrp 1 /cl; run;
2. Multiple Imputation Procedure
We conducted a sensitivity analysis using a multiple imputation (MI) approach that included additional variables beyond those in our random effects models to strengthen the MAR assumption. As a first step, we used t-tests and chi-square tests as appropriate to assess each potential variable’s association with missingness at month 12, and any variable with an association p-value of 0.25 or less was included in the imputation model along with variables included in our primary analysis model (treatment arm and stratification variables). Variables assessed included Age, Gender (Male/Female), High School education or less (yes/no), Married or living with partner (yes/no), Inadequate income (yes/no), Employed or student (yes/no), Disabled (yes/no), Fair or poor health (yes/no), BMI, Self-reported joints with OA (Knees only vs. Hips only vs Both Knees and Hips), Duration of symptoms, baseline SPPB score, baseline PHQ score, baseline CHAMPS scores, Number of content calls completed, MD Gender and MD type. Of these, the following were associated with missing status at month 12 and therefore included in the imputation model: Age, Inadequate income (yes/no), Disabled (yes/no), baseline PHQ score, and Number of content calls completed, The imputation model additionally included randomization arm, stratification variables (patient race, and dichotomous variable for percentage of female patients in provider panel (≤ 15% vs. > 15%) ), and all collected WOMAC scores at the 3 possible time points. Missing WOMAC measurements at any of the 3 time points were imputed using a Markov chain Monte Carlo (MCMC) algorithm with 10 imputations. The imputation provided results that were very similar to the main analysis. Models run on the imputed datasets estimated WOMAC to be 4.2 point lower (better) in the OA intervention arm than Usual Care (95% CI, −7.2, −1.1; p=0.007) at 12 months.
PROC MI Code
/* Main imputation with the covariates that are associated with month 12 missingness above */
proc mi data = out.allobs out = MIprep
nimpute =10 seed = 43765;
mcmc chain = single
initial = EM
niter = 200
nbiter = 400
acfplot(wlf) /* or plots=acf */
timeplot(wlf) /* or plots=trace */
;
var randgrp c_racestrata c_mdstrata agecalc disabled phqscore contentcalls_imp income womactotal1-womactotal3;
run;
/* Turn back into long dataset for modeling */
data out.milong;
set miprep2;
studyper=1;
month0=1;month6=0; month12=0;
womactotal=womactotal1; output;
studyper=2;
month0=0;month6=1;month12=0;
womactotal=womactotal2; output;
studyper=3;
month0=0; month6=0;month12=1;
womactotal=womactotal3; output;
run;
/* Analyze imputated data that was imputed with the additional covariates */ proc mixed data=milong; by _imputation_; class physicianid studyid studyper; model womactotal=c_mdstrata c_racestrata month6 month12 month6*Randgrp month12*Randgrp/ solution ddfm=kr outPm=OutPred cl; random physicianid; repeated studyper /subject=studyid type=un rcorr; estimate 'common baseline' intercept 1 /cl; estimate 'UC 6-month' intercept 1 month6 1 /cl; estimate 'TRT 6-month' intercept 1 month6 1 month6*Randgrp 1 /cl; estimate 'UC 12-month' intercept 1 month12 1 /cl; estimate 'TRT 12-month' intercept 1 month12 1 month12*Randgrp 1 /cl; estimate 'TRT 12 month - UC 12 month' month12*randgrp 1/cl; ods output SolutionF=MIMixedOutcome covB=mixcovBOutcome estimates=estOutcome; run;
proc sort data=estoutcome; by label _imputation_; run;
title 'Results of 10 imputations, with additional covariates in imputation model'; proc mianalyze data = estoutcome; by label; modeleffects estimate; stderr Stderr; run;
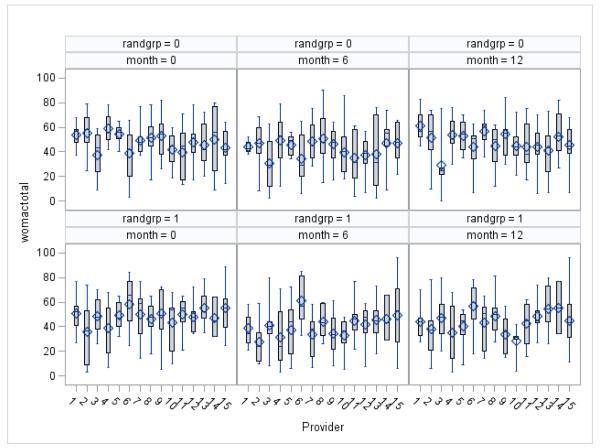
Figure A1C1.
Box plot of WOMAC by Provider, Randomization Arm, and Time Period^
^ Box on box plot indicates the interquartile range, dash is the median, diamond is the mean, upper and lower lines extend to the max and min values, respectively.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
BIBLIOGRAPHY
- 1.Song J, Chang RW, Dunlop D. Population impact of arthritis on disability in older adults. Arthritis & Rheumatism. 2006;55(2):248–55. doi: 10.1002/art.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis & Rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2007-2009. Morbidity and Mortality Weekly Report. 2010;59(39):1261–5. [PubMed] [Google Scholar]
- 4.McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. 2010;26(3):387–99. doi: 10.1016/j.cger.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Projected state-specific increases in self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitations--United States, 2005-2030. Morbidity and Mortality Weekly Report. 2007;56(17):423–5. [PubMed] [Google Scholar]
- 7.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154(4):217–26. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 10.Pasquale MK, Dufour R, Schaaf D, Reiners AT, Mardekian J, Joshi AV, et al. Pain conditions ranked by healthcare costs for members of a national health plan. Pain Pract. 2014;14(2):117–31. doi: 10.1111/papr.12066. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip and knee. Arthritis Care and Research. 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 12.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis & Cartilage. 2010;18(4):476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Richmond J, Hunter DH, Irrgang JJ, Jones MH, Snyder-Mackler L, Van Durme D, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guidline on the Treatment of Osteoarthritis (OA) of the Knee. Journal of Bone and Joint Surgery. 2010;92:990–3. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 15.Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, et al. Objective physical activity measurement in the osteoarthritis initiative: Are guidelines being met? Arthritis Rheum. 2011;63(11):3372–82. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colbert CJ, Almagor O, Chmiel JS, Song J, Dunlop D, Hayes KW, et al. Excess body weight and four-year function outcomes: comparison of African Americans and whites in a prospective study of osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(1):5–14. doi: 10.1002/acr.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz DA, Chang JT, Roth CP, Guan M, Kamberg CJ, Niu F, et al. Quality of osteoarthritis care for community-dwelling adults. Arthritis Care and Research. 2006;55(2):241–7. doi: 10.1002/art.21844. [DOI] [PubMed] [Google Scholar]
- 18.Asch SM, McGlynn EA, Hogan MM, Hayward RA, Shekelle P, Rubenstein L, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Annals of Internal Medicine. 2004;141:938–45. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 19.Mamlin LA, Melfi CA, Parchman ML, Gutierrez B, Allen DI, Katz BP, et al. Management of osteoarthritis of the knee by primary care physicians. Archives of Family Medicine. 1998;7(6):563–7. doi: 10.1001/archfami.7.6.563. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan A, Mather RC, 3rd, Karas V, Ellman MB, Young BB, Bach BR, Jr., et al. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthroscopy. 2014;30(1):65–71. doi: 10.1016/j.arthro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Allen KD, Bosworth HB, Brock DS, Chapman JG, Chatterjee R, Coffman CJ, et al. Patient and Provider Interventions for Managing Osteoarthritis in Primary Care: Protocols for Two Randomized Controlled Trials. BMC Musculoskelet Disord. 2012;13(1):60. doi: 10.1186/1471-2474-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R, Asch D, Bloch G, Bole D, Borenstein K, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis & Rheumatism. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Prevalence of self-reported arthritis or chronic joint symptoms among adults -- United States, 2001. Morbidity and Mortality Weekly Report. 2002;51(42):948–50. [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services . 2008 Physical Activity Guidelines for Americans. Washington, DC: 2008. [Google Scholar]
- 25.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed Guilford; New York: 2002. [Google Scholar]
- 26.Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. Journal of Nervous and Mental Disease. 1999;187(10):630–5. doi: 10.1097/00005053-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Altman RD, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis and Rheumatism. 2000;43(9):1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Romanova M, Liang LJ, Deng ML, Li Z, Heber D. Effectiveness of the MOVE! Multidisciplinary weight loss program for veterans in Los Angeles. Prev Chronic Dis. 2013;10:E112. doi: 10.5888/pcd10.120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthrits of the hip and knee. Clinical Rheumatology. 1986;5:231–41. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of Rheumatology. 1988;15:1833–40. [PubMed] [Google Scholar]
- 31.Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. The Journal of Rheumatology. 2002;29(12):2473–6. [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114(1-3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, RItter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Harada ND, Chiu V, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine and Science in Sports and Exercise. 2001;33(6):962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Hekler EB, Buman MP, Haskell WL, Conway TL, Cain KL, Sallis JF, et al. Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. J Phys Act Health. 2012;9(2):225–36. doi: 10.1123/jpah.9.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. The Journal of Rheumatology. 2000;27:2635–41. [PubMed] [Google Scholar]
- 38.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Weigl M, Angst F, Aeschlimann A, Lehmann S, Stucki G. Predictors for response to rehabilitation in patients with hip or knee osteoarthritis: a comparison of logistic regression models with three different definitions of responder. Osteoarthritis Cartilage. 2006;14(7):641–51. doi: 10.1016/j.joca.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Borm GF, Fransen J, Lemmens W. A simple sample size formula for analysis of covariance in randomized clinical trials. Journal of Clinical Epidemiology. 2007;60(12):1234–8. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Donner A, Klar N. Design and Analysis of Cluster Randomized Trials in Health Research. Oxford University Press; New York: 2000. [Google Scholar]
- 42.ICH E9 Expert Working Group ICH harmonised tripartite guideline - Statistical principles for clinical trials. Statistics in Medicine. 1999;18:1905–42. [PubMed] [Google Scholar]
- 43.Little RJA, Rubin DB. Statistical Analysis with Missing Datat. John Wiley & Sons, Inc; Hoboken, NJ: 2002. [Google Scholar]
- 44.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Wiley &Sons; Hoboken, NJ: 2006. [Google Scholar]
- 45.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med. 2009;28(20):2509–30. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 46.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Wiley-Interscience; Hoboken, N.J.: 2004. [Google Scholar]
- 47.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremity. The Journal of Rheumatology. 2002;29:131–8. [PubMed] [Google Scholar]
- 48.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- 49.Song J, Hochberg MC, Chang RW, Hootman JM, Manheim LM, Lee J, et al. Racial and ethnic differences in physical activity guidelines attainment among people at high risk of or having knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(2):195–202. doi: 10.1002/acr.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lubar D, White PH, Callahan LF, Chang RW, Helmick CG, Lappin DR, et al. A National Public Health Agenda for Osteoarthritis 2010. Semin Arthritis Rheum. 2010;39(5):323–6. doi: 10.1016/j.semarthrit.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 53.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–73. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes SL, Seymour RB, Campbell RT, Huber G, Pollak N, Sharma L, et al. Long-term impact of Fit and Strong! on older adults with osteoarthritis. Gerontologist. 2006;46(6):801–14. doi: 10.1093/geront/46.6.801. [DOI] [PubMed] [Google Scholar]
- 55.Bennell KL, Kyriakides M, Metcalf B, Egerton T, Wrigley TV, Hodges PW, et al. Neuromuscular versus quadriceps strengthening exercise in patients with medial knee osteoarthritis and varus malalignment: a randomized controlled trial. Arthritis Rheumatol. 2014;66(4):950–9. doi: 10.1002/art.38317. [DOI] [PubMed] [Google Scholar]
- 56.Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153(6):1199–209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain mangement in adults: a meta-analysis. Health Psychology. 2007;26(3):241–50. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 58.Zullig LL, Bosworth HB, Jeffreys AS, Corsino L, Coffman CJ, Oddone EZ, et al. The association of comorbid conditions with patient-reported outcomes in Veterans with hip and knee osteoarthritis. Clin Rheumatol. 2014 doi: 10.1007/s10067-014-2707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Black SA, Goodwin JS, Markides KS. The association between chronic diseases and depressive symptomatology in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 1998;53(3):M188–94. doi: 10.1093/gerona/53a.3.m188. [DOI] [PubMed] [Google Scholar]
- 60.Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(8):1042–52. doi: 10.1016/j.joca.2013.05.002. [DOI] [PubMed] [Google Scholar]