Abstract
Visual transduction begins with the detection of light within the photoreceptor cell layer of the retina. Within this layer, specialized cells, termed rods and cones, contain the proteins responsible for light capture and its transduction to nerve impulses. The phototransductive proteins reside within an outer segment region that is connected to an inner segment by a thin stalk rich in cytoskeletal elements. A unique property of the outer segments is the presence of an elaborate intracellular membrane system that holds the phototransduction proteins and provides the requisite lipid environment. The maintenance of normal physiological function requires that these postmitotic cells retain the unique structure of the outer segment regions—stacks of membrane saccules in the case of rods and a continuous infolding of membrane in the case of cones. Both photoreceptor rod and cone cells achieve this through a series of coordinated steps. As new membranous material is synthesized, transported, and incorporated into newly forming outer segment membranes, a compensatory shedding of older membranous material occurs, thereby maintaining the segment at a constant length. These processes are collectively referred to as ROS (rod outer segment) or COS (cone outer segment) renewal. We review the cellular and molecular events responsible for these renewal processes and present the recent but compelling evidence, drawn from molecular genetic, biochemical, and biophysical approaches, pointing to an essential role for a unique tetraspanning membrane protein, called peripherin/rds, in the processes of disk morphogenesis.
Keywords: Photoreceptor, Peripherin/rds, Disk Morphogenesis, Membrane fusion
I. Introduction
The process of vision begins with the detection of light by the retina. The retina can be broadly divided into two functionally distinct layers: the outer sensory layer, consisting of photoreceptors, and the inner neuronal layer containing non-photoreceptor cells including bipolar, amacrine, horizontal, and glial cells. The photoreceptor rods and cones, which contain photopigments responsible for light capture, are the initiators of nerve impulses carried to the brain via the optic nerve. There are approximately 100 million photoreceptors in an individual human retina, with rods localized mostly to the periphery and cones localized to the fovea (central region).
Rod photoreceptor cells are structurally and physiologically distinct from cone photoreceptor cells. Rod cells are elongated, cylindrical structures whereas cones are short and, as their name implies, conical at the apex. Rods are responsible for vision under low levels of light, discerning little detail. In most species, the number of rods exceeds that of cones in a given retina. Nocturnal animals, such as rats, have a preponderance of rods (>95%), whereas chickens have a 6:1 ratio of cones to rods. Cones are primarily involved in the detection of color and fine detail, which is discerned at higher light intensities. Three different types of cones, identified based on spectral responses, are responsible for coordinating the detection of colors. While numerous differences exist between rod and cone cells, the commonality between them is a unique structural feature consisting of two distinct subcellular compartments, termed inner and outer segments, which are connected by a thin stalk rich in cytoskeletal elements—a nonmotile cilium. The inner segments contain nuclei and other organelles required for protein synthesis and all the biochemical reactions needed for metabolic activity. Light capture (Hargrave and McDowell, 1992; Yarfitz and Hurley, 1994; Baylor, 1996) occurs exclusively in the outer segments, which contain the requisite photopigment(s): rhodopsin in rod outer segments (Applebury, 1994; Palczewski, 1994) and a family of proteins called iso-opsins in cones.
Photoreceptors are a nondividing, terminally differentiated neuronal cell type. The photopigment-containing membranes of rods and cones are continually being formed and removed. Formation occurs from an initial evagination of the plasma membrane at the junction between the inner and outer segments (morphogenesis); removal occurs at the apex of the outer segments (shedding). In this manner, the lipids and proteins of the outer segment are continuously turned over. The processes of disk morphogenesis and membrane shedding coordinate to maintain a constant average length for the cells. These processes, which are fundamental to the functioning of the visual system, share the common characteristic that each occurs through the tightly regulated fusion of two biological membranes.
Within the past decade, a 39–37-kDa glycoprotein with four transmembrane spanning domains (tetraspanning) called peripherin/rds (also known as peripherin-2, rds/peripherin) has been implicated to play an essential role in ROS renewal processes. More than 40 mutations within the peripherin/RDS gene have been associated with a variety of retinal degenerative diseases (Kohl et al., 1998); the majority map to the larger of two hydrophilic intradiskal loop regions of the protein. Although the basis of peripherin/rds action at the molecular level is not yet understood, several hypotheses have been proposed, including function as a membrane fusion protein. This review considers the cellular and biochemical processes that mediate the renewal process within the outer segment. We provide evidence supporting the view that peripherin/rds plays an essential role in these processes.
II. Organization of Vertebrate Photoreceptors
A. Structural Organization
A schematic representation of a rod cell is shown in Figure 1. Each cell type is composed of four morphologically and functionally distinct regions. Cone cells contain a cone outer segment (COS), a cone inner segment (CIS), soma, and the synaptic terminal. The rod cell contains similar distinct regions: a rod outer segment (ROS), a rod inner segment (RIS), soma, and the synaptic terminal. In both cell types the nucleus, synaptic terminal, and inner segment regions are similar whereas the outer segment portions are structurally and functionally distinct from each other. The rod outer segment contains a stack of closed, flattened membranous sacs called disks. Mammalian ROSs contain approximately 500–1000 disks with a diameter of 1–2 μm and a ROS length of 20–40 μm in humans. There are approximately 1700–2000 disks in a frog rod outer segment, resulting in ROS dimensions of 5–7 μm (diameter) × 35–50 μm (length) (Rosenkranz, 1977). In contrast, the COS region is formed entirely from repeated evaginations of the cone outer segment plasma membrane, resulting in a largely contiguous stack of parallel membrane sheets (Cohen, 1968; Laties et al., 1976). In COS these “disks”— or, more properly, “lamellae”—are in continuity with the plasma membrane and exposed to the extracellular environment of the outer retina (Young, 1967; Liebman and Entine, 1974; Anderson et al., 1986).
Fig. 1.
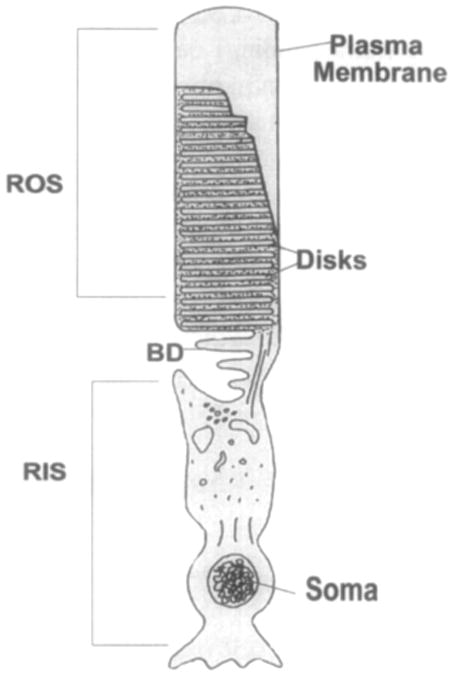
Schematic representation of rod photoreceptor cell. Rod cells are divided into two functionally distinct regions: the rod inner segment (RIS) containing the nucleus and intracellular organelles, and the rod outer segment (ROS) containing disks surrounding by the plasma membrane. BD, basal disks. (See also color insert.)
The outer segment region of the rod cell is connected to the inner segment through a nonmotile connecting cilium. This narrow region, only about 3 μm in diameter, provides the only contiguous connection between the inner and the outer segment. In the developing photoreceptor this cilium is divided into a proximal and a distal region. The proximal cilium corresponds to the mature connecting cilium, while the distal cilium develops into the outer segment of a mature cell (Besharse and Horst, 1990). It is within the ciliary region that newly synthesized materials exchange between the two compartments. New disks are formed at the distal end of the connecting cilium (designated in Fig. 1 as BD, basal disks). These basal disks, also referred to as basal infoldings or open disks, are evaginations of the plasma membrane. The addition of new membranous material at the base of the ROS displaces disks along the long axis of the outer segment. At the tip of the ROS, disks are shed and phagocytosed by a layer of polarized cells called the retinal pigment epithelium (RPE). The RPE is located at the very back of the retina, behind the photoreceptor cell layer and in front of the choroidal capillaries. Each RPE cell is in close contact with approximately 40 ROS (Newsome, 1983), although this number varies with position in the retina as the photoreceptor density changes. The RPE cells are in fact the “caretakers” of the ROS, designed to ingest old disks shed from the ROS. Defects in the RPE appear to generate retinal degeneration in the RCS (Royal College of Surgeons) rat due to the accumulation of membrane debris that is not phagocytosed. Under normal conditions, the transit time for a newly formed disk from base to tip of the rod outer segment is approximately 10 days in vertebrate retinas (Young, 1971) and 14–21 days in amphibians (Young and Bok, 1969).
B. Molecular Organization of Photoreceptor Membranes
1. Distribution of Proteins
The unique morphology of the rod outer segment is complemented by a highly organized distribution of lipids and proteins between the plasma membrane and disk membranes. Rhodopsin is the only membrane protein within the ROS that is known to be ubiquitously distributed. This prototypical G-protein receptor (Applebury, 1994) is the dominant protein by weight both in the plasma membrane (60% total protein; Molday, 1998) and, in slightly higher abundance, in disk membranes (85% total protein; Nir and Papermaster, 1983; Hicks and Molday, 1986; Polaws et al., 1986). ROS plasma membrane rhodopsin appears to be identical to the rhodopsin in disk membranes with respect to glycosylation, light-stimulated phosphorylation, and primary structure (Hsu et al., 1993). In contrast, the ion transport proteins responsible for maintaining normal retinal function—i.e., the Na+–Ca+ exchanger (Reid et al., 1990) and the cGMP-gated channel, α and β subunits (Cook et al., 1989)—are found exclusively in the plasma membrane. A minor component of the plasma membrane is the GLUT-1 glucose transporter (Hsu and Molday, 1991). Protein localization and distribution along the axial length of the plasma membrane are not homogeneous. In a series of microscopy studies, Bridges (Bridges and Fong, 1980; Bridges, 1981) reported a heterogeneous distribution of ricin-binding protein along the length of the plasma membrane. Heavier labeling of basal disks with ricin communis agglutinin 120 was observed by Hicks and Molday (1985). Since those studies, two ricin-binding proteins have been identified: the Na+–Ca2+ exchanger and a 103-kDa protein with an as yet undetermined function (Reid et al., 1990).
Individual disk membranes present a unique dumbbell-shaped structure that corresponds to a distinctive distribution of proteins. The lamellar (flat region) of the disk contains mostly rhodopsin, with a guanylate cyclase accounting for 1% of the remaining known membrane protein. Two other proteins, the peripherin/rds–rom-1 complex and the ABC transporter (Azarian and Travis, 1997; Illing et al., 1997; Sun and Nathans, 1997), are unique to the disk rim (Arikawa et al., 1992). The newly identified ATP-binding cassette (ABC) transporter (also known as the ABCR/RIM or ABCR) is a member of an extensive family of proteins with structural similarity to P-glycoprotein and the cystic fibrosis transmembrane regulator (CFTR) proteins (Higgins, 1992). Papermaster and colleagues (1978) initially identified this protein, called rim protein (RmP) at the time, as a 210-kDa glycoprotein located within the disk rims of frogs. This protein was localized to the incisures and disk margins and postulated to play a role in the maintenance of structure (Papermaster et al., 1982). A growing body of evidence suggests that the ABCR/RIM protein is involved in the transport of retinal derivatives across the disk membrane (Azarian and Travis, 1997; Sun et al., 1999; Sun and Nathans, 2001). The nucleotidase activity of the transporter is dependent on the content of phosphatidylethanolamine in reconstituted membranes (Ahn et al., 2000). Mutations with the ABCR/RIM have recently been linked to Stargardt's disease (Allikmets et al., 1997a,b; Lewis et al., 1999). A Stargardt's disease mutation (Leu 2027Phe) resulted in a decrease in hydrolytic activity (Biswas and Biswas, 2000).
2. Distribution of Lipids
The unique distribution of proteins within the various membranes of the ROS is mirrored by a heterogeneous distribution of lipid components. Certain lipids exhibit a preferential localization to the plasma membrane of the ROS compared to the disk membranes. For example, the plasma membrane is enriched in cholesterol (4-fold compared to phospholipid; Boesze-Battaglia and Albert, 1992), unsaturated fatty acids species, phosphatidylcholine (PC) relative to phosphatidylethanolamine (PE; Boesze-Battaglia and Albert, 1992), and squalene, a precursor of both isoprenyl groups and cholesterol (Fliesler et al., 1997), relative to the majority of mature disk membranes. The disk membranes also exhibit a heterogeneous lipid distribution; newly formed disks have 6-fold more cholesterol than disks at the apical tip of the ROS (Boesze-Battaglia et al., 1989; 1990), although there is little change in the phospholipid species. The predominant change occurs in the relative distribution of fatty acids within the various classes of phospholipids. In the case of PC, the fatty acid side chains become progressively more saturated as a function of disk spatial location (i.e., from base to tip of the ROS; Albert et al., 1998). The lipid constituents may also be arranged into membrane microdomains within individual disk membranes. Recent model membrane studies suggest that cholesterol is necessary for the recruitment of polyunsaturated phospholipids by rhodopsin into membrane microdomains (Polozova and Litman, 2000). Seno and colleagues (2001) have recently isolated ROS-specific membrane rafts containing PDE and transducin. Interestingly, PDE could be extracted from these rafts upon cholesterol depletion, while transducin showed no preferential extractability. The metabolism of lipids within the ROS and the effect of lipid composition on ROS function have been reviewed elsewhere (Fliesler and Anderson, 1983; Bazan and Rodriguez de Turco, 1994; Boesze-Battaglia and Schimmel, 1997).
III. Preservation of ROS Organization
A. Renewal of ROS Constituents
The complete renewal of the constituents of the ROS occurs through the following coordinated series of steps: (1) Formation of a new disk at the base of the outer segment (this step requires the proper delivery of lipid and protein components from RIS); (2) movement of disks up the length of the outer segment; (3) shedding of packets of disks at the apical tip; (4) recognition and binding of shed disks to the RPE; (5) ingestion, breakdown, and organization of lipid and protein components within the RPE cytoplasm (Nguyen-Legros and Hicks, 2000). The coordination and intricate biochemical regulation of these processes allow the continuous maintenance of the length of the ROS. The failure to maintain constant length is a hallmark of several retinal degenerative disease models.
The first three steps of the renewal process are shown schematically in Figure 2. Using 3H-labeled amino acids, autoradiographic studies have shown that rhodopsin remains associated with the individual disk into which it was incorporated (Young, 1967; Hall et al., 1969). When the radiolabel was followed in a pulse-chase style experiment, the radiolabel was detected as a distinct migrating band along the axial length of the ROS (Bibb and Young, 1974a). This band of newly formed protein migrates up the length of the outer segment toward the apex, where the labeled disks are shed and phagocytosed by the overlying pigment epithelium (Young, 1971). These studies developed the current mode of disk membrane apical displacement along the length of the ROS (Young, 1967; Bok, 1985). In contrast, when the distribution of tritiated fatty acids or glycerol was followed, diffuse labeling of the ROS was observed along the length of the outer segment (Bibb and Young, 1974a,b). These microscopy studies were consistent with biochemical studies suggesting that lipid turnover in photoreceptors occurs in a manner distinct from that of protein turnover (Anderson et al., 1980a–d). Collectively, these results suggested that although proteins remain associated with individual disks, lipids are freely exchangeable. Cone outer segments are also continuously renewed. Radiolabeled proteins inserted into cone outer segments do not form discrete bands, but diffusely label the entire outer segment of the cone; this confirms that the COS membrane does not form distinct disks, but is continuous, although a rim-like region enriched in peripherin/rds is also detected in cones (Arikawa et al., 1992).
Fig. 2.
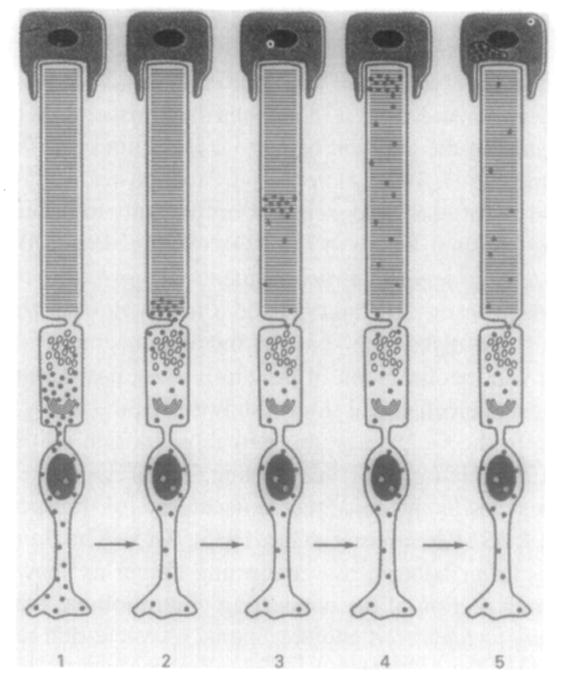
Schematic representation of the renewal of ROS protein constituents. The turnover of [3H]leucine-labeled membrane proteins in a rod cell is illustrated. The solid black dots indicate newly synthesized proteins (mostly the photopigment rhodopsin) that are initially seen around the Golgi (1). From there they pass to the base of the outer segment (2) where they are incorporated into newly formed disks. The newly formed disks are then apically displaced and phagocytosed by the pigment epithelium (3–5). (Reprinted with permission from Young, 1976, with minor modifications added for clarification.)
B. Transport of Membrane Constituents from the Inner to the Outer Segment
A prodigious amount of membranous material is incorporated into new disks; in bovine approximately 30 disks and in Xenopus laevis approximately 80 disks are added daily to the ROS. The daily biosynthesis of 80 new disks with an average diameter of 7 μm is equal to a net synthesis of 4500 μm2 of disk membrane surface, which translates to the synthesis of 80 × 106 molecules of rhodopsin in Xenopus (Papermaster et al., 1986) and approximately 10 × 106 molecules daily in bovine (Fliesler and Anderson, 1983). Thus the transport and polarized sorting of rhodopsin results in the addition of up to 3 μm2/min of ROS membrane material (Simons and Zerial, 1993; Deretic and Papermaster, 1995). Compensatory shedding of 10–15% of each outer segment occurs daily to balance incorporation and maintain the constant length of the ROS (Young, 1967). Because the ROS cannot synthesize lipid and protein constituents de novo, this membranous material is synthesized in the RIS and delivered to the ROS in transport vesicles. The formation of new disks at the base of the outer segment is dependent upon proper transport and incorporation of protein-bearing post-Golgi vesicles. Early autoradiographic and biochemical studies showed that the newly synthesized protein migrated from the Golgi, past the mitochondria-rich ellipsoid to the basal disks (Young, 1968; Hall et al., 1969; Young, 1976). Further studies identified opsin-bearing vesicles as the constituents necessary for transport of opsin form the Golgi to the ROS (Papermaster et al., 1975; 1985). The trans-Golgi network (TGN) vesicles cluster beneath the connecting cilium as shown in Figure 3A. In this longitudinal section of the connecting cilium joining the inner and outer segments of Xenopus laevis rod photoreceptors, a vesicle-rich region is observed beneath the cilium. These clustered vesicles are confluently labeled with anti-opsin ferritin, as are the rod outer segment disks. Very little labeling is observed in the contiguous inner segment plasma membrane. The TGN vesicles dock and subsequently fuse with the inner segment plasma membrane within the highly specialized region termed the periciliary ridge complex as shown in Figure 3B. In this cross section of the basal portion of a rod photoreceptor connecting cilium as it arises from the periciliary ridge complex, the vesicles are tightly clustered and docked within this region; occasionally, vesicles are shown to fuse with the plasma membrane, as indicted by the open arrow. Currently, the use of molecular motors to transport opsin-bearing vesicles to the base of the outer segment is proposed (Section III.C; see also rhodopsin trafficking as reviewed in Sung and Tai, 2000). Earlier work describing the morphogenesis and synthesis of ROS components is reviewed extensively in Papermaster and Schneider (1982).
Fig. 3.
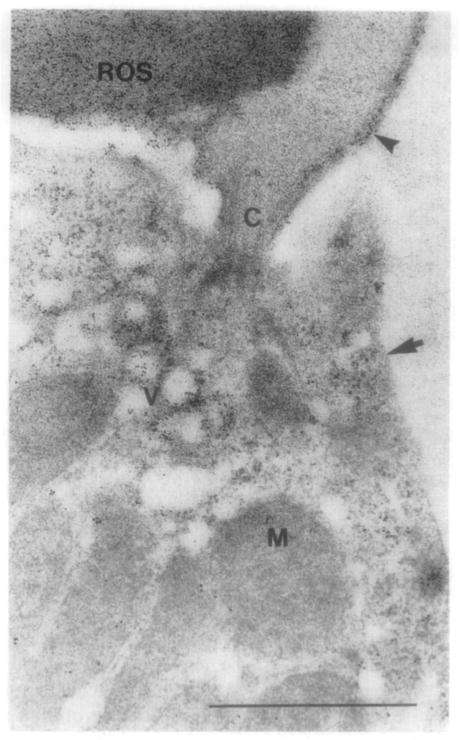
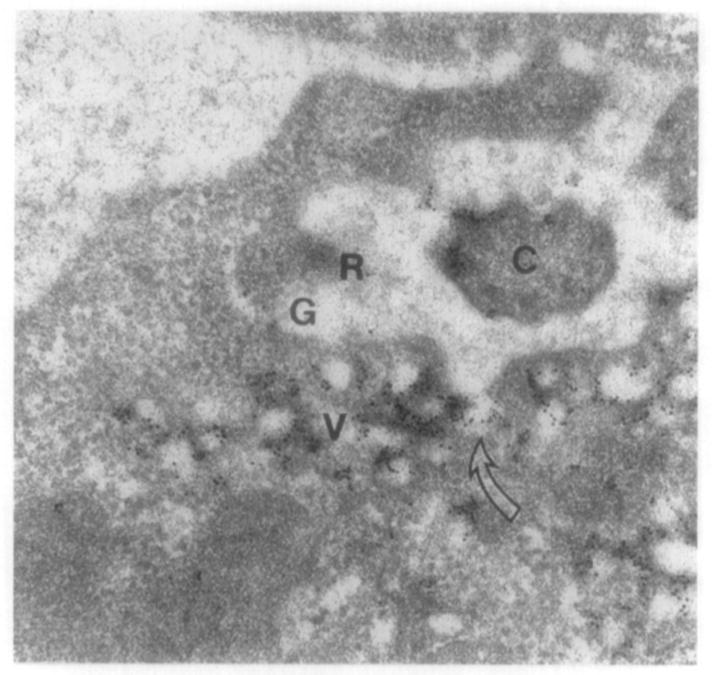
(A) Longitudinal section of the connecting cilium joining the inner and outer segments of Xenopus laevis rod photoreceptors. The section shown is of a Lowicryl-embedded retina labeled with antiopsin–ferritin complexes. The clustered vesicles beneath the cilium (C) and the rod outer segment disks (ROS) are confluently labeled. (See also color insert.) (B) Cross section of the basal portion of a rod photoreceptor connecting cilium as it arises from the periciliary ridge complex. The retina was obtained from an animal killed three hours after light onset; the retinal section was embedded in Lowicryl K4M and labeled with anti-opsin–ferritin complexes. The ridges (R) and grooves (G) form a deep invagination about the base of the cilium. Vesicles (V) are tightly clustered and docked within this region; occasionally, vesicles are shown to fuse with the plasma membrane as indicated by the open arrow. [This figure was generously provided by Dr. David Papermaster (Papermaster et al., 1985) and reprinted with permission from the Association for Research in Vision and Ophthalmology.]
1. Composition of Transport Vesicles
Much of our current understanding of the molecular mechanisms of TGN sorting and vectorial transport of rhodopsin to the ROS has come from studies utilizing a cell-free assay system (Deretic, 1998). Rhodopsin-bearing vesicles have been isolated from amphibian photoreceptors as a post-Golgi subcellular fraction of very low buoyant density (p = 1.09 g/ml) (Deretic and Papermaster, 1991). These vesicles contain newly synthesized rhodopsin which is sequestered and cotransported with docosahexaenoyl (DHA)-containing phosphatidylcholine and phosphatidylethanolamine (Rodriguez de Turco et al., 1997). The DHA-containing phospholipids remain associated with rhodopsin as the individual disk moves up the length of the outer segment and may make up a motionally restricted lipid environment (Albert et al., 1985). 31PNMR studies have identified two phospholipids headgroup domains, one of which appears to be under the influence of rhodopsin (Albert and Yeagle, 1983), and is postulated to interact with rhodopsin noncovalently (Gordon and Bazan, 1990).
Although the presence of acidic DHA-containing phospholipids is essential for the transport of rhodopsin-bearing post-Golgi vesicles (Rodriguez de Turco et al., 1997), the mechanism for this specific phospholipid composition has only recently been addressed (Deretic et al., 2001). In a cell-free assay system, designed to mimic TGN budding and fusion, propranolol was shown to inhibit the delivery of phospholipids to the ROS. Propranolol treatment appears to have no effect on post-Golgi vesicle budding but affects the subsequent fusion of these vesicles at the periciliary ridge. Since propranalol treatment results in an increase in phosphatidic acid and lysoPC and a concomitant decrease in PC, the vesicles may be rendered fusion-incompetent owing to increases in these lipids. In other membrane systems, lysoPC has been shown to be a potent fusion inhibitor, perhaps by increasing the membrane curvature (Yeagle et al., 1994). Collectively, these results suggest that DHA-PC is necessary for membrane fusion. The mechanism by which this occurs is somewhat speculative: This lipid may provide the necessary positive membrane curvature, it may aid in the docking process, or it may render the as yet unidentified fusion protein fusion-competent.
In addition to a specific membrane lipid composition, the proper formation (budding) and sorting (fusion) of these vesicles requires at least two and as many as six distinct small GTP-binding proteins (Deretic et al., 1995) and evk-1, a 25-kDa, protein with a pleckstrin homology domain. (Krappa et al., 1999). In photoreceptors, rab 6 (Deretic and Papermaster, 1993) and rab 8 (Deretic et al., 1995) are proposed to be involved sequentially with the post-Golgi membranes. Rab 6 is associated with the rhodopsin-bearing post-Golgi vesicles present, it is present at sites of disk morphogenesis but absent from mature disk membranes. It is present in a soluble form in the ROS cytoplasm. Collectively, these results have led to the suggestion that rab 6 has multiple roles. It is involved in rhodopsin sorting and delivery, but it may also play a role in disk morphogenesis, since it becomes soluble upon disk membrane morphogenesis. Some rab 8 is also associated with the rhodopsin-bearing post-Golgi vesicles (about 13 % of the membrane-associated fraction). More intriguing is the observation that anti-rab 8 antibody labels the actin bundles of the inner segment with a periodicity (i.e., at 1-μm intervals). The rhodopsin-bearing post-Golgi vesicles also cluster around the connecting cilium within the same region that rab 8 colocalizes with actin, suggesting that rab 8 may be involved in later steps of rhodopsin localization—specifically, microfilament-dependent disk morphogenesis (Deretic et al., 1995).
2. Localization Signal of Rhodopsin
The mislocalization of rhodopsin is involved in the pathology of various retinal degenerative diseases (Dryja et al., 1990; Fariss et al., 1993). Mutations and deletions within the distal eight amino acids of rhodopsin, later identified as the localization signal sequence, contribute to a disease cluster characterized by early onset and aggressive retinal degeneration (Sandberg et al., 1995). Transgenic mice, rats, and pigs carrying various COOH-terminal disease-linked mutations exhibit rhodopsin mislocalization and retinal degeneration (Sung et al., 1994; Li et al., 1998; Green et al., 2000). Abnormal protein distribution has been found in a Gln344ter rhodopsin-truncation mutant in transgenic mice (Sung et al., 1994). This mutant is lacking the last five C-terminal amino acids. Transgenic mice with a P347S mutation show an accumulation of extracellular vesicles between the inner and outer segments (Li et al., 1996). Recently, this mislocalization has been linked to the trigger of cAMP-mediated apoptotic events in the salamander retina (P. Alfinito and E. Townes-Anderson, unpublished observations).
The genetic models of C-terminal disease–linked rhodopsin mutants provided the framework for in vitro work, confirming the localization signal of rhodopsin. Using synthetic peptides as competitive inhibitors of rhodopsin trafficking in frog cell-free assays, Deretic and colleagues (1996; 1998) showed that the distal five amino acids of rhodopsin comprise a localization sequence of this protein. These residues, H2N–Q–V–S–(A)–P–A–COOH, are necessary for post-Golgi membrane formation and subcellular localization of rhodopsin. Structural studies based on the C terminus of rhodopsin have shown that the distal eight amino acids are part of a short stretch of β-sheet accessible to the cytoplasm (extradiskal side; Yeagle et al., 1996; 1997). In the recently reported crystal structure of rhodopsin (Palczewski et al., 2000), the B values (indices of the definition of atom positions) associated with this portion of the structure are very high, suggesting that structural determination from the cytoplasmic face of the protein lacks precision.
Additional evidence supporting the localization signal of rhodopsin within the amino acids of the C-terminal tail has been provided by an MDCK cell expression system. In stably transfected MDCK cells, Chuang and Sung (1998) have shown that WT rhodopsin was targeted to the apical plasma membrane through the TGN. A deletion of the terminal 32 amino acids showed a nonpolar steady-state distribution. Furthermore, when these terminal amino acids are linked to GST, an apical distribution of this protein is observed. The apical transport of this protein can be inhibited with the addition of Brefeldin A (an inhibitor of vesicle-mediated transport of nascent protein from the endoplasmic reticulum to the Golgi), suggesting a unique sorting pathway in MDCK cells. Consistent with the identification of the cytoplasmic tail as a targeting signal is the observation that the normally basolateral membrane protein CD7 is redirected to the apical domain with the addition of rhodopsin's 39 C-terminal amino acids.
In vivo confirmation of the C-terminal targeting signal of rhodopsin was recently provided using a transgenic Xenopus laevis model (Tam et al., 2000). Various rhodopsin C-terminal mutants were tagged with GFP, and expression was assessed in Xenopus using confocal microscopy (Moritz et al., 1999; Tam et al., 2000). The GFP-tagged rhodopsin was anchored to the membrane through palmitoylation or myristoylation sites, and membrane association was found to be necessary albeit not sufficient for proper localization of the GFP-C-terminus to the ROS. In an interesting series of studies, these authors made a number of rhodopsin-α adrenergic receptor chimeras, in which the terminal eight amino acids of rhodopsin were sufficient to localize the α-adrenergic receptor to the ROS rather than its normal localization to the RIS. Deletion and mutations of these eight amino acids resulted in a partial delocalization of the GFP-tagged regions to the inner segment. Expression of the transgene showed a variegated pattern analogous to position-effect variegation (Moritz et al., 2001). The GFP-tagged rhodopsin maintained characteristics of native protein when expressed in COS cells; it bound 11-cis retinal and activated transducin by 50% (Moritz et al., 2001).
3. Transport and Localization of Other ROS Proteins
The other membrane proteins found in the outer segment (i.e., the rim-localized, peripherin/rds, rom-1, the ABC transporter, and the plasma membrane–localized cGMP-gated calcium channel) are also transported to the outer segment, almost certainly in lipid vesicles as are other membrane proteins. The sorting of these proteins is independent of rhodopsin, as shown by Green and colleagues (2000) in a transgenic mouse model expressing a truncated rhodopsin (ser 334ter). In these immunocytolocalization studies, the investigators found that both peripherin/rds and the cGMP channel protein localize normally in the rod outer segment in transgenic rats expressing a truncated rhodopsin that was mislocalized to the inner segment region. The sorting of the cGMP channel only to the ROS plasma membrane and that of peripherin/rds only to disk membranes, their respective locations in vivo, was not addressed at the election micrograph level and should be considered further.
Earlier evidence supporting two transport pathways, one for opsin and one for peripherin/rds, comes from a series of confocal and electron microscopy studies (Fariss et al., 1993; 1997). Upon retinal detachment–induced degeneration, opsin was found to accumulate in the plasma membrane of the rod cell, while peripherin/rds was most prominent in cytoplasmic vesicles. These results led the authors to postulate that peripherin/rds and opsin are incorporated into different post-Golgi transport vesicles, although cotransport or peripherin/rds recycling from the plasma membrane into cytoplasmic vesicles cannot be ruled out.
Because the localization of peripherin/rds, rom-1, and the ABC transporter protein to the disk rim differs dramatically from that of rhodopsin, it is likely that transport of the rim proteins may not occur in the same vesicles that transport rhodopsin. Hence it is reasonable to infer the existence of two pools of transport vesicles, although only those vesicles bearing rhodopsin have been isolated and studied so far. The observation that peripherin/rds and rhodopsin are mislocalized in induced retinal detachment but remain segregated is consistent with this notion (Fariss et al., 1993). Some investigators have observed “cytoplasmic bridges” between the inner and outer segment (distinct from the connecting cilium), leading to the hypothesis that vesicles may bud from the apex of the inner segment to deliver components to the outer segment (Besharse and Horst, 1990). Interestingly, Besharse and Wetzel (1995) described a periciliary transport pathway in which vesicles bud from the apex of the rod inner segment, then migrate to and fuse with nascent disks of the outer segment, suggesting an additional or alternative pathway for rhodopsin transport to the outer segment. Alternatively, this periciliary transport pathway may be the means of transport of the rim-specific proteins to the outer segment, but direct support for this proposal, such as by the detection of rim proteins in these vesicles, has yet to be demonstrated.
It has been proposed that the localization sequence for peripherin/rds is in the C-terminal region of the protein. Evidence in support of this view comes from the development of a transgenic Xenopus laveis model in which the C-terminal region of peripherin/rds was tagged with the fluorescent probe GFP (Tam et al., 2001). The authors demonstrated that a deletion mutant removing the fusion domain of peripherin/rds (i.e., amino acid 311–325) was mislocalized at all levels of expression. In contrast, the rds 38 C-terminal construct localized to the outer segment. In similar experiments, Lee and Burnside (2001) expressed peripherin/rds in Xenopus laveis with the N-terminal domain tagged with the fluorescent GFP protein, however, removal of the entire C-terminal domain did not appear to result in mislocalization. Both the WT and a C-terminal deletion mutant were detected in the outer segment. Neither study addressed whether the mutant peripherin/rds had localized properly to the rim region or had mislocalized elsewhere in the outer segment.
4. Transport of Lipid Constituents
In contrast to the DHA-containing lipids, both the synthesis and the incorporation of other ROS membrane lipids (i.e., PI, PS, and DAG) into disks are rapid and not associated with the rhodopsin-bearing post-Golgi vesicles. These results suggest that 40–50% the lipids, those shown not to contain DHA, are delivered through a mechanism bypassing the Golgi. Mechanistically, the delivery of these lipids is likely mediated by various lipid carrier proteins (Cleves et al., 1991), which also contribute to molecular rearrangements of disk membrane DHA-containing phospholipids (Dudley and Anderson, 1978). The mode of cholesterol delivery to ROS is largely speculative. Rodriguez de Turco and colleagues (1997) have suggested that it occurs by a pathway(s) independent from that followed by integral membrane proteins (Urbani and Simoni, 1990; Voelker, 1991) and/or together with rhodopsin and the DHA-lipid–containing post-Golgi vesicles. As vesicles fuse with the plasma membrane adjacent to the connecting cilium, they could generate the cholesterol-enriched domains observed in frog photoreceptors surrounding the periciliary ridge complex and in nascent disks at the base of the ROS (Andrews and Cohen, 1981; 1983; Cohen, 1983). Collectively, these results suggest at least two distinct mechanisms that contribute to a complex polarized trafficking of DHA-PL and other phospholipids. These pathways involve vectorial and/or lipid-transfer protein-mediated transport to the ROS.
C. Role of Molecular Motors in Transport Pathway[s]
The rate of opsin synthesis and transport to the base of the ROS (Corless et al., 1976; Curcio et al., 1990; Guerin et al., 1993) is remarkably high and unlikely to occur solely by diffusion. Consequently, investigators have considered the role of motor proteins—proteins that utilize ATP to move “cargo” along actin and microtubule filaments as a necessity in this process (Williams, 2001). Recently, a number of motor proteins involved in the transport of the phototransductive proteins (“cargo”) of the ROS have been identified. In addition to the prodigious amount of material that must pass through the connecting cilium daily, support for the use of molecular motors in opsin transport comes from the demonstration of the binding of the dynein molecular motor Tctex-1 to the cytoplasmic tail of rhodopsin (Tai et al., 1999). Tctex-1 is proposed to participate in the delivery of rhodopsin-bearing vesicles to the distal inner segment. In addition to this dynein motor, the connecting cilium contains myosin VIIa (Hasson et al., 1995; Liu et al., 1997) and kinesin II. Myosin VIIa is the product of the Usher 1B syndrome gene (Weil et al., 1995), which includes an autosomal recessive form of retinitis pigmentosa and hearing impairment. Myosin VIIa localizes to the photoreceptor cell connecting cilium (Liu et al., 1997) and the apical RPE (Hasson et al., 1995). Shaker 1 mice possess a mutation of the myosin VIIa gene. The connecting cilia appear normal; however, an abnormal accumulation of opsin was detected upon immunogold electron microscopy (Liu et al., 1999). Since the motor properties of myosin VIIa have been confirmed in vitro (Udovichencko et al., 2001), investigators have proposed that myosin VIIa carries rhodopsin along the connecting cilium as cargo. Alternatively, myosin VIIa could participate in organization of the components of the connecting cilium. Recent evidence showing a temporal relationship between the expression of myosin VII and opsin (postnatal day 3), in combination with the localization of myosin VIIa to the proximal portion of the connecting cilium at day 4–5, is consistent with the “opsin as myosin cargo” hypothesis proposed by Williams (2001). Whether peripherin/rds and rom-1, the disk-rim-specific proteins, are associated with the myosin VIIa motor remains to be determined.
Kinesin II containing the KIF3A motor subunit has been detected in the synapse, the inner and outer segment, and the connecting cilium. KIF3 and KIF3B subunits of kinesin II have been localized to photoreceptors (Whitehead et al., 1999). Since KIF3A knockout mice die in utero, the KIF3A subunit was selectively deleted from photoreceptor cells using cre-loxP conditional mutagenesis. Photoreceptors with a deletion of KIF3A degenerated in a manner analogous to degenerations seen in retinitis pigmentosa (RP) mutations with a mislocalization of opsin and arrestin in these cells. The outer segment protein peripherin/rds, the ROS cytosolic protein transducin, and the synapse-specific proteins SV2, synaptotagmin, SNAP, and VAMP all appear to localize normally in less severely affected retinas. However, peripherin/rds was mislocalized in the most severely affected animals having retinal degeneration. Collectively, these studies have provided a model of opsin transport (for a schematic, see Marszalek et al., 2000) in which opsin-bearing post-Golgi vesicles are transported to the basal body through an interaction between the cytoplasmic tail of opsin and Tctex–dyenin (Tai et al., 1999). The opsin-bearing vesicles subsequently fuse with the plasma membrane and are transported to the outer segment. The mechanism of this transport is speculative; kinesin may interact directly with opsin, or the opsin may be transported in a membrane raft complex to the outer segment (for review, see Williams, 2001).
IV. Cellular Processes Coordinating Renewal
A. Disk Morphogenesis
The delivery and incorporation of the protein and lipid constituents into ROS plasma membrane comprise only one component of disk morphogenesis. Disk morphogenesis requires the organization of these constituents into a disk membrane. The development of a working model of disk membrane morphogenesis began when Sjostrand (1959) reported an apparent continuity between disk membranes and the surrounding plasma membrane as well as an apparent infolding of the membrane in longitudinal sections. When other investigators reported a similar pattern of disk membrane infolding, the hypothesis was advanced that disk membranes are formed by an infolding of the plasma membrane. Later studies by Nilsson and colleagues (1964) showed that only the newly formed disks were in continuity with the plasma membrane and that disks are oriented perpendicularly with respect to the long axis of the cilium. Autoradiographic studies (Young, 1967; Young and Bok, 1969) clearly showing a ROS renewal process led to a reevaluation of the morphology of newly forming disk at the base of the outer segment. A detailed microscopic study by Anderson and colleagues (1978) supported a new hypothesis that disk membranes form through a process of evagination or outfolding of plasma membrane at the base of the ROS. These studies were extended and showed that disks form through repeated evaginations of the plasma membrane of the connecting cilium (Kinney and Fischer, 1978a,b).
The currently accepted model for disk morphogenesis was first proposed by Steinberg and co-workers (1980) based upon a series of electron micrographs of tangential and longitudinal sections of adult and developing rod and cone cells in rhesus monkey, ground squirrel, and gray squirrel. Steinberg and co-workers (1977; 1980) proposed that the disk surface forms through a mechanism distinct from that of the disk rim. As shown in Figure 4A, the disk surface is formed at the very base of the outer segment at the ciliary stalk. The membrane of the inner face pushes outward, forming an evagination filled with cytoplasm. As membranous material is added, a series of successive evaginations is observed and the older evaginations are apically displaced. These evaginations expand in diameter and become thinner as they are apically displaced. Steinberg proposed that each evagination contributes only to disk surface formation. In Figure 4A, for example, the surface of evagination a becomes the apical surface of an individual disk and evagination b becomes the basal surface of that same disk. The space between the a and b surfaces is the intradiskal space.
Fig. 4.
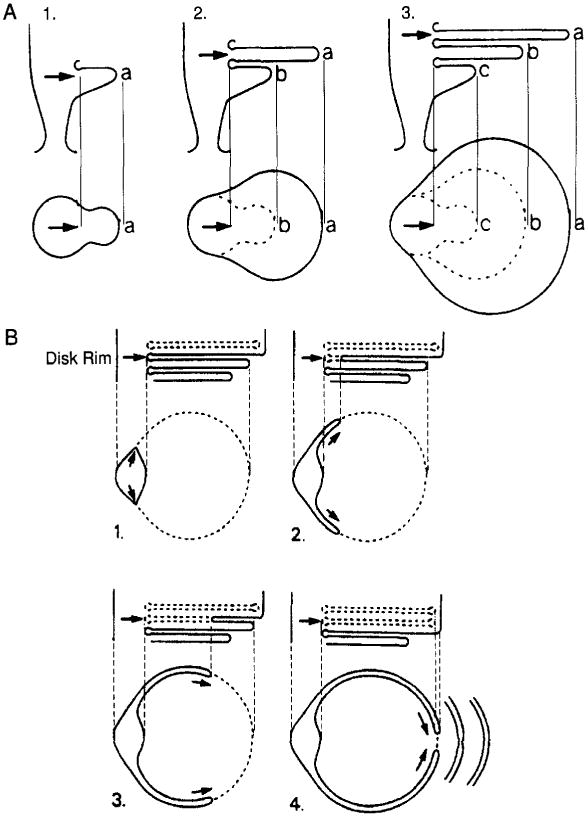
Model of disk membrane morphogenesis. (A) Diagram of disk surface formation. Drawings 1–3 (at top) show the early developmental stages of three evaginations (a, b, and c) at the base of the outer segment as they appear in longitudianal section. Each evagination appears as an outgrowth from the membrane of the inner face of the cilium and is filled with cytoplasm. As the evaginations expand in diameter, they become progressively thinner and the addition of new evaginations displaces the older evaginations apically. The lower portion of these same drawings illustrates their appearnce in tangential sections. In these sections each evagination appears as a circular bud that progressively expands in diameter. (B) Diagram of ROS disk membrane rim formation. The upper drawings (1–4) show the formation of a rim between adjacent disk surfaces in longitudinal sections. The lower drawings (1–4) show this same rim formation in tangential sections. The rim originates from the region of ciliary membranes between adjacent evaginations as indicated by the arrows in 1–4 (upper drawing). The growing region of the ciliary membrane is folded at the growth point, as indicated by the arrows in 1–4 (lower drawing), so that the inner portion is the rim while the outer portion is the new plasma membrane. (Reprinted from Steinberg et al., 1980, with permission from John Wiley and Sons.)
In contrast, the disk rim is derived from the region of ciliary membranes between adjacent evaginations, as shown in Figure 4B. The rim region grows bilaterally around the circumference of the adjacent disk. The growing membranes then meet at the disk perimeter opposite the cilium. The two opposing membranes then fuse to form a rim and a completed disk. It is important to note that the space between the two membranes of the fold becomes the space between the disk rim and the outer segment in the ROS. It is these two membranes that are proposed to fuse for a newly formed disk to seal, thus pointing to a disk–plasma membrane fusion event. Steinberg's model provides for the continuous renewal of the ROS plasma membrane and for an assembly process that alternately delivers membrane surface or rim-specific membranous materials. Furthermore, the rim region thus appears to be a rigid structure that will not evaginate and remains fixed, a structure consistent with biochemical evidence presented below.
A large body of work points to the importance of carbohydrates for proper ROS formation and organization. The structure of the ROS can be disrupted with the removal of the RPE. In a cell culture system the normal ROS structure (organized stacked flattened membrane disks) can, however, be maintained in a culture system supplemented with permissive sugars: galactose and lactose (Stiemke and Hollyfield, 1994). Early studies in which retinas were treated with tunicamycin (Fliesler et al., 1984; 1985; Ulshafer et al., 1986), an inhibitor of N-linked glycosylation, documented newly synthesized membrane material to form whorl-like or tubulovesicular structures rather than normal ROS disks (Mercurio and Holtzman, 1982). These vesicular structures are newly assembled opsin-containing membranes, which have been prepared for disk morphogenesis but are incapable of forming normal closed disks (Defoe et al., 1986). These vesicles are still sorted and transported to the outer segment (Plantner et al., 1980; Fliesler et al., 1985). In contast, tunicamycin did not inhibit lipid transport to the outer segment (Wetzel et al., 1993; Wetzel and Besharse, 1994), suggesting the absolute requirement of a protein component for normal disk assembly. Inhibition of Golgi function by monensin (Matheke et al., 1984; Matheke and Holtzman, 1984; Fliesler and Basinger, 1987) or inhibition of vesicle-mediated transport to the Golgi by Brefeldin A (Keller and Fliesler, 1990; Deretic and Papermaster, 1991) also inhibits disk morphogenesis with no discernible effect on the synthesis and transport of lipids to the outer segment. These studies provided further evidence that lipids and proteins are delivered to the outer segment through two distinct pathways. While it is generally believed that lipid transport does not depend on protein transport, protein transport may depend on lipid transport (Rodriguez de Turco, 1997).
Although protein glycosylation is necessary for disk morphogenesis, the post-translational trimming of the carbohydrate moieties is not essential, since treatment with castanospermine has no effect on disk morphogenesis (Fliesler et al., 1986). However, addition of lovastatin, a potent inhibitor of reactive isoprenyl units, did result in alterations of ROS morphology as well as COS and ROS degeneration (Pittler et al., 1995). These alterations consisted of whorl-like disorganized membrane structures analogous to those observed in the presence of tunicamycin. To distinguish between an effect of lovastatin on the synthesis of isoprenyl groups as opposed to a decrease in squalene, retinas were treated with monensin (squalene oxidase inhibitor), in which case disk morphogenesis was normal, suggesting that the inhibition of reactive isoprenyl groups was the causative effect of lovastatin on ROS morphology (Pittler et al., 1995). More recent in vivo models of cholesterol depletion of rod cell membranes suggest that a decrease in total cholesterol is not deleterious to ROS renewal rates, ultrastructure, opsin synthesis, or intracellular trafficking of the photoreceptor. It has been proposed that this effect may be due to sterol synergism between desmosterol (cholesterol precursor) and residual amounts of cholesterol (Fliesler et al., 2000). Although disk morphogenesis is controlled genetically (i.e., disks do not form in the absence of peripherin/rds), it is evident that a number of other factors influence this process, suggesting tight regulatory control.
B. Disk Shedding
Once new disk membranes are formed, they are displaced distally along the length of the ROS. The shed tips are engulfed by the retinal pigment epithelium (RPE) through a phagocytic process (Young and Bok, 1969; Bok and Young, 1979). Within the RPE, the engulfed tips form inclusion bodies, the components of which are subsequently broken down and eliminated from the RPE (Young, 1967; Ryter, 1985; Silverstein et al., 1977; 1989). Early morphological studies suggested that disk detachment from the ROS preceded engulfment by the RPE (Young, 1971; Hall et al., 1973; Anderson and Fischer, 1975; 1976; Bok and Young, 1979). In turn, an active process in which small packages of disks are delineated from the remainder of the ROS was proposed to precede detachment (Currie et al., 1978; Besharse and Dunis, 1982; Matsumoto and Besharse, 1985). Such delineations, also commonly referred to as disk packets, are inferred from Lucifer Yellow (Matsumoto and Besharse, 1985; Matsumoto et al., 1987) and Porcain Yellow (Laties et al., 1976) microscopy studies. While disk detachment and shedding are discussed as two distinct events, a clear distinction is ambiguous based on studies documenting the intrusion of RPE processes into the apical region of the outer segment upon shedding (Spitznas and Hogan, 1970; Steinberg et al., 1977). The formation of older disk membrane packets has been observed microscopically, and the isolation of these older disks is possible through the use of a cholesterol-dependent digitonin technique (Boesze-Battaglia et al., 1989; 1990). Disk shedding requires contact between the ROS and the RPE (Williams and Fischer, 1987); removal of the RPE results in photoreceptor degeneration and decreased disk morphogenesis over time (Hale et al., 1991). The phagocytosis of shed disks requires recognition of the disk packet by the RPE cells. A number of such phagocytosis receptors have been proposed [see Nguyen-Legros and Hicks (2000) for an extensive review].
Disk shedding is regulated by a daily rhythm (LaVail, 1973; 1976; 1980) involving a complex collection of environmental factors, including light (Besharse et al., 1977a,b) and second messengers. A unique circadian clock, synchronized by light, controls disk shedding (for review, see Cahill and Hasegawa et al., 1997; Van Gelder, 1998; Foster, 1998). The retinas use a circadian oscillator to control the levels of the endogenous neuromodulators dopamine and melatonin as signals of light and dark, respectively. This rhythmic circadian oscillator is controlled within the photoreceptors, most likely at the level of gene expression. Genes found to be regulated by such a circadian oscillator include genes coding for phototransduction proteins, proteins involved in melatonin synthesis, and transcriptional control genes (Cahill and Hasegawa, 1997). A number of second messengers generated within the RPE, including cAMP, a molecule that mimics darkness (Hall et al., 1993), PKC (Hall et al., 1989; 1991), and phosphoinosotides (Rodriguez de Turco, 1992), have been implicated in contributing to the control of ROS renewal by regulating phagocytosis. No clear mechanism by which shedding is regulated at the levels of signal transduction pathways is evident. Photoreceptor phagocytosis and the multiplicity of the regulatory factors and their mechanisms of action have been reviewed extensively (Nguyen-Legros and Hicks, 2000).
V. Molecular Basis of Disk Renewal
A. Peripherin/rds
Compelling arguments for the cellular function of peripherin/rds arrived with the identification (and subsequent transgenic rescue) of the genetic defect that produces the retinal degeneration slow (rds) mouse phenotype (Travis et al., 1989; 1992). These studies, in light of the previously described morphology of retinal photoreceptors from rds mice (Sanyal and Jansen, 1981; Hawkins et al., 1985), demonstrated that peripherin/rds is required to produce organelles recognizable as ROSs; a relative shortage of protein results in identifiable, though dysmorphic and shortened, ROSs. The discovery of the rds locus validated prior immunogold labeling studies that localized peripherin/rds to ROS disks and offered an initial suggestion of its importance for disk structure (Molday et al., 1987; Connell and Molday, 1990). The photoreceptor abnormalities produced by absent and reduced protein combined with its discrete subcellular localization argue forcefully that peripherin/rds is a disk rim component intimately involved in OS morphogenesis. Current studies are largely concerned with defining function at the protein level. In a remarkably prescient series of studies, morphometric TEM analyses of negatively stained ROSs discerned an electon-dense “terminal loop complex” within disk rims and postulated the existence of a transmembrane protein required for disk morphogenesis and disk rim formation (Corless and Fetter, 1987; Corless et al., 1987).
In humans the RDS gene has been localized to chromosome 6p21.2-cen. It is composed of three exons—U07147 (exon 1), U07148 (exon 2), and U07149 (exon 3) (Gene Bank accession numbers)—which are 821, 247 and 1909 bp, respectively (Farrar et al., 1991). The specific mechanisms controlling retinal-specific expression of peripherin/rds are currently under investigation. Two major mRNA transcripts (3 and 5.5 kb) are detected in human retinas and may result from alternate splicing or the use of alternate poly adenylation signals (Travis et al., 1991a). At present there is no conclusive evidence suggesting multiple isoforms of this protein. The highest level of peripherin/rds expression is in the midperipheral retina (a region of rod-photoreceptor dominance), as compared with the fovea. Peripherin/rds mRNA is downregulated in response to continuous light exposure in rat retinas (Yanagita et al., 1993).
B. Peripherin/rds Is Essential for Photoreceptor Morphogenesis
The most widely discussed notion for peripherin/rds molecular function is its direct participation in the morphogenesis of ROS disks (Molday, 1998). As reviewed above, formation of new disks is thought to result from a “zippering together” of evaginated sheets of ROS plasma membrane (Steinberg et al., 1980). The differentiation of disk from plasma membrane appears concomitantly with the formation of a disk rim—a region distinguished both morphologically and chemically from the central portion of the disk, the lamella. In TEM cross-sections, disk rims appear as distinct “hairpins” of membrane, both at the periphery and at the invaginating incisures. Dissolution of disk lamellae by high concentrations of osmium tetroxide leaves rim regions largely intact (Falk and Fatt, 1969). The alignment of stacked disks appears to be maintaned by bridging fibrils that connect rims of adjacent disks (Roof and Heuser, 1982; Corless et al., 1987). These demonstrations of discrete rim structures, in conjunction with studies that document high lateral fluidity of the lamellar region (Poo and Cone, 1974), led to the idea that ROS disk structure is constrained largely by a relatively rigid disk rim. Although details of the zippering process and organizational principles of the completed rim structure are currently sketchy, several lines of reasoning implicate peripherin/rds as a central player.
Studies of the part peripherin/rds plays in ROS morphogenesis have been hindered by the lack of a facile functional assay. Recalcitrance to transfection, combined with the failure of cultured photoreceptors to sustain ROSs of normal morphology, means that measuring recombinant-induced changes in disk morphogenesis is not feasible in cell or tissue culture. These challenges have sent investigators off in a variety of methodological directions, though generally to good effect. Recent information from molecular genetic, biochemical, and animal model studies is increasingly refining our knowledge of peripherin/rds function in disk morphogenesis.
1. Molecular Genetics Offers Clues to Key Features of Protein Structure and Function
In addition to the murine and bovine molecules originally described, homology cloning has identified roughly a dozen peripherin/rds orthologs in fish, amphibians, birds, and mammals (Begy and Bridges, 1990; Connell and Molday, 1990; Travis et al., 1989; 1991a,b; Gorin et al., 1993; Moghrabi et al., 1995; Kedzierski et al., 1996; Weng et al., 1998). They are highly conserved at the amino acid level (50–90% similarity) and share several distinctive features, shown schematically in Figure 5. Each predicts a protein of ∼346 amino acids that contains four transmembrane segments, a large intradiskal/extracellular loop (of ∼ 150 amino acids), seven conserved cysteine residues, and consensus sequence(s) for post-translational glycosylation (Molday, 1998). In several instances, regions of interest have been examined experimentally and the significance of putatively important features is known (discussed below).
Fig. 5.
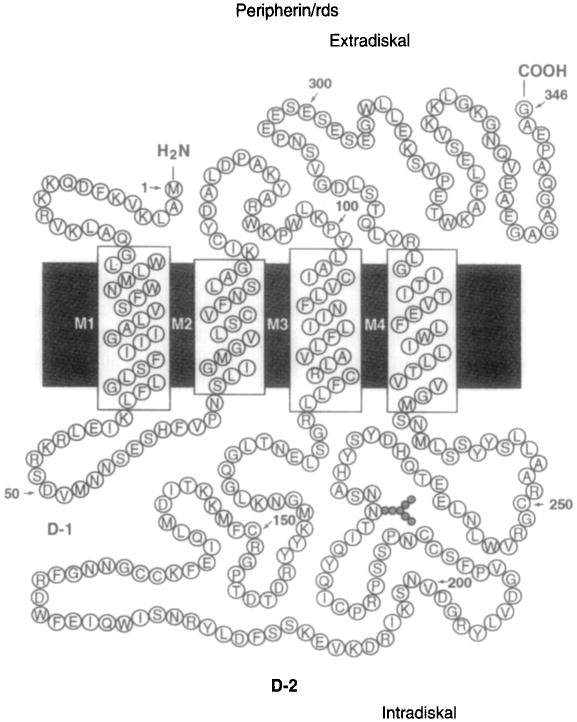
Schematic representation of human peripherin/rds. The model shows the orientation of human peripherin/rds within ROS disk membranes. The four transmembrane regions are designated M1 to M4 and the asparagine residue indicated with the attachment of carbohydrates.
A molecular genetics approach using differential hybridization to isolate conserved retinal-specific transcripts also lead to the identification of a peripherin/rds homolog, rom-1 (Bascom et al., 1992). Several orthologs of rom-1 have been cloned and show a high degree of similarity, both among themselves (∼85%) and to peripherin/rds (∼30%) (Moritz and Molday, 1996; Gould et al., 1997).
Human molecular genetics, and the candidate gene approach in particular, has produced a flurry of reports that emphasize the importance of periphrin/rds for human vision and add impetus to basic science investigations. Moreover, there has been a growing recognition that inherited defects can result in a wide heterogeneity of retinal disease phenotypes (Kohl et al., 1998). Although data from the human genetic studies have highlighted physiologically important regions of the protein and the wide variety of disease states produced, they generally leave us with several unanswered questions. In particular, we would like to know why particular regions or residues are significant and what the relationship is between disease phenotype and mutant genotype.
In at least one instance, human genetics provides partial answers. The discovery of digenic retinitis pigmentosa (RP) (Kajiwara et al., 1994) suggests that although peripherin/rds and rom-1 cooperate to generate healthy photoreceptors, they are not functionally equivalent and rom-1 plays a subsidiary role. More recent examples of both human disease and animal models support this conclusion (Milla et al., 1998; Kedzierski, 1999a; Clarke et al., 2000).
2. Biochemical Studies Defining Structure, Function, and Interactions
The current lack of a comprehensive scheme for making peripherin/rds genotype–phenotype correlations underscores the need to improve our understanding of both its normal function and the molecular consequences of pathogenic defects. For example, differentiating between a mutation that causes global protein misfolding and one that leaves protein structure largely intact but impairs function (such as disk morphogenesis) may help explain (and predict) variations in disease severity. Protein-level analyses of peripherin/rds from both vertebrate retina and heterologous cell-culture expression systems have generated several models for protein structure, function, and dysfunction.
Immunogold TEM studies make clear that peripherin/rds is not only limited to OS disk membranes, but is tightly confined to the high curvature rim region (Molday et al., 1987; Arikawa et al., 1992). This pattern of subcellular labeling provided the first suggestion for a role in disk rim formation and structure. Early biochemical investigations also established that peripherin/rds is an integral membrane protein that requires detergents for extraction from its native OS membranes (Connell and Molday, 1990; Travis et al., 1991b). Although peripherin/rds is found tightly associated with rom-1, no other OS proteins have been reported to interact stably with either polypeptide with the same avidity. A recent study reports a coim-munoprecipitation of ROS proteins containing glutamic acid–rich domains with peripherin/rds: namely, the cGMP-gated cation channel and glutamic acid-rich protein variants (GARPs) (Poetsch et al., 2001).
Peripherin/rds and rom-1 polypetides appear to be assembled at the biosynthetic level as homo- and heterotetrameric proteins (Bascom et al., 1992; Goldberg et al., 1995; Goldberg and Molday, 1996; Kedzierski et al., 1996), and evidence suggests that there is a stoichiometric excess of peripherin/rds over rom-1 (Kedzierski et al., 1999a; Loewen and Molday, 2000; Loewen et al., 2001). Subunits within the tetramers are held together noncovalently, yet tightly, as subunit exchange is not observed (Goldberg et al., 1995). Under nonreducing conditions, tetramers are extracted as larger (though still detergent-soluble) polymers of heterogeneous size; polymerization appears to be mediated by a single conserved cysteine residue (C150) in peripherin/rds (Goldberg et al., 1998; Loewen and Molday, 2000). This cysteine, like six other conserved cysteine residues, resides within the hydrophilic D2 loop. The D2 region appears particularly important for protein structure; a mutagenesis study found that substitution of any one of the six other conserved cysteines (C165, C166, C213, C214, C225, or C250) by serine caused protein misfolding and aggregation. This conclusion is reinforced by a recent report that insertional mutations in the D2 loop (but not other regions of the protein) generate heterogeneous structural perturbations, including defective subunit assembly and global misfolding (Goldberg et al., 2001). The finding that discrete determinants in D2 guide tetrameric assembly suggests that altered subunit assembly may be a general mechanism involved in generating instances of human retinal disease. One such example involving a digenic form of RP has been documented previously (Goldberg and Molday, 1996).
Although the majority of mutations associated with human retinal disease have been reported in the D2 region, attention is beginning to shift to the cytoplasmically oriented peripherin/rds C terminus. The C terminal region has been proposed as critical for catalyzing membrane fusion processes in the OS (discussed below). Also, the total number of tetramers in a single disk is reported to be comparable to the numbers of interdiskal filaments observed to connect adjacent disks, and it has been proposed that tetramers may serve as constitutents of the bridging filaments (Roof and Heuser, 1982; Corless et al., 1987; Goldberg and Molday, 1996). The recent finding that peripherin/rds can associate directly with the β-subunit of the cGMP-gatedcation channel suggests it may also serve as a component of anchoring filaments thai link disk rims to the adjacent plasma membrane (Roof and Heuser, 1982; Poetsch et al., 2001).
Finally, a recent brief communication offers hope that a facile assay for peripherin/rds function in rim formation is finally available. Flattening of canine pancreatic microsomes has been reported to occur in an in vitro expression system (Wrigley et al., 2000). The authors conclude that disk flattening is a redox-sensitive photoreceptor-autonomous process. It is not yet clear whether the flattened microsomes contain disk rim-like structures, or if peripherin/rds is localized within them. This report offers the promise of a tractable and efficient system to assess the effects of mutations on peripherin/rds function.
3. Murine Models
Nearly a dozen murine models have been reported to shed light on one or more aspects of peripherin/rds function (Sanyal et al., 1980; Hawkins et al., 1985; Kedzierski et al., 1997; 1998; 1999b; Weng et al., 1999; Nir et al., 2000; Ali et al., 2000; Clarke et al., 2000). The models that best address a role in disk morphogenesis contain relatively benign mutations; we expect that gene defects that disrupt protein structure globally will be null, like rds (Ma et al., 1995), and will be catastrophic for OS morphogenesis and produce photoreceptors of essentially similar appearance.
In fact, several models are primarily informative by way of arguing against particular hypotheses for molecular function and/or structure. For example, loss of a conserved glycosylation site (and subsequent lack of post-translational modification) has no observable effect on OS morphogenesis, thus undermining the idea that carbohydrate is required to maintain flattened disks (Kedzierski et al., 1999b). Likewise, results from a study of mice transgenic for a chimeric protein constructed from selected regions of rom-1 and peripherin/rds argue against the notion that functional efficacy is encoded within a single protein domain of peripherin/rds (Kedzierski et al., 1999a). Finally, two different investigations of knockout mouse models make obvious the fact that rom-1 plays a subsidiary role (Clarke et al., 2000), and ABCR (a rim-localized transporter protein) no essential role in disk morphogenesis (Weng et al., 1999).
A few murine models do offer evidence (albeit indirect) in support of a primary role in disk morphogenesis. First, detailed observations of rds heterozygotes show that OSs are produced, but with severe structural abnormalities (Hawkins et al., 1985). The observation that dysmorphic OSs appear within a normal developmental time course is consistent with the notion that the structural irregularity is a primary rather than a secondary effect of reduced peripherin/rds levels. Second, retroviral rescue of the postnatal rds mouse photoreceptor with a WT copy of the rds gene reiterates the concept that the gene product is a structural element used to build OSs; hence, delivery of this component at an abnormally late date is sufficient to partially restore normal OS structure and function (Ali et al., 2000).
Based on the literature discussed, we envision the following speculative model. Peripherin/rds polypetides are synthesized at the level of the inner segment and assembled into homo- and heterotetrameric proteins in the ER and Golgi; rom-1 is incorporated into a subset of these molecules, possibly as a regulatory subunit. The proteins are packaged into vesicles (distinct from that population containing rhodopsin), then sorted, and transported through the connecting cilium to the base of the OS. The addition of tetramers to expanding “saddle points” (Steinberg et al., 1980; Corless and Fetter, 1987) helps to maintain the alignment of adjacent disks during the rim maturation phase of disk formation; neighboring tetramers may polymerize via cysteine oxidation, providing a driving force for this reaction. We conjecture that both the restricted mobility of the tetramers and the high-curvature membrane at the rim region result from noncovalent protein–lipid interactions, and occur spontaneously as a function of thermodynamic considerations. Interaction of cytoplasmic regions of polymerized tetramers with GARP variants and the cGMP-gated cation channel may serve to stabilize the disk stack structure and close apposition to the plasma membrane.
C. Peripherin/rds as a Membrane Fusion Protein
1. Fusion in Photoreceptor Rod Cells
Cellular events as diverse as exocytosis, endocytosis, fertilization, viral entry into cells, and transcytosis in polarized cells are all dependent upon the fusion of two biological membranes. Fusion processes are also involved in maintaining the normal structure and physiological function of the photoreceptor rod and cone cells. Tightly controlled fusion events are involved in the fusion of transport vesicles from the trans-Golgi network (TGN) to the rod or cone outer segment, new disk morphogenesis, and, in rod cells, in membrane packet formation prior to disk shedding and phagocytosis. The coordinated action of these processes guarantees the timely delivery and proper sorting of new membranous material at the base of the ROS, the formation of new disk membranes, and the subsequent shedding and phagocytosis of older disks. Changes in outer segment membrane organization during disk membrane morphogenesis and shedding must require membrane fusion events.
Membrane fusion is supported by microscopic studies illustrating perturbations of ROS membrane structure in the form of vesiculation and tubulation (Young, 1976; Corless and Costello, 1981; Tsukamoto and Yamada, 1982). Further evidence for fusion of distal disks with plasma membrane comes from fluorescence microscopy studies, in which the aqueous interior of some disks becomes continuous with the external medium during disk packet formation. Lucifer Yellow staining was localized to a single band or multiple bands across the ROS, and staining did not spread throughout the entire ROS (Matsumoto and Besharse, 1985). Interestingly, the localization and frequency of this distal staining pattern was dependent upon environmental conditions (i.e., light). Staining was considered to be an active process since it could be inhibited with metabolic poisons and decreases in temperature. Collectively, these results suggest that the staining pattern may be reflecting a light-induced ROS event that is correlated with disk shedding, possibly disk packet formation (Matsumoto and Besharse, 1985). These authors have proposed that the confined pattern of staining in relatively narrow bands is consistent with disk plasma membrane fusion. Direct evidence for fusion was presented by Townes-Anderson (1995) in salamander photoreceptors, in which fusion between inner and outer segment was observed upon dissociation of photoreceptor rod cells from the remaining retina. The molecule(s) that regulate this fusion process have not been determined, although the salamander would provide a useful model in which to evaluate fusion processes in whole cells.
The role of peripherin/rds as a membrane fusion protein is also consistent with clinical phenotypes of peripherin/rds mutations. Mutations in peripherin/rds are associated with a variety of visual defects (Travis and Helper, 1993; Molday, 1994). In an animal model of retinal degeneration, the rds mouse, the peripherin/rds gene is defective (Connell et al., 1991). The ROS of rds mouse homozygotes does not develop and the photoreceptor cells eventually die (Sanyal and Jansen, 1981; Usukura and Bok, 1987; Connell and Molday, 1990). In adult heterozygotes, the rod outer segments are reduced in length and appear morphologically abnormal (Sanyal et al., 1980; Hawkins et al., 1985). These abnormalities begin at approximately two months of age and progress throughout life, eventually resulting in retinal degeneration and blindness (Hawkins et al., 1985). The retinal phenotype of the rds mouse provides corroborative clinically based evidence for the role of peripherin/rds in membrane fusion. These mice exhibit increases in the size and number of phagosomes, suggesting the involvement of normal peripherin/rds in the shedding process. Unregulated fusion between disk membranes of rds/+ mice and plasma membrane is likely to contribute to this phenomenon. A chimeric transgene consisting of the D-2 loop of peripherin/rds in the context of rom-1 was shown to be insufficient for proper ROS disk morphogenesis, suggesting that regions in addition to the D-2 loop (we hypothesize the C-terminal domain of peripherin/rds) contributes to functional efficacy.
2. Biochemical Analysis of Peripherin/rds as a Membrane Fusion Protein
The molecular basis of these fusion processes has been studied using an in vitro cell-free assay system based on paradigms from viral and egg–sperm fusion studies. Isolated bovine ROS disk membranes were shown to fuse with ROS plasma membrane vesicles using a well-characterized fluorescence lipid-mixing assay (for method review, see Boesze-Battaglia, 2000). Fusion was spontaneous, occurred upon the disruption of normal ROS structure by lysis (Boesze-Battaglia, 1997), required nanomolar levels of calcium (Boesze-Battaglia and Yeagle, 1992; Boesze-Battaglia, 1997), and was enhanced by retinal/ol at physiological concentrations (Boesze-Battaglia et al., 1992). The nature of the fusion protein remained unknown. In 1997, with the purification of bovine peripherin/rds to homogeneity and the isolation of population of ROS plasma membrane (ROS-PM) vesicles free from disks, experiments were undertaken which led to the identification of peripherin/rds as a photoreceptor-specific membrane fusion protein. Using native purified peripherin/rds reconstituted into disk lipid vesicles, this protein has been shown to promote fusion in an in vitro cell-free assay system. Fusion was measured fluorimetrically as an increase in the intensity of a lipophilic probe, R18, over time. Neither rhodopsin (Boesze-Battaglia et al., 1997) nor rom-1 (K. Boesze-Battaglia et al., unpublished observations) was able to promote membrane fusion with ROS-PM. When the fusion rate of the peripherin/rds–rom-1 complex with ROS-PM was accessed, it was found to be enhanced relative to peripherin/rds alone, suggesting an accessory role for rom-1 in peripherin/rds-mediated fusion.
Collectively, the data that provide the most compelling in vitro evidence for the hypothesis that peripherin/rds is a photoreceptor-specific membrane fusion protein are as follows: (1) Fusion between ROS-PM vesicles and disk membranes is inhibited by trypsinolysis of peripherin/rds, by preincubation of disk membranes with anti-peripherin/rds mAb 2B6, and by synthetic peptides to the C terminus of peripherin/rds. (2) Fusion can be initiated in a cell-free assay system consisting of ROS-PM and lipid recombinants containing purified peripherin/rds. (3) Fusion is specific for ROS plasma membrane vesicles oriented inside out (as would be seen in vivo). (4) A putative fusion peptide domain in peripherin/rds has been identified; designated PP-5, it corresponds at a minimal length to amino acids 311–325.
Peripherin/rds exhibits structural and functional homology to other well characterized membrane fusion proteins. Similar to other fusion proteins, peripherin/rds is membrane-anchored and the product of a single gene. The function of these membrane fusion proteins is imparted by the presence of fusogenic regions or patches, most commonly within the N terminus but in some cases in the C-terminal domains (for review, see White, 1992; Pecheur, 1999). These peptides are usually short stretches of relatively hydrophobic amino acids (16–25) residues within membrane-anchored domains and may be internal to the polypeptide chain. They have a hydrophobicity index between 0.50 and 0.70; and when modeled as α-helices, they display one face that is highly hydrophobic and a back face with high hydrogen binding capacity. Using a series of overlapping synthetic peptides to the C-terminal domain of peripherin/rds, we have shown that only a single peptide, called PP-5, is able to promote the prerequisite steps of fusion. This 15–amino acid residue was shown by FTIR to maintain an α-helical conformation (Boesze-Battaglia et al., 1998). When modeled as an α-helix, it is amphiphilic, a characteristic consistent with its role as a fusogenic domain. This fusion peptide domain is conserved among species, and is 100% homologous to an analogous region in human peripherin/rds. When compared to the analogous sequence in mouse and Xenopus peripherin/rds, there are six conserved residues corresponding to positions 311, 313, 314, 320, 323, and 325. In a comparison of bovine, human, and mouse peripherin/rds, only amino acid 322 is varied from a valine in human and bovine to a phenylanine in mouse. This single amino acid alteration does not substantially alter the hydropathy index of the fusion peptide. Phylogenetic homology is also observed between amino acids 294 and 314 (Kedzierski et al., 1997), a region upstream of the fusion peptide domain and the site of a recently characterized P296T human peripherin/rds mutation.
In addition to COS-1 cells (Goldberg et al., 1995; 1998), polarized Madin Darby canine kidney (MDCK) cells have also been transfected with peripherin/rds (Kim et al., 1997; Stefano et al., 2001). Using this MDCK cell expression system to express WT and mutant peripherin/rds, a higher initial rate of fusion between membranes containing the P296T mutant and ROS-PM was observed when compared to WT peripherin/rds. If peripherin/rds functions in a manner analogous to other fusion proteins, it is likely that the fusion-permissive form of peripherin/rds requires a coiled-coil structure (Bentz, 2000), which would require that a “hairpin” form be upstream of the fusion peptide (Weber et al., 1998). In this case an increase in hydrophilicity, as would occur with a substitution of proline to threonine, could alter the properties of the fusion protein. A similar mutation in gp-41 (fusion peptide of HIV) has been shown to favor the hairpin structure of the protein and enhance fusion (Liu et al., 2001). In this context, we propose that a region upstream of the fusion peptide domain of peripherin/rds aids in rendering the protein fusion-competent, most likely by aiding in a required change in conformation and possibly through the formation of a required “hairpin.” Disease-linked C-terminal mutations of peripherin/rds are assembled into two small but distinct clusters—a group within the homologous region and a smaller group within the fusion peptide domain (residues 311–325) (Kohl et al., 1998). The identification of three genetic polymorphisms in this region may make it more difficult to screen for defects in this area using conventional genetic approaches (Kohl et al., 1998). Such a cluster of mutations is consistent with high functional importance of these regions.
To date, photoreceptor peripherin/rds is the only fusion protein composed of four transmembrane domains; all other fusion proteins have two or fewer transmembrane domains. In addition, peripherin/rds may be unique in that both the C-terminal and the N-terminal regions may contribute to the fusion process. Data supporting a role for the N terminus include only a 30% decrease in disk–PM fusion upon trypsinolysis. On the basis of the sequence of peripherin/rds, both the N- and C-terminal domains of this protein are cleaved by trypsin. The role of the N terminus is unclear, since an N-terminal peptide NP-1 with sequence H2N–A– L–L–K–V–K–F–D–Q–K–K–R–V–COOH enhances membrane adhesion but has no effect on fusion between PM vesicles and disks or peripherin/rds LUVs. The simplest interpretation of these results is that the N-terminal region is not involved in a rate-limiting step of the process. However, it does not preclude the possibility that the N terminus may aid in rendering peripherin/rds fusion-competent. It is likely that the binding and fusion activity of peripherin/rds may reside in multiple regions of the protein and that either or both of these activities may be augmented by rom-1 or additional cofactors. Once the molecular mechanism of these fusion processes becomes clear, the triggers of fusion (i.e., second messengers, proteolytic events) can be elucidated and fusion localized to a distinct region within the ROS.
VI. Concluding Remarks
Whereas compelling evidence points to an important role for peripherin/rds in the membrane fusion processes that underlie disk morphogenesis and shedding, a number of unanswered questions remain. When these questions are addressed experimentally, the answers will shed much light on the regulation of disk formation and the molecular mechanism(s) by which disks are formed and proteins and lipids are sorted during disk morphogenesis. The complexity of ROS renewal processes and the absolute necessity for tightly controlled regulation of these processes lead us to propose that peripherin/rds has two functional domains, both of which are necessary in ROS renewal processes. The first and most well-characterized domain is the D-2 loop. This region is required for proper subunit assembly, without which ROS disk morphogenesis does not occur (Sanyal et al., 1980). The second and less well-characterized domain, is the C terminus, which contains the fusion domain(s) (Boesze-Battaglia et al., 1997; 1998) and is also proposed to contain the localization signal (Tarn et al., 2001). If tetrameric arrangement of the C terminus is needed to promote fusion, as proposed by in vitro model membrane studies (Boesze-Battaglia et al., 2000), is the fusion-competent form of peripherin/rds linked covalently as a tetramer or as two dimers that interact transiently for fusion to commence?
A role for rom-1 as a regulator of disk morphogenesis was proposed based on a series of electrophysiological and ultrastructural studies of Rom-1−/− mice. These mice showed an abnormal rod outer segment organization, which improved with age (Clarke et al., 2000). Rom-1 complexes noncovalently with peripherin/rds and it may act in two distinct capacities: rom-1 may retard fusion by keeping a highly fusogenic form of peripherin/rds in an inactive or nonfusogenic state, conversely, it may aid in the fusion process by acting as a SNARE-like protein (Gerst, 1999), binding to specific cell surface receptors and allowing peripherin/rds to promote fusion. Understanding how factors such as conformational change(s), and association with rom-1 render peripherin/rds fusion-competent will aid in elucidating the regulators of disk renewal at a molecular level. The recent work documenting a twofold excess of peripherin/rds over rom-1, with two distinct types of peripherin complexes—peripherin/rds homotetramers and disulfide-linked peripherin/rds dimers, complexing noncovalently with rom-1 to form heterotetramers (Loewen et al., 2000)—must be carefully considered in developing a working model of rom-1's role in fusion. In addition, the newly described interaction between peripherin/rds and GARP proteins (Poetsch et al., 2001) provides support for protein regulators of membrane fusion along the length of the ROS. The most essential series of studies should address how proper subunit assembly, as is required for normal disk morphogenesis, affects peripherin/rds fusogenic function through an analysis of peripherin/rds disease-linked mutants and fusogenic function.
From the point of view of protein transport and incorporation into the new disk in the outer segment, a number of unifying themes may aid in the design of future experiments. One theme in need of further consideration is the role of membrane rafts in the transport of lipid and protein constituents to the region of disk biogenesis. Kinesin has been shown to transport membrane rafts in other systems. The existence of both raft-like transport vesicle and nonraft opsin-bearing vesicles transported either through molecular motors (Section II.C) or through a periciliary pathway (Section II.B.4) is consistent with Steinberg's model (Section III.A), suggesting an assembly process that alternately delivers proteins to the rim region and disk lamellae. Still unresolved, however, are the mechanisms that establish the pattern of protein incorporation. The isolation of raft-like complexes from rod cells and the analysis of lipids and proteins associated with them will provide insight regarding the cargo (i.e., opsin and other ROS proteins) carried by these motors. Once the cargo arrives, are the constituents sorted to clearly define a disk rim and a lamellar region? And is sorting a consequence of distinct types of transport vesicles, distinct modes of transport (i.e., motors) either along the connecting cilium or through an alternative periciliary transport route? The possibility of retrograde transport along the connecting cilium as a regulatory mechanism signaling the down- or upregulation of various transcription factors remains an interesting possibility. Finally, once delivered, how are the components of a new disk assembled into a closed free-floating membrane, one that must remain intact until disk packet formation and shedding?
One property of newly formed disks is their high content of cholesterol relative to the plasma membrane (Andrews and Cohen, 1983;Boesze-Battaglia et al., 1989; 1990). Since membrane rafts are enriched in cholesterol, an attractive possibility is that the high cholesterol content of newly forming disks is derived from the import of cholesterol-rich raft-like domains which serve to transport ROS disk-specific proteins to the disks. The subsequent depletion of cholesterol from disks as the disks age could then cause a sorting of proteins to other regions of the disk. Indirect support for this possibility comes from the observation that retinal degeneration can be caused by treatment of retinas with lovastatin. This retinal degeneration is associated with decreases in isoprenylated proteins which have been shown to be raft-associated (Simons and Ikonen, 1997).
An important challenge for the future is to create genetic models with a phenotype distinct from that of RDS that will provide valuable information regarding the role of peripherin/rds in transport and ROS renewal. Such genetic models, including the recently developed GFP Xenopus laevis, coupled with cell-free assay systems designed to assay transport and fusion, in combination with in vitro model systems such as polarized MDCK cells, can collectively provide the information necessary to build a working model of protein and lipid transport and a molecular model of disk formation in the ROS.
Acknowledgments
We thank Dr. David Papermaster for providing the included images, Dr. David Williams for helpful discussions, Dr. Richard Schimmel for critical reading of the manuscript and discussions, and Janice Dispoto for all of her assistance with the references.
References
- Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- Albert AD, Lane SA, Yeagle PL. 2H and 31P Nuclear magnetic resonance studies of membranes containing bovine rhodopsin. J Membrane Biol. 1985;87:211–215. doi: 10.1007/BF01871220. [DOI] [PubMed] [Google Scholar]
- Albert AD, Yeagle PL. Phospholipid domains in bovine rod outer segment disk membranes. Proc Natl Acad Sci USA. 1983;80:7188–7191. doi: 10.1073/pnas.80.23.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AD, Young JE, Paw Z. Phospholipid fatty acyl spatial distribution in bovine rod outer segment disk membranes. Biochim Biophys Acta. 1998;1368:52–60. doi: 10.1016/s0005-2736(97)00200-9. [DOI] [PubMed] [Google Scholar]
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JWB, Munro PM, Fauser S, Reichel MB, Kinnon C, Hunt DM, Bhattacharya SS, Thrasher AJ. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Gene. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997a;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Berstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997b;277:1809–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK. Disc shedding in rodlike and conelike photoreceptors of tree squirrels. Science (Wash) 1975;187:953–955. doi: 10.1126/science.1145180. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fischer SK, Breding DJ. Exp Eye Res. 1986;42:267. doi: 10.1016/0014-4835(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: Disc shedding, phagocytosis and renewal. Invest Ophthalmol. 1978;17:117–133. [PubMed] [Google Scholar]
- Anderson RE, Kelleher PA, Maude MB. Metabolism of phosphatidylethanolamine in the frog retina. Biochim Biophys Acta. 1980a;620:227–235. doi: 10.1016/0005-2760(80)90204-0. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Kelleher PA, Maude MB, Maida TM. Synthesis and turnover of lipid and protein components of frog retinal rod outer segments. Neumchemistry. 1980b;1:29–42. doi: 10.1016/0197-0186(80)90048-0. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Maude MB, Kelleher PA. Metabolism of phosphatidylinositol in the frog retina. Biochim Biophys Acta. 1980c;620:236–246. doi: 10.1016/0005-2760(80)90205-2. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Maude MB, Kelleher PA, Maida TM, Basinger SF. Metabolism of phosphatidylcholine in the frog retina. Biochim Biophys Acta. 1980d;620:212–226. doi: 10.1016/0005-2760(80)90203-9. [DOI] [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. Exp Eye Res. 1981;33:1–10. doi: 10.1016/s0014-4835(81)80076-0. [DOI] [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. J Cell Biol. 1983;97:749–755. doi: 10.1083/jcb.97.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML. Relationships of G-protein-coupled receptors. A survey with the photoreceptor opsin subfamily. Soc Gen Phys Ser. 1994;49:235–248. [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: Relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt's disease (ABCR) FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Baylor D. How photons start vision. Proc Natl Acad Sci USA. 1996;93:560–565. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Rodriguez de Turco EB. Review. Pharmacological manipulation of docosahexaenoic-phospholipid biosynthesis in photoreceptor cells: Implications in retinal degeneration. J Ocular Pharm. 1994;10:591–604. doi: 10.1089/jop.1994.10.591. [DOI] [PubMed] [Google Scholar]
- Begy G, Bridges CD. Nucleotide and predicted protein sequence of rat retinal degeneration slow (rds) Nucleic Acids Res. 1990;18:3058. doi: 10.1093/nar/18.10.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz J. Membrane fusion mediated by coiled coils: A hypothesis. Biophys J. 2000;78:886–900. doi: 10.1016/S0006-3495(00)76646-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Rod photoreceptor disc shedding in vitro: Inhibition by cytochalasins and activation by colchicine. In: Hollyfield JG, editor. The Structure of the Eye. Elsevier North Holland; New York: 1982. pp. 85–96. [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Photoreceptor outer segments: Accelerated membrane renewal in rods after exposure to light. Science. 1977a;196:536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II Membrane addition and loss in relationship to light. J Cell Biol. 1977b;75:507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Horst CJ. The photoreceptor connecting cilium: A model for the transition zone. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. Plenum; New York: 1990. pp. 389–431. [Google Scholar]
- Besharse JC, Wetzel MG. Immunocytochemical localization of opsin in rod photoreceptors during periods of rapid disc assembly. J Neurocytol. 1995;24:371–388. doi: 10.1007/BF01189064. [DOI] [PubMed] [Google Scholar]
- Bibb C, Young RW. Renewal of fatty acids in the membranes of visual cells outer segments. J Cell Biol. 1974a;61:327–343. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C, Young RW. The renewal of glycerol in the visual cells and pigment epithelium of the frog retina. J Cell Biol. 1974b;62:378. doi: 10.1083/jcb.62.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas EE, Biswas SB. The C-terminal nucleotide binding domain of the human retinal ABCR protein is an adenosine triphosphotase. Biochemistry. 2000;39:15879–15886. doi: 10.1021/bi0015966. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K. Fusion of intracellular rod outer segment disk membranes with the surrounding plasma membrane. Invest Ophthalmol Vis Sci. 1997;38:18–26. [PubMed] [Google Scholar]
- Boesze-Battaglia K. Fusion between retinal rod outer segment membranes and model membranes: Functional assays and a role for peripherin/rds. Methods Enzymol. 2000;316:65–87. doi: 10.1016/s0076-6879(00)16717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp Eye Res. 1992;54:821–823. doi: 10.1016/0014-4835(92)90040-y. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Hennessey T, Albert AD. Cholesterol heterogeneity in bovine retinal rod outer segment disk membranes. J Biol Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Kong F, Lamba OP, Stefano FP, Williams DS. Purification and light dependent phosphorylation of a candidate fusion protein, photoreceptor peripherin/rds. Biochemistry. 1997;22:6835–6846. doi: 10.1021/bi9627370. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Lamba OP, Napoli A, Sinha S, Guo Y. Fusion between retinal rod outer segment membranes and model membranes; a role for photoreceptor peripherin/rds. Biochemistry. 1998;37:9477–9487. doi: 10.1021/bi980173p. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Schimmel RJ. Cell membrane lipid composition and distribution: Implications for cell function and lessons learned from photoreceptors and platelets. J Exp Biol. 1997;200:2927–2936. doi: 10.1242/jeb.200.23.2927. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Stefano FP, Fenner M, Napoli AA., Jr A peptide analogue to a fusion domain within photoreceptor peripherin/rds promotes membrane adhesion and destabilization. Biochim Biophys Acta. 2000;1463:343–354. doi: 10.1016/s0005-2736(99)00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Yeagle PL. Rod outer segment disc membranes are capable of fusion. Invest Ophthalmol Vis Sci. 1992;33:484–493. [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalomol Vis Sci. 1985;26:1659. [PubMed] [Google Scholar]
- Bok D, Young RW. Phagocytic properties of the retinal pigment epithelium. In: Zinn KM, Marmor MF, editors. The Retinal Pigment Epithelium. Harvard University Press; London: 1979. pp. 175–191. [Google Scholar]
- Bridges CDB. Lectin receptors of rods and cones visualization by fluorescent label. Invest Ophthalmol Vis Sci. 1981;20:8–16. [PubMed] [Google Scholar]
- Bridges CDB, Fong SL. Different distribution of receptors of peanut and ricin agglutinins between inner and outer segments of rod cells. Nature (London) 1979;282:513–515. doi: 10.1038/282513a0. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Hasegawa M. Circadian oscillators in vertebrate retinal photoreceptor cells. Biol Signals. 1997;6:191–200. doi: 10.1159/000109129. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday L, Rossant J, Szel A, Molday RS, Birch DG, McInnes RR. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- Cleves A, McGee T, Bankaitis V. Trends Cell Biol. 1991;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cohen AI. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J Cell Biol. 1968;37:424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI. Some cytological and initial biochemical observations on photoreceptors in retinas of rds mice. Invest Ophthalmol Vis Sci. 1983;24:832–834. [PubMed] [Google Scholar]
- Connell GJ, Bascom R, Molday L, Reid D, McInnes R, Molday RS. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci USA. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell GJ, Molday RS. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990;29:4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Cook NJ, Molday LL, Reid D, Kaupp UB, Molday RS. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989;264:6996–6999. [PubMed] [Google Scholar]
- Corless JM, Cobbs WH, Costello MJ, Robertson JD. On the asymmetry of frog of frog retinal rod outer segment disk membranes. Exp Eye Res. 1976;23:295–324. doi: 10.1016/0014-4835(76)90130-5. [DOI] [PubMed] [Google Scholar]
- Corless JM, Costello MJ. Paracrystalline inclusions associated with the disk membranes of frog retinal rod outer segments. Exp Eye Res. 1981;32:217–228. doi: 10.1016/0014-4835(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Corless JM, Fetter RD. Structural features of the terminal loop region of frog retinal rod outer segment disk membranes: III. Implications of the terminal loop complex for disk morphogenesis, membrane fusion, and cell surface interactions. J Comp Neurol. 1987;257:24–38. doi: 10.1002/cne.902570104. [DOI] [PubMed] [Google Scholar]
- Corless JM, Fetter RD, Zampighi OB, Costello MJ, Wall-Buford DL. Structural features of the terminal loop region of frog retinal rod outer segment disk membranes: II. Organization of the terminal loop complex. J Comp Neurol. 1987;257:9–23. doi: 10.1002/cne.902570103. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Currie JR, Hollyfield JG, Rayborn M. Rod outer segments elongate in constant light: Darkness is required for normal shedding. Vis Res. 1978;18:995–1003. doi: 10.1016/0042-6989(78)90027-5. [DOI] [PubMed] [Google Scholar]
- DeFoe DM, Besharse JC, Fliesler SJ. Tunicamycin-induced dysgenesis of retinal rod outer segment membranes. II Quantitative freeze-fracture analysis. Invest Ophthalmol Vis Sci. 1986;27:1595–1601. [PubMed] [Google Scholar]
- Deretic D. Post-Golgi trafficking of rhodopsin in retinal photoreceptors. Eye. 1998;(Pt. 3b):526–530. doi: 10.1038/eye.1998.141. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancicni M, Simons K, Papermaster D. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster DS. Polarized sorting of opsin on post-Golgi membranes in retinal photoreceptor cells. J Cell Biol. 1991;113:1281–1293. doi: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Papermaster D. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993;106:803–813. doi: 10.1242/jcs.106.3.803. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster D. In: Progress in Retinal and Eye Research. Osborne NN, Chader GJ, editors. Vol. 14. Pergamon Press; New York: 1995. pp. 249–265. [Google Scholar]
- Deretic D, Puleo-Scheppke B, Trippe C. Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Biol Chem. 1996;271:2279–2286. doi: 10.1074/jbc.271.4.2279. [DOI] [PubMed] [Google Scholar]
- Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci USA. 1998;95:10620–10625. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Dudley PA, Anderson RE. Phospholipid transfer protein from bovine retina with high activity towards retinal rod disc membranes. FEBS Lett. 1978;95:57–60. doi: 10.1016/0014-5793(78)80051-9. [DOI] [PubMed] [Google Scholar]
- Falk G, Fatt P. Distinctive properties of the lamellar and disk-edge structures of the rod outer segment. J Ultrastruct Res. 1969;28:41–60. doi: 10.1016/s0022-5320(69)90005-7. [DOI] [PubMed] [Google Scholar]
- Fariss RN, Matsumoto B, Fischer SK. Invest Ophthalmol Vis Sci. 1993;34:768. [Google Scholar]
- Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, Jordan SA, Kenna P, Humphries MM, Kumar-Singh R, McWilliam R, Allamand V, Sharp E, Humphries P. Autosomal dominant retinitis pigmentosa: Localization of a disease gene (RP6) to the short arm of chromosome 6. Genomics. 1991;11:870–874. doi: 10.1016/0888-7543(91)90009-4. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Basinger SF. Monensin stimulates glycerolipid incorporation into rod outer segment membranes. J Biol Chem. 1987;262:17516–17523. [PubMed] [Google Scholar]
- Fliesler SJ, Boesze-Battaglia K, Paw Z, Keller RK, Albert A. Squalene is localized to the plasma membrane in bovine retinal rod outer segments. Exp Eye Res. 1997;64:279–282. doi: 10.1006/exer.1996.0201. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Rapp LM, Hollyfield J. Photoreceptor specific degeneration caused by tunicamycin. Nature. 1984;311:575–577. doi: 10.1038/311575a0. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Rayborn ME, Hollyfield JG. Membrane morphogenesis in retinal rod outer segments: Inhibition by tunicamycin. J Cell Biol. 1985;100:574–587. doi: 10.1083/jcb.100.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Rayborn ME, Hollyfield JG. Inhibition of oligosaccharide processing and membrane morphogenesis in retinal rod photoreceptor cells. Proc Natl Acad Sci USA. 1986;83:6435–6439. doi: 10.1073/pnas.83.17.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Richards MJ, Miller CY, Cenedella RJ. Cholesterol synthesis in the vertebrate retina: Effects of U18666A on rat retinal structure, photoreceptor membrane assembly, and sterol metabolism and composition. Lipids. 2000;35:289–296. doi: 10.1007/s11745-000-0525-y. [DOI] [PubMed] [Google Scholar]
- Foster RG. Shedding light on the biological clock. Neuron. 1998;20:829–832. doi: 10.1016/s0896-6273(00)80464-x. [DOI] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AFX, Fales LM, Hurley JB, Khattree N. Folding and subunit assembly of photoreceptor peripherin/rds is mediated by determinants within the extracellular/intradiskal EC2 domain. J Biol Chem. 2001;276:42700–42706. doi: 10.1074/jbc.M107511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AE, Loewen CJ, Molday RS. Cysteine residues of photoreceptor peri pherin/rds: Role in subunit assembly and autosomal dominant retinitis pigmentosa. Biochemistry. 1998;37:680–685. doi: 10.1021/bi972036i. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: Hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry. 1996;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Moritz OL, Molday RS. Heterologous expression of photoreceptor peripherin/rds and Rom-1 in COS-1 cells: Assembly, interactions, and localization of multisubunit complexes. Biochemistry. 1995;34:14213–14219. doi: 10.1021/bi00043a028. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. J Neurosci. 1990;10:2190–2202. doi: 10.1523/JNEUROSCI.10-07-02190.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin MB, Snyder S, To A, Narfstrom K, Curtis R. The cat RDS transcript: Candidate gene analysis and phylogenetic sequence analysis. Mamm Genome. 1993;4:544–548. doi: 10.1007/BF00364792. [DOI] [PubMed] [Google Scholar]
- Gould DJ, Petersen-Jones SM, Lin CT, Sargan DR. Cloning of canine rom-1 and its investigation as a candidate gene for generalized progressive retinal atrophies in dogs. Anim Genet. 1997;28:391–396. doi: 10.1111/j.1365-2052.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- Green ES, Menz MD, LaVail MM, Flannery JG. Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:1546–1553. [PubMed] [Google Scholar]
- Guerin CJ, Lewis GP, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34:175–183. [PubMed] [Google Scholar]
- Hale IL, Fisher SK, Matsumoto B. Effects of retinal detachment on rod disc membrane assembly in cultured frog retinas. Investigative Ophthalmology & Visual Science. 1991;32(11):2873–2881. [PubMed] [Google Scholar]
- Hall MO, Abrams TA, Mittag TW. Protein kinase C activation by phorbol ester inhibits the phagocytosis of ROS by RPE cells. Invest Ophthalmol Vis Sci. 1989;30:118. [Google Scholar]
- Hall MO, Abrams TA, Mittag TW. ROS ingestion by the RPE is turned off by increased protein kinase C activity and by increased calcium. Exp Eye Res. 1991;52:591–598. doi: 10.1016/0014-4835(91)90061-i. [DOI] [PubMed] [Google Scholar]
- Hall MO, Abrams TA, Mittag TW. The phagocytosis of rod outer segments is inhibited by drugs linked to cyclic adensoine monophosphate production. Invest Ophthalmol Vis Sci. 1993;34:2392–2401. [PubMed] [Google Scholar]
- Hall MO, Basinger SR, Bok D. Studies on the assembly of rod outer segment disc membranes. In: Langer H, editor. Biochemistry and Physiology of the Visual Pigments. Springer-Verlag; New York: 1973. pp. 319–326. [Google Scholar]
- Hall MO, Bok D, Bacharach ADE. Biosynthesis and assembly of the rod outer segment membrane system. Formulation and fate of visual pigment in the frog retina. J Mol Biol. 1969;45:397–406. doi: 10.1016/0022-2836(69)90114-4. [DOI] [PubMed] [Google Scholar]
- Hargrave PA, McDowell JH. Rhodopsin and phototransduction. Int Rev Cytol. 1992;137B:49–97. doi: 10.1016/s0074-7696(08)62600-5. [DOI] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mosseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci USA. 1995;92:9815–9818. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RK, Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: Photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41:701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Hicks D, Molday RS. Localization of lectin receptor on bovine photoreceptor cells using dextran–gold markers. Invest Ophthalmol Vis Sci. 1985;7:1002–1013. [PubMed] [Google Scholar]
- Hicks D, Molday RS. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986;42:55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: From microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Connell GJ, Wong S, Molday RS. Structural and functional properties of rhodopsin from rod outer segment disk and plasma membrane. Biochim Biophys Acta. 1993;1145:85–92. doi: 10.1016/0005-2736(93)90384-c. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Molday RS. Glycolytic enzymes and a GLUT-1 glucose trasporter in the outer segments of rod and cone photoreceptor cells. J Biol Chem. 1991;266:21745–21752. [PubMed] [Google Scholar]
- Illing M, Molday LL, Molday RS. The 220 kDa Rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski W, Bok D, Travis GH. Transgenic analysis of rds/peripherin N-glycosylation: Effect on dimerization, interaction with rom1, and rescue of the rds null phenotype. J Neurochem. 1999b;72:430–438. doi: 10.1046/j.1471-4159.1999.0720430.x. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- Kedzierski W, Moghrabi WN, Allen AC, Jablonski-Stiemke MM, Azarian SM, Bok D, Travis GH. Three homologs of rds/perhipherin in Xenopus laevis photoreceptors that exhibit covalent and non-covalent interactions. J Cell Sci. 1996;109(Pt 10):2551–2560. doi: 10.1242/jcs.109.10.2551. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Weng J, Travis GH. Analysis of the rds/peripherin.rom1 complex in transgenic photoreceptors that express a chimeric protein. J Biol Chem. 1999a;274:29181–29187. doi: 10.1074/jbc.274.41.29181. [DOI] [PubMed] [Google Scholar]
- Keller RK, Fliesler SJ. Brefeldin A blocks transport of proteins, but not lipids, to the rod outer segment. Invest Ophthalmol Vis Sci. 1990;31:470. [Google Scholar]
- Kim R, Weimbs T, Bedolli M, Low S, Mostov KE. RDS/peripherin expressed in Madin-Darby Canine Kidney cells sorts to the plasma membrane. Invest Ophthalmol Vis Sci. 1998;39:S17. [Google Scholar]
- Kinney MS, Fischer SK. The photoreceptors and pigment epithelium of the adult Xenopus retina: Morphology and outer segment renewal. Proc R Soc Lond, Ser B. 1978a;201:149–167. doi: 10.1098/rspb.1978.0037. [DOI] [PubMed] [Google Scholar]
- Kinney MS, Fischer SK. The photoreceptors and pigment epithelium of the larval Xenopus retina: Morphology and outer segment renewal. Proc R Soc Lond, Ser B. 1978b;201:149–167. doi: 10.1098/rspb.1978.0037. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kohl S, Giddings I, Besch D, Apfelstedt-Sylla E, Zrenner E, Wissinger B. The role of the peripherin/RDS gene in retinal dystrophies. Acta Anat (Basel) 1998;162:75–84. doi: 10.1159/000046471. [DOI] [PubMed] [Google Scholar]
- Krappa R, Nyugen A, Burrola P, Deretic D, Lamke G. Evectins: Vesicular proteins that carry a pleckstrin homology domain and localize to post-golgi membranes. Proc Natl Acad Sci. 1999;96:4633–38. doi: 10.1073/pnas.96.8.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties AM, Bok D, Liebman P. Procion Yellow: A marker dye for outer segment disc patency and for rod renewal. Exp Eye Res. 1976;23:139–148. doi: 10.1016/0014-4835(76)90197-4. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973;58:650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retinal. Relationship to cyclic lighting. Science. 1976;194:1071. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407. [PubMed] [Google Scholar]
- Lee ES, Burnside BG. Trafficking of C-terminal truncated peripherin/rds in Transgenic xenopus laevis. Invest Ophthalmol Vis Sci. 2001;42(4) Abstract #1975. [Google Scholar]
- Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, Lupski JR, Leppert M, Dean M. Am J Hum Genet. 1999;64:422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: Evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci USA. 1996;93:14176–14181. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Wong F, Chang JH, Possin DE, Hao Y, Petters RM, Milam AH. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1998;39:808–819. [PubMed] [Google Scholar]
- Liebman PA, Entine G. Science. 1974;185:457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Liu J, Shu W, Fagan MB, Nunberg JH, Lu M. Structural and functional analysis of the HIV gp41 core containing an Ileu573 to Thr substitution: Implications for membrane fusion. Biochemistry. 2001;40:2797–2807. doi: 10.1021/bi0024759. [DOI] [PubMed] [Google Scholar]
- Liu XR, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Vansant G, Udovichenko IP, Wolfram U, Williams DS. Myosin Vila, the product of the Usher IB syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskel. 1997;37:240–252. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Liu X, Williams DS. Coincident onset of expression of myosin VIIa and opsin in the cilium of the developing photoreceptor cell. Exp Eye Res. 2001;72:351–355. doi: 10.1006/exer.2000.0963. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–5378. doi: 10.1074/jbc.275.8.5370. [DOI] [PubMed] [Google Scholar]
- Loewen C, Moritz O, Molday R. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J Biol Chem. 2001;276:22388–22396. doi: 10.1074/jbc.M011710200. [DOI] [PubMed] [Google Scholar]
- Ma J, Norton JC, Allen AC, Burns JB, Hasel KW, Burns JL, Sutcliffe JG, Travis GH. Retinal degeneration slow (rds) in mouse results from simple insertion of a t haplotype-specific element into protein-coding exon II. Genomics. 1995;28:212–219. doi: 10.1006/geno.1995.1133. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Lui X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LSB. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Matheke ML, Fliesler SJ, Basinger SF, Holtzman E. The effects of monensin on transport of membrane components in the frog retinal photoreceptor. I Light microscope autoradiography and biochemical analysis. J Neurosci. 1984;4:1086–1092. doi: 10.1523/JNEUROSCI.04-04-01086.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheke ML, Holtzman E. The effects of monensin and of puromycin on transport of membrane components in the frog retinal photoreceptor. II Electron microscopic autoradiography of proteins and glycerolipids. J Neurosci. 1984;4:1093–1103. doi: 10.1523/JNEUROSCI.04-04-01093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto B, Besharse JC. Light and temperature modulated staining of rod outer segment distal tips with Lucifer yellow. Invest Ophthalmol. 1985;26:628–635. [PubMed] [Google Scholar]
- Matsumoto B, DeFoe DM, Besharse JC. Membrane turnover in rod photoreceptors: Ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc R Soc Lond. 1987;230:339–354. doi: 10.1098/rspb.1987.0023. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Holtzman E. Ultrastructural localization of glycerolipid synthesis in rod cells of the isolated frog retina. J Neurocytol. 1982;11:295–322. doi: 10.1007/BF01258248. [DOI] [PubMed] [Google Scholar]
- Milla E, Heon E, Piguet B, Ducrey N, Butler N, Stone E, Schorderet DR, Munier F. Mutational screening of peripherin/RDS genes, rhodopsin and ROM-1 in 69 index cases with retinitis pigmentosa and other retinal dystrophies. Klin Monatsbl Augenheilkd. 1998;212:305–308. [PubMed] [Google Scholar]
- Moghrabi WN, Kedzierski W, Travis GH. Canine homolog and exclusion of retinal degeneration slow (rds) as the gene for early retinal degeneration (erd) in the dog [letter] Exp Eye Res. 1995;61:641–643. doi: 10.1016/s0014-4835(05)80059-4. [DOI] [PubMed] [Google Scholar]
- Molday RS. Peripherin/rds and rom-1: Molecular properties and role in photoreceptor cell degeneration. Prog Retinal Eye Res. 1994;13:271–299. [Google Scholar]
- Molday RS. Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 1998;39:2491–2513. [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Moritz OL, Molday RS. Molecular cloning, membrane topology, and localization of bovine rom-1 in rod and cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1996;37:352–362. [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Knox BE, Papermaster DS. Fluorescent photoreceptors of transgenic Xenopus laevis imaged in vivo by two microscopy techniques. Invest Ophthalmol Vis Sci. 1999;40:3276–3280. [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Papermaster DS, Nakayama T. A functional rhodopsin-GFP fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time dependent pattern. J Biol Chem. 2001;276:28242–28251. doi: 10.1074/jbc.M101476200. [DOI] [PubMed] [Google Scholar]
- Newsome DA. Retinal pigmented epithelium culture: Current applications. Trans Ophthalmol Soc UK. 1983;103:458–466. [PubMed] [Google Scholar]
- Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000;196:245–313. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- Nilsson SEG. Receptor cell outer segment development and ultrastructure of the disk membranes in the retina of the tadpole (Rana pipiens) J Ultrastruct Res. 1964;11:581–620. doi: 10.1016/s0022-5320(64)80084-8. [DOI] [PubMed] [Google Scholar]
- Nir I, Papermaster DS. Differential distribution of opsin in the plasma membrane of frog photoreceptors: An immunocytochemical study. Invest Ophthalmol Vis Sci. 1983;24:868–878. [PubMed] [Google Scholar]
- Nir I, Kedzierski W, Chen J, Travis GH. Expression of Bcl-2 protects against photoreceptor degeneration in retinal degeneration slow (rds) mice. J Neurosci. 2000;20:2150–2154. doi: 10.1523/JNEUROSCI.20-06-02150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. Is vertebrate phototransduction solved? New insights into the molecular mechanism of phototransduction. Invest Opthalmol Vis Sci. 1994;35:3577–3581. [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Reilly P, Schneider BG. Cone lamellae and red and green rod outer segment discs contain a large intrinsic membrane protein on their margins: An ultrastructural immunocytochemical study of frog retinas. Vis Res. 1982;22:1417–1428. doi: 10.1016/0042-6989(82)90204-8. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG. Biosynthesis and morphogenesis of outer segment membranes in vertebrate photoreceptor cells. In: McDevitt DS, editor. Cell Biology of the Eye. Academic Press; New York: 1982. pp. 475–531. [Google Scholar]
- Papermaster DS, Converse CA, Siuss J. Membrane biosynthesis in the frog retina: Opsin transport in the photoreceptor cell. Biochemistry. 1975;14(7):1343–52. doi: 10.1021/bi00678a001. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, DeFoe D, Besharse JC. Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem. 1986;34:5–16. doi: 10.1177/34.1.2934469. [DOI] [PubMed] [Google Scholar]
- Papermaster D, Schneider BG, Zorn MA, Kraehenbuhl JP. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978;78:415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecheur EI, Sainte-Marie J, Bienvenue A, Hoekstra D. Peptides and membrane fusion: Towards an understanding of the molecular mechanism of protein-induced fusion. J Mem Biol. 1999;167:1–17. doi: 10.1007/s002329900466. [DOI] [PubMed] [Google Scholar]
- Pittler SJ, Fliesler SJ, Fisher PL, Kennedy Keller R, Rapp LM. In vivo requirement of protein prenylation for maintenance of retinal cyloarchitecture and photoreceptor structure. J Cell Biol. 1995;130:431–439. doi: 10.1083/jcb.130.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantner JJ, Poncz L, Kean EL. Effect of tunicamycin on the glycosylation of rhodopsin. Arch Biochem Biophys. 1980;201:527–532. doi: 10.1016/0003-9861(80)90541-x. [DOI] [PubMed] [Google Scholar]
- Poetsch A, Molday L, Molday RS. The cGMP-gated channel and related glutamic acid rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001 doi: 10.1074/jbc.M108941200. In press. [DOI] [PubMed] [Google Scholar]
- Polaws AS, Altman LG, Papermaster DS. Immunocytochemical binding of anti-opsin N-terminal specific antibodies to the extracellular surface of rod outer segment plasma membranes. J Histochem Cytochem. 1986;34:659–664. doi: 10.1177/34.5.2939131. [DOI] [PubMed] [Google Scholar]
- Polozova A, Litman BJ. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys J. 2000;79:2632–2643. doi: 10.1016/S0006-3495(00)76502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Reid DM, Friedel U, Molday RS, Cook NJ. Identification of the sodium-calcium exchanger as the major ricin-binding glycoprotein of bovine rod outer segments and its localization to the plasma membrane. Biochemistry. 1990;29:1601–1607. doi: 10.1021/bi00458a035. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Gordon WC, Bazan NG. Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Experimental Eye Research. 1992;55(5):719–25. doi: 10.1016/0014-4835(92)90176-s. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Deretic D, Bazan N, Papermaster DS. Post-golgi vesicles co-transport docosahexanoyl-phospholipids and rhodopsin during frog photoreceptor membrane biogenesis. J Biol Chem. 1997;272:10491–10497. doi: 10.1074/jbc.272.16.10491. [DOI] [PubMed] [Google Scholar]
- Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: Integral membrane components. J Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz J. New Aspects of the ultrastructure of frog rod outer segments. Int Rev Cytol. 1977;50:25–15. doi: 10.1016/s0074-7696(08)60098-4. [DOI] [PubMed] [Google Scholar]
- Ryter A. Relationship between ultrastructure and specific functions of macrophages. Comp Immunol Microbiol Infect Dis. 1985;8:119. doi: 10.1016/0147-9571(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Sandberg MA, Weigel-DiFranco C, Dryja TP, Berson EL. Clinical expression correlates with location of rhodopsin mutation in dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1995;36:1934–1942. [PubMed] [Google Scholar]
- Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: Light microscopy. J Comp Neurol. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada M, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5′-3-O-(thio)triphosphate-sensitive localization of a G protein and its effect on detergent-resistant membrane rafts in rod photoreceptor outer segments. J Biol Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- Silverstein SC, Greenburg S, DiVirgilio F, Steinberg TH. Phagocytosis. In: Paul WP, editor. Fundamental Immunology. Raven Press; New York: 1989. p. 703. [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Sjostrand FS. Fine structure of cytoplasm. The organization of membrane layers. Rev Mod Physiol. 1959;31:301–318. [Google Scholar]
- Spitnas M, Hogan M. Outer segments of photoreceptors and retinal pigment epithelium. Arch Ophthalmol. 1970;84:810. doi: 10.1001/archopht.1970.00990040812022. [DOI] [PubMed] [Google Scholar]
- Stefano FP, Krouse J, Marta P, Boesze-Battaglia K. Heterologous expression of WT and mutant photoreceptor peripherin/rds in MDCK cells; an assessment of fusogenic function. Submitted for publication. Exp Eye Res. 2001 doi: 10.1006/exer.2001.1119. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Stenberg RH, Wood I, Hogan MJ. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Phil Trans R Soc Lond. 1977;277:459–474. doi: 10.1098/rstb.1977.0028. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Hollyfield JG. Outer segment disc membrane assembly in the absence of the pigment epithelium: The effect of exogenous sugars. Dev Brain Res. 1994;80:285–289. doi: 10.1016/0165-3806(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nature Genetics. 1997;17(1):15–6. doi: 10.1038/ng0997-15. [DOI] [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Tai AW. Rhodopsin trafficking and its role in retinal dystrophies. Int Rev Cytol. 2000;195:215–267. doi: 10.1016/s0074-7696(08)62706-0. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tarn MB, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster D. Are the COOH terminal regions for rod outer segment proteins potential targeting signals? Invest Opthalmol Vis Sci. 2001;42(4) Abstract #1974. [Google Scholar]
- Townes-Anderson E. Intersegmental fusion in vertebrate rod photoreceptors. Invest Opthamol Vis Sci. 1995;36:1918–1933. [PubMed] [Google Scholar]
- Travis GH, Brennan OL, Danielson PE, Kozak CA, Sutcliffe JG. Identification of a photorecepter-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Travis GH, Christerson L, Danielson PE, Klisak I, Sparkes RS, Hahn LB, Dryja TP, Sutcliffe JG. The human retinal degeneration slow (RDS) gene: Chromosome assignment and structure of the mRNA. Genomics. 1991a;10:733–739. doi: 10.1016/0888-7543(91)90457-p. [DOI] [PubMed] [Google Scholar]
- Travis GH, Groshan FR, Lloyd M, Bok D. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- Travis GH, Hepler JE. A medley of retinal dystrophies. Nat Genetics. 1993;3:191–192. doi: 10.1038/ng0393-191. [DOI] [PubMed] [Google Scholar]
- Travis GH, Sutcliffe JG, Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991b;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Yamada Y. Light-related morphological changes of outer segment membranes from lamellae to tubules and two kinds of wavy configurations in the frog visual cells. Exp Eye Res. 1982;34:675–694. doi: 10.1016/s0014-4835(82)80029-8. [DOI] [PubMed] [Google Scholar]
- Udovichenko IP, Gibbs D, Williams DS. Actin-based motor properties of native myosin VIIa. Submitted for publication. 2001 doi: 10.1242/jcs.115.2.445. [DOI] [PubMed] [Google Scholar]
- Ulshafer RJ, Allen CB, Fliesler SJ. Tunicamycin-induced dysgenesis of retinal rod outer segment membranes. I. A scanning electron microscopy study. Invest Ophthalmol Vis Sci. 1986;27:1587–1594. [PubMed] [Google Scholar]
- Urbani L, Simoni RD. J Biol Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- Usukura J, Bok D. Exp Eye Res. 1987;45:501–515. doi: 10.1016/s0014-4835(87)80061-1. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN. Circadian rhythms: Eyes of the clock. Curr Biol. 1998;8:798–801. doi: 10.1016/s0960-9822(07)00503-9. [DOI] [PubMed] [Google Scholar]
- Voelker DR. Microbiol Rev. 1991;55:543–560. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Soliner TH, Rothman JE. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson E, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD. Defective myosin VIIa gene responsible for Usher syndrome type 1. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Weng J, Belecky-Adams T, Adler R, Travis GH. Identification of two rds/peripherin homologs in the chick retina. Invest Ophthalmol Vis Sci. 1998;39:440–443. [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Wetzel MG, Bendala-Tufanisco E, Besharse JC. Tunicamycin does not inhibit transport of phosphatidylinositol to Xenopus rod outer segments. J Neurocytol. 1993;22:397–412. doi: 10.1007/BF01195560. [DOI] [PubMed] [Google Scholar]
- Wetzel MG, Besharse JC. Transport of phosphatidylcholine to Xenopus photoreceptor rod outer segments in the presence of tunicamycin. J Neurocytol. 1994;23:333–342. doi: 10.1007/BF01666523. [DOI] [PubMed] [Google Scholar]
- White J. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Whitehead JL, Wang SY, Bost-Usinger L, Hoang E, Frazer KA, Burnside B. Photoreceptor localization of the KIF3A and KIF3B subunits of the heterotrimeric microtubule motor kinesin II in vertebrate retina. Exp Eye Res. 1999;69:491–503. doi: 10.1006/exer.1999.0724. [DOI] [PubMed] [Google Scholar]
- Williams DS. Transport to photoreceptor outer segments by myosin VIIa and kinesin II. Vis Res. 2001 doi: 10.1016/s0042-6989(01)00228-0. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem. 2000;275:13191–13194. doi: 10.1074/jbc.c900853199. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Uehara F, Nakashima Y, Ozawa M, Muramatsu T, Ohba N. Demonstration of peripherin/rds mRNA in normal and light-damaged rat retinas by in situ hybridization histochemistry. Jpn J Ophthalmol. 1993;37:1–8. [PubMed] [Google Scholar]
- Yarfitz S, Hurley JB. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- Yeagle PL, Alderfer JL, Albert AD. Structure determination of the fourth cytoplasmic loop and carboxyl terminal domain of bovine rhodopsin. Mol Vis. 1996;2:12. [PubMed] [Google Scholar]
- Yeagle PL, Alderfer JL, Albert AD. Structure of the third cytoplasmic loop of bovine rhodopsin. Biochemistry. 1997;36:9649–9654. doi: 10.1021/bi970908a. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Passage of newly formed protein through the connecting cilium of retinal rods in the frog. J Ultrastruct Res. 1968;23:462–473. doi: 10.1016/s0022-5320(68)80111-x. [DOI] [PubMed] [Google Scholar]
- Young RW. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971;34:190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalomol Vis Sci. 1976;15:700–715. [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]


